Abstract
Cocoa shell is a by-product of the chocolate industry that is rich in dietary fiber and bioactive components. In this research, the influence of high voltage electric discharge (HVED) treatment on chemical and physical characteristics of the cocoa shell, i.e., the effects of applied time and frequencies on grinding ability, water binding capacity (WBC), dietary fibers and tannin content was investigated. HVED had a significant influence on the chemical and physical properties of cocoa shell, all of which could be linked to changes in fiber properties. Along with the fiber content, grinding ability and water binding capacity were increased. These properties have already been linked to fiber content and soluble/insoluble fiber ratio. However, this research implies that change in fiber properties could be linked to tannin formation via complexation of other polyphenolic components. Additional research is needed to verify this effect and to establish mechanisms of tannin formation induced by HVED and its influence on fiber quantification.
Keywords: cocoa shell, high voltage electrical discharge, tannin, dietary fiber, water binding capacity, grindability
1. Introduction
Cocoa shell is the major by-product of the cocoa processing industry. It is a part of the cocoa bean that is separated from cotyledon during pre-roasting or after the roasting of beans [1]. It has been reported that several tons of cocoa shell need to be disposed annually, which poses a large problem [2,3]. Cocoa beans are rich in bioactive compounds, which are stored in the cotyledon. During fermentation, these components diffuse into cocoa shell, which becomes rich in bioactive compounds [4]. In addition, cocoa shell is rich in dietary fiber, mainly consisting of cellulose, carbohydrates and pectic polysaccharides [2] and presents great material for use in food industry and enrichment of food poor in dietary fibers. In the last few years, cocoa shell has been used as a raw biomass material, feedstuff, adsorbent, soil conditioner, garden mulch or burnt for fuel [5,6].
High voltage electric discharge (HVED) is a non-thermal process that has been used for the treatment of waste products from the food industry in the last few years [7]. It is also used as an extraction method, because it can disrupt the cellular walls and increase the overall mass transfer of the cellular content [8]. HVED is an innovative technique that interjects energy directly in aqueous solution between electrodes that are submerged. Electric discharge in water consists of two phases: corona streamer discharge process and arc discharge process. For the streamer discharge process weak shock waves are characteristic, as well as small number of bubbles and active radicals. When transiting to arc discharge process, number of bubbles is increased, shock waves become stronger, turbulence and concentration of free radicals are increased [9]. These shock waves and explosions of cavitation bubbles can affect particle size by fragmentation of cell membranes [10]. Electric discharge directly in water leads to production of molecular oxygen and hydrogen, hydrogen peroxide, hydroxyl radicals and oxygen radical ion, all of which are very reactive species.
Since HVED can disrupt cellular walls, which are in cocoa shell predominantly composed of cellulose with lesser amounts of hemicellulose and pectin [3], the aim of this study was to evaluate HVED influence on cocoa shell dietary fiber content and properties related to it. For use of cocoa shell in food industry, dietary fiber content, grindability, water and oil binding capacity and content of bioactive components are very important.
2. Materials and Methods
2.1. Preparation of Cocoa Shell
Cocoa shell samples were obtained after roasting fermented cocoa beans (West Africa mix supplied by Huyser, Möller B.V., Edam, Holland) at 135 °C for 55 min in custom made roaster (Metal workshop “ILMA”, Požega, Croatia). After that, the cocoa shell was easily separated by hand from the cotyledon.
Untreated cocoa shell (UCS) sample was obtained by grinding cocoa shell attained after separation from the cotyledon. Control samples were obtained by mixing the unmilled cocoa shell in water for 15, 30 and 45 min at concentrations of 1.5% and 3.0%. After mixing, the shell was separated from water and dried in the laboratory oven (Memmert, UFE 500, Schwabach, Germany) at 40 °C. Dry samples were ground in the laboratory mill (IKA, M20, Staufen, Germany) (25 g for 2 min with cooling) to obtain a fine powder (composite sample obtained by repeated grinding) and as such were frozen and stored for analyses. The grinded untreated cocoa shell was also frozen and stored for analysis in the same way as a cocoa shell mixed in water.
2.2. HVED Treatment
High-voltage electrical discharge equipment which was described by Barišić et al. [11] includes a chamber connected to a high-voltage pulse generator of 30 kV (the device was custom made by Inganiare CPTS1, Osijek, Croatia for the Faculty of Food Technology Osijek). Treatment chamber contains a stainless steel cylindrical needle (diameter 2.5 mm), and the ground electrode in the form of a plate (diameter 45 mm). Mixing of samples is achieved by magnetic stirrer. The distance between the electrodes during all treatments was 2 cm. Electric field density was 15 kV/cm during all treatments. HVED energy input ranged between 13.11–79.80 kJ/kg.
Unmilled cocoa shell (same as control samples prepared in water) was treated in HVED device at concentrations of 1.5% (6 g in 400 mL of distilled water) and 3.0% (12 g in 400 mL of distilled water). The treatment time was 15, 30 and 45 min, and the used frequencies were 40 and 80 Hz. Each sample (HVED, control or untreated) was treated until 200 g of sample was gained which gave us uniform sample for all analyses. The cocoa shell treated with HVED was dried, grind and stored until analyses in the same way as the control samples. Control samples (mixed in water) and HVED treated samples were dried to a dry matter content of 86.00 ± 0.85%.
2.3. Tannin Content
2.3.1. Extraction
Each sample was weighed (2 g) and extracted three times with 10 mL of n-hexane (Carlo Erba Reagents, Val de Reuil, France) to remove lipids. Samples were dried at air over night and extracted with 5 mL 70% methanol (J. T. Baker, Deventer, Netherland) in ultrasound bath. After that, samples were centrifuged for 10 min at 3000 rpm (Sigma 2-16, Sigma, Osterode, Germany). Supernatant was decanted in 10 mL volumetric flask. That procedure was repeated twice after which flask with supernatant was filled up with 70% methanol.
2.3.2. Spectrophotometric Analysis
Tannin content was determined by method described by Amorim et al. [12]. Method is based on binding of tannins with casein. Calibration curve was created with the standard solutions of tannic acid (Sigma-Aldrich, St. Louis, USA) in the range of concentrations from 0.5 to 3 mg/mL (y = 0.9011x + 0.0095; R2 = 0.9993). Total phenol content and residual phenol content (obtained after complexation of tannin and casein) were determined spectrophotometrically at 760 nm according to the method of Singleton et al. [13]. Tannin content in prepared extracts was calculated Equation (1) as the difference between total phenol content and residual phenol content. Results are presented as mg of tannic acid per g of defatted sample (mg TA/g) and as a percentage of tannin in total phenol content (%).
| (1) |
2.4. Determination of Dietary Fibers
Dietary fibers were determined according to gravimetric AOAC method 991.43 [14]. Samples were treated with thermostable α-amylase, protease and amyloglucosidase (Megazyme Total Dietary Fiber Assay Kit, Megazyme Ltd., Bray, Ireland). The share of insoluble dietary fibers (IDF, %) was determined gravimetrically after filtration, and soluble dietary fibers (SDF, %) were determined by precipitation from the obtained filtrate. After correction for undigested protein (Kjeldahl method) and ash (mineralization at 525 °C), total dietary fibers were calculated Equations (2) and (3) as a sum of IDF and SDF. The values were calculated on the dry matter of the sample.
| (2) |
| (3) |
where: R1 = residue weight 1 from m1; R2 = residue weight 2 from m2; m1 = sample weight 1; m2 = sample weight 2; A = ash weight from R1; P = protein weight from R2; B = blank; BR = blank residue; BP = blank protein from BR1; BA = blank ash from BR2.
2.5. Grindability of Cocoa Shell
The grindability of cocoa shell was determined by sieving the powdered cocoa shell samples on analytical sieve shaker (Retsch GmbH, AS200, Haan, Germany) and measurement of mass of obtained fractions. A total of 50 g of the sample was sieved through six sieves (50, 71, 100, 125, 200 and 315 µm) during 15 min. After weighing each fraction, results were expressed as percentages of cocoa shell mass that was weighted on each sieve (%).
2.6. Water Binding Capacity (WBC) and Oil Binding Capacity (OBC)
For determination of WBC standard AACC Method 88-04 [15], was used. To 2.5 g of cocoa shell sample 30 mL of water was added. These solutions were left to stand at room temperature with periodic mixing. After that, the samples were centrifuged at 3000 rpm for 15 min (Centra-MP4R, IEC, Mumbai, India). The supernatant was decanted, and the remaining residue was weighted. The analysis was performed in two repetitions. The results were calculated Equation (4) and were expressed as grams of H2O absorbed per gram of cocoa shell (g/g).
| (4) |
For determination of OBC same procedure was used. The only difference was that for OBC instead of water cold pressed rapeseed oil was used. Results were expressed as grams of oil absorbed per gram of cocoa shell (%) obtained by formula Equation (5):
| (5) |
2.7. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflection (FTIR-ATR) Analysis
FTIR–ATR spectra were recorded with a Cary 630 spectrometer (Agilent, Santa Clara, CA, USA) in wavenumber range from 4000 to 650 cm−1. For each sample, 32 scans were recorded and averaged with a spectral resolution of 16 cm−1.
2.8. Statistical Analysis
Statistical analysis was conducted using Statistica®, Version 13.4.0.14 (1984–2018 TIBCO Software Inc, Palo Alto, CA, USA). To determine the statistically significant difference of treatment effects, main effects and factorial analysis of variance (ANOVA) were used. P-value that was considered significant was 0.05. In addition, Pearson’s correlation coefficients was determined (p < 0.05).
3. Results and Discussion
3.1. Tannin Content
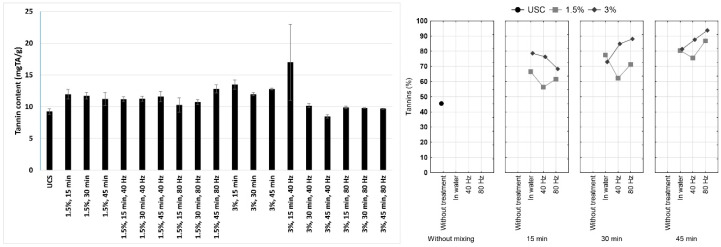
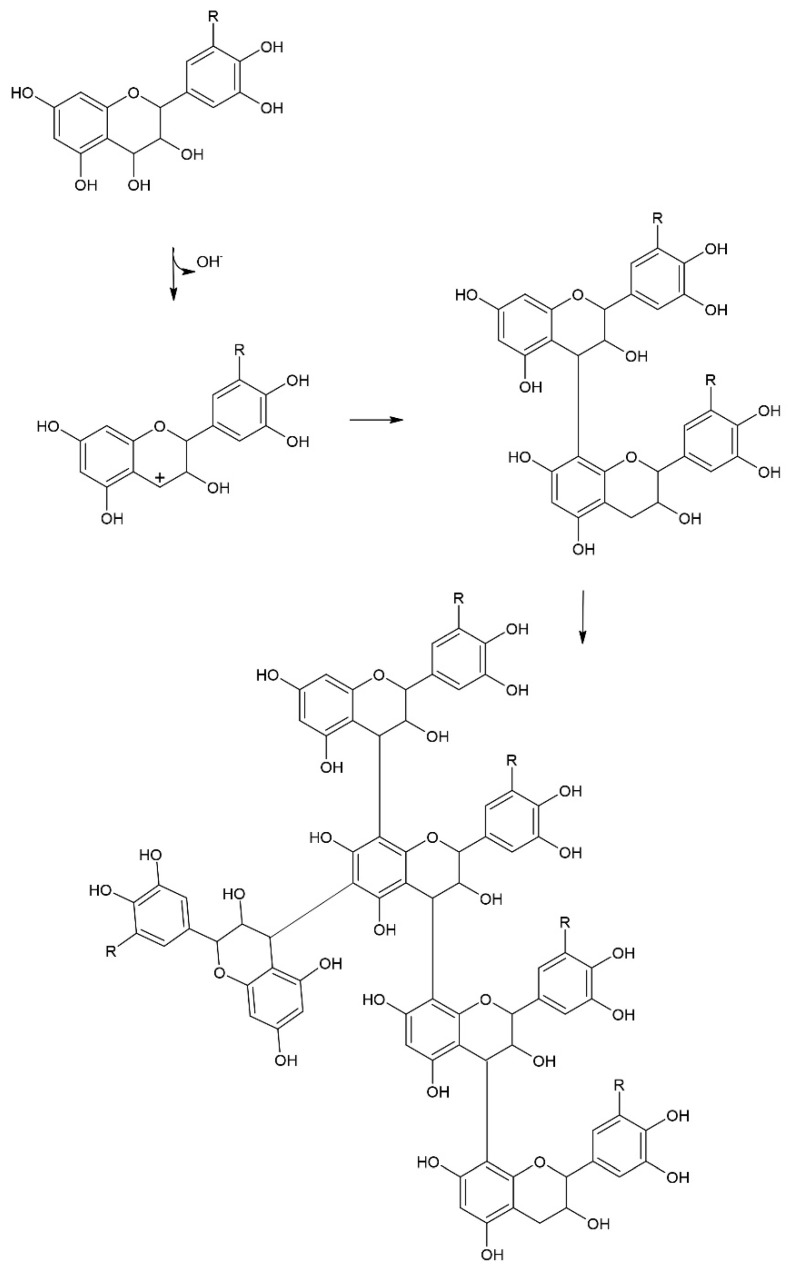
Tannin content of untreated cocoa shell and treated samples are shown in Figure 1 where results for tannin content (mg TA/g of defatted sample) and percentages of tannins in total phenols (%) are presented. It can be seen that the untreated shell had the lowest content of tannins, and the tannin content increased with all treatments. In samples treated with HVED, share of tannins in total phenols ranged from 45.03 to 65.09%. In our previous research [16], we have measured the decrease of content of all major polyphenolic compounds in cocoa shell treated with HVED (catechin, epicatechin, epicatechin gallate, gallic acid, caffeic acid and p-coumaric acid). These components are extractable by water, and the decrease in treated shell may have been the consequence of extraction, as reported by Jokić et al. [17], however, they are also prone to reactions of condensation in suitable conditions (Figure 2). Since the aim of this study was not to establish the effect of HVED treatment on extraction of bioactive compounds, cocoa shell was not milled before treatment, unlike in research of Jokić et al. [17]. Extraction yield is also dependent on electric field intensity, contact surface between material and solvent, liquid to solid ratio, etc. Considering above mentioned, HVED conditions applied in this research are not favorable for extraction [9]. Thus, extraction of polyphenolic compounds was aggravated. HVED generates different reactive species, which may have induced polymerization. Hence, HVED is a source of radicals that can easily oxidize tannins, which leads to an increase in their rigidity. Contrary to our results, Delsart et al. [18] and Lukić et al. [19] reported decrease of total tannin content in red wine treated by HVED and cold plasma, respectively, ascribing it to oxidation of tannins during treatment. However, one has to bear in mind that cold plasma and HVED treatment differ in that cold plasma includes gas introduction into liquid, and that there are major differences in chemical composition, mainly polyphenolic profile, of the treated samples. In addition, since treatment time in this research was significantly longer, oxidized tannins and other phenols might have been involved in mutual reactions, mainly because the oxidized phenols are hydrophobic. It has been reported that hydrophobic reactions can occur among polyphenols and induce their aggregation [20].
Figure 1.
Tannin content (expressed on defatted sample weight) in cocoa shell before and after the high voltage electric discharge (HVED) treatment and percent of tannin in total phenols.
Figure 2.
Condensation of polyphenols.
Results of tannin content, both in sample and in total phenols show that tannins are very resistant to HVED treatment. With the exception of 3.0% sample treated at 40 Hz, where significant reduction of tannin content occurred with the increase of treatment time, a slight increase of their content after treatment was observed (Figure 1), possibly due to oxidation and aggregation of tannins, but also due to the loss of a portion of soluble substances during treatment, which led to a change in the ratio of components in the samples.
Statistical analysis confirmed that tannins are very resistant to HVED treatment, since statistical significance was not established. Furthermore, factorial analysis of variance showed that influence of concentration and mixing time on percent of tannin in total phenols is statistically significant (Table 1). Coefficient of correlation is showing that tannin (%) is in relation with the smallest and largest particles, insoluble and total fibers. This may indicate that tannins have an impact on the proportion of fibers in cocoa shells, since the content of insoluble and total fibers increase as the proportion of tannin increases.
Table 1.
Factorial analysis of variance.
| Sum of Squares | DF | Mean Square | F-Value | p-Value | ||
|---|---|---|---|---|---|---|
| OBC (g/g) | Intercept | 392.4578 | 1 | 392.4578 | 533,103.9 | <0.001 * |
| Concentration (C) | 0.2885 | 1 | 0.2885 | 391.8 | <0.001 * | |
| Mixing (M) | 0.1274 | 2 | 0.0637 | 86.5 | <0.001 * | |
| Treatment (T) | 0.1614 | 2 | 0.0807 | 109.6 | <0.001 * | |
| C*M | 0.0200 | 2 | 0.0100 | 13.6 | <0.001 * | |
| C*T | 0.0474 | 2 | 0.0237 | 32.2 | <0.001 * | |
| M*T | 0.0516 | 4 | 0.0129 | 17.5 | <0.001 * | |
| C*M*T | 0.0821 | 4 | 0.0205 | 27.9 | <0.001 * | |
| Error | 0.0133 | 18 | 0.0007 | |||
| WBC (g/g) | Intercept | 2384.278 | 1 | 2384.278 | 313,752.4 | <0.001 * |
| Concentration (C) | 15.149 | 1 | 15.149 | 1993.6 | <0.001 * | |
| Mixing (M) | 3.762 | 2 | 1.881 | 247.6 | <0.001 * | |
| Treatment (T) | 0.040 | 2 | 0.020 | 2.6 | 0.101638 | |
| C*M | 0.130 | 2 | 0.065 | 8.5 | 0.002470 * | |
| C*T | 0.177 | 2 | 0.088 | 11.6 | <0.001 * | |
| M*T | 0.266 | 4 | 0.066 | 8.8 | <0.001 * | |
| C*M*T | 0.516 | 4 | 0.129 | 17.0 | <0.001 * | |
| Error | 0.137 | 18 | 0.008 | |||
| Tannin (mg TA/g of defatted sample) | Intercept | 4714.410 | 1 | 4714.410 | 1021.198 | <0.001 * |
| Concentration (C) | 0.030 | 1 | 0.030 | 0.006 | 0.936726 | |
| Mixing (M) | 13.343 | 2 | 6.671 | 1.445 | 0.261805 | |
| Treatment (T) | 17.066 | 2 | 8.533 | 1.848 | 0.186168 | |
| C*M | 24.240 | 2 | 12.120 | 2.625 | 0.099895 | |
| C*T | 10.946 | 2 | 5.473 | 1.186 | 0.328333 | |
| M*T | 29.441 | 4 | 7.360 | 1.594 | 0.218875 | |
| C*M*T | 24.668 | 4 | 6.167 | 1.336 | 0.294923 | |
| Error | 83.098 | 18 | 4.617 | |||
| Tannin (% of total polyphenols) | Intercept | 114,239.9 | 1 | 114,239.9 | 3614.463 | <0.001 * |
| Concentration (C) | 202.5 | 1 | 202.5 | 6.406 | 0.020914 * | |
| Mixing (M) | 453.1 | 2 | 226.5 | 7.167 | 0.005134 * | |
| Treatment (T) | 84.5 | 2 | 42.3 | 1.337 | 0.287391 | |
| C*M | 75.8 | 2 | 37.9 | 1.200 | 0.324306 | |
| C*T | 49.7 | 2 | 24.9 | 0.786 | 0.470508 | |
| M*T | 146.0 | 4 | 36.5 | 1.155 | 0.363192 | |
| C*M*T | 102.3 | 4 | 25.6 | 0.809 | 0.535434 | |
| Error | 568.9 | 18 | 31.6 |
OBC: oil binding capacity; WBC: water binding capacity; DF: degree of freedom; * p < 0.05 statistically significant.
3.2. Dietary Fibers
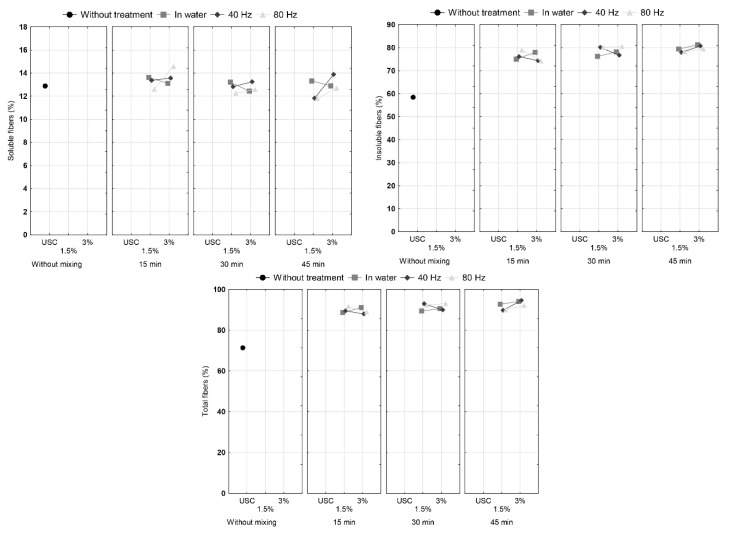
Proportions of soluble, insoluble and total fibers of cocoa shell samples are shown in Figure 3. It can be seen that content of insoluble and total fibers in treated samples is higher than in untreated cocoa shell. The effect of HVED on soluble dietary fibers was not statistically significant (Table 2). An increase of insoluble fiber share after treatment had statistical significance for mixing time and there is a visible trend. A greater effect on increase of insoluble fiber content was in HVED treated samples at 1.5% concentration than at 3.0% due to greater energy input at lower sample concentration.
Figure 3.
Content of insoluble, soluble and total fibers in cocoa shell before and after the HVED treatment.
Table 2.
Main effects analysis of variance.
| Effect | Sum of Squares | DF | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| 0–50 µm | Intercept | 169.1845 | 1 | 169.1845 | 59.27547 | 0.000006 * |
| Concentration | 20.7446 | 1 | 20.7446 | 7.26806 | 0.019455 * | |
| Mixing | 2.4918 | 2 | 1.2459 | 0.43652 | 0.656146 | |
| Treatment | 2.8862 | 2 | 1.4431 | 0.50561 | 0.615433 | |
| Error | 34.2505 | 12 | 2.8542 | |||
| 51–71 µm | Intercept | 2025.733 | 1 | 2025.733 | 451.2232 | <0.001 * |
| Concentration | 54.266 | 1 | 54.266 | 12.0875 | 0.004574 * | |
| Mixing | 12.171 | 2 | 6.085 | 1.3555 | 0.294609 | |
| Treatment | 1.952 | 2 | 0.976 | 0.2174 | 0.807671 | |
| Error | 53.873 | 12 | 4.489 | |||
| 72–100 µm | Intercept | 1371.127 | 1 | 1371.127 | 705.1271 | <0.001 * |
| Concentration | 18.601 | 1 | 18.601 | 9.5659 | 0.009312 * | |
| Mixing | 0.303 | 2 | 0.152 | 0.0780 | 0.925440 | |
| Treatment | 1.729 | 2 | 0.864 | 0.4446 | 0.651247 | |
| Error | 23.334 | 12 | 1.945 | |||
| 101–125 µm | Intercept | 525.4466 | 1 | 525.4466 | 970.2258 | <0.001 * |
| Concentration | 0.9016 | 1 | 0.9016 | 1.6648 | 0.221262 | |
| Mixing | 0.5083 | 2 | 0.2542 | 0.4693 | 0.636443 | |
| Treatment | 0.8918 | 2 | 0.4459 | 0.8234 | 0.462285 | |
| Error | 6.4989 | 12 | 0.5416 | |||
| 126–200 µm | Intercept | 3189.472 | 1 | 3189.472 | 1644.420 | <0.001 * |
| Concentration | 1.189 | 1 | 1.189 | 0.613 | 0.448736 | |
| Mixing | 2.527 | 2 | 1.264 | 0.651 | 0.538775 | |
| Treatment | 2.636 | 2 | 1.318 | 0.679 | 0.525389 | |
| Error | 23.275 | 12 | 1.940 | |||
| 201–315 µm | Intercept | 5128.784 | 1 | 5128.784 | 3745.356 | <0.001 * |
| Concentration | 0.147 | 1 | 0.147 | 0.107 | 0.748864 | |
| Mixing | 3.778 | 2 | 1.889 | 1.379 | 0.288933 | |
| Treatment | 4.432 | 2 | 2.216 | 1.618 | 0.238628 | |
| Error | 16.432 | 12 | 1.369 | |||
| >315 µm | Intercept | 31,757.57 | 1 | 31,757.57 | 1505.873 | <0.001 * |
| Concentration | 26.88 | 1 | 26.88 | 1.275 | 0.280959 | |
| Mixing | 45.68 | 2 | 22.84 | 1.083 | 0.369462 | |
| Treatment | 52.88 | 2 | 26.44 | 1.254 | 0.320301 | |
| Error | 253.07 | 12 | 21.09 | |||
| Insoluble fibers | Intercept | 109,774.9 | 1 | 109,774.9 | 30,704.36 | <0.001 |
| Concentration | 0.1 | 1 | 0.1 | 0.02 | 0.883234 | |
| Mixing | 36.8 | 2 | 18.4 | 5.15 | 0.024326 * | |
| Treatment | 2.7 | 2 | 1.4 | 0.38 | 0.691002 | |
| Error | 42.9 | 12 | 3.6 | |||
| Soluble fibers | Intercept | 3038.191 | 1 | 3038.191 | 7378.913 | <0.001 * |
| Concentration | 0.962 | 1 | 0.962 | 2.336 | 0.152303 | |
| Mixing | 2.116 | 2 | 1.058 | 2.570 | 0.117785 | |
| Treatment | 0.501 | 2 | 0.250 | 0.608 | 0.560488 | |
| Error | 4.941 | 12 | 0.412 | |||
| Total fibers | Intercept | 149,338.0 | 1 | 149,338.0 | 43,452.47 | <0.001 * |
| Concentration | 1.6 | 1 | 1.6 | 0.47 | 0.508137 | |
| Mixing | 21.7 | 2 | 10.9 | 3.16 | 0.079032 | |
| Treatment | 1.0 | 2 | 0.5 | 0.14 | 0.867489 | |
| Error | 41.2 | 12 | 3.4 |
DF: degree of freedom; * p < 0.05 statistically significant.
Increasing the fiber content in treated cocoa shells can be explained by the fact that during various treatments, fiber probably bonded with other components of the cocoa shell. However, some researches have shown that results obtained by gravimetric determination of fiber may be increased due to presence of insoluble proteins and condensed tannins [21,22,23]. The method used in this research has a step to exclude undigested proteins from the results for fiber content, however, condensed tannins may have an effect on the observed increase.
Condensed tannins are, along with resistant protein and Maillard reaction products, part of so-called Klason lignin [21], which is not always considered as a fiber. As shown by Perez et al. [24], roasted husk contains large amounts of free amino-acids and sugars, and our previous research [16] showed significant amounts of catechins. HVED generates free radicals and charged particles and highly reactive species (H+, OH-, H2O2), which may have induced advanced Maillard reactions and reactions of condensation of catechins to condensed tannins (Figure 2).
Our previous research showed that most likely 5-HMF and acrylamide are reacting with free radicals created by HVED and generating new compounds, which could be a part of Klason lignin [25]. This could contribute to increase of insoluble dietary fibers especially because condensed tannins and products of advanced Maillard reactions are insoluble in water.
In addition, we noticed that HVED treated samples had more undigested proteins than non-treated samples (results not shown). Decreased digestibility of proteins can be result of complex formation with tannins especially because HVED treatment generates change in pH and surface charge, which could be favorable conditions for complexation. Reduced in vitro and in vivo digestibility of proteins due to formation of complexes with tannins has already been reported for sorghum and several Acacia species. In addition, protein-protein complexation induced by tannins, and enzyme inhibition by tannins were also reported [26]. Although corrections for proteins were made, the other components that were bonded to them were not included here.
There are already some researches investigating the effect of electrical discharge on fibers. Yuan et al. [27] concluded that plasma improves the tensile strength and surface roughness, which leads to higher interfacial contact. In addition, during the treatment, it came to oxidation of fibers. Sinha and Panigrahi [28] observed increased hydrophobicity of jute fibers after plasma treatment, probably because of oxidation or decrease of phenolic and secondary alcoholic groups. Improved flexural strength of fibers occurred because of better adhesion between fibers and matrix. Bozaci et al. [29] and Karahan and Özdoğan [30] came to the conclusion that fibers have increased hydrophilicity, rougher surface and higher proportion of damaged fibers after plasma treatment.
Additional research is needed to reveal whether proposed mechanisms may be applicable to influence of HVED on fibers in cocoa shell.
3.3. Grindability of Cocoa Shell
The largest change in share of particles after HVED treatment was in the particle size ranges 0–50 μm and >315 μm (Table 3). Untreated cocoa shell had the largest percentage of particles between 0 and 50 μm and the smallest percentage of particles larger than 315 μm compared to treated and control samples. Any treatment, either only in water or with HVED, has led to an increase in the share of particles with size greater than 315 μm and a reduction in the share of particles with size less than 50 μm which was proven by coefficient of correlation (Table 4). There is a relation between the smallest and the largest particles. Main effect analysis of variance showed that there was a statistically significant difference between different sample concentrations during treatment for particle sizes of 0–50 μm, 51–71 μm and 72–100 μm (Table 2). In all treated samples decrease in the percentage of smaller particles and an increase in the percentage of larger particles was observed. The minimum change occurred in the sample 1.5%, 30 min, 40 Hz. Statistical analysis shows that there was a correlation between particle sizes and dietary fibers implying that difficulty to grind HVED treated cocoa shell can be caused by increased content of fibers.
Table 3.
Grindability of cocoa shell samples before and after the HVED treatment.
| Sample | 0–50 µm (%) | 51–71 µm (%) | 72–100 µm (%) | 101–125 µm (%) | 126–200 µm (%) | 201–315 µm (%) | >315 µm (%) |
|---|---|---|---|---|---|---|---|
| UCS | 15.19 | 21.89 | 11.83 | 7.94 | 18.24 | 14.10 | 10.81 |
| 1.5%, 15 min | 3.63 | 14.70 | 8.27 | 5.29 | 14.66 | 17.87 | 35.58 |
| 1.5%, 30 min | 3.12 | 12.64 | 7.83 | 5.07 | 13.12 | 16.76 | 41.47 |
| 1.5%, 45 min | 1.71 | 10.64 | 7.33 | 4.74 | 12.11 | 15.44 | 48.03 |
| 1.5%, 15 min, 40 Hz | 5.64 | 13.78 | 7.42 | 5.52 | 13.62 | 17.42 | 36.61 |
| 1.5%, 30 min, 40 Hz | 8.39 | 13.47 | 7.33 | 5.16 | 13.35 | 17.51 | 34.80 |
| 1.5%, 45 min, 40 Hz | 3.67 | 13.67 | 7.42 | 5.15 | 12.65 | 16.94 | 40.50 |
| 1.5%, 15 min, 80 Hz | 5.48 | 10.55 | 6.28 | 4.66 | 11.59 | 15.75 | 45.69 |
| 1.5%, 30 min, 80 Hz | 2.64 | 11.71 | 8.59 | 5.50 | 13.33 | 16.75 | 41.48 |
| 1.5%, 45 min, 80 Hz | 2.98 | 9.95 | 8.93 | 5.54 | 13.07 | 16.67 | 42.87 |
| 3.0%, 15 min | 3.52 | 9.98 | 7.22 | 4.64 | 11.72 | 15.36 | 47.56 |
| 3.0%, 30 min | 2.28 | 9.19 | 9.33 | 5.77 | 13.10 | 16.40 | 43.93 |
| 3.0%, 45 min | 2.16 | 9.26 | 10.98 | 6.40 | 15.05 | 17.31 | 38.84 |
| 3.0%, 15 min, 40 Hz | 1.36 | 8.89 | 11.45 | 5.79 | 15.12 | 18.76 | 38.64 |
| 3.0%, 30 min, 40 Hz | 0.64 | 4.04 | 11.23 | 7.40 | 15.23 | 18.69 | 42.77 |
| 3.0%, 45 min, 40 Hz | 2.08 | 8.84 | 10.14 | 5.24 | 12.78 | 16.18 | 44.75 |
| 3.0%, 15 min, 80 Hz | 1.50 | 11.73 | 10.83 | 6.07 | 15.48 | 19.18 | 35.20 |
| 3.0%, 30 min, 80 Hz | 1.32 | 6.51 | 9.05 | 4.92 | 12.47 | 15.72 | 50.01 |
| 3.0%, 45 min, 80 Hz | 3.07 | 11.41 | 7.46 | 4.41 | 11.17 | 15.14 | 47.33 |
UCS: untreated cocoa shell.
Table 4.
Pearson’s coefficients of correlation.
| Variable | 0–50 µm | 51–71 µm | 72–100 µm | 101–125 µm | 126–200 µm | 201–315 µm | >315 µm | WBC (g/g) | OBC (g/g) | Insoluble Fibers (%) | Soluble Fibers (%) | Total Fibers (%) | Tannin (mg TA/g) | Tannin (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–50 µm | 1.000 | |||||||||||||
| 51–71 µm | 0.839 | 1.000 | ||||||||||||
| 72–100 µm | 0.006 | −0.083 | 1.000 | |||||||||||
| 101–125 µm | 0.364 | 0.199 | 0.819 | 1.000 | ||||||||||
| 126–200 µm | 0.430 | 0.402 | 0.803 | 0.908 | 1.000 | |||||||||
| 201–315 µm | −0.456 | −0.329 | 0.312 | 0.210 | 0.303 | 1.000 | ||||||||
| >315 µm | −0.809 | −0.795 | −0.467 | −0.717 | −0.851 | 0.018 | 1.000 | |||||||
| WBC (g/g) | −0.252 | −0.106 | −0.473 | −0.422 | −0.391 | 0.096 | 0.349 | 1.000 | ||||||
| OBC (g/g) | −0.827 | −0.838 | −0.103 | −0.479 | −0.600 | 0.114 | −0.862 | 0.233 | 1.000 | |||||
| Insoluble fibers (%) | −0.765 | −0.714 | −0.455 | −0.674 | −0.751 | 0.264 | 0.883 | 0.450 | 0.776 | 1.000 | ||||
| Soluble fibers (%) | −0.519 | −0.351 | 0.098 | −0.179 | −0.023 | 0.582 | 0.268 | 0.306 | 0.334 | 0.334 | 1.000 | |||
| Total fibers (%) | −0.791 | −0.723 | −0.425 | −0.666 | −0.722 | 0.318 | 0.875 | 0.464 | 0.780 | 0.994 | 0.435 | 1.000 | ||
| Tannin (mg TA/g) | −0.244 | −0.154 | 0.058 | −0.089 | 0.035 | 0.357 | 0.097 | −0.118 | −0.100 | 0.123 | 0.065 | 0.127 | 1.000 | |
| Tannin (%) | −0.768 | −0.747 | 0.004 | −0.365 | −0.511 | 0.018 | 0.762 | −0.014 | 0.844 | 0.635 | 0.167 | 0.627 | 0.067 | 1.000 |
Bold values were considered significant at p < 0.05.
3.4. Water and Oil Binding Capacity
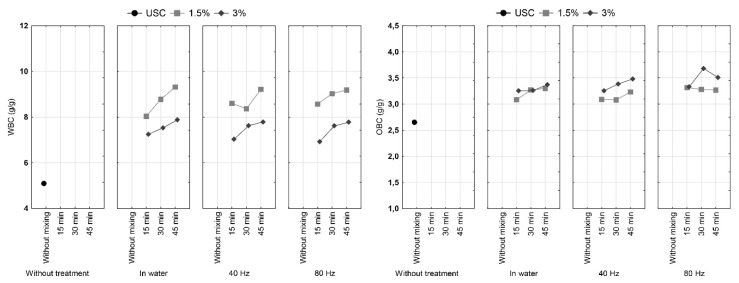
WBC and OBC are important parameters for processing of food and any change in these properties influences production process. Water binding capacity (WBC) and oil binding capacity (OBC) of cocoa shell samples are shown in Figure 4. It is visible that the sample of untreated cocoa shell had the lowest WBC and OBC. Samples treated at a concentration of 1.5% had the higher WBC compared to samples treated at a concentration of 3.0%. OBC showed the opposite trend, where samples treated at 3.0% had higher capacity for binding oil than samples treated at 1.5%. The largest increases can be observed in samples treated for 45 min in water and with HVED. Statistical analysis showed that there was a statistically significant difference between tested concentrations and shearing time but treatment (with or without HVED) did not show statistical significance. All combinations of these effects have proven to be significant (Table 1).
Figure 4.
Oil and water binding capacity of cocoa shell before and after the HVED treatment.
According to Sangnark and Noomhorm [31], water and oil binding capacity are correlated to particle size. This research also revealed correlation of OBC with particle sizes (Table 4). Additionally, porosity, overall charge density and hydrophobic properties of fibers, all of which may be changed by HVED treatment, can greatly affect WBC and OBC [31,32]. This may also be substantiated by correlation of OBC with total fiber, insoluble fiber and tannin content in this research.
3.5. FTIR-ATR
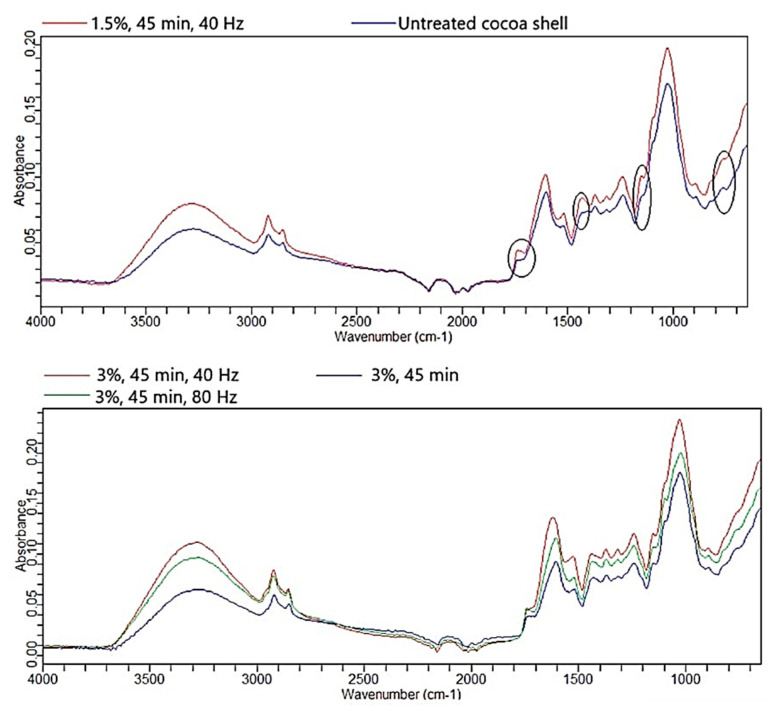
The changes in chemical composition by HVED treatment were supported by FTIR-ATR analysis. All the treatments had similar trend so only representative spectra are shown in Figure 5.
Figure 5.
Representative Fourier transform infrared spectroscopy with attenuated total reflection (FTIR-ATR) spectra of cocoa shell before and after the HVED treatment.
In untreated cocoa shell C=O stretching at 1737 cm−1 is presented only with a shoulder, and there is a peak at 1602.8 cm−1. After the treatment, a small peak appears at 1737 cm−1. Karahan and Özdoğan [30] ascribed this peak to ester groups of pectin. This is implying that increased content of soluble fibers may be linked to the appearance of this peak after the treatment. However, according to Günzler and Gremlich [33] and Grillo et al. [34], this is also C=O stretch in unconjugated esters, carboxylic acids, aldehydes and ketones.
C-H asymmetric deformation vibrations in untreated shell are presented through a shoulder at 1410 cm−1, whereas after the treatments peak appears at 1431.3 cm−1.
In the untreated cocoa shell there is a peak at 1028.7 cm−1 with two shoulders at 1096 cm−1 and 1148 cm−1. Treatments did not change shoulder at 1096 cm−1, unlike the other one that has shifted to 1155 cm−1 (C-H deformation) and a small peak appears there. This is also close to peak (1152 cm−1) of C-O-C asymmetric vibration in carbohydrates and glucosides according to Grillo et al. [34].
Untreated cocoa shell had small peak at 760 cm−1 (ring deformation vibrations). Treatments transfers this to shoulder.
These changes in spectra are the result of combined effect of changes in fiber composition (insoluble:soluble ratio) and phenol changes. Bozaci et al. [29] also observed shift of the bands after cold plasma treatment of jute fibers. They assigned this to reaction of fibers with active species from the plasma.
4. Conclusions
In essence, our study showed influence of HVED on fiber properties (soluble, insoluble and total fiber content) and related physical properties—occurrence of larger particle size and increase of water and oil binding capacity. In addition, it has been established that changes in fiber properties correlate to changes in tannin content. It is evident that HVED has a significant influence on the physical and chemical characteristics of cocoa shells due to formation of large number of reactive species, including free radicals and ions, and the reactions occurring during treatment need to be further examined in order to see why such changes are taking place and to reveal actual mechanisms that are involved. Other chemical characteristics of the modified cocoa shell in future studies should be considered as well. In addition, the effect of change in physical and chemical properties of shell on its applicability in different foods needs to be examined because its properties such as grinding, taste, color etc. are the main reasons for its non-use in food production. This research is valuable for future applications of untreated and cocoa shells treated with HVED in the food industry.
Author Contributions
Conceptualization, V.B. and Đ.A.; methodology, I.F., M.K.; investigation, V.B., I.F., M.B., A.J.; writing—original draft preparation, V.B., Đ.A.; writing—review and editing, A.J., J.B., D.Š., B.M., Đ.A.; visualization, A.J., K.D., M.J.; funding acquisition, Đ.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported in part by Croatian Science Foundation under the project UIP 2017-05-8709.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Arlorio M., Coisson J.D., Travaglia F., Varsaldi F., Miglio G., Lombardi G., Martelli A. Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res. Int. 2005;38:1009–1014. doi: 10.1016/j.foodres.2005.03.012. [DOI] [Google Scholar]
- 2.Martin-Cabrejas M.A., Valiente C., Esteban R.M., Molla E. Cocoa hull: A potential source of dietary fibre. J. Sci. Food Agric. 1994;66:307–311. doi: 10.1002/jsfa.2740660307. [DOI] [Google Scholar]
- 3.Redgwell R., Trovatoa V., Merinat S., Curti D., Hedigera S., Manez A. Dietary fibre in cocoa shell: Characterisation of component polysaccharides. Food Chem. 2003;81:103–112. doi: 10.1016/S0308-8146(02)00385-0. [DOI] [Google Scholar]
- 4.Hernández-Hernández C., Viera-Alcaide I., Morales-Sillero A.M., Fernández-Bolaños J., Rodríguez-Gutiérrez G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2017;240:831–839. doi: 10.1016/j.foodchem.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Chun K.S., Husseinsyah S., Osman H. Utilization of cocoa pod husk as filler in polypropylene biocomposites: Effect of maleated polypropylene. J. Compos. Mater. 2015;28:1507–1521. doi: 10.1177/0892705713513291. [DOI] [Google Scholar]
- 6.Panak Balentić J., Ačkar Đ., Jokić S., Jozinović A., Babić J., Miličević B., Šubarić D., Pavlović N. Cocoa shell: A by-product with great potential for wide application. Molecules. 2018;23:1404. doi: 10.3390/molecules23061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boussetta N., Turk M., De Taeye C., Larondelle Y., Lanoisellé J.L., Vorobiev E. Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Ind. Crop. Prod. 2013;49:690–696. doi: 10.1016/j.indcrop.2013.06.004. [DOI] [Google Scholar]
- 8.Yan L.-G., Deng Y., Ju T., Wu K., Xi J. Continuous high voltage electrical discharge extraction of flavonoids from peanut shells based on “annular gap type” treatment chamber. Food Chem. 2018;256:350–357. doi: 10.1016/j.foodchem.2018.02.129. [DOI] [PubMed] [Google Scholar]
- 9.Li Z., Fan Y., Xi J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019;277:246–260. doi: 10.1016/j.foodchem.2018.10.119. [DOI] [PubMed] [Google Scholar]
- 10.Boussetta N., Vorobiev E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. C. R. Chim. 2014;17:197–203. doi: 10.1016/j.crci.2013.11.011. [DOI] [Google Scholar]
- 11.Barišić V., Jozinović A., Flanjak I., Šubarić D., Babić J., Miličević B., Doko K., Ačkar Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability. 2020;12:3981. doi: 10.3390/su12103981. [DOI] [Google Scholar]
- 12.Amorim E.L.C., Nascimento J.E., Monteiro J.M., Sobrinho T.J.S.P., Araújo T.A.S., Albuquerque U.P. A simple and accurate procedure for the determination of tannin and flavonoid levels and some applications in ethnobotany and ethnopharmacology. Funct. Ecosyst. Communities. 2008;21:88–94. [Google Scholar]
- 13.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 14.AOAC Official Methods, Suppl. Association of Official Analytical Chemists; Rockville, MD, USA: Mar, 1995. AOAC 991.43. Total, soluble, and insoluble dietary fiber in foods: Enzymatic-gravimetric method, MES-TRIS buffer; pp. 7–9. Chapter 32. [Google Scholar]
- 15.AACC Methods Manual, Revised Ed. American Association of Cereal Chemists; St. Paul, MN, USA: 1983. AACC Method 88-04. [Google Scholar]
- 16.Barišić V., Flanjak I., Križić I., Jozinović A., Šubarić D., Babić J., Miličević B., Ačkar Đ. Impact of high-voltage electric discharge treatment on cocoa shell phenolic components and methylxanthines. J. Food Process Eng. 2020;43:e13057. doi: 10.1111/jfpe.13057. [DOI] [Google Scholar]
- 17.Jokić S., Pavlović N., Jozinović A., Ačkar Đ., Babić J., Šubarić D. High-voltage electric discharge extraction of bioactive compounds from the cocoa bean shell. Chem. Biochem. Eng. Q. 2019;33:271–280. doi: 10.15255/CABEQ.2018.1525. [DOI] [Google Scholar]
- 18.Delsart C., Grimi N., Boussetta N., Miot Sertier C., Ghidossi R., Vorobiev E., Mietton Peuchot M. Impact of pulsed-electric field and high-voltage electrical discharges on red wine microbial stabilization and quality characteristics. J. Appl. Microbiol. 2015;120:152–164. doi: 10.1111/jam.12981. [DOI] [PubMed] [Google Scholar]
- 19.Lukić K., Vukušić T., Tomašević M., Ćurko N., Gracin L., Kovačević Ganić K. The impact of high voltage electrical discharge plasma on the chromatic characteristics and phenolic composition of red and white wines. Innov. Food Sci. Emerg. Technol. 2019;53:70–77. doi: 10.1016/j.ifset.2017.11.004. [DOI] [Google Scholar]
- 20.Guyot S., Bernillon S., Poupard P., Renard M.G.C.C. Multiplicity of phenolic oxidation products in apple juices and ciders, from synthetic medium to commercial products. In: Daayf F., Lattanzio V., editors. Recent Advances in Polyphenol Research. Wiley-Blackwell; Hoboken, NJ, USA: 2008. pp. 278–293. [Google Scholar]
- 21.Lecumberri E., Mateos R., Izquierdo-Pulido M., Rupérez P., Goya L., Bravo L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.) Food Chem. 2007;104:948–954. doi: 10.1016/j.foodchem.2006.12.054. [DOI] [Google Scholar]
- 22.Bravo L., Grades N., Saura-Calixto F. Composition and potential uses of mesquite pods (Prosopis pallida L): Comparison with carob pods (Ceratonia siliqua L) J. Sci. Food Agric. 1994;65:303–306. doi: 10.1002/jsfa.2740650307. [DOI] [Google Scholar]
- 23.Saura-Calixto F. Dietary fibre complex in a sample rich in condensed tannins and uronic acids. Food Chem. 1987;23:95–103. doi: 10.1016/0308-8146(87)90003-3. [DOI] [Google Scholar]
- 24.Perez E., Mendez A., Leon M., Hernandez G., Sivoli L. Proximal composition and the nutritional and functional properties of cococa by-products (pods and husks) for their use in the food industry. In: Perez Sira E., editor. Chocolate-Cocoa Byproducts Technology, Rheology, Styling, and Nutrition. Nova; New York, NY, USA: 2015. pp. 219–230. [Google Scholar]
- 25.Barišić V., Flanjak I., Tot A., Budeč M., Benšić M., Jozinović A., Babić J., Šubarić D., Miličević B., Ačkar Đ. 5-hydroxymethylfurfural and acrylamide content of cocoa shell treated with high voltage electrical discharge. Food Cont. 2020:107043. doi: 10.1016/j.foodcont.2019.107043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozdal T., Capanoglu E., Altay F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013;51:954–970. doi: 10.1016/j.foodres.2013.02.009. [DOI] [Google Scholar]
- 27.Yuan X., Jayaraman K., Bhattacharyya D. Effects of plasma treatment in enhancing the performance of woodfibre-polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2004;35:1363–1374. doi: 10.1016/j.compositesa.2004.06.023. [DOI] [Google Scholar]
- 28.Sinha E., Panigrahi S. Effect of plasma treatment on structure, wettability of jute fiber and flexural strength of its composite. J. Compos. Mater. 2009;43:1791–1802. doi: 10.1177/0021998309338078. [DOI] [Google Scholar]
- 29.Bozaci E., Sever K., Demir A., Seki Y., Sarikanat M., Ozdogan E. Effect of the atmospheric plasma treatment parameters on surface and mechanical properties of jute fabric. Fibers Polym. 2009;10:781–786. doi: 10.1007/s12221-009-0781-6. [DOI] [Google Scholar]
- 30.Karahan H.A., Özdoğan E. Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. Fibers Polym. 2008;9:21–26. doi: 10.1007/s12221-008-0004-6. [DOI] [Google Scholar]
- 31.Sangnark A., Noomhorm A. Effect of dietary fibre from sugarcane bagasse and sucrose esteron doughand bread properties. LWT. 2004;37:697–704. doi: 10.1016/j.lwt.2004.02.015. [DOI] [Google Scholar]
- 32.Ulbrich M., Flöter E. Impact of high pressure homogenization modification of a cellulose based fiber product on water binding properties. Food Hydrocoll. 2014;41:281–289. doi: 10.1016/j.foodhyd.2014.04.020. [DOI] [Google Scholar]
- 33.Günzler H., Gremlich H. IR-Spektroskopie: Eine Einführung. 4th ed. WILEY-VCH GmbH & Co. KGaA; Weinheim, Germany: 2003. [Google Scholar]
- 34.Grillo G., Boffa L., Binello A., Mantegna S., Cravotto G., Chemat F., Dizhbite T., Lauberte L., Telysheva G. Analytical dataset of Ecuadorian cocoa shells and beans. Data Brief. 2019;22:56–64. doi: 10.1016/j.dib.2018.11.129. [DOI] [PMC free article] [PubMed] [Google Scholar]