Abstract
Improving the therapeutic efficacy of conventional anticancer drugs represents the best hope for cancer treatment. However, the shortage of druggable targets and the increasing development of anticancer drug resistance remain significant problems. Recently, membrane transport proteins have emerged as novel therapeutic targets for cancer treatment. These proteins are essential for a plethora of cell functions ranging from cell homeostasis to clinical drug toxicity. Furthermore, their association with carcinogenesis and chemoresistance has opened new vistas for pharmacology-based cancer research. This review provides a comprehensive update of our current knowledge on the functional expression profile of membrane transport proteins in cancer and chemoresistant tumours that may form the basis for new cancer treatment strategies.
Keywords: membrane transporters, pumps, ion channels, cancers, chemoresistance
1. Introduction
In mammalian cells, the plasma membrane is a selectively permeable barrier that creates an intracellular environment and maintains cell stability and homeostasis. The proper functioning of the plasma membrane is dependent on a group of membrane transport proteins that permit the selective transport of essential substances for the survival and development of the organism [1]. To date, three different types of membrane transport proteins have been described: (1) ATP-powered pumps or ATPases which actively transport solutes against their electrochemical gradients; (2) channel proteins which facilitate the passive diffusion of ions following their electrochemical gradients; and (3) facilitators which move solutes either up or down their gradients. When the gates of the transporters are open, the selective flux of metabolites and ions occurs that affects a wide range of cellular processes such as membrane potential (due to the ion exchange), cell volume (due to the water permeation coupled to ion transport), and cell signaling (due to the impact on the function of ions/metabolites or intracellular effectors). All of these events are critical in determining cell fate to survival, death, or malignant transformation [2]. Another important role of membrane transport proteins is to maintain a balance between toxicity and effectiveness of chemotherapeutics by controlling drug uptake, disposition, and clearance [3,4,5,6]. Therefore, disturbance in the expression profile of membrane transport proteins is often associated with tumourigenesis and/or chemoresistance [7,8]. In this review, we will discuss the correlations between membrane transporters (pumps and channels) and cancer progression as well as chemoresistance (Appendix A).
2. Membrane Pumps
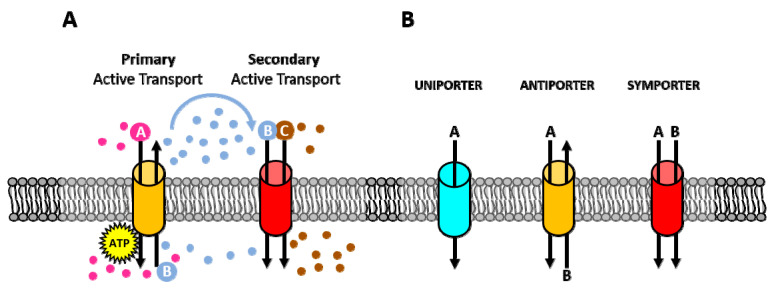
Membrane pumps are transmembrane proteins that facilitate the active transport of various substances against their electrochemical gradients. Mechanistically, membrane pumps can be divided into two main categories: primary and secondary active transporters. Through ATP hydrolysis, primary active transporters move solutes against their electrochemical gradients. These pumps are often uniporters which are involved in the active transport of a single molecule across the cell membrane. Instead, secondary active transporters utilize the energy stored in the electrochemical gradient of ions across the plasma membrane that was generated by the primary active transporters. Therefore, in this type of transport, the transfer of one molecule down its gradient is coupled to the movement of another molecule against its gradient (Figure 1A). Depending on the direction of transport, two types of secondary active transporters have been described: antiport pumps that transport two molecules in opposite directions and symport pumps that move both molecules in the same direction (Figure 1B) [9].
Figure 1.
Different types of ion transport. (A) Active and secondary transport: Primary active transporter uses ATP to move ions across the membrane [A and B], against their electrochemical gradients to create an electrochemical gradient. Secondary active transporter uses the electrochemical gradient generated by primary active transporters to move one molecule down its gradient [B] while transporting another molecule against its electrochemical gradient [C]. (B) Uniporter, antiporter, and symporter: Uniporter carries one molecule or ion in one direction. Antiporter carries two different molecules or ions in opposite directions. Symporter also carries two different molecules or ions in the same direction.
The crucial role of membrane pumps in conducting the active transport of a wide range of substrates including ions, amino acids, large polypeptides, and essential metabolites highlights their indispensable function in maintaining cellular homeostasis [10]. Moreover, membrane pumps are also involved in drug uptake and efflux that impact disposition and cytotoxic effects of anticancer drugs [11,12]. In this context, membrane transporters can act as importers and mediate the transport of drugs into the cell or function as exporters and pump substances outside the cell. In cancer, altered expression of membrane pumps often correlates with chemoresistance (Appendix A) [13,14,15]. The following sections will highlight the relationship between membrane pumps and cancer progression as well as chemoresistance.
2.1. Na+/K+-ATPase
The plasma membrane sodium pump (Na+/K+-ATPase) is a hetero-dimeric complex that consists of catalytic a- and regulatory b-subunits (Figure 2). Four different isoforms of a-subunit and three isoforms of b-subunit exist in human cells [16,17,18]. Functionally, Na+/K+-ATPase is a ubiquitous P-type ATPase transporter that exchanges three Na+ for two K+, thus establishing plasma membrane potential. The generated membrane potential is further required for accelerating the central cellular processes including secondary active transport of metabolites and cell excitability [19,20]. Na+/K+-ATPase is naturally activated and deactivated by ATP and cardiotonic steroids (e.g., ouabain, digitoxin), respectively [21,22]. Over the last decades, an association between Na+/K+-ATPase and etiology of several malignancies, including breast, non-small cell lung cancer, glioblastoma, and melanoma has been established [23,24]. For instance, the expression level of a-subunit (isoforms 1 and 3) is increased in various cancers, therefore its pharmacological inhibition has been proposed to improve cancer therapy [25,26,27]. Specifically, several studies demonstrated that the a1-subunit of Na+/K+-ATPase is highly expressed in glioblastomas and that its inhibition decreases cell proliferation and migration while increasing survival of the orthotopic patient-derived xenograft mouse model of human glioblastoma [28,29,30,31]. Similarly, pharmacological inhibition of a1-subunit reduces tumour progression and induces apoptosis in prostate cancer [32] and lung cancer cells [33]. Furthermore, Rajasekaran et al. reported that the expression level of β1-subunit is markedly reduced in renal cell carcinoma and that its ectopic expression inhibits the invasiveness and motility of these cells. For the mechanism, authors showed that elevated Na+/K+-ATPase positively impacts E-cadherin-mediated formation of tight junctions and epithelial cell polarity [34,35]. Likewise, reduced β1-subunit correlates with poorly differentiated breast (MDA435), colon (SW480), pancreas (MiaPaCa-2), and kidney (MSV-MDCK) cancer cells. This evidence suggests that the Na+/K+-ATPase β1-subunit plays a tumour-suppressor role in cancer [36,37].
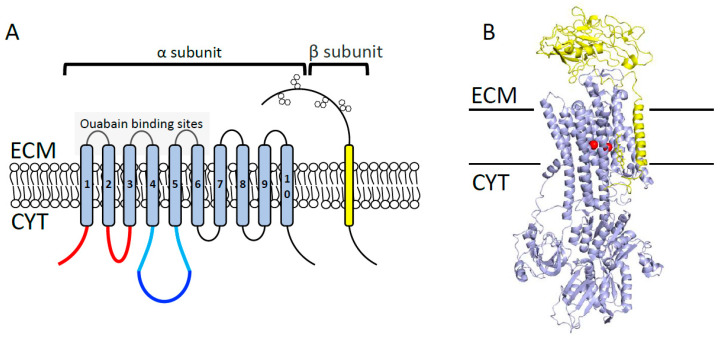
Figure 2.
Na+-, K+-ATPase overall structure. (A) Na+-, K+-ATPase consists of a catalytic α subunit and a regulatory β subunit. The α subunit consists of 10 transmembrane helices, harboring 3 different cytoplasmic domains: the actuator responsible for dephosphorylation (shown in red); the nucleotide-binding, responsible for ATP binding (shown in blue); and the phosphorylation domains (shown in cyan). The β subunit consists of one transmembrane helix with a large glycosylated extracellular domain (shown in hexagon orange boxes). ECM = extracellular milieu; CYT = cytoplasm. (B) Overall domain architecture of Na+/K+ transporter in the Na+-bound state (Protein Data Bank [PDB] code 4HQJ). Catalytic α subunit is colored in blue, β subunit is shown in yellow, and Na+ ions are shown in red.
In addition to its significance in cell proliferation and invasion, Na+/K+-ATPase is also emerging as an effective therapeutic target to overcome chemoresistance [38]. The first line of evidence was reported in 1991 when Andrews et al. showed that the pharmacological inhibition of Na+/K+-ATPase with ouabain reduced the intracellular accumulation of cisplatin by 50% in a 2008 ovarian carcinoma cell line [39], suggesting that low Na+/K+-ATPase is linked to cisplatin resistance. Further investigations demonstrated that inhibition of Na+/K+-ATPase expression and function promotes cisplatin-resistance in leukemia [40], non-small lung cancer [41,42], and prostate cancer cell lines [43]. For example, Na+/K+-ATPase is significantly reduced in cisplatin-resistant BHY oral squamous cell carcinoma cells, and its further inhibition exacerbates the cisplatin-resistant phenotype [44]. Similarly, Na+/K+-ATPase is considerably reduced in oxaliplatin-resistant ovarian carcinoma cells C10B, therefore its ectopic expression enhances oxaliplatin accumulation and promotes oxaliplatin-mediated cell death [45]. Together, these findings reveal that Na+/K+-ATPase may serve as a potential therapeutic target for the treatment of chemoresistant malignancies. However, further investigations are required for the development of a novel and high-affinity molecule to target Na+/K+-ATPase for cancer treatment.
2.2. SERCA
The Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) is a P-type ATPase located in the sarcoplasmic reticulum (SR) within myocytes [46]. In humans, three genes encoding three major paralogs of SERCA (SERCA 1, 2, and 3) have been described. In total, 11 different isoforms (SERCA1a–1b, SERCA2a–2c, and SERCA-3a–3f) of SERCA are variably expressed across human cells and tissues [47]. For example, SERCA2b is ubiquitously expressed while the expression of SERCA1a and SERCA 2a is restricted to skeletal and cardiac muscles, respectively [48]. Structurally, SERCA isoforms show a modular architecture consisting of three cytosolic domains responsible for ATP binding and hydrolysis and one transmembrane domain involved in Ca2+ binding and transport (Figure 3) [49]. Functionally, SERCA pumps two Ca2+ ions from the cytosol into the SR lumen coupled with the hydrolysis of a single ATP, therefore establishing a 1000-fold Ca2+ gradient across the SR and cytosolic compartments [50,51].
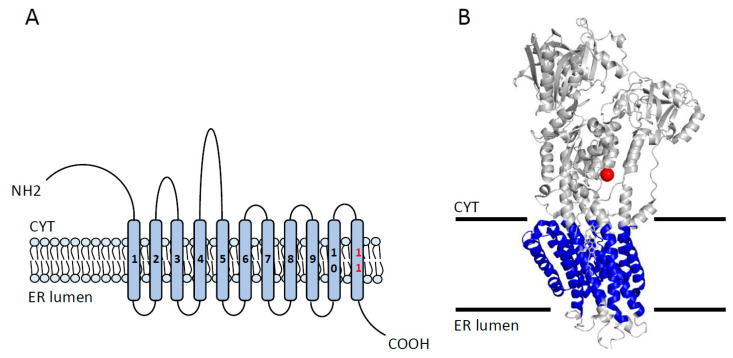
Figure 3.
The overall structure of SERCA (Sarco/endoplasmic reticulum Ca2+ ATPase) pump. (A) The topology of SERCA showing 10 transmembrane segments (TMS (transmembrane segment) 11 is found only in SERCA2b), with a large cytoplasmic N-terminal, large cytoplasmic loops, and a luminal C-terminal. (B) Overall 3D-architecture of SERCA transporter in the E2-state complexed with a Thapsigargin derivative Boc-(phi)Tg (Protein Data Bank [PDB] code 3NAN). Cytoplasmic and luminal loops are shown in gray, TMSs are shown in blue, and Tg inhibitor is shown in red. CYT = cytoplasm; ER lumen = endoplasmic reticulum lumen.
The proper maintenance of the SR Ca2+ gradient is vital in a vast array of cellular functions, such as cell proliferation, invasion, and cell death [52,53,54]. Given the significance of the above functions in cancer development, dysregulated SERCA is associated with various cancers [55]. For instance, Prasad et al. demonstrated that SERCA2 knockout mice are highly susceptible to developing squamous-cell carcinoma, emphasizing the link between impaired SERCA and carcinogenesis [56]. Furthermore, the expression profile of SERCAs is highly diverse in human carcinomas [57,58]. For example, downregulated SERCA2 plays a key role in the progression of lung and thyroid cancers [59,60]. On the contrary, the upregulation of SERCA2 in colorectal carcinoma is correlated with serosal invasion, lymph node metastasis, and advanced tumour stage [61]. Like SERCA2, SERCA3 expression is differentially altered in various cancer types. For instance, SERCA2 levels are reduced in colorectal carcinoma and breast cancers [62,63,64,65], while they increase in myeloid leukemia [66] and gastric cancer [63].
Interestingly, the aberrant expression of SERCAs is also associated with chemoresistance. In this aspect, several studies reported that SERCA1–3 are markedly decreased in cisplatin-resistant MDAH-2774 ovarian cancer cell lines [67], and in low-level cisplatin-resistant non-small-cell lung cancer cells H1339 [68]. A link between altered SERCA and cancer has led to the development of several modulators to either activate/restore or inhibit SERCA for the treatment of different cancers with dysregulated or impaired SERCA. The generated drugs have been used in cancer therapy either individually or in combination with chemotherapeutics [69,70]. Indeed, various inhibitors of SERCA such as thapsigargin, cyclopiazonic acid, and curcumin have been widely used as anticancer drugs in numerous cancers. Furthermore, short-chain fatty acids (e.g., butyrate, valerate, and caproate) and resveratrol have been shown to induce SERCA3 expression and inhibit cell survival in gastrointestinal carcinoma and breast cancer, respectively [63,71]. A curcumin analog F36 is an example of a SERCA inhibitor which has been shown to reduce the proliferation of colorectal cancer cells through inhibiting SERCA2 expression [72]. More recently, the small molecule CXL017 has been demonstrated as a potent anticancer drug in several chemoresistant leukemia cell lines [73,74,75]. However, further studies are required to confirm the effectiveness of CXL017 in other cancers and to test whether CXL017 can promote the effectiveness of conventional chemotherapeutics.
2.3. Vacuolar ATPase (V-ATPase)
Vacuolar ATPase (V-ATPase) is a large multi-subunit P-type ATPase, present in both vacuolar membranes and plasma membranes and which is involved in controlling cellular pH [76]. Structurally, V-ATPase consists of two domains. First is a peripherally associated domain V1 composed of eight (A–H) isoforms which is responsible for ATP hydrolysis. The second subunit is a membrane-associated domain V0 which is made of six different subunits (a, c, c’, c”, d, e) and is responsible for proton translocation (Figure 4) [77,78]. Among these subunits, V0a plays critical roles in membrane distribution and activity range as well as in fine-tuning of the pump [79,80]. Functionally, ATP binding and hydrolysis by the V1 domain are coupled with 360° rotation of the V0 domain and active transport of 2–4 cytosolic H+ across the membrane [81,82]. The proper functioning of the V-ATPase is essential for the control of cytosolic, organellar, and extracellular milieu pH which, in turn, is necessary for the appropriate regulation of cellular processes including cell survival and growth [83,84]. Hereby, disturbances in the expression and/or function of V-ATPase have been associated with many diseases, including cancer [85,86,87].
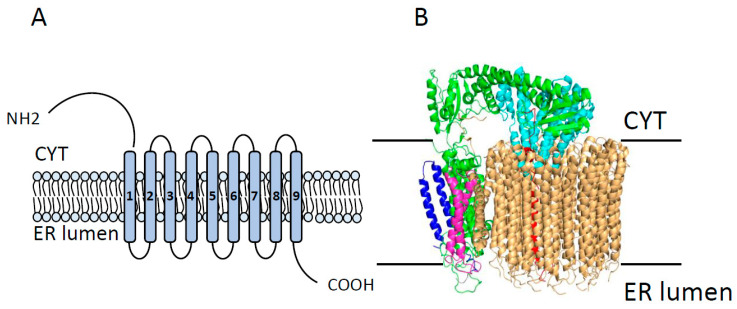
Figure 4.
Overall structure of the V0 domain of V-ATPase. (A) Topology of V0 domain showing its 9 transmembrane segments with large cytoplasmic N-terminal and C-terminal domains. (B) The overall architecture of the V0 domain of V-ATPase (Protein Data Bank [PDB] code 6C6L), showing all known components of the V0 domain, including subunits a (in red), d (in cyan), e (in blue), f (in pink), and the c-ring (in wheat). CYT = cytoplasm; ER lumen = endoplasmic reticulum lumen. Modified from Roh et al. 2018.
In tumours, the expression level of V-ATPase is often upregulated [83]. For instance, V-ATPase is highly expressed in cervical adenocarcinoma compared to normal tissues and is negatively correlated with patient survival [88]. In gastric cancer, overexpression of the V1A subunit is linked to tumour grade advancement, vascular invasion, and lymph node metastasis as well as reduced patient survival [89]. Similarly, V-ATPase is found upregulated in several aggressive cancers, including breast [90], melanoma [91,92], esophageal [93,94], and pancreatic cancers [95] that further highlights its potential as a prognostic biomarker for advanced metastatic cancers. Based on the subcellular location of the V-ATPase, two mechanisms have been proposed for promoting cancer progression and metastasis in different malignancies. First, in melanoma, breast, and prostate cancers, V-ATPase is located in the plasma membrane where it is responsible for creating an acidic extracellular environment critical for matrix metalloprotease- and protease-mediated cell growth and invasion [96,97,98,99]. Second, in bladder and breast cancers, the vacuolar V-ATPase promotes lysosomal acidification, lysosomal trafficking to the cell surface, and secretion of premetastatic peptides such as cathepsins A and B, leading to tumour metastasis [100,101].
Furthermore, overexpression of V-ATPase has been reported to be closely associated with the development of chemoresistance [102,103]. For example, overexpression of V-ATPase is associated with the development of cisplatin resistance in human epidermoid cancer cells (KB/PC4), human prostate cancer cells (P/CDP5) [104], and cisplatin- and vincristine-resistance in leukemia HL-60 cells [105]. Therefore, treatment with V-ATPase inhibitors restores the chemosensitivity of tumour cells through disrupting the pH gradient between the cytoplasm and lysosomal compartment [106,107,108]. It was shown that treatment with V-ATPase inhibitors omeprazole and esomeprazole restored sensitivity to cisplatin, vinblastine, and fluorouracil (5-FU) in chemoresistant melanoma and colon cancer cells [109]. Furthermore, the application of V-ATPase inhibitor Archazolid induced apoptosis in trastuzumab-resistant breast cancer cells [110]. Together, these findings proved the clinical potential of V-ATPases as both prognostic markers and therapeutic targets for cancer.
3. Ion Channels
Ion channels are gated aqueous pores involved in the selective movement of ions across biological membranes. In this type of transport, ions are passively moved down their electrochemical gradient [111]. Depending on the mode of activation, ion channels are classified into two classes, voltage-gated ion channels and ligand-gated ion channels. The voltage-gated ion channels open following changes in the membrane potential while the ligand-gated ion channels open in the presence of extracellular ligands, intracellular second messengers, or chemical factors [112]. Once gates of ion channels are open, ion exchanges across the cellular membranes occur that will result in the redistribution of membrane charges and/or activation of endogenous messengers. Therefore, activation of ion channels triggers various signaling pathways essential for cellular processes ranging from membrane excitability to cell survival [2]. Hereby, alterations in the expression or function of ion channels are associated with multiple human diseases, including cancer. In this regard, many ion channels are considered oncogenic proteins which are often associated with chemoresistance [7,8,113]. Recently, several excellent reviews have described the role of organellar channels in cancer progression and therapy [114,115,116,117]. Therefore, this review will focus on the significance of plasma membrane ion channels in cancer progression and chemoresistance.
3.1. Ca2+ Channels
Ca2+ is a universal second messenger that is vital for the proper functioning of the organism [118]. Thus, its cellular level is always subjected to tight regulations, mainly by the activity of three plasma membrane Ca2+ channels, voltage-gated Ca2+ channels, Orai-mediated store-operated channels, and transient receptor potential-mediated Ca2+ channels. It has been revealed that disruption in the expression or function of these channels is often correlated with carcinogenesis and/or chemoresistance [119,120]. Hence, targeting their expression level or function may serve as an effective strategy to improve cancer treatment. In this section, we will provide examples of the role of Ca2+ channels in cancer progression and chemoresistance.
3.1.1. Voltage-Gated Ca2+ Channels (VGCC)
VGCCs (CaV) are Ca2+ channels that open in response to membrane depolarization. Each CaV consists of a central a1 subunit and three auxiliary subunits, a2δ, b, and g, in a 1:1:1:1 ratio. In mammals, ten distinct members are grouped into three phylogenetic subfamilies: CaV1 (four different isoforms, CaV1.1–4), CaV2 (three different isoforms, CaV2.1–3), and CaV3 (three different isoforms, CaV3.1–3) [121]. Historically, VGCCs are restricted to exciting cells, however, several CaV channels are functionally expressed in non-excitable cancer cells [7]. Interestingly, alterations in the expression and/or function of different members of the CaV subfamilies have been observed in various cancers, suggesting their role in tumour progression, differentiation, and invasion [122,123,124,125]. For instance, CaV1.2 encoded by the human CACNA1C gene is predominantly expressed in oesophageal squamous cell carcinoma and is correlated with tumour cell differentiation [126]. Likewise, CaV1.3 encoded by the human CACNA1D gene is overexpressed in prostate and endometrial cancers [127,128]. Hereby, the CaV1 inhibitor BK10040 was reported to reduce proliferation and induce apoptosis in cancer cells such as A459 (lung adenocarcinoma) and MiaPaCa2 (pancreatic cancer cells) cell lines. Together, these pieces of evidence reveal the oncogenic role of the CaV1 subfamily in human cancers.
As members of the CaV1 subfamily, different CaV2 channels have been reported to be dysregulated in cancer. For example, CaV2.3 encoded by the human CACNA1E gene is upregulated in Wilm’s tumours (a rare childhood kidney cancer), and its expression level is associated with reduced relapse-free survival [129]. Similarly, CaV3.1 and CaV3.2 are highly expressed in human laryngeal carcinoma and glioblastoma, respectively, and their inhibition (using siRNA or mibefradil) causes cell cycle arrest and apoptosis [130,131]. Moreover, the functional expression of CaV channels has been also shown to be associated with chemoresistance. For example, overexpression of the regulatory subunit α2δ (encoded by human CACN12D3 gene) sensitized esophageal squamous cell carcinoma cell lines to cisplatin-induced cell death. Therefore, genetic silencing of α2δ promoted cisplatin resistance in vitro and in vivo [132]. Likewise, a combination of mibefradil (a blocker of CaV3 subfamily) and carboplatin synergistically inhibited the growth of platinum-resistant ovarian cancer cell lines A2780Cis and IGROV-1, suggesting that a combinatorial drug therapy using CaV inhibitors and conventional chemotherapeutics may serve as an effective treatment for ovarian cancer [133].
3.1.2. Orai-Mediated Store-Operated Ca2+ Entry
Orai proteins are highly selective Ca2+ channels that open in response to reduced ER Ca2+ levels. Hence, these channels are called store-operated channels (SOC). Currently, three Orai isoforms have been described (Orai1, Orai2, and Orai3), and each of them consists of six subunits that form a single pore [134]. In cancer, the altered expression profile of Orai isoforms is linked to cancer progression [120,135,136,137]. For instance, Orai1 is predominantly upregulated in gastrointestinal stromal tumours and its inhibition (using shRNA or 2-aminoethyl diphenylborate (2-APB) and SKF-96365) decreased proliferation and induced apoptosis in GIST-T1 cells [138]. Similarly, in esophageal squamous cell carcinoma, Orai1 upregulation is correlated with poor overall and recurrence-free survival, therefore its knockdown suppresses tumour growth and metastasis in nude mice xenograft [139]. Like Orai1, upregulated Orai3 has been reported in many cancers. For instance, in breast and non-small lung adenocarcinoma, elevated Orai3 plays essential roles in cell cycle progression, proliferation, apoptosis evasion, and invasion [140,141]. Similar observations have been made in prostate cancer where Orai3 overexpression is positively correlated with aggressive cancer phenotypes and poor clinical prognosis [137].
Furthermore, the expression profile of Orai channels has been associated with chemoresistance in many cancers [142]. For example, Orai1 expression is upregulated in cisplatin-resistant ovary carcinoma cells compared to their parental cells, and its pharmacological inhibition by 2-APB enhances cisplatin-induced cell death in resistant cell lines [143]. In hepatocellular carcinoma, blockade of Orai1 by SiRNA or SKF-96365 enhances cytotoxicity of 5-FU, whereas its ectopic expression induces resistance to 5-FU [144]. Similarly, in pancreatic adenocarcinoma cells, siRNA-mediated silencing of Orai1 enhances 5-FU- and gemcitabine-induced cell death [145]. Moreover, the Orai3 level is upregulated in breast cancer patients and is correlated with poor response to chemotherapy and poor patient outcome. Likewise, Orai3 overexpression in T47D breast cancer cells confers resistance to pro-apoptotic agents (thapsigargin and staurosporine) and chemotherapeutics (cisplatin, 5-FU, and paclitaxel) [146].
3.1.3. TRP-Mediated Ca2+ Transport
The transient receptor potential (TRP) family of ion channels consists of 28 distinct members divided into seven subfamilies: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPML (Mucolipin), TRPP (Polycystin), TRPA (Ankyrin), and TRPN (No mechanoreceptor potential C, nompC) [147]. Structurally, TRP channels show a modular architecture which consists of four concatenated subunits, each composed of six transmembrane segments (TMS) with a channel pore located between TMS 5/6 as well as cytoplasmic N- and C-termini (Figure 5) [148]. Functionally, TRP channels operate as sensory transduction ion channels, which means they sense and translate environmental stimuli into various signal transduction pathways essential for several cellular processes ranging from survival to cell death [149]. TRP channels mediate these effects mainly by changing the intracellular concentration of Ca2+, either directly by conducting Ca2+ entry or indirectly by changing membrane potential and providing a driving force for Ca2+ entry by other channels [150,151]. Therefore, disruptions in the functional expression of TRP channels have been associated with several diseases, including cancer [152,153,154,155,156]. The following sections will focus on the major subfamilies of TRP channels TRPC, TRPV, and TRPM.
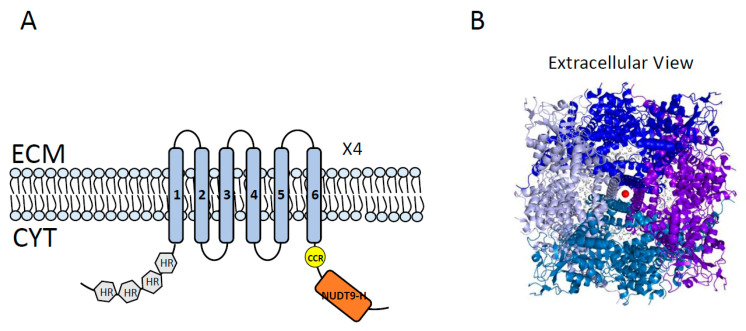
Figure 5.
Topology diagram and 3D structure of TRPM2. (A) Topology of TRPM2 showing its 6 transmembrane segments (TMS), a channel pore between TMS 5 and 6, and cytoplasmic N- and C-termini. N-terminal harbors 4 TRPM homology domains (shown in gray). C-terminal has a short coiled-coil region (CCR, shown in yellow) followed by the NUDT9H domain (shown in orange) which is the homolog of soluble mitochondrial ADPRase NUDT9. A functional TRPM2 is composed of four homotetramers TRPM2 (x4) and requires binding and hydrolysis of ADP-ribose (ADPR) by NUDT9-H. ECM = extracellular milieu; CYT = cytoplasm. (B) Overall domain architecture of TRPM2 (Protein Data Bank [PDB] code 6CO7), showing the extracellular view of four units of TRPM2 surrounding the channel pore (shown in different colors: blue, purple, marine, and light blue, “—” in a clockwise direction).
TRPC
The TRPC subfamily includes seven members (TRPC1–7) which are ubiquitously expressed and play important roles in the regulation of several Ca2+-dependent cellular processes [157]. In cancer, TRPCs display diverse functional expressions. For instance, increased expression of TRPC1 in human breast ductal adenocarcinoma samples compared to the adjacent non-tumoural tissues strongly correlates with tumour progression and invasion [158], therefore its silencing suppresses TRPC1-mediated Ca2+ entry and reduces cell proliferation [159,160]. Similar observations were established in other cancers including glioblastoma, pancreas, and colon cancers [161]. Moreover, TRPC3 is overexpressed in human ovarian cancer tissues, and its blockade decreases in vitro and in vivo growth of ovarian cancer cells [162]. Further studies show that TRPC3 has a predominant role in the proliferation and migration of a variety of tumour cells, including melanoma, lung, and bladder carcinoma cell lines [163]. Similarly, TRPC6 has been reported to be upregulated in various cancers, including glioma [164], gastric cancer [165], and breast cancer [166], whereby its silencing reduced growth and migration of cultured cells as well as tumour formation and metastasis in nude mice xenografts. Together, these findings highlight that the increased expression levels of TRPCs 1, 3, and 6 are strongly associated with malignant phenotypes of human cancers. On the contrary, TRPC4 is markedly downregulated in renal cell carcinoma cell lines and is correlated with tumour angiogenesis [167]. Therefore, pharmacological activation of TRPC4 by englerin A inhibits growth of A-498 and A-673 cells, suggesting that TRPC4 plays a tumour suppressor activity in renal cancer [168].
Moreover, the altered expression profile of various members of the TRPC subfamily has been associated with chemoresistance. For instance, TRPC1 expression is significantly decreased in cisplatin-resistant (A2780 and SKOV3) and carboplatin-resistant (A2780) ovarian cancer cell lines, suggesting that the reduced expression of TRPC1 is linked to chemoresistance [169]. On the contrary, TRPC5 has increased in 5-FU-resistant colorectal cancer cells HCT-8/5-FU and LoVo/5-FU cell lines, therefore blockade of TRPC5 promotes chemosensitivity in these cells [170]. Similarly, TRPC5 expression was induced following doxorubicin treatment in MCF-7, T47D, and MDA-MB-231 breast cancer cells, and its inhibition restored the cytotoxic effects of doxorubicin [171]. Furthermore, elevated TRPC5 in circulating exosomes negatively correlates with chemotherapy outcome in colorectal and breast cancer patients [172], suggesting that increased TRPC5 is associated with chemoresistance. Similarly, TRPC6 was induced by doxorubicin treatment in Huh7 and HepG2 hepatocellular carcinoma cells, therefore its inhibition enhanced doxorubicin-induced cell death [173], suggesting that high TRPC6 level is associated with chemoresistance in hepatocarcinoma cell lines.
TRPM
The TRPM subfamily consists of eight members, TRPM1–8, and each member represents different Ca2+ permeability, ranging from Ca2+- impermeable channels (TRPM4/5, see Na+ channels below) to highly Ca2+-permeable channels (TRPM6/7) [174]. Altered expression or function of TRPM channels is associated with the etiology of various cancers. For instance, decreased TRPM1 is linked to the aggressiveness of melanoma tumours and poor overall survival of melanoma patients, suggesting a tumour suppressor role for TRPM1 [175,176,177]. In contrast, upregulated TRPM2 is correlated with poor overall survival in patients with neuroblastoma and gastric cancer [178,179]. Furthermore, inhibition of TRPM2 expression or function decreased growth and invasion of various cancer cells, including breast, gastric, pancreatic, prostate, head and neck, melanoma, neuroblastoma, leukemia, and lung cancers [180,181,182]. Similarly, TRPM3 has been found upregulated in clear cell renal cell carcinoma cell lines 786-O or A498 and its knockdown or inhibition suppressed growth of tumours generated from renal carcinoma cells in orthotopic xenograft mouse models, suggesting an oncogenic role for TRPM3 in renal cancer [183]. Like TRPM2, TRPM7 is overexpressed in various malignancies [184]. For example, upregulated TRPM7 in pancreatic [185,186], breast [158], ovarian [187], and bladder [188] cancers is correlated with tumour progression and aggression as well as poor overall survival of cancer patients, suggesting a protumour effect of TRPM7. Similarly, TRPM8 is predominantly overexpressed in breast [158,189], pancreas [190,191], and prostate [192,193] cancers where its expression correlates with increased cancer cell proliferation and invasion as well as reduced apoptosis and poor patient survival. In contrast, TRPM8 activation by menthol (a natural ligand for TRPM8) reduced survival of melanoma cells, suggesting its anticancer role in melanoma [194].
Furthermore, the altered expression of several TRPM channels has been associated with anticancer drug resistance. For instance, inhibition of TRPM2 expression or function increased the cytotoxic effect of paclitaxel and doxorubicin in breast and gastric cancer cells [178,195]. A similar effect was observed in TRPM2-depleted neuroblastoma cell lines following treatment with doxorubicin [179]. On the contrary, TRPM7 is downregulated in doxorubicin-resistant colon cancer cell line LoVo-R, therefore its silencing confers further resistance against doxorubicin, suggesting that the reduced expression of TRPM7 is linked to doxorubicin resistance in these cells [196]. A similar chemoresistance-promoting effect was observed for TRPM8 in several cancers. For example, TRPM8 overexpression induces resistance to paclitaxel in prostate cancer cells [197]. Moreover, TRPM8 knockdown in osteosarcoma cells enhances the cytotoxic effect of epirubicin [198]. These pieces of evidence suggest that elevated TRPM8 promotes chemoresistance in prostate cancer and osteosarcoma cell lines.
TRPV
All six members of the TRPV subfamily show variable permeability to Ca2+. While TRPV1–V4 are modestly permeable to Ca2+, TRPV5 and TRPV6 represent high Ca2+ selectivity. In cancer, the expression profile of TRPV channels is highly contextualized; therefore, depending on the cell and tumour type, TRPV channels can act as both tumour promoters and suppressors [199]. For instance, TRPV1 expression is significantly decreased in melanoma tissues and is inversely related to patient survival. Hereby, activation of TRPV1 expression or function inhibits in vitro and in vivo proliferation of melanoma cells [200]. Similar observations have been made in colorectal and renal cancer cells, therefore TRPV1 activation in those cells inhibited proliferation and induced apoptosis [201,202,203]. Furthermore, TRPV1 depletion causes the spontaneous growth of intestinal tumours, highlighting the tumour suppressor function of TRPV1 in intestinal cancer [204]. On the contrary, TRPV1 was found overexpressed in prostate and breast cancers, therefore its inhibition decreased cancer cell survival [205,206,207]. Together, these findings suggest that TRPV1 can act as both a suppressor and an oncogene, based on the biological context.
Furthermore, enhanced expression of TRPV2 in triple-negative breast cancer [208], bladder cancer [209], and esophageal squamous cell carcinoma [210] is linked to cancer progression and poor patient survival. Conversely, reduced TRPV2 expression has been detected in advanced glioma, therefore its exogenous overexpression negatively affected the in vitro and in vivo proliferation of glioma cells, indicating that in different cancers, TRPV2 may function either as a tumour promoter or tumour suppressor [211]. TRPV3 was also found upregulated in colorectal and lung tumours [212,213] and its inhibition caused cell cycle arrest and decreased cancer cell proliferation [212]. Similarly, elevated levels of TRPV4 in breast, gastric, ovarian, and colon cancers correlates with increased cancer cell proliferation, invasion, and poor patient survival [214,215,216]. On the contrary, the expression level of TRPV4 is significantly reduced in advanced endothelial and skin cancers [211,217], suggesting that TRPV4 exhibits both oncogenic or tumour suppressor effects depend on the cancer type [218]. Moreover, TRPV5 was found downregulated in lung [219] and renal [220] tumours, and its reduced expression correlates with poor overall survival and short relapse-free survival of lung cancer patients [219]. On the other hand, upregulated TPPV6 in various cancers, including breast, colon, prostate, parathyroid, and thyroid cancers, enhanced tumour development and progression [221,222]. However, TRPV6 was reported to be downregulated in other cancers such as esophageal [223], lung [219], and renal [220] cancers, indicating a dual function for TRPV6 as a tumour suppressor or tumour promoter in different cancer types.
In addition to the biological roles and prognostic values, TRPVs have been reported to be involved in the regulation of chemoresistance. In this regard, activation of the TRPV1 channel has been shown to enhance the cytotoxic effects of 5-FU [224], cisplatin [225], and doxorubicin [226] in MCF-7 breast cancer cells. Similar effects were observed in bladder cancer cell lines 5637 and T24 after pirarubicin treatment in the presence of activated TRPV1 [227]. Using a molecular dynamic simulation of TRPV1, Ortega-Guerrero et al. demonstrated that TRPV1 channels can mediate doxorubicin diffusion and promote doxorubicin resistance [228], highlighting the therapeutic benefit of TRPV1 activation for improving the efficacy of the conventional chemotherapy drugs. Moreover, overexpression of TRPV2 in MZC glioma cells induces spontaneous chemoresistance [229]. Similarly, TRPV2 activation enhances the cytotoxic effects of temozolomide (TMZ), carmustine (BCNU), and doxorubicin in U87MG and MZC glioma cell lines [230]. TRPV2 activation also promotes bortezomib-induced cell death in RPMI and U166 melanoma-derived cell lines [231], suggesting that combinatorial treatments using TRPV2 activators and chemotherapeutics may represent an effective strategy to improve cancer therapy. In contrast, increased expression of TRPV6 in prostate cancer cell lines LNCaP and PC-3 correlates with resistance to cisplatin and thapsigargin, hence TRPV6 inhibition enhances cytotoxic effect of these drugs [232].
3.2. K+ Channels
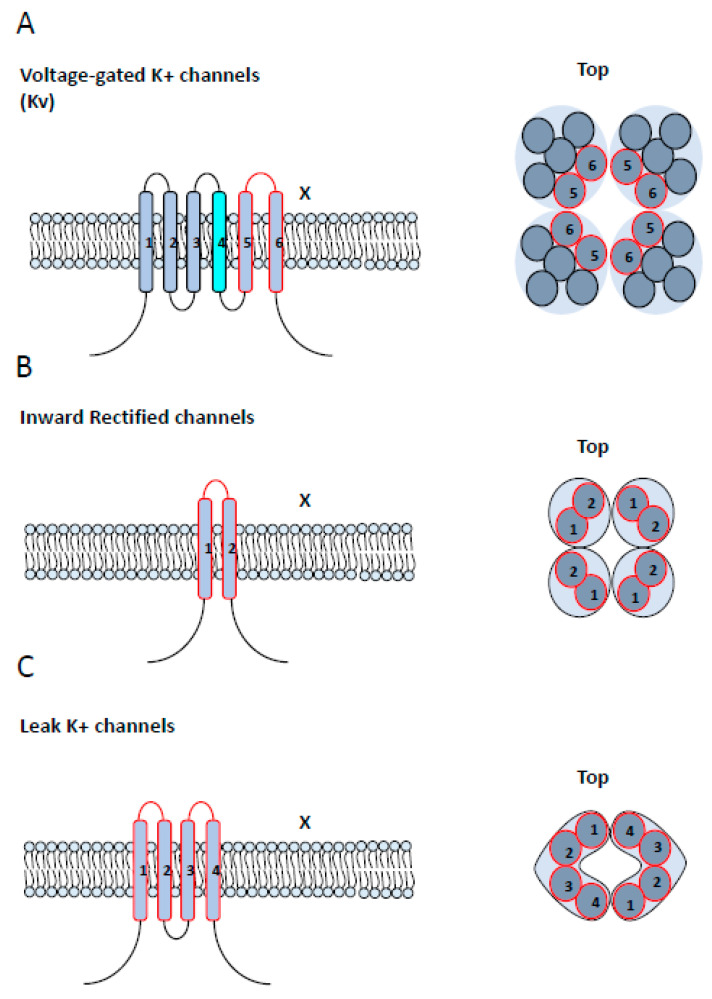
Potassium channels (K+ channels) are a diverse and ubiquitous group of ion channels involved in the maintenance and regulation of K+ gradients. Given the essential role of K+ in the control of cell homeostasis and functions, the proper functioning of K+ channels is crucial for a wide array of cellular functions, ranging from membrane excitability to cell proliferation, migration, and apoptosis [233]. Currently, 78 K+ channels have been identified and divided into four main classes based on their structural and biophysical characteristics: voltage-gated K+ channels (Kv) [234], Ca2+- activated K+ channels (KCa) [235], inwardly rectifying K+ channels (Kir) [236], and two-pore domain K+ channels (K2P) [237]. Kv, KCa, and Kir channels have a modular structure which consists of four subunits that contribute equally to the formation of a central tetrameric pore. The only observed structural difference is that each subunit of Kv and KCa consists of six transmembrane segments (TMSs), while Kir subunits possess two TMSs [238]. On the other hand, K2P channels consist of two subunits, each possessing four TMSs harboring two pore domains, which function as a dimer to form a pseudotetrameric pore (Figure 6) [239].
Figure 6.
Structural classification of K+ channels. (A) Left: the voltage-sensitive (Kv) and calcium-sensitive K+ (Kca) channels show a similar structure with six transmembrane segments (TMS) and a pore-domain formed by TMS 5 and 6, shown in red. The Kv channels differ in that they contain a voltage sensor in TMS4, shown in cyan. Right: A top view of Kv and Kca channels, showing the six TMS of each of the four subunits and their corresponding pore-forming loops, shown in red. The functional channel is a tetramer protein (x4). (B) Left: a lateral view of monomers of an inward rectifier potassium channel (Kir), showing two TMS connected by a pore-forming loop, shown in red. Right: a top view of Kir channel, showing the convergence of four units of Kir channels to the channel pore. The functional channel is a tetramer protein (x4). (C) Left: topology of a two-pore domain potassium channel (K2P), showing four TMS and two pore domains. Right: a top view of K2P channel, showing the convergence of two units of K2P to form the channel pore. The functional channel is a dimer protein (x2).
Alterations in the functional expression of K+ channels have been associated with the etiology of many cancers [240,241]. For instance, Kv1.1 is markedly upregulated in human medulloblastoma and its knockdown reduces in vitro cell growth and improves survival of tumour-bearing mice [242]. Similarly, KCa3.1 is upregulated in various cancers, including intrahepatic cholangiocarcinoma [243], breast cancer [244], and clear cell renal carcinomas [245], and its expression level correlates with tumour progression and poor patient survival. Likewise, elevated expression of Kir2.1 in advanced gastric cancer is associated with both in vitro and in vivo invasion and metastasis [246]. K2P2.1 is upregulated in prostate cancer and its knockdown induces cell cycle arrest and inhibits cell proliferation [247]. Furthermore, K+ channels show differential expression patterns between different cancers and within the same cancer. For instance, Kv11.1 is overexpressed in HT-29 colorectal cancer cells while it was found downregulated in lung carcinoma A549 cells [248]. Likewise, Kv1.3 is upregulated in LNCaP while its expression is reduced in PC3 prostate cancer cells [249].
Moreover, the expression profile of K+ channels can be used to predict cancer cell response to anticancer drugs. The expression level of KCa1.1 channel is reduced in cisplatin-resistant ovarian cancer cells and its knockdown further promotes resistance to cisplatin [250]. Similarly, downregulated KCa2.3 is correlated with platinum resistance in ovarian cancer tissues and poor overall survival of ovarian cancer patients [251]. Reduced expression of KCa3.1 is associated with cisplatin-resistant in epidermoid cancer cells, therefore KCa3.1 activation enhances cisplatin-induced apoptosis in these cells [252]. Furthermore, increased Kv1.5 enhances the cytotoxic effects of doxorubicin in gastric cancer cells, hereby its inhibition promotes chemoresistance [253]. This evidence indicates that decreased expression or activity of several K+ channels is positively correlated with chemoresistance, therefore K+ channel activators can enhance the therapeutic efficacy of conventional chemotherapy drugs. On the contrary, elevated expression of a few K+ channels has been shown to limit the efficacy of various chemotherapeutics. Therefore, inhibition of Kv10.1 promotes doxorubicin- and paclitaxel-induced cell death in breast cancer cell lines [254]. Likewise, Kv11.1 inhibition was reported to enhance the cytotoxic effects of cisplatin in colorectal cancer cells [255]. Together, these findings suggest that activation or inhibition of K+ channels may represent an effective therapeutic approach for improving cancer treatment.
3.3. Na+ Channels
Sodium channels (Na+ channels) are crucial for membrane excitability and cell communication. Depending on their mode of activation, two distinct classes of Na+ channels have been described, voltage-gated sodium channels (VGSC or NaV channels) which open in response to changes in membrane voltage, and ligand-gated sodium channels (LGSC or NaL channels) which are activated by the binding of specific ligands. The following sections focus on the significance of Na+ channels in cancer progression and their impacts on chemoresistance.
3.3.1. VGSCs (NaV Channels)
NaV channels consist of one pore-forming α1 subunit and one or more regulatory β subunits. There are nine different α1 subunits, NaV1.1 to NaV1.9, which all show a modular structure consisting of four domains (I–IV), each of which contains six transmembrane segments (TMSs) (Figure 7). The four domains form a pseudotetramer around a central pore. One or two out of four β subunits (β1–β4) can associate with α1 subunits to regulate biophysical properties and membrane stability of the channel [256]. Various combinations of α1 and β subunits generate nine functionally distinct NaV channels which are variably expressed across human cells and tissues [257]. In addition to the common role of NaV channels in excitable cells, the functional expression of NaV in various non-excitable cells contributes to the regulation of cell functions such as cell proliferation, invasion, and apoptosis [258].
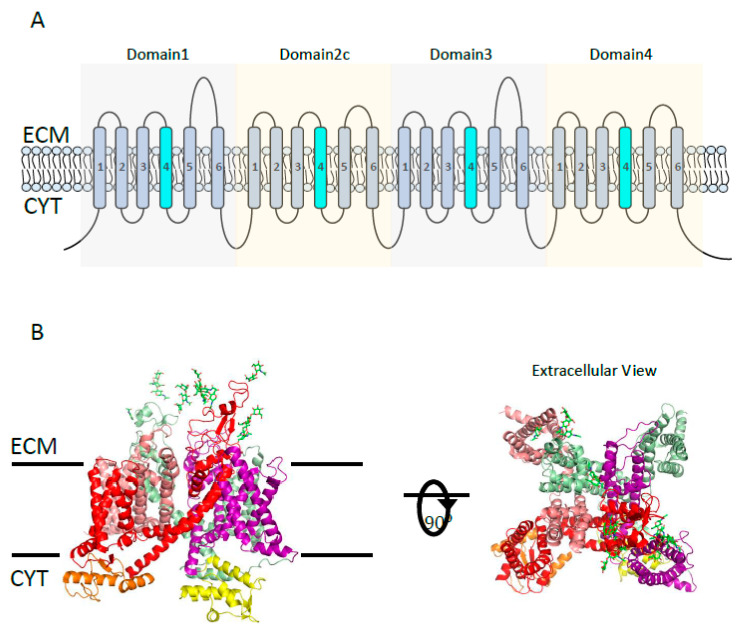
Figure 7.
NaV channel structure. (A) The topology of the α subunit of NaV channel, showing 24 transmembrane segments (TMS) and four domains (D1–4). Each domain consists of 6 TMS, a pore between the 5/6 TMS, and TMS4 as a voltage sensor (shown in cyan). (B) Overall 3D-architecture of the α-subunit of eukaryotic NaV channel (Protein Data Bank [PDB] code 5XOM). Left: the side view of the ΝaV channel domains D1 (red), D2 (salman), D3 (smudge), and D4 (purple) are shown with N- and C-terminal domains colored in orange and yellow, respectively. Glycosylations located in the extracellular loops of D1 and D3 are represented by green sticks. Right: the extracellular view of the NaV channel showing four domains surrounding the channel pore. ECM = extracellular milieu; CYT = cytoplasm.
In cancer, NaV expression is often upregulated, therefore inhibitors of NaV channels have been shown to decrease cancer cell invasion [259]. For example, NaV1.1 and NaV1.3 are highly expressed in ovarian cancer cells, while NaV1.2 and NaV1.4 are predominantly overexpressed in highly metastatic ovarian cancer cells compared to low-metastatic cells [260]. Furthermore, expression of NaV1.5 is increased in several cancers, including ovarian [260], colon [261], and breast cancers [262], therefore its inhibition significantly impairs in vitro and in vivo invasion of breast cancer cells [262]. Similarly, upregulated NaV1.6 was observed in primary cervical cancer cells, and its blockade inhibited invasion of those cells [263]. Furthermore, elevated expression of NaV1.7 induces growth and invasion of prostate [264], gastric [265], and endometrial [266] cancer cells. Like α1 subunits, the expression level of non-pore-forming β subunits of NaV channels is altered in various cancers. For instance, the expression level of the β1 subunit is negatively correlated with breast cancer cell migration, hence its overexpression promotes cell adhesion and reduces migration of MDA-MB-231 cells [267]. On the contrary, the β1 subunit was upregulated in breast cancer specimens compared to non-cancer tissues, where its overexpression promoted breast tumour growth and metastasis to the liver and lungs. This evidence suggests that the expression level of β1 subunits can differentially affect cancer progression depending on the tumour cells and tumour microenvironment [268]. A similar controversy has been observed for the β2 subunit, more particularly, overexpression of the β2 subunit induces migration and invasion of LNCaP prostate cancer cells while it inhibits in vivo tumour formation and reduces tumour volume [269]. This example further emphasizes the context-specific effects of β subunits in cancer. Furthermore, expression of β4 subunit is markedly decreased in thyroid [270], breast [271], and cervical [272] cancers, and its overexpression promotes growth and metastasis in MDA-MB-231 breast cancer cells, suggesting that β4 plays a tumour suppressor role in these cancers [271].
Furthermore, the functional expression of NaV channels has been also indicated to be associated with the chemosensitivity of cancer cells [273]. For instance, NaV inhibitor lidocaine enhances the inhibitory effects of cisplatin on breast tumour metastasis and suppresses in vivo formation of lung colonies [274,275]. Lidocaine also enhanced the cytotoxic effects of cisplatin in hepatocellular carcinoma while inhibiting both tumour growth and metastasis [276]. On the contrary, Tran et al. reported that increased expression of NaV channels sensitizes breast cancer to taxol [277]. Likewise, Adashi et al. showed that the expression level of the β3 subunit increased in colon cancer cells following doxorubicin treatment. Together, this evidence suggests that NaV channels can differentially alter cell responses to anticancer drugs [278].
3.3.2. LGSCs (NaL Channels)
NaL channels are activated by the binding of specific ligands. H+-NaL and Ca2+-NaL channels are the two well-known examples of NaL channels. H+-NaL channels are a group of voltage-insensitive Na+ channels called acid-sensing ion channels (ASICs). Currently, eight subunits of ASICs have been described: ASIC1a, ASIC1b1, ASIC1b2, ASIC2a, ASIC2b, ASIC3, ASIC4, and ASIC5. Each subunit consists of two transmembrane segments that assemble into homo or heterotrimeric complexes around a central pore [279]. ASICs are widely expressed in human cells and tissues, and their expression has been reported to be associated with the etiology of various cancers. For example, ASIC1 and ASIC3 are functionally expressed in the plasma membrane of lung cancer cells, where they contribute to the acidosis-induced cell proliferation and migration [280]. Upregulated ASIC1 and ASIC3 in prostate cancer cells promote in vitro migration and in vivo tumour metastasis [281]. Furthermore, expression levels of ASIC1 and ASIC2 correlate with the progression of low-grade gliomas to high-grade glioma; therefore, their inhibition decreases in vitro migration of glioma cells [282]. Increased ASIC1 has been also reported in glioblastoma, where its inhibition decreases cell migration [283]. In breast cancer cells, ASIC1 knockdown inhibits in vivo tumour growth and metastasis [284]. Together, this evidence suggests that ASIC channels function as tumour promoters in different cancers.
Furthermore, ASIC1a is highly expressed in 5-FU- and doxorubicin-resistant hepatocellular cancer cell lines (Bel7402/FU and HepG2/DOXO) compared to their parental cells (Bel7402 and HepG2). Hereby, inhibition of ASIC1a by amiloride sensitizes Bel7402/FU and HepG2/DOXO cells to 5-FU and doxorubicin, respectively. Furthermore, exogenous overexpression of ASIC1a in Bel7402 and HepG2 cell lines promotes resistance to 5-FU and doxorubicin. These findings suggest that inhibitors of ASIC channels may serve as potential anticancer drugs by improving chemotherapy [285].
Two common Ca2+-NaL channels are TRPM4 and TRPM5, which are monovalent-selective ion channels highly permeable to Na+. These channels open in response to increased intracellular Ca2+ levels. The altered expression profile of TRPM4 and TRPM5 has been associated with the etiology of several cancers. For instance, TRPM4 is increased in cervical and prostate cancers, and its downregulation reduces cancer cell proliferation and migration [286,287,288]. Similarly, upregulated TRPM5 in several cancers including gastric cancer is linked to poor patient survival. Furthermore, in highly metastatic melanoma cancer cells, TRPM5 expression promotes spontaneous lung metastasis [289]. Together, these studies provide evidence on the tumourigenic effect of Ca2+-NaL channels in human cancers.
4. Conclusions
Despite advancements in cancer therapy, cancer remains the second leading cause of death worldwide. Nevertheless, over the last few decades, targeted therapy has played a substantial role in improving the overall survival of cancer patients. Since the late 1990s, several small molecules and antibodies raised against specific tumourigenic proteins have been developed and approved by the U.S. Food and Drug Administration (FDA). However, the lack of druggable targets, compounded by severe toxicity profiles, has imposed a significant roadblock for anticancer drug discovery. Hereby, a growing number of studies are being conducted in order to discover novel and effective therapeutic targets for different malignancies. Recently, profound evidence has elucidated that proteins embedded in mammalian plasma membranes may unlock the fundamental basis for understanding carcinogenesis and disarming chemoresistance. Importantly, most ion channels and pumps are located in the plasma membrane and may serve as accessible and druggable targets for cancer treatment. In this regard, several studies have revealed a direct link between the functional dysregulation of membrane transporters and cancer development. Thus, many membrane transporters have been established as potential therapeutic candidates for cancer treatment. A growing body of evidence suggests that modulation of the expression and function of membrane transport proteins not only impacts cancer progression but also alters the cytotoxic effects of chemotherapeutics in different cancers. Given the diverse mechanisms of chemoresistance operating in human malignancies, discovering new therapeutic targets to enhance the efficacy of chemotherapy is crucial for improving patient outcomes. In this review, we emphasized the strong correlation between membrane transport proteins and carcinogenesis by focusing on three main aspects (Figure 3). First, the expression profile of membrane transporters is often altered in cancers, suggesting that membrane transporters may serve as valuable prognostic and diagnostic markers that can be clinically used to improve cancer detection and to monitor cancer progression. Second, the expression and functional patterns of membrane transporters correlate with response to chemotherapy and patient prognosis. Therefore, drugs that can modulate the expression or/and activity of membrane transporters may hold anticancer therapeutic potential, alone or in combination with conventional chemotherapeutics. Third, the strategic location of membrane transporters makes them easily accessible to pharmacological interventions.
Here we presented evidence on the impact of dysregulated membrane transporters on cancer growth, apoptosis, migration, and response to chemotherapy drugs. However, the extensive studies on the biological roles of membrane transporters in cancer are contrasted by a massive lack of information about their intrinsic properties and structural diversity. Hereby, recognizing the intrinsic regulation, gating kinetics, and structural diversity of membrane transporters is a key step toward uncovering their fundamental impacts on cancer progression and chemoresistance. Furthermore, the therapeutic approaches used to target different transporters have been discussed here; however, the lack of specific and potent drugs that can target distinct membrane transporters limits the therapeutic potential of these proteins. More importantly, developing novel drugs targeting membrane transporters requires further understanding of the biological function and structure of these proteins. Therefore, a better understanding of their structure and function may provide greater insights into their role in cancer progression and treatment as well as pave the way for the development of novel anticancer drugs and improvement of current chemotherapy efficacy.
Acknowledgments
We thank Xingjian Zhai for his insightful comments and edits on this review paper.
Appendix A
Table A1.
Summary of membrane transporter proteins in various cancers, their expression profile, and their role in chemotherapeutic resistance.
| Membrane Transport Protein | Type of Cancer | Cell Lines | Level of Expression or Activity | Chemoresistance |
|---|---|---|---|---|
| Na+/K+ ATPase | Ovarian | 2000, CB10 | Low | Cisplatin [39], oxaliplatin [45] |
| Leukemia | NIH/3T3 | Cisplatin [40] | ||
| Lung | PC-14, SBC-1 | Cisplatin [41,42] | ||
| Prostate | LNCaP | Cisplatin [43] | ||
| Squamous | BHY | Cisplatin [45] | ||
| SERCA | Ovarian | MDAH-2774 | Low | Cisplatin [67] |
| Lung | H1339 | Cisplatin [68] | ||
| V-ATPase | Epidermoid | KB/PC4 | Hight | Cisplatin [104] |
| Prostate | P/CDP5 | Cisplatin [104] | ||
| Leukemia | HL-60 | Cisplatin, vincristine [105] | ||
| Melanoma | Mel P, Mel M | Cisplatin, vinblastine, & 5-FU [109] | ||
| Colon | Colo1, Colo2 | |||
| Breast | SKRB3, JIMT-1 | Trastuzumab [110] | ||
| CaV3 | Ovarian | A2780 | Hight | Carboplatin [133] |
| Prostate | LNCaP, PC3 | Thapsigargin, Paclitaxel [290] | ||
| Orai1 | Ovarian | A2780 | Hight | Cisplatin [143] |
| Hepatocellular | HepG2 | 5-FU [144] | ||
| Pancreas | Panc1 | 5-FU, gemcitabine [145] | ||
| Orai3 | Breast cancer | T47D | Hight | Cisplatin, 5-FU, paclitaxel [146] |
| TRPC1 | Ovarian | A2780, SKOV3 | Low | Cisplatin, Carboplatin [169] |
| TRPC5 | Colorectal | HCT-8, LoVo | High | 5-FU [170] |
| Breast | MCF7, T47D, MDA231 | Doxorubicin [171] | ||
| TRPC6 | Hepatocellular | Huh7 and HepG2 | High | Doxorubicin [173] |
| TRPM2 | Breast | MCF7, MDA231 | High | Doxorubicin, paclitaxel [195] |
| Neuroblastoma | SH-SY5Y | Doxorubicin [179] | ||
| Gastric | AGS, MKN45 | Doxorubicin, paclitaxel [178] | ||
| TRPM7 | Colon | LoVo | Low | Doxorubicin [196] |
| TRPM8 | Prostate | LNCaP | High | Paclitaxel [197] |
| Bone | MG-63, U2OS | Epirubicin [198] | ||
| TRPV1 | Breast | MCF7 | High | Cisplatin [225], Doxorubicin [226], 5-FU [224] |
| Bladder | 5637, T24 | Pirarubicin [227] | ||
| TRPV2 | Glioma | U87MG, MZC | Low | Carmustine, temozolomide, doxorubicin [230] |
| Melanoma | RPMI and U166 | Bortezomib [231] | ||
| TRPV6 | Prostate | LNCaP | High | Cisplatin, thapsigargin [232] |
| KCa1.1 | Ovarian | A2780 | Low | Cisplatin [250] |
| KCa3.1 | Epidermoid | KB, KCP-4 | Low | Cisplatin [252] |
| Kv1.5 | Gastric | SGC7901 | Low | Doxorubicin [253] |
| Kv10.1 | Breast | MDA-MB-435S | High | Doxorubicin, paclitaxel [254] |
| Kv11.1 | Colorectal | HCT116 | High | Cisplatin [255] |
| NaV | Breast cancer | MCF7, MDA231, MDA468, 4T1 | High | Cisplatin [274,275], Taxol [277] |
| Hepatocellular | HepG2 | Cisplatin [276] | ||
| H+-NaL – ASIC1a | Hepatocellular | BEL-7402, HepG2 | High | Doxorubicin, 5-FU [285] |
The switch between the green and orange colors indicates a change in the type of membrane transport protein.
Author Contributions
S.A. and Y.E.H. conceived the original review. S.A. wrote the original draft of the review; Y.E.H. edited and approved the final draft of the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ResearchNS Establishment grant number 2019-2174 and NSERC Discovery grant 2018-05528.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kulbacka J., Choromanska A., Rossowska J., Wezgowiec J., Saczko J., Rols M.P. Cell membrane transport mechanisms: Ion channels and electrical properties of cell membranes. Adv. Anat. Embryol. Cell Biol. 2017;227:39–58. doi: 10.1007/978-3-319-56895-9_3. [DOI] [PubMed] [Google Scholar]
- 2.Prevarskaya N., Skryma R., Shuba Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018;98:559–621. doi: 10.1152/physrev.00044.2016. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita N., Hamada H., Tsuruo T., Ogata E. Enhancement of voltage-gated Na+ channel current associated with multidrug resistance in human leukemia cells. Cancer Res. 1987;47:3736–3741. [PubMed] [Google Scholar]
- 4.Lee S.C., Deutsch C., Beck W.T. Comparison of ion channels in multidrug-resistant and -sensitive human leukemic cells. Proc. Natl. Acad. Sci. USA. 1988;85:2019–2023. doi: 10.1073/pnas.85.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y., Sadee W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006;239:168–182. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y., Anderle P., Bussey K.J., Barbacioru C., Shankavaram U., Dai Z., Reinhold W.C., Papp A., Weinstein J.N., Sadee W. Membrane transporters and channels: Role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004;64:4294–4301. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]
- 7.Prevarskaya N., Skryma R., Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010;16:107–121. doi: 10.1016/j.molmed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Litan A., Langhans S.A. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front. Cell. Neurosci. 2015;9:86. doi: 10.3389/fncel.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadsby D.C. Ion channels versus ion pumps: The principal difference, in principle. Nat. Rev. Mol. Cell Biol. 2009;10:344–352. doi: 10.1038/nrm2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konig J., Muller F., Fromm M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharm. Rev. 2013;65:944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 11.Poguntke M., Hazai E., Fromm M.F., Zolk O. Drug transport by breast cancer resistance protein. Expert. Opin. Drug Metab. Toxicol. 2010;6:1363–1384. doi: 10.1517/17425255.2010.519700. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi T. Drug transporters as targets for cancer chemotherapy. Cancer Genom. Proteom. 2007;4:241–254. [PubMed] [Google Scholar]
- 13.Bosch T.M., Meijerman I., Beijnen J.H., Schellens J.H. Genetic polymorphisms of drug-metabolising enzymes and drug transporters in the chemotherapeutic treatment of cancer. Clin. Pharm. 2006;45:253–285. doi: 10.2165/00003088-200645030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Krisnamurti D.G., Louisa M., Anggraeni E., Wanandi S.I. Drug efflux transporters are overexpressed in short-term tamoxifen-induced MCF7 breast cancer cells. Adv. Pharm. Sci. 2016;2016:6702424. doi: 10.1155/2016/6702424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadlapatla R.K., Vadlapudi A.D., Pal D., Mitra A.K. Mechanisms of drug resistance in cancer chemotherapy: Coordinated role and regulation of efflux transporters and metabolizing enzymes. Curr. Pharm. Des. 2013;19:7126–7140. doi: 10.2174/13816128113199990493. [DOI] [PubMed] [Google Scholar]
- 16.Shinoda T., Ogawa H., Cornelius F., Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature. 2009;459:446–450. doi: 10.1038/nature07939. [DOI] [PubMed] [Google Scholar]
- 17.Morth J.P., Pedersen B.P., Toustrup-Jensen M.S., Sorensen T.L., Petersen J., Andersen J.P., Vilsen B., Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 18.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Castillo J.P., Rui H., Basilio D., Das A., Roux B., Latorre R., Bezanilla F., Holmgren M. Mechanism of potassium ion uptake by the Na(+)/K(+)-ATPase. Nat. Commun. 2015;6:7622. doi: 10.1038/ncomms8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skou J.C., Esmann M. The Na,K-ATPase. J. Bioenerg. Biomembr. 1992;24:249–261. doi: 10.1007/bf00768846. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa H., Shinoda T., Cornelius F., Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc. Natl. Acad. Sci. USA. 2009;106:13742–13747. doi: 10.1073/pnas.0907054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lingrel J.B., Arguello J.M., Van Huysse J., Kuntzweiler T.A. Cation and cardiac glycoside binding sites of the Na,K-ATPase. Ann. N. Y. Acad. Sci. 1997;834:194–206. doi: 10.1111/j.1749-6632.1997.tb52251.x. [DOI] [PubMed] [Google Scholar]
- 23.Rajasekaran S.A., Huynh T.P., Wolle D.G., Espineda C.E., Inge L.J., Skay A., Lassman C., Nicholas S.B., Harper J.F., Reeves A.E., et al. Na,K-ATPase subunits as markers for epithelial-mesenchymal transition in cancer and fibrosis. Mol. Cancer. 2010;9:1515–1524. doi: 10.1158/1535-7163.MCT-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mijatovic T., Dufrasne F., Kiss R. Na+/K+-ATPase and cancer. Pharm Pat. Anal. 2012;1:91–106. doi: 10.4155/ppa.12.3. [DOI] [PubMed] [Google Scholar]
- 25.Espineda C., Seligson D.B., James Ball W., Jr., Rao J., Palotie A., Horvath S., Huang Y., Shi T., Rajasekaran A.K. Analysis of the Na,K-ATPase alpha- and beta-subunit expression profiles of bladder cancer using tissue microarrays. Cancer. 2003;97:1859–1868. doi: 10.1002/cncr.11267. [DOI] [PubMed] [Google Scholar]
- 26.Sakai H., Suzuki T., Maeda M., Takahashi Y., Horikawa N., Minamimura T., Tsukada K., Takeguchi N. Up-regulation of Na(+),K(+)-ATPase alpha 3-isoform and down-regulation of the alpha1-isoform in human colorectal cancer. FEBS Lett. 2004;563:151–154. doi: 10.1016/S0014-5793(04)00292-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q., Li S.B., Zhao Y.Y., Dai D.N., Du H., Lin Y.Z., Ye J.C., Zhao J., Xiao W., Mei Y., et al. Corrigendum to “Identification of a sodium pump Na+/K+ ATPase alpha1-targeted peptide for PET imaging of breast cancer” [Journal of Controlled Release 281C (2018) 178–188] J. Control. Release. 2019;311:324–325. doi: 10.1016/j.jconrel.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Lefranc F., Mijatovic T., Kondo Y., Sauvage S., Roland I., Debeir O., Krstic D., Vasic V., Gailly P., Kondo S., et al. Targeting the alpha 1 subunit of the sodium pump to combat glioblastoma cells. Neurosurgery. 2008;62:211–221. doi: 10.1227/01.NEU.0000311080.43024.0E. [DOI] [PubMed] [Google Scholar]
- 29.Lan Y.L., Zou Y.J., Lou J.C., Xing J.S., Wang X., Zou S., Ma B.B., Ding Y., Zhang B. The sodium pump alpha1 subunit regulates bufalin sensitivity of human glioblastoma cells through the p53 signaling pathway. Cell Biol. Toxicol. 2019;35:521–539. doi: 10.1007/s10565-019-09462-y. [DOI] [PubMed] [Google Scholar]
- 30.Lan Y.L., Yu Z.L., Lou J.C., Ma X.C., Zhang B. Update on the effects of the sodium pump alpha1 subunit on human glioblastoma: From the laboratory to the clinic. Expert Opin. Investig. Drugs. 2018;27:753–763. doi: 10.1080/13543784.2018.1512582. [DOI] [PubMed] [Google Scholar]
- 31.Huang X., Lei Z., Li X.P., El-Mallakh R.S. Response of sodium pump to ouabain challenge in human glioblastoma cells in culture. World J. Biol. Psychiatry. 2009;10:884–892. doi: 10.1080/15622970902995620. [DOI] [PubMed] [Google Scholar]
- 32.Mobasheri A., Fox R., Evans I., Cullingham F., Martin-Vasallo P., Foster C.S. Epithelial Na, K-ATPase expression is down-regulated in canine prostate cancer; a possible consequence of metabolic transformation in the process of prostate malignancy. Cancer Cell Int. 2003;3:8. doi: 10.1186/1475-2867-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mijatovic T., Roland I., Van Quaquebeke E., Nilsson B., Mathieu A., Van Vynckt F., Darro F., Blanco G., Facchini V., Kiss R. The alpha1 subunit of the sodium pump could represent a novel target to combat non-small cell lung cancers. J. Pathol. 2007;212:170–179. doi: 10.1002/path.2172. [DOI] [PubMed] [Google Scholar]
- 34.Rajasekaran S.A., Ball W.J., Jr., Bander N.H., Liu H., Pardee J.D., Rajasekaran A.K. Reduced expression of beta-subunit of Na,K-ATPase in human clear-cell renal cell carcinoma. J. Urol. 1999;162:574–580. doi: 10.1016/S0022-5347(05)68629-6. [DOI] [PubMed] [Google Scholar]
- 35.Rajasekaran S.A., Palmer L.G., Quan K., Harper J.F., Ball W.J., Jr., Bander N.H., Peralta Soler A., Rajasekaran A.K. Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol. Biol. Cell. 2001;12:279–295. doi: 10.1091/mbc.12.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inge L.J., Rajasekaran S.A., Yoshimoto K., Mischel P.S., McBride W., Landaw E., Rajasekaran A.K. Evidence for a potential tumor suppressor role for the Na,K-ATPase beta1-subunit. Histol. Histopathol. 2008;23:459–467. doi: 10.14670/HH-23.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espineda C.E., Chang J.H., Twiss J., Rajasekaran S.A., Rajasekaran A.K. Repression of Na,K-ATPase beta1-subunit by the transcription factor snail in carcinoma. Mol. Biol. Cell. 2004;15:1364–1373. doi: 10.1091/mbc.e03-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mijatovic T., Jungwirth U., Heffeter P., Hoda M.A., Dornetshuber R., Kiss R., Berger W. The Na+/K+-ATPase is the Achilles heel of multi-drug-resistant cancer cells. Cancer Lett. 2009;282:30–34. doi: 10.1016/j.canlet.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 39.Andrews P.A., Mann S.C., Huynh H.H., Albright K.D. Role of the Na+, K(+)-adenosine triphosphatase in the accumulation of cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells. Cancer Res. 1991;51:3677–3681. [PubMed] [Google Scholar]
- 40.Shinohara N., Ogiso Y., Arai T., Takami S., Nonomura K., Koyanagi T., Kuzumaki N. Differential Na+,K(+)-ATPase activity and cisplatin sensitivity between transformants induced by H-ras and those induced by K-ras. Int. J. Cancer. 1994;58:672–677. doi: 10.1002/ijc.2910580510. [DOI] [PubMed] [Google Scholar]
- 41.Ohmori T., Nishio K., Ohta S., Kubota N., Adachi M., Komiya K., Saijo N. Ouabain-resistant non-small-cell lung-cancer cell line shows collateral sensitivity to cis-diamminedichloroplatinum(II) (CDDP) Int. J. Cancer. 1994;57:111–116. doi: 10.1002/ijc.2910570120. [DOI] [PubMed] [Google Scholar]
- 42.Bando T., Fujimura M., Kasahara K., Matsuda T. Significance of Na+,K(+)-ATPase on intracellular accumulation of cis-diamminedichloroplatinum(II) in human non-small-cell but not in small-cell lung cancer cell lines. Anticancer Res. 1998;18:1085–1089. [PubMed] [Google Scholar]
- 43.Blok L.J., Chang G.T., Steenbeek-Slotboom M., van Weerden W.M., Swarts H.G., De Pont J.J., van Steenbrugge G.J., Brinkmann A.O. Regulation of expression of Na+,K+-ATPase in androgen-dependent and androgen-independent prostate cancer. Br. J. Cancer. 1999;81:28–36. doi: 10.1038/sj.bjc.6690647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed Z., Deyama Y., Yoshimura Y., Suzuki K. Cisplatin sensitivity of oral squamous carcinoma cells is regulated by Na+,K+-ATPase activity rather than copper-transporting P-type ATPases, ATP7A and ATP7B. Cancer Chemother. Pharm. 2009;63:643–650. doi: 10.1007/s00280-008-0781-z. [DOI] [PubMed] [Google Scholar]
- 45.Tummala R., Wolle D., Barwe S.P., Sampson V.B., Rajasekaran A.K., Pendyala L. Expression of Na,K-ATPase-beta(1) subunit increases uptake and sensitizes carcinoma cells to oxaliplatin. Cancer Chemother. Pharm. 2009;64:1187–1194. doi: 10.1007/s00280-009-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyoshima C., Nakasako M., Nomura H., Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 47.Altshuler I., Vaillant J.J., Xu S., Cristescu M.E. The evolutionary history of sarco(endo)plasmic calcium ATPase (SERCA) PLoS ONE. 2012;7:e52617. doi: 10.1371/journal.pone.0052617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dally S., Corvazier E., Bredoux R., Bobe R., Enouf J. Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J. Mol. Cell. Cardiol. 2010;48:633–644. doi: 10.1016/j.yjmcc.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Inoue M., Sakuta N., Watanabe S., Zhang Y., Yoshikaie K., Tanaka Y., Ushioda R., Kato Y., Takagi J., Tsukazaki T., et al. Structural basis of sarco/endoplasmic reticulum Ca(2+)-ATPase 2b regulation via transmembrane helix interplay. Cell Rep. 2019;27:1221–1230. doi: 10.1016/j.celrep.2019.03.106. [DOI] [PubMed] [Google Scholar]
- 50.Periasamy M., Kalyanasundaram A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 51.Higgins E.R., Cannell M.B., Sneyd J. A buffering SERCA pump in models of calcium dynamics. Biophys. J. 2006;91:151–163. doi: 10.1529/biophysj.105.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo X., Luo J., Wang H., Chen J. SERCA regulates collective cell migration by maintaining cytoplasmic Ca(2+) homeostasis. J. Genet. Genom. 2019;46:451–454. doi: 10.1016/j.jgg.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Periasamy M., Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J. Mol. Cell. Cardiol. 2001;33:1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 54.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 55.Papp B., Brouland J.P., Arbabian A., Gelebart P., Kovacs T., Bobe R., Enouf J., Varin-Blank N., Apati A. Endoplasmic reticulum calcium pumps and cancer cell differentiation. Biomolecules. 2012;2:165–186. doi: 10.3390/biom2010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prasad V., Boivin G.P., Miller M.L., Liu L.H., Erwin C.R., Warner B.W., Shull G.E. Haploinsufficiency of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump, predisposes mice to squamous cell tumors via a novel mode of cancer susceptibility. Cancer Res. 2005;65:8655–8661. doi: 10.1158/0008-5472.CAN-05-0026. [DOI] [PubMed] [Google Scholar]
- 57.Monteith G.R., McAndrew D., Faddy H.M., Roberts-Thomson S.J. Calcium and cancer: Targeting Ca2+ transport. Nat. Rev. Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 58.Parkash J., Asotra K. Calcium wave signaling in cancer cells. Life Sci. 2010;87:587–595. doi: 10.1016/j.lfs.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korosec B., Glavac D., Rott T., Ravnik-Glavac M. Alterations in the ATP2A2 gene in correlation with colon and lung cancer. Cancer Genet. Cytogenet. 2006;171:105–111. doi: 10.1016/j.cancergencyto.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Pacifico F., Ulianich L., De Micheli S., Treglia S., Leonardi A., Vito P., Formisano S., Consiglio E., Di Jeso B. The expression of the sarco/endoplasmic reticulum Ca2+-ATPases in thyroid and its down-regulation following neoplastic transformation. J. Mol. Endocrinol. 2003;30:399–409. doi: 10.1677/jme.0.0300399. [DOI] [PubMed] [Google Scholar]
- 61.Chung F.Y., Lin S.R., Lu C.Y., Yeh C.S., Chen F.M., Hsieh J.S., Huang T.J., Wang J.Y. Sarco/endoplasmic reticulum calcium-ATPase 2 expression as a tumor marker in colorectal cancer. Am. J. Surg. Pathol. 2006;30:969–974. doi: 10.1097/00000478-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Xu X.Y., Gou W.F., Yang X., Wang G.L., Takahashi H., Yu M., Mao X.Y., Takano Y., Zheng H.C. Aberrant SERCA3 expression is closely linked to pathogenesis, invasion, metastasis, and prognosis of gastric carcinomas. Tumour Biol. 2012;33:1845–1854. doi: 10.1007/s13277-012-0444-x. [DOI] [PubMed] [Google Scholar]
- 63.Gelebart P., Kovacs T., Brouland J.P., van Gorp R., Grossmann J., Rivard N., Panis Y., Martin V., Bredoux R., Enouf J., et al. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. J. Biol. Chem. 2002;277:26310–26320. doi: 10.1074/jbc.M201747200. [DOI] [PubMed] [Google Scholar]
- 64.Azeez J.M., Vini R., Remadevi V., Surendran A., Jaleel A., Santhosh Kumar T.R., Sreeja S. VDAC1 and SERCA3 Mediate Progesterone-Triggered Ca2(+) Signaling in Breast Cancer Cells. J. Proteome Res. 2018;17:698–709. doi: 10.1021/acs.jproteome.7b00754. [DOI] [PubMed] [Google Scholar]
- 65.Papp B., Brouland J.P. Altered endoplasmic reticulum calcium pump expression during breast tumorigenesis. Breast Cancer Basic Clin. Res. 2011;5:163–174. doi: 10.4137/BCBCR.S7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Launay S., Gianni M., Kovacs T., Bredoux R., Bruel A., Gelebart P., Zassadowski F., Chomienne C., Enouf J., Papp B. Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood. 1999;93:4395–4405. doi: 10.1182/blood.V93.12.4395. [DOI] [PubMed] [Google Scholar]
- 67.Kucukkaya B., Basoglu H., Erdag D., Akbas F., Susgun S., Yalcintepe L. Calcium homeostasis in cisplatin resistant epithelial ovarian cancer. Gen. Physiol. Biophys. 2019;38:353–363. doi: 10.4149/gpb_2019013. [DOI] [PubMed] [Google Scholar]
- 68.Schrodl K., Oelmez H., Edelmann M., Huber R.M., Bergner A. Altered Ca2+-homeostasis of cisplatin-treated and low level resistant non-small-cell and small-cell lung cancer cells. Cell. Oncol. 2009;31:301–315. doi: 10.3233/CLO-2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chemaly E.R., Troncone L., Lebeche D. SERCA control of cell death and survival. Cell Calcium. 2018;69:46–61. doi: 10.1016/j.ceca.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casemore D., Xing C. SERCA as a target for cancer therapies. Integr. Cancer Sci. 2015;2:100–103. [Google Scholar]
- 71.Izquierdo-Torres E., Hernandez-Oliveras A., Meneses-Morales I., Rodriguez G., Fuentes-Garcia G., Zarain-Herzberg A. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019;113:37–47. doi: 10.1016/j.biocel.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 72.Fan L., Li A., Li W., Cai P., Yang B., Zhang M., Gu Y., Shu Y., Sun Y., Shen Y., et al. Novel role of Sarco/endoplasmic reticulum calcium ATPase 2 in development of colorectal cancer and its regulation by F36, a curcumin analog. Biomed. Pharm. 2014;68:1141–1148. doi: 10.1016/j.biopha.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Aridoss G., Zhou B., Hermanson D.L., Bleeker N.P., Xing C. Structure-activity relationship (SAR) study of ethyl 2-amino-6-(3,5-dimethoxyphenyl)-4-(2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (CXL017) and the potential of the lead against multidrug resistance in cancer treatment. J. Med. Chem. 2012;55:5566–5581. doi: 10.1021/jm300515q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bleeker N.P., Cornea R.L., Thomas D.D., Xing C. A novel SERCA inhibitor demonstrates synergy with classic SERCA inhibitors and targets multidrug-resistant AML. Mol. Pharm. 2013;10:4358–4366. doi: 10.1021/mp400458u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das S.G., Hermanson D.L., Bleeker N., Lowman X., Li Y., Kelekar A., Xing C. Ethyl 2-Amino-6-(3, 5-dimethoxyphenyl)-4-(2-ethoxy-2-oxoethyl)-4 H-chromene-3-carboxylate (CXL017): A Novel Scaffold That Resensitizes Multidrug Resistant Leukemia Cells to Chemotherapy. ACS Chem. Biol. 2013;8:327–335. doi: 10.1021/cb300460f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maxson M.E., Grinstein S. The vacuolar-type H(+)-ATPase at a glance-more than a proton pump. J. Cell Sci. 2014;127:4987–4993. doi: 10.1242/jcs.158550. [DOI] [PubMed] [Google Scholar]
- 77.Gruber G., Marshansky V. New insights into structure-function relationships between archeal ATP synthase (A1A0) and vacuolar type ATPase (V1V0) Bioessays. 2008;30:1096–1109. doi: 10.1002/bies.20827. [DOI] [PubMed] [Google Scholar]
- 78.Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol. Rev. 1989;69:765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- 79.Manolson M.F., Proteau D., Jones E.W. Evidence for a conserved 95–120 kDa subunit associated with and essential for activity of V-ATPases. J. Exp. Biol. 1992;172:105–112. doi: 10.1242/jeb.172.1.105. [DOI] [PubMed] [Google Scholar]
- 80.Manolson M.F., Proteau D., Preston R.A., Stenbit A., Roberts B.T., Hoyt M.A., Preuss D., Mulholland J., Botstein D., Jones E.W. The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H(+)-ATPase. J. Biol. Chem. 1992;267:14294–14303. [PubMed] [Google Scholar]
- 81.Cross R.L., Muller V. The evolution of A-, F-, and V-type ATP synthases and ATPases: Reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 2004;576:1–4. doi: 10.1016/j.febslet.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 82.Kettner C., Bertl A., Obermeyer G., Slayman C., Bihler H. Electrophysiological analysis of the yeast V-type proton pump: Variable coupling ratio and proton shunt. Biophys. J. 2003;85:3730–3738. doi: 10.1016/S0006-3495(03)74789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stransky L., Cotter K., Forgac M. The function of v-ATPases in cancer. Physiol. Rev. 2016;96:1071–1091. doi: 10.1152/physrev.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun-Wada G.H., Wada Y. Role of vacuolar-type proton ATPase in signal transduction. Biochim. Biophys. Acta. 2015;1847:1166–1172. doi: 10.1016/j.bbabio.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 85.Pamarthy S., Kulshrestha A., Katara G.K., Beaman K.D. The curious case of vacuolar ATPase: Regulation of signaling pathways. Mol. Cancer. 2018;17:41. doi: 10.1186/s12943-018-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hinton A., Bond S., Forgac M. V-ATPase functions in normal and disease processes. Pflügers Arch. Eur. J. Physiol. 2009;457:589–598. doi: 10.1007/s00424-007-0382-4. [DOI] [PubMed] [Google Scholar]
- 87.Sennoune S.R., Martinez-Zaguilan R. Vacuolar H(+)-ATPase signaling pathway in cancer. Curr. Protein Pept. Sci. 2012;13:152–163. doi: 10.2174/138920312800493197. [DOI] [PubMed] [Google Scholar]
- 88.Song T., Jeon H.K., Hong J.E., Choi J.J., Kim T.J., Choi C.H., Bae D.S., Kim B.G., Lee J.W. Proton pump inhibition enhances the cytotoxicity of paclitaxel in cervical cancer. Cancer Res. Treat. J. Korean Cancer Assoc. 2017;49:595–606. doi: 10.4143/crt.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu P., Chen H., Han L., Zou X., Shen W. Expression and role of V1A subunit of V-ATPases in gastric cancer cells. Int. J. Clin. Oncol. 2015;20:725–735. doi: 10.1007/s10147-015-0782-y. [DOI] [PubMed] [Google Scholar]
- 90.Cotter K., Liberman R., Sun-Wada G., Wada Y., Sgroi D., Naber S., Brown D., Breton S., Forgac M. The a3 isoform of subunit a of the vacuolar ATPase localizes to the plasma membrane of invasive breast tumor cells and is overexpressed in human breast cancer. Oncotarget. 2016;7:46142–46157. doi: 10.18632/oncotarget.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katara G.K., Kulshrestha A., Jaiswal M.K., Pamarthy S., Gilman-Sachs A., Beaman K.D. Inhibition of vacuolar ATPase subunit in tumor cells delays tumor growth by decreasing the essential macrophage population in the tumor microenvironment. Oncogene. 2016;35:1058–1065. doi: 10.1038/onc.2015.159. [DOI] [PubMed] [Google Scholar]
- 92.Katara G.K., Jaiswal M.K., Kulshrestha A., Kolli B., Gilman-Sachs A., Beaman K.D. Tumor-associated vacuolar ATPase subunit promotes tumorigenic characteristics in macrophages. Oncogene. 2014;33:5649–5654. doi: 10.1038/onc.2013.532. [DOI] [PubMed] [Google Scholar]
- 93.Chueca E., Apostolova N., Esplugues J.V., Garcia-Gonzalez M.A., Lanas A., Piazuelo E. Proton pump inhibitors display antitumor effects in barrett’s adenocarcinoma cells. Front. Pharm. 2016;7:452. doi: 10.3389/fphar.2016.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Son S.W., Kim S.H., Moon E.Y., Kim D.H., Pyo S., Um S.H. Prognostic significance and function of the vacuolar H+-ATPase subunit V1E1 in esophageal squamous cell carcinoma. Oncotarget. 2016;7:49334–49348. doi: 10.18632/oncotarget.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohta T., Numata M., Yagishita H., Futagami F., Tsukioka Y., Kitagawa H., Kayahara M., Nagakawa T., Miyazaki I., Yamamoto M., et al. Expression of 16 kDa proteolipid of vacuolar-type H(+)-ATPase in human pancreatic cancer. Br. J. Cancer. 1996;73:1511–1517. doi: 10.1038/bjc.1996.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Capecci J., Forgac M. The function of vacuolar ATPase (V-ATPase) a subunit isoforms in invasiveness of MCF10a and MCF10CA1a human breast cancer cells. J. Biol. Chem. 2013;288:32731–32741. doi: 10.1074/jbc.M113.503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cotter K., Capecci J., Sennoune S., Huss M., Maier M., Martinez-Zaguilan R., Forgac M. Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J. Biol. Chem. 2015;290:3680–3692. doi: 10.1074/jbc.M114.611210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michel V., Licon-Munoz Y., Trujillo K., Bisoffi M., Parra K.J. Inhibitors of vacuolar ATPase proton pumps inhibit human prostate cancer cell invasion and prostate-specific antigen expression and secretion. Int. J. Cancer. 2013;132:E1–E10. doi: 10.1002/ijc.27811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishisho T., Hata K., Nakanishi M., Morita Y., Sun-Wada G.H., Wada Y., Yasui N., Yoneda T. The a3 isoform vacuolar type H(+)-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol. Cancer Res. 2011;9:845–855. doi: 10.1158/1541-7786.MCR-10-0449. [DOI] [PubMed] [Google Scholar]
- 100.Kubisch R., Frohlich T., Arnold G.J., Schreiner L., von Schwarzenberg K., Roidl A., Vollmar A.M., Wagner E. V-ATPase inhibition by archazolid leads to lysosomal dysfunction resulting in impaired cathepsin B activation in vivo. Int. J. Cancer. 2014;134:2478–2488. doi: 10.1002/ijc.28562. [DOI] [PubMed] [Google Scholar]
- 101.Hendrix A., Sormunen R., Westbroek W., Lambein K., Denys H., Sys G., Braems G., Van den Broecke R., Cocquyt V., Gespach C., et al. Vacuolar H+ ATPase expression and activity is required for Rab27B-dependent invasive growth and metastasis of breast cancer. Int. J. Cancer. 2013;133:843–854. doi: 10.1002/ijc.28079. [DOI] [PubMed] [Google Scholar]
- 102.Whitton B., Okamoto H., Packham G., Crabb S.J. Vacuolar ATPase as a potential therapeutic target and mediator of treatment resistance in cancer. Cancer Med. 2018;7:3800–3811. doi: 10.1002/cam4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Torigoe T., Izumi H., Ishiguchi H., Uramoto H., Murakami T., Ise T., Yoshida Y., Tanabe M., Nomoto M., Itoh H., et al. Enhanced expression of the human vacuolar H+-ATPase c subunit gene (ATP6L) in response to anticancer agents. J. Biol. Chem. 2002;277:36534–36543. doi: 10.1074/jbc.M202605200. [DOI] [PubMed] [Google Scholar]
- 104.Murakami T., Shibuya I., Ise T., Chen Z.S., Akiyama S., Nakagawa M., Izumi H., Nakamura T., Matsuo K., Yamada Y., et al. Elevated expression of vacuolar proton pump genes and cellular PH in cisplatin resistance. Int. J. Cancer. 2001;93:869–874. doi: 10.1002/ijc.1418. [DOI] [PubMed] [Google Scholar]
- 105.Ma L., Center M.S. The gene encoding vacuolar H+-ATPase subunit C is overexpressed in multidrug resistant HL60 cells. Biochem. Biophys. Res. Commun. 1992;182:675–681. doi: 10.1016/0006-291X(92)91785-O. [DOI] [PubMed] [Google Scholar]
- 106.Ouar Z., Bens M., Vignes C., Paulais M., Pringel C., Fleury J., Cluzeaud F., Lacave R., Vandewalle A. Inhibitors of vacuolar H+-ATPase impair the preferential accumulation of daunomycin in lysosomes and reverse the resistance to anthracyclines in drug-resistant renal epithelial cells. Biochem. J. 2003;370:185–193. doi: 10.1042/bj20021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Supino R., Scovassi A.I., Croce A.C., Dal Bo L., Favini E., Corbelli A., Farina C., Misiano P., Zunino F. Biological effects of a new vacuolar-H,-ATPase inhibitor in colon carcinoma cell lines. Ann. N. Y. Acad. Sci. 2009;1171:606–616. doi: 10.1111/j.1749-6632.2009.04705.x. [DOI] [PubMed] [Google Scholar]
- 108.Petrangolini G., Supino R., Pratesi G., Dal Bo L., Tortoreto M., Croce A.C., Misiano P., Belfiore P., Farina C., Zunino F. Effect of a novel vacuolar-H+-ATPase inhibitor on cell and tumor response to camptothecins. J. Pharm. Exp. 2006;318:939–946. doi: 10.1124/jpet.106.103481. [DOI] [PubMed] [Google Scholar]
- 109.Luciani F., Spada M., De Milito A., Molinari A., Rivoltini L., Montinaro A., Marra M., Lugini L., Logozzi M., Lozupone F., et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J. Natl. Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 110.von Schwarzenberg K., Lajtos T., Simon L., Müller R., Vereb G., Vollmar A.M. V-ATPase inhibition overcomes trastuzumab resistance in breast cancer. Mol. Oncol. 2014;8:9–19. doi: 10.1016/j.molonc.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Molecular Biology of the Cell. 4th ed. Garland Science; New York, NY, USA: 2002. Ion channels and the electrical properties of membranes. [Google Scholar]
- 112.North R.A. Ligand-and Voltage-Gated Ion Channels. CRC Press; Boca Raton, FL, USA: 1995. [Google Scholar]
- 113.Bates E. Ion channels in development and cancer. Annu. Rev. Cell Dev. Biol. 2015;31:231–247. doi: 10.1146/annurev-cellbio-100814-125338. [DOI] [PubMed] [Google Scholar]
- 114.Leanza L., Biasutto L., Managò A., Gulbins E., Zoratti M., Szabò I. Intracellular ion channels and cancer. Front. Physiol. 2013;4:227. doi: 10.3389/fphys.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peruzzo R., Biasutto L., Szabò I., Leanza L. Impact of intracellular ion channels on cancer development and progression. Eur. Biophys. J. 2016;45:685–707. doi: 10.1007/s00249-016-1143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sterea A.M., Almasi S., El Hiani Y. The hidden potential of lysosomal ion channels: A new era of oncogenes. Cell Calcium. 2018;72:91–103. doi: 10.1016/j.ceca.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 117.Sterea A.M., El Hiani Y. Calcium Signaling. Springer; Berlin, Germany: 2020. The role of mitochondrial calcium signaling in the pathophysiology of cancer cells; pp. 747–770. [DOI] [PubMed] [Google Scholar]
- 118.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 119.Yang D., Kim J. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep. 2020;53:125–132. doi: 10.5483/BMBRep.2020.53.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chalmers S.B., Monteith G.R. ORAI channels and cancer. Cell Calcium. 2018;74:160–167. doi: 10.1016/j.ceca.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 121.Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fiske J.L., Fomin V.P., Brown M.L., Duncan R.L., Sikes R.A. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25:493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 123.Rao V.R., Perez-Neut M., Kaja S., Gentile S. Voltage-gated ion channels in cancer cell proliferation. Cancers. 2015;7:849–875. doi: 10.3390/cancers7020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiao X., Li B.X., Mitton B., Ikeda A., Sakamoto K.M. Targeting CREB for cancer therapy: Friend or foe. Curr. Cancer Drug Targets. 2010;10:384–391. doi: 10.2174/156800910791208535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Buchanan P.J., McCloskey K.D. Ca V channels and cancer: Canonical functions indicate benefits of repurposed drugs as cancer therapeutics. Eur. Biophys. J. 2016;45:621–633. doi: 10.1007/s00249-016-1144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi Z.-Z., Shang L., Jiang Y.-Y., Shi F., Xu X., Wang M.-R., Hao J.-J. Identification of genomic biomarkers associated with the clinicopathological parameters and prognosis of esophageal squamous cell carcinoma. Cancer Biomark. 2015;15:755–761. doi: 10.3233/CBM-150517. [DOI] [PubMed] [Google Scholar]
- 127.Hao J., Bao X., Jin B., Wang X., Mao Z., Li X., Wei L., Shen D., Wang J.-L. Ca2+ channel subunit α 1D promotes proliferation and migration of endometrial cancer cells mediated by 17β-estradiol via the G protein-coupled estrogen receptor. FASEB J. 2015;29:2883–2893. doi: 10.1096/fj.14-265603. [DOI] [PubMed] [Google Scholar]
- 128.Chen R., Zeng X., Zhang R., Huang J., Kuang X., Yang J., Liu J., Tawfik O., Thrasher J.B., Li B. Cav1. 3 channel α1D protein is overexpressed and modulates androgen receptor transactivation in prostate cancers. Urol. Oncol. 2014;32:524–536. doi: 10.1016/j.urolonc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 129.Natrajan R., Little S.E., Reis-Filho J.S., Hing L., Messahel B., Grundy P.E., Dome J.S., Schneider T., Vujanic G.M., Pritchard-Jones K. Amplification and overexpression of CACNA1E correlates with relapse in favorable histology Wilms’ tumors. Clin. Cancer Res. 2006;12:7284–7293. doi: 10.1158/1078-0432.CCR-06-1567. [DOI] [PubMed] [Google Scholar]
- 130.Yu W., Wang P., Ma H., Zhang G., Yulin Z., Lu B., Wang H., Dong M. Suppression of T-type Ca2+ channels inhibited human laryngeal squamous cell carcinoma cell proliferation running title: Roles of T-type Ca2+ channels in LSCC cell proliferation. Clin. Lab. 2014;60:621–628. doi: 10.7754/Clin.Lab.2013.130614. [DOI] [PubMed] [Google Scholar]
- 131.Valerie N.C., Dziegielewska B., Hosing A.S., Augustin E., Gray L.S., Brautigan D.L., Larner J.M., Dziegielewski J. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem. Pharmacol. 2013;85:888–897. doi: 10.1016/j.bcp.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 132.Nie C., Qin X., Li X., Tian B., Zhao Y., Jin Y., Li Y., Wang Q., Zeng D., Hong A. CACNA2D3 enhances the chemosensitivity of esophageal squamous cell carcinoma to cisplatin via inducing ca2+-mediated apoptosis and suppressing PI3K/Akt pathways. Front. Oncol. 2019;9:185. doi: 10.3389/fonc.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dziegielewska B., Casarez E.V., Yang W.Z., Gray L.S., Dziegielewski J., Slack-Davis J.K. T-type Ca2+ channel inhibition sensitizes ovarian cancer to carboplatin. Mol. Cancer Ther. 2016;15:460–470. doi: 10.1158/1535-7163.MCT-15-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hou X., Pedi L., Diver M.M., Long S.B. Crystal structure of the calcium release–activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shuba Y.M. Ca(2+) channel-forming ORAI proteins: Cancer foes or cancer allies? Exp. Oncol. 2019;41:200–206. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-3.13473. [DOI] [PubMed] [Google Scholar]
- 136.Fiorio Pla A., Kondratska K., Prevarskaya N. STIM and ORAI proteins: Crucial roles in hallmarks of cancer. Am. J. Physiol. Physiol. 2016;310:C509–C519. doi: 10.1152/ajpcell.00364.2015. [DOI] [PubMed] [Google Scholar]
- 137.Dubois C., Vanden Abeele F., Lehen’kyi V., Gkika D., Guarmit B., Lepage G., Slomianny C., Borowiec A.S., Bidaux G., Benahmed M., et al. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26:19–32. doi: 10.1016/j.ccr.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 138.Wang L., Hao J., Zhang Y., Yang Z., Cao Y., Lu W., Shu Y., Jiang L., Hu Y., Lv W. Orai1 mediates tumor-promoting store-operated Ca2+ entry in human gastrointestinal stromal tumors via c-KIT and the extracellular signal–regulated kinase pathway. Tumor Biol. 2017;39:1010428317691426. doi: 10.1177/1010428317691426. [DOI] [PubMed] [Google Scholar]
- 139.Zhu H., Zhang H., Jin F., Fang M., Huang M., Yang C.S., Chen T., Fu L., Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5:3455. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Faouzi M., Hague F., Potier M., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J. Cell. Physiol. 2011;226:542–551. doi: 10.1002/jcp.22363. [DOI] [PubMed] [Google Scholar]
- 141.Ay A.-S., Benzerdjerb N., Sevestre H., Ahidouch A., Ouadid-Ahidouch H. Orai3 constitutes a native store-operated calcium entry that regulates non small cell lung adenocarcinoma cell proliferation. PLoS ONE. 2013;8:e72889. doi: 10.1371/journal.pone.0072889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kischel P., Girault A., Rodat-Despoix L., Chamlali M., Radoslavova S., Abou Daya H., Lefebvre T., Foulon A., Rybarczyk P., Hague F., et al. Ion channels: New actors playing in chemotherapeutic resistance. Cancers. 2019:11. doi: 10.3390/cancers11030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schmidt S., Liu G., Liu G., Yang W., Honisch S., Pantelakos S., Stournaras C., Hönig A., Lang F. Enhanced Orai1 and STIM1 expression as well as store operated Ca2+ entry in therapy resistant ovary carcinoma cells. Oncotarget. 2014;5:4799. doi: 10.18632/oncotarget.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang B.D., Xia X., Lv X.F., Yu B.X., Yuan J.N., Mai X.Y., Shang J.Y., Zhou J.G., Liang S.J., Pang R.P. Inhibition of Orai1-mediated Ca2+ entry enhances chemosensitivity of HepG2 hepatocarcinoma cells to 5-fluorouracil. J. Cell. Mol. Med. 2017;21:904–915. doi: 10.1111/jcmm.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kondratska K., Kondratskyi A., Yassine M., Lemonnier L., Lepage G., Morabito A., Skryma R., Prevarskaya N. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843:2263–2269. doi: 10.1016/j.bbamcr.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 146.Hasna J., Hague F., Rodat-Despoix L., Geerts D., Leroy C., Tulasne D., Ouadid-Ahidouch H., Kischel P. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: The p53 connection. Cell Death Differ. 2018;25:691. doi: 10.1038/s41418-017-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li H. Transient Receptor Potential Canonical Channels and Brain Diseases. Springer; Berlin, Germany: 2017. TRP channel classification; pp. 1–8. [Google Scholar]
- 148.Owsianik G., D’hoedt D., Voets T., Nilius B. Structure-function relationship of the TRP channel superfamily. Rev. Physiol. Biochem. Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- 149.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 150.Gees M., Colsoul B., Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Minke B. TRP channels and Ca2+ signaling. Cell Calcium. 2006;40:261–275. doi: 10.1016/j.ceca.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Canales J., Morales D., Blanco C., Rivas J., Diaz N., Angelopoulos I., Cerda O. A TR(i)P to cell migration: New roles of trp channels in mechanotransduction and cancer. Front. Physiol. 2019;10:757. doi: 10.3389/fphys.2019.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shapovalov G., Ritaine A., Skryma R., Prevarskaya N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016;38:357–369. doi: 10.1007/s00281-015-0525-1. [DOI] [PubMed] [Google Scholar]
- 154.Chen J., Luan Y., Yu R., Zhang Z., Zhang J., Wang W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci. Trends. 2014;8:1–10. doi: 10.5582/bst.8.1. [DOI] [PubMed] [Google Scholar]
- 155.Lehen’kyi V., Prevarskaya N. Study of TRP Channels in Cancer Cells. In: Zhu M.X., editor. TRP Channels. CRC Press; Boca Raton, FL, USA: 2011. [PubMed] [Google Scholar]
- 156.Santoni G., Maggi F., Morelli M.B., Santoni M., Marinelli O. Transient receptor potential cation channels in cancer therapy. Med. Sci. 2019;7:108. doi: 10.3390/medsci7120108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Birnbaumer L. The TRPC class of ion channels: A critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu. Rev. Pharmacol. Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 158.Dhennin-Duthille I., Gautier M., Faouzi M., Guilbert A., Brevet M., Vaudry D., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: Correlation with pathological parameters. Cell. Physiol. Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 159.El Hiani Y., Ahidouch A., Lehen’kyi V., Hague F., Gouilleux F., Mentaverri R., Kamel S., Lassoued K., Brule G., Ouadid-Ahidouch H. Extracellular signal-regulated kinases 1 and 2 and TRPC1 channels are required for calcium-sensing receptor-stimulated MCF-7 breast cancer cell proliferation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2009;23:335–346. doi: 10.1159/000218179. [DOI] [PubMed] [Google Scholar]
- 160.El Hiani Y., Lehen’kyi V., Ouadid-Ahidouch H., Ahidouch A. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch. Biochem. Biophys. 2009;486:58–63. doi: 10.1016/j.abb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 161.Elzamzamy O.M., Penner R., Hazlehurst L.A. The role of TRPC1 in modulating cancer progression. Cells. 2020;9:388. doi: 10.3390/cells9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang S., Cao Q., Zhou K., Feng Y., Wang Y. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28:1320–1328. doi: 10.1038/onc.2008.475. [DOI] [PubMed] [Google Scholar]
- 163.Tiapko O., Groschner K. TRPC3 as a target of novel therapeutic interventions. Cells. 2018;7:83. doi: 10.3390/cells7070083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ding X., He Z., Zhou K., Cheng J., Yao H., Lu D., Cai R., Jin Y., Dong B., Xu Y. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J. Natl. Cancer Inst. 2010;102:1052–1068. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- 165.Cai R., Ding X., Zhou K., Shi Y., Ge R., Ren G., Jin Y., Wang Y. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int. J. Cancer. 2009;125:2281–2287. doi: 10.1002/ijc.24551. [DOI] [PubMed] [Google Scholar]
- 166.Aydar E., Yeo S., Djamgoz M., Palmer C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: A potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009;9:23. doi: 10.1186/1475-2867-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Veliceasa D., Ivanovic M., Hoepfner F.T.S., Thumbikat P., Volpert O.V., Smith N.D. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J. 2007;274:6365–6377. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]
- 168.Carson C., Raman P., Tullai J., Xu L., Henault M., Thomas E., Yeola S., Lao J., McPate M., Verkuyl J.M. Englerin A agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS ONE. 2015:10. doi: 10.1371/journal.pone.0127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu X., Zou J., Su J., Lu Y., Zhang J., Li L., Yin F. Down regulation of transient receptor potential cation channel, subfamily C, member 1 contributes to drug resistance and high histological grade in ovarian cancer. Int. J. Oncol. 2016;48:243–252. doi: 10.3892/ijo.2015.3254. [DOI] [PubMed] [Google Scholar]
- 170.Wang T., Chen Z., Zhu Y., Pan Q., Liu Y., Qi X., Jin L., Jin J., Ma X., Hua D. Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. J. Biol. Chem. 2015;290:448–456. doi: 10.1074/jbc.M114.590364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang P., Liu X., Li H., Chen Z., Yao X., Jin J., Ma X. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-03230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang T., Ning K., Lu T.X., Sun X., Jin L., Qi X., Jin J., Hua D. Increasing circulating exosomes-carrying TRPC 5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci. 2017;108:448–454. doi: 10.1111/cas.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wen L., Liang C., Chen E., Chen W., Liang F., Zhi X., Wei T., Xue F., Li G., Yang Q. Regulation of Multi-drug Resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huang Y., Fliegert R., Guse A.H., Lü W., Du J. A Structural overview of the ion channels of the TRPM family. Cell Calcium. 2019;85:102111. doi: 10.1016/j.ceca.2019.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Duncan L.M., Deeds J., Cronin F.E., Donovan M., Sober A.J., Kauffman M., McCarthy J.J. Melastatin expression and prognosis in cutaneous malignant melanoma. J. Clin. Oncol. 2001;19:568–576. doi: 10.1200/JCO.2001.19.2.568. [DOI] [PubMed] [Google Scholar]
- 176.Deeds J., Cronin F., Duncan L.M. Patterns of melastatin mRNA expression in melanocytic tumors. Hum. Pathol. 2000;31:1346–1356. doi: 10.1016/S0046-8177(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 177.Duncan L.M., Deeds J., Hunter J., Shao J., Holmgren L.M., Woolf E.A., Tepper R.I., Shyjan A.W. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- 178.Almasi S., Kennedy B.E., El-Aghil M., Sterea A.M., Gujar S., Partida-Sanchez S., El Hiani Y. TRPM2 channel-mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway. J. Biol. Chem. 2018;293:3637–3650. doi: 10.1074/jbc.M117.817635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bao L., Chen S.J., Conrad K., Keefer K., Abraham T., Lee J.P., Wang J., Zhang X.Q., Hirschler-Laszkiewicz I., Wang H.G., et al. Depletion of the human ion channel TRPM2 in neuroblastoma demonstrates its key role in cell survival through modulation of mitochondrial reactive oxygen species and bioenergetics. J. Biol. Chem. 2016;291:24449–24464. doi: 10.1074/jbc.M116.747147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miller B.A. TRPM2 in cancer. Cell Calcium. 2019;80:8–17. doi: 10.1016/j.ceca.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Almasi S., Long C.Y., Sterea A., Clements D.R., Gujar S., El Hiani Y. TRPM2 silencing causes G2/M arrest and apoptosis in lung cancer cells via increasing intracellular ROS and RNS levels and activating the JNK pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019;52:742–757. doi: 10.33594/000000052. [DOI] [PubMed] [Google Scholar]
- 182.Almasi S., Sterea A.M., Fernando W., Clements D.R., Marcato P., Hoskin D.W., Gujar S., El Hiani Y. TRPM2 ion channel promotes gastric cancer migration, invasion and tumor growth through the AKT signaling pathway. Sci. Rep. 2019;9:4182. doi: 10.1038/s41598-019-40330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hall D.P., Cost N.G., Hegde S., Kellner E., Mikhaylova O., Stratton Y., Ehmer B., Abplanalp W.A., Pandey R., Biesiada J. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell. 2014;26:738–753. doi: 10.1016/j.ccell.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yee N.S. Role of TRPM7 in cancer: Potential as molecular biomarker and therapeutic target. Pharmaceuticals. 2017;10:39. doi: 10.3390/ph10020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rybarczyk P., Gautier M., Hague F., Dhennin-Duthille I., Chatelain D., Kerr-Conte J., Pattou F., Regimbeau J.M., Sevestre H., Ouadid-Ahidouch H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer. 2012;131:E851–E861. doi: 10.1002/ijc.27487. [DOI] [PubMed] [Google Scholar]
- 186.Yee N.S., Kazi A.A., Li Q., Yang Z., Berg A., Yee R.K. Aberrant over-expression of TRPM7 ion channels in pancreatic cancer: Required for cancer cell invasion and implicated in tumor growth and metastasis. Biol. Open. 2015;4:507–514. doi: 10.1242/bio.20137088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang J., Xiao L., Luo C.-H., Zhou H., Hu J., Tang Y.-X., Fang K.-N., Zhang Y. Overexpression of TRPM7 is associated with poor prognosis in human ovarian carcinoma. Asian Pac. J. Cancer Prev. 2014;15:3955–3958. doi: 10.7314/APJCP.2014.15.9.3955. [DOI] [PubMed] [Google Scholar]
- 188.Gao S.-L., Kong C.-Z., Zhang Z., Li Z.-L., Bi J.-B., Liu X.-K. TRPM7 is overexpressed in bladder cancer and promotes proliferation, migration, invasion and tumor growth. Oncol. Rep. 2017;38:1967–1976. doi: 10.3892/or.2017.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu J., Chen Y., Shuai S., Ding D., Li R., Luo R. TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumor Biol. 2014;35:8969–8977. doi: 10.1007/s13277-014-2077-8. [DOI] [PubMed] [Google Scholar]
- 190.Yee N.S., Zhou W., Lee M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett. 2010;297:49–55. doi: 10.1016/j.canlet.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yee N.S., Li Q., Kazi A.A., Yang Z., Berg A., Yee R.K. Aberrantly over-expressed TRPM8 channels in pancreatic adenocarcinoma: Correlation with tumor size/stage and requirement for cancer cells invasion. Cells. 2014;3:500–516. doi: 10.3390/cells3020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Peng M., Wang Z., Yang Z., Tao L., Liu Q., Yi L., Wang X. Overexpression of short TRPM8 variant α promotes cell migration and invasion, and decreases starvation-induced apoptosis in prostate cancer LNCaP cells. Oncol. Lett. 2015;10:1378–1384. doi: 10.3892/ol.2015.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fuessel S., Sickert D., Meye A., Klenk U., Schmidt U., Schmitz M., Rost A.-K., Weigle B., Kiessling A., Wirth M.P. Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int. J. Oncol. 2003;23:221–228. doi: 10.3892/ijo.23.1.221. [DOI] [PubMed] [Google Scholar]
- 194.Yamamura H., Ugawa S., Ueda T., Morita A., Shimada S. TRPM8 activation suppresses cellular viability in human melanoma. Am. J. Physiol. Cell Physiol. 2008;295:C296–C301. doi: 10.1152/ajpcell.00499.2007. [DOI] [PubMed] [Google Scholar]
- 195.Koh D.W., Powell D.P., Blake S.D., Hoffman J.L., Hopkins M.M., Feng X. Enhanced cytotoxicity in triple-negative and estrogen receptor-positive breast adenocarcinoma cells due to inhibition of the transient receptor potential melastatin-2 channel. Oncol. Rep. 2015;34:1589–1598. doi: 10.3892/or.2015.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Castiglioni S., Cazzaniga A., Trapani V., Cappadone C., Farruggia G., Merolle L., Wolf F.I., Iotti S., Maier J.A. Magnesium homeostasis in colon carcinoma LoVo cells sensitive or resistant to doxorubicin. Sci. Rep. 2015;5:16538. doi: 10.1038/srep16538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yu S., Xu Z., Zou C., Wu D., Wang Y., Yao X., Ng C.F., Chan F.L. Ion channel TRPM8 promotes hypoxic growth of prostate cancer cells via an O2-independent and RACK1-mediated mechanism of HIF-1α stabilization. J. Pathol. 2014;234:514–525. doi: 10.1002/path.4413. [DOI] [PubMed] [Google Scholar]
- 198.Wang Y., Yang Z., Meng Z., Cao H., Zhu G., Liu T., Wang X. Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int. J. Biol. Sci. 2014;10:90. doi: 10.7150/ijbs.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Santoni G., Farfariello V., Amantini C. Transient Receptor Potential Channels. Springer; Berlin, Germany: 2011. TRPV channels in tumor growth and progression; pp. 947–967. [DOI] [PubMed] [Google Scholar]
- 200.Yang Y., Guo W., Ma J., Xu P., Zhang W., Guo S., Liu L., Ma J., Shi Q., Jian Z. Downregulated TRPV1 expression contributes to melanoma growth via the calcineurin-ATF3-p53 pathway. J. Investig. Dermatol. 2018;138:2205–2215. doi: 10.1016/j.jid.2018.03.1510. [DOI] [PubMed] [Google Scholar]
- 201.Hou N., He X., Yang Y., Fu J., Zhang W., Guo Z., Hu Y., Liang L., Xie W., Xiong H. TRPV1 induced apoptosis of colorectal cancer cells by activating calcineurin-NFAT2-p53 signaling pathway. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/6712536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wu Y.-Y., Liu X.-Y., Zhuo D.-X., Huang H.-B., Zhang F.-B., Liao S.-F. Decreased expression of TRPV1 in renal cell carcinoma: Association with tumor Fuhrman grades and histopathological subtypes. Cancer Manag. Res. 2018;10:1647. doi: 10.2147/CMAR.S166390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu T., Wang G., Tao H., Yang Z., Wang Y., Meng Z., Cao R., Xiao Y., Wang X., Zhou J. Capsaicin mediates caspases activation and induces apoptosis through P38 and JNK MAPK pathways in human renal carcinoma. BMC Cancer. 2016;16:790. doi: 10.1186/s12885-016-2831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.de Jong P.R., Takahashi N., Harris A.R., Lee J., Bertin S., Jeffries J., Jung M., Duong J., Triano A.I., Lee J. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J. Clin. Investig. 2014;124:3793–3806. doi: 10.1172/JCI72340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morelli M.B., Amantini C., Nabissi M., Liberati S., Cardinali C., Farfariello V., Tomassoni D., Quaglia W., Piergentili A., Bonifazi A. Cross-talk between alpha 1D-adrenoceptors and transient receptor potential vanilloid type 1 triggers prostate cancer cell proliferation. BMC Cancer. 2014;14:921. doi: 10.1186/1471-2407-14-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Weber L.V., Al-Refae K., Wölk G., Bonatz G., Altmüller J., Becker C., Gisselmann G., Hatt H. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer Targets Ther. 2016;8:243. doi: 10.2147/BCTT.S121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pecze L., Jósvay K., Blum W., Petrovics G., Vizler C., Oláh Z., Schwaller B. Activation of endogenous TRPV1 fails to induce overstimulation-based cytotoxicity in breast and prostate cancer cells but not in pain-sensing neurons. Biochim. Biophys. Acta Mol. Cell Res. 2016;1863:2054–2064. doi: 10.1016/j.bbamcr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 208.Elbaz M., Ahirwar D., Xiaoli Z., Zhou X., Lustberg M., Nasser M.W., Shilo K., Ganju R.K. TRPV2 is a novel biomarker and therapeutic target in triple negative breast cancer. Oncotarget. 2018;9:33459. doi: 10.18632/oncotarget.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Caprodossi S., Lucciarini R., Amantini C., Nabissi M., Canesin G., Ballarini P., Di Spilimbergo A., Cardarelli M.A., Servi L., Mammana G. Transient receptor potential vanilloid type 2 (TRPV2) expression in normal urothelium and in urothelial carcinoma of human bladder: Correlation with the pathologic stage. Eur. Urol. 2008;54:612–620. doi: 10.1016/j.eururo.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 210.Zhou K., Zhang S.-S., Yan Y., Zhao S. Overexpression of transient receptor potential vanilloid 2 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Med. Oncol. 2014;31:17. doi: 10.1007/s12032-014-0017-5. [DOI] [PubMed] [Google Scholar]
- 211.Santoni G., Amantini C., Maggi F., Marinelli O., Santoni M., Nabissi M., Morelli M.B. The TRPV2 cation channels: From urothelial cancer invasiveness to glioblastoma multiforme interactome signature. Lab. Investig. 2019;100:1–13. doi: 10.1038/s41374-019-0333-7. [DOI] [PubMed] [Google Scholar]
- 212.Li X., Zhang Q., Fan K., Li B., Li H., Qi H., Guo J., Cao Y., Sun H. Overexpression of TRPV3 correlates with tumor progression in non-small cell lung cancer. Int. J. Mol. Sci. 2016;17:437. doi: 10.3390/ijms17040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hoeft B., Linseisen J., Beckmann L., Müller-Decker K., Canzian F., Hüsing A., Kaaks R., Vogel U., Jakobsen M.U., Overvad K. Polymorphisms in fatty acid metabolism-related genes are associated with colorectal cancer risk. Carcinogenesis. 2010;31:466–472. doi: 10.1093/carcin/bgp325. [DOI] [PubMed] [Google Scholar]
- 214.Lee W.H., Choong L.Y., Mon N.N., Lu S., Lin Q., Pang B., Yan B., Krishna V.S.R., Singh H., Tan T.Z. TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci. Rep. 2016;6:27903. doi: 10.1038/srep27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fang Y., Liu G., Xie C., Qian K., Lei X., Liu Q., Liu G., Cao Z., Fu J., Du H. Pharmacological inhibition of TRPV4 channel suppresses malignant biological behavior of hepatocellular carcinoma via modulation of ERK signaling pathway. Biomed. Pharmacother. 2018;101:910–919. doi: 10.1016/j.biopha.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 216.Liu X., Zhang P., Xie C., Sham K.W., Ng S.S., Chen Y., Cheng C.H. Activation of PTEN by inhibition of TRPV4 suppresses colon cancer development. Cell Death Dis. 2019;10:1–14. doi: 10.1038/s41419-019-1700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Adapala R.K., Thoppil R.J., Ghosh K., Cappelli H., Dudley A.C., Paruchuri S., Keshamouni V., Klagsbrun M., Meszaros J.G., Chilian W.M. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35:314–322. doi: 10.1038/onc.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yu S., Huang S., Ding Y., Wang W., Wang A., Lu Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019;10:1–17. doi: 10.1038/s41419-019-1708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fan H., Shen Y.-X., Yuan Y.-F. Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac. J. Cancer Prev. 2014;15:2559–2563. doi: 10.7314/APJCP.2014.15.6.2559. [DOI] [PubMed] [Google Scholar]
- 220.Wu Y., Miyamoto T., Li K., Nakagomi H., Sawada N., Kira S., Kobayashi H., Zakohji H., Tsuchida T., Fukazawa M. Decreased expression of the epithelial Ca2+ channel TRPV5 and TRPV6 in human renal cell carcinoma associated with vitamin D receptor. J. Urol. 2011;186:2419–2425. doi: 10.1016/j.juro.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 221.Lehen’kyi V.Y., Raphaël M., Prevarskaya N. The role of the TRPV6 channel in cancer. J. Physiol. 2012;590:1369–1376. doi: 10.1113/jphysiol.2011.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stewart J.M. TRPV6 as a target for cancer therapy. J. Cancer. 2020;11:374. doi: 10.7150/jca.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang S.-S., Xie X., Wen J., Luo K.-J., Liu Q.-W., Yang H., Hu Y., Fu J.-H. TRPV6 plays a new role in predicting survival of patients with esophageal squamous cell carcinoma. Diagn. Pathol. 2016;11:14. doi: 10.1186/s13000-016-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Deveci H.A., Nazıroğlu M., Nur G. 5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in MCF-7 human breast cancer cells by TRPV1 channel activation but not Hypericum perforatum treatment. Mol. Cell. Biochem. 2018;439:189–198. doi: 10.1007/s11010-017-3147-1. [DOI] [PubMed] [Google Scholar]
- 225.Nur G., Nazıroğlu M., Deveci H.A. Synergic prooxidant, apoptotic and TRPV1 channel activator effects of alpha-lipoic acid and cisplatin in MCF-7 breast cancer cells. J. Recept. Signal. Transduct. 2017;37:569–577. doi: 10.1080/10799893.2017.1369121. [DOI] [PubMed] [Google Scholar]
- 226.Koşar P.A., Nazıroğlu M., Övey İ.S., Çiğ B. Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: Involvement of TRPV1 channels. J. Membr. Biol. 2016;249:129–140. doi: 10.1007/s00232-015-9855-0. [DOI] [PubMed] [Google Scholar]
- 227.Zheng L., Chen J., Ma Z., Liu W., Yang F., Yang Z., Wang K., Wang X., He D., Li L. Capsaicin enhances anti-proliferation efficacy of pirarubicin via activating TRPV1 and inhibiting PCNA nuclear translocation in 5637 cells. Mol. Med. Rep. 2016;13:881–887. doi: 10.3892/mmr.2015.4623. [DOI] [PubMed] [Google Scholar]
- 228.Ortega-Guerrero A., Espinosa-Duran J.M., Velasco-Medina J. TRPV1 channel as a target for cancer therapy using CNT-based drug delivery systems. Eur. Biophys. J. 2016;45:423–433. doi: 10.1007/s00249-016-1111-8. [DOI] [PubMed] [Google Scholar]
- 229.Nabissi M., Morelli M.B., Amantini C., Farfariello V., Ricci-Vitiani L., Caprodossi S., Arcella A., Santoni M., Giangaspero F., De Maria R. TRPV2 channel negatively controls glioma cell proliferation and resistance to Fas-induced apoptosis in ERK-dependent manner. Carcinogenesis. 2010;31:794–803. doi: 10.1093/carcin/bgq019. [DOI] [PubMed] [Google Scholar]
- 230.Nabissi M., Morelli M.B., Santoni M., Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34:48–57. doi: 10.1093/carcin/bgs328. [DOI] [PubMed] [Google Scholar]
- 231.Morelli M.B., Offidani M., Alesiani F., Discepoli G., Liberati S., Olivieri A., Santoni M., Santoni G., Leoni P., Nabissi M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer. 2014;134:2534–2546. doi: 10.1002/ijc.28591. [DOI] [PubMed] [Google Scholar]
- 232.Raphaël M., Lehen’kyi V.Y., Vandenberghe M., Beck B., Khalimonchyk S., Abeele F.V., Farsetti L., Germain E., Bokhobza A., Mihalache A. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc. Natl. Acad. Sci. USA. 2014;111:E3870–E3879. doi: 10.1073/pnas.1413409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Montgomery D.R., Buffington J.M. Channel Classification, Prediction of Channel Response, and Assessment of Channel Condition. University of Washington Seattle; Washington, DC, USA: 1993. [Google Scholar]
- 234.Kim D.M., Nimigean C.M. Voltage-gated potassium channels: A structural examination of selectivity and gating. Cold Spring Harb. Perspect. Biol. 2016;8:a029231. doi: 10.1101/cshperspect.a029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Berkefeld H., Fakler B., Schulte U. Ca2+-activated K+ channels: From protein complexes to function. Physiol. Rev. 2010;90:1437–1459. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- 236.Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 237.Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr. Pharm. Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- 238.Kuang Q., Purhonen P., Hebert H. Structure of potassium channels. Cell. Mol. Life Sci. 2015;72:3677–3693. doi: 10.1007/s00018-015-1948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Piechotta P.L., Rapedius M., Stansfeld P.J., Bollepalli M.K., Erhlich G., Andres-Enguix I., Fritzenschaft H., Decher N., Sansom M.S., Tucker S.J. The pore structure and gating mechanism of K2P channels. EMBO J. 2011;30:3607–3619. doi: 10.1038/emboj.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ouadid-Ahidouch H., Ahidouch A. K+ channel expression in human breast cancer cells: Involvement in cell cycle regulation and carcinogenesis. J. Membr. Biol. 2008;221:1–6. doi: 10.1007/s00232-007-9080-6. [DOI] [PubMed] [Google Scholar]
- 241.Comes N., Serrano-Albarras A., Capera J., Serrano-Novillo C., Condom E., y Cajal S.R., Ferreres J.C., Felipe A. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim. Biophys. Acta Biomembr. 2015;1848:2477–2492. doi: 10.1016/j.bbamem.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 242.Huang X., Dubuc A.M., Hashizume R., Berg J., He Y., Wang J., Chiang C., Cooper M.K., Northcott P.A., Taylor M.D. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Genes Dev. 2012;26:1780–1796. doi: 10.1101/gad.193789.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Song P., Du Y., Song W., Chen H., Xuan Z., Zhao L., Chen J., Chen J., Guo D., Jin C. KCa3. 1 as an effective target for inhibition of growth and progression of intrahepatic cholangiocarcinoma. J. Cancer. 2017;8:1568. doi: 10.7150/jca.18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Haren N., Khorsi H., Faouzi M., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. Intermediate conductance Ca2+ activated K+ channels are expressed and functional in breast adenocarcinomas: Correlation with tumour grade and metastasis status. Histol. Histopathol. 2010;25:1247–1255. doi: 10.14670/HH-25.1247. [DOI] [PubMed] [Google Scholar]
- 245.Rabjerg M., Oliván-Viguera A., Hansen L.K., Jensen L., Sevelsted-Møller L., Walter S., Jensen B.L., Marcussen N., Köhler R. High expression of KCa3. 1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival. PLoS ONE. 2015;10:e0122992. doi: 10.1371/journal.pone.0122992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ji C.-D., Wang Y.-X., Xiang D.-F., Liu Q., Zhou Z.-H., Qian F., Yang L., Ren Y., Cui W., Xu S.-L. Kir2. 1 Interaction with Stk38 Promotes Invasion and Metastasis of Human Gastric Cancer by Enhancing MEKK2–MEK1/2–ERK1/2 Signaling. Cancer Res. 2018;78:3041–3053. doi: 10.1158/0008-5472.CAN-17-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang G.-M., Wan F.-N., Qin X.-J., Cao D.-L., Zhang H.-L., Zhu Y., Dai B., Shi G.-H., Ye D.-W. Prognostic significance of the TREK-1 K2P potassium channels in prostate cancer. Oncotarget. 2015;6:18460. doi: 10.18632/oncotarget.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen S.-Z., Jiang M., Zhen Y.-S. HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother. Pharmacol. 2005;56:212–220. doi: 10.1007/s00280-004-0960-5. [DOI] [PubMed] [Google Scholar]
- 249.Laniado M.E., Fraser S.P., Djamgoz M.B. Voltage-gated K+ channel activity in human prostate cancer cell lines of markedly different metastatic potential: Distinguishing characteristics of PC-3 and LNCaP cells. Prostate. 2001;46:262–274. doi: 10.1002/1097-0045(20010301)46:4<262::AID-PROS1032>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 250.Samuel P., Pink R.C., Caley D.P., Currie J.M.S., Brooks S.A., Carter D.R.F. Over-expression of miR-31 or loss of KCNMA1 leads to increased cisplatin resistance in ovarian cancer cells. Tumor Biol. 2016;37:2565–2573. doi: 10.1007/s13277-015-4081-z. [DOI] [PubMed] [Google Scholar]
- 251.Liu X., Wei L., Zhao B., Cai X., Dong C., Yin F. Low expression of KCNN3 may affect drug resistance in ovarian cancer. Mol. Med. Rep. 2018;18:1377–1386. doi: 10.3892/mmr.2018.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee E.L., Hasegawa Y., Shimizu T., Okada Y. IK1 channel activity contributes to cisplatin sensitivity of human epidermoid cancer cells. Am. J. Physiol. Cell Physiol. 2008;294:C1398–C1406. doi: 10.1152/ajpcell.00428.2007. [DOI] [PubMed] [Google Scholar]
- 253.Han Y., Shi Y., Han Z., Sun L., Fan D. Detection of potassium currents and regulation of multidrug resistance by potassium channels in human gastric cancer cells. Cell Biol. Int. 2007;31:741–747. doi: 10.1016/j.cellbi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 254.Hartung F., Pardo L.A. Guiding TRAIL to cancer cells through Kv10. 1 potassium channel overcomes resistance to doxorubicin. Eur. Biophys. J. 2016;45:709–719. doi: 10.1007/s00249-016-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fortunato A. The role of hERG1 ion channels in epithelial-mesenchymal transition and the capacity of riluzole to reduce cisplatin resistance in colorectal cancer cells. Cell. Oncol. 2017;40:367–378. doi: 10.1007/s13402-017-0328-6. [DOI] [PubMed] [Google Scholar]
- 256.Catterall W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/S0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 257.Diss J., Fraser S., Djamgoz M. Voltage-gated Na+ channels: Multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur. Biophys. J. 2004;33:180–193. doi: 10.1007/s00249-004-0389-0. [DOI] [PubMed] [Google Scholar]
- 258.Black J.A., Waxman S.G. Noncanonical roles of voltage-gated sodium channels. Neuron. 2013;80:280–291. doi: 10.1016/j.neuron.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 259.Mao W., Zhang J., Korner H., Jiang Y., Ying S. The emerging role of voltage-gated sodium channels in tumour biology. Front. Oncol. 2019;9:124. doi: 10.3389/fonc.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gao R., Shen Y., Cai J., Lei M., Wang Z. Expression of voltage-gated sodium channel α subunit in human ovarian cancer. Oncol. Rep. 2010;23:1293–1299. doi: 10.3892/or_00000763. [DOI] [PubMed] [Google Scholar]
- 261.House C.D., Vaske C.J., Schwartz A.M., Obias V., Frank B., Luu T., Sarvazyan N., Irby R., Strausberg R.L., Hales T.G. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Nelson M., Yang M., Millican-Slater R., Brackenbury W.J. Nav1. 5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget. 2015;6:32914. doi: 10.18632/oncotarget.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hernandez-Plata E., Ortiz C.S., Marquina-Castillo B., Medina-Martinez I., Alfaro A., Berumen J., Rivera M., Gomora J.C. Overexpression of Nav1. 6 channels is associated with the invasion capacity of human cervical cancer. Int. J. Cancer. 2012;130:2013–2023. doi: 10.1002/ijc.26210. [DOI] [PubMed] [Google Scholar]
- 264.Shan B., Dong M., Tang H., Wang N., Zhang J., Yan C., Jiao X., Zhang H., Wang C. Voltage-gated sodium channels were differentially expressed in human normal prostate, benign prostatic hyperplasia and prostate cancer cells. Oncol. Lett. 2014;8:345–350. doi: 10.3892/ol.2014.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Xia J., Huang N., Huang H., Sun L., Dong S., Su J., Zhang J., Wang L., Lin L., Shi M. Voltage-gated sodium channel Nav1. 7 promotes gastric cancer progression through MACC1-mediated upregulation of NHE1. Int. J. Cancer. 2016;139:2553–2569. doi: 10.1002/ijc.30381. [DOI] [PubMed] [Google Scholar]
- 266.Liu J., Tan H., Yang W., Yao S., Hong L. The voltage-gated sodium channel Nav1. 7 associated with endometrial cancer. J. Cancer. 2019;10:4954. doi: 10.7150/jca.31544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chioni A.-M., Brackenbury W.J., Calhoun J.D., Isom L.L., Djamgoz M.B. A novel adhesion molecule in human breast cancer cells: Voltage-gated Na+ channel β1 subunit. Int. J. Biochem. Cell Biol. 2009;41:1216–1227. doi: 10.1016/j.biocel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nelson M., Millican-Slater R., Forrest L.C., Brackenbury W.J. The sodium channel β1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis. Int. J. Cancer. 2014;135:2338–2351. doi: 10.1002/ijc.28890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jansson K.H., Lynch J.E., Lepori-Bui N., Czymmek K.J., Duncan R.L., Sikes R.A. Overexpression of the VSSC-associated CAM, beta-2, enhances LNCaP cell metastasis associated behavior. Prostate. 2012;72:1080–1092. doi: 10.1002/pros.21512. [DOI] [PubMed] [Google Scholar]
- 270.Gong Y., Yang J., Wu W., Liu F., Su A., Li Z., Zhu J., Wei T. Preserved SCN4B expression is an independent indicator of favorable recurrence-free survival in classical papillary thyroid cancer. PLoS ONE. 2018;13:e0197007. doi: 10.1371/journal.pone.0197007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bon E., Driffort V., Gradek F., Martinez-Caceres C., Anchelin M., Pelegrin P., Cayuela M.-L., Marionneau-Lambot S., Oullier T., Guibon R. SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nat. Commun. 2016;7:1–18. doi: 10.1038/ncomms13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sanchez-Sandoval A.L., Gomora J.C. Contribution of voltage-gated sodium channel β-subunits to cervical cancer cells metastatic behavior. Cancer Cell Int. 2019;19:35. doi: 10.1186/s12935-019-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Djamgoz M., Fraser S.P., Brackenbury W.J. In vivo evidence for voltage-gated sodium channel expression in carcinomas and potentiation of metastasis. Cancers. 2019;11:1675. doi: 10.3390/cancers11111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li K., Yang J., Han X. Lidocaine sensitizes the cytotoxicity of cisplatin in breast cancer cells via up-regulation of RARβ2 and RASSF1A demethylation. Int. J. Mol. Sci. 2014;15:23519–23536. doi: 10.3390/ijms151223519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Freeman J., Crowley P.D., Foley A.G., Gallagher H.C., Iwasaki M., Ma D., Buggy D.J. Effect of perioperative lidocaine and cisplatin on metastasis in a murine model of breast cancer surgery. Anticancer Res. 2018;38:5599–5606. doi: 10.21873/anticanres.12894. [DOI] [PubMed] [Google Scholar]
- 276.Xing W., Chen D.-T., Pan J.-H., Chen Y.-H., Yan Y., Li Q., Xue R.-F., Yuan Y.-F., Zeng W.-A. Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiol. J. Am. Soc. Anesthesiol. 2017;126:868–881. doi: 10.1097/ALN.0000000000001528. [DOI] [PubMed] [Google Scholar]
- 277.Tran T.-A., Gillet L., Roger S., Besson P., White E., Le Guennec J.-Y. Non-anti-mitotic concentrations of taxol reduce breast cancer cell invasiveness. Biochem. Biophys. Res. Commun. 2009;379:304–308. doi: 10.1016/j.bbrc.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 278.Adachi K., Toyota M., Sasaki Y., Yamashita T., Ishida S., Ohe-Toyota M., Maruyama R., Hinoda Y., Saito T., Imai K. Identification of SCN3B as a novel p53-inducible proapoptotic gene. Oncogene. 2004;23:7791–7798. doi: 10.1038/sj.onc.1208067. [DOI] [PubMed] [Google Scholar]
- 279.Jasti J., Furukawa H., Gonzales E.B., Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 280.Wu Y., Gao B., Xiong Q.-J., Wang Y.-C., Huang D.-K., Wu W.-N. Acid-sensing ion channels contribute to the effect of extracellular acidosis on proliferation and migration of A549 cells. Tumor Biol. 2017;39:1010428317705750. doi: 10.1177/1010428317705750. [DOI] [PubMed] [Google Scholar]
- 281.Zhu S., Zhou H.-Y., Deng S.-C., Deng S.-J., He C., Li X., Chen J.-Y., Jin Y., Hu Z.-L., Wang F. ASIC1 and ASIC3 contribute to acidity-induced EMT of pancreatic cancer through activating Ca2+/RhoA pathway. Cell Death Dis. 2017;8:e2806. doi: 10.1038/cddis.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Berdiev B.K., Xia J., McLean L.A., Markert J.M., Gillespie G.Y., Mapstone T.B., Naren A.P., Jovov B., Bubien J.K., Ji H.-L. Acid-sensing ion channels in malignant gliomas. J. Biol. Chem. 2003;278:15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 283.Kapoor N., Bartoszewski R., Qadri Y.J., Bebok Z., Bubien J.K., Fuller C.M., Benos D.J. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J. Biol. Chem. 2009;284:24526–24541. doi: 10.1074/jbc.M109.037390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gupta S.C., Singh R., Asters M., Liu J., Zhang X., Pabbidi M.R., Watabe K., Mo Y.-Y. Regulation of breast tumorigenesis through acid sensors. Oncogene. 2016;35:4102–4111. doi: 10.1038/onc.2015.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhang Y., Zhang T., Wu C., Xia Q., Xu D. ASIC1a mediates the drug resistance of human hepatocellular carcinoma via the Ca(2+)/PI3-kinase/AKT signaling pathway. Lab. Investig. 2017;97:53–69. doi: 10.1038/labinvest.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Holzmann C., Kappel S., Kilch T., Jochum M.M., Urban S.K., Jung V., Stöckle M., Rother K., Greiner M., Peinelt C. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget. 2015;6:41783. doi: 10.18632/oncotarget.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Armisén R., Marcelain K., Simon F., Tapia J.C., Toro J., Quest A.F., Stutzin A. TRPM4 enhances cell proliferation through up-regulation of the β-catenin signaling pathway. J. Cell. Physiol. 2011;226:103–109. doi: 10.1002/jcp.22310. [DOI] [PubMed] [Google Scholar]
- 288.Narayan G., Bourdon V., Chaganti S., Arias-Pulido H., Nandula S.V., Rao P.H., Gissmann L., Dürst M., Schneider A., Pothuri B. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: Identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer. 2007;46:373–384. doi: 10.1002/gcc.20418. [DOI] [PubMed] [Google Scholar]
- 289.Maeda T., Suzuki A., Koga K., Miyamoto C., Maehata Y., Ozawa S., Hata R.-I., Nagashima Y., Nabeshima K., Miyazaki K. TRPM5 mediates acidic extracellular pH signaling and TRPM5 inhibition reduces spontaneous metastasis in mouse B16-BL6 melanoma cells. Oncotarget. 2017;8:78312. doi: 10.18632/oncotarget.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Weaver E.M., Zamora F.J., Puplampu-Dove Y.A., Kiessu E., Hearne J.L., Martin-Caraballo M. Regulation of T-type calcium channel expression by sodium butyrate in prostate cancer cells. Eur. J. Pharmacol. 2015;749:20–31. doi: 10.1016/j.ejphar.2014.12.021. [DOI] [PubMed] [Google Scholar]