Abstract
Tumorigenesis is correlated with abnormal expression and activity of G protein-coupled receptors (GPCRs) and associated G proteins. Oncogenic mutations in both GPCRs and G proteins (GNAS, GNAQ or GNA11) encoding genes have been identified in a significant number of tumors. Interestingly, uveal melanoma driver mutations in GNAQ/GNA11 were identified for a decade, but their discovery did not lead to mutation-specific drug development, unlike it the case for BRAF mutations in cutaneous melanoma which saw enormous success. Moreover, new immunotherapies strategies such as immune checkpoint inhibitors have given underwhelming results. In this review, we summarize the current knowledge on cancer-associated alterations of GPCRs and G proteins and we focus on the case of uveal melanoma. Finally, we discuss the possibilities that this signaling might represent in regard to novel drug development for cancer prevention and treatment.
Keywords: uveal melanoma, GNA mutations, G proteins, GPCR, therapy
1. Introduction
The G protein-coupled receptor (GPCR) family of proteins comprises more than 800 members and their seven-transmembrane domain structure allows, after binding to their ligands, the activation of heterotrimeric G proteins, which generates second messengers, as well as kinase cascades activation in the cytoplasm of the cells. These signals ultimately control gene transcription, cell survival, motility, and growth. It is nowadays clearly established that signals transmitted by GPCR/G proteins and downstream targets are involved in the initiation and progression of cancer. For example, overactivation of pathways such as AKT/mTOR, MAPK and Hippo will lead to altered cell growth and survival. In addition, invasion of cancer cells will be favored by GPCR-regulated RHO GTPases activation and changes in the cytoskeleton. GPCR/G proteins have also been described to play a role in angiogenesis and organization of tumor microenvironment.
Therefore, our knowledge of how GPCR/G proteins are responsible for the development of cancer transformation is crucial to identify new therapeutic targets. In this review, we describe first the G protein-encoding gene alterations that have been identified in malignancies. Second, we focus on the role of alterations in GNAQ/11 specifically in uveal melanoma. We finally discuss the opportunities offered by altered GPCR/G protein signaling in cancer to develop rational treatment strategies for patients with advanced uveal melanoma.
2. Heterotrimeric G Proteins
G proteins are guanine-nucleotide-binding proteins, which play a key role in signal transduction. When bound to GTP, G proteins are active, however an intrinsic GTPase activity allows their inactivation in the GDP-bound status. These heterotrimeric G proteins consist of α-, β- and γ-subunits. The activation of G proteins involves several mechanisms including the stimulation of seven-transmembrane domain receptors (GPCRs), of tyrosine kinases receptors (TKRs) or the activation of guanine-nucleotide exchange factors (GEFs) [1].
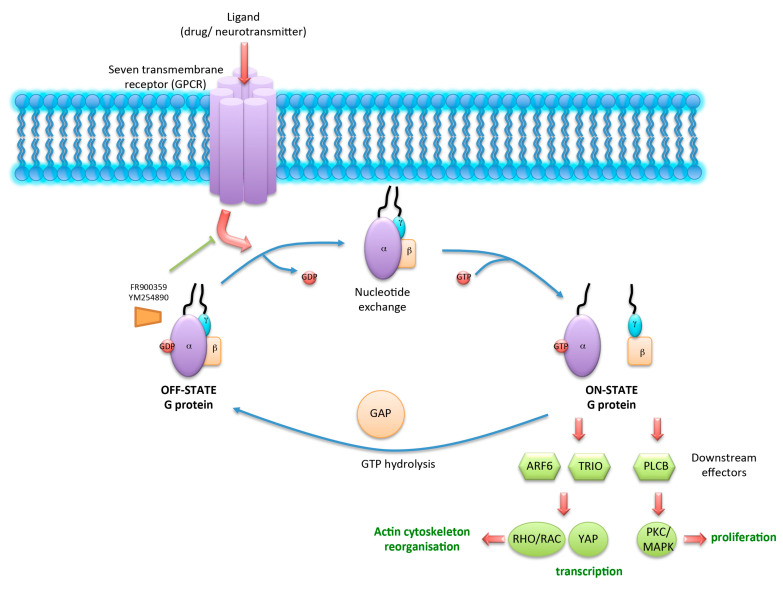
In the case of GPCRs stimulation and after conformation change of the receptors, the Gα unit will load GTP instead of GDP, leading to its release from the Gβγ unit and from the receptor. GTP-bound Gα and Gβγ will subsequently activate their cognate effectors. Activated G proteins will transmit the signal from several hormones and control many cell functions, including transcription, motility and secretion. This process is tightly regulated temporally and spatially, and leads to the activation of a panel a multiple G protein-specific targets (Figure 1). For example, ARF6 (ADP-ribosylation factor 6), TRIO, and PLCβ (phospholipase C β) represent downstream effectors which activate cellular pathways such as RHO/RAC (RAS-related C3 botulinum toxin) or YAP (yes-associated protein), which are involved in actin cytoskeleton reorganization. PKC (protein kinase C)/MAPK can be activated by PLCβ and controls cell proliferation [2,3]. Therefore, GPCRs are considered as molecular rheostats and represent potential targets for therapeutic inhibition [4].
Figure 1.
Schematic of G protein activation after G protein-coupled receptors (GPCR) binding to its ligand. Ligand-activated GPCR allows the release of GDP from OFF-STATE G proteins. “Empty pocket“ will be refilled promptly with GTP. This results in the disassembly of Gα from Gβγ subunits (ON-STATE) and activation of downstream effectors such as ARF6, TRIO, and PLCβ. Cellular pathways such as RHO/RAC or YAP are involved in actin cytoskeleton reorganization and cell growth. PKC/MAPK controls cell proliferation. GTPase function leads to GTP hydrolysis and reformation of the inactive heterotrimer. This step is regulated by GTPase-activating proteins (GAPs). FR and YM inhibitors block G protein signaling by preventing GDP release.
3. Mutations in Genes Encoding G Proteins
The GNA gene family contains several members, which encode for G proteins including GNAS (a complex locus that encodes Gαs subunits), GNA11 (encoding Gα11 subunits), and GNAQ (encoding Gαq subunits). Oncogenic mutations in these genes usually impair their GTPase activity, leading to constitutively active forms of GTP-bound proteins and to extended downstream signaling. For example, in the context of McCune–Albright syndrome, an active form of Gαs promotes cellular hyperproliferation [5]. Nonetheless, it was recently suggested that Gαs gain-of-function mutation can bypass the need for GTP binding and directly activate GDP-bound Gαs [6].
3.1. GNAS Mutants
Based on deep sequencing studies, it is known that mutations in GNAS occur in a wide range of tumors. Table 1 represents the frequency of alterations in this gene which were found in the The Cancer Genome Atlas TCGA PanCan 2018 [7,8] (Table 1). Most frequently mutated entities (approximately 10%) were colorectal, stomach and uterine cancers. Least mutated (<1%) were glioma, lymphoma and germinal cell cancers. With a 4% rate, GNAS is the most frequently altered G protein-encoding gene in human tumors. Most of these mutations were identified on two particular hotspot regions—R201 and Q227 [9,10,11].
Table 1.
Frequency of GNA alterations in cancers.
| Cancers | GNAS | |||||
|---|---|---|---|---|---|---|
| Alteration | Mutation | Fusion | Amplification | Deep deletion | Multiple Alterations | |
| Colorectal | 11.45% of 594 cases | 3.87% (23 cases) | 0.17% (1 case) | 7.24% (43 cases) | 0.17% (1 case) | |
| Stomach | 9.55% of 400 cases | 5.45% (24 cases) | 3.64% (16 cases) | 0.23% (1 case) | 0.23% (1 case) | |
| Uterine | 9.07% of 529 cases | 7.18% (38 cases) | 1.89% (10 cases) | |||
| Lung adeno | 7.77% of 566 cases | 3.71% (21 cases) | 3.89% (22 cases) | 0.18% (1 case) | ||
| Esophagus | 7.69% of 182 cases | 4.95% (9 cases) | 2.75% (5 cases) | |||
| Melanoma | 7.21% of 444 cases | 6.08% (27 cases) | 1.13% (5 cases) | |||
| Pancreas | 7.07% of 184 cases | 4.89% (9 cases) | 0.54% (1 case) | 1.63% (3 cases) | ||
| Sarcoma | 7.06% of 255 cases | 1.57% (4 cases) | 5.49% (14 cases) | |||
| Uterine CS | 7.02% of 57 cases | 3.51% (2 cases) | 3.51% (2 cases) | |||
| ACC | 6.59% of 91 cases | 5.49% (5 cases) | 1.1% (1 case) | |||
| BICB | 6.18% of 1084 cases | 1.01% (11 cases) | 0.37% (4 cases) | 4.61% (50 cases) | 0.18% (2 cases) | |
| Ovarian | 5.14% of 584 cases | 0.86% (5 cases) | 0.17% (1 case) | 3.94% (23 cases) | 0.17% (1 case) | |
| Cervical | 4.38% of 297 cases | 3.03% (9 cases) | 0.34% (1 case) | 1.01% (3 cases) | ||
| Bladder | 3.41% of 411 cases | 2.68% (11 cases) | 0.49% (2 cases) | 0.24% (1 case) | ||
| Head & Neck | 2.68% of 523 cases | 2.49% (13 cases) | 0.19% (1 case) | |||
| Lung squ | 2.46% of 487 cases | 1.85% (9 cases) | 0.21% (1 case) | 0.21% (1 case) | 0.21% (1 case) | |
| Liver | 2.42% of 372 cases | 1.34% (5 cases) | 1.08% (4 cases) | |||
| PCPG | 1.69% of 178 cases | 1.12% (2 cases) | 0.56% (1 case) | |||
| Thyroid | 1.2% of 500 cases | 0.8% (4 cases) | 0.4% (2 cases) | |||
| Mesothelioma | 1.15% of 87 cases | 1.15% (1 case) | ||||
| pRCC | 1.06% of 283 cases | 1.06% (3 cases) | ||||
| Prostate | 0.81% of 494 cases | 0.61% (3 cases) | 0.2% (1 case) | |||
| Testicular germ cell | 0.67% of 149 cases | 0.67% (1 case) | ||||
| ccRCC | 0.59% of 511 cases | 0.59% (3 cases) | ||||
| LGG | 0.58% of 514 cases | 0.19% (1 case) | 0.39% (2 cases) | |||
| GBM | 0.51% of 592 cases | 0.51% (3 cases) | ||||
| AML | 0.5% of 200 cases | 0.5% (1 case) | ||||
| Cholangiocarcinoma | ||||||
| DLBC | ||||||
| Kidney Chromophobe | ||||||
| Thymoma | ||||||
| Uveal melanoma | ||||||
| GNAQ | ||||||
| Alteration | Mutation | Fusion | Amplification | Deep deletion | Multiple Alterations | |
| Uveal melanoma | 50% of 80 cases | 50% (40 cases) | ||||
| Uterine | 3.97% of 529 cases | 2.84% (15 cases) | 0.38% (2 cases) | 0.76% (4 cases) | ||
| Melanoma | 3.38% of 444 cases | 3.38% (15 cases) | ||||
| Stomach | 2.5% of 440 cases | 0.91% (4 cases) | 0.23% (1 case) | 1.36% (6 cases) | ||
| Esophagus | 2.2% of 182 cases | 0.55% (1 case) | 0.55% (1 case) | 0.55% (1 case) | 0.55% (1 case) | |
| DLBC | 2.08% of 48 cases | 2.08% (1 case) | ||||
| Bladder | 1.95% of 411 cases | 0.73% (3 cases) | 0.24% (1 case) | 0.97% (4 cases) | ||
| Uterine CS | 1.75% of 57 cases | 1.75% (1 case) | ||||
| Sarcoma | 1.57% of 255 cases | 1.57% (4 cases) | ||||
| Colorectal | 1.52% of 594 cases | 1.35% (8 cases) | 0.17% (1 case) | |||
| Lung adeno | 1.41% of 566 cases | 0.88% (5 cases) | 0.53% (3 cases) | |||
| Ovarian | 1.37% of 584 cases | 0.68% (4 cases) | 0.68% (4 cases) | |||
| ACC | 1.1% of 91 cases | 1.1% (1 case) | ||||
| Pancreas | 1.09% of 184 cases | 0.54% (1 case) | 0.54% (1 case) | |||
| GBM | 1.01% of 592 cases | 0.17% (1 case) | 0.68% (4 cases) | 0.17% (1 case) | ||
| Cervical | 1.01% of 297 cases | 0.67% (2 cases) | 0.34% (1 case) | |||
| BICB | 1.01% of 1084 cases | 0.28% (3 cases) | 0.09% (1 case) | 0.18% (2 cases) | 0.37% (4 cases) | 0.09% (1 case) |
| Lung squ | 0.82% of 487 cases | 0.41% (2 cases) | 0.41% (2 cases) | |||
| Liver | 0.81% of 372 cases | 0.27% (1 case) | 0.27% (1 case) | 0.27% (1 case) | ||
| Thymoma | 0.81% of 123 cases | 0.81% (1 case) | ||||
| Head & Neck | 0.76% of 523 cases | 0.38% (2 cases) | 0.38% (2 cases) | |||
| Testicular germ cell | 0.67% of 149 cases | 0.67% (1 case) | ||||
| PCPG | 0.56% of 178 cases | 0.56% (1 case) | ||||
| AML | 0.5% of 200 cases | 0.5% (1 case) | ||||
| pRCC | 0.35% of 283 cases | 0.35% (1 case) | ||||
| Thyroid | 0.2% of 500 cases | 0.2% (1 case) | ||||
| Prostate | 0.4% (2 cases) | |||||
| LGG | ||||||
| Cholangiocarcinoma | ||||||
| Kidney Chromophobe | ||||||
| ccRCC | ||||||
| Mesothelioma | ||||||
| GNA11 | ||||||
| Alteration | Mutation | Fusion | Amplification | Deep deletion | Multiple Alterations | |
| Uveal melanoma | 46.25 % of 80 cases | 45% (36 cases) | 1.25% (1 case) | |||
| Sarcoma | 5.88% of 255 cases | 0.39% (1 case) | 0.39% (1 case) | 3.53% (9 cases) | 1.57% (4 cases) | |
| Cervical | 4.71% of 297 cases | 1.01% (3 cases) | 1.35% (4 cases) | 2.36% (7 cases) | ||
| Melanoma | 4.05% of 444 cases | 3.83% (17 cases) | 0.23% (1 case | |||
| Esophagus | 3.3% of 182 cases | 1.1% (2 cases) | 2.2% (4 cases) | |||
| Ovarian | 2.91% of 584 cases | 0.51% (3 cases) | 2.4% (14 cases) | |||
| Uterine | 2.84% of 529 cases | 1.89% (10 cases) | 0.95% (5 cases) | |||
| LGG | 2.33% of 514 cases | 0.19% (1 case) | 2.14% (11 cases) | |||
| Lung adeno | 1.59% of 566 cases | 0.71% (4 cases) | 0.18% (1 case) | 0.71% (4 cases) | ||
| Colorectal | 1.52% of 594 cases | 1.01% (6 cases) | 0.51% (3 cases) | |||
| Bladder | 1.46% of 411 cases | 0.73% (3 cases) | 0.73% (3 cases) | |||
| BICB | 1.29% of 1084 cases | 0.46% (5 cases) | 0.18% (2 cases) | 0.65% (7 cases) | ||
| Prostate | 1.21% of 494 cases | 0.2% (1 case) | 0.2% (1 case) | 0.81% (4 cases) | ||
| GBM | 1.18% of 592 cases | 0.17% (1 case) | 0.84% (5 cases) | 0.17% (1 case) | ||
| Mesothelioma | 1.15% of 87 cases | 1.15% (1 case) | ||||
| PCPG | 1.12% of 178 cases | 1.12% (2 cases) | ||||
| Pancreas | 1.09% of 184 cases | 0.54% (1 case) | 0.54% (1 case) | |||
| Liver | 0.81% of 372 cases | 0.27% (1 case) | 0.54% (2 cases) | |||
| Thymoma | 0.81% of 123 cases | 0.81% (1 case) | ||||
| Head & Neck | 0.57% of 523 cases | 0.38% (2 cases) | 0.19% (1 case) | |||
| AML | 0.5% of 200 cases | 0.5% (1 case) | ||||
| Lung squ | 0.41% of 487 cases | 0.21% (1 case) | 0.21% (1 case) | |||
| ccRCC | 0.39% of 511 cases | 0.39% (2 cases) | ||||
| ACC | ||||||
| Cholangiocarcinoma | ||||||
| DLBC | ||||||
| Kidney Chromophobe | ||||||
| pRCC | ||||||
| Stomach | ||||||
| Testicular germ cell | ||||||
| Thyroid | ||||||
| Uterine CS | ||||||
Data were obtained in cBioportal from the studies TCGA PANCAN2018 (cerami et al. 2012; gao et al. 2013) [12,24]. AML: Acute Myeloid Leukemia, ACC: Adrenocortical Carcinoma, LGG: Brain Lower Grade Glioma, DLBC: Diffuse Large B-Cell Lymphoma, GBM: Glioblastoma Multiforme, ccRCC: Kidney Renal Clear Cell Carcinoma, pRCC: Kidney Renal Papillary Cell Carcinoma, PCPG: Pheochromocytoma and Paraganglioma, BICB: Breast Invasive Carcinoma Breast.
Interestingly, GNAS is described as a driver oncogene in a subset of colon adenomas and adenocarcinomas [12], and mutations in this gene can promote hyperplasia of endocrine cells in thyroid and pituitary tumors [9]. In addition, Gαs was shown to regulate inflammatory mediators such as cyclooxygenase 2 (COX2)-derived prostaglandins [13]. Since COX2 has a known protumorigenic role in colon neoplasia, oncogenic mutations in GNAS may therefore activate a proinflammatory response, which favors tumor development [14,15].
3.2. GNAQ and GNA11 Mutants
GNAQ and GNA11 genes are two paralogs and share 90% sequence homology. GNAQ and GNA11 mutations are mutually exclusives and occur in about 2% of human cancers. Mutations in GNAQ and GNA11 were mostly identified at residues involved in GTPase activity (Q209 or R183) [16,17]. Apart from being found in the meninges (59%), in blue naevi (83%), and in a subset of cutaneous melanomas linked to chronic sun-induced damage (3–4%), GNAQ or GNA11 represent driver oncogenes in around 50% uveal melanoma [17,18,19]. Details on the role of these mutations in uveal melanoma will be discussed in the next paragraph.
3.3. Other GNA Mutants
Several mutations in other Gα encoding genes have been identified at a much lower frequency in human cancers and might not be activating. Therefore, their exact consequence is not yet known. As an example, GNA15 (encoding Gαq subunits) is significantly mutated in skin melanomas that do not often carry GNAQ or GNA11 mutations [20].
Interestingly, very few mutations on Gβ and Gγ subunits have been identified compared to Gα subunits. However, mutations in GPCRs encoding genes were identified in almost 20% human cancers. Although many of these mutations are still uncharacterized regarding the impact on tumorigenesis, the most frequent were found in the thyroid-stimulating hormone receptor (TSHR), smoothened (SMO), glutamate metabotropic receptors (GRMs), the lysophosphatidic acid receptor (LPA) and the sphingosine-1-phosphate (S1P) receptor. More details on mutations in GPCRs encoding genes are described in the review of Kan et al. [21]. At the molecular level, the Hippo-YAP pathway was described to be essential to tumorigenesis downstream the activation of GPCRs [2,22]. A CRISPR/Cas9-based systematic analysis showed that cell sensitivity to the morphogen Sonic Hedgehog (SHH) can be regulated by GPCR-induced signals (i.e., SMO), leading to GNAS and PKA activation [23].
In sum, hotspot mutations in GNAS, GNAQ and GNA11 have been identified in human tumors; however, a better characterization of the mutations on the other G protein-encoding genes is still needed. Nevertheless, common signaling between G proteins and their activating GPCRs could represent therapeutic opportunities for cancer treatment.
4. Recently Described Roles of G Protein Mutations in Tumorigenesis
Cellular migration and motility was recently described as a new role of mutations of G proteins. Cervantes-Villagrana and colleagues demonstrated that Gαq and Gα13 proteins can directly regulate the signaling of Gβγ to PREX1 [25]. Since RAC and RHO GTPases exert opposing effects, the authors dissected their respective activating signalings via Gα12/13 or Gαq. By means of pharmacological inhibition, they showed that Gαq activates PREX1 more efficiently under the activation of lysophosphatidic acid receptors. Moreover, by using different Gα variants in which GTPase activity was lost, they could observe the formation of stable complexes with Gβγ and a prevention of its downstream interaction with PREX1. In sum, the authors identified a new mechanism of the prioritization of RHO over RAC mediated by Gαq and Gα13.
Another study showed that Giα2 plays a role in the cell migration downstream of PI3K/AKT and RAC1 [26]. Silencing of Giα2 in pancreatic cancer cells reduced the migration dependent on TGFβ, oxytocin, SDF-1α, and EGF. In addition, silencing of Giα2 in cells that overexpressed active RAC1 abolished their migration without affecting the basal RAC1 activation.
Metabolism of pancreas and in particular lipid reprogramming has recently been described to play a key role in GNAS-driven pancreatic tumorigenesis [27]. Indeed, genetically engineered mouse models could show cooperation between GnasR201C and KrasG12D mutations to promote the initiation of intraductal papillary mucinous neoplasms (IPMNs), the latter progressing into pancreatic ductal adenocarcinomas (PDAs) after additional Tp53 loss. The authors not only observed an essential role of mutant Gnas in tumor maintenance but also a mechanism of protein kinase A-mediated suppression of salt-inducible kinases (Sik1-3), in association with lipid remodeling.
As increased intracellular ROS levels are known to induce cell cycle arrest, senescence, and apoptosis, the deregulation of ROS levels may lead to tumorigenesis. Hydrogen peroxide, and superoxide are the main products of NADPH oxidase (NOX enzymes) and are considered as signaling molecules [28]. Interestingly, angiotensin II receptor 1 (AT1R) has been shown to activate NOX1 likely via Gαq and PLCβ which activate PKC [29]. Evidence showed that thrombin can increase NOX1-dependent ROS generation by mechanisms involving PAR4 or EGFR transactivation [24,30,31]. Several studies have reported an upregulation of NOX4 in response to GPCR ligands such as angiotensin II (AT-II), urotensin-II, β-adrenergic agonists, renin and thrombin [32,33,34,35,36,37]. Interestingly, a direct link between GPCR and NOX4 transcription was described and involved transcription factors HIF1 and FoxO3a [35,38]. Similar to NOX1, NOX4-dependent ROS generation activated by AT-II involves Gαq/11, PLC activation, an increase in intracellular calcium levels and activation of PKC [39]. GPCR-induced cytosolic calcium levels are expected to regulate NOX5-dependent ROS generation. Thrombin, AT-II and endothelin 1 were reported to activate NOX5 by a mechanism which involved calmodulin recruitment and CAMKII activation [40,41,42,43]. NOX5 was also shown to be induced by bile acid activation of the TGR5 receptor and Gα [44,45]. GPCR can also generate ROS via small G-proteins (RAC) and activate JAK /STAT-dependent transcription [46].
Nevertheless, the precise identification of which G-protein is involved in GPCR-mediated ROS production is still unclear. A better understanding is expected in the near future based on the current engagement in G-protein targeted drug discovery.
Epigenetic mechanisms such as GNA gene promoter hypermethylation was proposed as a promising biomarker for hepatocellular carcinoma (HCC) diagnosis and targeted therapy [47]. Downregulation of GNAO1 is associated with the early process of HCC and is a consequence of its promoter’s methylation, as observed by 5-Aza-2’-deoxycytidine (DAC) and trichostatin A (TSA) treatment. Moreover, this mechanism seems to be regulated by methyltransferase 1 (DNMT1).
Interestingly, a new study based on clinical data and next-generation sequencing (NGS) on a cohort of 1348 patients with a wide range of cancers presented the most frequent coalterations in the presence of GNA alterations (GNAS, GNAQ, and GNA11) to be in AURKA, SRC, CBL and LYN genes [48]. In sum, multiple recent studies indicate a key role of GNA mutations in processes such as cell migration, promoter hypermethylation and ultimately tumorigenesis.
5. G protein Mutations in Uveal Melanoma
The Cancer Genome Atlas (TCGA) has classified cutaneous melanoma tumors based on the most frequent genetic subtypes. Four main groups have been proposed as follows: mutant BRAF, mutant RAS, mutant NF1, and triple BRAF/RAS/NF1 wildtype (which includes mutations in other genes such as GNA).
Several recent studies bring evidence supporting a key role of GNA mutations specifically in the transformation of the melanocyte lineage. For example, several zebrafish models of uveal melanoma have shown that melanocyte-specific expression of driver mutations GNAQ/GNA11(Q209L) led to considerable changes in the melanocyte biology of the fish [49]. Moreover, the additional loss of tumor suppressor Tp53 induced the development of melanocytic tumors including uveal melanoma, with almost complete penetrance. As observed in human uveal melanoma, the authors could find a nuclear localization of YAP, which could lead to transcription of genes involved in tumor growth [2,3]. Finally, they observed hyperpigmentation and altered melanocyte migration and survival, independently of Tp53. In a mouse model of leptomeningeal melanocytic neoplasms, the inducible expression of Gnaq(Q209L) variant at the neural crest stage before melanocyte differentiation, could favor the development of blue nevus-like lesions in the dermis, various melanocytic neoplasms in the cranium and spine but also melanoma of the central nervous system [50]. Interestingly, the authors observed different phenotypes depending on the time window of mutant expression or depending on several melanocyte precursor-specific promoters used to express it. Therefore they conclude that melanocytes become sensitive to the oncogenic effect of GNAQ(Q209L) only during certain temporary phases of their development. Thus, these results suggest an essential role of GNAQ mutations in the tumorigenesis of the melanocyte lineage.
Nevertheless, these data do not explain the GNAQ/GNA11 selective mutational pressure observed in uveal melanoma compared to cutaneous melanoma or why they are considered to be oncogenic [17,19]. Uveal melanoma is a subtype of melanoma which develops from melanocytes located in the eye, and its incidence is generally much lower than that of cutaneous melanoma [51]. GNAQ and GNA11 mutations were amplified by real-time PCR in the circulating DNA from the plasma of 22 patients with uveal melanoma, and Q209 mutations were detected by ultradeep sequencing in 9 of these [52]. More recently, the identification of additional somatic mutations in uveal melanoma which could not be detected by classical NGS led to a tumor classification in four molecular and clinical subgroups that will allow a stratification of uveal melanoma patient management. Moreover, “secondary” driver mutations in the GNA pathway have also been detected in uveal melanoma at low frequencies, but might account for tumor development and progression [53,54]. Of note, a retrospective study identified 18 patients with metastatic GNAQ/11 mutant nonuveal melanoma, which showed lower tumor mutational burden and fewer ultraviolet signature mutations than cutaneous melanomas. In contrast to uveal melanoma, these tumors frequently metastasized lymphatically and concurrent mutations (EIF1AX, SF3B1 and BAP1) were not associated with patient prognosis. The authors concluded that primary GNAQ/11 mutant nonuveal melanoma is a subtype of melanoma that is clinically and genetically distinct from both cutaneous and uveal melanoma [55].
The selective mutational rate in uveal melanoma compared to cutaneous melanoma is not well understood. One hypothesis could be a particular oncogenic activity of these mutations in ocular melanocytes, since most cutaneous melanocytes tend to become senescent when carrying GNA mutations. Indeed, as discussed above, GNAQ or GNA11 are mutated in nearly 83% blue naevi [17,19].
In uveal melanoma, hot spot somatic mutations in GNAQ/GNA11 lead to amino acid substitutions in exon 5 (p.Q209L or p.Q209P) or in exon 4 (p.R183C). While the first group of mutations prevents the intrinsic GTPase activity and therefore constitutively activates the protein, the second group leads to only a partial loss of GTPase activity.
In the first described uveal melanoma mouse model, an oncogenic Gnaq mutant under the control of the Rosa26 promoter was conditionally expressed by the cre recombinase under the control of melanocyte specific-promoter Mitf, and could initiate tumors which progressed to intravasation and metastases in 100% offspring [56]. In addition, the YAP protein (Hippo pathway) could play a role in this process. Interestingly, the overexpression of mutant Gnaq(Q209L) led to a loss of cutaneous melanocytes in adult mice, which could explain why this mutation is not found in cutaneous melanoma.
In another mouse model, melanocyte-specific expression of Gna11(Q209L) led to pigmented neoplastic lesions from melanocytes of the skin and noncutaneous organs, including the eye and leptomeninges [57]. Additional loss of Brca-1-associated protein (Bap1) increased the size of cutaneous melanoma tumors. BAP1 is a chromatin-associated deubiquitinase, and the function of which is involved in DNA double-strand breaks repair [58,59]. However, BAP1-inactivating mutations appear in 40% of uveal melanoma. The author also identified RasGRP3 expression specifically in Gnaq/Gna11-driven melanoma and observed that RasGRP3 is required for Gnaq/Gna11-driven RAS activation.
Interestingly, other members in the GNA signaling have also been identified with mutations specifically in uveal melanoma. One example is the GPCR cysteinyl leukotriene receptor 2 (CYSLTR2), a member of the rhodopsin-like family that responds to purinergic or pyrimidinergic nucleotides (P2Ys) [60]. Recurrent mutations on codon 129 and 136 have been identified in uveal melanoma, but only the first one is oncogenic [61]. CYSLTR2 is activated by lipid mediators called leukotrienes, which can not only induce a strong cutaneous melanocyte proliferation but also induce cancer [62,63]. The phospholipase C (PLC) family represents one of the downstream targets of GNA proteins. The role of phospholipases is to hydrolyze PIP2 into the following second messengers: IP3, which controls intracellular calcium concentration and DAG, which activates PKC (protein kinase C), which in turn activates MAPK to control cell proliferation. Mutations in members of this family, PLCB4 and PLCB3, were identified in patient-derived uveal melanoma samples [61,64,65]. However, their exact function as driver oncogenes is not yet clear. Another example of the downstream target is the identification of TRIO (GEF) in a genome-wide RNAi screen being essential in the signal transduction from GNAQ to the nucleus, independently of PLCB. Moreover, this signal transduction activated RHO- and RAC-regulated pathways acting on JNK and p38 [66]. Although TRIO is found altered in 10% cutaneous melanoma, its potential role in the development of this cancer type is not yet demonstrated.
6. Targeting the GNA Pathway as a Therapeutic Option for Uveal Melanoma
Uveal melanoma is a very resistant cancer to classical chemotherapy or to radiotherapy. Immune checkpoint inhibitors which have greatly improved the overall survival of patients with advanced cutaneous melanoma, unfortunately showed very little efficacy in metastatic uveal melanoma [67,68,69,70]. However, combined checkpoint blockade represents the most effective treatment option available so far for metastatic uveal melanoma outside of clinical trials [71,72].
The location of the eye as an immune privilege organ may account for the loss of specific immune response [73]. In addition, the low mutational burden of uveal melanoma (and therefore the weak generation of neoantigens) might also play a role in this lack of response to immunotherapies [64,74]. Since no efficient treatment for patients with metastatic uveal melanoma is available at the moment, immunotherapy treatments in combination with targeted therapies could be an alternative worthy of clinical trials.
The combination of BRAF/MEK inhibitors is not possible due to the absence of BRAF mutations in uveal melanoma [75,76,77]. Clinical trials for MEK inhibitors in combination with chemotherapy or other candidate targets (PKC, AKT) have shown no significant improvement in the progression free survival [78,79]. Indeed, the observed heterogeneity of MAPK activation in uveal melanoma with GNAQ/11 mutations could explain at least in part this phenomenon and, therefore, it does not allow this mutational status to be an efficient biomarker of MEK inhibitors’ sensitivity [80].
Although a direct link between the activation of G proteins/GPCRs and tumor development has been proven, no therapeutic strategy targeting the function of G proteins is currently available in clinics [81]. Nevertheless, in light of the data discussed above, it appears quite obvious that the inhibition of oncogenic G proteins and their downstream targets should lead to the efficient killing of tumors that developed from driver GNA mutations [82].
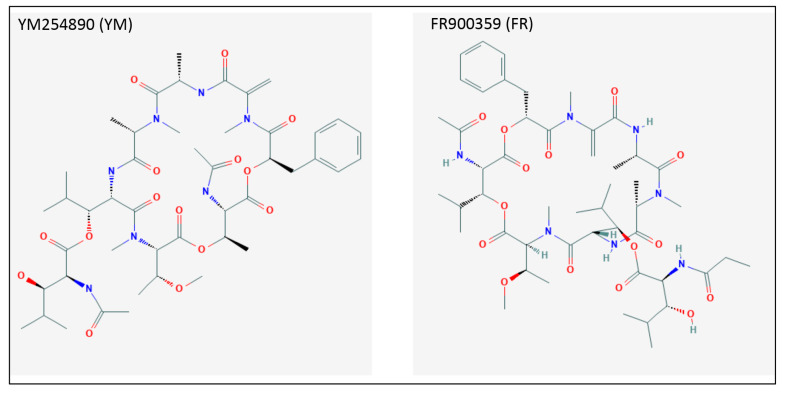
The so-called guanine nucleotide dissociation inhibitors (GDI) are molecules that are able to maintain G proteins in their GDP-bound inactive state (Figure 1). Of these, GNAQ family-specific inhibitors FR900359 (FR) and YM254890 (YM) were described to block the downstream signaling of GNAQ variants in cancer cells with high GNAQ activity [83]. Several studies showed under FR treatment a significant reduction in the aggressive phenotype in skin melanoma cells, proliferation of which was dependent on GNAQ [22,84,85,86]. The chemical structure of FR and YM are represented in Figure 2.
Figure 2.
Chemical structure of GNAQ inhibitors FR900359 (FR) and YM254890 (YM).
In uveal melanoma-specific mutations, both identified hotspots are located in the GTPase domain and play a key role in stabilizing the transition state for GTP hydrolysis. As discussed above, these variants differ because the R183C variant is still able to be regulated by receptor stimulation, whereas Q209L/P variants are uncoupled from GPCRs [87,88].
Based on this result, one should assume a different sensitivity to GDIs depending on which variant is expressed in the tumor cells. One advantage of GDIs could be their dual role of blocking signaling downstream wildtype but also oncogenic G proteins.
The path of GDI into the clinic still needs to cross several steps. Safety issues such as the ubiquitous expression of G proteins could be overcome by local treatment instead of systemic (for example, directly into the eye for uveal melanoma). Documentation on the long term toxicity in humans is still missing. Finally, this “mutation-nonspecific” type of inhibitor will block both oncogenic and wildtype forms of the protein, which will require precise adjustment of the moelcule’s dosage.
CYSLTR2-mutated uveal melanoma usually presents an overactivation of the GNAQ pathway and an insensitivity to any ligand [89]. Specific antagonists of CYSLTR2 have been generated in 2010 [90] and CYSLTR1/2 inhibitors have been tested in clinical trials for the condition of asthma. The latter led to a significant attenuation of allergen-induced inflammation in the tested cohort [91].
It is therefore likely that these inhibitors will be tested in uveal melanoma with GPCR mutations in the future. GDIs such as FR or YM are also expected to have an impact on tumors with aberrant activation of the G proteins, whether they carry a mutation on GNA genes or upstream their receptor.
Mutations in GNAQ and CYSTR2 represent the vast majority of uveal melanoma tumors and they activate downstream targets such as TRIO or ARF6. Inhibitors of these two proteins have been developed, and it would make sense to test them in uveal melanoma [92,93,94]
7. Conclusions
The observation that one of the direct causes for cancer development is the alteration of genes encoding for G proteins, leading to inactivate their GTPase function, has been known for a while. Nonetheless, trials for inhibiting these oncoproteins have brought very little success for biochemical reasons. Indeed, unlike cell membrane receptors, G proteins are intracellular and more difficult to access. In addition, unlike the success of ATP binding competition in the case of kinase inhibitors, the high affinity of GTP/GDP to the protein together with their high intracellular levels renders the chemical competition difficult. The development of specific inhibitors for GNAQ, efficacy of which was shown in vitro and in vivo, has paved the road for a rational therapeutic approach in cancers carrying alterations in this particular protein.
The field of melanoma research has made unprecedented significant advances in the last decade (targeted and immunotherapies), which resulted in a prolongation of the survival for patients with advanced cutaneous melanoma. However, in the case of uveal melanoma, these new therapeutic strategies have not yet led to major improvements due to two main reasons. First, the major risk factor for melanoma, UV radiation from the sun, does not play a big role in the development of uveal melanoma. Second, uveal melanoma transformation generates from different oncogenic drivers than cutaneous melanoma. The vast majority of tumors carry activating mutations in GNAQ/11, which leads to the overactivation of signaling such as ARF6/TRIO/RHO/RAC/YAP and PLCB/PKC/ERK. In addition, 40% uveal melanoma present genetic alterations in BAP1, which are associated with metastasis [95].
Based on the late advances in both the molecular mechanisms of uveal melanoma development and possibilities of targeting G protein signaling in this cancer type, we expect to observe an improvement in therapeutic strategies for patients with advanced uveal melanoma in the near future.
Acknowledgments
We would like to thank the German Research Council (RTG2099) for financial support.
Funding
This research was funded by Deutsche Forschungsgemeinschaft, grant number 259332240/RTG 2099.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Algazi A.P., Tsai K.K., Shoushtari A.N., Munhoz R.R., Eroglu Z., Piulats J.M., Ott P.A., Johnson D.B., Hwang J., Daud A.I., et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–3353. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annala S., Feng X., Shridhar N., Eryilmaz F., Patt J., Yang J., Pfeil E.M., Cervantes-Villagrana R.D., Inoue A., Häberlein F., et al. Direct targeting of Gαq and Gα11 oncoproteins in cancer cells. Sci. Signal. 2019;12:eaau5948. doi: 10.1126/scisignal.aau5948. [DOI] [PubMed] [Google Scholar]
- 3.Bánfi B., Tirone F., Durussel I., Knisz J., Moskwa P., Molnár G.Z., Krause K.-H., Cox J.A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J. Biol. Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 4.BelAiba R.S., Djordjevic T., Petry A., Diemer K., Bonello S., Banfi B., Hess J., Pogrebniak A., Bickel C., Görlach A. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 5.Berman D.M., Wilkie T.M., Gilman A.G. GAIP and RGS4 Are GTPase-Activating Proteins for the Gi Subfamily of G Protein α Subunits. Cell. 1996;86:445–452. doi: 10.1016/S0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 6.Blangy A., Bouquier N., Gauthier-Rouvière C., Schmidt S., Debant A., Leonetti J.-P., Fort P. Identification of TRIO-GEFD1 chemical inhibitors using the yeast exchange assay. Biol. Cell. 2006;98:511–522. doi: 10.1042/BC20060023. [DOI] [PubMed] [Google Scholar]
- 7.Boru G., Cebulla C.M., Sample K.M., Massengill J.B., Davidorf F.H., Abdel-Rahman M.H. Heterogeneity in Mitogen-Activated Protein Kinase (MAPK) Pathway Activation in Uveal Melanoma With Somatic GNAQ and GNA11 Mutations. Investig. Ophthalmol. Vis. Sci. 2019;60:2474–2480. doi: 10.1167/iovs.18-26452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caggia S., Chunduri H., Millena A.C., Perkins J.N., Venugopal S.V., Vo B.T., Li C., Tu Y., Khan S.A. Novel role of Giα2 in cell migration: Downstream of PI3-kinase-AKT and Rac1 in prostate cancer cells. J. Cell Physiol. 2018;234:802–815. doi: 10.1002/jcp.26894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvajal R.D., Piperno-Neumann S., Kapiteijn E., Chapman P.B., Frank S., Joshua A.M., Piulats J.M., Wolter P., Cocquyt V., Chmielowski B., et al. Selumetinib in Combination With Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT) J. Clin. Oncol. 2018;36:1232–1239. doi: 10.1200/JCO.2017.74.1090. [DOI] [PubMed] [Google Scholar]
- 10.Case A.J., Li S., Basu U., Tian J., Zimmerman M.C. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H19–H28. doi: 10.1152/ajpheart.00974.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellone M.D., Teramoto H., Williams B.O., Druey K.M., Gutkind J.S. Prostaglandin E2 Promotes Colon Cancer Cell Growth Through a Gs-Axin- -Catenin Signaling Axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 12.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceraudo E., Horioka M., Mattheisen J.M., Hitchman T.D., Moore A.R., Kazmi M.A., Chi P., Chen Y., Sakmar T.P., Huber T. Uveal Melanoma Oncogene CYSLTR2 Encodes a Constitutively Active GPCR Highly Biased Toward Gq Signaling. bioRxiv. 2019 doi: 10.1101/663153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cervantes-Villagrana R.D., Adame-García S.R., García-Jiménez I., Color-Aparicio V.M., Beltrán-Navarro Y.M., König G.M., Kostenis E., Reyes-Cruz G., Gutkind J.S., Vázquez-Prado J. Gβγ signaling to the chemotactic effector P-REX1 and mammalian cell migration is directly regulated by Gαq and Gα13 proteins. J. Biol. Chem. 2019;294:531–546. doi: 10.1074/jbc.RA118.006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan A.T., Ogino S., Fuchs C.S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay C., Kim D.W., Gombos D.S., Oba J., Qin Y., Williams M.D., Esmaeli B., Grimm E.A., Wargo J.A., Woodman S.E., et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer. 2016;122:2299–2312. doi: 10.1002/cncr.29727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croce M., Ferrini S., Pfeffer U., Gangemi R. Targeted therapy of uveal melanoma: Recent failures and new perspectives. Cancers. 2019;11:846. doi: 10.3390/cancers11060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danielli R., Ridolfi R., Chiarion-Sileni V., Queirolo P., Testori A., Plummer R., Boitano M., Calabrò L., De Rossi C., Di Giacomo A.M., et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: Safety and clinical efficacy. Cancer Immunol. Immunother. 2012;61:41–48. doi: 10.1007/s00262-011-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 20.Decatur C.L., Ong E., Garg N., Anbunathan H., Bowcock A.M., Field M.G., Harbour J.W. Driver Mutations in Uveal Melanoma. JAMA Ophthalmol. 2016;134:728–733. doi: 10.1001/jamaophthalmol.2016.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaney M.K., Kim K., Estevez B., Xu Z., Stojanovic-Terpo A., Shen B., Ushio-Fukai M., Cho J., Du X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arter. Thromb. Vasc. Biol. 2016;36:846–854. doi: 10.1161/ATVBAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeWire S.M., Ahn S., Lefkowitz R.J., Shenoy S.K. β-Arrestins and Cell Signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 23.Diebold I., Petry A., Burger M., Hess J., Görlach A. NOX4 mediates activation of FoxO3a and matrix metalloproteinase-2 expression by urotensin-II. Mol. Biol. Cell. 2011;22:4424–4434. doi: 10.1091/mbc.e10-12-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J., Aksoy B.B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diebold I., Petry A., Hess J., Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell. 2010;21:2087–2096. doi: 10.1091/mbc.e09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drews R.T., Gravel R.A., Collu R. Identification of G protein α subunit mutations in human growth hormone (GH)- and GH/prolactin-secreting pituitary tumors by single-strand conformation polymorphism (SSCP) analysis. Mol. Cell. Endocrinol. 1992;87:125–129. doi: 10.1016/0303-7207(92)90240-7. [DOI] [PubMed] [Google Scholar]
- 27.Fazeli G., Stopper H., Schinzel R., Ni C.-W., Jo H., Schupp N. Angiotensin II induces DNA damage via AT1 receptor and NADPH oxidase isoform Nox4. Mutagenesis. 2012;27:673–681. doi: 10.1093/mutage/ges033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng X., Arang N., Rigiracciolo D.C., Lee J.S., Yeerna H., Wang Z., Lubrano S., Kishore A., Pachter J.A., König G.M., et al. A Platform of Synthetic Lethal Gene Interaction Networks Reveals that the GNAQ Uveal Melanoma Oncogene Controls the Hippo Pathway through FAK. Cancer Cell. 2019;35:457–472. doi: 10.1016/j.ccell.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng X., Degese M.S., Iglesias-Bartolome R., Vaque J.P., Molinolo A.A., Rodrigues M., Zaidi M.R., Ksander B.R., Merlino G., Sodhi A., et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes S.A., Bindal N., Bamford S., Cole C., Kok C.Y., Beare D., Jia M., Shepherd R., Leung K., Menzies A., et al. COSMIC: Mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2010;39:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauvreau G.M., Boulet L.-P., FitzGerald J.M., Cockcroft D.W., Davis B.E., Leigh R., Tanaka M., Fourre J.A., Tanaka M., Nabata T., et al. A dual CysLT1/2 antagonist attenuates allergen-induced airway responses in subjects with mild allergic asthma. Allergy. 2016;71:1721–1727. doi: 10.1111/all.12987. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R.A., DuBois R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 33.Heppt M.V., Amaral T., Kähler K.C., Heinzerling L., Hassel J.C., Meissner M., Kreuzberg N., Loquai C., Reinhardt L., Utikal J., et al. Combined immune checkpoint blockade for metastatic uveal melanoma: A retrospective, multi-center study. J. Immunother. Cancer. 2019;7:299. doi: 10.1186/s40425-019-0800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Q., Shokat K.M. Disease-Causing Mutations in the G Protein Gαs Subvert the Roles of GDP and GTP. Cell. 2018;173:1254–1264. doi: 10.1016/j.cell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J.L.-Y., Urtatiz O., Van Raamsdonk C.D. Oncogenic G Protein GNAQ Induces Uveal Melanoma and Intravasation in Mice. Cancer Res. 2015;75:3384–3397. doi: 10.1158/0008-5472.CAN-14-3229. [DOI] [PubMed] [Google Scholar]
- 36.Diebold I., Flügel D., Becht S., BelAiba R.S., Bonello S., Hess J., Kietzmann T., Görlach A. The Hypoxia-Inducible factor-2alpha Is Stabilized by Oxidative Stress Involving NOX4. Antioxid. Redox Signal. 2010;13:425–436. doi: 10.1089/ars.2009.3014. [DOI] [PubMed] [Google Scholar]
- 37.Ismail I.H., Davidson R., Gagne J.-P., Xu Z.Z., Poirier G.G., Hendzel M.J. Germline Mutations in BAP1 Impair Its Function in DNA Double-Strand Break Repair. Cancer Res. 2014;74:4282–4294. doi: 10.1158/0008-5472.CAN-13-3109. [DOI] [PubMed] [Google Scholar]
- 38.Jie H., Dan L., Weibiao C. Rho Kinase ROCK2 Mediates Acid-Induced NADPH Oxidase NOX5-S Expression in Human Esophageal Adenocarcinoma Cells. PLoS ONE. 2016;11:e0149735. doi: 10.1371/journal.pone.0149735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson P., Aoude L.G., Wadt K., Glasson W.J., Warrier S.K., Hewitt A.W., Kiilgaard J.F., Heegaard S., Isaacs T., Franchina M., et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget. 2016;7:4624–4631. doi: 10.18632/oncotarget.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalinec G., Nazarali A.J., Hermouet S., Xu N., Gutkind J.S. Mutated alpha subunit of the Gq protein induces malignant transformation in NIH 3T3 cells. Mol. Cell. Biol. 1992;12:4687–4693. doi: 10.1128/MCB.12.10.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kan Z., Jaiswal B.S., Stinson J., Janakiraman V., Bhatt D., Stern H.M., Yue P., Haverty P.M., Bourgon R., Zheng J., et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 42.Kostenis E., Pfeil E.M., Annala S. Heterotrimeric Gq proteins as therapeutic targets? J. Boil. Chem. 2020;295:5206–5215. doi: 10.1074/jbc.REV119.007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Küsters-Vandevelde H.V.N., van Engen-van Grunsven I.A.C.H., Küsters B., van Dijk M.R.C.F., Groenen P.J.T.A., Wesseling P., Blokx W.A.M. Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol. 2010;120:755–764. doi: 10.1007/s00401-010-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landis C.A., Masters S.B., Spada A., Pace A.M., Bourne H.R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 45.Lapadula D., Farias E., Randolph C.E., Purwin T.J., McGrath D., Charpentier T.H., Zhang L., Wu S., Terai M., Sato T., et al. Effects of Oncogenic Gαq and Gα11 Inhibition by FR900359 in Uveal Melanoma. Mol. Cancer Res. 2019;17:963–973. doi: 10.1158/1541-7786.MCR-18-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li D., Cao W. Bile acid receptor TGR5, NADPH Oxidase NOX5-S and CREB Mediate Bile Acid-Induced DNA Damage In Barrett’s Esophageal Adenocarcinoma Cells. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livingstone E., Zaremba A., Horn S., Ugurel S., Casalini B., Schlaak M., Hassel J.C., Herbst R., Utikal J.S., Weide B., et al. GNAQ and GNA11 mutant nonuveal melanoma: A subtype distinct from both cutaneous and uveal melanoma. Br. J. Derm. 2020:1–12. doi: 10.1111/bjd.18947. [DOI] [PubMed] [Google Scholar]
- 48.Long G.V., Flaherty K.T., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017;28:1631–1639. doi: 10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu T., Zhang D.-M., Wang X.-L., He T., Wang R.-X., Chai Q., Katusic Z.S., Lee H.-C. Regulation of coronary arterial BK channels by caveolae-mediated angiotensin II signaling in diabetes mellitus. Circ. Res. 2010;106:1164–1173. doi: 10.1161/CIRCRESAHA.109.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu X., Wang F., Liu M., Yang K.T., Nau A., Kohan D.E., Reese V., Richardson R.S., Yang T. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: Involvement of Nox4-derived hydrogen peroxide. Am. J. Physiol. Ren. Physiol. 2016;310:F1243–F1250. doi: 10.1152/ajprenal.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyon A.M., Tesmer J.J.G. Structural insights into phospholipase C-β function. Mol. Pharm. 2013;84:488–500. doi: 10.1124/mol.113.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marty C., Ye R.D. Heterotrimeric G protein signaling outside the realm of seven transmembrane domain receptors. Mol. Pharm. 2010;78:12–18. doi: 10.1124/mol.110.063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKenna K.C., Chen P.W. Influence of Immune Privilege on Ocular Tumor Development. Ocul. Immunol. Inflamm. 2010;18:80–90. doi: 10.3109/09273941003669950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metz C.H., Scheulen M., Bornfeld N., Lohmann D., Zeschnigk M. Ultradeep sequencing detects GNAQ and GNA11 mutations in cell-free DNA from plasma of patients with uveal melanoma. Cancer Med. 2013;2:208–215. doi: 10.1002/cam4.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mezhybovska M., Wikström K., Ohd J.F., Sjölander A. Pro-inflammatory mediator leukotriene D4 induces transcriptional activity of potentially oncogenic genes. Biochem. Soc. Trans. 2005;33:698–700. doi: 10.1042/BST0330698. [DOI] [PubMed] [Google Scholar]
- 56.Miller F.J., Chu X., Stanic B., Tian X., Sharma R.V., Davisson R.L., Lamb F.S. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid. Redox Signal. 2010;12:583–593. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mong S., Miller J., Wu H.L., Crooke S.T. Leukotriene D4 receptor-mediated hydrolysis of phosphoinositide and mobilization of calcium in sheep tracheal smooth muscle cells. J. Pharm. Exp. 1988;244:508–515. [PubMed] [Google Scholar]
- 58.Montezano A.C., Burger D., Paravicini T.M., Chignalia A.Z., Yusuf H., Almasri M., He Y., Callera G.E., He G., Krause K.-H., et al. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ. Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore A.R., Ceraudo E., Sher J.J., Guan Y., Shoushtari A.N., Chang M.T., Zhang J.Q., Walczak E.G., Kazmi M.A., Taylor B.S., et al. Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat. Genet. 2016;48:675–680. doi: 10.1038/ng.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore A.R., Ran L., Guan Y., Sher J.J., Hitchman T.D., Zhang J.Q., Hwang C., Walzak E.G., Shoushtari A.N., Monette S., et al. GNA11 Q209L Mouse Model Reveals RasGRP3 as an Essential Signaling Node in Uveal Melanoma. Cell Rep. 2018;22:2455–2468. doi: 10.1016/j.celrep.2018.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morelli J.G., Yohn J.J., Lyons M.B., Murphy R.C., Norris D.A. Leukotrienes C4 and D4 as potent mitogens for cultured human neonatal melanocytes. J. Investig. Derm. 1989;93:719–722. doi: 10.1111/1523-1747.ep12284392. [DOI] [PubMed] [Google Scholar]
- 62.Carrim N., Arthur J.F., Hamilton J.R., Gardiner E.E., Andrews R.K., Moran N., Berndt M.C., Metharom P. Thrombin-induced Reactive Oxygen Species Generation in Platelets: A Novel Role for Protease-Activated Receptor 4 and GPIbα. Redox Biol. 2015;6:640–647. doi: 10.1016/j.redox.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Hayre M., Degese M.S., Gutkind J.S. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr. Opin. Cell Biol. 2014;27:126–135. doi: 10.1016/j.ceb.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Onken M.D., Makepeace C.M., Kaltenbronn K.M., Kanai S.M., Todd T.D., Wang S., Broekelmann T.J., Rao P.K., Cooper J.A., Blumer K.J. Targeting nucleotide exchange to inhibit constitutively active G protein α subunits in cancer cells. Sci. Signal. 2018;11:eaao6852. doi: 10.1126/scisignal.aao6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paletta-Silva R., Rocco-Machado N., Meyer-Fernandes J.R. NADPH oxidase biology and the regulation of tyrosine kinase receptor signaling and cancer drug cytotoxicity. Int. J. Mol. Sci. 2013;14:3683–3704. doi: 10.3390/ijms14023683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandey D., Gratton J.-P., Rafikov R., Black S.M., Fulton D.J.R. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol. Pharm. 2011;80:407–415. doi: 10.1124/mol.110.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parish A.J., Nguyen V., Goodman A.M., Murugesan K., Frampton G.M., Kurzrock R. GNAS, GNAQ, and GNA11 alterations in patients with diverse cancers. Cancer. 2018;124:4080–4089. doi: 10.1002/cncr.31724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patra K.C., Kato Y., Mizukami Y., Widholz S., Boukhali M., Revenco I., Grossman E.A., Ji F., Sadreyev R.I., Liss A.S., et al. Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nat. Cell Biol. 2018;20:811–822. doi: 10.1038/s41556-018-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pelletier S., Duhamel F., Coulombe P., Popoff M.R., Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol. Cell. Biol. 2003;23:1316–1333. doi: 10.1128/MCB.23.4.1316-1333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez D.E., Henle A.M., Amsterdam A., Hagen H.R., Lees J.A. Uveal melanoma driver mutations in GNAQ/11 yield numerous changes in melanocyte biology. Pigment Cell Melanoma Res. 2018;31:604–613. doi: 10.1111/pcmr.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piaggio F., Tozzo V., Bernardi C., Croce M., Puzone R., Viaggi S., Patrone S., Barla A., Coviello D., Jager M.J., et al. Secondary Somatic Mutations in G-Protein-Related Pathways and Mutation Signatures in Uveal Melanoma. Cancers. 2019;11:1688. doi: 10.3390/cancers11111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pusapati G.V., Kong J.H., Patel B.B., Gouti M., Sagner A., Sircar R., Luchetti G., Ingham P.W., Briscoe J., Rohatgi R. G protein–coupled receptors control the sensitivity of cells to the morphogen Sonic Hedgehog. Sci. Signal. 2018;11:eaao5749. doi: 10.1126/scisignal.aao5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robert C., Karaszewska B., Schachter J., Rutkowski P., Mackiewicz A., Stroiakovski D., Lichinitser M., Dummer R., Grange F., Mortier L., et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 74.Robertson A.G., Shih J., Yau C., Gibb E.A., Oba J., Mungall K.L., Hess J.M., Uzunangelov V., Walter V., Danilova L., et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell. 2017;32:204–220. doi: 10.1016/j.ccell.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Royer-Bertrand B., Torsello M., Rimoldi D., El Zaoui I., Cisarova K., Pescini-Gobert R., Raynaud F., Zografos L., Schalenbourg A., Speiser D., et al. Comprehensive Genetic Landscape of Uveal Melanoma by Whole-Genome Sequencing. Am. J. Hum. Genet. 2016;99:1190–1198. doi: 10.1016/j.ajhg.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt S., Debant A. The Enzymes. Volume 33. Academic Press; Waltham, MA, USA: 2013. Aptamer-Derived Peptide Inhibitors of Rho Guanine Nucleotide Exchange Factors; pp. 147–168. [DOI] [PubMed] [Google Scholar]
- 77.Schrage R., Schmitz A.-L., Gaffal E., Annala S., Kehraus S., Wenzel D., Büllesbach K.M., Bald T., Inoue A., Shinjo Y., et al. The experimental power of FR900359 to study Gq-regulated biological processes. Nat. Commun. 2015;6:1–17. doi: 10.1038/ncomms10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh A.D., Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:962–965. doi: 10.1016/S0161-6420(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 79.Kim S.-M., Kim Y.-G., Jeong K.-H., Lee S.-H., Lee T.-W., Ihm C.-G., Moon J.-Y. Angiotensin II-induced Mitochondrial Nox4 Is a Major Endogenous Source of Oxidative Stress in Kidney Tubular Cells. PLoS ONE. 2012;7:e39739. doi: 10.1371/journal.pone.0039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., Kobori M. A novel Galphaq/11-selective inhibitor. J. Biol. Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- 81.Theccanat T., Philip J.L., Razzaque A.M., Ludmer N., Li J., Xu X., Akhter S.A. Regulation of cellular oxidative stress and apoptosis by G protein-coupled receptor kinase-2; The role of NADPH oxidase 4. Cell. Signal. 2016;28:190–203. doi: 10.1016/j.cellsig.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Urtatiz O., Cook C., Huang J.L.Y., Yeh I., Van Raamsdonk C.D. GNAQQ209L expression initiated in multipotent neural crest cells drives aggressive melanoma of the central nervous system. Pigment Cell Melanoma Res. 2020;33:96–111. doi: 10.1111/pcmr.12843. [DOI] [PubMed] [Google Scholar]
- 83.van der Kooij M.K., Joosse A., Speetjens F.M., Hospers G.A.P., Bisschop C., de Groot J.W.B., Koornstra R., Blank C.U., Kapiteijn E. Anti-PD1 treatment in metastatic uveal melanoma in the Netherlands. Acta Oncol. 2017;56:101–103. doi: 10.1080/0284186X.2016.1260773. [DOI] [PubMed] [Google Scholar]
- 84.Van Raamsdonk C.D., Bezrookove V., Green G., Bauer J., Gaugler L., O’Brien J.M., Simpson E.M., Barsh G.S., Bastian B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Raamsdonk C.D., Griewank K.G., Crosby M.B., Garrido M.C., Vemula S., Wiesner T., Obenauf A.C., Wackernagel W., Green G., Bouvier N., et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaqué J.P., Dorsam R.T., Feng X., Iglesias-Bartolome R., Forsthoefel D.J., Chen Q., Debant A., Seeger M.A., Ksander B.R., Teramoto H., et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol. Cell. 2013;49:94–108. doi: 10.1016/j.molcel.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weinstein L.S., Shenker A., Gejman P.V., Merino M.J., Friedman E., Spiegel A.M. Activating Mutations of the Stimulatory G Protein in the McCune–Albright Syndrome. N. Engl. J. Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 88.Wiesner T., Obenauf A.C., Murali R., Fried I., Griewank K.G., Ulz P., Windpassinger C., Wackernagel W., Loy S., Wolf I., et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat. Genet. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson C.H., McIntyre R.E., Arends M.J., Adams D.J. The activating mutation R201C in GNAS promotes intestinal tumourigenesis in ApcMin/+ mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene. 2010;29:4567–4575. doi: 10.1038/onc.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wood L.D., Parsons D.W., Jones S., Lin J., Sjöblom T., Leary R.J., Shen D., Boca S.M., Barber T., Ptak J., et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 91.Wunder F., Tinel H., Kast R., Geerts A., Becker E., Kolkhof P., Hütter J., Ergüden J., Härter M. Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT2) receptor. Br. J. Pharm. 2010;160:399–409. doi: 10.1111/j.1476-5381.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu D., Du M., Zhang J., Xiong P., Li W., Zhang H., Xiong W., Liu F., Liu J. DNMT1 mediated promoter methylation of GNAO1 in hepatoma carcinoma cells. Gene. 2018;665:67–73. doi: 10.1016/j.gene.2018.04.080. [DOI] [PubMed] [Google Scholar]
- 93.Yoo J.H., Shi D.S., Grossmann A.H., Sorensen L.K., Tong Z., Mleynek T.M., Rogers A., Zhu W., Richards J.R., Winter J.M., et al. ARF6 Is an Actionable Node that Orchestrates Oncogenic GNAQ Signaling in Uveal Melanoma. Cancer Cell. 2016;29:889–904. doi: 10.1016/j.ccell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu F.-X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H., Zhao J., Yuan H., Tumaneng K., Li H., et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zimmer L., Vaubel J., Mohr P., Hauschild A., Utikal J., Simon J., Garbe C., Herbst R., Enk A., Kämpgen E., et al. Phase II DeCOG-Study of Ipilimumab in Pretreated and Treatment-Naïve Patients with Metastatic Uveal Melanoma. PLoS ONE. 2015;10:e0118564. doi: 10.1371/journal.pone.0118564. [DOI] [PMC free article] [PubMed] [Google Scholar]