Abstract
Confinement due to the COVID-19 pandemic can influence dietary profiles, especially those of adolescents, who are highly susceptible to acquiring bad eating habits. Adolescents’ poor dietary habits increase their subsequent risk of degenerative diseases such as obesity, diabetes, cardiovascular pathologies, etc. Our aim was to study nutritional modifications during COVID-19 confinement in adolescents aged 10 to 19 years, compare them with their usual diet and dietary guidelines, and identify variables that may have influenced changes. Data were collected by an anonymous online questionnaire on food intake among 820 adolescents from Spain, Italy, Brazil, Colombia, and Chile. The results show that COVID-19 confinement did influence their dietary habits. In particular, we recorded modified consumption of fried food, sweet food, legumes, vegetables, and fruits. Moreover, gender, family members at home, watching TV during mealtime, country of residence, and maternal education were diversely correlated with adequate nutrition during COVID-19 confinement. Understanding the adolescents’ nutrition behavior during COVID-19 lockdown will help public health authorities reshape future policies on their nutritional recommendations, in preparation for future pandemics.
Keywords: COVID-19, adolescents, diet, questionnaire, lifestyle, e-survey
1. Introduction
On December 2019, an outbreak of pneumonia of then unknown etiology emerged in Wuhan City, Hubei Province in China, alerting the medical and scientific communities [1]. The causal agent was later identified in a new betacoronavirus called SARS-CoV-2, which can affect the lower respiratory tract and provoke bilateral pneumonia in humans [1]. This pathology—termed COVID-19 by the World Health Organization (WHO)—infected and killed thousands of people throughout the world; extraordinary measures have been taken in most countries, including Spain, Italy, Brazil, Chile, and Colombia. One of the containment measures was the total confinement of the population in their homes, also known as lockdown. This led to the disruption of most daily activities [2]. Different governments took different measures, yet all promulgated lockdown policies. On 9 March 2020, a national quarantine was imposed for Italy. A state of alarm and national lockdown was imposed on 14 March in Spain. A nationwide quarantine started in Colombia on 24 March 2020. On 27 March, Brazil announced a temporary ban on foreign air travelers and most state governors have imposed isolation policies. No national lockdown has been established in Chile, but some communities and urban areas did declare a mandatory quarantine at different times. However, on 16 March 2020, schools were closed in that country too (Supplementary Materials Table S1).
Confinement influences lifestyle, especially diet and physical activity. The World Health Organization and the Spanish Academy of Nutrition and Dietetics (2020) indicate that a healthy diet can help in the prevention and treatment of the disease [3]. Thus, recommendations have been published for food and nutrition during the period of confinement of the population, because there is a close relationship between the quality of a population’s food and its health [4]. Adequate nutrition is considered a potential factor for health in the early stages of life and adolescence [5]. At this stage, i.e., the transition period from childhood to adulthood, it is essential to acquire good eating behaviors that can concomitantly influence current health status and predisposition to diseases, e.g., obesity, diabetes, cardiovascular pathologies, etc., in adulthood. It is worth mentioning that the WHO implements and maintains health risk factor monitoring systems in adolescents [6].
It should be noted that, during confinement, it could become difficult to shop for fresh groceries and shortages of certain food products might happen. As recognized by The Food and Agriculture Organization (FAO), the COVID-19 pandemic has caused disruptions in food chains around the world, affecting both supply and demand [7]. Further, COVID-19 has made visible and magnified social inequalities, with the poorest families being the most affected ones [7].
On the other hand, there is the possibility that closer contact with family members and more home cooking due to COVID-19 confinement could teach adolescents skills that could improve their nutrition knowledge and behaviors, as several studies have indeed reported [8,9].
Therefore, in view of the current pandemic—when the population is suffering from social isolation—it is necessary to carry out research on the influence of this confinement on the quality of adolescents’ diet, considering some markers of healthy food intake. This could help public health authorities shape their recommendations, in terms of nutrition policies for adolescents, for future lockdown policies. Indeed, lifestyle lessons from the COVID-19 pandemic should prepare the whole population for the next one [10].
We aimed to assess the effects of COVID-19-induced confinement policies on self-reported nutritional habit modifications in adolescents from the five above-mentioned countries compared with their usual diet and with the dietary guidelines. Moreover, we aimed to identify potential variables that may have influenced this change.
2. Materials and Methods
2.1. Participants
This project was undertaken between 17 April 2020 and 25 May 2020. The target population was adolescents aged 10 to 19 years from several regions of Spain, Italy, Brazil, Colombia, and Chile (Supplementary Materials Table S2). The participants consented to participate in the study, with a digital informed consent form.
2.2. Study Design
This cross-sectional study used data collected via an anonymous online questionnaire consisting of more than 30 questions about dietary habits during COVID-19 confinement and the previous period. We distributed the questionnaire via social media, e.g., Twitter, WhatsApp or others (see below). In addition, researchers involved in this project distributed the survey to work colleagues.
Dietary practices were evaluated using a standardized adolescent questionnaire, the National School Health Survey–PeNSE; Pesquisa Nacional de Saúde do Escolar [11], which was slightly modified. Data collection was performed through a questionnaire divided into modules: sociodemographic and family features and dietary practices before and during confinement. The adolescent recorded the number of days on which they consumed the following foods or food groups during the week before confinement (BEFORE) and one week during confinement (COVID19): legumes; vegetables; fruit; sweet food; fried food (including packaged potatoes); processed meat (burger, sausage, mortadella, salami, ham, chicken nuggets, or sausages); sugar-sweetened beverages (SSB), and fast food. The PeNSE survey allows us to compare international indicators, especially those of the Global School-Based Student Health Survey [12], developed by the WHO, used in more than 90 countries around the world.
2.3. Data Collection
Data collection was carried out through a structured questionnaire created in Google Forms (Google LLC, Menlo Park, CA, USA). The questionnaire was divided into modules by subject: sociodemographic characteristics, dietary and lifestyle practices. The invitation to participate in the survey was made by social media (Facebook, Instagram and WhatsApp) or by e-mail to municipal authorities’ parents. The flow chart of participants of the study is depicted in Supplementary Figure S1.
2.4. Data Analysis
Initially, we compared the average intake of different food groups among the participants during COVID-19 confinement (COVID19) versus the previous period (BEFORE) by paired two-way Student’s t-test. The comparison of mean intake of different food groups during COVID-19 confinement classified by sociodemographic and family variables was assessed by two-way ANOVA. The independent categorical variables in Table of percentage of adolescents that maintain adequate food intake according to dietary guidelines by sociodemographic and familiar variables were assessed by chi-square test. A 95% confidence interval (95%CI) was adopted. To do this, two variables were created: a variable quantifying servings of legume, fruit, vegetables, fried food, sugary drinks, processed meat, and fast food intake per week, with seven categories (once, two, three, four, five, six and seven times per week) (this was the more important variable of this study); and a binary variable indicating if these adolescents met the WHO recommended diet during self-quarantine or longer home stays (yes/no). Socio-demographic variables collected were categorized as: gender: female and male; age: ≤14 years, 15–16 years and ≥17 years; number of people living at home: ≤3 people, 4–6 people and ≥7 people; watching TV during mealtimes: always, sometimes and never; and maternal education: none, primary, secondary, professional formation and complete university. A significance level of p < 0.05 was applied to all statistical analyses. GraphPad Prism 8 (version 8.3.0; Graph Pad Software Inc. San Diego, CA, USA) was used for all statistical analyses.
2.5. Ethical Approval
Ethical approval was obtained by the appropriate Ethical Committees of each country where the survey was performed. Specifically, by the IMDEA Food Research Ethics Committee in Spain (code IMD: PI-043); by the Ethical Committee of Human Inspired Technology Research Centre-Padova University in Italy (HIT Ethical Committee 33035 22 April 2020); by the Research Ethics Committee of IPPMG–Federal University of Rio de Janeiro in Brazil (approval number: 3,975,744); by the Cartagena committee and the University of Cartagena in Colombia (acta N° 134) and by the University of Concepción School of Medicine Bioethical Committee in Chile (CEBB 646-2020). The study is in accordance with the ethical principles of non-maleficence, beneficence, justice and autonomy, contained in the ethical resolutions of each country, according to the Helsinki declaration. An informed consent form was signed digitally by one of the participants’ guardians before initiating the survey.
3. Results and Discussion
3.1. Socio-Demographic Characteristics
A total of 820 adolescents from several regions of Spain, Italy, Brazil, Colombia, and Chile participated in this study (Supplementary Materials Table S2). The average age of adolescents was 15 years (18.7%), with more girls (61.1%) than boys (38.9%). As for maternal education, 43.3% reported that their mothers did not have a university degree. The sample was about equally divided between the five countries. All sociodemographic variables are included in Table 1.
Table 1.
Socio-demographic characteristics of the 820adolescents who filled out the questionnaire.
| Variables | Sample% | Variables | Sample% |
|---|---|---|---|
| Gender (N = 810) | Maternal education (N = 779) | ||
| Boys | 38.89 | None | 1.54 |
| Girls | 61.11 | Primary | 10.14 |
| Age (years) (N = 820) | Secondary | 15.92 | |
| ≤14 | 27.68 | Professional formation | 17.97 |
| 15–16 | 30.98 | Complete university | 54.43 |
| ≥17 | 41.34 | Family members (N = 820) | |
| Country (N = 820) | ≤3 | 25.73 | |
| Spain | 18.54 | 4–6 | 67.80 |
| Brazil | 14.02 | ≥7 | 6.46 |
| Colombia | 19.63 | Viewing TV during mealtimes (N = 820) | |
| Chile | 26.22 | Always | 46.71 |
| Italy | 21.59 | Sometimes | 19.88 |
| Never | 33.41 |
3.2. Confinement Due to COVID-19 Changed Dietary Trends among Adolescents
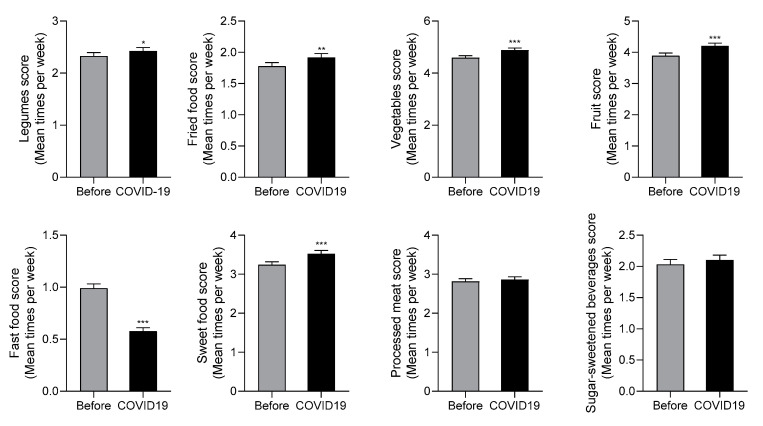
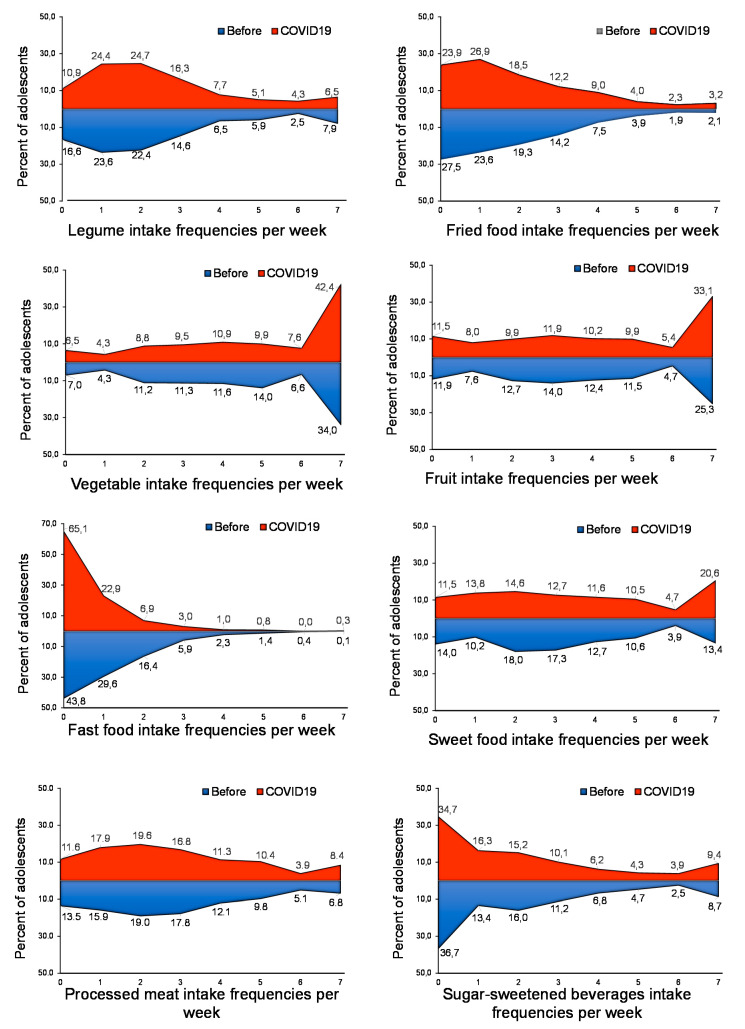
Figure 1 reports the mean food score per week during and before the COVID-19 period. Legumes, vegetables, and fruit intakes were significantly increased during COVID-19 confinement. In addition, the distribution intake frequencies show an increase in the number of adolescents who consume the recommended weekly servings of legumes during confinement (2, 3, or 4 servings per week; from 22.7, 15.4 and 6.1% before to 25.0%, 16.0% and 7.4% during COVID-19 confinement) (Figure 2).
Figure 1.
Comparison of average dietary intake among adolescents during COVID-19 confinement (COVID19) and the previous period (Before). Data are means ± SEM. Comparison between groups by paired two-way Student’s t-test. * p < 0.05, ** p < 0.001, *** p < 0.0001. N = 820.
Figure 2.
Food intake frequencies to compare dietary patterns during COVID-19 confinement (COVID19, red area) and the previous period (BEFORE, blue area), expressed by percent of adolescents according to weekly intake frequency of each food group. N = 820.
It is also important to highlight the changes in the pattern of vegetable and fruit consumption of the adolescents of this survey. Forty-three percent of adolescents consumed vegetables every day during confinement versus 35.2% who did it before (Figure 2). Similarly, only 25.5% of adolescents surveyed consumed at least one piece of fruit per day before COVID-19 versus 33.2% during confinement (Figure 2). These results are not surprising because the sale of this type of food has increased since the beginning of confinement [13] and the population has more time to cook at home. Further, the WHO recommends legumes, fruits and vegetables as the best food items during self-quarantine or longer home stays [14].
In addition, we report that fast food intake was dramatically reduced in adolescents during COVID-19 confinement (Figure 1). While before confinement only 44.6% of adolescents consumed fast food less than once a week, this figure increased to 64% during confinement (Figure 2). It is possible that home cooking could reduce the incidence of chronic diseases [15], but any long-term improvements caused by increased cooking might be small compared to the more problematic and enduring effects of this crisis on children and adolescents [16,17].
By contrast, fried and sweet food average intakes increased significantly during COVID-19 confinement (Figure 1). Fourteen percent of adolescents consumed sweet food every day before COVID-19, which increased to 20.7% during confinement (Figure 2). Similarly, Figure 2 shows the increase of adolescents who consumed fried foods 4–7 days per week, from 7.4%, 3.7%, 1.8% and 2.1% before to 8.8%, 3.8%, 2.2% and 2.9% during confinement. These results confirm previous studies that suggested that the confinement could lead to irregular eating patterns and frequent snacking in adolescents due to boredom and stress [17,18]. It is also important to highlight that these dietary habits are associated with a higher caloric intake and an increased risk of obesity [19].
There were no changes in self-reported processed meat and sugar-sweetened beverage intakes (SSB) in these populations during COVID-19 isolation (Figure 1 and Figure 2). In contrast, in a recent study of Italian adults, purchases of ready-made meals were reduced during the lockdown [20].
3.3. Adolescents’ Dietary Habits during COVID-19 Confinement According to Sociodemographic and Family Characteristics and the Modifications Versus the Previous Period
Table 2 shows the changes in adolescents’ dietary trends classified by sociodemographic and family characteristics due to COVID-19 confinement. Only the most notable results will be discussed. The highest rates of adherence to the weekly food intake recommendation were in females among adolescents living in Europe and those who reported a higher maternal education (Table 3).
Table 2.
Comparison of adolescents’ dietary trends before and during COVID-19 confinement classified by sociodemographic and familiar characteristics.
| Variables | Legumes | Fried Food | Vegetables | Sweet Food | Fruits | Sugar-Sweetened Beverages | Processed Meat | Fast Food | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% IC | p-Value | Mean | 95% IC | p-Value | Mean | 95% IC | p-Value | Mean | 95% IC | p-Value | Mean | 95% IC | p-Value | Mean | 95% IC | p-Value | Mean | 95% IC | p-Value | Mean | 95% IC | p-Value | |
| Gender (N = 810) | ||||||||||||||||||||||||
| Male | 2.3–2.4 | (2.1–2.6)–(2.2–2.6) | 0.1223 | 1.8–1.9 | (1.6–2.0)–(1.8–2.1) | 0.0803 | 4.3–4.6 | (4.1–4.6)–(4.3–4.8) | 0.0028 ** | 3.1–3.3 | (2.8–3.3)–(3.0–3.6) | 0.0174 * | 3.7–3.9 | (3.5–4.0)–(3.7–4.2) | 0.0556 | 2.3–2.4 | (2.1–2.7)–(2.0–2.6) | 0.193 | 2.9–3.0 | (2.6–3.1)–(2.8–3.3) | 0.0182 * | 0.9–0.6 | (0.8–1.0)–(0.5–0.7) | <0.0001 *** |
| Female | 2.3–2.4 | (2.1–2.5)–(2.3–2.6) | 0.0768 | 1.7–1.9 | (1.6–1.8)–(1.7–2.0) | 0.0224 * | 4.8–5.1 | (4.6–5.0)–(4.9–5.3) | <0.0001 *** | 3.3–3.6 | (3.2–3.5)–(3.4–3.8) | 0.0067 ** | 4.0–4.4 | (3.7–4.2)–(4.1–4.6) | <0.0001 *** | 1.9–1.9 | (1.7–2.1)–(1.7–2.1) | 0.9232 | 2.8–2.8 | (2.6–3.0)–(2.6–2.9) | 0.3263 | 1.0–0.6 | (0.9–1.1)–(0.5–0.6) | <0.0001 *** |
| Age (years) (N = 820) | ||||||||||||||||||||||||
| ≤14 | 2.3–2.3 | (2.0–2.6) – (2.0–2.6) | >0.9999 | 1.3–1.6 | (1.1–1.5)–(1.4–1.8) | 0.0025 ** | 4.9–5.0 | (4.5–5.2)–(4.7–5.3) | 0.0707 | 3.2–3.5 | (2.9–3.3)–(3.1–3.8) | 0.0386 * | 4.1–4.2 | (3.8–4.4)–(3.9–4.5) | 0.4900 | 1.8–2.0 | (1.5–2.1)–(1.7–2.3) | 0.0518 | 2.6–2.8 | (2.4–2.9)–(2.5–3.0) | 0.2457 | 0.8–0.6 | (0.6–0.9)–(0.4–0.7) | 0.0056 ** |
| 15–16 | 2.4–2.5 | (2.1–2.6) – (2.3–2.7) | 0.0954 | 1.9–1.9 | (1.7–2.2)–(1.7–2.2) | 0.9258 | 4.4–4.9 | (4.1–4.7)–(4.6–5.1) | <0.0001 *** | 3.6–3.8 | (3.4–3.9)–(3.5–4.1) | 0.2576 | 4.1–4.3 | (3.8–4.4)–(4.0–4.7) | 0.0139 * | 2.2–2.1 | (1.9–2.5)–(1.8–2.4) | 0.469 | 2.7–2.8 | (2.5–3.0)–(2.5–3.0) | 0.8028 | 1.0–0.5 | (0.9–1.2)–(0.4–0.6) | <0.0001 *** |
| ≥17 | 2.4–2.5 | (2.1–2.6) – (2.3–2.7) | 0.0237 * | 2.0–2.1 | (1.8–2.1)–(1.9–2.3) | 0.0448 * | 4.6–4.8 | (4.3–4.8)–(4.6–5.1) | 0.002 ** | 3.0–3.4 | (2.8–3.2)–(3.2–3.6) | 0.001 ** | 3.6–4.1 | (3.8–3.9)–(3.8–4.4) | <0.0001 *** | 2.1–2.2 | (1.9–2.3)–(1.9–2.4) | 0.5048 | 3.0–3.0 | (2.8–3.2)–(2.8–3.3) | 0.9426 | 1.1–0.6 | (0.9–1.2)–(0.6–0.7) | <0.0001 *** |
| Country (N = 820) | ||||||||||||||||||||||||
| Spain | 2.5–2.5 | (2.3–2.7) – (2.3–2.8) | 0.9375 | 2.2–2.3 | (2.0–2.5)–(2.0–2.5) | 0.7728 | 4.5–4.9 | (4.1–4.8)–(4.6–5.2) | <0.0001 *** | 2.7–3.1 | (2.4–3.0)–(2.8–3.5) | 0.0075 ** | 4.5–5.1 | (4.1–4.9)–(4.7–5.5) | 0.0008 *** | 1.4–1.5 | (1.1–1.6)–(1.2–1.8) | 0.2383 | 2.7–3.0 | (2.4–3.0)–(2.7–3.3) | 0.0534 | 0.8–0.2 | (0.7–1.0)–(0.1–0.3) | <0.0001 *** |
| Italy | 1.6–1.8 | (1.4–1.8) – (1.5–2.0) | 0.0308 * | 1.2–1.4 | (1.0–1.4)–(1.2–1.6) | 0.0375 * | 4.5–4.7 | (4.2–4.9)–(4.4–5.1) | 0.0723 | 3.7–4.2 | (3.4–4.0)–(3.9–4.5) | <0.0001 *** | 4.2–4.5 | (3.9–4.6)–(4.2–4.9) | 0.0172 * | 1.8–1.9 | (1.4–2.0)–(1.6–2.2) | 0.1321 | 3.0–3.0 | (2.7–3.3)–(2.7–3.3) | 0.6935 | 0.7–0.5 | (0.6–0.9)–(0.4–0.6) | 0.0114 * |
| Brazil | 5.1–4.7 | (4.7–5.5) – (4.3–5.1) | 0.0031 ** | 1.6–1.7 | (1.4–1.7)–(1.5–1.9) | 0.1613 | 4.4–4.6 | (4.1–4.6)–(4.3–4.9) | 0.0039 ** | 3.1–4.0 | (2.9–3.4)–(3.7–4.3) | <0.0001 *** | 3.6–3.9 | (3.3–3.9)–(3.6–4.2) | 0.0097 ** | 1.6–2.0 | (1.2–1.9)–(1.5–2.4) | 0.03 * | 3.2–3.3 | (3.0–3.4)–(3.0–3.5) | 0.3427 | 1.4–0.8 | (1.2–1.6)–(0.5–1.0) | <0.0001 *** |
| Colombia | 2.2–2.6 | (2.0–2.5)–(2.3–2.9) | 0.0026 ** | 2.7–2.7 | (2.4–3.0)–(2.4–3.1) | 0.7284 | 3.9–4.0 | (3.6–4.3)–(3.7–4.4) | 0.4793 | 3.4–3.4 | (3.1–3.6)–(3.2–3.7) | 0.7301 | 3.5–3.8 | (3.2–3.7)–(3.5–4.1) | 0.0195 * | 3.0–2.5 | (2.6–3.3)–(2.2–2.8) | 0.0008 *** | 3.0–3.0 | (2.7–3.2)–(2.7–3.1) | 0.4084 | 1.3–0.7 | (1.1–1.4)–(0.5–0.8) | <0.0001 *** |
| Chile | 1.4–1.6 | (1.3–1.5)–(1.4–1.7) | 0.0021 | 1.4–1.8 | (1.2–1.6)–(1.6–2.0) | <0.0001 *** | 4.9–5.2 | (4.6–5.1)–(4.9–5.4) | 0.0019 ** | 3.0–3.3 | (2.7–3.2)–(3.0–3.6) | 0.0381 * | 4.1–4.2 | (3.7–4.4)–(3.9–4.5) | 0.2454 | 2.0–2.3 | (1.7–2.3)–(1.9–2.6) | 0.0598 | 2.6–2.6 | (2.4–2.9)–(2.4–2.9) | 0.5145 | 0.8–0.7 | (0.6–1.0)–(0.5–0.8) | 0.0392 * |
| Maternal education (N = 779) | ||||||||||||||||||||||||
| None | 3.0–3.3 | (1.7–4.9)–(1.5–4.5) | 0.3388 | 1.8–2.2 | (1.2–3.1)–(0.5–3.2) | 0.6326 | 2.9–3.3 | (2.1–4.6)–(1.6–4.3) | 0.2098 | 3.4–4.2 | (2.8–3.9)–(3.6–4.8) | 0.0143 * | 3.3–3.6 | (1.7–4.9)–(2.1–5.1) | 0.6332 | 3.1–3.8 | (2.0–5.6)–(1.2–5.0) | 0.3625 | 2.9–3.3 | (1.6–4.3)–(1.8–4.9) | 0.4699 | 1.6–1.8 | (0.8–2.4)–(0.2–3.3) | 0.7126 |
| Primary | 2.0–2.2 | (1.6–2.4)–(1.8–2.6) | 0.3204 | 2.0–2.0 | (1.7–2.3)–(1.7–2.4) | 0.754 | 4.5–4.9 | (4.1–5.0)–(4.4–5.4) | 0.0883 | 3.2–3.2 | (2.7–3.7)–(2.7–3.7) | 0.8764 | 3.6–3.9 | (3.0–4.1)–(3.4–4.5) | 0.0575 | 1.9–2.0 | (1.5–2.4)–(1.5–2.5) | 0.8761 | 2.8–2.7 | (2.4–3.2)–(2.3–3.1) | 0.6177 | 1.0–0.5 | (0.7–1.2)–(0.3–0.8) | 0.0007 *** |
| Secondary | 2.1–2.2 | (1.–2.4)–(1.8–2.6) | 0.3204 | 1.9–2.1 | (1.6–2.2)–(1.7–2.4) | 0.2244 | 3.8–4.0 | (3.4–4.3)–(3.5–4.4) | 0.2896 | 3.5–3.5 | (3.1–3.9)–(3.0–3.9) | 0.9364 | 2.9–3.1 | (2.5–3.3)–(2.7–3.5) | 0.352 | 2.5–2.6 | (2.1–2.9)–(2.2–3.0) | 0.7131 | 2.9–3.1 | (2.8–3.5)–(2.6–3.4) | 0.226 | 1.0–0.5 | (0.8–1.2)–(0.4–0.7) | <0.0001 *** |
| Professional formation | 2.0–2.3 | (1.7–2.3)–(2.0–2.6) | 0.004** | 1.9–2.3 | (1.7–2.3)–(2.1–2.7) | 0.0025** | 4.8–5.3 | (4.4–5.2)–(5.0–5.7) | 0.0006*** | 3.1–3.5 | (2.7–3.4)–(3.1–3.8) | 0.0321 * | 4.0–4.6 | (3.6–4.4)–(4.2–5.0) | 0.0002 *** | 2.4–2.6 | (2.0–2.8)–(2.2–3.0) | 0.4696 | 2.8–3.1 | (2.5–3.2)–(2.7–3.4) | 0.1009 | 1.0–0.7 | (0.8–1.2)–(0.5–0.8) | 0.0006 *** |
| Complete univesity | 2.5–2.5 | (2.3–2.7)–(2.3–2.7) | 0.7896 | 1.6–1.7 | (1.5–1.8)–(1.5–1.8) | 0.3039 | 4.8–5.2 | (4.6–5.1)–(5.0–5.4) | <0.0001*** | 3.2–3.6 | (3.0–3.4)–(3.4–3.9) | <0.0001 *** | 4.2–4.5 | (4.0–4.5)–(4.3–4.8) | 0.0054 ** | 1.6–1.7 | (1.4–1.8)–(1.5–1.9) | 0.1633 | 2.8–2.8 | (2.6–3.0)–(2.6–3.0) | 0.7943 | 0.9–0.5 | (0.8–1.0)–(0.4–0.6) | <0.0001 *** |
| Family members (N = 820) | ||||||||||||||||||||||||
| ≤3 | 2.3–2.5 | (2.1–2.7)–(2.2–2.8) | 0.0826 | 1.7–1.9 | (1.5–2.0)–(1.7–2.2) | 0.0326 * | 4.6–4.9 | (4.3–4.9)–(4.6–5.2) | 0.0042 ** | 3.3–4.0 | (3.0–3.6)–(3.7–4.3) | <0.0001 *** | 3.7–4.0 | (3.4–4.1)–(3.6–4.3) | 0.0808 | 2.1–2.2 | (1.8–2.4)–(1.9–2.6) | 0.2819 | 2.9–3.0 | (2.6–3.1)–(2.7–3.2) | 0.356 | 1.2–0.7 | (1.0–1.3)–(0.5–0.9) | <0.0001 *** |
| 4–6 | 2.3–2.4 | (2.2–2.5)–(2.3–2.6) | 0.1148 | 1.8–1.9 | (1.6–1.9)–(1.7–2.0) | 0.0354* | 4.6–4.9 | (4.5–4.8)–(4.7–5.1) | <0.0001 *** | 3.3–3.4 | (3.0–3.4)–(3.2–3.6) | 0.0926 | 4.0–4.3 | (3.8–4.2)–(4.0–4.5) | 0.0018 ** | 2.1–2.1 | (1.9–2.2)–(1.9–2.3) | 0.6841 | 2.8–2.8 | (2.6–3.0)–(2.6–3.0) | 0.7992 | 0.9–0.5 | (0.8–1.1)–(0.5–0.6) | <0.0001 *** |
| ≥7 | 2.3–2.4 | (1.8–2.8)–(1.9–3.0) | 0.308 | 2.2–2.2 | (1.7–2.6)–(1.8–2.6) | 0.9168 | 4.1–4.5 | (3.6–4.6)–(4.0–5.0) | 0.0045 ** | 3.0–3.0 | (2.3–3.6)–(2.3–3.7) | 0.9234 | 3.4–4.5 | (2.8–4.0)–(3.9–5.2) | 0.0017 ** | 1.7–1.9 | (1.2–2.2)–(1.4–2.4) | 0.499 | 2.8–2.9 | (2.3–3.3)–(2.4–3.5) | 0.6483 | 0.1–0.3 | (0.6–1.2)–(0.1–0.5) | <0.0001 *** |
| TV during mealtimes (N = 820) | ||||||||||||||||||||||||
| Always | 2.4–2.4 | (2.2–2.6)–(2.3–2.6) | 0.3225 | 2.0–2.3 | (1.9–2.2)–(2.1–2.5) | 0.0003 *** | 4.4–4.6 | (4.2–4.6)–(4.4–4.8) | 0.0208 * | 3.4–3.9 | (3.1–3.6)–(3.6–4.1) | <0.0001 *** | 3.5–3.8 | (3.3–3.8)–(3.5–4.0) | 0.025 * | 2.5–2.5 | (2.2–2.7)–(2.2–2.7) | 0.7866 | 3.0–3.1 | (2.8–3.2)–(2.9–3.3) | 0.2836 | 1.0–0.7 | (0.9–1.1)–(0.6–0.8) | <0.0001 *** |
| Sometimes | 2.0–2.2 | (1.7–2.3)–(2.0–2.4) | 0.067 | 1.6–1.7 | (1.3–1.8)–(1.4–1.9) | 0.2683 | 4.7–5.0 | (4.3–5.0)–(4.6–5.3) | 0.0488 * | 3.3–3.1 | (2.9–3.6)–(2.8–3.5) | 0.3887 | 3.8–4.3 | (3.8–4.6)–(4.1–4.8) | 0.0631 | 2.0–2.0 | (1.6–2.3)–(1.7–2.4) | 0.43 | 2.7–2.8 | (2.4–3.0)–(2.5–3.1) | 0.3175 | 1.0–0.6 | (0.8–1.2)–(0.4–0.8) | 0.0004 *** |
| Never | 2.4–2.5 | (2.2–2.7)–(2.3–2.8) | 0.1096 | 1.5–1.5 | (1.4–1.7)–(1.3–1.7) | 0.5086 | 4.8–5.2 | (4.5–5.0)–(5.0–5.5) | <0.0001 *** | 3.1–3.3 | (2.8–3.3)–(3.0–3.6) | 0.0459 * | 4.2–4.6 | (3.9–4.5)–(4.3–4.9) | 0.0005 *** | 1.5–1.6 | (1.2–1.7)–(1.3–1.9) | 0.3032 | 2.6–2.6 | (2.4–2.9)–(2.3–3.8) | 0.4801 | 0.9–0.4 | (0.7–1.1)–(0.3–0.5) | <0.0001 *** |
Paired two-way Student’s t-test. Data are shown as mean of food intakes and respective 95% confidence intervals (95%CI). * p < 0.05, ** p < 0.001, *** p < 0.0001. N = 820.
Table 3.
Percentage of adolescents that maintain adequate food intake according to dietary guidelines by sociodemographic and familiar variables.
| Variables | Legumes | Fried Food | Vegetables | Sweet Food | Fruits | Sugar-Sweetened Beverages | Processed Meat | Fast Food | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% IC | p-Value | % | 95% IC | p-Value | % | 95% IC | p-Value | % | 95% IC | p-Value | % | 95% IC | p-Value | % | 95% IC | p-Value | % | 95% IC | p-Value | % | 95% IC | p-Value | |
| Gender (N = 810) | 0.241 | 0.4004 | 0.0109* | 0.0277 * | 0.4586 | <0.0001 *** | 0.4586 * | 0.2628 | ||||||||||||||||
| Boys | 21.3 | 18.5–24.0 | 48.9 | 45.5–52.3 | 37.8 | 34.5–41.1 | 29.5 | 26.4–32.0 | 31.4 | 28.3–34.6 | 42.9 | 39.5–46.2 | 25.1 | 22.4–28.6 | 86.0 | 83.7–88.4 | ||||||||
| Girls | 24.8 | 21.9–27.8 | 51.9 | 48.5–55.3 | 46.9 | 43.5–50.3 | 22.6 | 19.8–25.5 | 33.9 | 30.8–37.1 | 57.2 | 53.8–60.6 | 32.3 | 29.1–36.0 | 88.7 | 86.5–90.9 | ||||||||
| Age (years) (N = 820) | 0.0063 ** | 0.0006 *** | 0.0585 | 0.3118 | 0.3931 | 0.4846 | 0.523 | 0.013 * | ||||||||||||||||
| ≤14 | 15.86 | 1.3.4–18.3 | 58.59 | 55.2–62.2 | 49.78 | 46.4–53.2 | 27.31 | 24.3–30.4 | 29.96 | 36.8–33.1 | 54.63 | 51.2–58.0 | 28.19 | 25.1–31.0 | 88.55 | 86.4–91.7 | ||||||||
| 15–16 | 25.59 | 22.6–28.6 | 53.15 | 49.7–56.7 | 41.34 | 38.0–44.7 | 21.65 | 18.8–24.5 | 35.83 | 32.5–39.1 | 49.21 | 45.8–52.6 | 31.89 | 28.7–35.0 | 90.94 | 89.0–92.9 | ||||||||
| ≥17 | 26.84 | 23.8–29.9 | 42.77 | 39.4–46.1 | 40.12 | 36.8–43.5 | 25.96 | 23.0–29.0 | 33.04 | 29.8–36.2 | 51.03 | 47.6–54.4 | 28.61 | 25.5–32.0 | 84.37 | 81.9–86.9 | ||||||||
| Country (N = 820) | <0.0001 *** | <0.0001 *** | <0.0001 *** | 0.0006 ** | <0.0001 *** | 0.0002 ** | 0.0219 * | 0.0012 * | ||||||||||||||||
| Spain | 34.87 | 31.6–38.1 | 42.11 | 38.7–45.5 | 33.55 | 30.1–36.8 | 38.82 | 35.5–42.2 | 49.34 | 45.9–52.8 | 64.47 | 61.2–67.8 | 25.66 | 22.7–29.0 | 98.03 | 97.1–99.0 | ||||||||
| Brazil | 24.35 | 21.4–27.3 | 63.48 | 60.2–67.8 | 35.65 | 32.4–38.9 | 13.04 | 10.7–15.3 | 23.48 | 20.6–26.4 | 53.04 | 49.6–56.5 | 20.00 | 17.3–23.0 | 81.74 | 79.1–84.4 | ||||||||
| Colombia | 30.43 | 27.3–33.6 | 34.78 | 31.5–38.0 | 29.81 | 26.7–32.9 | 31.06 | 27.9–34.2 | 22.98 | 20.1–25.9 | 36.02 | 32.7–39.3 | 37.27 | 34.0–41.6 | 81.99 | 79.4–846.6 | ||||||||
| Chile | 10.70 | 8.6–12.8 | 52.09 | 48.7–55.5 | 55.814 | 52.4–59.2 | 26.51 | 23.5–29.5 | 28.37 | 25.3–31.5 | 51.16 | 47.7–54.6 | 33.49 | 30.3–37.6 | 85.58 | 83.2–88.0 | ||||||||
| Italy | 22.03 | 19.2–24.9 | 61.02 | 57.7–64.4 | 53.11 | 49.7–56.5 | 13.56 | 11.2–15.9 | 40.11 | 36.8–42.5 | 53.67 | 50.3–57.1 | 27.12 | 24.1–30.2 | 89.83 | 87.8–91.9 | ||||||||
| Maternal education (N = 779) | 0.6915 | 0.0103* | <0.0001 *** | 0.9074 | 0.0015 ** | 0.0007 *** | 0.8702 | 0.0427 * | ||||||||||||||||
| None | 25.00 | 22.0–28.0 | 66.67 | 63.4–70.0 | 8.33 | 6.4–10.3 | 25.00 | 22.0–28.0 | 16.67 | 14.0–19.3 | 41.67 | 38.2–45.1 | 25.00 | 22.0–28.0 | 66.67 | 63.4–70.0 | ||||||||
| Primary | 25.32 | 22.3–28.4 | 45.57 | 42.1–49.1 | 40.51 | 37.1–44.0 | 27.85 | 24.7–31.0 | 26.58 | 23.5–29.7 | 51.90 | 48.4–55.4 | 29.11 | 25.9–32.3 | 92.41 | 90.5–94.3 | ||||||||
| Secondary | 18.548 | 15.8–21.3 | 54.03 | 50.5–57.5 | 33.06 | 29.8–36.4 | 26.61 | 23.5–29.7 | 20.97 | 18.1–23.8 | 38.71 | 35.3–42.1 | 30.65 | 27.4–33.9 | 89.52 | 87.4–91.7 | ||||||||
| Professional formation | 25.71 | 22.6–28.8 | 39.29 | 35.9–42.7 | 47.86 | 44.3–51.4 | 22.86 | 19.9–25.8 | 35.00 | 31.7–38.3 | 45.00 | 41.5–48.5 | 25.71 | 22.6–28.8 | 83.57 | 81.0–86.2 | ||||||||
| Complete university | 22.88 | 19.9–25.8 | 55.19 | 51.7–58.7 | 47.64 | 44.1–51.1 | 24.06 | 21.1–27.1 | 38.92 | 35.5–42.3 | 58.49 | 55.0–62.0 | 30.19 | 27.0–33.4 | 88.92 | 86.7–91.1 | ||||||||
| Family members (N = 820) | 0.7388 | 0.164 | 0.0003 *** | <0.0001 *** | 0.6579 | 0.859 | 0.3438 | 0.0163 * | ||||||||||||||||
| ≤3 | 5.61 | 4.1–7.5 | 13.17 | 10.9–15.5 | 10.37 | 8.3–12.5 | 3.41 | 2.2–4.7 | 8.05 | 6.2–9.9 | 12.93 | 10.6–15.2 | 6.71 | 5.0–8.4 | 21.34 | 18.5–24.1 | ||||||||
| 4–6 | 16.10 | 13.6–19.0 | 34.76 | 31.5–38.0 | 30.61 | 27.5–33.8 | 19.02 | 16.3–21.7 | 22.56 | 19.7–25.4 | 35.24 | 32.0–38.5 | 21.10 | 18.3–23.9 | 21.10 | 56.6–63.4 | ||||||||
| ≥7 | 1.71 | 0.9–2.9 | 2.44 | 1.4–3.5 | 2.20 | 1.2–3.2 | 2.56 | 1.5–3.6 | 2.44 | 1.4–3.5 | 3.29 | 2.1–4.5 | 1.71 | 0.8–2.6 | 6.22 | 4.6–7.9 | ||||||||
| Viewing TV during mealtimes (N = 820) | 0.4672 | <0.0001 *** | 0.0003 *** | 0.1811 | 0.0001 *** | <0.0001 *** | 0.2881 | 0.4256 | ||||||||||||||||
| Always | 25.07 | 22.1–28.0 | 41.51 | 38.1–44.9 | 36.29 | 33.0–39.6 | 22.19 | 19.3–25.0 | 25.85 | 22.9–28.8 | 43.86 | 40.5–47.3 | 27.15 | 24.1–30.2 | 84.07 | 81.6–86.6 | ||||||||
| Sometimes | 20.25 | 17.5–23.0 | 57.06 | 53.7–60.4 | 44.79 | 41.4–48.2 | 25.77 | 22.8–28.8 | 36.20 | 32.9–39.5 | 53.37 | 50.0–56.8 | 29.45 | 26.3–32.6 | 29.45 | 86.1–90.5 | ||||||||
| Never | 22.99 | 21.0–25.9 | 58.76 | 55.4–62.1 | 51.82 | 48.4–55.2 | 28.47 | 25.4–31.6 | 41.24 | 37.9–44.6 | 60.95 | 57.6–64.3 | 32.85 | 29.6–36.1 | 91.97 | 90.1–93.8 | ||||||||
Chi-square test. Data are shown as percent of adolescents and respective 95% confidence intervals (95%CI) for the outcome weighted for these variables. * p < 0.05, ** p < 0.001, *** p < 0.0001. N = 820.
3.3.1. Gender
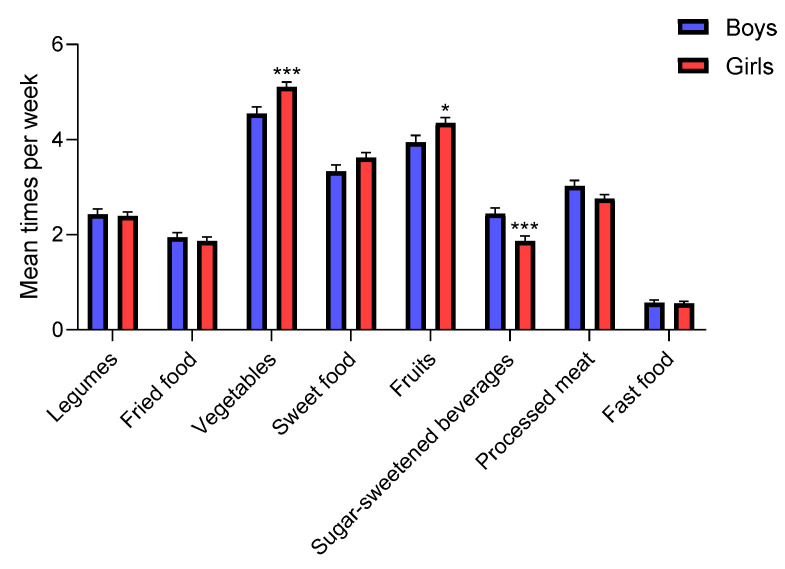
The gender classification results show that females significantly increased their vegetable (from 4.8 before to 5.1 times per week, p < 0.0001) and fruit intake (from 4.0 before to 4.4 times per week, p < 0.0001) during confinement (Table 2). On the other hand, males also showed an increase in vegetable consumption (from 4.0 before to 4.4 times per week during COVID-19, p = 0.0007), and processed meat intake (from 2.9 before to 3.0 time per week during COVID-19, p = 0.0182) but did not change their average fruit consumption (Table 2). Moreover, when comparing the average food intake by gender, we confirm that girls consumed significantly more fruits and vegetables during COVID-19 confinement and fewer SSB than boys, in our survey (Figure 3). These results are in concordance with previous observational studies about nutritional behaviors and differences between sexes [21].
Figure 3.
Comparison of average dietary intake among adolescents during COVID-19 confinement by gender. Data are means ± SEM. Comparison between groups by paired two-way ANOVA. * p < 0.05, *** p < 0.0001.
The potential correlates of adequate nutrition with sociodemographic and family variables are shown in Table 3. The most evident differences between girls and boys (in terms of dietary guidelines) were found for SSB (p < 0.0001). Only 42.9% of males versus almost 57.2% of females occasionally drank SSB, adhering to the recommendations of limiting the use of such beverages [22] (Table 3). This result is very relevant because SSB and sweet food increase the risk of overweight and obesity, type 2 diabetes mellitus, cardiovascular disease, among others noxious health effects [22].
3.3.2. Age
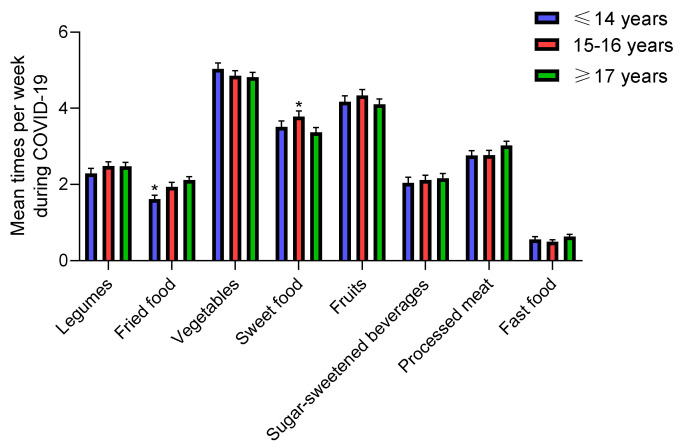
Overweight and obesity in childhood lead to a high risk of these conditions in adolescence and adulthood, and earlier-onset obesity is associated with a greater risk of adult overweight or obesity [23]. Therefore, it is important to evaluate changes in dietary patterns by age due to confinement to allow one to identify young people at risk of nutritional inadequacies and the development of eating disorders.
Only adolescents over the age of 14 significantly increased vegetable and fruit intake during COVID-19 confinement versus before confinement (Table 2). Adolescents under the age of 14 significantly increased the average consumption of fried and sweet foods (from 1.3 and 3.2 before to 1.6 and 3.5 during the COVID-19 pandemic, respectively; p = 0.0025 and p = 0.0386) (Table 2). It is important to note that we recorded a dramatic increase in sweet food consumption in those over 17 years of age due to COVID-19 confinement (p = 0.001), but all teens, regardless of age, consumed sweets 3 to 4 times per week. There were no differences in adolescents’ mean food intake during COVID-19 confinement by age (Figure 4), but we found significant differences by age in legume, fried food, vegetable and fast food average intake during confinement compared with the recommended dietary allowance (legumes: 3 or 4 servings per week [24], fried food: occasional [14], vegetables: diary [25] and fast food: occasional [26,27,28] (Table 3). However, in relation to age, no significant association with dietary patterns was identified, possibly because the sample consisted only of adolescents and with a low range of age variation (10 to 19 years).
Figure 4.
Mean dietary intake among adolescents during COVID-19 confinement by age. Data are means ± SEM. Comparison between groups by paired two-way ANOVA.
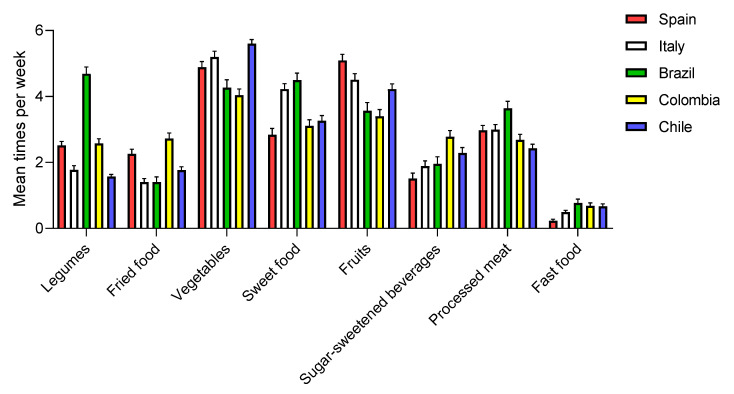
3.3.3. Country
The country of residence is strongly related to mean food intake during the COVID-19 pandemic (Figure 5) and to the modification of dietary trend among adolescents in this period versus habitual diet (Table 2). Many factors, such as socio-economic status, fad diets, religion, and traditions of each country, influence dietary trends [29,30].
Figure 5.
Mean dietary intake among adolescents during COVID-19 confinement by country. Data are means ± SEM.
Brazilian adolescents had a higher average legume intake (Figure 5) versus the other countries. In addition, all adolescents except the Spanish one significantly increased the consumption of legumes during confinement (Table 2).
Adolescents from Spain, Brazil, and Chile, but not Italy and Colombia, increased vegetable consumption during confinement (Table 2). In addition, the Colombian adolescents had low overall fruit and vegetable consumption, despite having increased their fruit intake in a significant way during confinement. These results are consistent with previous studies that show that the Colombian population does not consume the recommended amount of fruits and vegetables [31]. In our study, Spain and Italy were the countries with the greatest mean consumption of fruits (4–5 times per week), and significantly increased their consumption during COVID-19 confinement (Table 2). A total of 49.3% of Spanish adolescents met the fruit intake recommendations versus the almost 23% of Colombian subjects (Table 3).
Colombian adolescents significantly decreased sugar-sweetened beverage intakes during the COVID-19 pandemic, but this country remained the biggest consumer of SSB as compared with Spain, Italy, Brazil, and Chile (Table 2). Only 36.0% of Colombian adolescents drank SBB only occasionally during the COVID-19 lockdown, versus 64.5% of Spanish adolescents (Table 3).
Chileans significantly increased fried food intake during COVID-19 (p < 0.0001), but again Colombia was the biggest overall consumer of fried food (almost three times per week versus 1.4 to 2.3 times per week for the other countries (Table 2)). It should be noted that all adolescents decreased their weekly fast food consumption and all countries showed the same average fast food intake during confinement (0.2–0.8 times per week) (Figure 5 and Table 2). Detailed statistical analysis of Figure 5 is described in Table 4.
Table 4.
Analysis of mean dietary intake among adolescents during COVID-19 confinement by country.
| Legumes | Fried food | Vegetables | Sweet Food | Fruits | Sugar-Sweetened Beverages | Processed Meat | Fast Food | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p Value | p Value | p Value | p Value | p Value | p Value | p Value | p Value | |||||||||
| Spain vs. Italy | 0.0065 | ** | 0.001 | *** | 0.6246 | ns | <0.0001 | *** | 0.0621 | ns | 0.4204 | ns | >0.9999 | ns | 0.745 | ns |
| Spain vs. Brazil | <0.0001 | *** | 0.0048 | ** | 0.0882 | ns | <0.0001 | *** | <0.0001 | *** | 0.3736 | ns | 0.0513 | ns | 0.177 | ns |
| Spain vs. Colombia | 0.999 | ns | 0.2521 | ns | 0.0015 | ** | 0.7525 | ns | <0.0001 | *** | <0.0001 | *** | 0.6983 | ns | 0.2613 | ns |
| Spain vs. Chile | <0.0001 | *** | 0.1303 | ns | 0.0073 | ** | 0.2396 | ns | 0.0004 | *** | 0.0023 | ** | 0.0734 | ns | 0.2284 | ns |
| Italy vs. Brazil | <0.0001 | *** | >0.9999 | ns | 0.001 | *** | 0.7779 | ns | 0.0007 | *** | 0.9989 | ns | 0.0513 | ns | 0.7745 | ns |
| Italy vs. Colombia | 0.002 | ** | <0.0001 | *** | <0.0001 | *** | <0.0001 | *** | <0.0001 | *** | 0.0004 | *** | 0.606 | ns | 0.9124 | ns |
| Italy vs. Chile | 0.8563 | ns | 0.3835 | ns | 0.283 | ns | <0.0001 | *** | 0.6211 | ns | 0.2878 | ns | 0.0409 | * | 0.9137 | ns |
| Brazil vs. Colombia | <0.0001 | *** | <0.0001 | *** | 0.8752 | ns | <0.0001 | *** | 0.9641 | ns | 0.0062 | ** | 0.0008 | *** | 0.9959 | ns |
| Brazil vs. Chile | <0.0001 | *** | 0.5245 | ns | <0.0001 | *** | <0.0001 | *** | 0.0347 | * | 0.6008 | ns | <0.0001 | *** | 0.9914 | ns |
| Colombia vs. Chile | <0.0001 | *** | <0.0001 | *** | <0.0001 | *** | 0.9335 | ns | 0.0008 | *** | 0.121 | ns | 0.7343 | ns | >0.9999 | ns |
Data are means ± SEM. In the table, comparison between groups by paired two-way ANOVA. * p < 0.05, ** p < 0.001, *** p < 0.0001, ns: non-significant.
These results are especially relevant because experiences from previous epidemics have shown that there is a need to maintain optimal nutrition at individual and global levels, in order to improve the physical and mental health of the population [32]. In this sense, knowing the dietary habits in each country is necessary to encourage a healthy lifestyle after COVID-19 confinement or the development of future reactions to unavoidable pandemics.
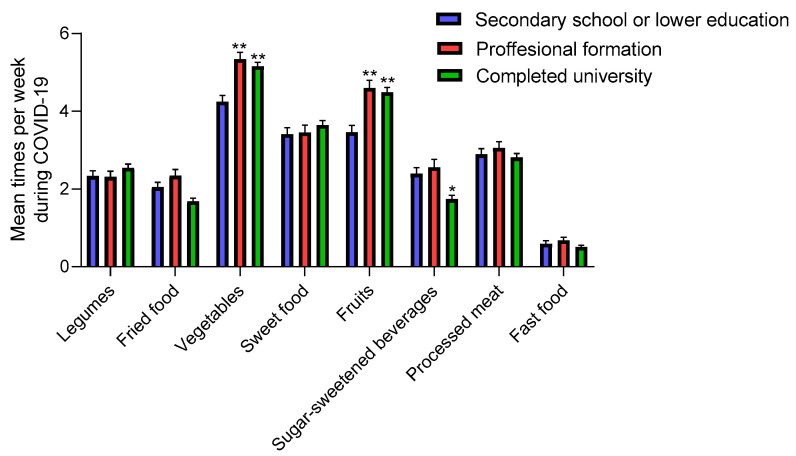
3.3.4. Maternal Education
Adolescents whose mothers had education levels higher than secondary school level significantly increased their consumption of fruits and vegetables during confinement (Table 2), and were the ones who consumed the most fruits and vegetables during and before compared to the rest of the adolescents (Figure 6). By contrast, adolescents who reported high maternal education also significantly increased their consumption of sweet food (Table 2). In spite of this, there were no significant differences in mean sweet food intake between them (Figure 6). It is important to highlight the low consumption of SSB among adolescents whose mother had a university degree versus other groups (p < 0.001) during COVID-19 (Figure 5). Our results suggest that there was greater adherence to the unhealthy eating pattern during COVID-19 among adolescents whose mothers had low education. Whether this is related to the family income is not known and deserves further investigation.
Figure 6.
Mean dietary intake among adolescents during COVID-19 confinement by maternal education. Data are means ± SEM. Comparison between groups by paired two-way ANOVA. * p < 0.001, ** p < 0.0001 compared to secondary school or lower education group.
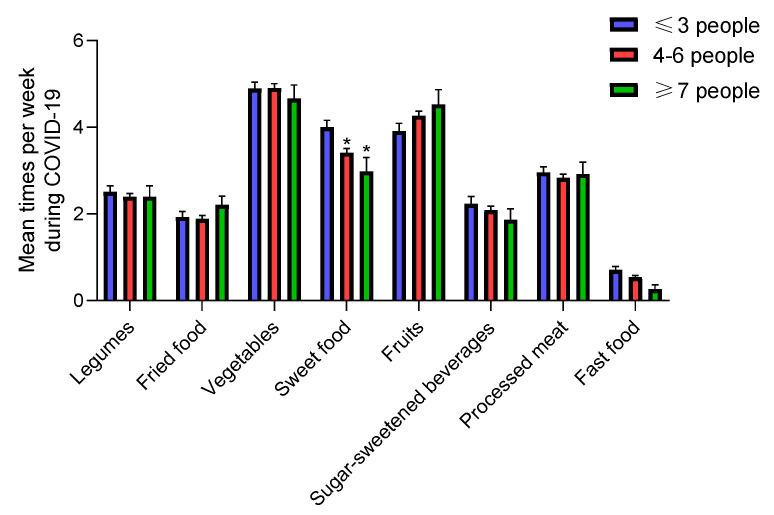
3.3.5. Number of Family Members at Home
There were differences in adolescents’ sweet food intake during COVID-19 confinement as stratified by family members. The ≤3 people group had a higher average sweet food intake (Figure 7) versus the other groups (p < 0.001), and significantly increased their consumption during COVID-19 confinement, unlike the other groups (Table 2). Furthermore, only 3.4% of adolescents of ≤3 people group and 2.6% of ≥7 people group ate only occasionally sweet food versus the 19% of 4–5 people group (p < 0.001) (Table 3). It is important to highlight that the lowest rates of adherence to the weekly vegetables, sweet food and fast food intake recommendation were in those adolescents who belong to the ≥7 people group (Table 3).This could be because larger families reduce the amount of resources, such as time, energy, or money available to each child [33]. However, we cannot prove beyond reasonable doubt that larger family size influences diet quality, either directly or indirectly.
Figure 7.
Mean dietary intake among adolescents during COVID-19 confinement by family members. Data are means ± SEM. Comparison between groups by paired two-way ANOVA. * p < 0.001 compared to the ≤3 people group.
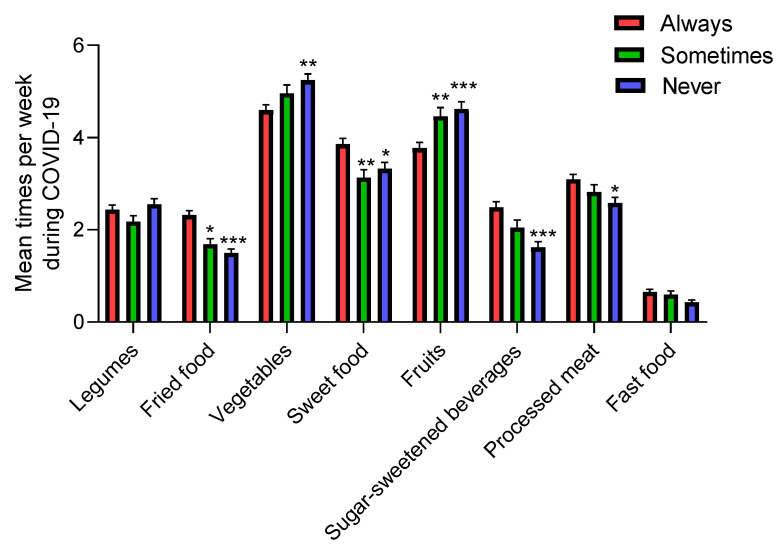
3.3.6. Watching TV during Mealtimes
The results show that TV viewing during mealtimes is related to lower consumption of vegetables and fruits during the COVID-19 period and a higher fried food, sweet food, and SSB consumption (Figure 8). Further, those adolescents who always watched TV during mealtimes significantly increased their fried and sweet food intake during COVID-19 confinement versus before, and those adolescents who never watched TV during mealtimes significantly increased their vegetable and fruit intake (Table 2). We found significant differences in vegetable, fruit, fried food, sweet food, and SSB intake during confinement compared with the recommended dietary allowance in those adolescents who always watch TV during mealtimes versus the other two groups, proving that watching TV during mealtimes was associated with poorer dietary quality among adolescents (Table 3).
Figure 8.
Mean dietary intake among adolescents during COVID-19 confinement by viewing TV during mealtimes. Data are means ± SEM. Comparison between groups by paired two-way ANOVA. * p < 0.05, ** p < 0.001, *** p < 0.0001 compared to the Always group.
As a limitation of this study, we did not investigate modifications of adolescent’s dietary trends as correlated to economic status, daily food intake times, and amount and availability of food in each region or country. We also do not have data regarding sleep, physical activity, and time spent TV watching.
4. Conclusions
In conclusion, our findings provide the first description of how the COVID-19 pandemic has modified dietary trends of adolescents from Spain, Italy, Brazil, Colombia, and Chile. These new habits could be acquired and have some later impact on health. Due to confinement, it appears that families had more time to cook and improve eating habits by increasing legume, fruit, and vegetable intake, even though this, apparently, did not increase the overall diet quality. Further, adolescents also exhibited a higher sweet food consumption, likely due to boredom and stress produced by COVID-19 confinement. This study shows the association between gender, country of residence, family members at home, watching TV during mealtimes and maternal education variables with adequate nutrition during COVID-19 confinement. Therefore, it is important to generate future large-scale studies that analyze eating habits to encourage the adoption of healthy diets among adolescents, especially after this period of confinement. Understanding the present adolescent’s nutrition behavior during Covid-19 lockdown will help public health authorities reshape future policies on adolescents’ nutritional recommendations, when new pandemics arrive and lockdown policies are implemented.
Acknowledgments
P.d.C.P., W.A.F.P. and A.D. would like to acknowledge Print/CAPES Proc. 88887.470197/2019-00. P.d.C.P. would like to acknowledge National Council for Scientific and Technological Development (CNPq, Portuguese: Conselho Nacional de Desenvolvimento Científico e Tecnológico). M.B.R.-R was recipients of contracts from the Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid, Fondo Social Europeo, and Iniciativa de Empleo Juvenil YEI (PEJD-2018-POST/BIO-8933). D.C.M.-E. is a fellow of “Centro de Estudios Interdisciplinarios Básicos y Aplicados” (CEIBA), Colombia, through the program “Bolívar Gana con Ciencia”.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/6/1807/s1, Table S1: National responses to the COVID-19 pandemic, Table S2: Percentage of adolescent participating in this survey classified by countries and regions, Figure S1: Flow chart of participants of the study.
Author Contributions
Conceptualization, M.B.R.-R., A.D. and P.d.C.P.; investigation, M.B.R.-R., D.C.M.-E., A.D., P.d.C.P., W.A.F.P., M.T.A., N.U., F.C.-M., M.M., P.B., G.B., A.P., X.T., D.A.-C., K.P.-S., J.E.R.-M., L.d.O.C., and P.M.M.; data curation, M.B.R.-R.; writing—original draft preparation, M.B.R.-R.; writing—review and editing, A.D., F.V., P.d.C.P.; supervision, A.D.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
A.D. lab is funded by Fundación Ramón Areces (CIVP18A3888) and by the Spanish “Agencia Estatal de Investigación” and European FEDER Funds (AGL2016-78922-R).
Conflicts of Interest
The authors have no conflicts of interest to declare.
Footnotes
Sample Availablity: Samples of the compounds are not available from the authors.
References
- 1.Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: What we know so far. Pathogens. 2020;9:231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.W.H.O Coronavirus Disease (COVID-19) Pandemic. [(accessed on 24 May 2020)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 3.Lana R.M., Coelho F.C., Gomes M., Cruz O.G., Bastos L.S., Villela D.A.M., Codeco C.T. The novel coronavirus (SARS-CoV-2) emergency and the role of timely and effective national health surveillance. Cad. Saude Publica. 2020;36:e00019620. doi: 10.1590/0102-311x00019620. [DOI] [PubMed] [Google Scholar]
- 4.Muscogiuri G., Barrea L., Savastano S., Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur. J. Clin. Nutr. 2020;74:850–851. doi: 10.1038/s41430-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glabska D., Guzek D., Groele B., Gutkowska K. Fruit and vegetables intake in adolescents and mental health: A systematic review. Rocz. Państwowego Zakładu Hig. 2020;71:15–25. doi: 10.32394/rpzh.2019.0097. [DOI] [PubMed] [Google Scholar]
- 6.W.H.O Global Accelerated Action for the Health of Adolescents (AA-HA!): Guidance to Support Country Implementation. [(accessed on 24 May 2020)]; Available online: https://www.who.int/maternal_child_adolescent/topics/adolescence/framework-accelerated-action/en/2017.
- 7.F.A.O COVID-19 and the Risk to Food Supply Chains: How to Respond? [(accessed on 7 June 2020)]; Available online: http://www.fao.org/3/ca8388en/CA8388EN.pdf.
- 8.Fulkerson J.A., Friend S., Horning M., Flattum C., Draxten M., Neumark-Sztainer D., Gurvich O., Garwick A., Story M., Kubik M.Y. Family home food environment and nutrition-related parent and child personal and behavioral outcomes of the healthy home offerings via the mealtime environment (home) plus program: a randomized controlled trial. J. Acad. Nutr. Diet. 2017;118:240–251. doi: 10.1016/j.jand.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons D., Chapman G.E. The significance of home cooking within families. Br. Food J. 2012;114:1184–1195. doi: 10.1108/00070701211252110. [DOI] [Google Scholar]
- 10.Galli F., Reglero G., Bartolini D., Visioli F. Better prepare for the next one. Lifestyle lessons from the COVID-19 pandemic. PharmaNutrition. 2020;12:100193. doi: 10.1016/j.phanu.2020.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira M.M., Campos M.O., Andreazzi M.A.R., Malta D.C. Characteristics of the National Adolescent School-based Health Survey—PeNSE, Brazil. Epidemiol. Serviços Saúde. 2017;26:605–616. doi: 10.5123/S1679-49742017000300017. [DOI] [PubMed] [Google Scholar]
- 12.W.H.O Global School-Based Student Health Survey (GSHS) [(accessed on 24 May 2020)]; Available online: https://www.who.int/ncds/surveillance/gshs/en/
- 13.The Centre for the Promotion of Imports from Developing Countries All Hands on Deck for the Fresh Sector (COVID-19) [(accessed on 24 May 2020)]; Available online: https://www.cbi.eu/news/all-hands-deck-fresh-sector-covid-19/2020.
- 14.W.H.O Food and Nutrition Tips during Self-Quarantine. [(accessed on 24 May 2020)]; Available online: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/technical-guidance/food-and-nutrition-tips-during-self-quarantine.
- 15.Liu J., Rehm C.D., Micha R., Mozaffarian D. Quality of meals consumed by us adults at full-service and fast-food restaurants, 2003–2016: Persistent low quality and widening disparities. J. Nutr. 2020;150:873–883. doi: 10.1093/jn/nxz299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkumar R.P. COVID-19 and mental health: A review of the existing literature. Asian J. Psychiatry. 2020;52:102066. doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G., Zhang Y., Zhao J., Zhang J., Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet. 2020;395:945–947. doi: 10.1016/S0140-6736(20)30547-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moynihan A.B., van Tilburg W.A., Igou E.R., Wisman A., Donnelly A.E., Mulcaire J.B. Eaten up by boredom: Consuming food to escape awareness of the bored self. Front. Psychol. 2015;6:369. doi: 10.3389/fpsyg.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scully M., Dixon H., Wakefield M. Association between commercial television exposure and fast-food consumption among adults. Public Health Nutr. 2009;12:105–110. doi: 10.1017/S1368980008002012. [DOI] [PubMed] [Google Scholar]
- 20.Scarmozzino F., Visioli F. Covid-19 and the subsequent lockdown modified dietary habits of almost half the population in an Italian sample. Foods. 2020;9:675. doi: 10.3390/foods9050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzymisławska M., Puch E.A., Zawada A., Grzymisławski M. Do nutritional behaviors depend on biological sex and cultural gender? Adv. Clin. Exp. Med. 2020;29:165–172. doi: 10.17219/acem/111817. [DOI] [PubMed] [Google Scholar]
- 22.Mis N.F., Braegger C., Bronsky J., Campoy C., Domellöf M., Embleton N., Hojsak I., Hulst J., Indrio F., Lapillonne A., et al. Sugar in infants, children and adolescents: A position paper of the european society for paediatric gastroenterology, hepatology and nutrition committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2017;65:681–696. doi: 10.1097/MPG.0000000000001733. [DOI] [PubMed] [Google Scholar]
- 23.Choquet H., Meyre D. Genomic insights into early-onset obesity. Genome Med. 2010;2:36. doi: 10.1186/gm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Heart, Lung, and Blood Institute DASH Eating Plan. [(accessed on 24 May 2020)]; Available online: https://www.nhlbi.nih.gov/health-topics/dash-eating-plan.
- 25.Rosi A., Paolella G., Biasini B., Scazzina F. Dietary habits of adolescents living in North America, Europe or Oceania: A review on fruit, vegetable and legume consumption, sodium intake, and adherence to the Mediterranean Diet. Nutr. Metab. Cardiovasc. Dis. 2019;29:544–560. doi: 10.1016/j.numecd.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Costa C.S., Del-Ponte B., Assuncao M.C.F., Santos I.S. Consumption of ultra-processed foods and body fat during childhood and adolescence: A systematic review. Public Health Nutr. 2017;21:148–159. doi: 10.1017/S1368980017001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poti J.M., Braga B., Qin B. Ultra-processed food intake and obesity: What really matters for health-processing or nutrient content? Curr. Obes. Rep. 2017;6:420–431. doi: 10.1007/s13679-017-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadbeigi A., Asgarian A., Moshir E., Heidari H., Afrashteh S., Khazaei S., Ansari H. Fast food consumption and overweight/obesity prevalence in students and its association with general and abdominal obesity. J. Prev. Med. Hyg. 2018;59:E236–E240. doi: 10.15167/2421-4248/jpmh2018.59.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarnowiecki D.M., Dollman J., Parletta N. Associations between predictors of children’s dietary intake and socioeconomic position: A systematic review of the literature. Obes. Rev. 2014;15:375–391. doi: 10.1111/obr.12139. [DOI] [PubMed] [Google Scholar]
- 30.Richter M., Vereecken C.A., Boyce W., Maes L., Gabhainn S.N., Currie C.E. Parental occupation, family affluence and adolescent health behaviour in 28 countries. Int. J. Public Health. 2009;54:203–212. doi: 10.1007/s00038-009-8018-4. [DOI] [PubMed] [Google Scholar]
- 31.Herran O.F., Patino G.A., Gamboa E.M. Socioeconomic inequalities in the consumption of fruits and vegetables: Colombian National Nutrition Survey, 2010. Cad. Saúde Pública. 2019;35:e00031418. doi: 10.1590/0102-311x00031418. [DOI] [PubMed] [Google Scholar]
- 32.Naja F., Hamadeh R. Nutrition amid the COVID-19 pandemic: A multi-level framework for action. Eur. J. Clin. Nutr. 2020 doi: 10.1038/s41430-020-0634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucera B., McIntosh W.A. Family size as a determinant of children’s dietary intake: A dilution model approach. Ecol. Food Nutr. 1991;26:127–138. doi: 10.1080/03670244.1991.9991196. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.