Abstract
Charcot-Marie-Tooth neuropathy type 1 (CMT1) is an inherited demyelinating neuropathy characterized by distal muscle weakness and atrophy. Charcot-Marie-Tooth disease type 1C (CMT1C) is a rare form of CMT1 caused by mutations in the lipopolysaccharide-induced tumor necrosis factor (LITAF) or small integral membrane protein of the lysosome/late endosome (SIMPLE) gene. Phenotypically, CMT1C is characterized by sensory loss and slow conduction velocity, and is typically slowly progressive and often associated with pes cavus foot deformity and bilateral foot drop. A 42-year-old female presented with a 10-year history of slowly progressive bilateral calf pain and cramps. After multiple electromyography/nerve conduction studies (EMG/NCS) and genetic testing, the patient was revealed to have CMT1C with a heterozygous pathogenic variant, c.334G>A (p.Gly112Ser). However, the presentation of the patient's CMT1C phenotype was unusual compared to patients with similar diagnosis in a previous study, including a normal sensory exam with the exception of high arches and mildly reduced vibratory sense. Additionally, the patient's teenage son already started showing symptoms of CMT1C despite the fact that the onset of the disease typically occurs at an older age. This particular case further highlights the idea that the phenotype related to CMT1C may have a wide spectrum of disease severity.
Keywords: neurology, charcot-marie-tooth, pes cavus, demyelinating diseases, sensorimotor neuropathy, genetic mutation
Introduction
Charcot-Marie-Tooth disease type 1C (CMT1C) is a rare form of an inherited demyelinating neuropathy, Charcot-Marie-Tooth neuropathy type 1 (CMT1). It is caused by mutations in the lipopolysaccharide-induced tumor necrosis factor (LITAF) or small integral membrane protein of the lysosome/late endosome (SIMPLE) gene [1]. This autosomal dominant disease is marked by sensory loss and slow conduction velocity, and is typically slowly progressive and often associated with pes cavus foot deformity and bilateral foot drop [2]. However, given the rarity of this condition, with a prevalence of <1/1,000,000, the exact phenotype is not very well characterized.
Case presentation
A 42-year-old female presented with a 10-year history of slowly progressive bilateral calf pain and cramps. The patient denied paresthesias or weakness. She also denied a history of trauma in the area of the pain. She described the pain as a dull pain that limited her daily activities and reported similar complaints in her teenage son. To help with the pain, she had applied a topical cream and used compression stockings. She had also tried physical therapy but had not found it helpful.
Motor examination revealed posterior calf atrophy with normal bulk elsewhere. Examination showed normal muscle tone, strength, and reflexes in all extremities. The patient had decreased vibration sensation in her toes bilaterally with intact proprioception. Further examination revealed bilateral pes cavus with hammertoe.
A previous electromyography/nerve conduction study (EMG/NCS) performed in December of 2016 had suggested moderately-severe demyelinating sensorimotor polyneuropathy with conduction velocities ranging between 20-30 m/s. A repeat EMG/NCS was requested in 2018 as the patient’s exam did not fit the previous EMG/NCS findings. The repeat EMG/NCS showed slightly worse changes than the previous EMG/NCS study with moderate to severe demyelinating sensorimotor polyneuropathy with evidence of secondary axonal changes (Tables 1, 2).
Table 1. Sensory nerve conduction study showing moderate to severe demyelinating sensorimotor neuropathy with evidence of secondary axonal changes.
Lat: latency; Amp: amplitude; NR: not reported
| Nerve/Sites | Rec. site | Onset Lat | Peak Lat | NP Amp | PP Amp | Segments | Distance | Velocity | Temp. |
| ms | ms | µV | µV | cm | m/s | °C | |||
| R Ulnar - Digit V (Antidromic) | |||||||||
| Wrist | Dig V | 3.91 | 5.52 | 9.2 | 11.5 | Wrist - Dig V | 11 | 28 | |
| A.Elbow - Wrist | |||||||||
| R Radial - Anatomical Snuff Box (Forearm) | |||||||||
| Forearm | Wrist | 2.45 | 3.65 | 23.3 | 16.3 | Forearm - Wrist | 10 | 41 | |
| R Sural - Ankle (Calf) | |||||||||
| Calf | Ankle | NR | NR | NR | NR | Calf - Ankle | 14 | NR | 23.3 |
| R Superficial Peroneal - Ankle | |||||||||
| Lateral Leg | Ankle | NR | NR | NR | NR | Lateral Leg - Ankle | 14 | NR | 23.4 |
Table 2. Motor nerve conduction studies showing severe demyelinating sensorimotor neuropathy.
APB: abductor pollicis brevis; ADM: abductor digiti minimi; EDB: extensor digitorum brevis; AH: abductor hallucis; Tib Ant: tibialis anterior; NR: not reported
| Nerve/Sites | Muscle | Latency | Amplitude | Rel Amplitude | Duration | Segments | Distance | Lat Diff | Velocity | Temp. |
| ms | mV | % | ms | cm | ms | m/s | °C | |||
| R Median - APB | ||||||||||
| Wrist | APB | 4.69 | 1.5 | 100 | 5.73 | Wrist - APB | 7 | 23.6 | ||
| Elbow | APB | 14.27 | Elbow - Wrist | 24.5 | 9.58 | 26 | 23.7 | |||
| R Ulnar - ADM | ||||||||||
| Wrist | ADM | 5 | 5 | 100 | 11.56 | Wrist - ADM | 7 | 23.6 | ||
| B.Elbow | ADM | 11.61 | 2.9 | 58.4 | B.Elbow - Wrist | 19.5 | 6.61 | 29 | 23.6 | |
| A.Elbow | ADM | 14.22 | 3.1 | 105 | A.Elbow - B.Elbow | 9 | 2.6 | 35 | 23.4 | |
| A.Elbow - Wrist | 9.22 | 23.4 | ||||||||
| R Peroneal - EDB | ||||||||||
| Ankle | EDB | NR | NR | NR | NR | Ankle - EDB | 8 | 23.3 | ||
| Pop Fossa - Ankle | NR | |||||||||
| R Tibial - AH | ||||||||||
| Ankle | AH | 6.3 | 2.6 | 100 | 7.66 | Ankle - AH | 8 | 23.4 | ||
| Pop Fossa | AH | NR | Pop Fossa - Ankle | 23.4 | ||||||
| R Peroneal - Tib Ant | ||||||||||
| Fib Head | Tib Ant | 5.16 | 4.3 | 100 | 11.61 | Fib Head - Tib Ant | 12 | 23.4 | ||
| Pop Fossa | Tib Ant | 7.5 | 5.2 | 121 | 12.34 | Pop Fossa - Fib Head | 8 | 2.34 | 34 | 23.6 |
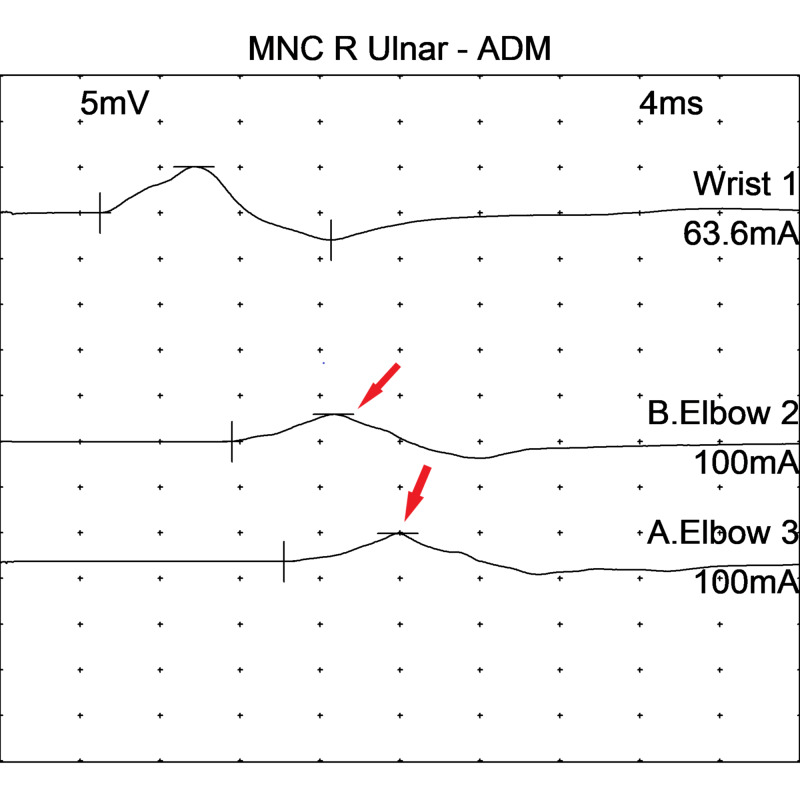
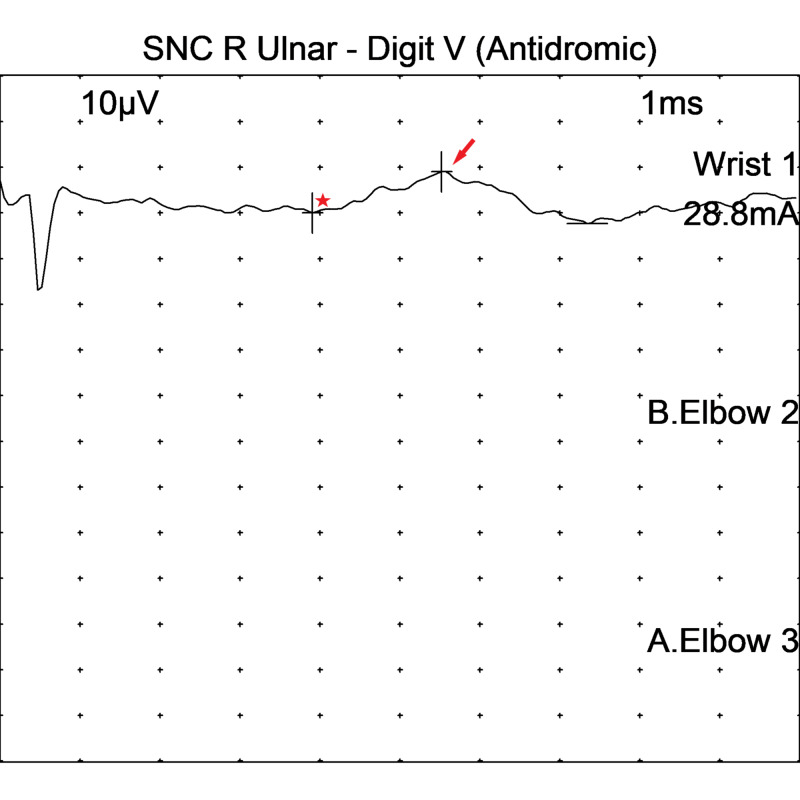
EMG findings on motor NCS in the right ulnar nerve are shown in Figure 1, while sensory NCS from the right ulnar nerve can be seen in Figure 2.
Figure 1. Motor nerve conduction studies in the right ulnar nerve - abductor digiti minimi showing decreased conduction velocities and a 2 mv drop in amplitude across the elbow (arrows).
MNC: motor nerve conduction; ADM: abductor digiti minimi
Figure 2. Sensory nerve conduction studies in the right ulnar sensory nerve showed prolonged peak latency (*) and decreased amplitude (arrow).
SNC: sensory nerve conduction
A repeat EMG/NCS performed one year later showed that previously present sensory responses were absent. The findings were suggestive of hereditary motor and sensory neuropathy. Furthermore, MRI and CT scans of the lower extremities were unremarkable.
Genetic testing using sequence analysis revealed a heterozygous pathogenic variant, c.334G>A (p.Gly112Ser) (rs104894519), in LITAF consistent with the diagnosis of CMT1C. Another heterozygous variant of uncertain significance, c.559C>T (p.Arg187Cys) (rs768929130, ExAC 0.2%), was also identified in the periaxin gene (PRX), which is associated with autosomal recessive Charcot-Marie-Tooth disease type 4F (CMT4F) [3]. The clinical significance of this second variant is uncertain at this time and is of uncertain significance. We further plan to arrange genetic testing for CMT1C in the patient’s children as her son has reported similar symptoms.
Discussion
A combination of EMG/NCS and genetic testing revealed that the patient presented to the clinic had CMT1C, a rare form of CMT1 marked by mutations in LITAF or the SIMPLE gene. In particular, the patient was characterized by a heterozygous pathogenic variant, c.334G>A (p.Gly112Ser), in LITAF. The LITAF gene encodes for an endosomal transmembrane protein, which is highly expressed in the myelinating Schwann cells [4]. The mutation is reported to result in mislocalization of the LITAF protein from the late endosome or lysosome to the mitochondria. The severity could also vary based on this degree of mislocalization [5]. CMT1C has been described as a mild form of neuropathy in a previous small case series, including in one of 10 patients with Gly112Ser mutations in LITAF [6]. The previous small case series report two distinguishable groups of CMT1C patients. The first group labeled as “CMT-like” incorporated patients resembling the CMT phenotype such as motor weakness, distal motor deficit, and diminished reflexes. The second group labeled as “sensory form” encompassed patients with predominantly sensory symptoms or asymptomatic complaints of mild transient paraesthesia, transient pain in feet or fingers, and cramps and repeated ankle sprains. The patients had either motor weakness, diminished reflexes, or an abnormal sensory exam. However, our patient had a normal exam with the exception of high arches and mildly reduced vibratory sense. Although the symptoms usually begin in childhood in the majority of the patients, some variability in age has been observed [1,7]. Our patient presented with the symptoms in the third decade. However, our patient’s son is beginning to show symptoms at the age of 13 years. Since the patient’s son is showing symptoms at such an early age, we plan to see him in our clinic to characterize his phenotype and send for genetic testing.
This article was previously presented as a meeting abstract at the 144th Annual Meeting of the American Neurological Association on 4/2/2019.
Conclusions
The phenotypic variation presented in our patient demonstrates additionalities of already known CMT1C-related phenotype and highlights the wide range of disease severity related to the phenotype of CMT1C. The case presented to our clinic also underlines the importance of early diagnosis for symptomatic treatment and the need for optimal genetic counseling.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Charcot-Marie-Tooth disease type 1C: clinical and electrophysiological findings for the c.334G>a (p.Gly112Ser) Litaf/Simple mutation. Jerath NU, Shy ME. Muscle Nerve. 2017;56:1092–1095. doi: 10.1002/mus.25600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird TD. GeneReviews. Seattle, WA: University of Washington, Seattle; 1998. Charcot-Marie-Tooth neuropathy X type 1. [Google Scholar]
- 3.A mutation in periaxin is responsible for CMT4F, an autosomal recessive form of Charcot-Marie-Tooth disease. Guilbot A, Williams A, Ravisé N, et al. Hum Mol Genet. 2001;10:415–421. doi: 10.1093/hmg/10.4.415. [DOI] [PubMed] [Google Scholar]
- 4.Motor and sensory neuropathy due to myelin infolding and paranodal damage in a transgenic mouse model of Charcot-Marie-Tooth disease type 1C. Lee SM, Sha D, Mohammed AA, Asress S, Glass JD, Chin LS, Li L. Hum Mol Genet. 2013;22:1755–1770. doi: 10.1093/hmg/ddt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LITAF mutations associated with Charcot-Marie-Tooth disease 1C show mislocalization from the late endosome/lysosome to the mitochondria. Lacerda AF, Hartjes E, Brunetti CR. PLoS One. 2014;9:0. doi: 10.1371/journal.pone.0103454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phenotypic spectrum of Charcot-Marie-Tooth disease due to LITAF/SIMPLE mutations: a study of 18 patients. Guimarães-Costa R, Iancu Ferfoglia R, Leonard-Louis S, et al. Eur J Neurol. 2017;24:530–538. doi: 10.1111/ene.13239. [DOI] [PubMed] [Google Scholar]
- 7.A novel LITAF/SIMPLE mutation within a family with a demyelinating form of Charcot-Marie-Tooth disease. Ciotti P, Luigetti M, Geroldi A, et al. J Neurol Sci. 2014;343:183–186. doi: 10.1016/j.jns.2014.05.029. [DOI] [PubMed] [Google Scholar]