Abstract
The use of chemical insecticides has had several side-effects, such as environmental contamination, foodborne residues, and human health threats. The utilization of plant-derived essential oils as efficient bio-rational agents has been acknowledged in pest management strategies. In the present study, the fumigant toxicity of essential oil isolated from Satureja intermedia was assessed against cosmopolitan stored-product insect pests: Trogoderma granarium Everts (khapra beetle), Rhyzopertha dominica (Fabricius) (lesser grain borer), Tribolium castaneum (Herbst) (red flour beetle), and Oryzaephilus surinamensis (L.) (saw-toothed grain beetle). The essential oil had significant fumigant toxicity against tested insects, which positively depended on essential oil concentrations and the exposure times. Comparative contact toxicity of S. intermedia essential oil was measured against Aphis nerii Boyer de Fonscolombe (oleander aphid) and its predator Coccinella septempunctata L. (seven-spot ladybird). Adult females of A. nerii were more susceptible to the contact toxicity than the C. septempunctata adults. The dominant compounds in the essential oil of S. intermedia were thymol (48.1%), carvacrol (11.8%), p-cymene (8.1%), and γ-terpinene (8.1%). The high fumigant toxicity against four major stored-product insect pests, the significant aphidicidal effect on A. nerii, and relative safety to the general predator C. septempunctata make terpene-rich S. intermedia essential oil a potential candidate for use as a plant-based alternative to the detrimental synthetic insecticides.
Keywords: Aphis nerii, Coccinella septempunctata, plant-based insecticide, Oryzaephius surinamensis, Rhyzopertha dominica, Tribolium castaneum, Trogoderma granarium
1. Introduction
The Khapra Beetle {Trogoderma granarium Everts (Coleoptera: Dermestidae)}, lesser grain borer {Rhyzopertha dominica (Fabricius) (Coleoptera: Bostrichidae)}, red flour beetle {Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae)}, and saw-toothed grain beetle {Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae)} are among the most well-known and economically-important stored-product pests with world-wide distribution. Along with direct damage due to feeding on various stored products, the quality of products is strictly diminished because of their residues and mechanically associated microbes [1,2,3,4,5].
Oleander aphid {Aphis nerii Boyer de Fonscolombe (Hemiptera: Aphididae)}, as a cosmopolitan obligate parthenogenetic aphid, is a common insect pest of many ornamental plants comprising several species of Asclepiadaceae, Apocynaceae, Asteraceae, Convolvulaceae, and Euphorbiaceae, especially in greenhouse conditions. Along with direct damage, A. nerii is able to transmit pathogenic viruses to many plants [6,7,8]. The seven-spot ladybird beetle {Coccinella septempunctata L. (Coleoptera: Coccinellidae)} is a natural enemy of various soft-bodied pests like aphids, thrips, and spider mites, and is considered an important biocontrol agent for greenhouse crops [9,10,11].
The utilization of chemical insecticides is the main strategy in the management of insect pests. However, there is a global concern about their numerous side effects including environmental pollution, insecticide resistance, resurgence of secondary pests, and toxicity to non-target organisms ranging from soil microorganisms to pollinator, predator and parasitoid insects, fish, and even humans [12,13,14]. Therefore, the search for eco-friendly and efficient alternative agents for insect pest management is urgent.
Based on the low toxicity to mammals, rapid biodegradation in the environment, and very low chance of insect pest resistance, the use of essential oils extracted from different aromatic plants has been the motivating subject of many researchers in pest management strategies over the past decade [15,16,17,18].
Sixteen species of the Satureja genus from the Lamiaceae have been reported in the Iranian flora, of which S. atropatana Bunge, S. bachtiarica Bunge, S. edmondi Briquet, S. intermedia C. A. Mey, S. isophylla Rech., S. kallarica Jamzad, S. khuzistanica Jamzad, S. macrosiphonia Bornm., S. sahendica Bornm., and S. rechingeri Jamzad are endemic to Iran [19]. S. intermedia, as a small delicate perennial plant growing on rock outcrops, is among aromatic plants with considerable amount (1.45% (w/w)) of essential oil [20]. The essential oil of S. intermedia is rich in terpenes such as 1,8-cineole, p-cymene, limonene, γ-terpinene, α-terpinene, thymol, and β-caryophyllene, which are classified in four main groups; monoterpene hydrocarbons, oxygenated monoterpenoids, sesquiterpene hydrocarbons, and oxygenated sesquiterpenoids [20,21,22]. Some important biological effects of S. intermedia essential oil include antifungal, antibacterial, and antioxidant effects, and cytotoxic effects have been reported in previous studies [21,22,23]. Although the susceptibility of insect pests to the essential oils isolated from some Satureja species such as S. hortensis, S. montana L., S. parnassica Heldr. & Sart ex Boiss., S. spinosa L., and S. thymbra L. was documented in recent years [24,25,26], the insecticidal effects of S. intermedia essential oil have not reported yet.
As part of a screening program for eco-friendly and efficient plant-derived insecticides, the evaluation of the fumigant toxicity against four major Coleopteran stored-product insect pests O. surinamensis, R. dominica, T. castaneum and T. granarium and the contact toxicity against a greenhouse insect pest Aphis nerii of the essential oil of S. intermedia was the main objective of the present study. Because of the importance of studying the effects of insecticides on the natural enemies of insect pests, the toxicity of S. intermedia essential oil against C. septempunctata was also investigated.
2. Materials and Methods
2.1. Plant Materials and Essential Oil Extraction
Aerial parts (3.0 kg) of S. intermedia were gathered from the Heiran regions, Ardebil province, Iran (38°23′ N, 48°35′ E, elevation 907 m). It was identified according to the keys provided by Jamzad [27]. The voucher specimen was deposited in the Department of Plant Production, Moghan College of Agriculture and Natural Resources, Ardabil, Iran. The fresh leaves and flowers were separated and dried under shade within a week. One hundred grams of the specimen were poured into a 2-L round-bottom flask and subjected to hydrodistillation using a Clevenger apparatus for 3 h. The extraction was repeated in triplicate and the obtained essential oil was dried over anhydrous Na2SO4 and stored in a refrigerator at 4 °C.
2.2. Essential Oil Characterization
The chemical profile of the S. intermedia essential oil was evaluated using gas chromatography (Agilent 7890B) coupled with mass-spectrometer (Agilent 5977A). The analysis was carried out by a HP-5 ms capillary column (30 m × 0.25 mm × 0.25 µm). The temperature of the injector was 280 °C and the column temperature adjusted from 50 to 280 °C using the temperature program: 50 °C (hold for 1 min), increase to 100 °C at 8°/min, increase to 185 °C at 5°/min, increase to 280 °C at 15°/min, and hold at 280 °C for 2 min. The carrier gas was helium (99.999%) with flow rate of 1 mL/min. Essential oil was diluted in methanol, and 1 µL solution was injected (split 1:10 at 0.75 min). The identification of components was performed by comparing mass spectral fragmentation patterns and retention indices with those reported in the databases [28,29,30].
2.3. Insects
The required colonies of Oryzaephilus surinamensis and Rhyzopertha dominica were reared on wheat grains for several generations at the Department of Plant Production, Moghan College of Agriculture and Natural Resources, University of Mohaghegh Ardabili (Ardabil province, Iran). Tribolium castaneum and Trogoderma granarium adults were collected from infested stored wheat grains in Moghan region (Ardabil province, Iran). Insects were identified by Asgar Ebadollahi. Fifty unsexed pairs of adult insects were separately released onto wheat grains and removed from breeding container after 48 h. Wheat grains contaminated with insect eggs were separately kept in an incubator at 25 ± 2 °C, 65 ± 5% relative humidity and a photoperiod of 14:10 (L:D) h. Finally, one to fourteen-day-old adults of O. surinamensis, R. dominica, T. castaneum and T. granarium were designated for fumigant bio-assays.
Aphis nerii and its natural predator Coccinella septempunctata were used to evaluate the contact toxicity of the S. intermedia essential oil. Cohorts of apterous adult females of A. nerii and unsexed adults of C. septempunctata were taken directly from homegrown oleander (Nerium oleander L.) and a chemically untreated alfalfa (Medicago sativa L.) field (Moghan region, Ardabil province, Iran), respectively.
2.4. Fumigant Toxicity
The fumigant toxicity of S. intermedia essential oil was tested on adults of O. surinamensis, R. dominica, T. castaneum, and T. granarium. To determine the fumigant toxicity of the essential oil, filter papers (Whatman No. 1, 2 × 2 cm) were impregnated with essential oil concentrations and were attached to the under surface of the screw cap of glass containers (340-mL) as fumigant chambers. A series of concentrations (4.71–14.71, 7.06–20.88, 20.59–58.82, and 8.82–35.29 µL/L for O. surinamensis, R. dominica, T. castaneum, and T. granarium, respectively) was organized to assess the toxicity of S. intermedia essential oil after an initial concentration setting experiment for each insect species. Twenty unsexed adults (1–14 days old) of each insect species were separately put into glass containers and their caps were tightly affixed. The same conditions without any essential oil concentration were used for control groups and each treatment was replicated five times. Insects mortality was documented 24, 48 and 72 h after initial exposure to the essential oil. Insects were considered dead when no leg or antennal movements were observed [31].
2.5. Contact Toxicity
The contact toxicity of S. intermedia essential oil against the apterous adult females of A. nerii and unsexed adults of C. septempunctata was tested through filter paper discs (Whatman No. 1), 9 cm diameter, positioned in glass petri dishes (90 × 10 mm). Range-finding experiments were established to find the proper concentrations for each insect. Concentrations ranging from 200 to 750 µg/mL for A. nerii and from 500 to 1400 µg/mL for C. septempunctata were prepared via 1.00% aqueous Tween-80 as an emulsifying agent. Each solution (200 µL) was applied to the surface of the filter paper. Ten insects were separately released onto each treated disc, the dishes sealed with Parafilm® and kept at 25 ± 2 °C, 65 ± 5% relative humidity and a photoperiod of 16:8 h (light:dark). Except for the addition of essential oil concentrations, all other procedures were unchanged for the control groups. Four replications were made for each treatment and mortality was documented after 24 h. Aphids and ladybirds were considered dead if no leg or antennal movements were detected when softly prodded [32,33].
2.6. Data Analysis
The mortality percentage was corrected using Abbott’s formula: Pt = [(Po − Pc)/(100 − Pc)] × 100, in which Pt is the corrected mortality percentage, Po is the mortality (%) caused by essential oil concentrations and Pc is the mortality (%) in the control groups [34].
Analysis of variance (ANOVA) and Tukey’s test at p = 0.05 were used to statistically identify the effects of independent factors (essential oil concentration and exposure time) on insect mortality and the differences among mean mortality percentage of insects, respectively. Probit analysis was used to estimate LC50 and LC95 values with 95% fiducial limits, the data heterogeneity and linear regression information using SPSS 24.0 software package (Chicago, IL, USA).
3. Results
3.1. Chemical Composition of Essential Oil
The chemical composition of S. intermedia essential oil is presented in Table 1. A total of 47 compounds were identified in the essential oil, in which the phenolic monoterpenoids thymol (48.1%) and carvacrol (11.8%), along with p-cymene (8.1%), γ-terpinene (8.1%), carvacryl methyl ether (4.0%), α-pinene (2.7%), and β-caryophyllene (2.4%) were dominants. Terpenoids were the most abundant components (98.6%), especially monoterpene hydrocarbons (20.5%) and oxygenated monoterpenoids (68.4%) with only minor amounts of phenylpropanoids or fatty acid-derived compounds.
Table 1.
Chemical composition of the essential oil isolated from aerial parts of Satureja intermedia.
| RIcalc | RIdb | Compound | % | RIcalc | RIdb | Compound | % |
|---|---|---|---|---|---|---|---|
| 929 | 932 | α-Pinene | 2.7 | 1384 | 1387 | β-Bourbonene | 0.1 |
| 984 | 974 | 1-Octen-3-ol | 0.3 | 1389 | 1379 | Geranyl acetate | tr |
| 990 | 988 | Myrcene | 0.4 | 1423 | 1417 | β-Caryophyllene | 2.4 |
| 1016 | 1020 | p-Cymene | 8.1 | 1428 | 1431 | β-Gurjunene | 0.1 |
| 1034 | 1024 | Limonene | 0.5 | 1432 | 1442 | α-Maaliene | 0.1 |
| 1037 | 1026 | 1,8-Cineole | 1.7 | 1438 | 1439 | Aromadendrene | 0.7 |
| 1060 | 1054 | γ-Terpinene | 8.1 | 1454 | 1452 | α-Humulene | 0.3 |
| 1066 | 1065 | cis-Sabinene hydrate | 0.4 | 1476 | 1478 | γ-Muurolene | 0.5 |
| 1083 | 1086 | Terpinolene | 0.2 | 1487 | 1489 | β-Selinene | 0.2 |
| 1083 | 1089 | p-Cymenene | 0.2 | 1496 | 1496 | Viridiflorene | 0.7 |
| 1092 | 1095 | Linalool | 0.2 | 1500 | 1500 | α-Muurolene | 0.2 |
| 1094 | 1098 | trans-Sabinene hydrate | 0.1 | 1510 | 1505 | β-Bisabolene | 1.3 |
| 1121 | 1128 | allo-Ocimene | 0.2 | 1515 | 1513 | γ-Cadinene | 0.3 |
| 1164 | 1165 | Borneol | 0.4 | 1523 | 1522 | δ-Cadinene | 0.7 |
| 1176 | 1174 | Terpinen-4-ol | 0.8 | 1530 | 1533 | trans-Cadina-1,4-diene | 0.1 |
| 1187 | 1191 | Hexyl butyrate | 0.1 | 1535 | 1537 | α-Cadinene | tr |
| 1239 | 1241 | Carvacryl methyl ether | 4.0 | 1540 | 1544 | α-Calacorene | 0.3 |
| 1284 | 1282 | (E)-Anethole | 0.7 | 1557 | 1553 | Thymohydroquinone | 0.5 |
| 1290 | 1289 | Thymol | 48.1 | 1578 | 1577 | Spathulenol | 0.9 |
| 1298 | 1298 | Carvacrol | 11.8 | 1581 | 1582 | Caryophyllene oxide | 0.8 |
| 1340 | 1340 | Piperitenone | tr | Monoterpene hydrocarbons | 20.5 | ||
| 1346 | 1346 | α-Terpinyl acetate | 0.1 | Oxygenated monoterpenoids | 68.4 | ||
| 1349 | 1349 | Thymyl acetate | 0.2 | Sesquiterpene hydrocarbons | 8.0 | ||
| 1357 | 1356 | Eugenol | 0.1 | Oxygenated sesquiterpenoids | 1.7 | ||
| 1365 | 1373 | α-Ylangene | 0.1 | Phenylpropanoids | 0.8 | ||
| 1371 | 1374 | α-Copaene | 0.2 | Others | 0.4 | ||
| 1376 | 1372 | Carvacryl acetate | 0.1 | Total identified | 99.8 |
3.2. Fumigant Toxicity
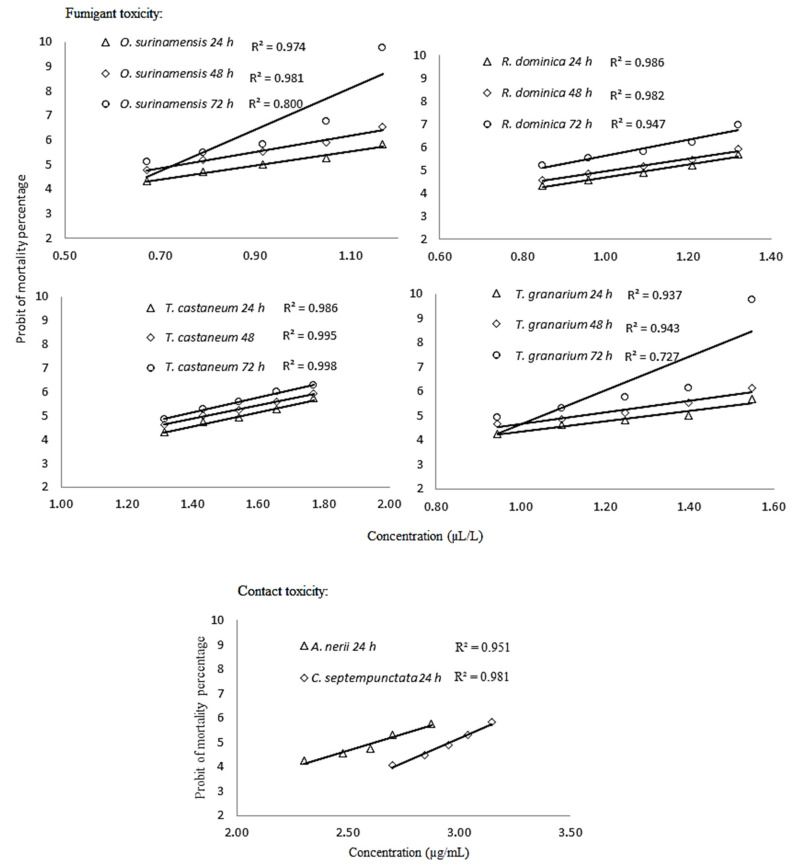
Analysis of variance (ANOVA) revealed that the tested concentrations of S. intermedia essential oil (F = 239.462 and p < 0.0001 for O. surinamensis, F = 223.629 and p < 0.0001 for R. dominica, F = 169.615 and p < 0.0001 for T. castaneum, and F = 89.032 and p < 0.0001 for T. granarium with df = 4, 45) and the considered exposure times (F = 212.855 and p < 0.0001 for O. surinamensis, F = 281.180 and p < 0.0001 for R. dominica, F = 84.705 and p < 0.0001 for T. castaneum, and F = 84.501 and p < 0.0001 for T. granarium with df = 2, 45) had significant effects on the mortality of all insect pests. According to Figure 1 and relatively high R2 values, there is a positive correlation between the fumigation of essential oil concentrations and the mortality of four storage insect pests at all exposure times. Furthermore, the steep slopes indicate a homogenous toxic response among beetles to the essential oil.
Figure 1.
Concentration–response lines of contact and fumigant toxicity of Satureja intermedia essential oil against Aphis nerii and Coccinella septempunctata, and Oryzaephilus surinamensis, Rhyzopertha dominica, Tribolium castaneum, and Trogoderma granarium, respectively.
According to Table 2, an obvious difference in the mean mortality percentage of all tested storage insect pests was detected, as essential oil concentration and exposure time were increased. For example, 25.00% mortality of O. surinamensis adults was observed at 4.71 µL/L and 24-h exposure time, which had increased to 80.00% and 100% at 14.71 µL/L after 24 and 72 h, respectively. It is apparent that the essential oil of S. intermedia gave at least 90% mortality against all tested stored-product insect pests at 58.82 µL/L after 72 h (Table 2).
Table 2.
Mean mortality ± SE of the adults of Oryzaephilus surinamensis, Rhyzopertha dominica, Tribolium castaneum, and Trogoderma granarium exposed to the fumigation of Satureja intermedia essential oil after 24, 48, and 72 h.
| Insect | Time (h) | Concentration (µL/L) | ||||
|---|---|---|---|---|---|---|
| 4.71 | 6.18 | 8.24 | 11.18 | 14.71 | ||
| O. surinamensis | 24 | 25.00 ± 0.41 j | 38.75 ± 0.63 i | 50.00 ± 0.41 g | 60.00 ±0.41 f | 80.00 ± 0.41 d |
| 48 | 41.25 ± 0.48 h | 57.50 ± 0.29 f,g | 70.00 ± 0.41 e | 81.25 ± 0.48 d | 93.75 ± 0.48 c | |
| 72 | 53.75 ± 0.48 g | 68.75 ± 0.48 e | 80.00 ± 0.58 d | 96.25 ± 0.48 b | 100.00 ± 0.00 a | |
| 7.06 | 9.12 | 12.35 | 16.18 | 20.88 | ||
| R. dominica | 24 | 25.00 ± 0.41 l | 33.75 ± 0.48 k | 46.25 ± 0.48 i | 58.75 ± 0.29 h | 75.00 ± 0.58 e |
| 48 | 33.75 ± 0.48 k | 43.75 ± 0.48 j | 56.25 ± 0.48 h | 67.50 ± 0.29 g | 82.50 ± 0.29 c | |
| 72 | 57.50 ± 0.29 h | 70.00 ± 0.41 f | 78.75 ± 0.25 d | 88.75 ± 0.48 b | 97.50 ± 0.29 a | |
| 20.59 | 27.06 | 34.71 | 45.29 | 58.82 | ||
| T. castaneum | 24 | 23.75 ± 0.48 k | 38.75 ± 0.48 i | 46.25 ± 0.48 g | 60.00 ±0.41 e | 76.25 ± 0.25 c |
| 48 | 35.00 ± 0.58 j | 50.00 ± 0.58 f | 58.75 ± 0.63 e | 71.25 ± 0.48 d | 82.50 ± 0.50 b | |
| 72 | 43.75 ± 0.48 h | 60.00 ± 0.41 e | 71.25 ± 0.25 d | 83.75 ± 0.63 b | 90.00 ± 0.50 a | |
| 8.82 | 12.53 | 17.68 | 25.00 | 35.29 | ||
| T. granarium | 24 | 22.50 ± 0.48 j | 35.00 ± 0.29 i | 42.50 ± 0.25 h | 50.00 ± 0.41 g | 75.00 ± 0.29 c |
| 48 | 37.50 ± 0.25 i | 45.00 ± 0.29 h | 55.00 ± 0.29 f | 70.00 ± 0.41 d | 87.50 ± 0.48 b | |
| 72 | 47.50 ± 0.25 g | 62.50 ± 0.48 e | 77.50 ± 0.48 c | 87.50 ± 0.48 b | 100.00 ± 0.00 a | |
Data that do not have the same letters are statistically significant different at p = 0.05 based on Tukey’s test. Each datum represents mean ± SE of four replicates with eighty adult insects.
Based on lower LC50 values of those stored-product insect pests tested, O. surinamensis was significantly the most susceptible insect to the essential oil of S. intermedia at all time intervals. In contrast, the adults of T. castaneum with highest LC50 and LC95 values were the most tolerant to fumigation with S. intermedia essential oil. Furthermore, the susceptibility of insect pests to the fumigation of S. intermedia essential oil followed in the order: O. surinamensis > R. dominica > T. granarium > T. castaneum (Table 3).
Table 3.
Probit analysis of the data obtained from fumigation of Satureja intermedia essential oil on the adults of Oryzaephilus surinamensis, Rhyzopertha dominica, Tribolium castaneum, and Trogoderma granarium.
| Insect | Time (h) | LC50 with 95% Confidence Limits (µL/L) | LC90 with 95% Confidence Limits (µL/L) | χ2 (df = 3) |
Slope ± SE | Sig. * |
|---|---|---|---|---|---|---|
| O. surinamensis | 24 | 8.151 (7.396–8.970) | 23.177 (18.675–32.578) | 1.99 | 2.824 ± 0.344 | 0.574 |
| 48 | 5.542 (4.853–6.119) | 13.710 (11.971–16.756) | 1.288 | 3.258 ± 0.378 | 0.732 | |
| 72 | 4.716 (4.143–5.174) | 9.200 (8.413–10.405) | 5.134 | 4.415 ± 0.504 | 0.162 | |
| R. dominica | 24 | 12.825 (11.661–14.189) | 36.901 (29.147–54.0970) | 0.885 | 2.792 ± 0.356 | 0.829 |
| 48 | 10.398 (9.265–11.454) | 30.455 (24.687–42.838) | 1.056 | 2.746 ± 0.358 | 0.788 | |
| 72 | 6.358 (5.126–7.296) | 15.970 (14.160–19.138) | 2.488 | 3.204 ± 0.432 | 0.477 | |
| T. granarium | 24 | 20.489 (18.114–23.612) | 81.507 (58.604–140.911) | 4.233 | 2.137 ± 0.283 | 0.237 |
| 48 | 13.654 (11.811–15.364) | 49.192 (38.852–71.499) | 3.978 | 2.302 ± 0.289 | 0.264 | |
| 72 | 9.785 (6.082–12.258) | 24.075 (18.870–42.027) | 5.842 | 3.277 ± 0.360 | 0.12 | |
| T. castaneum | 24 | 35.612 (32.538–39.070) | 95.948 (77.352–135.744) | 0.967 | 2.977 ± 0.376 | 0.809 |
| 48 | 28.048 (24.747–30.916) | 80.251 (65.751–111.454) | 0.297 | 2.807 ± 0.378 | 0.961 | |
| 72 | 22.861 (19.648–25.415) | 57.584 (50.068–71.481) | 0.139 | 3.194 ± 0.405 | 0.987 |
* Since the significance level is greater than 0.05, no heterogeneity factor is used in the calculation of confidence limits. The number of insects for calculation of LC50 values is 200 for T. granarium and 400 for other insects in each time.
3.3. Contact Toxicity
The tested concentrations of S. intermedia essential oil demonstrated significant contact toxicity on both A. nerii (F = 27.682, df = 4, 15; p < 0.0001) and C. septempunctata (F = 35.607, df = 4, 15; p < 0.0001). A positive correlation between essential oil concentrations and the mortality of A. nerii and C. septempunctata in the contact assay is also apparent, based on the high R2 values (Figure 1). Comparisons of the mean mortality percentage of A. nerii and its predator C. septempunctata caused by S. intermedia essential oil are shown in Table 4. The mortality percentages of both insects increased with increasing essential oil concentrations, but their susceptibility to the essential oil was noticeably different. For example, 62.50% mortality was documented for A. nerii at 500 µg/mL essential oil concentration while its predator C. septempunctata was more tolerant and exhibited only 17.50% mortality at this concentration (Table 4).
Table 4.
Mean mortality ± SE of the adults of Aphis nerii and Coccinella septempunctata exposed to the different concentration of Satureja intermedia essential oil after 24 h.
| Insect | Concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| 200 | 300 | 400 | 500 | 750 | |
| A. nerii | 22.50 ± 0.25 e | 32.50 ± 0.25 d | 40.00 ± 0.41 c | 62.50 ± 0.25 b | 77.50 ± 0.75 a |
| 500 | 700 | 900 | 1100 | 1400 | |
| C. septempunctata | 17.50 ± 0.48 e | 30.00 ± 0.41 d | 45.00 ± 0.29 c | 62.50 ± 0.48 b | 80.00 ± 0.41 a |
Data that do not have the same letters are statistically significant different at p = 0.05 based on Tukey’s test. Each datum represents mean ± SE of four replicates with eighty adult insects.
The results of the probit analysis for the contact toxicity of S. intermedia essential oil against A. nerii and C. septempunctata adults are shown in Table 5. According to low LC50 and LC95 values, the adult females of A. nerii were more susceptible to contact toxicity of S. intermedia essential oil than the adults of C. septempunctata.
Table 5.
Probit analysis of the data obtained from contact toxicity of Satureja intermedia essential oil on the adults of Aphis nerii and Coccinella septempunctata.
| Insect | LC50 with 95% Confidence Limits (μg/mL) |
LC90 with 95% Confidence Limits (μg/mL) |
χ2 (df = 3) |
Slope ± SE | Sig. * |
|---|---|---|---|---|---|
| A. nerii | 418.379 (379.586–464.130) | 1224.788 (975.704–1738.840) | 4.363 | 2.747 ± 0.318 | 0.225 |
| C. septempunctata | 913.722 (853.739–980.799) | 1908.099 (1652.748–2352.473) | 1.932 | 4.008 ± 0.413 | 0.587 |
* Since the significance level is greater than 0.05, no heterogeneity factor is used in the calculation of confidence limits. The number of insects for calculation of LC50 values is 240 for each insect.
4. Discussion
The susceptibility of O. surinamensis, R. dominica, T. castaneum and T. granarium adults to the essential oil of S. intermedia with 24-h LC50 values of 8.151, 12.825, 20.489, and 35.612 µL/L, respectively, was distinguished in the present study. The fumigant toxicity of some plant-derived essential oils against O. surinamensis, R. dominica, T. castaneum and T. granarium has been documented in previous studies; it was found that the essential oils of Agastache foeniculum (Pursh) Kuntze, Achillea filipendulina Lam., and Achillea millefolium L. with respective 24-h LC50 values of 18.781, 12.121, and 17.977 µL/L, had high toxicity on the adults of O. surinamensis [31,34,35,36]. The adults of R. dominica were also susceptible to the fumigation of essential oils extracted from Eucalyptus globulus Labill (24-h LC50 = 3.529 μL/L), Lavandula stoechas L. (24-h LC50 = 5.660 μL/L), and Apium graveolens L. (24-h LC50 = 53.506 μL/L) [37,38]. The fumigation of the essential oils of Lippia citriodora Kunth (24-h LC50 = 37.349 μL/L), Melissa officinalis L. (24-h LC50 = 19.418 μL/L), and Teucrium polium L. (24-h LC50 = 20.749 μL/L) resulted in significant mortality in T. castaneum [39,40,41]. The essential oils of Schinus molle L. (48-h LC50 = 806.50 μL/L) and Artemisia sieberi Besser (24-h LC50 = 33.80 μL/L) also had notable fumigant toxicity against the adults of T. granarium [42,43]. The toxicity of all the above-mentioned essential oils was augmented when the exposure time was prolonged. These findings support the results regarding the time-dependent susceptibility of O. surinamensis, R. dominica, T. castaneum and T. granarium to plant essential oils. The differences in observed LC50 values are likely due to the differences in the essential oil compositions from the different plant species and possibly to differences in the experimental conditions. Furthermore, the S. intermedia essential oil with low 24-h LC50 value was more toxic on O. surinamensis than A. foeniculum, A. filipendulina, and A. millefolium essential oils, on R. dominica than A. graveolens essential oil, on T. castaneum than Lippia citriodora essential oil, and on T. granarium than S. molle essential oil.
The terpenes, especially thymol, carvacrol, p-cymene and γ-terpinene, were recognized as the main components of S. intermedia essential oil in the present study. In the study of Sefidkon and Jamzad, thymol (32.3%), γ-terpinene (29.3%), p-cymene (14.7%), elemicin (4.8%), limonene (3.3%), and α-terpinene (3.3%) were the main components of S. intermedia essential oil [20]. In another study, thymol (34.5%), γ-terpinene (18.2%), p-cymene (10.5%), limonene (7.3%), α-terpinene (7.1%), carvacrol (6.9%), and elemicin (5.3%) were found to be major components in the essential oil of S. intermedia [23]. In the present study, however, limonene was a minor component (0.5%), and neither elemicin nor α-terpinene were detected. Ghorbanpour et al. reported the terpenes thymol (32.3%), p-cymene (14.7%), γ-terpinene (3.3%), and carvacrol (1.0%), and the phenylpropanoid elemicin (4.8%) as the main components in the essential oil of S. intermedia [22], while the concentrations of γ-terpinene and carvacrol were much lower compared to the present findings. The differences in the chemical profile of the plant essential oils are likely due to the internal and external factors such as seasonal variation, geographical features, plant growth stage, and different extraction conditions [19,44,45]. The insecticidal properties of several terpenes, especially monoterpene hydrocarbons and monoterpenoids, which accounted for 88.9% of the S. intermedia essential oil in the present study, have been documented in recent investigations. For example, insecticidal activities of p-cymene, α-pinene, γ-terpinene, 1,8-cineole, and limonene have been demonstrated against several detrimental insect pests [46,47,48,49,50]. Previous studies have also indicated that the monoterpenoids thymol and carvacrol had significant toxicity against insect pests [46,51,52]. Accordingly, the insecticidal efficiency of S. intermedia essential oil can be attributed to such components.
The contact toxicity of the essential oil of Eucalyptus globulus Labill. against A. nerii has been reported by Russo et al. [53]. Although this is the only previous study to investigate the susceptibility of A. nerii to a plant essential oil, its findings confirm the results of the present study about the possibility of A. nerii management through plant essential oils. Indeed, the toxicity of S. intermedia essential oil was evaluated for the first time in the present study against A. nerii and its natural enemy C. septempunctata. The essential oil of S. intermedia was more toxic on A. nerii (LC50: 418 µg/mL) than the predator ladybird C. septempunctata (LC50: 914 µg/mL), suggesting that the predator was more tolerant than the aphid to S. intermedia essential oil, which is very valuable in terms of predator protection. Similar results were obtained for controlling aphids [54,55] and some other insect pests [56,57,58] using plant-derived essential oils along with protecting their predators. However, the destructive side-effects of some essential oils on parasitoids have been reported [59,60,61]. Therefore, it is important to select efficient pesticides with lower side effects on natural enemies at operative concentrations to the pests, which has been achieved in the current study.
5. Conclusions
In conclusion, the terpene-rich essential oil of S. intermedia has significant fumigant toxicity against the adults of O. surinamensis, R. dominica, T. castaneum, and T. granarium, and may be considered as a natural effective fumigant on stored products. This bio-rational agent also has significant contact toxicity on the adult females of A. nerii, one of the cosmopolitan insect pests of ornamental plants. Furthermore, the predator ladybird C. septempunctata was more tolerant to the essential oil than the aphid. Accordingly, S. intermedia essential oil can be nominated as an eco-friendly efficient insecticide by decreasing the risks associated with the application of synthetic chemicals. However, the exploration of any side-effects of the essential oil on other useful insects such as parasitoids and pollinators, its phytotoxicity on the treated plants and crops, any adverse tastes or odors on stored products, and the preparation of novel formulations to increase its stability in the environment for practical utilization are needed.
Acknowledgments
W.N.S. participated in this work as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/). This study received financial support from the University of Mohaghegh Ardabili, which is greatly appreciated.
Author Contributions
Conceptualization, A.E.; methodology, A.E. and W.N.S.; validation, A.E. and W.N.S.; formal analysis, A.E. and W.N.S.; investigation, A.E.; resources, A.E.; data curation, A.E.; writing—original draft preparation, A.E.; writing—review and editing, A.E. and W.N.S.; project administration, A.E.; funding acquisition, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Mohaghegh Ardabili.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Edde P.A. A review of the biology and control of Rhyzopertha dominica (F.) the lesser grain borer. J. Stored Prod. Res. 2012;48:1–18. doi: 10.1016/j.jspr.2011.08.007. [DOI] [Google Scholar]
- 2.Bosly H.A., Kawanna M.A. Fungi species and red flour beetle in stored wheat flour under Jazan region conditions. Toxicol. Ind. Health. 2014;30:304–310. doi: 10.1177/0748233712457449. [DOI] [PubMed] [Google Scholar]
- 3.Garcia D., Girardi N.S., Passone M.A., Nesci A., Etcheverry M. Harmful effects on Oryzaephilus surinamensis (L.) and Tribolium castaneum by food grade antioxidants and their formulations in peanut kernel. J. Food Chem. Nanotechnol. 2017;3:86–92. doi: 10.17756/jfcn.2017-042. [DOI] [Google Scholar]
- 4.Özberk F. Impacts of khapra beetle (T. granarium Everts) onto marketing price and relevant traits in bread wheat (T. aestivum L.) Appl. Ecol. Environ. Res. 2018;16:6143–6153. doi: 10.15666/aeer/1605_61436153. [DOI] [Google Scholar]
- 5.Athanassiou C.G., Phillips T.W., Wakil W. Biology and control of the khapra beetle, Trogoderma granarium, a major quarantine threat to global food security. Annu. Rev. Entomol. 2019;64:131–148. doi: 10.1146/annurev-ento-011118-111804. [DOI] [PubMed] [Google Scholar]
- 6.Blackman R.L., Eastop V.F. Aphids on the World’s Crops. 2nd ed. John Wiley & Sons Ltd.; Chichester, UK: 2000. [Google Scholar]
- 7.Zehnder C.B., Hunter M.D. A comparison of maternal effects and current environment on vital rates of Aphis nerii, the milkweed-oleander aphid. Ecol. Entomol. 2007;32:172–180. doi: 10.1111/j.1365-2311.2007.00853.x. [DOI] [Google Scholar]
- 8.Colvin S.M., Yeargan K.V. Predator fauna associated with oleander aphids on four milkweed species and the effect of those host plants on the development and fecundity of Cycloneda munda and Harmonía axyridis. J. Kans. Entomol. Soc. 2014;87:280–298. doi: 10.2317/JKES130628.1. [DOI] [Google Scholar]
- 9.Gupta G., Kumar N.R. Growth and development of ladybird beetle Coccinella septempunctata L. (Coleoptera: Coccinellidae), on plant and animal based protein diets. J. Asia Pac. Entomol. 2017;20:959–963. doi: 10.1016/j.aspen.2017.07.008. [DOI] [Google Scholar]
- 10.Cheng Y., Zhi J., Li F., Jin J., Zhou Y. An artificial diet for continuous maintenance of Coccinella septempunctata adults (Coleoptera: Coccinellidae) Biocontrol Sci. Technol. 2018;28:242–252. doi: 10.1080/09583157.2018.1439450. [DOI] [Google Scholar]
- 11.Liu T., Wang Y., Zhang L., Xu Y., Zhang Z., Mu W. Sublethal effects of four insecticides on the seven-spotted lady beetle (Coleoptera: Coccinellidae) J. Econ. Entomol. 2019;112:2177–2185. doi: 10.1093/jee/toz146. [DOI] [PubMed] [Google Scholar]
- 12.Damalas C.A., Eleftherohorinos I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health. 2011;8:1402–1419. doi: 10.3390/ijerph8051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulé R., Sabella G., Robba L., Manachini B. Systematic review of the effects of chemical insecticides on four common butterfly families. Front. Environ. Sci. 2017;5:32. doi: 10.3389/fenvs.2017.00032. [DOI] [Google Scholar]
- 14.Zikankuba V.L., Mwanyika G., Ntwenya J.E., James A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric. 2019;5:1601544. doi: 10.1080/23311932.2019.1601544. [DOI] [Google Scholar]
- 15.Isman M.B., Grieneisen M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014;19:140–145. doi: 10.1016/j.tplants.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Chau D.T.M., Chung N.T., Huong L.T., Hung N.H., Ogunwande I.A., Dai D.N., Setzer W.N. Chemical compositions, mosquito larvicidal and antimicrobial activities of leaf essential oils of eleven species of Lauraceae from Vietnam. Plants. 2020;9:606. doi: 10.3390/plants9050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavela R., Benelli G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21:1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Spochacz M., Chowański S., Walkowiak-Nowicka K., Szymczak M., Adamski Z. Plant-derived substances used against beetles–Pests of stored crops and food–and their mode of action: A review. Compr. Rev. Food Sci. Food Saf. 2018;17:1339–1366. doi: 10.1111/1541-4337.12377. [DOI] [PubMed] [Google Scholar]
- 19.Alizadeh A. Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages. Zeitschrift für Naturforschung C. 2015;70:51–58. doi: 10.1515/znc-2014-4121. [DOI] [PubMed] [Google Scholar]
- 20.Sefidkon F., Jamzad Z. Chemical composition of the essential oil of three Iranian Satureja species (S. mutica, S. macrantha and S. intermedia) Food Chem. 2005;91:1–4. doi: 10.1016/j.foodchem.2004.01.027. [DOI] [Google Scholar]
- 21.Shahnazi S., Khalighi-Sigaroodi F., Ajani Y., Yazdani D., Taghizad-Farid R., Ahvazi M., Abdoli M. The chemical composition and antimicrobial activity of essential oil of Satureja intermedia CA Mey. J. Med. Plants. 2008;1:85–92. [Google Scholar]
- 22.Ghorbanpour M., Hadian J., Hatami M., Salehi-Arjomand H., Aliahmadi A. Comparison of chemical compounds and antioxidant and antibacterial properties of various Satureja species growing wild in Iran. J. Med. Plants. 2016;3:58–72. [Google Scholar]
- 23.Sadeghi I., Yousefzadi M., Behmanesh M., Sharifi M., Moradi A. In vitro cytotoxic and antimicrobial activity of essential oil from Satureja intermedia. Iran. Red Crescent Med. J. 2013;15:70–74. doi: 10.5812/ircmj.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaelakis A., Theotokatos S.A., Koliopoulos G., Chorianopoulos N.G. Essential oils of Satureja species: Insecticidal effect on Culex pipiens larvae (Diptera: Culicidae) Molecules. 2007;12:2567–2578. doi: 10.3390/12122567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tozlu E., Cakir A., Kordali S., Tozlu G., Ozer H., Akcin T.A. Chemical compositions and insecticidal effects of essential oils isolated from Achillea gypsicola, Satureja hortensis, Origanum acutidens and Hypericum scabrum against broadbean weevil (Bruchus dentipes) Sci. Hortic. 2011;130:9–17. doi: 10.1016/j.scienta.2011.06.019. [DOI] [Google Scholar]
- 26.Magierowicz K., Górska-Drabik E., Sempruch C. The insecticidal activity of Satureja hortensis essential oil and its active ingredient carvacrol against Acrobasis advenella (Zinck.) (Lepidoptera, Pyralidae) Pestic. Biochem. Physiol. 2019;153:122–128. doi: 10.1016/j.pestbp.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Jamzad Z. Thymus and Satureja Species of Iran. 1st ed. Research Institute of Forests and Rangelands; Tehran, Iran: 2009. [Google Scholar]
- 28.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 29.Mondello L. FFNSC 3. Shimadzu Scientific Instruments; Columbia, MD, USA: 2016. [Google Scholar]
- 30.NIST . NIST17. National Institute of Standards and Technology; Gaithersburg, MD, USA: 2017. [Google Scholar]
- 31.Alkan M. Chemical composition and insecticidal potential of different Origanum spp. (Lamiaceae) essential oils against four stored product pests. Turk. Entomol. Derg. 2020;44:149–163. doi: 10.16970/entoted.620387. [DOI] [Google Scholar]
- 32.Behi F., Bachrouch O., Boukhris-Bouhachem S. Insecticidal activities of Mentha pulegium L., and Pistacia lentiscus L., essential oils against two citrus aphids Aphis spiraecola Patch and Aphis gossypii Glover. J. Essent. Oil Bear. Plants. 2019;22:516–525. doi: 10.1080/0972060X.2019.1611483. [DOI] [Google Scholar]
- 33.Ikbal C., Pavela R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019;92:971–986. doi: 10.1007/s10340-019-01089-6. [DOI] [Google Scholar]
- 34.Islam R., Khan R.I., Al-Reza S.M., Jeong Y.T., Song C.H., Khalequzzaman M. Chemical composition and insecticidal properties of Cinnamomum aromaticum (Nees) essential oil against the stored product beetle Callosobruchus maculatus (F.) J. Sci. Food Agric. 2009;89:1241–1246. doi: 10.1002/jsfa.3582. [DOI] [Google Scholar]
- 35.Ebadollahi A., Safaralizadeh M.H., Pourmirza A.A., Gheibi S.A. Toxicity of essential oil of Agastache foeniculum (Pursh) Kuntze to Oryzaephilus surinamensis L. and Lasioderma serricorne F. J. Plant Prot. Res. 2010;50:215–219. doi: 10.2478/v10045-010-0037-x. [DOI] [Google Scholar]
- 36.Ebadollahi A. Essential oil isolated from Iranian yarrow as a bio-rational agent to the management of saw-toothed grain beetle, Oryzaephilus surinamensis (L.) Korean J. Appl. Entomol. 2017;56:395–402. [Google Scholar]
- 37.Ebadollahi A., Safaralizadeh M.H., Pourmirza A.A. Fumigant toxicity of essential oils of Eucalyptus globulus Labill and Lavandula stoechas L., grown in Iran, against the two coleopteran insect pests; Lasioderma serricorne F. and Rhyzopertha dominica F. Egypt. J. Biol. Pest Control. 2010;20:1–5. [Google Scholar]
- 38.Ebadollahi A. Fumigant toxicity and repellent effect of seed essential oil of celery against lesser grain borer, Rhyzopertha dominica. J. Essent. Oil Bear. Plants. 2018;21:146–154. doi: 10.1080/0972060X.2018.1445036. [DOI] [Google Scholar]
- 39.Ebadollahi A., Ashrafi Parchin R., Farjaminezhad M. Phytochemistry, toxicity and feeding inhibitory activity of Melissa officinalis L. essential oil against a cosmopolitan insect pest; Tribolium castaneum Herbst. Toxin Rev. 2016;35:77–82. doi: 10.1080/15569543.2016.1199572. [DOI] [Google Scholar]
- 40.Ebadollahi A., Razmjou J. Chemical composition and toxicity of the essential oils of Lippia citriodora from two different locations against Rhyzopertha dominica and Tribolium castaneum. Agric. For. 2019;65:135–146. doi: 10.17707/AgricultForest.65.3.11. [DOI] [Google Scholar]
- 41.Ebadollahi A., Taghinezhad E. Modeling and optimization of the insecticidal effects of Teucrium polium L. essential oil against red flour beetle (Tribolium castaneum Herbst) using response surface methodology. Inf. Process. Agric. 2019 doi: 10.1016/j.inpa.2019.08.004. in press. [DOI] [Google Scholar]
- 42.Abdel-Sattar E., Zaitoun A.A., Farag M.A., El Gayed S.H., Harraz F.M.H. Chemical composition, insecticidal and insect repellent activity of Schinus molle L. leaf and fruit essential oils against Trogoderma granarium and Tribolium castaneum. Nat. Prod. Res. 2010;24:226–235. doi: 10.1080/14786410802346223. [DOI] [PubMed] [Google Scholar]
- 43.Nouri-Ganbalani G., Borzoui E. Acute toxicity and sublethal effects of Artemisia sieberi Besser on digestive physiology, cold tolerance and reproduction of Trogoderma granarium Everts (Col.: Dermestidae) J. Asia Pac. Entomol. 2017;20:285–292. doi: 10.1016/j.aspen.2017.01.002. [DOI] [Google Scholar]
- 44.Sefidkon F., Abbasi K., Khaniki G.B. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem. 2006;99:19–23. doi: 10.1016/j.foodchem.2005.07.026. [DOI] [Google Scholar]
- 45.Hadian J., Ebrahimi S.N., Salehi P. Variability of morphological and phytochemical characteristics among Satureja hortensis L. accessions of Iran. Ind. Crop. Prod. 2010;32:62–69. doi: 10.1016/j.indcrop.2010.03.006. [DOI] [Google Scholar]
- 46.Lee B.-H., Choi W.-S., Lee S.-E., Park B.-S. Fumigant toxicity of essential oils and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.) Crop Prot. 2001;20:317–320. doi: 10.1016/S0261-2194(00)00158-7. [DOI] [Google Scholar]
- 47.Lee B.-H., Lee S.-E., Annis P.C., Pratt S.J., Park B.-S., Tumaalii F. Fumigant toxicity of essential oils and monoterpenes against the red flour beetle, Tribolium castaneum Herbst. J. Asia Pac. Entomol. 2002;5:237–240. doi: 10.1016/S1226-8615(08)60158-2. [DOI] [Google Scholar]
- 48.Yildirim E., Emsen B., Kordali S. Insecticidal effects of monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) J. Appl. Bot. Food Qual. 2013;86:198–204. [Google Scholar]
- 49.Saad M.M.G., Abou-Taleb H.K., Abdelgaleil S.A.M. Insecticidal activities of monoterpenes and phenylpropenes against Sitophilus oryzae and their inhibitory effects on acetylcholinesterase and adenosine triphosphatases. Appl. Entomol. Zool. 2018;53:173–181. doi: 10.1007/s13355-017-0532-x. [DOI] [Google Scholar]
- 50.Liu T.-T., Chao L.K.-P., Hong K.-S., Huang Y.-J., Yang T.-S. Composition and insecticidal activity of essential oil of Bacopa caroliniana and interactive effects of individual compounds on the activity. Insects. 2020;11:23. doi: 10.3390/insects11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youssefi M.R., Tabari M.A., Esfandiari A., Kazemi S., Moghadamnia A.A., Sut S., Acqua S.D., Benelli G., Maggi F. Efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the West Nile vector Culex pipiens. Molecules. 2019;24:1867. doi: 10.3390/molecules24101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu X., Weng H., Li C., He J., Zhang X., Ma Z. Efficacy of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and its three main components against insect pests. Ind. Crop. Prod. 2020;147:112237. doi: 10.1016/j.indcrop.2020.112237. [DOI] [Google Scholar]
- 53.Russo S., Yaber Grass M.A., Fontana H.C., Leonelli E. Insecticidal activity of essential oil from Eucalyptus globulus against Aphis nerii (Boyer) and Gynaikothrips ficorum (Marchal) AgriScientia. 2018;35:63–67. doi: 10.31047/1668.298x.v1.n35.20458. [DOI] [Google Scholar]
- 54.Abramson C.I., Wanderley P.A., Wanderley M.J.A., Miná A.J.S., de Souza O.B. Effect of essential oil from citronella and alfazema on fennel aphids Hyadaphis foeniculi Passerini (Hemiptera: Aphididae) and its predator Cycloneda sanguinea L. (Coleoptera: Coccinelidae) Am. J. Environ. Sci. 2007;3:9–10. doi: 10.3844/ajessp.2007.9.10. [DOI] [Google Scholar]
- 55.Kimbaris A.C., Papachristos D.P., Michaelakis A., Martinou A.F., Polissiou M.G. Toxicity of plant essential oil vapours to aphid pests and their coccinellid predators. Biocontrol Sci. Technol. 2010;20:411–422. doi: 10.1080/09583150903569407. [DOI] [Google Scholar]
- 56.Faraji N., Seraj A.A., Yarahmadi F., Rajabpour A. Contact and fumigant toxicity of Foeniculum vulgare and Citrus limon essential oils against Tetranychus turkestani and its predator Orius albidipennis. J. Crop Prot. 2016;5:283–292. doi: 10.18869/modares.jcp.5.2.283. [DOI] [Google Scholar]
- 57.Ribeiro N., Camara C., Ramos C. Toxicity of essential oils of Piper marginatum Jacq. against Tetranychus urticae Koch and Neoseiulus californicus (McGregor) Chil. J. Agric. Res. 2016;76:71–76. doi: 10.4067/S0718-58392016000100010. [DOI] [Google Scholar]
- 58.Seixas P.T.L., Demuner A.J., Alvarenga E.S., Barbosa L.C.A., Marques A., Farias E.D.S., Picanço M.C. Bioactivity of essential oils from Artemisia against Diaphania hyalinata and its selectivity to beneficial insects. Sci. Agric. 2018;75:519–525. doi: 10.1590/1678-992x-2016-0461. [DOI] [Google Scholar]
- 59.Titouhi F., Amri M., Messaoud C., Haouel S., Youssfi S., Cherif A., Mediouni Ben Jemâa J. Protective effects of three Artemisia essential oils against Callosobruchus maculatus and Bruchus rufimanus (Coleoptera: Chrysomelidae) and the extended side-effects on their natural enemies. J. Stored Prod. Res. 2017;72:11–20. doi: 10.1016/j.jspr.2017.02.007. [DOI] [Google Scholar]
- 60.Haouel-Hamdi S., Abdelkader N. Combined use of Eucalyptus salmonophloia essential oils and the parasitoid Dinarmus basalis for the control of the cowpea seed beetle Callosobruchus maculatus. Tunis. J. Plant Prot. 2018;13:123–137. [Google Scholar]
- 61.De Souza M.T., de Souza M.T., Bernardi D., Krinski D., de Melo D.J., Oliveira D.C., Rakes M.G., Zarbin P.H., de Noronha Sales Maia B.H.L., Zawadneak M.A.C. Chemical composition of essential oils of selected species of Piper and their insecticidal activity against Drosophila suzukii and Trichopria anastrephae. Environ. Sci. Pollut. Res. 2020;27:13056–13065. doi: 10.1007/s11356-020-07871-9. [DOI] [PubMed] [Google Scholar]