Abstract
We investigated the influence of corn steep liquor (CSL) and cassava waste water (CWW) as carbon and nitrogen sources on the morphology and production of biomass and chitosan by Mucor subtilissimus UCP 1262 and Lichtheimia hyalospora UCP 1266. The highest biomass yields of 4.832 g/L (M. subtilissimus UCP 1262) and 6.345 g/L (L. hyalospora UCP 1266) were produced in assay 2 (6% CSL and 4% CWW), factorial design 22, and also favored higher chitosan production (32.471 mg/g) for M. subtilissimus. The highest chitosan production (44.91 mg/g) by L. hyalospora (UCP 1266) was obtained at the central point (4% of CWW and 6% of CSL). The statistical analysis, the higher concentration of CSL, and lower concentration of CWW significantly contributed to the growth of the strains. The FTIR bands confirmed the deacetylation degree of 80.29% and 83.61% of the chitosan produced by M. subtilissimus (UCP 1262) and L. hyalospora (UCP 1266), respectively. M. subtilissimus (UCP 1262) showed dimorphism in assay 4–6% CSL and 8% CWW and central point. L. hyalospora (UCP 1266) was optimized using a central composite rotational design, and the highest yield of chitosan (63.18 mg/g) was obtained in medium containing 8.82% CSL and 7% CWW. The experimental data suggest that the use of CSL and CWW is a promising association to chitosan production.
Keywords: agro-industrial wastes, biopolymer, Mucorales, morphology, pellets
1. Introduction
In recent times, chitosan is one of the most important biopolymers on the market, due to its properties such as biocompatibility, biodegradability, adsorption ability, and antimicrobial activity, with wide applications in several areas. However, special attention has been paid to the development of new processes and sustainable methods of synthesis of this polysaccharide [1].
The chitosan is a heterogeneous polysaccharide containing 2-amino-2-deoxy-D-glucose linked by β 1-4 bonds (glycosamine) and N-acetyl-2-amino-2-deoxy-D-glucose (N-acetyl-glycosamine), consisting of more than 60% of glycosamine [1,2]. Its cationic nature, chelating ion-binding capacity, protein immobilization, film and gel formation characteristics, chemical reactivity, modifiability, biocompatibility and antimicrobial activity are all suggested due to their heterogeneous structure and the presence of protonatable amino group of d-glycosamine [3,4,5].
Commercial chitosan is derived from the deacetylation of chitin from crustacean residues, where the process requires a strong alkaline treatment with high temperature due to the complex organization of chitin with other components in the exoskeleton crustacean, and is a slow process with a high cost, causing environmental pollution due to chemical treatments [6]. In addition, this raw material is seasonally and geographically limited and has highly variable characteristics, which may interfere with product yield and molecular product characteristics [5].
Filamentous fungi, mainly Mucoromycota, formerly belonging to a polyphiletic phylum Zigomycota [7], are known to synthesize natural chitosan in their cell wall, produced by enzymatic deacetylation of the chitin chain, a modification of sugar chains [6,8,9,10,11,12,13,14].
The production of chitosan from the fungal cell walls presents advantages over the traditional process for deacetylation of shrimp residues. The bioprocess of chitosan extraction does not require high temperatures and strong alkaline solutions using simple chemical procedures, and moreover has a low fungal biomass content which compared with inorganic materials compared to crustacean wastes. The chemical process used to crustacean require the demineralization as well as heavy metals such as nickel and copper contamination the environment. The chitosan-producing fungal species have approximately the same deacetylation of degree and lower viscosity than chitosan obtained from the deacetylation chitin from crustaceans [5,6,11,15]. In addition, fungal chitosan is produced in a controlled and seasonally independent environment [1,5,16].
The fungal cell wall suggested sustainable route for chitosan production using agroindustrial residues. This article evaluated chitosan production by Mucor subtilissimus UCP 1262 and Lichtheimia hyalospora (UCP 1266) cultivated by submerged fermentation using agroindustrial residues, cassava waste water, and corn steep liquor; alternative sources; as well as the effect on dimorphism. In addition, the chitosan produced were characterized in terms of their acetylation degree and viscosity, and their properties were compared with those of a commercial chitosan.
The microbiological bioprocess investigation of chitosan production by fungi was based on problems with commercial sources from shrimp and crabshell chitin deacetylated by strong alkali solution at high temperatures for long periods of time, being a source of seasonal and limited supply, as well as causing environmental pollution if inadequately discarded. Thus, it is of fundamental importance that Mucorales fungi order be used as natural chitin and chitosan sources, which is produced in their cell wall during their growth, for the purpose of the development, innovation, and generation of green technology using agro-industrial substrates, contributing to the reduction of these residues in the environment. The production of a bioactive biopolymer may partially satisfy a part of the need for innovative products in the biomedicine, pharmaceutical, and environmental areas, as it is a natural resource and has low toxicity, moreover guaranteeing positive impacts for the economy, as well as the possibility of a new process being applied to industry in the future.
2. Results and Discussion
2.1. Elemental Analysis of Cassava Waste Water (CWW) and Corn Steep Liquor (CSL)
The carbon, nitrogen, oxygen, and sulfur concentrations of CWW and CSL are shown in Table 1. CSL is the main source of carbon and nitrogen and with CWW provide essential nutrients for the growth of microorganisms.
Table 1.
Percentage of nitrogen, carbon, oxygen, and sulfur present in the composition of cassava waste water (CWW) and corn steep liquor (CSL).
| Substrate | Carbon | Nitrogen | Oxygen | Sulfur |
|---|---|---|---|---|
| Cassava Waste Water (CWW) | 33.35 | 2.04 | 6.74 | 0 |
| Corn Steep Liquor (CSL) | 34.84 | 7.06 | 6.59 | 1.18 |
2.2. Effect of Substrate Concentrations on Fungal Morphology
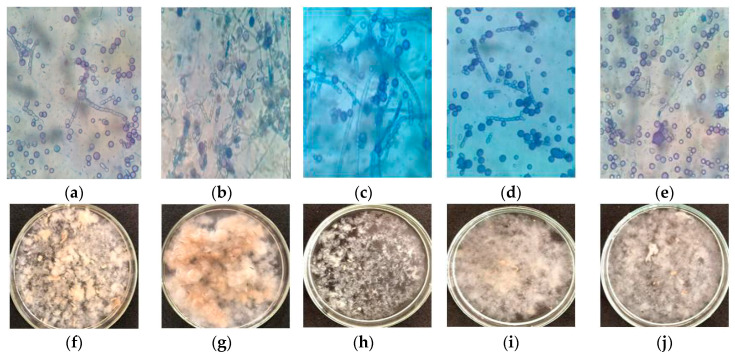
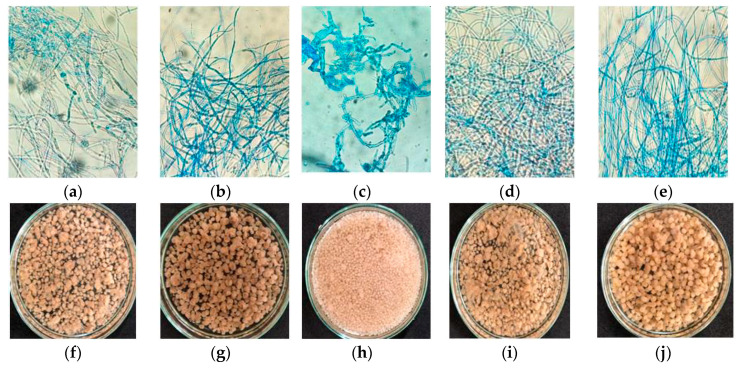
In this study, the effect of the concentrations of corn steep liquor (CSL) and cassava waste water (CWW) used in 22 factorial design (Table 2) on fungal morphology was evaluated. Microscopic and macroscopic images showed cellular differentiation in M. subtilissimus (UCP 1262) (Figure 1) and L. hyalospora (UCP 1266) (Figure 2).
Table 2.
Independents variables and levels used in the 22 full-factorial design for growth of M. subtilissimus and L. hyalospora.
| Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Corn Steep Liquor—CSL (%, v/v) | 2 | 4 | 6 |
| Cassava Waste Water—CWW (%, v/v) | 4 | 6 | 8 |
Minimum level (−1); intermediate level (0); Maximum level (+1).
Figure 1.
Morphological aspects of Mucor subtilissimus UCP 1262 cultivated in different concentrations of CSL and CWW according to 22 factorial design for 120 h. (a,f): Assay 1; (b,g): assay 2; (c,h): assay 3; (d,i): assay 4; (e,j): center point. Assay 1: 2% CSL and 4% CWW; assay 2: 6% CSL and 4% CWW; assay 3: 2% CSL and 8% CWW; assay 4: 6% CSL and 8% CWW; center point: 4% CSL and 6% CWW. Bars: 10 μm.
Figure 2.
Morphological aspects of Lichtheimia hyalospora UCP 1266 cultivated in different concentrations of CSL and CWW according to the 22 factorial design for 120 h. (a,f): Assay 1; (b,g): assay 2; (c,h): assay 3; (d,i): assay 4; (e,j): center point. Assay 1: 2% CSL and 4% CWW; assay 2: 6% CSL and 4% CWW; assay 3: 2% CSL and 8% CWW; assay 4: 6% CSL and 8% CWW; center point: 4% CSL and 6% CWW. Bars: 10 μm.
For M. subtilissimus (UCP 1262), the yeast phase was evident in assays 3 (Figure 1c) and 4 (Figure 1d) (higher concentrations of CWW, 8%). In the remaining assays, the transition phase (presence of globose cells, longs/shorts, and arthroposporous hyphae) was observed (Figure 1). In the assays 1 (Figure 1a) and point central (Figure 1e), yeast-like cells and fragments of short arthroposporous hyphae were observed. In assay 2 (Figure 1b), we observed yeast cells and long arthroposporous hyphae. In addition to mycelium formation, different filamentous fungal species can grow in yeast, depending on environmental conditions. This transition is called dimorphism [17].
Karimi and Zamani [18] stated that in addition to factors and mechanisms such as initial spore concentration, sugar concentration, and atmospheric factors evaluated in several studies [18,19,20,21,22], fungal morphology may be affected by the addition of certain chemical compounds. Among them, cyanide, acriflavine, and cycloheximide induce yeast morphology due to the inhibition of synthesis or action of cytochrome oxidase. These results can be corroborated in the current study, considering that CWW presents high cyanide concentrations (444.0 mg of cyanide per liter of CWW) [23,24].
In submerged cultivation with constant agitation, filamentous fungi exhibit three macroscopic morphologies—scattered mycelia, clumps (small pellets), and pellets (spherical masses of hyphae) [25,26]. However, due to the predominance of the yeast and transition phase, for M. subtilissimus (UCP 1262), the granular aspect was observed under experimental conditions (Figure 1f–j). In terms of the predominance of granular mycelium, a few areas of differentiation/fragmentation were observed in assays 1 (Figure 1f) and 2 (Figure 1g). Assay 3 (Figure 1i) and center point (Figure 1j) presented granular mycelium with fragmentation areas. Presence of scattered mycelia with significant presence of areas of differentiation and viscous liquid were observed in assay 3 (Figure 1h).
Unlike M. subtilissimus (UCP 1262), L. hyalospora (UCP 1266) showed no dimorphism. No microscopically relevant differences in agro-industrial waste concentrations were observed (Figure 2). L. hyalospora (UCP 1266) produced different sizes and shapes of pellets that differed with agro-industrial waste concentrations. Hollow pellets over 2 mm in diameter were predominant in all assays (Figure 2f–j), and microscopically with hyphae without cell lysis (Figure 2a–e), except assay 3 (Figure 2h; highest CWW level and lower CSL level), with compact and smooth pellets ranging from 1 to 2 mm in diameter and the presence of fragmented hyphae (Figure 2c). Large and hollow pellets have been observed in several studies [12,27,28,29], where it has been suggested that autolysis in the center of these clusters of hyphae is caused by difficulties in oxygen transfer.
According to [25], the composition of the medium can influence the pellet structure, as high concentrations of nitrogen induce pellet formation. Our results differ from those obtained by [30], who showed that CSL did not influence pellet formation in Penicillium chrysogenum. However, Metz and Kossen [25] reported that the type of fungal strain is an important factor influencing pellet formation.
2.3. Effect of Substrates on Biomass and Chitosan Production by M. subtilissimus (UCP 1262) and L. hyalospora (UCP 1266)
Although both fungi showed different patterns in macro-morphology, both showed high potentials in the assimilation of alternative sources of carbon and nitrogen.
Table 3 presents the comparative analysis of the results obtained for biomass and chitosan production in each 22 factorial design. The highest biomass yield produced by M. subtilissimus (UCP 1262; 4.832 mg/L) and L. hyalospora (UCP 1266; 6.540 mg/L) was in the culture medium supplemented with CSL 6% and CWW 4%, in which M. subtilissimus (UCP 1262) presented predominance of long and arthrosporous hyphae and L. hyalospora (UCP 1266) formed pellets of 4 to 5 mm in diameter. The result suggests that media with higher concentrations of CSL favor the growth of the fungi studied. Similar results were reported by Berger et al. (2014), when using the CSL and CWW in the culture medium for growth by Cunninghamella elegans. CSL is a carbohydrate- and amino acid-rich residue that favors the growth of filamentous and unicellular fungi [23,31,32].
Table 3.
Factorial design applied to production of biomass and chitosan by Mucor subtilissimus (UCP 1262) and Lichtheimia hyalospora (UCP 1266) using corn steep liquor (CSL) and wastewater (CWW) as alternatives substrates.
| Assays | Substrates | Mucor subtilissimus (UCP 1262) | Lichtheimia hyalospora (UCP 1266) | |||||
|---|---|---|---|---|---|---|---|---|
| CSL | CWW | pH | Biomass (g/L) | Chitosan (mg/g) | pH | Biomass (g/L) | Chitosan(mg/g) | |
| 1 | 2 | 4 | 7.1 | 1.725 | 18.87 | 5.8 | 2.675 | 23.31 |
| 2 | 6 | 4 | 6.2 | 4.832 * | 32.41 * | 5.2 | 6.540 * | 29.84 |
| 3 | 2 | 8 | 6.9 | 2.963 | 13.87 | 5.8 | 3.661 | 23.49 |
| 4 | 6 | 8 | 6.9 | 2.140 | 14.96 | 5.6 | 4.982 | 42.56 |
| 5 | 4 | 6 | 6.4 | 4.752 | 19.36 | 5.1 | 6.345 | 44.60 |
| 6 | 4 | 6 | 6.2 | 4.527 | 19.02 | 5.2 | 6.298 | 44.91 |
| 7 | 4 | 6 | 6.4 | 4.269 | 18.92 | 5.2 | 6.291 | 44.86 |
| 8 | 4 | 6 | 6.4 | 4.582 | 19.18 | 5.1 | 6.319 | 45.03 * |
(*) Maximum yield.
An increase in pH from 6.0 (beginning of fermentation) to 7.1–6.2 was observed in M. subtilissimus (UCP 1262) cultivation, a result similar to that of [23,33,34]. Lower pH resulted in higher biomass yields, in line with that described by [23].
However, at the end of L. hyalospora (UCP 1266) cultivation there was a decrease in pH from 6.0 (initial pH) to 5.1. Similar results were observed by [35,36]. Just as low pH favors fungal growth, it also favors chitin deacetylase activity, increasing chitin deacetylation in chitosan [37,38].
As for biomass production, assay 2 provided the best chitosan yields by M. subtilissimus (UCP 1262) (32.47 mg/g), unlike [23], which obtained the best chitosan yield by C. elegans in higher concentration of CWW and lower concentration of CSL [39] when cultivating C. elegans in medium, with higher concentration of corn steep liquor—CSL (7%) obtaining the best chitosan yield. The study by [40], when cultivating Syncephalastrum racemosum in higher concentrations of CSL (8%), obtained the highest yield of biomass and chitosan.
Similar to M. subtilissimus (UCP 1262), assay 2 (6% CSL and 4% CWW) provided the highest biomass production (6.540 g/L) by L. hyalospora (UCP 1266). However, it obtained the highest chitosan production (45.03 mg/g) at the central point of the fractional factorial design-FFD (4% of CSL and 6% of CWW). Previously, Rhizopus arrhizus obtained a higher production of the biopolymer also using 4% of CSL [41].
Higher concentrations of cassava waste water negatively influenced the chitosan production by M. subtilissimus (UCP 1262), as well as fungal morphology, predominantly forming individual spherical cells and budding multiplication. This yeast phase probably contributed negatively to chitosan production. This assumption is shown in Figure 1 in assay 3 (2% corn steep liquor and 8% cassava waste water) and assay 4 (6% of corn steep liquor and 8% cassava waste water). The influence of dimorphism on chitosan yield was also observed by [20], who detected in the cell wall of M. rouxii (now Rhizopus arrhizus) that the filament form had higher chitosan yields (9.4%) compared to the yeast phase, which had lower chitosan contents (8.4%).
The same condition that stimulated the M. subtilissimus (UCP 1262) yeast phase, resulting in low chitosan yields, influenced the formation of compact and smooth pellets by L. hyalospora (UCP 1266), also causing low biomass and chitosan yields. The best pellet diameter range for chitosan production was 4 to 5 mm, with a content of 45.03 g/kg of dry biomass. In the study of Sparringa et al. [42] the highest production of glycosamine (107 g/kg), obtained from chitosan deacetylated produced by Rhizopus oligosporus NRRL 2710, resulted in pellet formation with 16–35 mm. Sparringa and Owens [43] analyzed the pellet size of affected R. oryzae ATCC 20,344 glycosamine production. In addition, high glycosamine content (0.19 g/g) was obtained with pellet formation of 5.0 mm, and reduction to 1 mm negatively influenced glycosamine yield to 0.15 g/g.
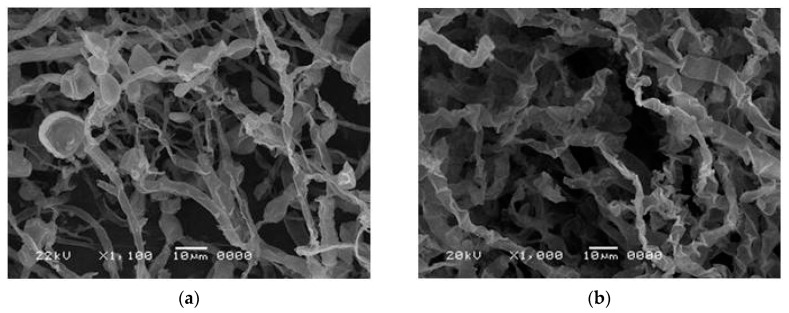
Ultrastructural aspects of biomasses with higher chitosan yields were also observed by scanning electron microscopy. Figure 3 shows the mycelial branches of the Mucor subtilissimus (UCP 1262) (6% CSL/4% CWW) with loose mycelium and hyphae with a thickness that was thinner, tubular, contorted, and with morphological aspect of yeast cells (Figure 3a) and Lichtheimia hyalospora (UCP 1266) (assay central point: 4% CSL/6% CWW) with compact mycelium hyphae with a thickness that was thinner, tubular and contorted. (Figure 3b). This study suggests that L. hyalospora (UCP 1266) mycelium fragmentation is a result of pellet formation. As other genera of the order Mucorales, they grow rapidly and have tubular-shaped hyphae and no septation. Liao et al. [44] observed morphological aspects of Rhizopus oryzae by SEM grown in agroindustrial wastes (soybean meal, wheat, and rice) to understand the relationship between morphology and production of glycosamine, lipids, and amino acids. According to [45], the culture medium composition and culture conditions influence the metabolic regulation and, consequently, the morphology. The increase in biomass and chitosan is a reliable indicator of the development of the studied fungi.
Figure 3.
Scanning electron micrograph of mycelium of Mucor subtilissimus (UCP 1262) and Lichtheimia hyalospora (UCP 1266). (a) M. subtilissimus (UCP 1262) after cultivation in assay 2 of the factorial design (CSL 6% and CWW 4%) and (b) L. hyalospora (UCP 1266) after cultivation in assay central point of the factorial design (CSL 4% and 6% CWW).
Table 4 presents the best results from various studies for biomass and chitosan production by Mucoralean fungi, which suggests that the chitosan content of the fungi depends on the fermentation time, culture medium, and cultivation conditions.
Table 4.
Comparison of biomass and chitosan produced by Mucor subtilissimus UCP 1262 and Lichtheimia hyalospora UCP 1266 cultivated on agro-industrial substrates with the results described by the literature on Mucoralean fungi.
| Fungal Strain | Medium Composition | Cultural Conditions | Biomass (g/L) | Chitosan (mg/g) | References |
|---|---|---|---|---|---|
| Mucor subtilissimus UCP 1262 | 6% CSL and 4% CWW | SmF, 28 °C, 150 rpm, 120 h | 4.83 | 32.41 | Present study |
| Lichtheimia hyalospora UCP 1266 | 4% CSL and 6% CWW | SmF, 28 °C, 150 rpm, 120 h | 6.298 | 44.91 | Present study |
| L. hyalospora UCP 1266 | 1% CSL and 25% papaya peel juice | SmF, 28 °C 150 rpm, 96 h | - | 12.04 | Kroll et al. [46] |
| Cunninghamella elegans UCP 0542 | 9.43% CSL and 42.5% papaya peel juice | SmF, 28 °C, 150 rpm, 96 h | - | 37.25 | Kroll et al. [46] |
| C. elegans UCP 0542 | 10% CWW and 4% CSL | SmF, 28 °C, 150 rpm, 72 h | 5.67 | 57.82 | Sharifia et al. [23] |
| Rhizopu sarrhizus UCP 0402 | 6% CSL and 13.24% honey | SmF, 28 °C, 150 rpm, 96 h | 11.71 | 29.30 | Berger et al. [32] |
| Syncephalastrum racemosum UCP 1302 | 8% CSL and 2% sugarcane bagasse | SSF, 28 °C, 96 h | 32.0 | 25.0 | Oliveira et al. [40] |
| Mucor circinelloides UCP 0050 | Yam bean medium | SmF, 28 °C, 150 rpm, 96 h | 20.7 | 64.00 | Berger et al. [47] |
| Mucor rouxii ATCC 24905 | Soybean meal | SSF, 25 °C, 144 h | - | 34.40 | Mondala et al. [5] |
| Rhizomucor miehei (ATCC 26282) | Sabouraud broth | SmF, 28 °C, 120 rpm, 168 h | 4.1 | 13.67 | Fai et al. [48] |
| Mucor racemosus | Sabouraud broth | SmF, 28 °C, 120 rpm, 168 h | 3.8 | 11.72 | Fai et al. [48] |
SmF: submerged fermentation; SSF: solid-state fermentation.
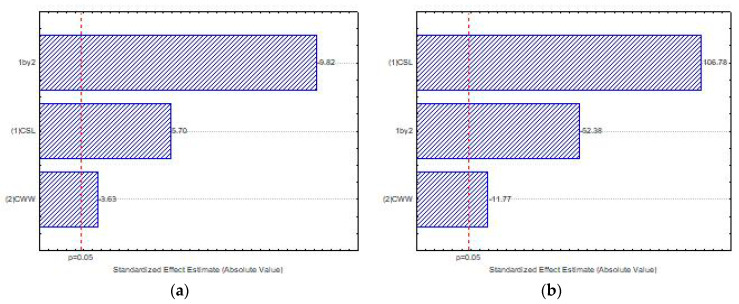
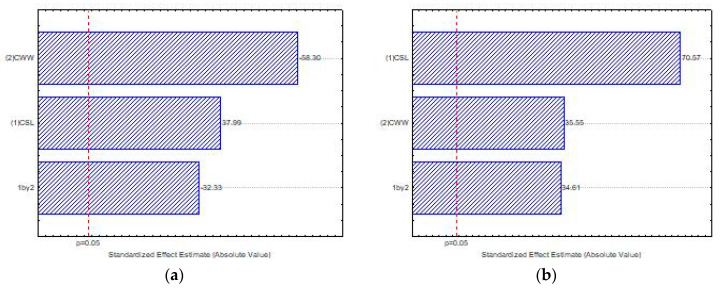
Figure 4 presents the influence of the independent variables, corn steep liquor—CSL (1) and cassava waste water-CWW (2), and the interaction between these variables (1 × 2) on biomass production by Mucor subtilissimus (UCP 1262) and Lichtheimia hyalospora (UCP 1266), using factorial design, with statistical significance of p < 0.05. The Pareto chart illustrates that the increase in the concentration of CSL (1) positively influenced the growth of the microorganisms. However, lower levels of CWW (2) were suggested for biomass production (Figure 4a,b). The interaction of the independent variables (1 × 2) showed that the maximum level of CSL (1) and the minimum level of CWW (2) had an antagonistic interaction, with significant influence on the biomass production in both microorganisms (Figure 4a,b).
Figure 4.
Pareto chart of standardized effects of corn steep liquor (CSL) (1) and cassava waste water (CWW) (2) on the biomass production by Mucor subtilissimus (UCP 1262) (a) and Lichtheimia hyalospora (UCP 1266) (b).
The Pareto graph (Figure 5a) proves that higher levels of the independent variable CSL (1) significantly influenced the chitosan production. However, lower levels of the CWW (2) significantly influenced the production of chitosan by M. subtilissimus (UCP 1262). The interaction of the independent variables (1 × 2) significantly influenced, antagonistically, the chitosan production by M. subtilissimus.
Figure 5.
Pareto chart of standardized effects of CSL (1) and CWW (2) on the chitosan production by Mucor subtilissimus (UCP 1262) (a) and Lichtheimia hyalospora (UCP 1266) (b).
The Pareto (Figure 5b) shows that higher concentrations of both independent variables—CSL and CWW—exhibited a significant influence on the chitosan production by L. hyalospora (UCP 1266). Consequently, the interaction of the variables (1 × 2) was significant, suggesting that the synergic effect of these substrates stimulated the biopolymer production.
2.4. Characterization of Chitosan Extracted from M. subtilissimus (UCP 1262) and L. hyalospora (UCP 1266)
Degree of Deacetylation
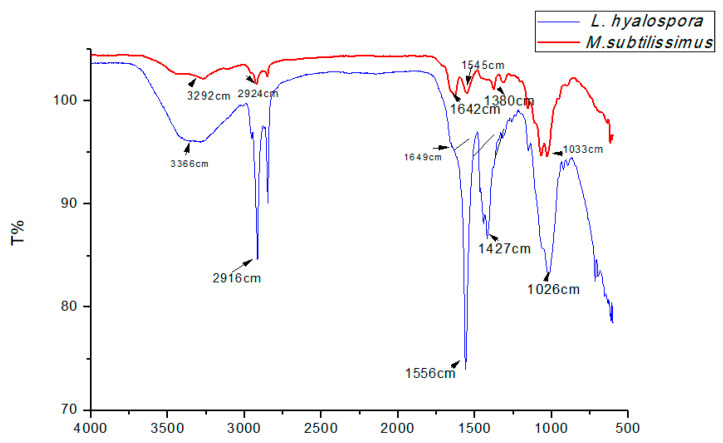
Chitosan extracted from the fungal biomass of M. subtilissimus (UCP 1262) and L. hyalospora (UCP 1266) were characterized by FTIR spectroscopy. The data obtained allowed the identification of extracted chitosan as well as the estimation of the degree of deacetylation (DD%), a fundamental parameter that influences the biological and physicochemical properties of the biopolymer. The degree of deacetylation of the fungal chitosan is shown in Table 5 and Figure 6. The amide I and amine bands of chitosans extracted from M. subtilissimus (UCP 1262) were found at 1642-1545 cm−1. In the case chitosan obtained from L. hyalospora (UCP 1266), the analysis from the FTIR spectrum showed that the amide I peak was observed at 1649 cm−1, and the amine peak was 1556 cm−1. The values are consistent with those reported by the literature [8,9,10,23,31].
Table 5.
Infrared spectroscopy, degree of deacetylation (DD%), and viscosity of chitosan samples produced by Mucor subtilissimus (UCP 1262) and Lichtheimia hyalospora (UCP 1266) in (CWW) and corn steep liquor (CSL).
| Biopolymers | Infrared Spectroscopy | Degree of Deacetylation (DD%) | Viscosity (cP) |
|---|---|---|---|
| Chitosan from L. hyalospora (UCP 1266) | 1649–1556 | 83.61 | 2.78 |
| Chitosan from M. subtilissimus (UCP 1262) | 1642–1545 | 80.28 | 3.06 |
Figure 6.
Infrared spectrum of chitosan produced by M.subtilissimus (UCP 1262) and L. hyalospora (UCP 1266).
As is observable from Figure 6, the bands at 1545 cm−1 (M. subtilissimus UCP 1262) and 1556 cm−1 (L. hyalospora UCP 1266) showed significant intensities, suggesting stable deacetylation in the chitosan of microorganisms. When chitin deacetylation occurs, the amide I band (C = O-NHR) decreases while amide II growth occurs, indicating the prevalence of NH2 groups [49]. According to [50], when the range of 1500–1700 cm−1 is stressed, it suggests an intensification of deacetylation. The infrared spectrum chitosan of L. hyalospora (UCP 1266) showed the amide bands to be the most significant.
The higher DD% fungal chitosan is more appropriate for food industrial application, principally when it is used as an antimicrobial [51].
The viscosity of chitosan of M. subtilissimus (UCP 1262) (3.06 cP) was considerably higher that the of L. hyalospora (UCP 1266) (2.78 cP). Similar results were obtained in studies of the viscosity of fungal chitosan, where the viscosity of fungal chitosan ranged from 1.02 to 2.67 cP [52,53,54]. The commercial chitosan, extracted from natural shellfish exoskeleton, may have a deacetylation degree of 80.0–95.0% and viscosity of 20–500 cP [54].
2.5. Optimization of Chitosan Production by L. hyalospora (UCP 1266)
Due to the results obtained from the previous factorial design, L. hyalospora (UCP 1266) was selected for a further DCCR 22, as it presented the highest chitosan productivity in the culture media with CSL and CWW. For this, the levels of CSL were increased and the higher levels of CWW around the central point of the previous factorial design were maintained. Since the studied concentrations may have allowed a higher activity of chitin deacetylase, the enzyme was responsible for the deacetylation of chitin in cell wall [55].
The results of the experiments that evaluated the influence of different concentrations of CSL and CWW on the production of biomass and chitosan by L. hyalospora (UCP 1266) are shown in Table 6.
Table 6.
Central composite rotatable design applied to the production of biomass and chitosan by L. hyalospora (UCP 1266) using CSL and CWW as alternatives substrates.
| Assays | CSL (%) | CWW (%) | pH | Biomass (g/L) | Chitosan (mg/g) | Chitosan (mg/L) |
|---|---|---|---|---|---|---|
| 1 | 4 | 6 | 5.4 | 6.32 | 44.88 | 283.64 |
| 2 | 4 | 8 | 5.6 | 5.59 | 46.83 | 261.78 |
| 3 | 8 | 6 | 5.4 | 9.34 | 51.98 | 485.49 |
| 4 | 8 | 8 | 5.4 | 8.79 | 54.03 | 474.92 |
| 5 | 3.17 | 7 | 5.8 | 5.48 | 46.89 | 256.96 |
| 6 | 8.82 | 7 | 4.8 | 11.87 | 63.18 | 749.95 |
| 7 | 6 | 5.58 | 5.4 | 7.81 | 48.09 | 375.58 |
| 8 | 6 | 8.41 | 5.4 | 7.26 | 57.81 | 437.04 |
| 9 | 6 | 7 | 4.8 | 9.14 | 58.46 | 534.32 |
| 10 | 6 | 7 | 5.1 | 9.09 | 58.71 | 533.67 |
| 11 | 6 | 7 | 5.2 | 8.91 | 58.42 | 520.52 |
| 12 | 6 | 7 | 4.8 | 9.19 | 58.90 | 541.29 |
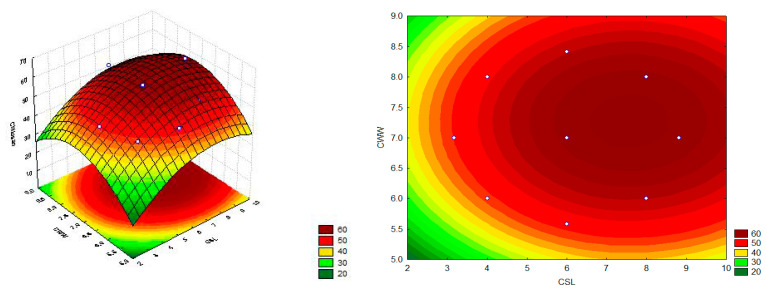
From the calculation of the coefficients, we obtained an equation adjusted to the experimental data, with the effects of the two independent variables x and y (CSL and CWW) on the production of chitosan Equation (1).
| ZChitosan(mg/g) = −191.65 + 11.04x − 0.73x2 + 57.81y – 3.97y2 + 0.012xy | (1) |
According to the response surface graph (Figure 7) and Equation (1), the highest chitosan production by L. hyalospora (UCP 1266) (63.18 mg/g) was achieved in medium containing the highest concentration of CSL (+1.41) and intermediate concentration of CWW (center point—0) (assay 6). The coefficient of determination (R2) of the obtained model was 0.85, showing good suitability of the experimental data.
Figure 7.
Response surface graphs of the chitosan production by L. hyalospora (UCP 1266) grown in medium formulated with CSL and CWW for 120 h at 28 °C.
The analysis of these results suggests a sustainable culture medium with concentrations of cassava waste water and corn steep liquor as nutritional sources of carbon and nitrogen for optimized production of biomass and chitosan by L. hyalospora UCP 1266. The use of low cost substrates as nutritional sources in culture media decreases the final value of the by-products, mainly in industrial production. Microbial biomass offers economic advantages for industrial scale production on chitosan obtained from crustacean shells, as it does not require high solvent amounts and high temperatures during the extraction process, in addition to obtaining the polysaccharide in a short time.
3. Materials and Methods
3.1. Microorganisms
M. subtilissimus UCP 1262 and L. hyalospora UCP 1266 were isolated from the soil of the city of São José do Belmonte (S 07° 51′37″ and W 038°45′35″; Pernambuco, Brazil). Fungi were identified and deposited in the Culture Collection of the Nucleus of Research in Environmental Sciences and Biotechnology, Catholic University of Pernambuco, Brazil, and registered in the World Federation Culture for Collection (WFCC). The strains were kept in Sabouraud agar medium (consisting of agar 20 g, peptone 10 g, glucose 40 g, distilled water 1000 mL, and pH adjusted to 6.0), at 5 °C.
3.2. Substrates
The substrates used for lipid and chitosan production were cassava waste water (CWW), kindly provided by the cassava processing plant located in the municipality of Carnaíba, Pernambuco, and corn steep liquor (CSL), obtained from Corn Products do Brasil Ltd. (Cabo de Santo Agostino, Pernambuco, Brazil). Aliquots of CSL were subjected to elemental analysis to determine the amounts of carbon, hydrogen, and nitrogen (%) using an EA 1110 analyzer (Carlo Erba Instruments). Table 1 shows the chemical composition of the cassava waste water (CWW) [23] and CSL.
3.3. Conditions of Culture and Biomass Production
The fungi were grown in Petri dishes (9 cm in diameter) containing Sabouraud agar medium, and were incubated at 28 °C for 5 days until sporulation. Then, the spores were transferred to Erlenmeyer flasks (250 mL) containing sterile distilled water to prepare a suspension of 107 spores/mL. Aliquots (1 mL) of these spore suspensions were used as inoculum and transferred to Erlenmeyer flasks (250 mL) with 100 mL of the media containing CSL and CWW, according to the factorial design (Table 1).
The flasks were incubated at 28 °C on an orbital shaker at 150 rpm for 120 h. At the end of this period, the pH of the media was terminated using a potentiometer. The biomass was centrifuged and washed with distilled water, and mycelia fragments were removed from the biomass, stained with blue cotton dye, and observed under the optical microscope. The remaining biomass was lyophilized and kept in a desiccator to determine the constant weight by gravimetry.
3.4. Morphological Analysis in Scanning Electron Microscopy (SEM)
The biomass was washed in phosphate-buffered saline (PBS), pH 7.2, and fixed with glutaraldehyde 2.5% in fosfate buffer, 0.1 M, pH 7.4, for 1 h at room temperature. In the post-fixation, malachite green 0.05% was solubilized in phosphate buffer for 1 h at room temperature in dark conditions. They were then subjected to the dehydration process with ethanol in proportions of 50%, 70%, 90%, and 100%. Samples were then placed on aluminum supports and analyzed by scanning electron microscopy (LSM JEOL 5600 LV).
3.5. Extration Chitosan
Chitosan was extracted as described by [56]. Briefly, the biomass was deproteinized with the addition of sodium hydroxide solution (1 M, 30 mL, v/v) and subjected to 121 °C for 15 min. The insoluble alkali fraction was separated by centrifugation (4000 rpm, 15 min). The remaining biomass was washed several times with saline solution (0.85%) and cold distilled water to reach pH 7.0. The obtained residue was treated with acetic acid (2%, 30 mL, v/v) for 15 min, 100 °C; centrifuged (4000 rpm); and filtered. The supernatant was alkalized to pH 9.0, stored at 4 °C for 24 h, and centrifuged (4000 rpm, 15 min) until chitosan precipitated. The chitosan was washed with cold distilled water and saline solution at pH 7.0 and then lyophilized.
3.6. Characterization of Chitosan
3.6.1. Infrared Spectroscopy (FTIR)
The chitosan was previously dried overnight at 60 °C under reduced pressure and homogenized with 100 mg potassium bromide (KBr). Discs prepared with potassium bromide were placed to dry for 24 h at 110 °C under reduced pressure. Infrared ray spectroscopy was performed using a Fourier transform (FTIR) BRUKER Mod. IFS. Discs with only potassium bromide were used as reference. The maximum intensity of the absorption bands was measured by the baseline.
3.6.2. Deacetylation Degree
The absorbance bands 1320 cm−1 and 1420 cm−1 were used to measure the degree of acetylation, according to [57], to later obtain the deacetylation degree of chitosan, as shown in the following Equation (2):
| DD% = A1320/A1420 = 0.3822 + 0.03133 | (2) |
where A1320 is absorbances of chitosan at 1320 cm−1, and A1420 is absorbances of chitosan 1420 cm−1.
3.6.3. Viscosity Determination
The viscosity of 1% chitosan in 1% acetic acid solution was determined using the automatic viscometer (Brookfield, Middleboro, MA, USA; TC 500).
3.7. Selection of Waste Concentrations in Culture Using Factory Design
To study the effect of the independent variables (CSL and CWW) and their interaction on the response variables (biomass and chitosan), 22 factorial design was performed, consisting of eight assays with four central points (Table 2).
3.8. Central Composite Rotational Design (CCRD) for Optimization of Chitosan Production by Lichtheimia hyalospora (UCP 1266)
To establish the optimal conditions for chitosan production by Lichtheimia hyalospora (UCP 1266), we performed a CCRD 22 to analyze the effects of CWW (5.58–8.41%; v/v) and CSL (3.58–6.41%; v/v) (independent variables). The experimental design consisted of 12 runs that included 4 repetitions at the center point. The 12 assays were prepared in standard order. In each experiment, we calculated the biomass and chitosan production. Inoculum, incubation period, and biomass yield are described in Section 3.3.
4. Conclusions
The results of this study showed the potential for Mucor subtilissimus (UCP 1262) and Lichtheimia hyalospora (UCP 1266) to convert agro-industrial residues (CWW and CSL) for biomass and chitosan production. The association of CSL and CWW are promising substrates that reduce the cost to obtain effective chitosanpolymer production from Mucoralean strains. The dimorphism negatively influenced the biomass and chitosan productions by M. subtilissimus (UCP 1262). However, this is the first time that the effect of cassava waste water (CWW) and corn steep liquor (CSL) levels on the presence of budding cells, yeast-like cells, and arthropod short hyphae has been described. The big pellets’ diameter ranges were related to higher chitosan yields by L. hyalospora (UCP 1266). The investigation with Mucoralean strains showed a quality source for chitosan that was produced by L. hyalospora (UCP 1266), as well as the degree of deacetylation and its ease of manipulation in the laboratory.
Acknowledgments
We thank the Nucleus of Research in Environmental Sciences and Biotechnology, Catholic University of Pernambuco, Brazil, and Center of Strategic Technologies of the Northeast (CETENE), Brazil, for the use of their laboratories.
Author Contributions
G.M.d.C.-T. conceived the study. G.M.d.C.-T., N.B.G., and A.F.d.S. designed the experiments. A.F.d.S., H.M.G., D.M.R., R.F.d.S.A., and M.A.B.d.L. performed the experiments. A.F.d.S., N.B.G., and G.M.d.C.-T. interpreted the data analysis. M.A.B.d.L., A.F.d.S., and D.R.R. performed microscopy analysis. G.M.d.C.-T. contributed reagents and materials. A.F.d.S., N.B.G., and G.M.d.C.-T. wrote the manuscript. All authors read, reviewed, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Council for Scientific and Technological Development (CNPq)-under process no. 314422/2018-8, the Coordination for the Improvement of Higher Level Education Personnel (CAPES) under processes D.R.R. and R.F.S.A., and the Foundation for the Support of Science and Technology of the State of Pernambuco (FACEPE) process no. APQ-0291-2.12/15.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tyliszczak B., Drabczyk A., Kudłacik-Kramarczyk S., Sobczak-Kupiec A. Sustainable Production: Novel Trends in Energy, Environment and Material Systems. Springer; Cham, Switzerland: 2020. Sustainable Production of Chitosan; pp. 45–60. [Google Scholar]
- 2.Kaur S., Dhillon G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014;40:155–175. doi: 10.3109/1040841X.2013.770385. [DOI] [PubMed] [Google Scholar]
- 3.Dutta P.K., Dutta J., Tripathi V.S. Chitin and Chitosan: Chemistry, Properties and Applications. J. Sci. Ind. Res. 2004;63:20–31. [Google Scholar]
- 4.Badawy M.E., Rabea E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011;2011:460381. doi: 10.1155/2011/460381. [DOI] [Google Scholar]
- 5.Mondala A., Al-Mubarak R., Atkinson J., Shields S., Young B., Senger Y.D.S., Pekarovic J. Direct Solid-State Fermentation of Soybean Processing Residues for the Production of Fungal Chitosan by Mucorrouxii. J. Mater. Sci. Chem. Eng. 2015;3:11. [Google Scholar]
- 6.Vaingankar P.N., Juvekar A.R. Fermentative production of mycelial chitosan from zygomycetes: Media optimization and physico-chemical characterization. Adv. Biosci. Biotechnol. 2014;5:940. doi: 10.4236/abb.2014.512108. [DOI] [Google Scholar]
- 7.Spatafora J.W., Chang Y., Benny G.L., Lazarus K., Smith M.E., Berbee M.L., James T.Y. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia. 2016;108:1028–1046. doi: 10.3852/16-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amorim R.V.S., Souza W., Fukushima K., Campos-Takaki G.M. Faster chitosan production by Mucorelean strains in submerged culture. Braz. J. Microbiol. 2001;32:20–23. doi: 10.1590/S1517-83822001000100005. [DOI] [Google Scholar]
- 9.Naghdi M., Zamani A., Karimi K. A sulfuric-lactic acid process for efficient purification of fungal chitosan with intact molecular weight. Int. J. Biol. Macromol. 2014;63:158–162. doi: 10.1016/j.ijbiomac.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Amorim R.V.S., Ledingham W.M., Kennedy J.F., Campos-Takaki G.M. Chitosan from Syncephalastrum racemosum using sugar cane substrates as inexpensive carbon sources. Food Biotechnol. 2006;20:43–53. doi: 10.1080/08905430500524028. [DOI] [Google Scholar]
- 11.Akila R.M. Fermentative production of fungal Chitosan, a versatile biopolymer (perspectives and its applications) Adv. Appl. Sci. Res. 2014;5:157–170. [Google Scholar]
- 12.Satari B., Karimi K., Zamani A. Oil, chitosan, and ethanol production by dimorphic fungus Mucorindicus from different lignocelluloses. J. Chem. Technol. Biotechnol. 2016;91:1835–1843. doi: 10.1002/jctb.4776. [DOI] [Google Scholar]
- 13.Zininga J.T., Puri A.K., Govender A., Singh S., Permaul K. Concomitant production of chitosan and lipids from a newly isolated Mucor circinelloides ZSKP for biodiesel production. Bioresour. Technol. 2019;272:545–551. doi: 10.1016/j.biortech.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Saini S., Dhania G. Bioremediation of Industrial Waste for Environmental Safety. Springer; Singapore: 2020. Cadmium as an Environmental Pollutant: Ecotoxicological Effects, Health Hazards, and Bioremediation Approaches for Its Detoxification from Contaminated Sites; pp. 357–387. [Google Scholar]
- 15.Tan S.C., Tan T.K., Wong S.M., Khor E. The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr. Polym. 1996;30:239–242. doi: 10.1016/S0144-8617(96)00052-5. [DOI] [Google Scholar]
- 16.White S.A., Farina P.R., Fulton I. Production and isolation of chitosan from Mucorrouxii. Appl. Environ. Microbiol. 1979;38:323–328. doi: 10.1128/AEM.38.2.323-328.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Herrera J. Fungal Cell Wall: Structure, Synthesis, and Assembly. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 18.Karimi K., Zamani A. Mucorindicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013;31:466–481. doi: 10.1016/j.biotechadv.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Bartnicki-Garcia S., Nickerson W. JInduction of yeastlike development in Mucor by carbon dioxide. J. Bacteriol. 1962;84:829–840. doi: 10.1128/JB.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartnicki-Garcia S., Nickerson W.J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucorrouxii. Biochim. Biophys. 1962;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- 21.Bartnicki-Garcia S. Control of dimorphism in Mucor by hexoses: Inhibition of hyphal morphogenesis. J. Bacteriol. 1968;96:1586–1594. doi: 10.1128/JB.96.5.1586-1594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharifia M., Karimi K., Taherzadeh M.J. Production of ethanol by filamentous and yeast-like forms of Mucorindicus from fructose, glucose, sucrose, and molasses. J. Ind. Microbiol. Biotechnol. 2008;35:1253–1259. doi: 10.1007/s10295-008-0422-x. [DOI] [PubMed] [Google Scholar]
- 23.Berger L., Stamford T., Stamford-Arnaud T., de Oliveira Franco L., do Nascimento A., Cavalcante H., de Campos-Takaki G. Effect of corn steep liquor (CSL) and cassava waste water (CWW) on chitin and chitosan production by Cunninghamella elegans and their physicochemical characteristics and cytotoxicity. Molecules. 2014;19:2771–2792. doi: 10.3390/molecules19032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araújo N.C.D., Lima V.L.A., Ramos J.G., Andrade E.M.G., Lima G.S.D., Oliveira S.J.C. Contents of macronutrients and growth of ‘BRS Marataoã’cowpeafertigated with yellow water and cassava waste water. Environ. Water. 2019;14 doi: 10.4136/ambi-agua.2309. [DOI] [Google Scholar]
- 25.Metz B., Kossen N.W.F. The growth of molds in the form of pellets—A literature review. Biotechnol. Bioeng. 1977;19:781–799. doi: 10.1002/bit.260190602. [DOI] [Google Scholar]
- 26.Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004;22:189–259. doi: 10.1016/j.biotechadv.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Kim W.J., Lee W.G., Theodore K., Chang H.N. Optimization of culture conditions and continuous production of chitosan by the fungi, Absidiacoerulea. Biotechnol. Bioprocess Eng. 2001;6:6–10. doi: 10.1007/BF02942243. [DOI] [Google Scholar]
- 28.Gow N.A., Gadd G.M., editors. Growing Fungus. Chapman & Hall; London, UK: 2007. [Google Scholar]
- 29.Sitanggang A.B., Wu H.S., Wang S.S., Ho Y.C. Effect of pellet size and stimulating factor on the glucosamine production using Aspergillus sp. BCRC 31742. Bioresour. Technol. 2010;101:3595–3601. doi: 10.1016/j.biortech.2009.12.084. [DOI] [PubMed] [Google Scholar]
- 30.Pirt S.J., Callow D.S. Continuous-flow culture of the filamentous mould Penicillium chrysogenum and the control of its morphology. Nature. 1959;184:307–310. doi: 10.1038/184307a0. [DOI] [PubMed] [Google Scholar]
- 31.Berger L.R.R., Cardoso A., Stamford T.C.M., Cavalcante H.M.M., Macedo R.O., Campos-Takaki G.M. Agroindustrial waste as alternative medium in the production of chitin and chitosan by Rhizopus arrhizus—A factorial design. Asian Chitin J. 2011;7:83–90. [Google Scholar]
- 32.Souza A., Rodriguez D., Ribeaux D., Luna M., Lima e Silva T., Andrade R., Campos-Takaki G. Waste soybean oil and corn steep liquor as economic substrates for bioemulsifier and biodiesel production by Candida lipolytica UCP 0998. Int. J. Mol. Sci. 2016;17:1608. doi: 10.3390/ijms17101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardoso A., Lins C.I.M., dos Santos E.R., Silva M.C.F., Campos-Takaki G.M. Microbial enhance of chitosan production by Rhizopusarrhizus using agroindustrial substrates. Molecules. 2012;17:4904–4914. doi: 10.3390/molecules17054904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardoso A., Marques A., Campos Marinho P.H., Shiosaki R.K., Campos Takaki G.M. Microorganisms in Industry and Environment: From Scientific and Industrial Research to Consumer Products. World Scientific; Singapore: 2011. Influence of the heavy metals on chitosan production by Absidiacorymbifera UCP 0134; pp. 176–180. [Google Scholar]
- 35.Santos E., da Silva M., de Souza P., da Silva A., de Paiva S., Albuquerque C., Campos-Takaki G. Enhancementof Cunninghamella elegans UCP/WFCC 0542 biomassa and chitosanwith amino acidsupply. Molecules. 2013;18:10095–10107. doi: 10.3390/molecules180910095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger L., Stamford T., Stamford-Arnaud T., de Alcântara S., da Silva A., da Silva A., Campos-Takaki G. Green conversion of agroindustrial wastes into chitin and chitosan by Rhizopusarrhizus and Cunninghamella elegans strains. Int. J. Mol. Sci. 2014;15:9082–9102. doi: 10.3390/ijms15059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nwe N., Chandrkrachang S., Stevens W.F., Maw T., Tan T.K., Khor E., Wong S.M. Production of fungal chitosan by solid state and submerged fermentation. Carbohydr. Polym. 2002;49:235–237. doi: 10.1016/S0144-8617(01)00355-1. [DOI] [Google Scholar]
- 38.Zhao Y., Park R.D., Muzzarelli R.A. Chitin deacetylases: Properties and applications. Mar. Drugs. 2010;8:24–46. doi: 10.3390/md8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira C.E.V., Magnani M., de Sales C.V., de Souza Pontes A.L., Campos-Takaki G.M., Stamford T.C.M., de Souza E.L. Effects of chitosan from Cunninghamella elegans on virulence of post-harvest pathogenic fungi in table grapes (Vitislabrusca L.) Int. J. Food Microbiol. 2014;171:54–61. doi: 10.1016/j.ijfoodmicro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Leite M.V., Stamford T.C., Stamford-Arnaud T.M., Lima J.M.N., Silva A.M., Okada K., Campos-Takaki G.M. Conversion of agro-industrial wastes to chitosan production by Syncephalastrum racemosum UCP 1302. Int. J. Appl. Res. Nat. Prod. 2008;8:5–11. [Google Scholar]
- 41.Lins C.I.M., Cardoso A., Silva M.C.F., Batista A.C.L., Jara A.M.A.T., Berger L.R.R., Campos-Takaki G.M. Evaluation of chitin and chitosan by different extraction methods from mucoralean fungi biomass. Asian Chitin J. 2010;6:5–8. [Google Scholar]
- 42.Sparringa R.A., Owens J.D. Glucosamine content of tempemould, Rhizopusoligosporus. Int. J. Food Microbiol. 1999;47:153–157. doi: 10.1016/S0168-1605(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 43.Liao W., Liu Y., Frear C., Chen S. Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material–dairy manure–using a pelletized filamentous fungus Rhizopusoryzae ATCC 20344. Bioresour. Technol. 2008;99:5859–5866. doi: 10.1016/j.biortech.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Denardi-Souza T., Massarolo K.C., Tralamazza S.M., Badiale-Furlong E. Monitoring of fungal biomass changed by Rhizopusoryzae in relation to amino acid and essential fatty acids profile in soybean meal, wheat and rice. CyTA-J. Food. 2018;16:156–164. doi: 10.1080/19476337.2017.1359676. [DOI] [Google Scholar]
- 45.Kroll K., Paehtz V., Kniemeyer O. Elucidating the fungal stress response by proteomics. J. Proteom. 2014;97:151–163. doi: 10.1016/j.jprot.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Berger L.R.R., Stamford T.C.M., de Oliveira K.Á.R., Pessoa A.D.M.P., de Lima M.A.B., Pintado M.M.E., de Souza E.L. Chitosan produced from Mucorales fungi using agroindustrial by-products and its efficacy to inhibit Colletotrichum species. Int. J. Biol. Macromol. 2018;108:635–641. doi: 10.1016/j.ijbiomac.2017.11.178. [DOI] [PubMed] [Google Scholar]
- 47.Fai A.E.C., Stamford T., Stamford-Arnaud T.M., Santa-Cruz P.D., Silva M.C., Campos-Takaki G.M., Stamford T.L. Physico-chemical characteristics and functional properties of chitin and chitosan produced by Mucorcircinelloides using yam bean as substrate. Molecules. 2011;16:7143–7154. doi: 10.3390/molecules16087143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tajdini F., Amini M.A., Nafissi-Varcheh N., Faramarzi M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucormiehei and Mucorracemosus. Int. J. Biol. Macromol. 2010;47:180–183. doi: 10.1016/j.ijbiomac.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Kumari S., Rath P.K. Extraction and characterization of chitin and chitosan from (Labeorohit) fish scales. Procedia Mater. Sci. 2014;6:482–489. doi: 10.1016/j.mspro.2014.07.062. [DOI] [Google Scholar]
- 50.Zvezdova D. Synthesis and characterization of chitosan from marine sources in Black Sea. Annu. Proc. Angel Kanchev Univ. Ruse. 2010;49:65–69. [Google Scholar]
- 51.Dakia P.A., Blecker C., Robert C., Wathelet B., Paquot M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocoll. 2008;22:807–818. doi: 10.1016/j.foodhyd.2007.03.007. [DOI] [Google Scholar]
- 52.Tayel A.A., Moussa S., Opwis K., Knittel D., Schollmeyer E., Nickisch-Hartfiel A. Inhibition of microbial pathogens by fungal chitosan. Int. J. Biol. Macromol. 2010;47:10–14. doi: 10.1016/j.ijbiomac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Dhillon G.S., Kaur S., Sarma S.J., Brar S.K. Integrated process for fungal citric acid fermentation using apple processing wastes and sequential extraction of chitosan from waste stream. Ind. Crops Prod. 2013;50:346–351. doi: 10.1016/j.indcrop.2013.08.010. [DOI] [Google Scholar]
- 54.Yang L., Li X., Lai C., Fan Y., Ouyang J., Yong Q. Fungal chitosan production using xylose rich of corn stoverprehydrolysate by Rhizopusoryzae. Biotechnol. Biotechnol. Equip. 2017;31:1160–1166. doi: 10.1080/13102818.2017.1370678. [DOI] [Google Scholar]
- 55.Latha S., Suresh G. Studies on chitosan production from different fungal mycelium. Growth. 2013;564:189. [Google Scholar]
- 56.Hu K.J., Hu J.L., Ho K.P., Yeung K.W. Screening of fungi for chitosan producers, and copper adsorption capacity of fungal chitosan and chitosanaceous materials. Carbohydr. Polym. 2004;58:45–52. doi: 10.1016/j.carbpol.2004.06.015. [DOI] [Google Scholar]
- 57.Brugnerotto J., Lizardi J., Goycoolea F.M., Argüelles-Monal W., Desbrieres J., Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001;42:3569–3580. doi: 10.1016/S0032-3861(00)00713-8. [DOI] [Google Scholar]