Abstract
Titanium dioxide (TiO2) is a material of diverse applications commonly used as a food additive or cosmetic ingredient. Its prevalence in products of everyday use, especially in nanosize, raises concerns about safety. Current findings on the safety of titanium dioxide nanoparticles (TiO2 NPs) used as a food additive or a sunscreen compound are reviewed and systematized in this publication. Although some studies state that TiO2 NPs are not harmful to humans through ingestion or via dermal exposure, there is a considerable number of data that demonstrated their toxic effects in animal models. The final agreement on the safety of this nanomaterial has not yet been reached among researchers. There is also a lack of official, standardized guidelines for thorough characterization of TiO2 NPs in food and cosmetic products, provided by international authorities. Recent advances in the application of ‘green-synthesized’ TiO2 NPs, as well as comparative studies of the properties of ‘biogenic’ and ‘traditional’ nanoparticles, are presented. To conclude, perspectives and directions for further studies on the toxicity of TiO2 NPs are proposed.
Keywords: nanoparticles, titanium(IV) oxide, toxicity, titania, E171, exposure
1. Introduction
1.1. Properties and Applications of Titanium Dioxide
Titanium dioxide (TiO2, titania, titanium(IV) oxide) is a material with a plethora of practical and possible applications. Commonly called ‘titanium white’, the fine white powder is mainly used as a pigment because of its brightness and opacifying strength (hiding power). TiO2 is resistant to chemical attack and displays excellent thermal stability, but most importantly, has the ability to both absorb and scatter the UV light (thanks to its high refractive index). These properties render the titanium pigment an irreplaceable ingredient in the production of paints, surface coatings, plastics, and paper [1]. The global production of titanium dioxide worldwide is continuously rising [2].
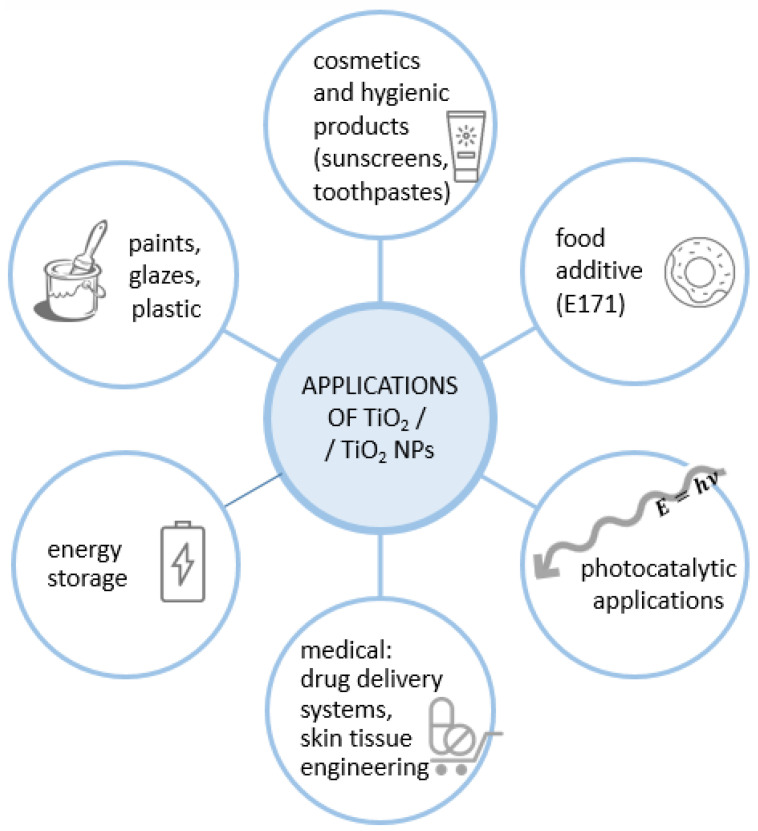
Titanium dioxide comes in three distinct crystal polymorphs—anatase, rutile, and brookite. Rutile is the most thermally stable polymorph, as both brookite and anatase are transformed into rutile when exposed to a temperature above 800 °C. All crystal forms of TiO2 offer photoactive properties. The differences in these properties can be characterized by different band gaps in TiO2 electron structures. Anatase was found the most photoactive form, as the bandgap, in this case, is higher compared to other polymorphs [1,3,4]. Manufacturing of the nanoscaled TiO2 particles, where at least one diameter is below 100 nm, has expanded the range of TiO2 utility (Figure 1). Titanium dioxide in the form of nanoparticles (TiO2 NPs) has become a common additive in paints, plastics, personal care products (cosmetics, sunscreens) and food—as the additive E171 [5,6]. Due to the properties which stem only from significantly decreased particle size (comparing between macro- or microparticles), titanium(IV) oxide nanoparticles are of great interest to many research groups [7]. In medical sciences, TiO2 was tested as a new effective drug carrier (for example, as TiO2 nanotubes) [8] or in skin tissue engineering and wound dressing [9,10,11]. Nanoscale TiO2 particles also have interesting photocatalytic properties, such as the ability to mediate photodegradation of pharmaceuticals, bacteria inactivation, the photooxidative killing effect on cancer cells, energy storage, as well as air and water purification [6,12]. Nowadays, TiO2 NPs are one of the most manufactured nanomaterials in the world [5,13].
Figure 1.
Various applications of titanium dioxide/titanium dioxide nanoparticles (TiO2/TiO2 NPs).
Particles of size in the nanoscale have a higher surface-to-volume ratio, as compared to macro- or micro-particles. This fact affects their properties, such as reactivity of surface area, the degree to which the NPs aggregate, or bioavailability [14,15]. It is generally known that an increase in surface area accelerates the dissolution processes. Higher dissolution rates and smaller size of particles enhance their absorption through membranes [16], which leads to their deposition within tissues and organs after oral administration, while the insoluble material is mostly excreted with feces [17]. However, TiO2 has very low dissolution rate when compared to other metallic nanoparticles [18]. Brun et al. demonstrated that there was no visible dissolution of TiO2 particles for as long as 24 h after the uptake by human gut epithelial cells grown in in vitro monocultures [19]. As the dissolution rates achieved by TiO2 are very low [20], the cytotoxic effects caused by TiO2-NPs are more closely related to their size rather than due to metallic ions being released from the particles absorbed by cells. Such assumption was confirmed in the study by Gurr et al., where they demonstrated that very fine TiO2-NPs (<20 nm diameter) induced genotoxicity through oxidative stress in human bronchial epithelial cells, even without photoactivation of the nanomaterial [21]. Noteworthy, the same material sized >200 nm showed no sign of genotoxicity without irradiation.
If a substance additionally accumulates in biological tissues, its increased uptake may lead to adverse effects. This issue does not apply to larger forms of the same substance [14,22]. Upon introduction to biological systems, nanoparticles are exposed to a complex mixture of molecules, forming a so-called ‘corona’. This layer constitutes the interface between the nanomaterial and the environment and is often regarded as a biological identity of the particle. The corona plays a significant role in the bioactivity of a nanomaterial. It has been shown to mediate cellular responses (uptake, accumulation, intracellular localization, distribution and degradation) [23,24]. The protein corona (PC) is the most extensively studied nano–bio interface type [25]. With regard to titanium dioxide nanoparticles, Khan et al. recently assessed the impact of the surface chemistry on the behavior of the nanoparticle in an in vitro study, using adenocarcinomic human alveolar basal epithelial (A549) cells. Uncoated TiO2 NPs were compared with particles modified with PVP, Dispex AA4040, and Pluronic F127. The results revealed differences in terms of the tendency to form agglomerates, the rate of dissociation from corona proteins, dispersion of the particles and their degradation. Dispex AA4040, and Pluronic F127 coatings were found to influence the retention of PC and additionally exhibited an exchange between corona and intracellular proteins [26]. As the biocorona of the TiO2 NP notably affects its biological fate and therefore its potential toxicity, the studies on this interfacial layer should be included in the safety assessment of the nano-TiO2 used as food and cosmetic additive. These issues will be discussed later in this review.
1.2. Effect of TiO2 NPs Shape on Their Toxicity
So far, only a few studies have focused on the effect of the shape of the titania nanoparticles on their toxicity, mainly inhalation based, and much is yet to study on the subject. Allegri et al. compared the toxicity of TiO2 P25 nanoparticles with TiO2 nanofibers towards alveolar carcinoma epithelial cells [27]. The study concluded that although the nanoparticles exhibited a significant toxic effect, the nanofibers revealed a stronger impact on the tested cell viability and hemolysis. Worth noting is the fact that the TiO2 nanofibers caused more severe changes than P25 when either dose or surface area are taken into account. Additionally, nanofibers induced similar inflammatory response as crocidolite, a known cancer-inducing mineral. Such results correlate well with the study by Porter et al. in which mice exposed to different titania nanoparticles by inhalation showed more significant lung damage and inflammation in the case of nanobelt-shaped particles when compared to nanospheres [28]. These effects were linked to the length of the particles, as longer nanobelts induced a stronger response. Similar results, pinpointing the anatase nanobelts as more hazardous after inhalation compared to P25 nanospheres and anatase nanospheres, were also reported [29]. In another study, it was found that differently shaped anatase nanoparticles (nanotubes, nanocubes, nanospheres) caused similar effects when dosing was based on the surface area of the materials [30]. However, the nanotubes were associated with the alveolar proteinosis and occurrence of the inflammatory response. On the other hand, titania nanotubes were found to be more cytotoxic than P25 only at the concentration of 2.5 µg/mL when tested on cardiomyocytes in vitro [31], with much higher internalization (by diffusion and endocytosis) into the cells. A comparison of P25 and food grade titania with TiO2-based bipyramids, rods and platelets indicated that only the food grade titania and platelets were genotoxic to human epithelial cells in vitro [32]. TiO2 nanorods demonstrated a dose-dependent toxicity in alveolar adenocarcinoma cells in vitro but were not compared to differently shaped TiO2 nanoparticles [33]. In the same study, the rats that inhaled the nanorods were found to exhibit tissue damage, acute and chronic lung inflammation, and increased levels of titanium were measured not only in lungs but also in the bloodstream. Simon et al. attempted to find a relationship between toxicity and shape of the used titania nanoparticles [34]. In this study however, it was found that different nanoparticles were decreasing cell viability in a different manner, depending on the cell line used. Human umbilical vein endothelial cells (HUVEC cells) were affected by P25 spheres, sol-gel based isotropic nanoparticles and nanosheets, with no effect observed when nanoneedles were used. HEKn cells were most affected by nanosheets and in a smaller manner by P25 spheres, sol-gel based isotropic nanoparticles and nanosheets. Interestingly, HeLa cell proliferation was decreased slightly at high doses of sol-gel based isotropic nanoparticles and nanosheets but not by P25 nanospheres or nanoneedles.
All these cited studies indicate that the shape of the particles plays an important role in the toxicity of the titania nanoparticles. The biological effects observed for various TiO2 NPs are enhanced by the elongated form (tubes or fibers). Their increased internalization to the cells results in higher accumulation, which in turn explains its hampered clearance from the lungs.
1.3. The Role of Oxidative Stress in the Toxicity of Nanoparticles
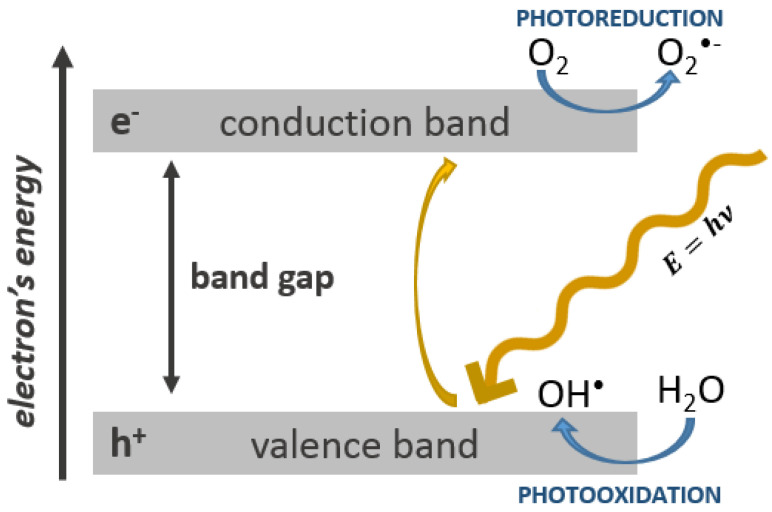
There is a plethora of studies that associate cyto- and geno-toxicity with their photocatalytic activity [4,13]. As mentioned before, TiO2 NPs can both scatter and absorb the UV radiation. UV light absorption is possible due to the semiconducting properties of TiO2 (Figure 2). The electrons from the valence band are promoted to the conduction band which photogenerates holes in the valence band. These holes and electrons can recombine or migrate to the NP surface where different redox processes take place, which causes reactive oxygen species (ROS) production.
Figure 2.
Bandgap in a semiconducting material. A valence band electron (e−) is excited to the conduction band upon light absorption (of ≥ bandgap energy) and leaves a hole in the valence band (h+) (according to [4,13]).
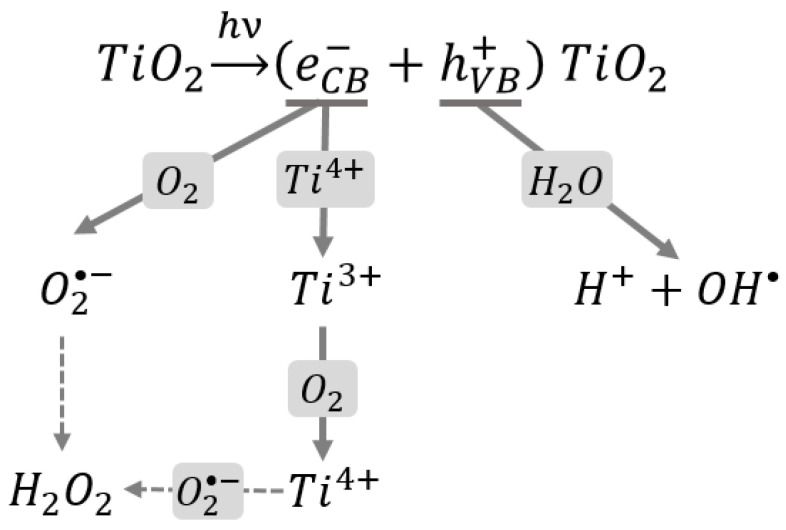
The valence band holes react mainly with the moisture on the surface of particles, which results in the production of hydroxyl radicals. However, the conduction band electrons can interact with oxygen molecules (also present on the surface of particles) or be captured at TiIV sites, and later react with oxygen. As a consequence, it leads to the formation of hydrogen peroxide and superoxide anion radicals (Figure 3). All of the products mentioned above, such as hydroxyl radical, hydrogen peroxide, and superoxide anion radical, constitute a group of reactive oxygen species, which may impair the cell function [4,13,35].
Figure 3.
Photo-excitation of TiO2 and generation of reactive oxygen species (according to [4,13]).
Regarding the molecular mechanisms of the in vivo toxicity of NPs in general, the oxidative stress plays the most crucial role. For example, Nel et al., in their study, presented a direct relationship between the surface area, ROS-generating capability, and proinflammatory effects of nanoparticles in the lung [22]. ROS generated by mitochondria in cells are normally quickly neutralized by antioxidant substances. However, an excessive generation of oxidants, such as ROS, causes an imbalance between oxidants and antioxidant processes, which is called oxidative stress [14,15,22,36]. Oxidative stress has been proven to contribute to many types of human chronic diseases, such as cancer, as well as inflammatory, neurodegenerative or cardiovascular diseases [37]. According to Aillon et al., the organs that are the most exposed to oxidative stress are the liver and spleen, due to the accumulation of NPs capable of generating ROS. In this regard, high blood flow and slow clearance make kidneys and lungs very vulnerable to oxidative stress [14].
Taking into consideration these properties and the prevalence of TiO2 NPs in the products of everyday use, the recently emerging concerns seem to be understandable. Although a considerable amount of literature on the toxicity of TiO2 NPs is available nowadays—including excellent reviews by Skocaj et al. [38] and Shakeel et al. [39]—there is still much uncertainty, as some findings are inconsistent. Moreover, it is difficult to establish the intake levels of TiO2 NPs, as they depend on the type of the product consumed or used, its formulation, route of exposure, and the rate of consumption or usage of the product by a person [40]. The purpose of this paper is to review recent reports on the toxicity of titanium dioxide nanoparticles as food and cosmetic additives, to systematize these findings, and to point out perspectives for their further development.
2. Routes of Exposure and Toxicity of TiO2 NPs
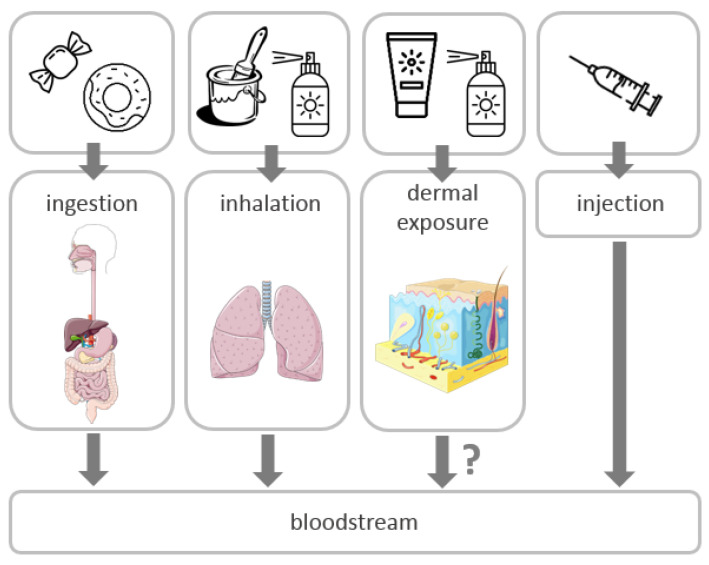
It is now a well-established fact, based on a variety of studies, that there are four main routes of exposure to titanium dioxide nanoparticles in humans: ingestion, pulmonary absorption (mainly through inhalation), dermal exposure and injection (Figure 4) [39].
Figure 4.
Routes of exposure and distribution of TiO2 NPs in the human body.
Researchers agree that the ingestion, inhalation, and injection of TiO2 NPs lead to their systemic disposal. However, in case of dermal exposure, the findings are inconsistent. The following section of this paper attempts to indicate the main uncertainties related to the toxicity of TiO2 NPs as additives in food and cosmetic products, which are in the review of current chemical and toxicological studies.
2.1. Ingestion—TiO2 NPs as a Food Additive (E171)
In the food industry, TiO2 has been applied as an additive to enhance the white color of certain products, such as sweets or milk-based products [6,41,42]. In 2012, Weir et al. measured and compared the amount of titanium in common food products [6]. The obtained data, normalized to the titanium per serving, proved that the highest titanium contents could be found in chewing gums, candies, powdered sugar toppings, or products with white icing. The difference in TiO2 consumption between women and men in the United States was negligible. However, the difference in consumption between children and adults was found significant. Children are susceptible to consume up to four times more TiO2 per kilogram of body weight (kgbw) than an adult person. This fact can be simply explained by their consumer preferences, generally based on the taste in sweet snacks, among which many contain E171. Therefore, exposure to TiO2 depends also on dietary habits [6].
As the daily exposure to E171 may reach several hundreds of milligrams of which a considerable part appears in the nano range (about 36%) [6], there are concerns that a long-term exposition to this substance may lead to harmful effects in the human body. In response to this growing public worldwide health problem, the European Food Safety Authority (EFSA) published a ‘Re-evaluation of titanium dioxide (E171) as a food additive’, based on documentation on usage levels and safety of titanium dioxide provided by various international associations, councils and committees. The EFSA panel concluded that both the absorption and the bioavailability of orally administered micro- and nano-TiO2 is low. Most of the TiO2 ingested dose is eliminated unchanged in the feces, except for a tiny amount (not exceeding 0.1%), which is absorbed by the gut-associated lymphoid tissue and distributed to various organs. The panel stated that the micro- and nano-sized particles are unlikely to cause a genotoxic hazard in vivo [43]. However, a year later, a study conducted by Bettini et al. [44] proved that orally administered food-grade TiO2 containing nanoscale particles impaired immune homeostasis and induced carcinogenesis in rats. Based on this publication, the French ANSES (Agency for Food, Environmental and Occupational Health) published their opinion on TiO2 NPs [45], in which the necessity of conducting thorough research on the possible dangers connected with the usage of E171 was underlined. France is the first country to ban using the E171 food additive because of the possible harmful effects on humans and a lack of scientific data to confirm its safety. The restrictions became effective in 2020 [46].
The genotoxic potential of E171 has already been proven in several studies. In 2016, Proquin et al. [47] used an in vitro model with human Caco-2 and HCT116 cells to research the potentially toxic effects of E171, containing fractions of micro- and nano-particles (MPs and NPs, respectively). Their findings proved the highest capability to induce ROS generation in a cell-free environment for E171 (defined as a mixture of 39% TiO2 NPs and 61% MPs), followed by NPs and MPs. However, in a cellular environment, only MPs revealed the capacity to produce ROS, which, as they suggested, can lead to a proinflammatory response. On the other hand, the NPs did not stimulate ROS production, which was explained by the fact that following internalization, they react with cellular structures blocking ROS formation. This study also provided evidence for single-strand DNA breaks in Caco-2 cells induced by all E171, NPs, and MPs. The researchers suggested that the E171 was more toxic to Caco-2 cells than NPs or MPs alone. Harmful effects of TiO2 NPs were also confirmed by Grissa et al. [48]. The assumption of this study was to simulate long-term, low dose ingestion of E171 in humans. For this purpose, anatase NPs (5–12 nm in size) were administered intragastrically to Wistar rats for 60 days. As a result of the performed study, there were noted changes in the hematopoietic parameters, as well as a genotoxic effect of TiO2 NPs in vivo at 100 and 200 mg/kgbw. On the other hand, the particles that were used in this study were generally smaller than those found commonly in foodstuffs [6], and the crystalline phase of anatase is known to be much more toxic than rutile. In a study by Talamini et al., a material exhibiting foodstuff-grade particle size distribution was used [49]. The researchers studied a repeated 3-week oral administration of E171 to mice (E171 suspension dripping into the mouth of mice, 5 mg/kgbw for 3 days per week). The results were related not only to toxic outcomes, such as an inflammatory response and increased superoxide production in the digestive tract, but also to the deposition of TiO2 in the internal organs, especially in the liver and large intestine, where a three-fold increase in TiO2 NPs was noted [49].
Oral exposure to TiO2 NPs is associated not only with the ingestion of E171, but also with the consumption of pharmaceuticals. TiO2 is a common pharmaceutical excipient, mostly used as a white pigment, but in its nanoform, it can also be an effective carrier of antibiotics, which additionally enhances or prolongs the action of the drug [50,51,52]. Evidence for genotoxic effects of nano-TiO2 drug carrier administered orally was recently provided by Mottola et al., who researched the influence of nano-TiO2 and lincomycin coexposure on human amniocytes. The results of this in vitro study demonstrate that the exposure to TiO2 NPs induced an increase in DNA strand breaks, a loss of DNA stability and apoptosis, as well as reduced cells viability, whereas the exposure to lincomycin itself had no toxic/genotoxic effects on amniotic cells. The authors suggested that the underlying molecular mechanism of the DNA damage may be the production of ROS by the NPs, notably the ●OH radical [50,53]. To date, researchers usually associate the genotoxicity of TiO2 NPs with the formation of oxidants [54,55,56,57,58,59].
Toothpaste is another source of TiO2 (also in a nanoform) which may be ingested. Therefore, it is not surprising that attention has now turned to this personal care product. The review of scientific data on this subject, which was carried out by national and international agencies, led to a prohibition of E171 usage in food production [60,61]. Usually the amount of toothpaste used is small, so the ingestion of TiO2 NPs is possible only in case of unwanted swallowing. Thus, taking into consideration the low absorption of TiO2 administered orally, the appearance of toxic effects is rather unlikely [43].
As mentioned earlier, nanomaterials can interact with molecules, which are present in biological fluids, for example, bacterial lipopolysaccharide (LPS), which is a proinflammatory compound present in the gastrointestinal tract. Bianchi et al. indicated that LPS included in the biocorona of the titania P25 particle displays enhanced proinflammatory effects [62]. The biological fate of nanomaterials should be also evaluated with regard to food ingredient effects. As an example, model food ingredients, bovine serum albumin and sucrose were able to stabilize TiO2 NPs and induced a decrease in their agglomerate sizes [63]. It has been also shown that the adsorption of proteins on the food grade TiO2 nanoparticles is inhibited in the presence of oxalate, a dicarboxylic acid, or phosphates [64]. As TiO2 NPs are largely utilized in dairy-based products, Cao et al. focused on their interactions with milk proteins. The researchers observed dissociation of casein micelles and formation of NP-protein complexes. It was suggested that this interaction may have altered the shielding of the peptide bonds. Therefore, it could be supposed that the amount of undigested protein, which may reach the colon and affect the intestinal microflora, would be significantly changed [65].
2.2. Local Effects of Tio2 NPs on the Intestinal Barrier and Changes in the Gut Microbiota
The safety assessment of the food-grade nano-TiO2 should be also regarded from the perspective of the local effects that may appear. This issue should not be omitted, because even if the TiO2 NPs may not provoke toxic effects due to their penetration, local damages in the gastrointestinal tract may disrupt the essential nutrient absorption. An overview of studies from the last six years shows a consensus among researchers on the detrimental effects caused by TiO2 NPs, both in vitro and in vivo. In 2014, Botelho et al. conducted a study on human gastric epithelial cells and stated that titania NPs provoked tumor-like phenotypes. Briefly, they observed an increase in the proliferation of the cells and a decrease in their apoptosis. They also detected increased glutathione levels, which is a sign of oxidative stress-mediated toxicity, as well as DNA lesions [66]. Urrutia-Ortega et al. observed that intragastric E171 exposure increased tumor progression markers (COX2, Ki67 and β-catenin included) and enhanced tumor formation in the distant colon in a murine model [67]. They noted that TiO2 did not induce tumor formation itself, but led to dysplastic changes in colonic epithelium and a decrease in goblet cells. Moreover, they concluded that the exposure to E171 may worsen pre-existing intestinal disorders. This was confirmed by Ruiz et al., who noticed an aggravation of acute colitis in a mouse model following oral gavage, as well as accumulation of titania crystals in the spleen. Moreover, the in vitro experiments proved that the particles were taken up by the human epithelial cells and macrophages and activated the NLRP3 inflammasome. Additionally, after the assessment of titanium levels in blood samples from human volunteers, they discovered increased titanium levels in samples from patients with ulcerative colitis, compared with healthy donors and patients with inflammatory bowel disease [68]. An important conclusion in this case is that the exposure to E171 is strongly contraindicated in patients with pre-existing inflammatory conditions or an impaired intestinal barrier function. A few recent experiments on a well-established cell line Caco-2 support former studies, indicating detrimental effects on the intestinal epithelium layer. Taken together, the results point out that exposure to TiO2 NPs has the following local effects:
increases the release of mucins and the expression of some efflux pumps [69];
increases ROS generation [70];
induces morphological changes or decreases the number of intestinal microvilli, which in turn decreases the surface area needed for optimal nutrients absorption [70,72,73];
leads to their internalization and entrapment by Caco-2 monolayers [71].
Another interesting explanation for the local toxicity of TiO2 NPs has been recently suggested by Yao et al., who remarked that it may be caused by an imbalance between the Th1 and Th2 cells, resulting in the tight junction barrier damage [73].
As far as proper intestinal function is concerned, the importance of the gut microbiota cannot be ignored. The human gut microbiome is a complex ecosystem and its imbalance may lead to pathogenesis or progression of a large spectrum of diseases [74]. Recent studies, both in vitro and in vivo, provide an insight into the influence of nano-TiO2 on the gut microbiota. Dudefoi et al. employed a defined human gut bacterial community, microbial ecosystem therapeutic-1 (MET-1) to evaluate the impact of two food-grade TiO2 additives. MET-1 contains 33 bacterial strains which can be cultured as an ecosystem. The researchers did not observe a significant alteration of the human gut microbiota, however, they were concerned about the cumulative effects of chronic ingestion of the nanoscale titania [75]. A limited impact on microbial communities has also been observed by Agans et al. [76]. Alterations in intestinal microbiota composition were noted by Radziwill-Bienkowska et al. [77]. They presented the data on the changes occurring in response to some factors such as intestinal disorders, diet variations and microbial challenges. In turn, Pinget et al. performed an in vivo study in mice and confirmed that food grade TiO2 had minimal influence on the gut microbiota composition. In addition, they found that it can still significantly impair the gut homeostasis. The impact of TiO2 included colonic inflammation, increased inflammatory response and altered release of bacterial metabolites [78]. Chen et al. also associated the disorders of gut microbiota with an inflammatory response and suggested that the oxidative stress may contribute to the underlying mechanism [79]. Overall, further investigation is needed to determine the effects of chronic exposure to the food-grade TiO2, particularly in vulnerable subpopulations.
2.3. Dermal Exposure—TiO2 NPs as a Sunscreen Compound
Sunscreens are another type of commonly used personal care products with a relatively high content of TiO2 NPs. Formulations with nanoscale TiO2 are useful in terms of light scattering and UV absorption. Moreover, when applied on the skin, they look more transparent, which is a desirable property for many consumers [13,80]. Although TiO2 NPs in sunscreens have already been studied for nearly two decades [81], some questions and uncertainties remain still unresolved, and regulation of the usage and safety of TiO2 NPs in these products is needed [82].
As mentioned before, smaller particles are more effective in terms of light scattering and absorption. However, the small size also increases possible absorption through the skin. It has not yet been determined which size of titania nanoparticles in sunscreen provides the best protection against UV radiation. Because the ozone layer almost entirely absorbs the UV-C radiation, skin should be protected in the UV-B (290–320 nm) and UV-A (320–400 nm) regions. More insight into this topic was given by Popov et al., who tested TiO2 NPs of six different sizes for their ability to stop the 307–311 nm light [83]. The study performed on six healthy volunteers with the so-called tape-stripping technique was applied keeping a proper timeline in order to assess the in-depth distribution of the fine TiO2 particles. Scattering and absorption coefficients for a medium containing TiO2 particles of different volume concentrations were calculated using Monte Carlo simulations. The Monte Carlo method was also developed to simulate UV-B propagation within the horny layer containing the embedded TiO2 particles. The results obtained in their study indicated that TiO2 NPs of 62 nm diameter revealed the optimal protective properties. Interestingly, the diameter of 62 nm was neither the smallest nor the largest one tested. Additionally, the researchers experimented on the concentration of TiO2 in subsequent layers of the stratum corneum, which revealed that titania could be found even 15 µm deep [83].
To assure effective protection against UV radiation, it is essential to determine not only the size of TiO2 particles, but also their shape. To date, several studies have conducted a thorough analysis of TiO2 particles extracted from a sunscreen formulations [84,85,86]. Interesting results were recently reported by Ilić et al., who evaluated the in vitro effect of TiO2 nanomaterials of three morphologies on human keratinocytes (HaCaT). They discovered that nanowires and nanoplates were significantly more effective in protecting human skin cells from UV-B induced damage. It can be concluded that TiO2 NPs can be designed specifically in order to enhance the quality and efficacy of a sunscreen product [87].
It is essential to thoroughly control the sunscreen formulations in order to verify the size of the NPs, their size distribution, aggregation rate, and the concentration of the NPs. According to the opinion of the Scientific Committee on Consumer Safety of the European Commission (SCCS), TiO2 NPs used in sunscreens up to a concentration of 25% can be considered to not pose any risk of adverse effects in humans after application on healthy, intact or sunburnt skin [88,89]. The parameters of NPs ought to be thoroughly controlled to ensure complete safety of usage for every sunscreen product. Although a few national and international institutions have proposed recommendations for labeling sunscreen products and testing their effectiveness [89,90,91,92,93], there is still a lack of official guidelines for a thorough characterization of TiO2 NPs in sunscreen formulations, which would set the standards for the quality control methods. Recently, the U.S. Food and Drug Administration (FDA) has issued a rule in order to update the regulatory requirements and put into effect the final monograph for over-the-counter sunscreen drug products. This rule applies to sunscreens marketed without FDA-approved applications. The FDA proposes to mark two ingredients, TiO2 and ZnO, as ‘generally recognized as safe and effective’ [94].
Recent literature highlights some modern techniques of the physicochemical characterization of nanosized TiO2, which could be implemented in common industrial practice through official guidelines [80,95,96]. Contado and Pagnoni presented flow field-flow fractionation (FlFFF) combined with inductively coupled plasma-atomic emission spectrometer (ICP-AES) as a relatively simple, low-cost, yet powerful tool for determining the TiO2 content and particle-mass size distribution (PSD) in sunscreen lotions [96]. A recent study by Bocca et al. used ICP-MS (inductively coupled plasma-mass spectrometry) and its modification SP ICP-MS (single particle inductively coupled plasma-mass spectrometry) to determine and compare the concentration and particle size distribution of TiO2 NPs in commercial sunscreens [80]. In that study, ICP-MS was used both as a direct technique, SP ICP-MS, and as a detector combined with the asymmetric flow-field flow fractionation (AF4-FFF), for preseparation, on-line coupled to the multi-angle light scattering (MALS). The results of that study indicated that the concentration of TiO2 NPs in creamy applications did not exceed the SCCS limit of 25%, and therefore, their usage can be considered safe [80]. Despite this, in 2001, Serpone et al. cast doubt upon the biological safety of the TiO2-containing sunscreens [81]. In the presence of fine TiO2 particles, they observed their harmful effects on DNA after illuminating supercoiled plasmids with simulated sunlight. The researchers also tried to fabricate photocatalytically inactive TiO2 specimens by modifying the particle surface. It should also be noted that the deleterious effects of TiO2 on DNA were possible due to the penetration of these NPs through the cell membranes. This issue remains a matter of argument. Thus far, several studies have reported that the TiO2 NPs do not cross the stratum corneum (SC), the outermost epidermal layer, and that the number of the particles passing through the SC is insignificant. SC is generally an effective barrier against the transfer of chemicals through the skin. It consists of dead cells incapable of active transport of substances. It has been already shown that after a two-hour exposure to sunscreens containing TiO2 and ZnO NPs, their levels in human viable epidermal layers were too low to be tested [97]. Thus, these results confirmed that the penetration through the SC is unlikely. Another study, in which sunscreen formulations containing 5% TiO2 (coated and noncoated NPs) were applied topically to Yucatan minipigs, also reported no significant penetration through normal, unharmed skin [98].
In contrast to the findings mentioned above, some researchers claim that nanosized TiO2 can penetrate the skin and induce tissue damages, even in major organs. An in vitro and in vivo study by Wu et al. demonstrated that TiO2 NPs do not pass through the SC of isolated porcine skin after 24 h exposure, but after 30 days of topical application, the NPs were found in deeper layers of the epidermis [99]. Moreover, subchronic (60 days) dermal exposure in hairless mice proved that TiO2 NPs could not only penetrate through the SC but also reach different tissues and induce pathological lesions, among which the most severe ones were displayed in the skin and liver. The authors also detected an elevated malondialdehyde level and a decreased superoxide dismutase level, which proved that these NPs induce oxidative stress processes. In conclusion, they also stated that TiO2 NPs topically applied on skin for a prolonged time can induce skin aging [99]. A very recent experiment by Pelclova et al. confirmed that TiO2 NPs could penetrate skin [100]. Detectable levels of nano-TiO2 were found in blood and urine of the human volunteers up to one week after using the sunscreen formulation. Furthermore, it was found that although the TiO2-based sunscreens prevented sunburns, they did not decrease the systemic oxidative stress, as evaluated by the tested biomarkers.
The results of various dermal exposure studies, both confirming and disproving the penetration of TiO2 NPs through the SC, are summed up in Table 1.
Table 1.
Examples of in vitro and in vivo studies to assess dermal exposure to TiO2 NPs in animals and humans.
| References Year |
Properties of the Formulation (Type of Emulsion, Size, Structure of TiO2 NPs) | Type of Study | Penetration through the SC? Observations |
|---|---|---|---|
| Pelclova et al. [100] 2019 |
43 nm, oil-free formulation, crystalline structure not specified | in vivo, human participants | Yes Absorption of TiO2 NPs through human skin—detectable levels in blood and urine |
| Zhang et al. [101] 2019 |
15–40 nm, for in vivo study nano-TiO2 solution was dripped on the skin of the mice | in vitro—HUVEC, in vivo—Balb/c mice |
not indicated in vitro—increase in ROS and sICAM-1 levels, a decrease in cell viability; in vivo—increase in ROS-dependent markers concentration in mouse serum Protective effects of vitamin E demonstrated |
| Crosera et al. [102] 2015 |
38 nm, suspension of commercial TiO2 nanopowder dispersed in synthetic sweat | in vitro, human abdominal skin (intact and damaged by needle-abrasion technique) | No
No penetration of TiO2 NPs in either intact or damaged skin |
| Xie et al. [103] 2015 |
20 nm, rod-shaped rutile-type TiO2 NPs radiolabeled solution (1 mg/mL) | in vitro, rat skin: intact and slightly damaged with sodium lauryl sulphate (SLS) solution | No No penetration of TiO2 NPs in either intact or damaged skin, both in vitro and in vivo |
| Miquel-Jeanjean et al. [104] 2012 |
20–30 nm × 50–150 nm, needle-shaped particles, water-in-oil commercial emulsion | in vitro, four specimens of domestic pig ear skin: intact, damaged (stripped), irradiated, damaged and irradiated | No TiO2 NPs remained in the uppermost layers of the SC, even if the skin barrier function was impaired |
| Monteiro-Riviere et al. [105] 2011 |
10 × 50 nm, mean agglomerates 200 nm; o/w and w/o commercial formulations; rutile | in vitro—skin in flow-through diffusion cells; in vivo—weanling white Yorkshire pig skin |
Minimal penetration of TiO2 NPs into the upper epidermal layers: in vitro—epidermal penetration, minimal transdermal absorption; in vivo—Ti within the epidermis and superficial dermis, no transdermal absorption detected; UV-B sunburned skin slightly enhanced the SC penetration |
| Sadrieh et al. [98] 2010 |
Sunscreen formulation with: uncoated NPs (anatase and rutile): 30–50 nm, coated NPs (rutile): 20–30 nm in diameter and 50–150 in length, submicron particles (rutile): 300–500 nm |
in vivo, Yucatan minipig skin | No No structural abnormalities in the skin cells observed |
| Filipe et al. [97] 2009 |
Sunscreen (hydrophobic) formulation with: TiO2: not indicated TiO2 and ZnO: not indicated Coated rutile TiO2 material: 20 nm |
in vivo, human participants | No Levels of TiO2 NPs too low for detection beneath the SC, no toxic effects |
| Senzui et al. [106] 2009 |
Rutile TiO2 NPs, noncoated and coated; 35, 10 × 100, and 250 nm; 10% cyclopentasiloxane suspension | in vitro, Yucatan micropig skin: intact, stripped and hairless | No No penetration through viable skin, however, TiO2 particles penetrated relatively deeply into the skin, possibly via empty hair follicle |
| Wu et al. [99] 2009 |
TiO2 powders suspensions: anatase: 4 and 10 nm, rutile: 25, 60, 90 nm, anatase/rutile: 21 nm (P25) |
in vitro—porcine skin,
in vivo—hairless mice |
Yes Toxic effects after subchronic exposure |
| Gontier et al. [107] 2008 |
Formulations: carbomergel with Degussa P25 (mixture of rutile and anatase, NPs of average size 21 nm, uncoated, approximately spherical platelets), hydrophobic basisgel with Eusolex T-200 (rutile, 20 × 100 nm, coated with Al2O3 and SiO2, lanceolate shape), polyacrylategel with Eusolex T-2000, a commercial sunscreen |
Samples of: porcine skin; human skin (dorsal region and buttocks); human skin grafted to SCID-mice |
No Porcine skin: TiO2 NPs found only on the surface of the outermost SC layer; human skin: penetration of NPs only into 10 μm layer of the SC; human skin grafted to SCID-mice: TiO2 NPs attached to the corneocytes |
| Mavon et al. [108] 2007 |
Formulation: w/o emulsion containing 3% TiO2 NPs with a mean diameter of 20 nm | in vitro—abdominal/face skin from human donors, in vivo—upper arms skin of human donors |
No No TiO2 NPs detected in the follicle, viable epidermis or dermis. TiO2 NPs accumulation in the uppermost layers of the SC (also in opened infundibulum) |
| Pinheiro et al. [109] 2007 |
Commercial sunscreen formulation | samples of human skin: healthy and psoriatic, from sacral-lumbal region | No In normal skin, TiO2 NPs were retained at the outermost layers of SC, in psoriatic skin, the penetration was slightly facilitated, but in both types of skin, the NPs did not reach living cell layers |
Abbreviations: sICAM-1—soluble intercellular adhesion molecule-1; HUVEC—human umbilical vein endothelial cells; ROS—reactive oxygen species; NPs—nanoparticles; SC—stratum corneum.
A considerable amount of studies have also examined the influence of TiO2 NPs on human cell lines in in vitro experiments. For the human keratinocyte cell line HaCaT (human adult low calcium high-temperature keratinocytes), following exposure to TiO2 NPs, decreased cell viability and induction of the cell cycle arrest have been demonstrated [110]. Rutile TiO2 NPs with <100 nm particle size were also tested on a human metastatic melanoma cell line, where a reduction in cell metabolic activity and cytotoxic response were observed. Especially interesting was a study of the influence of nano-TiO2 on the expression of mRNA of the ABCB5 transmembrane protein. The researchers presented that the studied nanomaterial might influence cell invasiveness and aggressiveness as the protein ABCB5 is closely linked to tumorigenicity, progression, and disease recurrence of some human malignancies [111].
The debate continues also on the potential penetration of titania NPs through damaged skin. Sunscreens are often applied on skin which is already sunburnt, dried out by UV irradiation, affected by beauty procedures (e.g., hair removal) or irritated by environmental factors (wind, salt and sand). In general, it should be noted that any changes in the composition of lipids caused by skin damages may impair the barrier function of the skin and therefore facilitate the penetration of NPs [4,112]. To date, the results of most comparative studies, both for commercial sunscreen formulations and nano-TiO2 suspensions, indicate that slight skin damages do not enhance its permeability [102,103,104,105,106]. However, it has to be emphasized that sunscreens definitely should not be applied on mechanically injured skin or an open wound. Besides, many authors have remarked that sunscreens are often used in sprayable forms, and this way of application may cause potential health risks in another manner—by inhaling TiO2 NPs. A variety of sunscreens is available in such a form. This issue concerns emulsions or oil sprays, foams, as well as mists. Sprayable forms have become increasingly common among consumers because of their ease of use. Inhalation exposure to TiO2 has been evaluated in several epidemiological analyses [113,114,115,116,117,118]. In these studies, as well as in case reports on human exposure to inhaled TiO2 [119,120], it has conclusively been shown that there is no positive correlation between the occurrence of carcinogenic effects and the occupational exposure to titania. However, it has to be emphasized that most of these studies provided no indication on the size of TiO2 particles.
Taking this into consideration, the International Agency for Research on Cancer (IARC) stated that the exposure to titanium dioxide is not directly associated with an increased cancer risk. Nevertheless, after assessment of the data derived from animal model studies, the IARC decided that there exists sufficient evidence to claim carcinogenicity of titanium dioxide to animals [1]. However, these data must be interpreted with caution, as various methodological approaches were adopted. Experiments concerned both the micro- and the nano-form of titanium dioxide. What is more, the results cannot be easily extrapolated to humans, because concentrations employed in some cases exceeded maximum human exposure. Overall, the IARC includes TiO2 in the group of substances which are possibly carcinogenic to humans (Group 2B). The ongoing discussion about the potential deposition and toxic effects of the nanoparticles caused by their inhalation needs to be resolved. Currently, the IARC advises against using sprayable sunscreen products. It should also be remarked that children are particularly susceptible to an unintended inhalation of TiO2 NPs since many sunscreen formulations for children come in the form of a spray or a foam, as these methods render the formulation easier to dispense and spread.
Recently, the attention of researchers studying TiO2 toxicity in sunscreens has turned to the surface and the entourage of TiO2 nanoparticles. For example, Y2O3-decorated TiO2 nanoparticles were found to display enhanced UV attenuation and reduced photoactivity and consequently, cytotoxicity, compared with a commercial TiO2 sample. The authors suggested the inclusion of these materials into sunscreen products [121]. In another study, coating of TiO2 NPs with dihydroxyphenyl benzimidazole carboxylic acid (Oxisol) not only led to photolytic activity reduction, but also boosted its antioxidant effects and stabilization of the formulation [122]. By modifying the surface of TiO2 NPs, it is also possible to improve the appearance of a sunscreen formulation, as formulations containing TiO2 NPs modified with a complexing compound, p-toluene sulfonic acid, were found to be more transparent [123].
The aforementioned issue of the protein corona should also be considered regarding the safety of nano-TiO2 as a sunscreen formulation compound. Serum proteins around the surface of the NP may undergo oxidation, even upon low generation of ROS, which provokes an oxidative stress response [124]. Future development of sunscreen ingredients should therefore comprise a proper design of their chemical surface. Furthermore, Sanches et al. stated that different contents of proteins, as well as other molecules (such as calcium or phosphorus) present in the biological medium, conceal TiO2 NPs and may influence their uptake and distribution [125]. An important remark is that a thorough analysis of TiO2 nanomaterial for sunscreen products should be performed also with regard to the nano–bio interactions. Additionally, Filipe et al. suggest that ROS or lipid peroxidation products appearing on the surface of the skin are prone to diffuse underneath the SC and subsequently lead to oxidative damage [97]. Moreover, ROS generated by nano-TiO2 may affect the transformation of other commonly used compounds of sunscreen formulations, including parabens, and increase their bioavailability and toxicity [126]. Several studies postulated that extreme stability and very poor aqueous solubility of TiO2 [127] could render its insolubility in sunscreens, making it biologically inert [13]. Nevertheless, some sunscreen formulations contain hydroxyacids (for example, citric or salicylic acid) which have the ability to chelate TiIV, leading to its dissolution [13].
Regarding dermal exposure of TiO2 NPs on human skin, it should be underlined that cream formulations containing these nanoparticles also reveal an impact on human cutaneous bacteria strains. Interestingly, this influence highly depends on the surface properties of NPs, mostly changes in polarity and charge, but also on the timescale of emulsions aging [128].
3. ’Green’ TiO2 NPs—Safer Perspective for the Future?
Numerous studies have already demonstrated that various metallic and metallic oxide NPs may be fabricated in compliance with green chemistry assumptions. ‘Green synthesis’ is often preferred over traditional methods for its many advantages, such as effectiveness, eco-friendliness, ease of characterization, fewer chances of failure, fast performance, and low cost [129]. It has been suggested that the materials used to fabricate the NPs greatly influence their morphology and physicochemical properties, which may have an impact on their further utilization [129]. ‘Green synthesized’ NPs, often called ‘biogenic NPs’ are generally considered safe, and in some cases, they display better properties to those synthesized with ‘traditional’ methods [130,131,132]. Currently, several methods have been developed for the synthesis of green NPs. Some of those technologies include the use of vitamins, like vitamin B2 or ascorbic acid, as well as enzymes from various plant extracts. They are in accordance with bio-based methods, which may involve the use of plants, bacteria, fungi or algae [129].
A considerable amount of studies has already described the green synthesis of TiO2 NPs and their characterization. Different approaches towards fabrication techniques of biogenic TiO2 NPs have been demonstrated and summed up in recent reviews and are beyond the scope of this paper [133,134]. However, compared with the amount of publications on various synthesis methods for ‘green’ TiO2 NPs, a relatively small body of literature touches upon their properties and compares their effectiveness with chemically derived TiO2 NPs [135,136,137]. For instance, there were published studies pointing out their antimicrobial activity. TiO2 NPs prepared with the use of Hibiscus rosa sinensis flower extract displayed not only significant activity against pathogenic bacteria but also acted more effectively than those synthesized by chemical synthesis [134]. In another study, ‘green’ TiO2 NPs, obtained by rapid synthesis using Moringa oleifera aqueous leaf extract, were found to exhibit significant wound healing activity in rats when compared with standard antibiotic drug for treatment of local infections [137]. A different approach was undertaken by Yu et al., who used lignosulfonate (LS), a natural macromolecular sun-blocker, to modify the surface of TiO2 NPs and therefore enhance the UV-blocking efficiency of the nanoparticles. Sunscreens containing TiO2@LS nanocomposites exhibited 30–60% higher SPF values than creams with the same dosage of nanograde TiO2 [138].
Taken together, these findings recommend the employment of ‘green’ TiO2 NPs for dermal applications. Continued efforts are needed to implement the use of safe and eco-friendly TiO2 NPs into sun-blocking formulations.
4. Conclusions and Perspectives
This paper has raised important questions on the current state of knowledge on the toxicity of titanium dioxide nanoparticles—a chemical compound commonly used in various everyday applications. In general, current findings seem to be inconsistent and highlight the necessity of establishing safety recommendations for TiO2 in its nanoform, regarding its applications as a food additive and cosmetic ingredient (Table 2).
Table 2.
Conclusions on the review of the literature regarding the safety of titanium(IV) oxide nanoparticles.
| Type of Usage/Application of TiO2 NPs | Conclusions | |
|---|---|---|
Food additive E171
|
The absorption of TiO2 from the digestive tract. | Generally considered as extremely low. |
| Safety of the long-term dietary exposure to TiO2 NPs. | Still uncertain: potential toxic effects may concern the absorption, distribution, and accumulation of TiO2 NPs. | |
| Potential risks caused by TiO2 NPs. | Genotoxicity, inflammatory response, carcinogenesis. | |
| Lack of established, acceptable daily intake limits. | ||
Sunscreen formulation
|
Penetration of TiO2 NPs through the SC. | Lack of certainty. |
| Generation of ROS on the skin surface and underneath—potential penetration through the skin and harmful effects? | Some evidence on the increase in oxidative stress marker levels has been reported. | |
| Lack of standardized guidelines for the quality control of sunscreens. | ||
There are few long-term exposure studies on the safety of TiO2 NPs usage as a food additive or a sunscreen ingredient. Despite the EFSA statement that the absorption of TiO2 after oral administration is extremely low [43], the usage of this ingredient, especially in nanoform, raises questions concerning its complete safety. This is because nanoparticles are generally more soluble and have a better ability to pass through the intestinal wall than larger particles, which in turn may lead to unwanted, harmful effects. It is still not sure if the food-grade TiO2 is involved in inducing toxic (e.g., proinflammatory or carcinogenic) processes in humans. Therefore, it is essential to conduct more studies on the toxicity of TiO2 NPs, taking into consideration the newest data. It is suggested that the further usage of E171 should be reconsidered, as it offers neither nutritional value nor extended shelf life. If the manufacturers persist in using E171, maximum daily intake levels ought to lead.
Referring to the application of TiO2 NPs as a sunscreen component, probably the most significant uncertainty concerns the penetration of these nanoparticles through the outermost layer of the skin, the stratum corneum, and their potential further passage to the bloodstream, which may result in their appearance in biological fluids. A few in vivo studies demonstrate the induction of oxidative stress processes and pathological lesions in different organs, such as the liver. Although the European Commission and FDA provided recommendations for testing and labeling of sunscreens, there is still a lack of official, standardized, binding guidelines for the manufacturers.
We suggest that in the penetration studies of TiO2 NPs in UV-filters, scientists should always take into consideration the type of formulation (cream/lotion/oil and type of the emulsion), way of application (cream/spray), the size of NPs and their surface properties. Further studies are necessary to determine whether TiO2 NPs passage through the SC and underlying parts of the skin leads to their presence in the bloodstream and the distribution to various organs (Table 3). In recent years, there has been an increasing amount of literature published on the surface modifications of various NPs. It seems that changing surface properties might be the key to obtain TiO2 NPs which are biologically inert but effective in terms of UV-blocking. The proper coating might decrease the penetration rate from the skin, as well as the absorption rate from the digestive tract. Noteworthy, developing green synthesis methods may also lead to the improvement of TiO2 NPs properties, as well as their stability.
Table 3.
Most probable perspectives in future studies regarding the safety of titanium(IV) oxide nanoparticles in everyday products.
| Type of Usage/Application of TiO2 NPs | Perspectives |
|---|---|
Food additive E171
|
Conducting a thorough safety assessment of E171 (especially its nanofraction). |
| Developing surface modification methods (e.g., to decrease the absorption rate) as well as green synthesis technologies. | |
| Establishing E171 daily intake levels considering different particle sizes, polymorphic structures, surface modifications, etc. | |
Sunscreen formulation
|
Providing complete assessment of the risk associated with the usage of TiO2 NPs-containing sunscreens. |
| Improving the action of TiO2 NPs by their surface modification and green synthesis. | |
| Providing official guidelines for sunscreen manufacturers. |
Funding
This research was funded by the National Science Centre, Poland, grant number 2016/21/B/NZ9/00783.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Carbon Black, Titanium Dioxide, and Talc. Volume 93. International Agency for Research on Cancer; Lyon, France: 2010. [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly T.D., Matos G.R. Historical Statistics for Mineral and Material Commodities in the United States. U.S. Geological Survey; Reston, VA, USA: 2006. Titanium dioxide end-use statistics, 1975-2004. (Data Series 140). [Google Scholar]
- 3.Zhang M., Chen T., Wang Y. Insights into TiO2 polymorphs: Highly selective synthesis, phase transition, and their polymorph-dependent properties. RSC Adv. 2017;7:52755–52761. doi: 10.1039/C7RA11515F. [DOI] [Google Scholar]
- 4.Smijs T.G., Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011;4:95–112. doi: 10.2147/NSA.S19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccinno F., Gottschalk F., Seeger S., Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012;14:1109. doi: 10.1007/s11051-012-1109-9. [DOI] [Google Scholar]
- 6.Weir A., Westerhoff P., Fabricius L., von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziental D., Czarczynska-Goslinska B., Mlynarczyk D.T., Glowacka-Sobotta A., Stanisz B., Goslinski T., Sobotta L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials. 2020;10:387. doi: 10.3390/nano10020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q., Huang J.-Y., Li H.-Q., Chen Z., Zhao A.Z.-J., Wang Y., Zhang K.-Q., Sun H.-T., Al-Deyab S.S., Lai Y.-K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016;11:4819–4834. doi: 10.2147/IJN.S108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babitha S., Korrapati P.S. Biodegradable zein-polydopamine polymeric scaffold impregnated with TiO2 nanoparticles for skin tissue engineering. Biomed. Mater. 2017;12:055008. doi: 10.1088/1748-605X/aa7d5a. [DOI] [PubMed] [Google Scholar]
- 10.Stan M.S., Nica I.C., Dinischiotu A., Varzaru E., Iordache O.G., Dumitrescu I., Popa M., Chifiriuc M.C., Pircalabioru G.G., Lazar V., et al. Photocatalytic, antimicrobial and biocompatibility features of cotton knit coated with Fe-N-Doped titanium dioxide nanoparticles. Materials. 2016;9:789. doi: 10.3390/ma9090789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seisenbaeva G.A., Fromell K., Vinogradov V.V., Terekhov A.N., Pakhomov A.V., Nilsson B., Ekdahl K.N., Vinogradov V.V., Kessler V.G. Dispersion of TiO2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15792-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng D., Kim M.G., Lee J.Y., Cho J. Green energy storage materials: Nanostructured TiO2 and Sn-based anodes for lithium-ion batteries. Energy Envron. Sci. 2009;2:818–837. doi: 10.1039/b823474d. [DOI] [Google Scholar]
- 13.Sharma S., Sharma R.K., Gaur K., Cátala Torres J.F., Loza-Rosas S.A., Torres A., Saxena M., Julin M., Tinoco A.D. Fueling a hot debate on the application of TiO2 nanoparticles in sunscreen. Materials. 2019;12:2317. doi: 10.3390/ma12142317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aillon K.L., Xie Y., El-Gendy N., Berkland C.J., Forrest M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009;61:457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanone S., Boczkowski J. Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr. Mol. Med. 2006;6:651–663. doi: 10.2174/156652406778195026. [DOI] [PubMed] [Google Scholar]
- 16.Merisko-Liversidge E.M., Liversidge G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008;36:43–48. doi: 10.1177/0192623307310946. [DOI] [PubMed] [Google Scholar]
- 17.Cho W.-S., Kang B.-C., Lee J.K., Jeong J., Che J.-H., Seok S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013;10:9. doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koltermann-Jülly J., Keller J.G., Vennemann A., Werle K., Müller P., Ma-Hock L., Landsiedel R., Wiemann M., Wohlleben W. Abiotic dissolution rates of 24 (nano) forms of 6 substances compared to macrophage-assisted dissolution and in vivo pulmonary clearance: Grouping by biodissolution and transformation. NanoImpact. 2018;12:29–41. doi: 10.1016/j.impact.2018.08.005. [DOI] [Google Scholar]
- 19.Brun E., Barreau F., Veronesi G., Fayard B., Sorieul S., Chanéac C., Carapito C., Rabilloud T., Mabondzo A., Herlin-Boime N., et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014;11:13. doi: 10.1186/1743-8977-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller J.G., Peijnenburg W., Werle K., Landsiedel R., Wohlleben W. Understanding dissolution rates via continuous flow systems with physiologically relevant metal ion saturation in lysosome. Nanomaterials. 2020;10:311. doi: 10.3390/nano10020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurr J.-R., Wang A.S.S., Chen C.-H., Jan K.-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Nel A. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 23.Foroozandeh P., Aziz A.A. Merging worlds of nanomaterials and biological environment: Factors governing protein corona formation on nanoparticles and its biological consequences. Nanoscale Res. Lett. 2015;10:221. doi: 10.1186/s11671-015-0922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saptarshi S.R., Duschl A., Lopata A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013;11:26. doi: 10.1186/1477-3155-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chetwynd A.J., Zhang W., Thorn J.A., Lynch I., Ramautar R. The nanomaterial metabolite corona determined using a quantitative metabolomics approach: A pilot study. Small. 2020:2000295. doi: 10.1002/smll.202000295. [DOI] [PubMed] [Google Scholar]
- 26.Khan A.O., Di Maio A., Guggenheim E.J., Chetwynd A.J., Pencross D., Tang S., Belinga-Desaunay M.-F.A., Thomas S.G., Rappoport J.Z., Lynch I. Surface chemistry-dependent evolution of the nanomaterial corona on TiO2 nanomaterials following uptake and sub-cellular localization. Nanomaterials. 2020;10:401. doi: 10.3390/nano10030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allegri M., Bianchi M.G., Chiu M., Varet J., Costa A.L., Ortelli S., Blosi M., Bussolati O., Poland C.A., Bergamaschi E. Shape-related toxicity of titanium dioxide nanofibres. PLoS ONE. 2016;11:e0151365. doi: 10.1371/journal.pone.0151365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter D.W., Wu N., Hubbs A.F., Mercer R.R., Funk K., Meng F., Li J., Wolfarth M.G., Battelli L., Friend S., et al. Differential mouse pulmonary dose and time course responses to titanium dioxide nanospheres and nanobelts. Toxicol. Sci. 2013;131:179–193. doi: 10.1093/toxsci/kfs261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva R.M., Teesy C., Franzi L., Weir A., Westerhoff P., Evans J.E., Pinkerton K.E. Biological response to nano-scale titanium dioxide (TiO2): Role of particle dose, shape, and retention. J. Toxicol. Environ. Health Part A. 2013;76:953–972. doi: 10.1080/15287394.2013.826567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danielsen P.H., Knudsen K.B., Štrancar J., Umek P., Koklič T., Garvas M., Vanhala E., Savukoski S., Ding Y., Madsen A.M., et al. Effects of physicochemical properties of TiO2 nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice. Toxicol. Appl. Pharm. 2020;386:114830. doi: 10.1016/j.taap.2019.114830. [DOI] [PubMed] [Google Scholar]
- 31.Papa A.-L., Dumont L., Vandroux D., Millot N. Titanate nanotubes: Towards a novel and safer nanovector for cardiomyocytes. Nanotoxicology. 2013;7:1131–1142. doi: 10.3109/17435390.2012.710661. [DOI] [PubMed] [Google Scholar]
- 32.Gea M., Bonetta S., Iannarelli L., Giovannozzi A.M., Maurino V., Bonetta S., Hodoroaba V.-D., Armato C., Rossi A.M., Schilirò T. Shape-engineered titanium dioxide nanoparticles (TiO2-NPs): Cytotoxicity and genotoxicity in bronchial epithelial cells. Food Chem. Toxicol. 2019;127:89–100. doi: 10.1016/j.fct.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Horváth T., Papp A., Igaz N., Kovács D., Kozma G., Trenka V., Tiszlavicz L., Rázga Z., Kónya Z., Kiricsi M., et al. Pulmonary impact of titanium dioxide nanorods: Examination of nanorod-exposed rat lungs and human alveolar cells. Int. J. Nanomed. 2018;13:7061–7077. doi: 10.2147/IJN.S179159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon M., Saez G., Muggiolu G., Lavenas M., Le Trequesser Q., Michelet C., Devès G., Barberet P., Chevet E., Dupuy D., et al. In situ quantification of diverse titanium dioxide nanoparticles unveils selective endoplasmic reticulum stress-dependent toxicity. Nanotoxicology. 2017;11:134–145. doi: 10.1080/17435390.2017.1278803. [DOI] [PubMed] [Google Scholar]
- 35.Brezová V., Gabčová S., Dvoranová D., Staško A. Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (an EPR study) J. Photochem. Photobiol. B Biol. 2005;79:121–134. doi: 10.1016/j.jphotobiol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCord J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108:652–659. doi: 10.1016/S0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 38.Skocaj M., Filipic M., Petkovic J., Novak S. Titanium dioxide in our everyday life; Is it safe? Radiol. Oncol. 2011;45:227–247. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakeel M., Jabeen F., Shabbir S., Asghar M.S., Khan M.S., Chaudhry A.S. Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: A review. Biol. Trace. Elem. Res. 2016;172:1–36. doi: 10.1007/s12011-015-0550-x. [DOI] [PubMed] [Google Scholar]
- 40.Jovanović B. Critical review of public health regulations of titanium dioxide, a human food additive. Integr. Environ. Assess. Manag. 2015;11:10–20. doi: 10.1002/ieam.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudefoi W., Terrisse H., Popa A.F., Gautron E., Humbert B., Ropers M.-H. Evaluation of the content of TiO2 nanoparticles in the coatings of chewing gums. Food Addit. Contam. Part A. 2018;35:211–221. doi: 10.1080/19440049.2017.1384576. [DOI] [PubMed] [Google Scholar]
- 42.Geiss O., Ponti J., Senaldi C., Bianchi I., Mehn D., Barrero J., Gilliland D., Matissek R., Anklam E. Characterisation of food grade titania with respect to nanoparticle content in pristine additives and in their related food products. Food Addit. Contam. Part A. 2020;37:239–253. doi: 10.1080/19440049.2019.1695067. [DOI] [PubMed] [Google Scholar]
- 43.EFSA-European Food Safety Authority Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016;14:e04545. doi: 10.2903/j.efsa.2016.4545. [DOI] [Google Scholar]
- 44.Bettini S., Boutet-Robinet E., Cartier C., Coméra C., Gaultier E., Dupuy J., Naud N., Taché S., Grysan P., Reguer S., et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (ANSES) Avis Relatif à Une Demande d’Avis Relatif à l’Exposition Alimentaire Aux Nanoparticules de Dioxyde de Titane. ANSES; Paris, France: 2017. [Google Scholar]
- 46.Arrêté du 17 avril 2019 Portant Suspension de la Mise sur le Marché des Denrées Contenant l’Additif E 171 (dioxyde de titane-TiO2) [(accessed on 24 May 2020)]; Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000038410047&categorieLien=id.
- 47.Proquin H., Rodríguez-Ibarra C., Moonen C.G.J., Urrutia Ortega I.M., Briedé J.J., de Kok T.M., van Loveren H., Chirino Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis. 2016;32:139–149. doi: 10.1093/mutage/gew051. [DOI] [PubMed] [Google Scholar]
- 48.Grissa I., Elghoul J., Ezzi L., Chakroun S., Kerkeni E., Hassine M., El Mir L., Mehdi M., Ben Cheikh H., Haouas Z. Anemia and genotoxicity induced by sub-chronic intragastric treatment of rats with titanium dioxide nanoparticles. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015;794:25–31. doi: 10.1016/j.mrgentox.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Talamini L., Gimondi S., Violatto M.B., Fiordaliso F., Pedica F., Tran N.L., Sitia G., Aureli F., Raggi A., Nelissen I., et al. Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology. 2019;13:1087–1101. doi: 10.1080/17435390.2019.1640910. [DOI] [PubMed] [Google Scholar]
- 50.Mottola F., Iovine C., Santonastaso M., Romeo M.L., Pacifico S., Cobellis L., Rocco L. NPs-TiO2 and lincomycin coexposure induces DNA damage in cultured human amniotic cells. Nanomaterials. 2019;9:1511. doi: 10.3390/nano9111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seyed-Talebi S.M., Kazeminezhad I., Motamedi H. TiO2 hollow spheres as a novel antibiotic carrier for the direct delivery of gentamicin. Ceram. Int. 2018;44:13457–13462. doi: 10.1016/j.ceramint.2018.03.276. [DOI] [Google Scholar]
- 52.Rowe R.C., Sheskey P.J., Quinn M.E. Handbook of Pharmaceutical Excipients. 6th ed. Pharmaceutical Press; London, UK: 2009. [Google Scholar]
- 53.Patel S., Patel P., Bakshi S.R. Titanium dioxide nanoparticles: An in vitro study of DNA binding, chromosome aberration assay, and comet assay. Cytotechnology. 2017;69:245–263. doi: 10.1007/s10616-016-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carriere M., Arnal M.-E., Douki T. TiO2 genotoxicity: An update of the results published over the last six years. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis. 2020:503198. doi: 10.1016/j.mrgentox.2020.503198. [DOI] [PubMed] [Google Scholar]
- 55.Liao F., Chen L., Liu Y., Zhao D., Peng W., Wang W., Feng S. The size-dependent genotoxic potentials of titanium dioxide nanoparticles to endothelial cells. Environ. Toxicol. 2019;34:1199–1207. doi: 10.1002/tox.22821. [DOI] [PubMed] [Google Scholar]
- 56.Santonastaso M., Mottola F., Colacurci N., Iovine C., Pacifico S., Cammarota M., Cesaroni F., Rocco L. In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO2) in human sperm cells. Mol. Reprod. Dev. 2019;86:1369–1377. doi: 10.1002/mrd.23134. [DOI] [PubMed] [Google Scholar]
- 57.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zijno A., De Angelis I., De Berardis B., Andreoli C., Russo M.T., Pietraforte D., Scorza G., Degan P., Ponti J., Rossi F., et al. Different mechanisms are involved in oxidative DNA damage and genotoxicity induction by ZnO and TiO2 nanoparticles in human colon carcinoma cells. Toxicol. Vitr. 2015;29:1503–1512. doi: 10.1016/j.tiv.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Chen T., Yan J., Li Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014;22:95–104. doi: 10.1016/j.jfda.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agir pour l’Environnement . Rapport d’Enquête Sur la Présence de Dioxyde de Titane Dans les Dentifrices. Agir pour l’Environnement; Paris, France: 2019. [Google Scholar]
- 61.Streit um Weißmacher -Plusminus -ARD |Das Erste. [(accessed on 19 January 2020)]; Available online: https://www.daserste.de/information/wirtschaft-boerse/plusminus/sendung/hr/streit-um-titandioxid-100.html.
- 62.Bianchi M.G., Allegri M., Chiu M., Costa A.L., Blosi M., Ortelli S., Bussolati O., Bergamaschi E. Lipopolysaccharide adsorbed to the bio-corona of TiO2 nanoparticles powerfully activates selected pro-inflammatory transduction pathways. Front. Immunol. 2017;8:866. doi: 10.3389/fimmu.2017.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yusoff R., Nguyen L.T.H., Chiew P., Wang Z.M., Ng K.W. Comparative differences in the behavior of TiO2 and SiO2 food additives in food ingredient solutions. J. Nanopart. Res. 2018;20:76. doi: 10.1007/s11051-018-4176-8. [DOI] [Google Scholar]
- 64.Kim J., Doudrick K. Emerging investigator series: Protein adsorption and transformation on catalytic and food-grade TiO2 nanoparticles in the presence of dissolved organic carbon. Environ. Sci. Nano. 2019;6:1688–1703. doi: 10.1039/C9EN00130A. [DOI] [Google Scholar]
- 65.Cao X., Han Y., Li F., Li Z., McClements D.J., He L., Decker E.A., Xing B., Xiao H. Impact of protein-nanoparticle interactions on gastrointestinal fate of ingested nanoparticles: Not just simple protein corona effects. NanoImpact. 2019;13:37–43. doi: 10.1016/j.impact.2018.12.002. [DOI] [Google Scholar]
- 66.Botelho M.C., Costa C., Silva S., Costa S., Dhawan A., Oliveira P.A., Teixeira J.P. Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro. Biomed. Pharmacother. 2014;68:59–64. doi: 10.1016/j.biopha.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Urrutia-Ortega I.M., Garduño-Balderas L.G., Delgado-Buenrostro N.L., Freyre-Fonseca V., Flores-Flores J.O., González-Robles A., Pedraza-Chaverri J., Hernández-Pando R., Rodríguez-Sosa M., León-Cabrera S., et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016;93:20–31. doi: 10.1016/j.fct.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz P.A., Morón B., Becker H.M., Lang S., Atrott K., Spalinger M.R., Scharl M., Wojtal K.A., Fischbeck-Terhalle A., Frey-Wagner I., et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut. 2017;66:1216–1224. doi: 10.1136/gutjnl-2015-310297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dorier M., Béal D., Tisseyre C., Marie-Desvergne C., Dubosson M., Barreau F., Houdeau E., Herlin-Boime N., Rabilloud T., Carriere M. The food additive E171 and titanium dioxide nanoparticles indirectly alter the homeostasis of human intestinal epithelial cells in vitro. Environ. Sci. Nano. 2019;6:1549–1561. doi: 10.1039/C8EN01188E. [DOI] [Google Scholar]
- 70.Guo Z., Martucci N.J., Moreno-Olivas F., Tako E., Mahler G.J. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact. 2017;5:70–82. doi: 10.1016/j.impact.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pedata P., Ricci G., Malorni L., Venezia A., Cammarota M., Volpe M.G., Iannaccone N., Guida V., Schiraldi C., Romano M., et al. In vitro intestinal epithelium responses to titanium dioxide nanoparticles. Food Res. Int. 2019;119:634–642. doi: 10.1016/j.foodres.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 72.Sohal I.S., DeLoid G.M., O’Fallon K.S., Gaines P., Demokritou P., Bello D. Effects of ingested food-grade titanium dioxide, silicon dioxide, iron (III) oxide and zinc oxide nanoparticles on an in vitro model of intestinal epithelium: Comparison between monoculture vs. a mucus-secreting coculture model. NanoImpact. 2020;17:100209. doi: 10.1016/j.impact.2020.100209. [DOI] [Google Scholar]
- 73.Yao L., Tang Y., Chen B., Hong W., Xu X., Liu Y., Aguilar Z.P., Xu H. Oral exposure of titanium oxide nanoparticles induce ileum physical barrier dysfunction via Th1/Th2 imbalance. Environ. Toxicol. 2020:22934. doi: 10.1002/tox.22934. [DOI] [PubMed] [Google Scholar]
- 74.Kho Z.Y., Lal S.K. The human gut microbiome-A potential controller of wellness and disease. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dudefoi W., Moniz K., Allen-Vercoe E., Ropers M.-H., Walker V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017;106:242–249. doi: 10.1016/j.fct.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 76.Agans R.T., Gordon A., Hussain S., Paliy O. Titanium dioxide nanoparticles elicit lower direct inhibitory effect on human gut microbiota than silver nanoparticles. Toxicol. Sci. 2019;172:411–416. doi: 10.1093/toxsci/kfz183. [DOI] [PubMed] [Google Scholar]
- 77.Radziwill-Bienkowska J.M., Talbot P., Kamphuis J.B.J., Robert V., Cartier C., Fourquaux I., Lentzen E., Audinot J.-N., Jamme F., Réfrégiers M., et al. Toxicity of food-grade TiO2 to commensal intestinal and transient food-borne bacteria: New insights using nano-SIMS and synchrotron UV fluorescence imaging. Front. Microbiol. 2018;9:794. doi: 10.3389/fmicb.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinget G., Tan J., Janac B., Kaakoush N.O., Angelatos A.S., O’Sullivan J., Koay Y.C., Sierro F., Davis J., Divakarla S.K., et al. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Front. Nutr. 2019;6:57. doi: 10.3389/fnut.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Z., Han S., Zhou D., Zhou S., Jia G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale. 2019;11:22398–22412. doi: 10.1039/C9NR07580A. [DOI] [PubMed] [Google Scholar]
- 80.Bocca B., Caimi S., Senofonte O., Alimonti A., Petrucci F. ICP-MS based methods to characterize nanoparticles of TiO2 and ZnO in sunscreens with focus on regulatory and safety issues. Sci. Total Environ. 2018;630:922–930. doi: 10.1016/j.scitotenv.2018.02.166. [DOI] [PubMed] [Google Scholar]
- 81.Serpone N., Salinaro A., Emeline A. In: Deleterious Effects of Sunscreen Titanium Dioxide Nanoparticles on DNA: Efforts to Limit DNA Damage by Particle Surface Modification, Proceedings of the BiOS 2001 The International Symposium on Biomedical Optics, San Jose, CA, USA, 2001. Murphy C.J., editor. SPIE; Bellingham, WA, USA: 2001. pp. 86–98. [Google Scholar]
- 82.Solaiman S.M., Algie J., Bakand S., Sluyter R., Sencadas V., Lerch M., Huang X.-F., Konstantinov K., Barker P.J. Nano-sunscreens-a double-edged sword in protecting consumers from harm: Viewing Australian regulatory policies through the lenses of the European Union. Crit. Rev. Toxicol. 2019;49:122–139. doi: 10.1080/10408444.2019.1579780. [DOI] [PubMed] [Google Scholar]
- 83.Popov A.P., Priezzhev A.V., Lademann J., Myllylä R. TiO2 nanoparticles as an effective UV-B radiation skin-protective compound in sunscreens. J. Phys. D Appl. Phys. 2005;38:2564–2570. doi: 10.1088/0022-3727/38/15/006. [DOI] [Google Scholar]
- 84.Lu P.J., Fang S.W., Cheng W.L., Huang S.C., Huang M.C., Cheng H.F. Characterization of titanium dioxide and zinc oxide nanoparticles in sunscreen powder by comparing different measurement methods. J. Food Drug Anal. 2018;26:1192–1200. doi: 10.1016/j.jfda.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Philippe A., Košík J., Welle A., Guigner J.-M., Clemens O., Schaumann G.E. Extraction and characterization methods for titanium dioxide nanoparticles from commercialized sunscreens. Environ. Sci. Nano. 2018;5:191–202. doi: 10.1039/C7EN00677B. [DOI] [Google Scholar]
- 86.De la Calle I., Menta M., Klein M., Maxit B., Séby F. Towards routine analysis of TiO2 (nano-)particle size in consumer products: Evaluation of potential techniques. Spectrochim. Acta Part B At. Spectrosc. 2018;147:28–42. doi: 10.1016/j.sab.2018.05.012. [DOI] [Google Scholar]
- 87.Ilić K., Selmani A., Milić M., Glavan T.M., Zapletal E., Ćurlin M., Yokosawa T., Vrček I.V., Pavičić I. The shape of titanium dioxide nanomaterials modulates their protection efficacy against ultraviolet light in human skin cells. J. Nanopart. Res. 2020;22:71. doi: 10.1007/s11051-020-04791-0. [DOI] [Google Scholar]
- 88.European Commission. Directorate General for Health & Consumers . Opinion on Titanium Dioxide (Nano Form): COLIPA n° S75. European Commission; Luxembourg: 2013. [Google Scholar]
- 89.European Commission. Directorate-General for Health and Food Safety . Opinion on Titanium Dioxide (Nano Form) as UV-Filter in Sprays. European Commission; Luxembourg: 2018. [Google Scholar]
- 90.Health Canada Guidance Document-Sunscreen 2012. [(accessed on 19 January 2020)]; Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/consultation/natur/sunscreen-ecransolaire-eng.pdf.
- 91.Center for Drug Evaluation and Research FDA labeling and effectiveness testing: Sunscreen drug products for over-the-counter human use—Small entity compliance Guide 2012. Fed. Regist. 2012;77:27591–27593. [PubMed] [Google Scholar]
- 92.Center for Drug Evaluation and Research. FDA ”FDA/CDER/”Beitz Nonprescription Sunscreen Drug Products—Safety and Effectiveness Data Guidance for Industry. [(accessed on 19 January 2020)];2016 Available online: https://www.fda.gov/media/94249/download.
- 93.BVL-Sonnenschutzmittel. [(accessed on 19 January 2020)]; Available online: https://www.bvl.bund.de/DE/Arbeitsbereiche/03_Verbraucherprodukte/02_Verbraucher/03_Kosmetik/06_Sonnenschutzmittel/bgs_kosmetik_sonnenschutzmittel_node.html.
- 94.US Food and Drug Administration FDA Advances New Proposed Regulation to Make Sure that Sunscreens Are Safe and Effective. [(accessed on 6 November 2019)]; Available online: http://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective.
- 95.Melquiades F.L., Ferreira D.D., Appoloni C.R., Lopes F., Lonni A.G., Oliveira F.M., Duarte J.C. Titanium dioxide determination in sunscreen by energy dispersive X-ray fluorescence methodology. Anal. Chim. Acta. 2008;613:135–143. doi: 10.1016/j.aca.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 96.Contado C., Pagnoni A. TiO2 in commercial sunscreen lotion: Flow field-flow fractionation and ICP-AES together for size analysis. Anal. Chem. 2008;80:7594–7608. doi: 10.1021/ac8012626. [DOI] [PubMed] [Google Scholar]
- 97.Filipe P., Silva J.N., Silva R., de Castro C.J.L., Marques Gomes M., Alves L.C., Santus R., Pinheiro T. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharm. Physiol. 2009;22:266–275. doi: 10.1159/000235554. [DOI] [PubMed] [Google Scholar]
- 98.Sadrieh N., Wokovich A.M., Gopee N.V., Zheng J., Haines D., Parmiter D., Siitonen P.H., Cozart C.R., Patri A.K., McNeil S.E., et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano-and submicron-size TiO2 particles. Toxicol. Sci. 2010;115:156–166. doi: 10.1093/toxsci/kfq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu J., Liu W., Xue C., Zhou S., Lan F., Bi L., Xu H., Yang X., Zeng F.-D. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009;191:1–8. doi: 10.1016/j.toxlet.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 100.Pelclova D., Navratil T., Kacerova T., Zamostna B., Fenclova Z., Vlckova S., Kacer P. NanoTiO2 sunscreen does not prevent systemic oxidative stress caused by UV radiation and a minor amount of nanoTiO2 is absorbed in humans. Nanomaterials. 2019;9:888. doi: 10.3390/nano9060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Q., Liu Z., Du J., Qin W., Lu M., Cui H., Li X., Ding S., Li R., Yuan J. Dermal exposure to nano-TiO2 induced cardiovascular toxicity through oxidative stress, inflammation and apoptosis. J. Toxicol. Sci. 2019;44:35–45. doi: 10.2131/jts.44.35. [DOI] [PubMed] [Google Scholar]
- 102.Crosera M., Prodi A., Mauro M., Pelin M., Florio C., Bellomo F., Adami G., Apostoli P., De Palma G., Bovenzi M., et al. Titanium dioxide nanoparticle penetration into the skin and effects on HaCaT cells. Int. J. Environ. Res. Public Health. 2015;12:9282–9297. doi: 10.3390/ijerph120809282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie G., Lu W., Lu D. Penetration of titanium dioxide nanoparticles through slightly damaged skin in vitro and in vivo. J. Appl. Biomater. Funct. Mater. 2015;13:356–361. doi: 10.5301/jabfm.5000243. [DOI] [PubMed] [Google Scholar]
- 104.Miquel-Jeanjean C., Crépel F., Raufast V., Payre B., Datas L., Bessou-Touya S., Duplan H. Penetration study of formulated nanosized titanium dioxide in models of damaged and sun-irradiated skins: Photochemistry and photobiology. Photochem. Photobiol. 2012;88:1513–1521. doi: 10.1111/j.1751-1097.2012.01181.x. [DOI] [PubMed] [Google Scholar]
- 105.Monteiro-Riviere N.A., Wiench K., Landsiedel R., Schulte S., Inman A.O., Riviere J.E. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: An in vitro and in vivo study. Toxicol. Sci. 2011;123:264–280. doi: 10.1093/toxsci/kfr148. [DOI] [PubMed] [Google Scholar]
- 106.Senzui M., Tamura T., Miura K., Ikarashi Y., Watanabe Y., Fujii M. Study on penetration of titanium dioxide (TiO2) nanoparticles into intact and damaged skin in vitro. J. Toxicol. Sci. 2010;35:107–113. doi: 10.2131/jts.35.107. [DOI] [PubMed] [Google Scholar]
- 107.Gontier E., Ynsa M.-D., Bíró T., Hunyadi J., Kiss B., Gáspár K., Pinheiro T., Silva J.-N., Filipe P., Stachura J., et al. Is there penetration of titania nanoparticles in sunscreens through skin? A comparative electron and ion microscopy study. Nanotoxicology. 2009 doi: 10.1080/17435390802538508. [DOI] [Google Scholar]
- 108.Mavon A., Miquel C., Lejeune O., Payre B., Moretto P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. SPP. 2007;20:10–20. doi: 10.1159/000096167. [DOI] [PubMed] [Google Scholar]
- 109.Pinheiro T., Pallon J., Alves L.C., Veríssimo A., Filipe P., Silva J.N., Silva R. The influence of corneocyte structure on the interpretation of permeation profiles of nanoparticles across skin. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007;260:119–123. doi: 10.1016/j.nimb.2007.02.014. [DOI] [Google Scholar]
- 110.Montalvo-Quiros S., Luque-Garcia J.L. Combination of bioanalytical approaches and quantitative proteomics for the elucidation of the toxicity mechanisms associated to TiO2 nanoparticles exposure in human keratinocytes. Food Chem. Toxicol. 2019;127:197–205. doi: 10.1016/j.fct.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 111.Zdravkovic B., Zdravkovic T.P., Zdravkovic M., Strukelj B., Ferk P. The influence of nano-TiO2 on metabolic activity, cytotoxicity and ABCB5 mRNA expression in WM-266-4 human metastatic melanoma cell line. J. BUON. 2019;24:338–346. [PubMed] [Google Scholar]
- 112.Elder A., Vidyasagar S., DeLouise L. Physicochemical factors that affect metal and metal oxide nanoparticle passage across epithelial barriers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:434–450. doi: 10.1002/wnan.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen J.L., Fayerweather W.E. Epidemiologic study of workers exposed to titanium dioxide. J. Occup. Med. 1988;30:937–942. doi: 10.1097/00043764-198812000-00011. [DOI] [PubMed] [Google Scholar]
- 114.Ellis E.D., Watkins J.P., Tankersley W.G., Phillips J.A., Girardi D.J. Occupational exposure and mortality among workers at three titanium dioxide plants. Am. J. Ind. Med. 2013;56:282–291. doi: 10.1002/ajim.22137. [DOI] [PubMed] [Google Scholar]
- 115.Boffetta P., Gaborieau V., Nadon L., Parent M.-E., Weiderpass E., Siemiatycki J. Exposure to titanium dioxide and risk of lung cancer in a population-based study from Montreal. Scand. J. Work Environ. Health. 2001;27:227–232. doi: 10.5271/sjweh.609. [DOI] [PubMed] [Google Scholar]
- 116.Boffetta P., Soutar A., Cherrie J.W., Granath F., Andersen A., Anttila A., Blettner M., Gaborieau V., Klug S.J., Langard S., et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control. 2004;15:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- 117.Fryzek J.P., Chadda B., Marano D., White K., Schweitzer S., McLaughlin J.K., Blot W.J. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J. Occup. Environ. Med. 2003;45:400–409. doi: 10.1097/01.jom.0000058338.05741.45. [DOI] [PubMed] [Google Scholar]
- 118.Ramanakumar A.V., Parent M.-É., Latreille B., Siemiatycki J. Risk of lung cancer following exposure to carbon black, titanium dioxide and talc: Results from two case-control studies in Montreal. Int. J. Cancer. 2008;122:183–189. doi: 10.1002/ijc.23021. [DOI] [PubMed] [Google Scholar]
- 119.Rode L.E., Ophus E.M., Gylseth B. Massive pulmonary deposition of rutile after titanium dioxide exposure. Acta Pathol. Microbiol. Scand. Sect. A Pathol. 1981;89:455–461. doi: 10.1111/j.1699-0463.1981.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 120.Ophus E.M., Rode L., Gylseth B., Nicholson D.G., Saeed K. Analysis of titanium pigments in human lung tissue. Scand. J. Work Environ. Health. 1979;5:290–296. doi: 10.5271/sjweh.3104. [DOI] [PubMed] [Google Scholar]
- 121.Chaki Borrás M., Sluyter R., Barker P.J., Konstantinov K., Bakand S. Y2O3 decorated TiO2 nanoparticles: Enhanced UV attenuation and suppressed photocatalytic activity with promise for cosmetic and sunscreen applications. J. Photochem. Photobiol. B Biol. 2020;207:111883. doi: 10.1016/j.jphotobiol.2020.111883. [DOI] [PubMed] [Google Scholar]
- 122.Battistin M., Dissette V., Bonetto A., Durini E., Manfredini S., Marcomini A., Casagrande E., Brunetta A., Ziosi P., Molesini S., et al. A new approach to UV protection by direct surface functionalization of TiO2 with the antioxidant polyphenol dihydroxyphenyl benzimidazole carboxylic acid. Nanomaterials. 2020;10:231. doi: 10.3390/nano10020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abuçafy M.P., Manaia E.B., Kaminski R.C.K., Sarmento V.H., Chiavacci L.A. Gel Based Sunscreen containing surface modified TiO2 obtained by sol-gel process: Proposal for a transparent UV inorganic filter. J. Nanomater. 2016;2016:e8659240. doi: 10.1155/2016/8659240. [DOI] [Google Scholar]
- 124.Jayaram D.T., Runa S., Kemp M.L., Payne C.K. Nanoparticle-induced oxidation of corona proteins initiates an oxidative stress response in cells. Nanoscale. 2017;9:7595–7601. doi: 10.1039/C6NR09500C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanches P.L., Souza W., Gemini-Piperni S., Rossi A.L., Scapin S., Midlej V., Sade Y., Leme A.F.P., Benchimol M., Rocha L.A., et al. Rutile nano-bio-interactions mediate dissimilar intracellular destiny in human skin cells. Nanoscale Adv. 2019;1:2216–2228. doi: 10.1039/C9NA00078J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De la Vega S.A.C., Molins-Delgado D., Barceló D., Díaz-Cruz M.S. Nanosized titanium dioxide UV filter increases mixture toxicity when combined with parabens. Ecotoxicol. Environ. Saf. 2019;184:109565. doi: 10.1016/j.ecoenv.2019.109565. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt J., Vogelsberger W. Aqueous long-term solubility of titania nanoparticles and titanium (IV) hydrolysis in a sodium chloride system studied by adsorptive stripping voltammetry. J. Solut. Chem. 2009;38:1267–1282. doi: 10.1007/s10953-009-9445-9. [DOI] [Google Scholar]
- 128.Rowenczyk L., Duclairoir-Poc C., Barreau M., Picard C., Hucher N., Orange N., Grisel M., Feuilloley M. Impact of coated TiO2-nanoparticles used in sunscreens on two representative strains of the human microbiota: Effect of the particle surface nature and aging. Colloids Surf. B Biointerfaces. 2017;158:339–348. doi: 10.1016/j.colsurfb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 129.Gour A., Jain N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019;47:844–851. doi: 10.1080/21691401.2019.1577878. [DOI] [PubMed] [Google Scholar]
- 130.Velmurugan P., Hong S.-C., Aravinthan A., Jang S.-H., Yi P.-I., Song Y.-C., Jung E.-S., Park J.-S., Sivakumar S. Comparison of the physical characteristics of green-synthesized and commercial silver nanoparticles: Evaluation of antimicrobial and cytotoxic effects. Arab. J. Sci. Eng. 2017;42:201–208. doi: 10.1007/s13369-016-2254-8. [DOI] [Google Scholar]
- 131.Ramteke C., Chakrabarti T., Sarangi B.K., Pandey R.-A. Synthesis of silver nanoparticles from the aqueous extract of leaves of ocimum sanctum for enhanced antibacterial activity. J. Chem. 2012;2013:278925. doi: 10.1155/2013/278925. [DOI] [Google Scholar]
- 132.Singh J., Dutta T., Kim K.-H., Rawat M., Samddar P., Kumar P. ’Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018;16 doi: 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nabi G., Aain Q., Khalid N.R., Tahir M.B., Rafique M., Rizwan M., Hussain S., Iqbal T., Majid A. A review on novel eco-friendly green approach to synthesis TiO2 nanoparticles using different extracts. J. Inorg. Organomet. Polym. 2018;28:1552–1564. doi: 10.1007/s10904-018-0812-0. [DOI] [Google Scholar]
- 134.Nadeem M., Tungmunnithum D., Hano C., Abbasi B.H., Hashmi S.S., Ahmad W., Zahir A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018;11:492–502. doi: 10.1080/17518253.2018.1538430. [DOI] [Google Scholar]
- 135.Kumar P.S.M., Francis A.P., Devasena T. Biosynthesized and chemically synthesized titania nanoparticles: Comparative analysis of antibacterial activity. J. Environ. Nanotechnol. 2014;3:73–81. doi: 10.13074/jent.2014.09.143098. [DOI] [Google Scholar]
- 136.Jayaseelan C., Rahuman A.A., Roopan S.M., Kirthi A.V., Venkatesan J., Kim S.-K., Iyappan M., Siva C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;107:82–89. doi: 10.1016/j.saa.2012.12.083. [DOI] [PubMed] [Google Scholar]
- 137.Sivaranjani V., Philominathan P. Synthesize of titanium dioxide nanoparticles using moringa oleifera leaves and evaluation of wound healing activity. Wound Med. 2016;12:1–5. doi: 10.1016/j.wndm.2015.11.002. [DOI] [Google Scholar]
- 138.Yu J., Li L., Qian Y., Lou H., Yang D., Qiu X. Facile and green preparation of high UV-blocking lignin/titanium dioxide nanocomposites for developing natural sunscreens. Ind. Eng. Chem. Res. 2018;57:15740–15748. doi: 10.1021/acs.iecr.8b04101. [DOI] [Google Scholar]