Abstract
A large number of metallic nanoparticles have been successfully synthesized by using different plant extracts and microbes including bacteria, fungi viruses and microalgae. Some of these metallic nanoparticles showed strong antimicrobial activities against phytopathogens. Here, we summarized these green-synthesized nanoparticles from plants and microbes and their applications in the control of plant pathogens. We also discussed the potential deleterious effects of the metallic nanoparticles on plants and beneficial microbial communities associated with plants. Overall, this review calls for attention regarding the use of green-synthesized metallic nanoparticles in controlling plant diseases and clarification of the risks to plants, plant-associated microbial communities, and environments before using them in agriculture.
Keywords: green synthesis, microorganisms, plant extracts, metallic nanoparticles, plant pathogens
1. Introduction
Nanotechnology is an emerging field of science with a wide range of applications in various areas including medicine and agriculture. In agriculture, nanotechnology can be exploited by the use of natural resources in the conservation, production and protection of crops and livestock [1]. Recently, biosynthesis of nanoparticles (NPs) or green synthesis of NPs has received much attention due to the biocompatibility, low toxicity, and eco-friendly nature of the process and NP products [2]. The use of biological materials, such as bacteria, yeast, mold, microalgae and plant extracts, to synthesize NPs has some advantages like less energy consumption and moderate technology without using toxic chemicals [3,4]. The application of nanotechnology in plant disease control is just emerging [5]. NPs can be used directly or as carriers of various pesticides for plant protection. Most of the studies have been done in laboratory conditions [6]. It is crucial for us to know the effects on plants, microbes associated with the plants in fields and overall ecosystems before the application of green-synthesized NPs in plant disease management.
2. Green Synthesis of Metallic Nanoparticles
Green synthesis of NPs is a cost-effective and eco-friendly technique that does not use toxic chemicals. This technique employs a number of reducing and stabilizing agents like microbes, plants and other natural resources to produce NPs for sustainable in manner [7,8]. The green synthesis of NPs has gained much attention due to it being eco-friendly, cost effective and highly stable [9]. Several studies have reported the production of NPs using plants and microorganisms [10,11]. The green synthesis methods of NPs are diversified, but organisms or their extracts are simply reacted with a metallic salt and then biological reduction is carried out to convert the metal to NPs. The produced NPs are readily available to use after proper characterization [10,12].
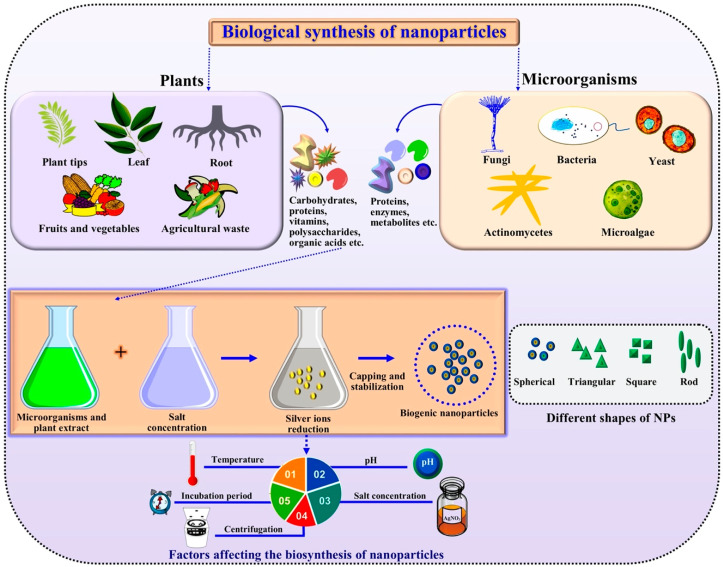
Microbe-mediated synthesis of NPs is a green approach that utilizes bacteria, fungi, viruses and their products for the production of NPs. These microbes provide templates for synthesis and organization of well-defined, structured NPs [13,14]. In comparison to microbial synthesis as a potential technique, plants can be used in convenient manner for NPs production. The synthesis of NPs can be scaled up easily by using plant extracts. In addition, the plant extracts can reduce metallic ions more quickly than microbes and produce stable metallic NPs [10,15]. In plants extracts, many compounds like polysaccharides, proteins, amino acids, organic acids and phytochemicals like polyphenols, flavonoids, terpenoids, alkaloids, tannins, and alcoholic substances are present that can reduce and stabilize the NPs [12,16]. A generalized schematic representation of green synthesis of NPs is shown in Figure 1.
Figure 1.
Generalized schematic representation of green synthesis of metallic nanoparticles (NPs).
2.1. Microbe-Based Synthesis
The green synthesis mediated by microbes has been raised as an alternative method of NPs design and development [17,18]. Microbes can be used as safe and cheap tools for synthesis of metallic NPs like gold, silver, copper, zinc, titanium, palladium, and nickel. The synthesis of NPs can be carried out both extracellularly and intracellularly using microbes [19]. For extracellular synthesis, the culture filtrate is collected by centrifugation and mixed with an aqueous metallic salt solution. Synthesis of NPs is monitored by the color change of the mixed solution. For example, the light yellow to dark brown color is an indicator of synthesis of silver NPs (AgNPs) [8,20]. For intracellular synthesis, the biomass is washed thoroughly with sterile water after culturing microorganisms under optimum growth conditions and incubated with metal ion solution. As mentioned above, the color change serves as an indicator of NPs synthesis. Then NPs are collected by ultra sonication, centrifugation and washing [21]. Here, we review various metallic NPs synthesis through the utilization of microorganisms.
A diverse group of bacteria are living in soil, water, plants and animals. They can live in various soil pH, salinity, temperature and nutrient conditions. In aquatic environment, bacteria can be found in normal to highly saline water in deep-sea and even in the ice with a freezing temperature. Some of them can be occurred in heavily contaminated or hyper accumulated soils and plants. Pseudomonas stutzeri and Pseudomonas aeruginosa can survive even in high concentrated metal ion conditions [22,23]. Thiobacillus ferrooxidans, T. thiooxidans and Sulfolobus acidocaldarius can reduce ferric to the ferrous ion while living on elemental sulfur as an energy source [24]. Therefore, bacteria possess their own mechanisms by which they can survive and uptake nutrients for their growth and multiplication. They can reduce the metallic substances and utilize energy for themselves. Bacteria are evolving many defense mechanisms like sequestration intracellularly, pumping efflux, changing concentration of metal ions and precipitation extracellularly to overcome various stresses [25]. These types of mechanisms of bacteria can be applied in the green synthesis of NPs.
Recently, bacterial strains belonging to Acinetobacter calcoaceticus, Bacillus amyloliquefaciens, Bacillus megaterium, Bacillus licheniformis, Escherichia coli, Lactobacillus sp. and Pseudomonas stutzeri have been used in for the biosynthesis of AgNPs [26]. Silver NPs can be produced by both intracellular and extracellular biosynthesis and these NPs have shown antimicrobial activity against many pathological organisms [27]. The culture supernatant of Pseudomonas rhodesiae was incubated with AgNO3 solution and AgNPs were synthesized. A clear surface plasmon resonance peak at 420–430 nm in the range of 350–450 nm was confirmed as a featured peak of AgNPs by UV–Visible spectroscopy. The reduction of Ag+ and stabilization of the AgNPs was identified by Fourier-transform infrared spectroscopy. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were performed to measure the size of the AgNPs synthesized with supernatant of P. rhodesiae. The AgNPs were generally spherical and uniform with a range of 20–100 nm in diameter. X-ray diffraction analysis was used to observe the crystalline nature of the P. rhodesiae mediated AgNPs [8]. An extracellular biosynthesis of AgNPs was carried out using B. cereus SZT1, isolated from wastewater-contaminated soil. The AgNPs were spherical shapes and their particle size ranged from 18 to 39 nm [18]. The culture filtrate of endophytic Pseudomonas poae strain CO was used to synthesize AgNPs with the size of 19.8–44.9 nm [20]. The AgNPs were biosynthesized using the culture supernatant of Stenotrophomonas sp. BHU-S7, which was isolated from agricultural farm soil [28]. The synthesis of gold NPs (AuNPs) using Bacillus subtilis isolated from Hatti Gold Mine was reported in a study. The microorganisms isolated from gold mine might be highly resistant to gold ions toxicity and could be used to synthesize AuNPs efficiently. The synthesis of ultra-small palladium and platinum NPs were done by using Shewanella loihica PV-4 within the size range of 2–7 nm [29]. Ochrobactrum sp. was used to synthesize tellurium NPs and this strain might serve as an effective nanofactory to convert the toxic tellurite oxyanions into useful NPs [30].
In addition to the isolation of bacteria from terrestrial environments some marine bacterial cultures have been utilized as nanofactories for synthesis of NPs. A novel bacterium Stenotrophomonas was used for green synthesis of AgNPs and AuNPs. Here, the secretory proteins with low molecular weight present in the supernatant play a key role for biosynthesis of AgNPs and AuNPs [31]. Another marine strain, Kocuria flava, was able to synthesize copper NPs with a size of 5 to 30 nm [32].
In a previous study, AgNPs were synthesized from Pseudomonas stutzeri AG259 through the process involving NADH-dependent reductase enzyme which provides electrons to oxidize NADH to NAD+. The donation of electron from NADH causes the bioreduction of Ag ions to AgNPs [33]. Pseudomonas aeruginosa SM1 can synthesize various NPs intracellularly, such as Ag, Fe, Co, Ni, Li, Pd, Pt and Rh NPs [34]. Moreover, some researchers have shown the synthesis of NPs without involving biological enzymes. For example, dead or inactive cells of Corynebacterium glutamicum were used to synthesize AgNPs. A large amount of reduction was found on the surface of the inactive cells resulting in the formation of AgNPs with irregular shape and size of 5 to 50 nm [35]. Zinc oxide NPs are also promising as antimicrobial agents, drug delivery and bioimaging probes in next-generation biological applications. Zinc oxide NPs were synthesized using a bacterium Aeromonas hydrophila in simple and cost-effective method. The crystalline nature of the NPs was observed by atomic force microscopy (AFM), which showed that the NPs were spherical and oval with an average size of 57.72 nm [36].
The conversion of metallic ions into NPs through reduction is dependent on functional groups of biomolecules present in the organisms which induce biomineralization, and other environmental factors, such as pH, media composition, concentration of metallic salts and temperature [33]. The size, shape and composition can be highly determined by these environmental factors [37]. For example, at the optimum growth temperature of 20 °C, spherical AgNPs were produced with an average diameter of 2–5 nm using Morganella psychrotolerans, while at 25 °C, a mixture of triangular and hexagonal nanoplates along with spherical NPs were obtained [38].
Actinomycetes, a group of filamentous bacteria, are known for their metabolic versatility. These bacteria can survive in stressful environmental conditions by using the bioactive potentials [39]. Actinomycetes consist of a significant composition of the microbial population in soils and produce extracellular enzymes to decompose materials. Their enzymes have received more attention than enzymes from other sources due to their high stability and uncommon substrate specificity. These are found in extreme habitats and produce enzymes with high commercial value [40]. Among the 22,000 discovered microbial secondary metabolites, 70% are from actinomycetes while two-thirds of them are originated from the genus Streptomyces [41]. Both extracellular and intracellular synthesis of NPs can be undertaken, but extracellular synthesis is a popular method and has been used commercially in various fields. Biomass extracts of Streptomyces zaomyceticus Oc-5 and Streptomyces pseudogriseolus Acv-11 were used for synthesis of copper oxide NPs (CuONPs). Green synthesized CuONPs were with surface plasmon resonance absorption band at 400 nm, crystalline, spherical with an average size of 78 nm and 80 nm for strain Oc-5 and Acv-11, respectively [42]. In another study, the free-biomass filtrates with metabolites from three endophytic actinomycetes of Streptomyces capillispiralis Ca-1, Streptomyces zaomyceticus Oc-5, and Streptomyces pseudogriseolus Acv-11 served as biocatalysts for green synthesis of AgNPs [43]. An actinobacteria Rhodococcus sp. was used to reduce aqueous AgNO3 for the green synthesis of AgNPs [44]. The extracellular synthesis of gold (Au) NPs was carried out using culture supernatant of soil isolated Streptomyces griseoruber with a size 5–50 nm [45]. The green synthesized metallic NPs show higher antimicrobial potentials than conventionally synthesized NPs because some biomolecules act as capping and stabilizing agents during synthesis of the NPs [14].
Fungi are excellent sources of many bioactive compounds that can be utilized in various sectors. The microscopic filamentous fungi (ascomycetes and imperfect fungi) and other fungal species are reported to produce about 6400 bioactive compounds [46]. These microorganisms possess tolerance to the heavy metals and are capable of internalizing as well as bioaccumulating the metals. So, these organisms have been used for reduction and stabilization during the synthesis of NPs. Moreover, large-scale cultivation of fungi is very easy and can be used to synthesize NPs with uniform shape and size [47,48,49,50]. Fungi are more convenient compared to other microbes due to their production of high quantities of enzymes and proteins for NPs synthesis [51,52]. The fungi mediated synthesis of NPs can be extracellular or intracellular [53]. For extracellular synthesis, the aqueous culture filtrates consisting of biomolecules are added to metal precursor, and free NPs are formed in the dispersion. This is a commonly used method, as no techniques are needed to get cell-free NPs [49,54,55,56]. During intracellular synthesis, a metal precursor is added to the mycelial culture and internalized in the biomass followed by the extraction of NPs. The extraction of the NPs is performed to disrupt the biomass by chemical treatment, centrifugation, and filtration and then release the synthesized NPs [57,58,59].
The fungal synthesis of metallic NPs is dependent on culture conditions. In a previous study, the culture conditions of Trichothecium sp. reduced Au ions resulting extracellular NPs synthesis but produced NPs intracellularly when cultured with agitations. The possible mechanism involved here is the non-agitation condition, but not the agitation condition, enhancing the release of enzymes and proteins [60]. The desired characteristics of NPs from different fungal species can be obtained through the adjustment of some factors like temperature, agitation, light, and culture and synthesis times. These parameters should be maintained during the fungal culture and NPs synthesis to control of the size and shape of NPs [61]. It was also found that differences in pH, temperature, culture medium, biomass quantity and concentration of the metal precursor can be used to determine physicochemical characteristics of NPs [58,61,62,63]. AgNPs were synthesized by using the filtrate of Rhizopus stolonifer with NPs size of 2.86, 25.89, and 48.43 nm under the temperature regime of 40, 20, and 60 °C, respectively, but not at 10 or 80 °C. At a very low or high temperature, enzymes and active molecules may be denatured or inactivated which are needed in AgNPs biogenesis [64].
Husseiny et al. reported the biosynthesis of AgNPs using Fusarium oxysporum and reported the effect of substrate concentration and incubation temperature [65]. Most of the AgNPs were smaller at 50 °C and, at higher temperature, particle size increased. The amount of biomass played a key role in synthesis or complete reduction of Ag+ to Ag0. The optimum weight of fungal biomass was 11 g for the smallest particle size. For the AgNPs synthesis by F. oxysporum, pH was found to be an important factor and the smallest size particles were obtained at pH 6. Due to the lower pH, protein structure might be affected or denatured and its potential may have been lost; thus, NP size was found to be large [66]. In alkaline conditions, the catalyzing activity of reductase enzyme for the synthesis might be gradually deactivated, and reduced synthesis and increase in size of the particles at higher pH. A similar phenomenon was observed during AgNPs synthesis using Penicillium fellutanum [67]. A seven-day old fungus used as a young culture for 72 h was better than a fifteen-day old culture for s similar incubation time in the case of AgNPs [65].
Yeast cells act as one of the most important agents for bioremediation of heavy metals. Yeasts are easily cultured in low-cost media and capable of removing various heavy metals. Yeasts have the adaptive capacity to extreme environmental conditions like pH, temperature and high concentrated organic and inorganic contaminants. Most of the available studies concern Ascomycota such Saccharomyces cerevisiae, Schizosaccharomyces pombe and Candida sp. Yeasts may have evolved some mechanisms for detoxification such as mobilization, immobilization or transformation of metals [68,69]. The immobilization mechanisms involve biosorption, biotransformation and bioaccumulation of metal ions by living microorganisms [70]. These bioremediation properties of yeasts can be exploited for the green synthesis of NPs to be applied in fields.
Saccharomyces cerevisiae was used for biosynthesis of AgNPs by biotransformation. Both the dried and fresh culture S. cerevisiae was used as the biocatalyst. More AgNPs were obtained from freshly cultured yeast than dried culture. The AgNPs were spherical with a size of 2–20 nm in diameter, and 5.4 nm sized particles were mostly found. AgNPs were found inside the cells, within the membrane of cells, attached to the cell membrane, and outside of the yeast cells [11]. A marine yeast Yarrowia lipolytica strain was used for the biosynthesis of AgNPs in a cell associated manner. This study suggested that the brown pigment (melanin) might be the possible reason for biomineralization of metallic ions [71]. Pichia jadinii was used for intracellular synthesis of AuNPs ranging from 1–100 nm. In this study, the growth and cellular activities of P. jadinii were controlled easily to regulate AuNPs size and shape [72]. The green synthesis AgNPs was obtained in an extracellular process by using Candida utilis NCIM 3469 with a size 20–80 nm [73]. In another study, Saccharomy cescerevisiae was capable of synthesizing copper NPs (CuNPs) extracellularly, where more than 70% of the particles were about 10–12 nm [74].
Several studies illustrate that viruses are considered to be a suitable group which serves as a biotemplate for material synthesis at the nanoscale to microscale [75]. Recently, material science researchers were using the viral NPs (VNPs) as templates or scaffolds for the synthesis of novel hybrid nanomaterials [76]. A number of plant viruses were employed as nano-factories because of their special structural integrity, easy manipulation and lower infectivity to human [76,77]. Furthermore, due to structural diversity, viral capsids are exploited as a biotemplate for material synthesis [78,79,80,81]. The viral NPs can be engineered genetically, chemically and also utilized as nano-templates at three levels of their structure [82]. The capsids of viruses are arranged by repeating protein subunits to form highly precise three-dimensional symmetrical structures with uniform shape and size [83,84].
The synthesis of nanomaterials using viruses is a clean, nontoxic and environmentally-friendly method which provides a broad range of sizes, shapes, compositions, and physicochemical properties [83,85]. In a study, a notorious plant pathogenic virus, Squash leaf curl China virus (SLCCNV) was used as biotemplate to fabricate silver and gold nanomaterials. The SLCCNV was exposed to HAuCl4 and AgNO3 precursors in presence of sunlight and quick (∼5 min) formation of SLCCNV-metallic-hybrid nanomaterials in an eco-friendly way was observed [86]. A wild type bacteriophage P22 was utilized for synthesis of cadmium sulfide (CdS) nanocrystal quantum dots on its ∼60 nm procapsid. The bacteriophage P22 shell possessed capsomers composed of hexameric and pentameric clusters. The pre-synthesized CdS quantum dots resemble the hexameric and pentameric patterns of assembly on the P22 shells, which might be due to interaction with particular protein pockets [87]. In another research, tobacco mosaic virus and bovine papilloma virus were used as additive materials with plant extracts Avena sativa, Hordeumvulgare, Musa pradisiaca and Nicotiana benthamiana. These two viruses promoted the reduction and increase the NPs number remarkably as compared to a control without virus [14,88]. These viral synthesized nanomaterials have a wide range of applications in biomedicine and serve as catalysts to biosensors [89]. Similarly, M13 bacteriophage can be used as versatile template for engineering various nanomaterials [90].Although a number of viruses and bacteriophages have been exploited for green synthesis of metallic NPs, no study is available regarding their application in the control of phytopathogens.
2.2. Nanoparticles from Microalgae
The synthesis of microalgae-based NPs, termed “phyconanotechnology”, has become an emerging area with wide scope in recent years. A large number of photoautotrophic microorganisms belong to microalgae which contain secondary metabolites, pigments and proteins [91,92]. These microorganisms can act as nano biofactories for synthesis of metallic NPs [93,94,95,96,97]. A number of methodologies have been developed for metallic NPs synthesis using microalgae from their corresponding aqueous salt solutions which can determine the size and shape of NPs with good quality. Synthesis of microalgae driven NPs can be obtained by using extracted biomolecules from disrupted cells of microalgae [92,98].
Microalgae can be exploited as an efficient bionanofactory, capable of producing metallic NPs by reducing various metal ions such as silver, gold, cadmium, and [98,99]. Both the live and died dried biomass of microalgae can be used to synthesize metallic NPs. Several microalgae such as Chlorella vulgaris, Spirulina platensis, and Lyngbya majuscule have been utilized for biosynthesis of AGNPs [93,100]. The biosynthesis of AgNPs extracellularly using a marine cyanobacterium, Oscillatoriawillei NTDM01 which reduced silver ions and stabilized the AgNPs by a secreted protein. The extracted biomolecules of Chlorella vulgaris, a single-celled green microalga was used to synthesize AgNPs [101]. In another study, living cells Chlorella vulgaris were incubated along with gold chloride solution and after incubation cells were harvested followed by centrifugation. NPs were detected inside intact cells by TEM and assigned to metallic gold by synchrotron-based X-ray powder diffraction and X-ray absorption spectroscopy. The sizes of the intracellular AuNPs were 40–60 nm in diameter [102]. Arsiya et al. reported the green synthesis of palladium NPs by Chlorella vulgaris aqueous extract [103]. The biomass was dried, powdered homogeneously, boiled in water and the crude extract was filtered. Aqueous solution of PdCl2 was mixed with crude filtrate of Chlorella vulgaris and solution color was changed to yellow dark brown indicating formation of PdNPs [103]. Marine microalgae such as Chaetoceros calcitrans, Chlorella salina, Isochrysis galbana and Tetraselmis gracilis were used to synthesize AgNPs [104]. A large number of studies of algal NPs are available but their use in phytopathogenic control is yet to be determined. A list of NPs have been synthesized from various microbes are given in Table 1.
Table 1.
Microbes mediated synthesized metallic NPs against plant pathogens.
| Microbes | Sources of Isolation | MetalNPs | Features | Major Application | Plant Disease Management | |||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Shape | Pathogen | Host | References | ||||
| Bacteria | ||||||||
| Pseudomonas rhodesiae | Rhizospheric soil of cotton | Ag | 20–100 | Spherical | Antibacterial agent | Dickeyadadantii | Sweet potato | [8] |
| Bacillus siamensis | Coriandrumsativum | Ag | 25–50 | Spherical | Antibacterial agent | Xanthomonas oryzae pv. oryzae | Rice | [105] |
| Bacillus cereus | Wastewater contaminated soil | Ag | 18–39 | Spherical | Antibacterial agent | Xanthomonas oryzae pv. oryzae | Rice | [18] |
| Pseudomonas poae | Garlic | Ag | 20–45 | Spherical | Antifungal agent | Fusarium graminearum | Wheat | [20] |
| Bacillus sp. | Soil | Ag | 7–21 | Spherical | Antifungal agent | Fusarium oxysporum | Tomato | [106] |
| Serratia sp. | Soil | Ag | 10–20 | Spherical | Antifungal agent | Bipolaris sorokiniana | Wheat | [107] |
| Stenotrophomonas sp. | Soil | Ag | 12 | Spherical | Antifungal agent | Sclerotium rolfsii | Chickpea | [28] |
| Pseudomonas sp., and Achromobacter sp. | Rhizospheric soil of chickpea | Ag | 20–50 | Spherical | Antifungal agent | Fusarium oxysporum f. sp. ciceri | Chickpea | [108] |
| Aeromonas hydrophila | Missing | ZnO | 57–72 | Crystalline | Aspergillus flavus | Maize | [36] | |
| Streptomyces spp. | Oxalis corniculata leaves | CuO | 78–80 | Spherical | Antifungal agent | Alternaria alternata, Fusarium oxysporum, Pythium ultimum, and Aspergillus niger | Multiple crops | [42] |
| Streptomyces capillispiralis | Convolvulus arvensis leaves | Cu | 4–59 | Spherical | Antifungal agent | Alternaria spp., Aspergillus niger, Pythium spp., and Fusarium spp. | Multiple crops | [109] |
| Streptomyces griseus | Rhizospheric soil of tea | Cu | 5–50 | Spherical | Antifungal agent | Poria hypolateritia | Tea | [110] |
| Bacillus thuringensis | Soil | Ag | 10–20 | Polymorphic | Antiviral agent | Sun hemp rosette virus | Cluster bean | [111] |
| Bacillus licheniformis | Soil | Ag | 77–92 | Polymorphic | Antiviral agent | Bean yellow mosaic virus | Faba bean | [112] |
| Fungi | ||||||||
| Trichiderma hazarium | Tomato | Ag | 11–13 | Spherical | Antifungal agent | Helminthosporium sp., Alternaria alternata, Phytophthora arenaria, and Botrytis sp. | Multiple crops | [113] |
| Aspergillus niger | Grape | Ag | 10–100 | Spherical | Antifungal agent | Penicillin digitatum, Aspergillus flavus, and Fusarium oxysporum | Multiple crops | [114] |
| Penicillium duclauxii | Corn seeds | Ag | 3–32 | Spherical | Antifungal agent | Bipolaris sorghicola | Sorghum | [115] |
| Setosphaeria rostrata | Solanum nigrum leaves | Ag | 2–50 | Spherical | Antifungal agent | Aspergillus niger, Rhizoctonia solani, Fusarium graminearum, and Fusarium udum | Multiple crops | [116] |
| Trichoderma longibrachiatum | Cucumber | Ag | 1–25 | Spherical | Antifungal agent | Alternaria alternata, Pyricularia grisea, Fusarium verticillioides, Helminthosporium oryzae, and Penicillium glabrum | Multiple crops | [117] |
| Trichoderma harzianum | Soil | Ag | 20–30 | Spherical | Antifungal agent | Sclerotinia sclerotiorum | Cabbage | [118] |
| Guignardia mangiferae | Leaves of medicinal plants | Ag | 5–30 | Spherical | Antifungal agent | Rhizoctonia solani | Rice | [119] |
| Arthroderma fulvum | Soil | Ag | 13–18 | Spherical | Antifungal agent | Aspergillus flavus | Maize | [120] |
| Aspergillus versicolor | Soil | Ag | 5–39 | Spherical | Antifungal agent | Sclerotinia sclerotiorum and Botrytis cinerea | Strawberry | [121] |
| Fusarium solani | Wheat grain | Ag | 5–30 | Spherical | Antifungal agent | Fusarium spp., Aspergillus spp., Alternaria spp. and Rhizopus stolonifer | wheat, barley and corn | [122] |
| Cephalosporium sp. and Trichoderma sp. | Rhizospheric soil of chickpea | Ag | 20–50 | Antifungal agent | Fusarium oxysporum f. sp. ciceri | Chickpea | [108] | |
2.3. Nanoparticles from Plant Extracts
Plants are well known for their ability to reduce metal ions on the surface and different organs or tissues at distance from the penetration sites of ions. The study of the accumulation of metal ions in plants suggested the transformation of metals to NPs [123]. For example, Medicago sativa and Brassica juncea grown withAgNO3 accumulated 12.4 wt. % and 13.6% wt. % silver, respectively, as AgNPs with a size of 50 nm [124]. In other studies, gold icosahedra of 4 nm in size were observed in M. sativa [125], and 2 nm of semi-spherical copper particles were observed in Iris pseudacorus [126] grown on the respective metal salt-containing substrates.
In recent years, various in vitro approaches have been developed in which plants extracts are being used as reducing agents for synthesis of NPs [123]. For this green synthesis of NPs, extracts of different plant species along with a variety of acids and metal salts, such as copper, gold, silver, platinum, iron have been used [127,128,129].The plant materials that have been used for NPs synthesis are more beneficial than microbial or chemical methods as there are no effects of microbes or hazardous chemical contamination. Moreover, it requires less energy and has easy and broader implications [130]. The green synthesis of NPs mediated by plant extracts involves the alleviation of metal ions [131] due to the presence of biomolecules such as phenols, terpenoids, ketones, carboxylic acids, aldehydes, enzymes, amides, and flavonoids [130,132,133,134,135]. The plant extracts prepared from their different parts such as roots, stems, barks, leaves, flowers, fruits and seeds have been used for green synthesis of NPs [136,137,138,139,140,141,142]. The plant extracts can function as reducing and stabilizing agents in the green synthesis of NPs [133].
A variety of green synthesized NPs from different plant species have been reported in last few years. For example, AgNPs have been synthesized from fruit extract of Phyllanthus emblica [130], leaves extract of Citrus limon [143], green tea (Camellia sinensis) [144], Coffea Arabica [145], neem (Azadirachta indica) [146], Acalypha indica [147], Aloe vera plant extract [148], latex of Jatropha gossypifolia [149], root extract of Morinda citrifolia [150], Phoenix dactylifera [151], inflorescence extract of Mangifera indica [152]. Zinc oxide NPs (ZnONPs) and titanium dioxide NPs (TiO2NPs) synthesized from extract of fresh lemon fruits (Citrus limon) showed antibacterial activity [133]. Zinc oxide NPs (ZnONPs) synthesized from extract of chamomile flower (Matricaria chamomilla), olive leave (Olea europaea) and red tomato fruit (Lycopersicon esculentum) showed antibacterial actions. Aqueous Rosemary extract was used to synthesize the MgO nano-flowers (MgONFs) having antibacterial potentials [132]. The quality, size and shape of these green synthesized NPs are dependent on various factors like plant extract concentrations and their compositions, metal salt concentration, reaction pH, reaction temperature [12,13,153]. Some NPs recently synthesized in a green way from plant materials used for plant pathogen management are listed in the Table 2.
Table 2.
Plants mediated synthesized metallic NPs against plant pathogens.
| Plants | Plant Parts Used | Metal NPs | Features | Plant Disease Management | References | ||
|---|---|---|---|---|---|---|---|
| Size (nm) | Shape | Pathogen | Host | ||||
| Citrus limon | Fruits | ZnO and TiO2 | 20–200 | Polymorphic | Dickeya dadantii | Sweet potato | [133] |
| Phyllanthu semblica | Fruits | Ag | 20–93 | Spherical | Acidovorax oryzae | Rice | [130] |
| Rosmarinus officinalis | Flowers | MgO | <20 | Flower | Xanthomonas oryzae pv. oryzae | Rice | [132] |
| Matricaria chamomilla | Flowers | MgO and MnO2 | 9–112 | Disk-shapedSpherical | Acidovorax oryzae | Rice | [134] |
| Matricaria chamomilla | Flowers | ZnO | 50–192 | Crystalline | Xanthomonas oryzae pv. oryzae | Rice | [7] |
| Olea europaea | Leaves | ZnO | 41–124 | Crystalline | Xanthomonas oryzae pv. oryzae | Rice | [7] |
| Lycopersicon esculentum | Fruits | ZnO | 66–133 | Crystalline | Xanthomonas oryzae pv. oryzae | Rice | [7] |
| Piper nigrum | Stem | Ag | 9–30 | crystalline | Erwinia cacticida | Watermelon | [154] |
| Citrus maxima | Fruits | Ag | 11–13 | Spherical | Acidovorax oryzae | Rice | [155] |
| Artemisia absinthium | Leaves | Ag | 5–100 | Spherical | Phytophthora parasitica | Citrus | [156] |
| Trachyspermum ammi | Leaves | Ni | 68 | Missing | Colletotrichum musae | Banana | [157] |
| Abelmoschus esculentus | Seed | Au | 45–75 | Spherical | Puccinia graminis pv. tritci | Wheat | [158] |
| Parthenium hysterophorus | Leaves | ZnO | 28–84 | Spherical andHexagonal | Fusarium culmorum | Barley | [159] |
| Syzygium aromaticum | Bud | Cu | 15 | Spherical | Aspergillus niger, Aspergillus flavus, and Penicillium spp. | Multiple crops | [160] |
3. Green Synthesized Metallic Nanoparticles for Control of Phytopathogens
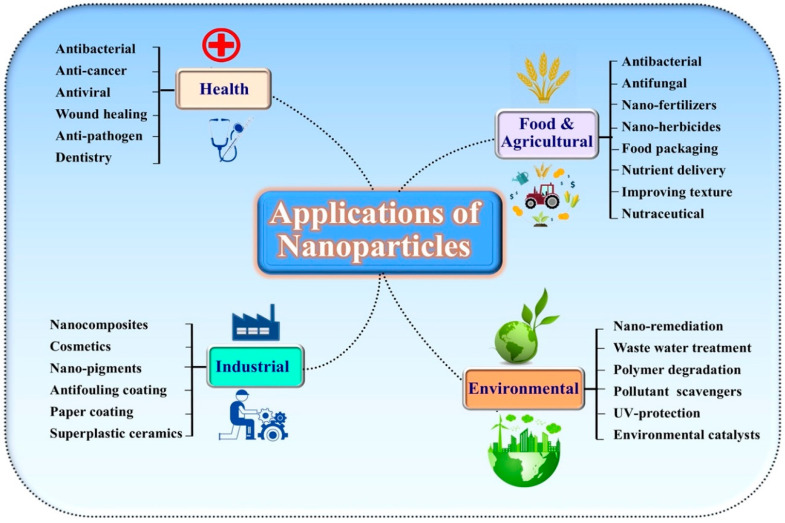
NPs have been used in various fields including plant diseases control [130,151] (Figure 2). Generally, pesticides are used selectively to the pests of the crop plants without affecting on plants themselves along with the symbiotic flora, fauna and human beings [161]. However, the indiscriminate applications of these pesticides tend to be the serious public concerns and environmental issues [162]. Moreover, the occurrence and development of new pathogenic strains is a continuous problem, the chemical application is expensive and also not always effective. Recently, the progress in the area of green synthesis has inspired scientists and researchers to utilize its potentials against the pathogenic microorganisms. Biosynthesized metallic NPs such as silver, copper, gold, and zinc, have been reported to act against both Gram-positive and Gram-negative bacteria such as B. subtilis, E. coli, and Staphylococcus aureus. These NPs showed inhibitory actions against some pathogenic fungi including A. niger, F. oxysporum, A. fumigatus, and inhibitory effects against other pathogenic microbes [163]. The silver NPs have received more attention due to “green synthesis” from plants, bacteria, fungi or yeasts [164]. Fouda et al. [43] observed the antimicrobial activity of green synthesized AgNPs from endophytic Streptomyces against four plant pathogenic fungi represented by Alternaria alternata, Fusarium oxysporum, Pythiumultimum, and Aspergillus niger. The green synthesized ZnONPs and TiO2NPs with lemon fruit extract at room temperature showed their antibacterial activities against Dickeya dadantii, a causal bacterium of sweet potato stem and root rot disease [133]. The MgO and MnO2 NPs synthesized using chamomile flower extract showed activity against Acidovorax oryzae strain RS-2, a bacterium causing bacterial brown stripe disease of rice. Nanotechnology has potential prospects in plant pathogenic management in various ways. The most commonly used method is the direct application of NPs to seeds or foliar spray against the plant pathogens, which are more greatly suppressed compared to chemical pesticides. In another method, nanomaterials are used as carrier materials of chemical active ingredients for controlled release of pesticides [1]. Here, we list the green synthesized metallic NPs with their size, shape, and targeting plant pathogens in Table 1 and Table 2.
Figure 2.
Application of nanoparticles in various fields.
4. Mechanisms of Action of Nanoparticles against Phytopathogens
Although green synthesized metallic NPs have been studied for their potentials against phytopathogens, the exact mode of actions of NPs is not completely understood [165]. To date, some mechanisms have been reported, such as protein dysfunction (e.g., oxidation of cysteine in Fe-binding site, destruction of Fe–S cluster, exchange of catalytic metal, and exchange of structural metal), production of reactive oxygen species(ROS) and antioxidant depletion, impaired membrane function (e.g., membrane damage, loss of membrane potential), interference with nutrient uptake (e.g., inhibition of Fe (III) transporter gene expression) and genotoxicity (e.g., double-strand breaks) [166]. These mechanisms may not operate individually, but function in combination against various phytopathogens [52,166].
The adhesion of NPs with microbial cell membrane occurs due to the electrostatic attraction between the negatively charged cell membrane of microbes and NPs with positive or low negative charges. The morphological structures of the membrane are disturbed by the NPs and the membrane depolarization causes the disruption of membrane permeability and respiratory actions, and ultimately damages the cell structures, leading to cell death. This disruption of cell structure leads to the leakage of internal cell content including proteins, enzymes, DNA, and metabolites. In addition, the NPs may cause irregular pits on the microbial cell wall that facilitate NPs’ entry into periplasmic space and inside the cells. NP actions on membrane damage and pit formation on the cell surface can be observed by using TEM and SEM [14]. Masum et al. observed the green synthesized AgNPs effects on Acidovorax oryzae strain RS-2 using TEM and showed highly ruptured cell walls, leakage of cytoplasmic and nucleic contents, swollen structure leading to bacterial death [112]. In another study, biosynthesized AgNPs were applied to Fusarium graminearum strain PH-1 and antifungal activities of distortion of hyphae and damaging cell walls were observed by both SEM and TEM [20]. Similar effects of AgNPs on several other phytopathogenic fungi such as Alternaria alternata, Botrytis cinera and Trichosporon asahii were also observed [167].
The toxicity of NPs may be caused by the formation of ROS [143]. Free radicals can damage the cell wall and various biomolecules such as proteins, lipids and DNA. DNA damages, such as mutations, deletions, single-strand breaks, double-strand breaks, and cross-linking with proteins, may occur [168]. Zhang et al. revealed that damaging cell membrane and generating ROS are involved in the antibacterial mechanisms [169]. They observed damage of cell membrane and AgNPs inside Azotobacter vinelandii cells using TEM and revealed AgNP-induced hydroxyl radicals inside bacterial cells using electron spin resonance.
5. Environmental Consequences of Metallic Nanoparticles
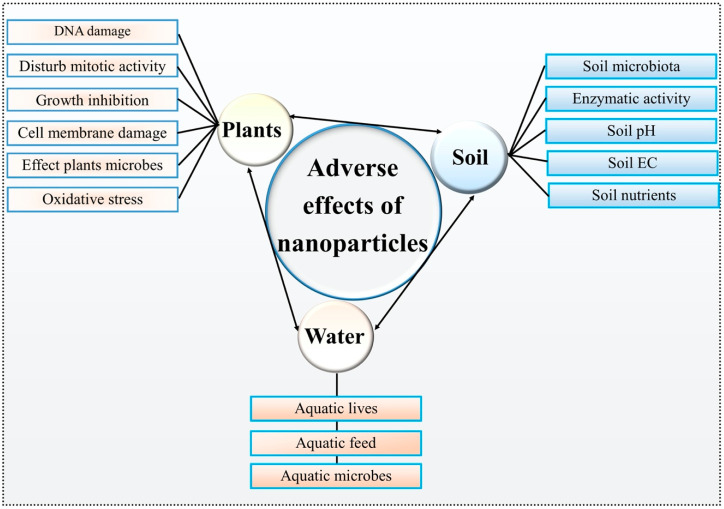
Several studies have revealed that NPs can be used to manage or control plant pathogens [5,6]. Besides this benefit, there may be some toxic or adverse effects of these NPs on various components of the environments (Figure 3). The NPs that have been used for plant pathogen management may be dispersed from the crop lands to the soil, water and atmosphere. The dispersion may take place through leaching, surface run-off by rain, and transport by air current or trophic transfer [170]. Different studies on this subject have suggested that NPs may be absorbed by microbes in the soils, sediments and plant roots. Later, these NPs are migrated from roots to other parts of the plants, and accumulation occurs [171]. Shifting of NPs from one trophic level to another trophic level takes place as the microbes, plant products or their waste materials are utilized or consumed by various organisms such as protozoa, arthropods, annelids, mollusks, fish, insects, birds and mammals [172,173]. This demonstrates that the adverse effects may be inherited by their offspring [174]. This scenario has also been observed in marine organisms [175] and also in food chains of plant-herbivore-carnivore [172,173,176,177]. Therefore, a standard application of nanomaterials in crop plants is needed for safe and sustainable use of nanotechnology in agriculture. For the management of plant pathogens, the application of NPs should be accompanied with the knowledge of possible risks for direct or indirect application to crop plants, and the ecosystem where the crop plants have interacted with microorganisms, animals or human beings. Below, we discuss the toxicity or adverse effects of metallic NPs on plants and beneficial microbes in more detail.
Figure 3.
Adverse effects of metallic NPs on major elements (plants, soil and water) in agroecosystems.
6. Toxicity of Metallic Nanoparticles on Plants
The ultra-small size of the NPs is the main cause to facilitate the inhibitory actions against the various plant pathogens. Indeed, this is the similar basic phenomenon of adverse effects of NPs on the surrounding environment, animals, plants, human and beneficial microorganisms [1]. The NPs can be applied to both the above-ground parts and below-ground parts of the plants to manage the plant diseases. In addition, seeds may be dipped into biosynthesized NPs solution to manage the seedling diseases and the pathogenic attack at a later stage. The applied NPs may inhibit the plant pathogens locally in various plant parts and also may become translocated throughout the plant system, thus reducing the occurrences of the disease. However, diversified NPs were subjected to plant toxicity due to their uptake, translocation and accumulation in the plant cells or organs [178]. The NPs uptake and translocation depends on multiple factors such as properties of NPs (size, composition and surface area), dose of application, delivery techniques, and plant species. The bioaccumulation may alter the plant physiology affecting the growth and development of plants [179]. Metal NPs are known to be highly toxic, even at low concentrations, although the NPs are rarely dispersed in the environment [180]. Research into NPs toxicity in crop plants is still in its infancy, but the application of innovative agro nanotechnology tools and products is crucial for agricultural development [181]. In most studies, for cultivated crop plants, such as tomato, wheat, onion, and zucchini, the excess metallic NPs may trigger oxidative burst through the interference of electron transport chain. It also can impair the ROS detoxifying process, resulting in genotoxic implications [182,183,184,185]. As a result, production of secondary metabolites and phytohormones are affected, and retarded plant growth occurs [186].
The toxicity and adverse effects that have been reported in plants include constraints in water transport in plant systems, decrease in the growth hormone production, metabolic disorder, oxidative stress, chromosomal abnormalities decreased growth, deviation in transcriptional profile of several genes, raising susceptibility to natural toxins (e.g., Arsenic) [170].
7. Toxicity of Metallic Nanoparticles on Beneficial Microbes Associated with Plants
Microbes are epiphytically and endophytically associated with plants, in rhizosphere soil, and bulk soil near the plant root system, and may promote plant growth through the production of phytohormone (e.g., auxin) and siderophore, nitrogen fixation, and phosphate solubilization [187]. NPs applied to plants and soils may be toxic to these beneficial microbes in the same manner as they are to the plant pathogens. The effects on soil microbial community can be assessed by the observation of respiration and enzymatic activities in soils [188]. For example, CuONPs and TiO2NPs reduced biomass, total phospholipid fatty acids and activities of enzymes, such as urease, phosphatase and dehydrogenase, of soil microbiota in a flooded rice field. The principle component analysis of phospholipid fatty acids and diversity indices indicated that CuONPs affected the microbial composition and diversity [189]. Similarly, CeO2, Fe3O4, TiO2 and ZnONPs had effects on bacterial community and activities of enzymes (catalase, invertase, phosphatase, and urease) in saline alkali and black soils [190]. ZnONPs and CeO2NPs reduced thermogenic metabolism, numbers of Azotobacter, phosphate solubilizing and potassium solubilizing bacteria, and enzyme activities in soil; TiO2NPs reduced the enzymatic activities and abundance of soil bacteria [191].
NPs can affect plant-associated and soil microbes beneficial to plants at the time for phytopathogen control. Chavan and Nadanathangam evaluated the effects of AgNPs and ZnONPs on nitrogen fixers (Rhizobium leguminosarum MTCC 10096, Sinorhizobium meliloti clsxc_S_SNF, Azotobacter chroococcum clsxc_A_FLNF), phosphate solubilizers (Arthrobacter sp. MTCC 8160, Bacillus sp. clsxc_ NPS, Serratia marcescens MTCC 7642, Pantoea dispersaclsxc_PSD) and biofilm formers (Bacillus subtilis MTCC 441, Bacillus sp. clsxc_TYA, Pseudomonas aeruginosa MTCC 7763, Klebsiellapneumonia clsxc_AZ2). AgNPs showed bactericidal effects at lower concentrations of 2–22 µg/mL whereas ZnONPs showed a bacteriostatic effects up to concentrations of 3000 µg/mL in some cases. Moreover, AgNPs significantly changed the bacterial community in soil [192]. Another study assessed the toxicity of ZnONPs and CeO2NPs against Sinorhizobium meliloti, a symbiotic alfalfa inhabiting bacterium using advanced microscopic and spectroscopic techniques. ZnONPs were more toxic than CeO2NPs according to the viable cell counts and inhibition of growth dynamics [193]. Microbial community analysis using culture-dependent and independent methods showed that ZnONPs altered the microbial community in soil [194].
8. Conclusions and Perspectives
Recent studies show that the biogenic or green-synthesized metallic NPs using microbes and plants without hazardous chemicals are promising in control plant pathogens including bacteria, fungi, oomycetes, and viruses. On the other hand, the biogenic metallic NPs have toxic potentials to plants and plant-associated beneficial microbes and ultimately to human beings. Therefore, the positive and negative effects of these NPs on agriculture and environments should be ascertained before their commercial use in plant disease management in the field.
To achieve the green control of plant diseases with the green-synthesized NPs, we should pay attention to the following aspects in the near future. (1) AgNPs is the major metallic NPs tested on plant pathogens and shows toxicities to plants and plant-associated microbes. Less phytotoxic metals, such as Zn, Fe, Mg, and Mn, should be tested. (2) Minimum inhibitory concentration of metallic NPs on pathogens should be determined in vitro and in vivo. (3) Metallic NP effects on plant growth and development at the working concentration should be determined. (4) Metallic NPs at the working concentration in vivo should be less toxic to other plant-associated microbes than the target pathogens; Metallic NP effects on plant microbiota should be determined. (5) The dynamic of the metallic NPs and their effects on soil microbiota should be determined. Few studies on plant disease control in fields by metallic NPs have been done. Future studies should determine the efficiency of metallic NPs in fields along with the assessment of their potential risks to environments and human health.
Author Contributions
Conceptualization, M.A.A., T.A., A.H., R.H. and B.L.; writing—original draft preparation, M.A.A.; writing—review and editing, M.A.A., T.A., W.W. and M.M.I.M., Y.W., G.S. and Q.A.; supervision, B.L.; funding acquisition, B.L., W.W. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research work is financially supported by Shanghai Agriculture Applied Technology Development Program (2019-02-08-00-08-F01150), Zhejiang Provincial Natural Science Foundation of China (LZ19C140002), Zhejiang Provincial Project (2017C02002, 2019C02006, 2020C02006), National Natural Science Foundation of China (31872017, 31571971, 31371904, 31801787, 31901925), National Key Research and Development Program of China (2017YFD0201104; 2018YFD0300900), Key Scientific Technological Project of Ningbo (2016C11017), the Fundamental Research Funds for the Central Universities, Dabeinong Funds for Discipline Development and Talent Training in Zhejiang University, State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products (grant number 2010DS700124-ZZ2014), Key Research and Development Program of Ningxia Hui Autonomous Region (2020BBF03004).
Conflicts of Interest
The authors declared that they have no conflict of interest to this work.
References
- 1.Mujeebur R.K., Tanveer F.R. Nanotechnology: Scope and Application in Plant Disease Management. Plant Pathol. J. 2014;13:214–231. [Google Scholar]
- 2.Mohammadlou M., Maghsoudi H., Jafarizadeh-Malmiri H. A review on green silver nanoparticles based on plants: Synthesis, potential applications and eco-friendly approach. Int. Food Res. J. 2016;232:446–463. [Google Scholar]
- 3.Mie R., Samsudin M.W., Din L.B., Ahmad A., Ibrahim N., Adnan S.N. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2014;9:121–127. doi: 10.2147/IJN.S52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaviya S., Santhanalakshmi J., Viswanathan B., Muthumary J., Srinivasan K. Biosynthesis of silver nanoparticles using Citrussinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011;79:594–598. doi: 10.1016/j.saa.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Elmer W., White J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018;56:111–133. doi: 10.1146/annurev-phyto-080417-050108. [DOI] [PubMed] [Google Scholar]
- 6.Worrall E.A., Hamid A., Mody K.T., Mitter N., Pappu H.R. Nanotechnology for Plant Disease Management. Agronomy. 2018;8:285. doi: 10.3390/agronomy8120285. [DOI] [Google Scholar]
- 7.Ogunyemi S.O., Abdallah Y., Zhang M., Fouad H., Hong X., Ibrahim E., Masum M.M.I., Hossain A., Mo J., Li B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019;47:341–352. doi: 10.1080/21691401.2018.1557671. [DOI] [PubMed] [Google Scholar]
- 8.Hossain A., Hong X., Ibrahim E., Li B., Sun G., Meng Y., Wang Y., An Q. Green Synthesis of Silver nanoparticles with Culture Supernatant of a Bacterium Pseudomonas rhodesiae and Their Antibacterial Activity against Soft Rot Pathogen Dickeya dadantii. Molecules. 2019;24:2303. doi: 10.3390/molecules24122303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhuper S., Panda D., Nayak P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano Trends J. Nanotechnol. Appl. 2012;13:16–22. [Google Scholar]
- 10.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. doi: 10.1039/c1gc15386b. [DOI] [Google Scholar]
- 11.Korbekandi H., Mohseni S., Mardani Jouneghani R., Pourhossein M., Iravani S. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif. Cells Nanomed. Biotechnol. 2016;44:235–239. doi: 10.3109/21691401.2014.937870. [DOI] [PubMed] [Google Scholar]
- 12.Mittal A.K., Chisti Y., Banerjee U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013;31:346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Shah M., Fawcett D., Sharma S., Tripathy S.K., Poinern G.E.J. Green Synthesis of Metallic nanoparticles via Biological Entities. Materials (Basel) 2015;8:7278–7308. doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gahlawat G., Choudhury A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019;9:12944–12967. doi: 10.1039/C8RA10483B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakkar K.N., Mhatre S.S., Parikh R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6:257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed S., Chaudhry S.A., Ikram S. A review on biogenic synthesis of ZnO nanoparticles usingv plant extracts and microbes: A prospect towards green chemistry. J. Photoch. Photobiol. B. 2017;166:272–284. doi: 10.1016/j.jphotobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Singh J., Dutta T., Kim K.H., Rawat M., Samddar P., Kumar P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018;16:84. doi: 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed T., Shahid M., Noman M., Niazi M.B.K., Mahmood F., Manzoor I., Zhang Y., Li B., Yang Y., Yan C., et al. Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens. 2020;9:160. doi: 10.3390/pathogens9030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh P., Kim Y.J., Zhang D., Yang D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim E., Zhang M., Zhang Y., Hossain A., Qiu W., Chen Y., Wang Y., Wu W., Sun G., Li B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusariumgraminearum. Nanomaterials (Basel) 2020;10:219. doi: 10.3390/nano10020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manish S., Priya M., Anjali S., Girish K.G. Green Nanoparticles: Synthesis and Applications. IOSR J. Biotechnol. Biochem. 2018;4:78–83. [Google Scholar]
- 22.Bridges K., Kidson A., Lowbury E.J., Wilkins M.D. Gentamicin- and silver-resistant Pseudomonas in a burns unit. Br. Med. J. 1979;1:446–449. doi: 10.1136/bmj.1.6161.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haefeli C., Franklin C., Hardy K. Plasmid-determined silver resistance in Pseudomonasstutzeri isolated from a silver mine. J. Bacteriol. 1984;158:389–392. doi: 10.1128/JB.158.1.389-392.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brock T.D., Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl. Environ. Microbiol. 1976;32:567–571. doi: 10.1128/AEM.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iravani S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014;2014:359316. doi: 10.1155/2014/359316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad T.N.V.K.V., Kambala V.S.R., Naidu R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophoramoniliformis and their characterisation. J. Appl. Phycol. 2013;25:177–182. doi: 10.1007/s10811-012-9851-z. [DOI] [Google Scholar]
- 27.Saratale R.G., Karuppusamy I., Saratale G.D., Pugazhendhi A., Kumar G., Park Y., Ghodake G.S., Bharagava R.N., Banu J.R., Shin H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces. 2018;170:20–35. doi: 10.1016/j.colsurfb.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 28.Mishra S., Singh B.R., Naqvi A.H., Singh H.B. Potential of biosynthesized silver nanoparticles using Stenotrophomonas sp. BHU-S7 (MTCC 5978) for management of soil-borne and foliar phytopathogens. Sci. Rep. 2017;7:45154. doi: 10.1038/srep45154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed E., Kalathil S., Shi L., Alharbi O., Wang P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J. Saudi Chem. Soc. 2018;22:919–929. doi: 10.1016/j.jscs.2018.02.002. [DOI] [Google Scholar]
- 30.Zonaro E., Piacenza E., Presentato A., Monti F., Dell’Anna R., Lampis S., Vallini G. Ochrobactrum sp. MPV1 from a dump of roasted pyrites can be exploited as bacterial catalyst for the biogenesis of selenium and tellurium nanoparticles. Microb. Cell Fact. 2017;16:215. doi: 10.1186/s12934-017-0826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhotra A., Dolma K., Kaur N., Rathore Y.S., Ashish Mayilraj S., Choudhury A.R. Biosynthesis of gold and silver nanoparticles using a novel marine strain of Stenotrophomonas. Bioresour. Technol. 2013;142:727–731. doi: 10.1016/j.biortech.2013.05.109. [DOI] [PubMed] [Google Scholar]
- 32.Kaur H., Dolma K., Kaur N., Malhotra A., Kumar N., Dixit P., Sharma D., Mayilraj S., Choudhury A.R. Marine microbe as nano-factories for copper biomineralization. Biotechnol. Bioprocess. Eng. 2015;20:51–57. doi: 10.1007/s12257-014-0432-7. [DOI] [Google Scholar]
- 33.Klaus-Joerger T., Joerger R., Olsson E., Granqvist C. Bacteria as workers in the living factory: Metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001;19:15–20. doi: 10.1016/S0167-7799(00)01514-6. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava S.K., Constanti M. Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li) by Pseudomonasaeruginosa SM1. J. Nanopart. Res. 2012;14:831. doi: 10.1007/s11051-012-0831-7. [DOI] [Google Scholar]
- 35.Sneha K., Sathishkumar M., Mao J., Kwak I.S., Yun Y.S. Corynebacteriumglutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. 2010;162:989–996. doi: 10.1016/j.cej.2010.07.006. [DOI] [Google Scholar]
- 36.Jayaseelan C., Rahuman A.A., Kirthi A.V., Marimuthu S., Santhoshkumar T., Bagavan A., Gaurav K., Karthik L., Rao K.V. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012;90:78–84. doi: 10.1016/j.saa.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Hulkoti N.I., Taranath T.C. Biosynthesis of nanoparticles using microbe—A review. Colloids Surf. B Biointerfaces. 2014;121:474–483. doi: 10.1016/j.colsurfb.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Ramanathan R., O’Mullane A.P., Parikh R.Y., Smooker P.M., Bhargava S.K., Bansal V. Bacterial kinetics-controlled shape-directed biosynthesis of silver nanoplates using Morganella psychrotolerans. Langmuir. 2011;27:714–719. doi: 10.1021/la1036162. [DOI] [PubMed] [Google Scholar]
- 39.Nawani N., Aigle B., Mandal A., Bodas M., Ghorbel S., Prakash D. Actinomycetes: Role in biotechnology and medicine. BioMed Res. Int. 2013;2013:687190. doi: 10.1155/2013/687190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash D., Nawani N., Prakash M., Bodas M., Mandal A., Khetmalas M., Kapadnis B. Actinomycetes: A repertory of green catalysts with a potential revenue resource. Biomed. Res. Int. 2013;2013:264020. doi: 10.1155/2013/264020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramani R., Albersberg W. Marine actinomycetes: An ongoing source of novel bioactive metabolites. Microbiol. Res. 2012;167:571–580. doi: 10.1016/j.micres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Hassan S.E., Fouda A., Radwan A.A., Salem S.S., Barghoth M.G., Awad M.A., Abdo A.M., El-Gamal M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019;24:377–393. doi: 10.1007/s00775-019-01654-5. [DOI] [PubMed] [Google Scholar]
- 43.Fouda A., Hassan S.E., Abdo A.M., El-Gamal M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020;195:707–724. doi: 10.1007/s12011-019-01883-4. [DOI] [PubMed] [Google Scholar]
- 44.Otari S.V., Patil R.M., Nadaf N.H., Ghosh S.J., Pawar S.H. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012;72:92–94. doi: 10.1016/j.matlet.2011.12.109. [DOI] [Google Scholar]
- 45.Ranjitha V.R., Rai V.R. Actinomycetes mediated synthesis of gold nanoparticles from the culture supernatant of Streptomyces griseoruber with special reference to catalytic activity. 3 Biotech. 2017;7:299. doi: 10.1007/s13205-017-0930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bérdy J. Bioactive microbial metabolites. J. Antibiot. (Tokyo) 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 47.Gade A.K., Bonde P., Ingle A.P., Marcato P.D., Durán N., Rai M.K. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy. 2008;2:243–247. doi: 10.1166/jbmb.2008.401. [DOI] [Google Scholar]
- 48.Ahluwalia V., Kumar J., Sisodia R., Shakil N.A., Walia S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bioefficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crops Prod. 2014;55:202–206. doi: 10.1016/j.indcrop.2014.01.026. [DOI] [Google Scholar]
- 49.Azmath P., Baker S., Rakshith D., Satish S. Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharm. J. 2016;24:140–146. doi: 10.1016/j.jsps.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan N.T., Khan M.J., Jameel J., Jameel N., Rheman S.U.A. An overview: Biological organisms that serves as nanofactories for metallic nanoparticles synthesis and fungi being the most appropriate. Bioceram Dev. Appl. 2017;7:101. doi: 10.4172/2090-5025.1000101. [DOI] [Google Scholar]
- 51.Vahabi K., Mansoori G.A., Karimi S. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei. Insci. J. 2011;1:65–79. doi: 10.5640/insc.010165. [DOI] [Google Scholar]
- 52.Alghuthaymi M.A., Almoammar H., Rai M., Said-Galiev E., AbdElsalam K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015;29:221–236. doi: 10.1080/13102818.2015.1008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guilger-Casagrande M., de Lima R. Synthesis of Silver nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019;7:287. doi: 10.3389/fbioe.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabri M.A., Umer A., Awan G.H., Hassan M.F., Hasnain A. Selection of suitable biological method for the synthesis of silver nanoparticles. Nanomater. Nanotechnol. 2016;6:1–20. doi: 10.5772/62644. [DOI] [Google Scholar]
- 55.Costa Silva L.P., Oliveira J.P., Keijok W.J., Silva A.R., Aguiar A.R., Guimarães M.C.C., Braga F.R. Extracellular biosynthesis of silver nanoparticles using the cell-free filtrate of nematophagus fungus Duddingtonia flagans. Int. J. Nanomed. 2017;12:6373–6381. doi: 10.2147/IJN.S137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gudikandula K., Vadapally P., Charya M.A.S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano. 2017;2:64–78. doi: 10.1016/j.onano.2017.07.002. [DOI] [Google Scholar]
- 57.Castro-Longoria E., Vilchis-Nestor A.R., Avalos-Borja M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B Biointerfaces. 2011;83:42–48. doi: 10.1016/j.colsurfb.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 58.Rajput S., Werezuk R., Lange R.M., Mcdermott M.T. Fungal isolate optimized for biogenesis of silver nanoparticles with enhanced colloidal stability. Langmuir. 2016;32:8688–8697. doi: 10.1021/acs.langmuir.6b01813. [DOI] [PubMed] [Google Scholar]
- 59.Molnár Z., Bódai V., Szakacs G., Erdélyi B., Fogarassy Z., Sáfrán G., Varga T., Kónya Z., Tóth-Szeles E., Szűcs R., et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018;8:3943. doi: 10.1038/s41598-018-22112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmad A., Senapati S., Khan M.I., Kumar R., Sastry M. Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J. Biomed. Nanotechnol. 2005;1:47–53. doi: 10.1166/jbn.2005.012. [DOI] [Google Scholar]
- 61.Birla S.S., Gaikwad S.C., Gade A.K., Rai M.K. Rapid Synthesis of Silver Nanoparticles from Fusarium oxysporum by Optimizing Physicocultural Conditions. Sci. World J. 2013;2013:796018. doi: 10.1155/2013/796018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena J., Sharma P.K., Sharma M.M., Singh A. Process optimization for green synthesis of silver nanoparticles by Sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. Springerplus. 2016;5:861. doi: 10.1186/s40064-016-2558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang M., Wei S., Jian-Xin L., Xiao-Xi Z., Zhi H., Wen L., Liu Z.C., Tang J.X. Optimization for extracellular biosynthesis of silver nanoparticles by Penicilliumaculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater. Sci. Eng. 2017;C77:963–971. doi: 10.1016/j.msec.2017.03.294. [DOI] [PubMed] [Google Scholar]
- 64.AbdelRahim K., Mahmoud S.Y., Ali A.M., Almaary K.S., Mustafa A.E.-Z.M.A., Husseiny S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopusstolonifer. Saudi J. Biol. Sci. 2017;24:208–216. doi: 10.1016/j.sjbs.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Husseiny S.M., Salah T.A., Anter H.A. Biosynthesis of size controlled silver nanoparticles by Fusariumoxysporum, their antibacterial and antitumor activities. Beni-Suef Univ. J. Basic Appl. Sci. 2015;4:225–231. doi: 10.1016/j.bjbas.2015.07.004. [DOI] [Google Scholar]
- 66.Banu A., Rathod V. Synthesis and characterization of silver nanoparticles by Rhizopus stolonier. Int. J. Biomed. Adv. Res. 2011;2:148–158. doi: 10.7439/ijbar.v2i5.30. [DOI] [Google Scholar]
- 67.Kathiravan V., Ravi S., Ashokkumar S. Synthesis of silver nanoparticles from Meliadubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;130:116–121. doi: 10.1016/j.saa.2014.03.107. [DOI] [PubMed] [Google Scholar]
- 68.Dameron C.T., Reese R.N., Mehra R.K., Kortan A.R., Carroll P.J., Steigerwald M.L., Brus L.E., Winge D.R. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature. 1989;338:596–597. doi: 10.1038/338596a0. [DOI] [Google Scholar]
- 69.Kowshik M., Deshmukh N., Vogel W., Urban J., Kulkarni S.K., Paknikar K.M. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol. Bioeng. 2002;78:583–588. doi: 10.1002/bit.10233. [DOI] [PubMed] [Google Scholar]
- 70.Siddique S., Rovina K., Al Azad S., Naher L., Suryani S., Chaikaew P. Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: A review. J. Microb. Biochem. Technol. 2015;7:384–393. doi: 10.4172/1948-5948.1000243. [DOI] [Google Scholar]
- 71.Apte M., Sambre D., Gaikawad S., Joshi S., Bankar A., Kumar A.R., Zinjarde S. Psychrotrophic yeast Yarrowialipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express. 2013;3:32. doi: 10.1186/2191-0855-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gericke M., Pinches A. Microbial production of gold nanoparticles. Gold Bull. 2006;39:22–28. doi: 10.1007/BF03215529. [DOI] [Google Scholar]
- 73.Waghmare S.R., Mulla M.N., Marathe S.R., Sonawane K.D. Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech. 2015;5:33–38. doi: 10.1007/s13205-014-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.do Nascimento J.M., de Oliveira J.D., de Lima Rizzo A.C., Leite S.G.F. Biogenic Production of Copper nanoparticles by Saccharomycescerevisiae. J. Bionanosci. 2018;12:689–693. doi: 10.1166/jbns.2018.1583. [DOI] [Google Scholar]
- 75.Khan A.A., Fox E.K., Górzny M.Ł., Nikulina E., Brougham D.F., Wege C., Bittner A.M. pH Control of the Electrostatic Binding of Gold and Iron Oxide nanoparticles to Tobacco Mosaic Virus. Langmuir. 2013;29:2094–2098. doi: 10.1021/la3044126. [DOI] [PubMed] [Google Scholar]
- 76.Steinmetz N.F., Manchester M. Viral Nanoparticles: Tools for Materials Science and Biomedicine. Pan Stanford Publishing; Singapore: 2011. [Google Scholar]
- 77.Douglas T., Young M. Host–guest encapsulation of materials by assembled virus protein cages. Nature. 1998;393:152–155. doi: 10.1038/30211. [DOI] [Google Scholar]
- 78.Lee Y.J., Yi H., Kim W.J., Kang K., Yun D.S., Strano M.S., Ceder G., Belcher A.M. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus gene. Science. 2009;324:1051–1055. doi: 10.1126/science.1171541. [DOI] [PubMed] [Google Scholar]
- 79.Nam K.T., Kim D.-W., Yoo P.J., Chiang C.-Y., Meethong N., Hammond P.T., Chiang Y.-M., Belcher A.M. Virus-Enabled Synthesis and Assembly of Nanowires for Lithium Ion Battery Electrodes. Science. 2006;312:885–888. doi: 10.1126/science.1122716. [DOI] [PubMed] [Google Scholar]
- 80.Mao C., Solis D.J., Reiss B.D., Kottmann S.T., Sweeney R.Y., Hayhurst A., Georgiou G., Iverson B., Belcher A.M. Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires. Science. 2004;303:213–217. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- 81.Merzlyak A., Lee S.-W. Phage as templates for hybrid materials and mediators for nanomaterial synthesis. Curr. Opin. Chem. Biol. 2006;10:246–252. doi: 10.1016/j.cbpa.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Douglas T., Young M. Viruses: Making Friends with Old Foes. Science. 2006;312:873–875. doi: 10.1126/science.1123223. [DOI] [PubMed] [Google Scholar]
- 83.Sirotkin S., Mermet A., Bergoin M., Ward V., Van Etten J.L. Viruses as Nanoparticles: Structure versus collective dynamics. Phys. Rev. E. 2014;90:022718. doi: 10.1103/PhysRevE.90.022718. [DOI] [PubMed] [Google Scholar]
- 84.Bothner B., Dong X.F., Bibbs L., Johnson J.E., Siuzdak G. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. J. Biol. Chem. 1998;273:673–676. doi: 10.1074/jbc.273.2.673. [DOI] [PubMed] [Google Scholar]
- 85.Blum A.S., Soto C.M., Wilson C.D., Cole J.D., Kim M. Cowpea mosaic virus as a scaffold for 3-D patterning of gold nanoparticles. Nano Lett. 2004;4:867–870. doi: 10.1021/nl0497474. [DOI] [Google Scholar]
- 86.Thangavelu R.M., Ganapathy R., Ramasamy P., Krishnan K. Fabrication of virus metal hybrid nanomaterials: An ideal reference for bio semiconductor. Arabian J. Chem. 2020;13:2750–2765. doi: 10.1016/j.arabjc.2018.07.006. [DOI] [Google Scholar]
- 87.Kale A., Bao Y., Zhou Z., Prevelige P.E., Gupta A. Directed self-assembly of CdS quantum dots on bacteriophage P22 coat protein templates. Nanotechnology. 2013;24:045603. doi: 10.1088/0957-4484/24/4/045603. [DOI] [PubMed] [Google Scholar]
- 88.Love A.J., Makarov V., Yaminsky I., Kalinina N.O., Taliansky M.E. The use of tobacco mosaic virus and cowpea mosaic virus for the production of novel metal nanomaterials. Virology. 2014;449:133–139. doi: 10.1016/j.virol.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y., Dong Y., Zhou J., Li X., Wang F. Application of Plant Viruses as a Biotemplate for Nanomaterial Fabrication. Molecules. 2018;23:2311. doi: 10.3390/molecules23092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park J.P., Do M., Jin H.E., Lee S.W., Lee H. M13 bacteriophage displaying DOPA on surfaces: Fabrication of various nanostructured inorganic materials without time-consuming screening processes. ACS Appl. Mater. Interfaces. 2014;6:18653–18660. doi: 10.1021/am506873g. [DOI] [PubMed] [Google Scholar]
- 91.Khanna P., Kaur A., Goyal D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods. 2019;163:105656. doi: 10.1016/j.mimet.2019.105656. [DOI] [PubMed] [Google Scholar]
- 92.Dahoumane S.A., Mechouet M., Alvarez F.J., Agathos S.N., Jeffryes C. Microalgae: An outstanding tool in nanotechnology. Bionatura. 2016;1:196–201. doi: 10.21931/RB/2016.01.04.7. [DOI] [Google Scholar]
- 93.Ali D.M., Sasikala M., Gunasekaran M., Thajuddin N. Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, Oscillatoria willei NTDM01. Dig. J. Nanomater. 2011;6:285–290. [Google Scholar]
- 94.Prasad R., Pandey R., Barman I. Engineering tailored nanoparticles with microbes: Quo vadis? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:316–330. doi: 10.1002/wnan.1363. [DOI] [PubMed] [Google Scholar]
- 95.Prasad T.N.V.K.V., Elumalai E.K. Marine Algae Mediated Synthesis of Silver Nanopaticles using Scaberia agardhiiGreville. J. Biol. Sci. 2013;13:566–569. doi: 10.3923/jbs.2013.566.569. [DOI] [Google Scholar]
- 96.Namvar F., Azizi S., Ahmad M.B., Shameli K., Mohamad R., Mahdavi M., Tahir P.M. Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum. Res. Chem. Intermed. 2015;41:5723–5730. doi: 10.1007/s11164-014-1696-4. [DOI] [Google Scholar]
- 97.Patel V., Berthold D., Puranik P., Gantar M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015;5:112–119. doi: 10.1016/j.btre.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agarwal P., Gupta R., Agarwal N. Advances in Synthesis and Applications of Microalgal nanoparticles for Wastewater Treatment. J. Nanotechnol. 2019;2019:7392713. doi: 10.1155/2019/7392713. [DOI] [Google Scholar]
- 99.Brayner R., Barberousse H., Hemadi M., Djedjat C., Yéprémian C., Coradin T., Livage J., Fiévet F., Couté A. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J. Nanosci. Nanotechnol. 2007;7:2696–2708. doi: 10.1166/jnn.2007.600. [DOI] [PubMed] [Google Scholar]
- 100.El-Sheekh M.M., El-Kassas H.Y. Algal production of nano-silver and gold: Their antimicrobial and cytotoxic activities: A review. J. Genet. Eng. Biotechnol. 2016;14:299–310. doi: 10.1016/j.jgeb.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie J., Lee J.Y., Wang D.I., Ting Y.P. Silver nanoplates: From biological to biomimetic synthesis. ACS Nano. 2007;1:429–439. doi: 10.1021/nn7000883. [DOI] [PubMed] [Google Scholar]
- 102.Luangpipat T., Beattie I.R., Chisti Y., Haverkamp R.G. Gold nanoparticles produced in a microalga. J. Nanopart. Res. 2011;13:6439–6445. doi: 10.1007/s11051-011-0397-9. [DOI] [Google Scholar]
- 103.Arsiya F., Sayadi M.H., Sobhani S. Green synthesis of palladium nanoparticles using Chlorellavulgaris. Mater. Lett. 2017;186:113–115. doi: 10.1016/j.matlet.2016.09.101. [DOI] [Google Scholar]
- 104.Merin D.D., Prakash S., Bhimba B.V. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac. J. Trop. Med. 2010;3:797–799. doi: 10.1016/S1995-7645(10)60191-5. [DOI] [Google Scholar]
- 105.Ibrahim E., Fouad H., Zhang M., Zhang Y., Qiu W., Yan C., Li B., Mo J., Chen J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019;9:29293–29299. doi: 10.1039/C9RA04246F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gopinath V., Velusamy P. Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusariumoxysporum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;106:170–174. doi: 10.1016/j.saa.2012.12.087. [DOI] [PubMed] [Google Scholar]
- 107.Mishra S., Singh B.R., Singh A., Keswani C., Naqvi A.H., Singh H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE. 2014;9:e97881. doi: 10.1371/journal.pone.0097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaur P., Thakur R., Duhan J.S., Chaudhury A. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicerarietinum) J. Chem. Technol. Biotechnol. 2018;93:3233–3243. doi: 10.1002/jctb.5680. [DOI] [Google Scholar]
- 109.Hassan S.E.L.D., Salem S.S., Fouda A., Awad M.A., El-Gamal M.S., Abdo A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018;11:262–270. doi: 10.1016/j.jrras.2018.05.003. [DOI] [Google Scholar]
- 110.Ponmurugan P., Manjukarunambika K., Elango V., Gnanamangai B.M. Antifungal activity of biosynthesised copper nanoparticles evaluated against red root-rot disease in tea plants. J. Exp. Nanosci. 2016;11:1019–1031. doi: 10.1080/17458080.2016.1184766. [DOI] [Google Scholar]
- 111.Jain D., Kothari S. Green synthesis of silver nanoparticles and their application in plant virus inhibition. J. Mycol. Plant Pathol. 2014;44:21–24. [Google Scholar]
- 112.Elbeshehy E.K.F., Elazzazy A.M., Aggelis G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp.; nanoparticle characterization and their activity against Bean Yellow Mosaic Virus and human pathogens. Front. Microbiol. 2015;6:453. doi: 10.3389/fmicb.2015.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El-Moslamy S.H., Elkady M.F., Rezk A.H., Abdel-Fattah Y.R. Applying Taguchi design and large-scale strategy for mycosynthesis of nano-silver from endophytic Trichoderma harzianum SYA.F4 and its application against phytopathogens. Sci. Rep. 2017;7:45297. doi: 10.1038/srep45297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Al-Zubaidi S., Alayafi A.A., Abdelkader H.S. Biosynthesis, Characterization and Antifungal Activity of Silver Nanoparticles by Aspergillus niger Isolate. J. Nanotechnol. Res. 2019;1:23–36. doi: 10.26502/jnr.2688-8521002. [DOI] [Google Scholar]
- 115.Almaary K.S., Sayed S.R.M., Abd-Elkader O.H., Dawoud T.M., El Orabi N.F., Elgorban A.M. Complete green synthesis of silver- nanoparticles applying seed-borne Penicilliumduclauxii. Saudi J. Biol. Sci. 2020;27:133–1339. doi: 10.1016/j.sjbs.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akther T., Hemalatha S. Mycosilver Nanoparticles: Synthesis, Characterization and its Efficacy against Plant Pathogenic Fungi. BioNanoScience. 2019;9:296–301. doi: 10.1007/s12668-019-0607-y. [DOI] [Google Scholar]
- 117.Elamawi R.M., Al-Harbi R.E., Hendi A.A. Biosynthesis and characterization of silver nanoparticles using Trichodermalongibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control. 2018;28:28. doi: 10.1186/s41938-018-0028-1. [DOI] [Google Scholar]
- 118.Guilger M., Pasquoto-Stigliani T., Bilesky-Jose N., Grillo R., Abhilash P.C., Fraceto L.F., Lima R. Biogenic silver nanoparticles based on Trichodermaharzianum: Synthesis, characterization, toxicity evaluation and biological activity. Sci. Rep. 2017;7:44421. doi: 10.1038/srep44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balakumaran M.D., Ramachandran R., Kalaichelvan P.T. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 2015;178:9–17. doi: 10.1016/j.micres.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 120.Xue B., He D., Gao S., Wang D., Yokoyama K., Wang L. Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016;11:1899–1906. doi: 10.2147/IJN.S98339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elgorban A.M., Aref S.M., Seham S.M., Elhindi K.M., Bahkali A.H., Sayed S.R., Manal M.A. Extracellular synthesis of silver nanoparticles using Aspergillusversicolor and evaluation of their activity on plant pathogenic fungi. Mycosphere. 2016;7:844–852. doi: 10.5943/mycosphere/7/6/15. [DOI] [Google Scholar]
- 122.Abd El-Aziz A.R.M., Al-Othman M.R., Mahmoud M.A., Metwaly H.A. Biosynthesis of silver nanoparticles using Fusariumsolani and its impact on grain borne fungi. Dig.J. Nanomater. Biostruct. 2015;10:655–662. [Google Scholar]
- 123.Makarov V.V., Love A.J., Sinitsyna O.V., Makarova S.S., Yaminsky I.V., Taliansky M.E., Kalinina N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014;6:35–44. doi: 10.32607/20758251-2014-6-1-35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harris A.T., Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanoparticle Res. 2008;10:691–695. doi: 10.1007/s11051-007-9288-5. [DOI] [Google Scholar]
- 125.Gardea-Torresdey J.L., Parsons J.G., Gomez E., Peralta-Videa J., Troiani H.E., Santiago P., Yacaman M.J. Formation and Growth of Au nanoparticles inside Live Alfalfa Plants. Nano Lett. 2002;2:397–401. doi: 10.1021/nl015673+. [DOI] [Google Scholar]
- 126.Manceau A., Nagy K.L., Marcus M.A., Lanson M., Geoffroy N., Jacquet T., Kirpichtchikova T. Formation of metallic copper nanoparticles at the soil-root interface. Environ. Sci. Technol. 2008;42:1766–1772. doi: 10.1021/es072017o. [DOI] [PubMed] [Google Scholar]
- 127.Ghosh S., Patil S., Ahire M., Kitture R., Gurav D.D., Jabgunde A.M., Kale S., Pardesi K., Shinde V., Bellare J., et al. Gnidiaglauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J. Nanobiotechnol. 2012;10:17. doi: 10.1186/1477-3155-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khan M., Khan M., Adil S.F., Tahir M.N., Tremel W., Alkhathlan H.Z., Al-Warthan A., Siddiqui M.R. Green synthesis of silver nanoparticles mediated by Pulicariaglutinosa extract. Int. J. Nanomed. 2013;8:1507–1516. doi: 10.2147/IJN.S43309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rai M., Yadav A. Plants as potential synthesiser of precious metal nanoparticles: Progress and prospects. IET Nanobiotechnol. 2013;7:117–124. doi: 10.1049/iet-nbt.2012.0031. [DOI] [PubMed] [Google Scholar]
- 130.Masum M.M.I., Siddiqa M.M., Ali K.A., Zhang Y., Abdallah Y., Ibrahim E., Qiu W., Yan C., Li B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019;10:820. doi: 10.3389/fmicb.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rai M., Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- 132.Abdallah Y., Ogunyemi S.O., Abdelazez A., Zhang M., Hong X., Ibrahim E., Hossain A., Fouad H., Li B., Chen J. The Green Synthesis of MgO Nano-Flowers Using Rosmarinusofficinalis L. (Rosemary) and the Antibacterial Activities against Xanthomonas oryzaepv. oryzae. BioMed Res. Int. 2019;2019:5620989. doi: 10.1155/2019/5620989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hossain A., Abdallah Y., Ali M.A., Masum M.M.I., Li B., Sun G., Meng Y., Wang Y., An Q. Lemon-Fruit-Based Green Synthesis of Zinc Oxide nanoparticles and Titanium Dioxide nanoparticles against Soft Rot Bacterial Pathogen Dickeyadadantii. Biomolecules. 2019;9:863. doi: 10.3390/biom9120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ogunyemi S.O., Zhang F., Abdallah Y., Zhang M., Wang Y., Sun G., Qiu W., Li B. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria chamomilla L. extract and its inhibitory effect on Acidovoraxoryzae strain RS-2. Artif. Cells Nanomed. Biotechnol. 2019;47:2230–2239. doi: 10.1080/21691401.2019.1622552. [DOI] [PubMed] [Google Scholar]
- 135.Prabhu S., Poulose E.K. Silver Nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012;2:32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- 136.Raju D., Mehta U.J., Ahmad A. Simple Recovery of Intracellular Gold nanoparticles from Peanut Seedling Roots. J. Nanosci. Nanotechnol. 2015;15:1575–1581. doi: 10.1166/jnn.2015.9046. [DOI] [PubMed] [Google Scholar]
- 137.Karthiga P. Preparation of silver nanoparticles by Garciniamangostana stem extract and investigation of the antimicrobial properties. Biotechnol. Res. Innov. 2018;2:30–36. doi: 10.1016/j.biori.2017.11.001. [DOI] [Google Scholar]
- 138.Rajeshkumar S. Synthesis of silver nanoparticles using fresh bark of Pongamia pinnata and characterization of its antibacterial activity against gram positive and gram negative pathogens. Resour. Eff. Technol. 2016;2:30–35. doi: 10.1016/j.reffit.2016.06.003. [DOI] [Google Scholar]
- 139.Yasir M., Singh J., Tripathi M.K., Singh P., Shrivastava R. Green Synthesis of Silver Nanoparticles Using Leaf Extract of Common Arrowhead Houseplant and Its Anticandidal Activity. Pharmacogn. Mag. 2018;13:S840–S844. doi: 10.4103/pm.pm_226_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moteriya P., Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpiniapulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif. Cells Nanomed. Biotechnol. 2017;45:1556–1567. doi: 10.1080/21691401.2016.1261871. [DOI] [PubMed] [Google Scholar]
- 141.Pilaquinga F., Morejón B., Ganchala D., Morey J., Piña N., Debut A., Neira M. Green synthesis of silver nanoparticles using Solanum mammosum L. (Solanaceae) fruit extract and their larvicidal activity against Aedes aegypti L. (Diptera: Culicidae) PLoS ONE. 2019;14:e0224109. doi: 10.1371/journal.pone.0224109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rautela A., Rani J., Debnath M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019;10:5. doi: 10.1186/s40543-018-0163-z. [DOI] [Google Scholar]
- 143.Vankar P.S., Shukla D. Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl. Nanosci. 2012;2:163–168. doi: 10.1007/s13204-011-0051-y. [DOI] [Google Scholar]
- 144.Nakhjavani M., Mohsen Sarafraz M., Nikkhah V., Shoja S., Sarafraz M. Green synthesis of silver nanoparticles using green tea leaves: Experimental study on the morphological, rheological and antibacterial behaviour. Heat Mass Transfer. 2017;53:3201–3209. doi: 10.1007/s00231-017-2065-9. [DOI] [Google Scholar]
- 145.Dhand V., Soumya L., Bharadwaj S., Chakra S., Bhatt D., Sreedhar B. Green synthesis of silver nanoparticles using Coffeaarabica seed extract and its antibacterial activity. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016;58:36–43. doi: 10.1016/j.msec.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 146.Ahmed S., Saifullah, Ahmad M., Swami B.L., Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Rad. Res. Appl. Sci. 2016;9:1–7. doi: 10.1016/j.jrras.2015.06.006. [DOI] [Google Scholar]
- 147.Krishnaraj C., Ramachandran R., Mohan K., Kalaichelvan P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012;93:95–99. doi: 10.1016/j.saa.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 148.Tippayawat P., Phromviyo N., Boueroy P., Chompoosor A. Green synthesis of silver nanoparticles in Aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ. 2016;4:e2589. doi: 10.7717/peerj.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Borase H.P., Patil C.D., Salunkhe R.B., Suryawanshi R.K., Salunke B.K., Patil S.V. Transformation of aromatic dyes using green synthesized silver nanoparticles. Bioprocess. Biosyst. Eng. 2014;37:1695–1705. doi: 10.1007/s00449-014-1142-4. [DOI] [PubMed] [Google Scholar]
- 150.Suman T.Y., Radhika Rajasree S.R., Kanchana A., Elizabeth S.B. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morindacitrifolia root extract. Colloids Surf. B Biointerfaces. 2013;106:74–78. doi: 10.1016/j.colsurfb.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 151.Oves M., Aslam M., Rauf M.A., Qayyum S., Qari H.A., Khan M.S., Alam M.Z., Tabrez S., Pugazhendhi A., Ismail I.M.I. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenixdactylifera. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;89:429–443. doi: 10.1016/j.msec.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 152.Qayyum S., Oves M., Khan A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE. 2017;12:e0181363. doi: 10.1371/journal.pone.0181363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dwivedi A.D., Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. 2010;369:27–33. doi: 10.1016/j.colsurfa.2010.07.020. [DOI] [Google Scholar]
- 154.Paulkumar K., Gnanajobitha G., Vanaja M., Rajeshkumar S., Malarkodi C., Pandian K., Annadurai G. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci. World J. 2014;2014:829894. doi: 10.1155/2014/829894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ali K.A., Yao R., Wu W., Masum M.M.I., Luo J., Wang Y., Zhang Y., An Q., Sun G., Li B. Biosynthesis of silver nanoparticle from pomelo (Citrus Maxima) and their antibacterial activity against Acidovorax oryzae RS-2. Mater. Res. Express. 2020;7:015097. doi: 10.1088/2053-1591/ab6c5e. [DOI] [Google Scholar]
- 156.Ali M., Kim B., Belfield K.D., Norman D., Brennan M., Ali G.S. Inhibition of Phytophthoraparasitica and P. capsici by silver nanoparticles synthesized using aqueous extract of Artemisia absinthium. Phytopathology. 2015;105:1183–1190. doi: 10.1094/PHYTO-01-15-0006-R. [DOI] [PubMed] [Google Scholar]
- 157.Jagana D., Hegde Y.R., Lella R. Green nanoparticles—A Novel Approach for the Management of Banana Anthracnose Caused by Colletotrichum musae. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:1749–1756. doi: 10.20546/ijcmas.2017.610.211. [DOI] [Google Scholar]
- 158.Jayaseelan C., Ramkumar R., Rahuman A.A., Perumal P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschusesculentus and its antifungal activity. Ind. Crops Prod. 2013;45:423–429. doi: 10.1016/j.indcrop.2012.12.019. [DOI] [Google Scholar]
- 159.Rajiv P., Rajeshwari S., Venckatesh R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L.; its size-dependent antifungal activity against plant fungal pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;112:384–387. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 160.Rajesh K.M., Ajitha B., Reddy Y.A.K., Suneetha Y., Reddy P.S. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik. 2018;154:593–600. doi: 10.1016/j.ijleo.2017.10.074. [DOI] [Google Scholar]
- 161.Hassall K.A. Pesticides: Their Properties, Uses and Disadvantages: Part I: General Introduction; Insecticides and Related Compounds. Br. Vet. J. 1965;121:105–118. doi: 10.1016/S0007-1935(17)41302-9. [DOI] [PubMed] [Google Scholar]
- 162.Kutz F.W., Wood P.H., Bottimore D.P. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev. Environ. Contam. Toxicol. 1991;120:1–82. doi: 10.1007/978-1-4612-3080-9_1. [DOI] [PubMed] [Google Scholar]
- 163.Nisar P., Ali N., Rahman L., Ali M., Shinwari Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. J. Biol. Inorg. Chem. 2019;24:929–941. doi: 10.1007/s00775-019-01717-7. [DOI] [PubMed] [Google Scholar]
- 164.Rafique M., Sadaf I., Rafique M.S., Tahir M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017;45:1272–1291. doi: 10.1080/21691401.2016.1241792. [DOI] [PubMed] [Google Scholar]
- 165.Hajipour M.J., Fromm K.M., Akbar Ashkarran A., Jimenez de Aberasturi D., de Larramendi I.R., Rojo T., Serpooshan V., Parak W.J., Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 166.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 167.Xia Z.-K., Ma Q.-H., Li S.-Y., Zhang D.-Q., Cong L., Tian Y.-L., Yang R.-Y. The antifungal effect of silver nanoparticles on Trichosporon asahii. J. Microbiol. Immunol. Infect. 2016;49:182–188. doi: 10.1016/j.jmii.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 168.Soenen S., Rivera-Gil P., Montenegro J.-M., Parak W.J., De Smedt S., Braeckmans K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. 2011;6:446–465. doi: 10.1016/j.nantod.2011.08.001. [DOI] [Google Scholar]
- 169.Zhang L., Wu L., Si Y., Shu K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: Growth inhibition, cell injury, oxidative stress and internalization. PLoS ONE. 2018;13:e0209020. doi: 10.1371/journal.pone.0209020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morales-Díaz A.B., Ortega-Ortíz H., Juárez-Maldonado A., Cadenas-Pliego G., González-Morales S., Benavides-Mendoza A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017;8:013001. doi: 10.1088/2043-6254/8/1/013001. [DOI] [Google Scholar]
- 171.Gardea-Torresdey J.L., Rico C.M., White J.C. Trophic Transfer, Transformation, and Impact of Engineered Nanomaterials in Terrestrial Environments. Environ. Sci. Technol. 2014;48:2526–2540. doi: 10.1021/es4050665. [DOI] [PubMed] [Google Scholar]
- 172.Kim J.I., Park H.-G., Chang K.-H., Nam D.H., Yeo M.-K. Trophic transfer of nano-TiO2 in a paddy microcosm: A comparison of single-dose versus sequential multi-dose exposures. Environ. Pollut. 2016;212:316–324. doi: 10.1016/j.envpol.2016.01.076. [DOI] [PubMed] [Google Scholar]
- 173.De la Torre Roche R., Servin A., Hawthorne J., Xing B., Newman L.A., Ma X., Chen G., White J.C. Terrestrial Trophic Transfer of Bulk and Nanoparticle La2O3 Does Not Depend on Particle Size. Environ. Sci. Technol. 2015;49:11866–11874. doi: 10.1021/acs.est.5b02583. [DOI] [PubMed] [Google Scholar]
- 174.Shimizu M., Tainaka H., Oba T., Mizuo K., Umezawa M., Takeda K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part. Fibre Toxicol. 2009;6:20. doi: 10.1186/1743-8977-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bielmyer-Fraser G.K., Jarvis T.A., Lenihan H.S., Miller R.J. Cellular Partitioning of Nanoparticulate versus Dissolved Metals in Marine Phytoplankton. Environ. Sci. Technol. 2014;48:13443–13450. doi: 10.1021/es501187g. [DOI] [PubMed] [Google Scholar]
- 176.Zhu X., Wang J., Zhang X., Chang Y., Chen Y. Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere. 2010;79:928–933. doi: 10.1016/j.chemosphere.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 177.Werlin R., Priester J.H., Mielke R.E., Krämer S., Jackson S., Stoimenov P.K., Stucky G.D., Cherr G.N., Orias E., Holden P.A. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat. Nanotechnol. 2011;6:65–71. doi: 10.1038/nnano.2010.251. [DOI] [PubMed] [Google Scholar]
- 178.Kurepa J., Paunesku T., Vogt S., Arora H., Rabatic B.M., Lu J., Wanzer M.B., Woloschak G.E., Smalle J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010;10:2296–2302. doi: 10.1021/nl903518f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang S., Kurepa J., Smalle J.A. Ultra-small TiO nanoparticles disrupt microtubular networks in Arabidopsis thaliana. Plant Cell Environ. 2011;34:811–820. doi: 10.1111/j.1365-3040.2011.02284.x. [DOI] [PubMed] [Google Scholar]
- 180.Ali S.H., Ali S.A. Nanotechnology Is the Potential Cause of Phytotoxicity. J. Biomater. 2019;3:1–6. [Google Scholar]
- 181.Servin A.D., White J.C. Nanotechnology in agriculture: Next steps for understanding engineered nanoparticle exposure and risk. NanoImpact. 2016;1:9–12. doi: 10.1016/j.impact.2015.12.002. [DOI] [Google Scholar]
- 182.Dimkpa C.O., McLean J.E., Martineau N., Britt D.W., Haverkamp R., Anderson A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013;47:1082–1090. doi: 10.1021/es302973y. [DOI] [PubMed] [Google Scholar]
- 183.Faisal M., Saquib Q., Alatar A.A., Al-Khedhairy A.A., Hegazy A.K., Musarrat J. Phytotoxic hazards of NiO- nanoparticles in tomato: A study on mechanism of cell death. J. Hazard. Mater. 2013;250:318–332. doi: 10.1016/j.jhazmat.2013.01.063. [DOI] [PubMed] [Google Scholar]
- 184.Pakrashi S., Jain N., Dalai S., Jayakumar J., Chandrasekaran P.T., Raichur A.M., Chandrasekaran N., Mukherjee A. In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS ONE. 2014;9:e87789. doi: 10.1371/journal.pone.0087789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pagano L., Servin A.D., De La Torre-Roche R., Mukherjee A., Majumdar S., Hawthorne J., Marmiroli M., Maestri E., Marra R.E., Isch S.M., et al. Molecular Response of Crop Plants to Engineered Nanomaterials. Environ. Sci. Technol. 2016;50:7198–7207. doi: 10.1021/acs.est.6b01816. [DOI] [PubMed] [Google Scholar]
- 186.Sanzari I., Leone A., Ambrosone A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019;7:120. doi: 10.3389/fbioe.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Abdallah Y., Yang M., Zhang M., Masum M.M.I., Ogunyemi S.O., Hossain A., An Q., Yan C., Li B. Plant growth promotion and suppression of bacterial leaf blight in rice by Paenibacilluspolymyxa Sx3. Lett. Appl. Microbiol. 2019;68:423–429. doi: 10.1111/lam.13117. [DOI] [PubMed] [Google Scholar]
- 188.Simonin M., Richaume A. Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: A review. Environ. Sci. Pollut. Res. 2015;22:13710–13723. doi: 10.1007/s11356-015-4171-x. [DOI] [PubMed] [Google Scholar]
- 189.Xu C., Peng C., Sun L., Zhang S., Huang H., Chen Y., Shi J. Distinctive effects of TiO2 and CuO nanoparticles on soil microbes and their community structures in flooded paddy soil. Soil Biol. Biochem. 2015;86:24–33. doi: 10.1016/j.soilbio.2015.03.011. [DOI] [Google Scholar]
- 190.You T., Liu D., Chen J., Yang Z., Dou R., Gao X., Wang L. Effects of metal oxide nanoparticles on soil enzyme activities and bacterial communities in two different soil types. J. Soils Sedim. 2018;18:211–221. doi: 10.1007/s11368-017-1716-2. [DOI] [Google Scholar]
- 191.Chai H., Yao J., Sun J., Zhang C., Liu W., Zhu M., Ceccanti B. The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull. Environ. Contam. Toxicol. 2015;94:490–495. doi: 10.1007/s00128-015-1485-9. [DOI] [PubMed] [Google Scholar]
- 192.Chavan S., Nadanathangam V. Effects of nanoparticles on plant growth-promoting bacteria in Indian agricultural soil. Agronomy. 2019;9:140. doi: 10.3390/agronomy9030140. [DOI] [Google Scholar]
- 193.Bandyopadhyay S., Peralta-Videa J.R., Plascencia-Villa G., José-Yacamán M., Gardea-Torresdey J.L. Comparative toxicity assessment of CeO2 and ZnO nanoparticles towards Sinorhizobium meliloti, a symbiotic alfalfa associated bacterium: Use of advanced microscopic and spectroscopic techniques. J. Hazard. Mater. 2012;241:379–386. doi: 10.1016/j.jhazmat.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 194.Collins D., Luxton T., Kumar N., Shah S., Walker V.K., Shah V. Assessing the Impact of Copper and Zinc Oxide Nanoparticles on Soil: A Field Study. PLoS ONE. 2012;7:e42663. doi: 10.1371/journal.pone.0042663. [DOI] [PMC free article] [PubMed] [Google Scholar]