Abstract
Background
High costs of direct-acting antivirals (DAAs) have led health-care insurers to limit access worldwide. Using a natural experiment, we evaluated the impact of removing fibrosis stage restrictions on hepatitis C (HCV) treatment initiation rates among people living with human immunodeficiency virus (HIV), and then examined who was left to be treated.
Methods
Using data from the Canadian HIV-HCV Coinfection Cohort, we applied a difference-in-differences approach. Changes in treatment initiation rates following the removal of fibrosis stage restrictions were assessed using a negative binomial regression with generalized estimating equations. The policy change was then specifically assessed among people who inject drugs (PWID). We then identified the characteristics of participants who remained to be treated using a modified Poisson regression.
Results
Between 2010–2018, there were a total of 585 HCV initiations among 1130 eligible participants. After removing fibrosis stage restrictions, DAA initiations increased by 1.8-fold (95% confidence interval [CI] 1.3–2.4) controlling for time-invariant differences and secular trends. Among PWID the impact appeared even stronger, with an adjusted incidence rate ratio of 3.6 (95% CI 1.8–7.4). However, this increased treatment uptake was not sustained. At 1 year following universal access, treatment rates declined to 0.8 (95% CI .5–1.1). Marginalized participants (PWID and those of indigenous ethnicity) and those disengaged from care were more likely to remain HCV RNA positive.
Conclusions
After the removal of fibrosis restrictions, HCV treatment initiations nearly doubled immediately, but this treatment rate was not sustained. To meet the World Health Organization elimination targets, the minimization of structural barriers and adoption of tailored interventions are needed to engage and treat all vulnerable populations.
Keywords: HIV–hepatitis C coinfection, direct-acting antivirals, people who inject drugs, unrestricted access, quasi-experimental methods
People coinfected with human immunodeficiency virus and hepatitis C virus were 1.8 times more likely to initiate treatments after fibrosis stage restrictions were removed, after controlling for temporal trends. Marginalized populations and those disengaged from care remain to be treated.
Deaths due to viral hepatitis are soon projected to surpass those due to human immunodeficiency virus (HIV), tuberculosis, and malaria [1]. Given the advent of direct-acting antivirals (DAAs) and the significant public health burden of viral hepatitis, the World Health Organization (WHO) set targets to eliminate viral hepatitis by 2030. Countries “on track to hepatitis C virus [HCV] elimination” have (1) unrestricted access to DAAs; and (2) treat at least 7% of their overall infected population per year [2]. Only 12 of 194 countries are on track to meeting these targets [2]. The high costs of DAAs have led health authorities to continue to restrict access. Despite clinical guidelines, a common eligibility criteria for treatment reimbursement globally remains the presence of significant liver fibrosis [3–6]. An unintentional consequence of fibrosis stage restrictions may be that younger people who inject drugs (PWID) and men who have sex with men (MSM)—who are clinically less advanced but at ongoing risk of transmitting HCV—may differentially face barriers to treatment.
Globally, an estimated 2.3 million people living with HIV (PLWH) are coinfected with HCV, of whom 80% are PWID or people who previously injected drugs [7]. Despite the rapid advances in HCV treatment, barriers to elimination remain across each step of the care continuum. In high-income settings, people coinfected with HIV-HCV are generally well identified and most are already engaged in HIV care; therefore, the only remaining step to curing HCV is initiating treatment. This is an ideal “micro-population” in which to achieve the WHO targets [8].
Health-care services in Canada are universal, but medication coverage is not. The decisions as to what medications are reimbursed and under what circumstances are made independently by provincial health authorities. When DAAs were first approved for use. most provinces required people living with HCV to have significant liver fibrosis to access treatment. This restriction has been variably removed over time and geography creating a natural experiment and an opportunity to estimate the impact of unrestricted access to DAAs on treatment initiation rates. We then examined the characteristics of participants that remained to be treated to estimate how close we are to eliminating HCV among PLWH.
METHODS
Study Population
The Canadian Coinfection Cohort Study (CCC) is an open, publicly funded prospective cohort of PLWH with evidence of HCV infection who were recruited from 18 centers [9]. Details on study procedures and the representativeness of the cohort have been published elsewhere [9]. CCC participants who were HCV RNA positive with 1 visit as of 24 March 2010 (time 0) from either British Columbia, Ontario, or Quebec were included in this analytic sample. The CCC is approved by a community advisory committee and by all institutional ethics boards of the participating centers.
Primary Analysis
Outcome
HCV treatment initiation was the primary outcome. The study period spanned between 2010 and 2018; therefore, treatments included both pegylated interferon (in combination with ribavirin or DAAs) and interferon-free regimens. DAAs included boceprevir, telaprevir, simeprevir, sofosbuvir, sofosbuvir/simeprevir, sofosbuvir/ledipasvir, ombitasvir/paritaprevir/ritonavir with and without dasabuvir, sofosbuvir/daclatasvir, sofosbuvir/velpatasvir, and elbasvir/grazoprevir. Eligible participants who did not achieve the outcome were censored on the date they were considered lost to follow-up (no study visits for at least 18 months), died, withdrew from the study, spontaneously cleared HCV infection, or at the administrative end date (23 March 2018). Since participants could initiate HCV treatment multiple times (ie, failure, reinfection), treatment initiation was treated as a repeatable outcome. Once participants initiated HCV treatment and achieved a sustained virologic response, they were censored unless they became reinfected, at which time they could again contribute person-time at risk for treatment.
Exposure
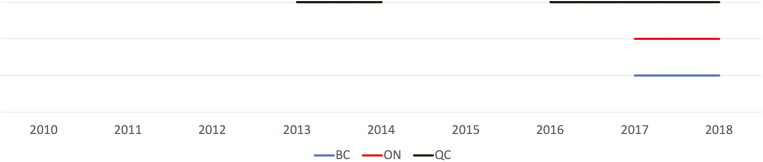
The exposure of interest was the change in provincial policies that removed the criterion requiring the presence of a “significant liver fibrosis stage” for DAAs to be reimbursed by 3 provincial health plans. Figure 1 illustrates when the policies changed in each province. Briefly, for PLWH in Quebec, between June 2014-July 2015 and from July 2016 onwards there were no restrictions based on fibrosis stage. In Ontario and British Columbia, fibrosis restrictions were removed as of March 2017. There were never any sobriety restrictions for the reimbursement of DAAs in any province [5].
Figure 1.
Time-varying policy changes by province. The solid lines represent calendar time when no fibrosis stage restrictions were in place. No line represents that either significant (>F2) or advanced (>F3) liver fibrosis stage restrictions were required for reimbursement of direct-acting antivirals (DAAs). Before 2013, due to the lower efficacy and higher toxicity of interferon-based therapies in human immune deficiency virus (HIV)-hepatitis C virus (HCV) coinfection, only a very few patients—typically, only people with advanced fibrosis and at increased risk for short-term adverse outcomes—were treated with these agents. In Quebec, in 2014, simeprevir and sofosbuvir were unrestricted for patients living with HCV. Although HIV infection was a listed restriction, coinfected patients were usually granted access on a case-by-case basis through the “patient d’exception” process. As of 2016, people coinfected with HIV and HCV were considered a priority population and sofosbuvir/ledipasvir and ombitasvir/paritaprevir/ritonavir dasabuvir were available without fibrosis stage restrictions; sofosbuvir/velpatasvir was available without restrictions from 2017. In British Columbia and Ontario, in 2017, after the pan-Canadian Pharmaceutical Alliance used collective bargaining to reduce DAA drug prices across Canada, provinces removed fibrosis stage restrictions as a criterion for treatment reimbursement. No sobriety restrictions were present in Canada. Abbreviations: BC, British Columbia; ON, Ontario; QC, Quebec.
Statistical Analysis
We applied a quasi-experimental method known as a difference-in-differences (DD) approach to estimate the impact of removing significant liver fibrosis restrictions on HCV treatment initiation [10, 11]. We chose this design because of the need to control for secular trends in HCV treatment uptake that co-occurred with policy changes (DAA approval in 2013). Details of the DD design and model are available in Supplementary Appendix 1.
The base DD model includes 3 main variables: (1) group (defined by province of residence: British Columbia [reference], Ontario, or Quebec); (2) time (calendar years from 24 March 2010–23 March 2018 and reference year of 24 March 2013–2014); and (3) interaction between group and time, which is equal to 1 in provinces and years when fibrosis stage restrictions were not in place. The coefficient on this interaction term provides the DD estimate of the policy effect [12]. The adjusted DD model also included both individual-level fixed and time-varying predictors of HCV treatment initiation. Fixed covariates included age, sex, MSM, indigenous ethnicity, and HCV genotype. Time-varying covariates included income (<$18 000 CAD) [13], injection drug use (within the prior 6 months), undetectable HIV RNA (<50 copies/mL), and significant liver fibrosis (determined using a hierarchical classification, based on availability of a liver biopsy, clinical diagnosis of cirrhosis, fibroscan [>7.2 kPa] [14], or aspartate aminotransferase [AST] to platelet ratio index ≥1.5).
We next evaluated the impact of the policy change among PWID by restricting the sample to participants who reported active injection drug use in the 6 months prior (time updated).
All DD models were fit using negative binomial regression. The natural logarithm of each participant’s time at risk was used as the offset. Generalized estimating equations with robust standard errors were used to adjust for clustering. Results are presented as adjusted incidence rate ratios (IRRs).
We conducted several robustness checks. We evaluated: (1) the parallel trends assumption for DD study design; (2) whether the policy reached the intended population (ie, effect modification based on not having significant liver fibrosis); (3) the “lead effect,” to assess whether HCV treatment uptake predated the policy change; (4) the “lagged effect,” to assess the sustainability of the policy change; and (5) the “falsification test,” to assess whether omitted variables affecting decisions to initiate DAAs were driving our results. Here, we used serum creatinine levels.
Secondary Analysis: Assessment of Who is Left to be Treated?
Based on the eligibility criteria above, we summarized the proportion of participants who initiated treatment and those who remained eligible for treatment, by calendar year, significant fibrosis, and active injection drug use. We then performed a cross-sectional analysis, using a modified Poisson regression model with robust standard errors to assess the predictors of remaining HCV RNA positive at each participant’s final visit. Predictors included: (1) socio-demographic factors, including age, indigenous ethnicity, women or MSM (compared to heterosexual men), income, homelessness, incarceration (in the prior 6 months), and province of residence (with British Columbia as a reference); (2) behavioral factors, including active injection drug and alcohol use; (3) clinical factors, including an undetectable HIV RNA level, significant liver fibrosis, HCV genotype, and psychiatric diagnosis; and (4) disengagement in care, via being lost to follow-up, which was defined as not having a cohort visit within 18 months of our administrative censoring date (excluding those who had formally withdrawn from the study and those who died).
All analyses were performed using Stata 15/IC (StataCorp LP, College Station, TX).
RESULTS
Primary Analysis
As of March 2018, 1843 coinfected individuals had been recruited to the CCC. After applying the eligibility criteria, a total of 1130 CCC participants from British Columbia (n = 414), Ontario (n = 326) and Quebec (n = 390) were HCV RNA+ as of 24 March 2010 (Supplementary Figure 1). Between 24 March 2010 and 23 March 2018, there were 585 HCV treatment initiations by 543 participants (458 participants achieved a sustained virologic response). The majority (n = 390, 67%) of treatment initiations were all-oral DAA regimens, while 100 (17%) were with first- and second-generation DAAs in combination with pegylated-interferon and 72 (12%) were with pegylated-interferon regimens alone. There were 23 (4%) regimens that could not be classified because patients were enrolled in blinded clinical trials or information on their regimens was missing. Censoring reasons were similar across provinces (Supplementary Table 1). Table 1 illustrates the baseline characteristics of the analytic sample, comparing the 3 provinces. Clinical factors were comparable across provinces, but sociodemographic characteristics—such as the proportion of participants who were of indigenous ethnicity, women, and had a low income—differed between the provinces. Behavioral characteristics, such as the proportion of active PWID and MSM, also differed at baseline, but the proportions did not vary over time (results not shown).
Table 1.
Baseline Characteristics
| British Columbia, n = 414 | Ontario, n = 326 | Quebec, n = 390 | |
|---|---|---|---|
| Time at risk, person-years | 1426 | 1230 | 1476 |
| Age, years (IQR) | 47 (42, 53) | 48 (41, 52) | 48 (42, 53) |
| Indigenous | 136 (33%) | 54 (17%) | 11 (3%) |
| Heterosexual men | 190 (46%) | 129 (40%) | 208 (53%) |
| Female | 140 (34%) | 72 (22%) | 84 (22%) |
| Men who have sex with men | 80 (19%) | 125 (38%) | 96 (25%) |
| Injection drug use, prior 6 months | 174 (42%) | 79 (24%) | 141 (36%) |
| Income <$18 000 CAD/year | 323 (79%) | 207 (64%) | 326 (84%) |
| Homelessness | 43 (10) | 17 (5) | 67 (17) |
| Incarceration, prior 6 months | 172 (41) | 97 (30) | 144 (37) |
| Alcohol use, prior 6 months | 200 (48) | 194 (60) | 246 (63) |
| Significant liver fibrosisa | 119 (29%) | 113 (35%) | 134 (34%) |
| HCV genotype | |||
| 1 | 279 (67%) | 221 (68%) | 252 (64%) |
| 2 | 19 (5%) | 12 (4%) | 14 (4%) |
| 3 | 73 (17%) | 38 (12%) | 69 (18%) |
| 4 | 2 (<1%) | 17 (5%) | 15 (4%) |
| Missing | 41 (10%) | 36 (11%) | 40 (10%) |
| HIV RNA undetectableb | 270 (76%) | 240 (82%) | 274 (79%) |
| CD4, cells/uL (IQR) | 420 (250, 620) | 480 (284, 690) | 442 (280, 640) |
Data represent baseline characteristics of Canadian Coinfection Cohort Study participants included in our analytical sample. The median date of entry was 13 July 2011 (IQR, 5 Aug 2010 to 12 May 2014).Abbreviations: AST, aspartate aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
aSignificant fibrosis was determined using a hierarchical classification, based on availability of a liver biopsy, clinical diagnosis, fibroscan (>7.2 KPa), or AST to Platelet Ratio Index (≥1.5).
bProportion based on people on combined antiretroviral therapy.
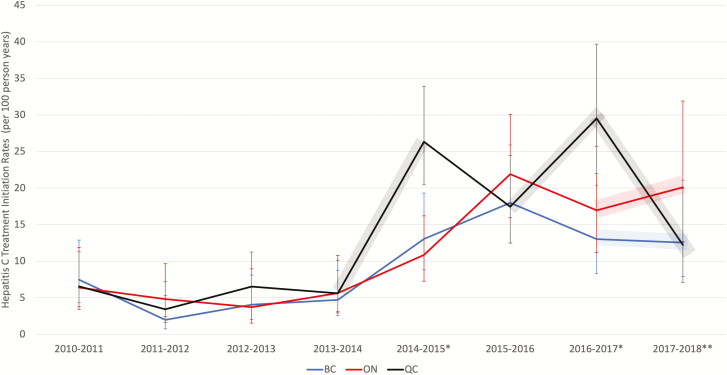
Figure 2 illustrates the temporal trends in HCV treatment initiations between 2010–2018. Before second-generation DAAs were available, treatment rates were low across all provinces. Following the introduction of oral DAAs, treatment initiation rates rose appreciably in all provinces, but rates began to diverge. Treatment rates in Quebec followed a distinct trajectory, compared to those in British Columbia and Ontario. Uniquely, in Quebec between 2014 and 2015, there were no restrictions to reimburse DAAs by fibrosis stage, and rates rose compared to the other provinces. Between 2015 and 2016, temporary restrictions were put in place in Quebec, and treatment rates declined to levels comparable to Ontario and British Columbia, where restrictions to treatment persisted. As restrictions were permanently removed in Quebec in July 2016, rates increased once more, but then dropped considerably between 2017 and 2018. Treatment rates in Ontario and British Columbia followed similar trends to each other. There was an initial increase between 2013 and 2016, followed by a slight decline in rates between 2016 and 2017. At 1 year following unrestricted access to DAAs (March 2017), treatment uptake appeared to be rising in Ontario but plateauing in British Columbia.
Figure 2.
Hepatitis C treatment initiation trends by Canadian provinces between 2010 and 2018. The shaded areas represent a time when the access to direct-acting antivirals were not restricted by fibrosis stage in QC (gray) and in BC (blue) and ON (red). *Data are from QC. **Data are from BC and ON. Abbreviations: BC, British Columbia; ON, Ontario; QC, Quebec.
Accounting for shared temporal trends and time-invariant differences between the provinces, removing fibrosis stage restrictions increased HCV treatment rates by 1.8 times (95% confidence interval [CI] 1.4–2.4) overall. Among PWID, the effect of the policy change was even more pronounced, with an IRR of 3.8 (95% CI 2.0–7.3). Adjustment for covariates did not change the impact of the policy change (Table 2; Supplementary Table 2).
Table 2.
Relative Impact in Hepatitis C Virus Treatment Initiation Rates
| Relative impact of removing significant liver fibrosis restrictions (IRR) | Adjusteda relative impact of removing significant liver fibrosis restrictions (IRR) | |
|---|---|---|
| All CCC participants | 1.8 (1.4, 2.4) | 1.8 (1.3, 2.5) |
| PWIDb | 3.8 (2.0, 7.3) | 3.6 (1.8, 7.4) |
Results following removal of significant liver fibrosis restrictions overall (n = 1130) and specifically among PWID (n = 460). Each cell represents a separate regression analysis, for each we included fixed effects for province and year. The natural logarithm of each participant’s time at risk (in years) was used as an offset. Standard errors are clustered by individuals. Full regression tables for each analysis are available in Supplementary Table 2. Abbreviations: CCC, Canadian Coinfection Cohort Study; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IRR, incident rate ratio; PWID, people who inject drugs.
aAdjusted models included fixed covariates included age (centered at mean), sex, men who have sex with men, and HCV genotype. The time-varying covariates included income (<$18 000 CAD) [13], injection drug use (within the 6 months), undetectable HIV RNA (<50 copies/mL), and significant fibrosis.
bPWID were defined as those self-reporting injection drug use within the 6 months of cohort visits. This was treated as a time-varying variable.
The results of the sensitivity analyses are presented in Supplementary Tables 3–5. The parallel trends assumption was verified visually and statistically (Supplementary Figure 2). We found evidence of an effect modification by the presence of significant liver fibrosis: following the removal of restrictions, those without significant fibrosis were 1.5 times more likely to initiate treatment (Supplementary Table 3). A lead indicator for provinces implementing the removal of fibrosis stage restrictions 1 year before the actual policy change was not associated with treatment initiations, with an IRR of 1.0 (95% CI .7–1.4). A 1-year lagged indicator for provinces removing fibrosis stage restrictions indicated that treatment initiation rates might not be sustainable, with an IRR of 0.8 (95% CI .5–1.1; Supplementary Table 4). Our falsification test demonstrated that changes in the reimbursement policy were not associated with changes in serum creatinine (Supplementary Table 5).
Secondary Analysis
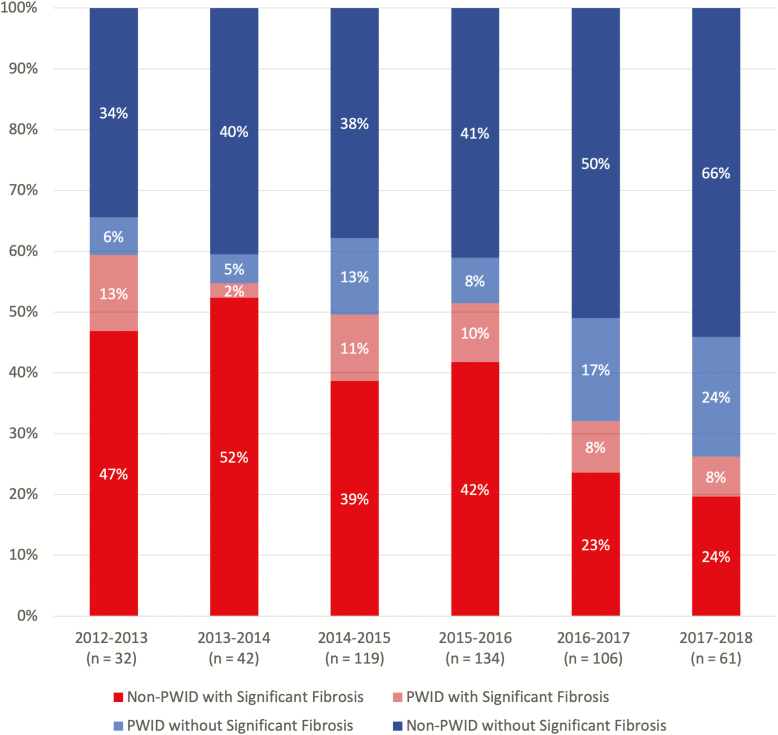
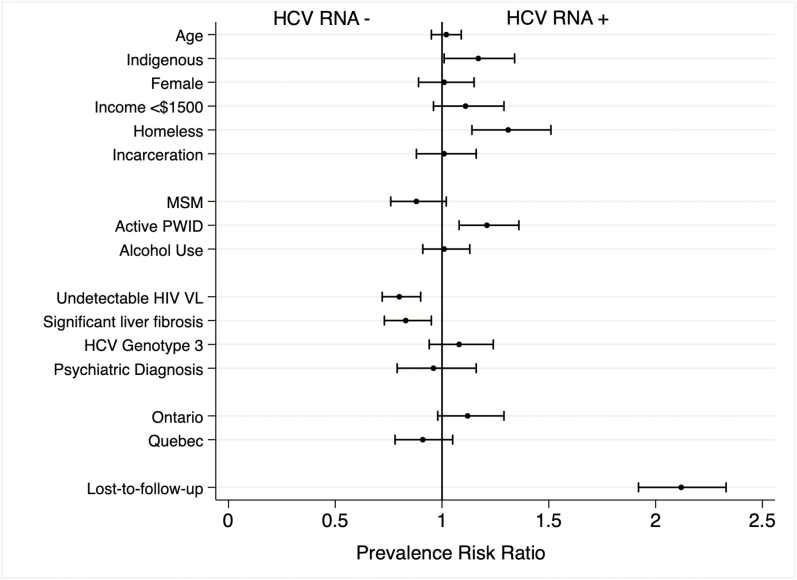
Figure 3 illustrates the increase in the proportion of treatments occurring among people without significant fibrosis and among PWID over time. Using data from each participant’s last visit, we evaluated predictors of remaining HCV RNA positive and found associations of sociodemographic, behavioral, and clinical characteristics (Supplementary Table 6 summarizes participants’ characteristics). Figure 4 illustrates the adjusted prevalence risk ratios (aPRRs) of all covariates analyzed. We found that people of indigenous ethnicity, compared to any other ethnicity (aPRR 1.17, 95% CI 1.01–1.34); those reporting homelessness (aPRR 1.31, 95% CI 1.14–1.51); PWID, compared to people who did not report actively injecting drugs within the prior 6 months (aPRR 1.21, 95% CI 1.08–1.36); and those who disengaged from care (aPRR 2.12, 95% CI 1.92–2.33) were more likely to remain HCV RNA positive and, therefore, still require HCV treatment. Factors associated with achieving an HCV cure were: self-identification as an MSM (aPRR 0.88, 95% CI .76–1.02), having an undetectable HIV RNA (aPRR 0.80, 95% CI .72–.90), and having significant liver fibrosis (aPRR 0.83, 95% CI .73–.95).
Figure 3.
Treatment initiations by fibrosis stage and active injection drug use between 2012 and 2018. Abbreviations: PWID, people who inject drugs.
Figure 4.
Predictors of remaining HCV RNA+ (prevalence risk ratios) among people living with HIV between 2010–2018. The circles are point estimates, and the bars are 95% confidence intervals. The vertical line indicates the null value of 1. Incarceration rates and active PWID data are based on the prior 6 months at time of data collection. An undetectable HIV VL was defined as <50 copies/mL. Data for HCV genotype 3 was compared with genotype 1, 2, or 4. The Ontario and Quebec data were compared with the province of British Columbia. Those lost to follow-up had no visit within 18 months of the administrative censoring date. Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; MSM, men who have sex with men; PWID, people who inject drugs; VL, viral load.
DISCUSSION
The cost of DAAs has led many payers worldwide to restrict access: this remains a significant impediment to universal access to treatment. In this study, we capitalized on a natural experiment occurring in Canada, which has international implications. We found that coinfected people were nearly 2 times more likely to initiate treatment after fibrosis stage restrictions were removed, after controlling for fixed differences across provinces and shared temporal trends. Among the population already engaged in health care, we found annual treatment rates peaked at 25% (between 2015 and 2016) but, by 2018, had decreased to 17%. If maintained, this rate could be sufficient to achieve micro-elimination among HCV coinfected PLWH in Canada. However, it is unclear if these rates can be sustained, as we also found that the population that remains to be treated is marginalized and largely disengaged from care.
Reimbursement Restrictions
Despite advocacy efforts and threats of legal action, a recent study suggests that both public and private health insurers in the United States continue to deny coverage for DAAs at increasingly high rates [6, 15, 16]. While these studies did not examine the reasons for the increases in absolute denials, authors have speculated that the constrained budgets of payers continue to contribute to insurers having to prioritize treatments. Similar reviews of DAA coverage were conducted in Canada and Europe, where it was found that fewer restrictions were in place than in the United States (specifically in regards to sobriety), but the majority of countries still required patients to have significant liver fibrosis [3–5, 16, 17].
Most strikingly, PWID were 3.6 times more likely to initiate treatment following unrestricted coverage. In addition to patient-level benefits of treatment initiation and HCV viral clearance, there is a particular public health impact of treating PWID. Modeling studies have shown that, in high-prevalence settings, treatment can also act as prevention [18]. These studies indicate that restricting access to treatment by advanced fibrosis and/or by drug use status would likely limit the impact on preventing transmission among PWID [19, 20].
Warehousing Effect
Removing structural barriers, such as medication access, is an important step in HCV elimination. However, we found unrestricted DAA access alone may not lead to sustained treatment rates. After the initial surge in treatment initiation following unrestricted access in Quebec, treatment rates declined considerably. An explanation for this decline may be a “warehousing effect”: that is, physicians were aware of those existing patients who were eligible and likely to adhere to treatment, and treated them as soon as access was expanded. Once most of the “warehoused patients” had been treated, the people remaining were those who continued to be more difficult to reach, inconsistently engaged in health care, or perceived to be socially unstable, resulting in a reluctance to initiate treatment. Our results are consistent with the decreased treatment rates observed in countries such as Australia, where access to treatment has been universal since 2016 [21]. As the prices of DAAs continue to decrease and as generic treatments become broadly licensed, our study suggests that universal access may not be enough to meet WHO targets.
Who is Left to Treat?
The underlying principle of the WHO response to viral hepatitis is the promotion of health-care equity. As we report, following unrestricted access to treatment, PWID were more likely to access treatments. However, this was not sufficient to achieve equity (Figure 4). The objective of the secondary analysis was to identify the characteristics of those participants engaged in health care who had not yet accessed treatment in the DAA era. We found that the strongest predictor of remaining HCV RNA positive was becoming disengaged from care; of disengaged patients, 90% (228/254) remained HCV RNA positive. Consistent with previous studies, among those remaining in care, people of indigenous ethnicity and those who reported injecting drugs were still more likely to be HCV RNA positive at the end of this study [22–24]. Although this analysis could not elicit the reasons why participants with these characteristics had not accessed treatment, a recent survey of Canadian providers identified poor access to harm-reduction services and mental-health treatments as the most important barriers to initiating HCV treatments [25].
The strengths of our study were the leveraging of detailed longitudinal data on a generalizable HIV-HCV coinfected population, in combination with the use of quasi-experimental methodology. The time-varying changes before and after DAA reimbursements within provinces allowed us to make plausible causal conclusions of the impact of removing fibrosis stage restrictions on treatment uptake among PLWH. Several sensitivity analyses confirmed the result of the primary analysis.
Our study also has limitations. Results are based on participation in the CCC study, which may not reflect those who had yet to be linked to health care, possibly representing up to 15% of the total coinfected population in Canada [8]. This unengaged population most likely represents people who were more marginalized and vulnerable, meaning our results may be optimistic if we generalized to the broader coinfected population. While our secondary analysis provides insight as to who remains eligible for treatment, this analysis was not designed to attribute causality. Further research is needed to elucidate the individual-level barriers to accessing DAA treatment. Furthermore, our study, and specifically the exposure of interest, coincided with the emerging opioid epidemic [26]. While this crisis had not yet directly impacted death rates in our study population (Supplementary Table 1), it is possible that physicians may also have been more hesitant to treat active PWID if they believed an overdose or reinfection was inevitable. Finally, the rates of losses to follow-up were high, although nondifferential between the provinces. If participants who disengaged from care were less likely to have initiated treatment, censoring could be informative, which would lead to an overestimation of our estimates. In contrast, it is also possible that people who were lost to follow-up may have been treated outside of the CCC. Finally, the impact of universal access to HCV treatments on treatment uptake rates was limited to 3 provinces, with averages of 3 years post–policy change in Quebec and only 1 year in British Columbia and Ontario. More follow-up time is required to evaluate whether this impact is sustainable.
CONCLUSION
Using a quasi-experimental design, we show that the removal of fibrosis restrictions markedly increased treatment access in the short term, particularly for the priority population of PWID, to levels that could result in HCV elimination in people coinfected with HIV and HCV. However, these rates may not be sustainable. To reach elimination, an emphasis on finding innovative ways to address persistent disparities in treatment uptake rates among vulnerable populations is needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. The Canadian coinfection cohort investigators (CTN222) are Drs. Lisa Barrett, Queen Elizabeth II Health Sciences Centre Health Science Center for Clinical Research, Halifax, Nova Scotia; Jeff Cohen, Windsor Regional Hospital Metropolitan Campus, Ontario; Brian Conway, Vancouver Infectious Diseases Research and Care Centre, British Columbia; Curtis Cooper, the Ottawa Hospital Research Institute, Ontario; Pierre Côté, Clinique du Quartier Latin, Montréal, Quebec; Joseph Cox, McGill University Health Centre, Division of Infectious Diseases and Chronic Viral Illness Service, Montreal, Quebec; John Gill, Southern Alberta Human Immunodeficiency Virus (HIV) Clinic, Calgary; Shariq Haider, McMaster University, Hamilton, Ontario; Mark Hull, British Columbia Centre for Excellence in HIV/Acquired Immunodeficiency Syndrome, Vancouver; Marina Klein, McGill University Health Centre, Division of Infectious Diseases and Chronic Viral Illness Service, Montreal, Quebec; Julio Montaner, St. Paul’s Hospital, Vancouver, British Columbia; Erica Moodie, McGill University, Montreal, Quebec; Neora Pick, Oak Tree Clinic, Children’s and Women’s Health Centre of British Columbia, University of British Columbia, Vancouver; Anita Rachlis, Sunnybrook & Women’s College Health Sciences Centre, Toronto, Ontario; Danielle Rouleau, Centre Hospitalier de l’Université de Montréal, Quebec; Aida Sadr, Native British Columbia Health Center, St-Paul’s Hospital, Vancouver, British Columbia; Steve Sanche, Saskatchewan HIV/AIDS Research Endeavour University of Saskatchewan, Saskatoon; Roger Sandre, HAVEN Program, Sudbury, Ontario; Mark Tyndall, Department of Medicine, Infectious Diseases Division, University of Ottawa, Ontario; Marie-Louise Vachon, Centre Hospitalier Universitaire de Québec; Sharon Walmsley, University Health Network, Toronto, Ontario; and Alex Wong, Regina Qu’Appelle Health Region, Regina General Hospital, Saskatchewan. M. B. K. obtained the funding, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. S. S. created the study concept and design, drafted the manuscript, and conducted the statistical analysis. All authors participated in the acquisition, analysis, or interpretation of data, as well as critical revisions of the manuscript. M. B. K., E. S., and E. E. M. M. supervised the study.
Acknowledgments. The authors thank all study coordinators and nurses for their assistance with study coordination, participant recruitment, and care.
Financial support. This work was supported by Fonds de recherche du Québec –Santé; Réseau syndrome d’immunodéficience acquise/maladies infectieuses, the Canadian Institute for Health Research (CIHR; grant number FDN-143270); and the CIHR Canadian Human Immunodeficiency Virus Trials Network (grant number CTN222). S. S. is supported by a PhD award from the Canadian Institute for Health Research and the Canadian Network for Hepatitis. C. E. S., E. E. M. M., and V. M.-L. are supported by career awards from the Fonds de recherche du Québec –Santé. M. B. K. is supported by a Tier I Canada Research Chair.
Potential conflicts of interest. M. B. K. is has received research support from ViiV, Abbvie, Merck and Gilead; and has received honoraria for lectures from Janssen, ViiV and Merck. M.-L. V. has received personal fees from AbbVie, Merck, and Gilead. A. W. has received personal fees from Merck, Gilead, Bristol Myers Squibb, and AbbVie. V. M.-L. has received grants and personal fees from Merck, Gilead, and AbbVie. M. T. has received grants from Gilead, Bristol Myers Squibb, and Janssen, and personal fees from Gilead. B. C. has received grants, personal fees, and non-financial support from Gilead, Merck, and Abbvie. S. W. received grants, consulting fees, lecture fees, nonfinancial support and fees for the development of educational presentations from Merck, ViiV Healthcare, GlaxoSmithKline, Pfizer, Gilead, Abbvie, Bristol-Myers Squibb, and Janssen. J. G. received personal fees for being a member of the national advisory boards of Abbvie, Gilead, Merck, Janssen, ViiV Healthcare, and Bristol-Myers Squibb. M. H. has served as a consultant for Gilead, Merck, Vertex Pharmaceuticals, Pfizer, Viiv Healthcare and Ortho-Jansen; has received grants from the National Institute on Drug Abuse; and has received payment for lectures from Merck and Ortho-Janssen. J. C. received grants from Merck, ViiV Healthcare, and Gilead and personal fees from Bristol-Myers Squibb, ViiV Healthcare, Merck, and Gilead. C. C. has received personal fees for being a member of the national advisory boards of Gilead, Merck, Janssen, Abbvie, and Bristol-Myers Squibb and has received grants from Abbvie and Gilead. L. W. has no conflicts of interest to declare. None of the authors feels in conflict of interest with regards to this study and there was no pharmaceutical industry support to conduct this study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Canadian Coinfection Cohort Study Investigators:
Lisa Barrett, Jeff Cohen, Brian Conway, Curtis Cooper, Pierre Côté, Joseph Cox, John Gill, Shariq Haider, Mark Hull, Marina Klein, Julio Montaner, Erica Moodie, Neora Pick, Anita Rachlis, Danielle Rouleau, Aida Sadr, Steve Sanche, Roger Sandre, Mark Tyndall, Marie-Louise Vachon, Sharon Walmsley, Alex Wong, and M B K Saskatchewan
References
- 1. Stanaway JD, Flaxman AD, Naghavi M, et al. . The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016; 388:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polaris Observatory Center for Disease Analysis Foundation. Just 12 countries worldwide on track to eliminate hepatitis C infection by 2030, with United Kingdom, Italy and Spain among those joining the list. Available at: www.cdafound.org. Accessed 9 July 2019. [Google Scholar]
- 3. Marshall AD, Cunningham EB, Nielsen S, et al. ; International Network on Hepatitis in Substance Users (INHSU) Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol 2018; 3:125–33. [DOI] [PubMed] [Google Scholar]
- 4. Marshall AD, Pawlotsky JM, Lazarus JV, Aghemo A, Dore GJ, Grebely J. The removal of DAA restrictions in Europe - one step closer to eliminating HCV as a major public health threat. J Hepatol 2018; 69:1188–96. [DOI] [PubMed] [Google Scholar]
- 5. Marshall AD, Saeed S, Barrett L, et al. ; Canadian Network on Hepatitis C (CanHepC) Restrictions for reimbursement of direct-acting antiviral treatment for hepatitis C virus infection in Canada: a descriptive study. CMAJ Open 2016; 4:E605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 7. Platt L, Easterbrook P, Gower E, et al. . Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 8. Sacks‐Davis R, Doyle JS, Rauch A, et al. . Linkage and retention in HCV care for HIV‐infected populations: early data from the DAA era. J Int AIDS Soc 2018; 21(S2):e25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein MB, Saeed S, Yang H, et al. . Cohort profile: the Canadian HIV-hepatitis C co-infection cohort study. Int J Epidemiol 2010; 39:1162–9. [DOI] [PubMed] [Google Scholar]
- 10. Saeed S, Moodie EEM, Strumpf EC, Klein MB. Evaluating the impact of health policies: using a difference-in-differences approach. Int J Public Health 2019; 64:637–42. [DOI] [PubMed] [Google Scholar]
- 11. Abadie A. Semiparametric difference-in-differences estimators. Rev Econ Stud 2005; 72:1–19. [Google Scholar]
- 12. Wing C, Simon K, Bello-Gomez RA. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health 2018; 39:453–69. [DOI] [PubMed] [Google Scholar]
- 13. Statistics Canada. Income in Canada. Available at: www.statcan.gc.ca/pub/75-202-x/2009000/know-savoir-eng.htm. Accessed 9 July 2019. [Google Scholar]
- 14. Hull M, Shafran S, Wong A, et al. . CIHR Canadian HIV trials network coinfection and concurrent diseases core research group: 2016 updated Canadian HIV/hepatitis C adult guidelines for management and treatment. Can J Infect Dis Med Microbiol 2016; 2016:34. Article ID 4385643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gowda C, Lott S, Grigorian M, et al. . Absolute insurer denial of direct-acting antiviral therapy for hepatitis C: a national specialty pharmacy cohort study. Open Forum Infect Dis 2018; 5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lo Re V 3rd, Gowda C, Urick PN, et al. . Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol 2016; 14:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Younossi ZM, Bacon BR, Dieterich DT, et al. . Disparate access to treatment regimens in chronic hepatitis C patients: data from the TRIO network. J Viral Hepat 2016; 23:447–54. [DOI] [PubMed] [Google Scholar]
- 18. Hickman M, De Angelis D, Vickerman P, Hutchinson S, Martin NK. Hepatitis C virus treatment as prevention in people who inject drugs: testing the evidence. Curr Opin Infect Dis 2015; 28:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris RJ, Martin NK, Rand E, et al. . New treatments for hepatitis C virus (HCV): scope for preventing liver disease and HCV transmission in England. J Viral Hepat 2016; 23:631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin NK, Boerekamps A, Hill AM, Rijnders BJA. Is hepatitis C virus elimination possible among people living with HIV and what will it take to achieve it? J Int AIDS Soc 2018; 21(Suppl 2):e25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajarizadeh B, Grebely J, Matthews GV, Martinello M, Dore GJ. Uptake of direct-acting antiviral treatment for chronic hepatitis C in Australia. J Viral Hepat 2018; 25:640–8. [DOI] [PubMed] [Google Scholar]
- 22. Janjua NZ, Islam N, Wong J, et al. . Shift in disparities in hepatitis C treatment from interferon to DAA era: A population-based cohort study. J Viral Hepat 2017; 24:624–30. [DOI] [PubMed] [Google Scholar]
- 23. Kanwal F, Kramer JR, El-Serag HB, et al. . Race and gender differences in the use of direct acting antiviral agents for hepatitis C virus. Clin Infect Dis 2016; 63:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saeed S, Strumpf EC, Moodie EE, et al. . Disparities in direct acting antivirals uptake in HIV-hepatitis C co-infected populations in Canada. J Int AIDS Soc 2017; 20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan J, Young J, Cox J, Nitulescu R, Klein MB. Patterns of practice and barriers to care for hepatitis C in the direct-acting antiviral (DAA) era: a national survey of Canadian infectious diseases physicians. Can Liver J 2018; 1:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Special Advisory Committee on the Epidemic of Opioid Overdoses. National report: apparent opioid-related deaths in Canada (January 2016 to December 2017) web-based report. Ottawa, Canada: Public Health Agency of Canada, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.