Abstract
Shortening derived from palm oil is widely used in baking applications. However, palm oil and the related products are reported to contain high levels of monochloropropandiol (MCPD) ester and glycidyl ester (GE). MCPD and glycidol are known as process contaminants, which are carcinogenic and genotoxic compounds, respectively. The objective was to evaluate the effects of antioxidant addition in palm olein and stearin to the content of MCPD esters and GE in baked cake. Butylated hydroxyanisole (BHA), rosemary extract and tocopherol were used to fortify the samples at 200 mg/kg and in combinations (400, 600 and 800 mg/kg rosemary or tocopherol combined with 200 mg/kg BHA). The MCPD esters and GE content, radical formation and the quality of the fats portion were analyzed. The results showed that palm olein fortified with rosemary extract yielded less 2-MCPD ester. The GE content was lower when soft stearin was fortified with rosemary. ESR spectrometry measurements showed that the antioxidants were effective to reduce radical formation. The synergistic effects of combining antioxidants controlled the contaminants formation. In conclusion, oxidation stability was comparable either in the single or combined antioxidants. Tocopherol in combination with BHA was more effective in controlling the MCPD esters and GE formation.
Keywords: radical, palm-based shortening, baking system, MCPD esters mitigation, GE mitigation
1. Introduction
Palm oil and the derivative products from palm oil are well-recognized as an important raw material in the food industry [1]. Palm oil is unique due to the balance in its saturated and unsaturated fatty acid composition [2]. Each fraction derived from palm oil serves a specific function in the food industry [3]. Fractionation of palm oil yields olein and stearin fractions. The olein fraction is used as a frying medium due to its remarkable oxidation stability. On the other hand, stearin (solid fraction) provides consistency, portability and texture to the products [4].
Palm oil in the baking industry functions as a shortening. A shortening is a pure fat product containing animal and/or vegetable oils that is formulated and processed according to functionality [3]. The stearin fraction of palm oil performs as a good shortening due to its high solid fat content (SFC) and β’ polymorphic form [5]. In addition, a recent study showed that the application of soft fraction of palm oil can deliver a higher volume in baked cakes [6]. Generally, a highly functional specialty fat is achievable by blending, hydrogenation, and interesterification process [7].
Generally, palm oil and fats are known to contain higher monochloropropandiol (MCPD) esters and glycidyl esters (GE) compared to other types of refined vegetable oils [8]. Furthermore, 2- and 3-MCPD esters are heat-induced food contaminants, and they are carcinogenic [9]. In addition, GE is often considered a coexisting contaminant with MCPD esters in refined vegetable oils and is shown to be genotoxic in animal studies [10].
Furthermore, MCPD esters and/or GE can be induced by processing under suitable conditions. Water, salt, glycerol or lipid precursors undergoing thermal processing can trigger the formation of MCPD esters [11]. Studies have reported that glycerol can act as a strong precursor to form the 3-MCPD ester on a weight basis [12]. Other important precursors, such as monoacylglycerol (MAG), diacylglycerol (DAG) and triacylglycerol (TAG), also play roles in the formation of MCPD esters [13]. Four MCPD ester formation pathways were reported by Rahn and Yaylayan, which are directly responsible for the nucleophilic attack at the glycerol carbon carrying an ester group or a hydroxyl group, formation of the acyloxonium ion and formation of the glycidol ester with an epoxide ring [14]. Apart from these well-known mechanisms, 3-MCPD ester (di-ester form) is believed to be formed using a DAG undergoing free radical reaction mechanisms [15].
Since the generation of free radicals is also related to heating processes, investigation of the effects of antioxidant on the changes in MCPD esters and GE during conventional baking using palm-based shortening is the main focus of this study. Antioxidants used in food applications can extend the fatty food storage time by slowing down the oxidation process [16] For example, butylated hydroxyanisole (BHA) is used in fried food, and the application of two or more antioxidants can act synergistically to maximize the functional properties [17,18]. It is also reported that antioxidant extracts incorporated into the food or food packaging material can hinder the lipid deterioration within a meat product [19,20]. Generally, the antioxidants prevent the generation of free radical (e.g., hydroxyl radicals) by acting as a hydrogen or electron donor [21,22]. Additionally, a previous study of the changes in 3-MCPD ester and GE content of a frying system suggested addition of antioxidants to the palm olein was effective to reduce the level of MCPD ester and GE contaminations [23]. As MCPD ester formation can be triggered via a radical mechanism [24], reducing radical formations by antioxidant fortification in baking recipes is a potential mitigation tool that is a straightforward and low-cost alternative.
Indeed, the maximum level of GE has been set at 1 mg/kg for vegetables fats and oils as final consumers oil or as food ingredient. A more stringent limit, which is 0.5 mg/kg of GE, is allowed when the vegetable fats and oils are destined for baby food. At the same time, the European Commission has suggested the maximum level of 3-MCPD esters of palm oil is 2.5 mg/kg and the limit from a vegetable oil derived from coconut, maize, rapeseed, sunflower, soybean and palm kernel oil should not have 3-MCPD esters of more than 1.25 mg/kg. Meanwhile, the tolerable daily intake (TDI) of 3-MCPD is 2 µg/kg body weight [25,26].
In this study, palm olein and soft stearin were chosen as the shortening in a recipe based on an evaluation study of quality characteristics attributed to the finished products in our laboratory [6]. The shortening was fortified with BHA, rosemary extract and tocopherol, either in a single dosage or in combination with natural and synthetic antioxidants. Effects of different antioxidants on the changes in the radicals present in the samples, MCPD esters, GE, oxidation stage (specific extinction values) and acylglycerol composition of the fats portion were studied.
2. Materials and Methods
2.1. Materials and Chemicals
All the baking ingredients were purchased from a local supplier (JC Rainbow, Selangor, Malaysia), except the whole egg powder was obtained from AgroEgg (Singapore). Palm olein and soft stearin were donated by a local palm oil refinery company. Baking materials were kept in an air-tight container at room temperature, while the egg powder and fats portion were kept under a chilled temperature (4 °C) until used. All the chemicals were purchased from a local supplier (Merck, Selangor, Malaysia). MCPD esters and GE standards were purchased from Toronto Research Chemicals, Inc. (North York, ON, Canada). All chemicals were conserved in suitable condition according to the material safety data sheet (MSDS) while the MCPD ester and GE standards were kept in amber bottles at −20 °C prior to the analysis.
2.2. Fortification of Palm Olein and Soft Stearin with Antioxidants
Each of the antioxidants, BHA (butylated hydroxyanisole, 96%, Fisher Scientific Acros, Pittsburgh, PA 15275, USA), rosemary extract (Oleoresin Rosemary, Herbalox brand, Type O) and tocopherol (DL-α-Tocopherol, 99%, Merck, Selangor, Malaysia), was measured at the weight needed and added to the palm olein or stearin to make a final concentration of antioxidants in the oil of 200 mg/kg. The fats and antioxidants were placed in a 1000-mL beaker and heated over a hotplate stirrer, stirred at 200 rpm, at 50 °C for 10 min, to homogenize the sample. A similar procedure was applied with different concentrations of the natural antioxidants (400, 800 and 1200 mg/kg) and a fixed amount of BHA (200 mg/kg).
2.3. Sample Preparation
The cake samples were prepared based on a modified Madeira cake recipe from a previous study. The recipe was measured in weight basis instead of percentage to fix the number of the sample size during each preparation. Each portion of the recipe was made up of 40 g fat fortified with the antioxidant, 120 g wheat flour, 50 g granulated sugar, 100 g egg powder mix (ratio of egg powder to water, 1:3) and 5 g leavening agent (sodium bicarbonate) [27].
The preparation began with mixing the fats and granulated sugar using a kitchen mixer for 10 min until homogenized. Whole egg powder was mixed with water at given ratio, and it was added to mix for another 5 min. Finally, the flour and baking powder were added together. The mixing was continued for another 5 min. The speed of the kitchen mixer was maintained at medium speed throughout the mixing processes. The well-mixed batter was distributed into a 5 cm × 5 cm × 5 cm paper cup at 40 ± 1 g. Each bake in the oven produced 25 pieces of cake in the middle tray. The baking temperature was fixed at 160 °C, and the baking duration was 20 min throughout the sample preparation. The described design during baking was adapted from a previous study in order to stabilize the heat distribution during each batch of the baking process [6].
2.4. Fat Extraction
The fats portion from the cake sample were extracted using a fat extraction system (SoxtecTM 8000, Foss, Hilleroed, Denmark). The sample was pre-dried in an oven for 24 h at 98 °C. Every 2 g of sample was extracted with 80 mL petroleum ether (boiling point 40–60 °C, AR grade, Merck, Selangor, Malaysia) as the extraction solvent.
2.5. Free Fatty Acid Analysis (Acidity Value)
The free fatty acid content was determined by a modified AOCS Official Method Ca 5a-40. Samples were titrated against alkaline (NaOH) to the phenolphthalein end-point. Every 2 g of test sample was diluted with 10 mL of hot neutralized alcohol (95% ethanol) and 0.5 mL of phenolphthalein indicator. The normality of the NaOH solution was 0.0005 M. The FFA content was expressed as the palmitic acid equivalent in percentage per gram of oil sample [28,29].
2.6. Electron Spin Resonance Measurements
The electron spin resonance (ESR) spectral analysis and G-value measurements were performed using an ESR spectrometer (JES-FA 2000, JEOL, Tokyo, Japan). A sample portion of 10 mg was placed in a quartz sample tube. To visualize the free radical activities, the measurement conditions of the first derivative spectrum used a 9181.6 MHz microwave frequency at 1 mW power, 1.2 mT modulation amplitude, 0.03 s time constant and 60 s sweep time. The free radical was compared to the signal intensity, expressed in an arbitrary unit per unit sample weight (AU/mg).
2.7. Total Chlorine Analysis
The total chlorine was analyzed using energy dispersive X-ray (EDX) fluorescence spectrometry (EDX-8000, Shimadzu, Kyoto, Japan). Briefly, a 2 g dried cake sample was lightly crushed and placed into a sample holder (31 mm X-Cell) and covered by an EDX film (3525 Ultralene, 4 µm thickness). Then, the cells were placed on the sample tray for measurement. Measurement was started by internal calibration against a blank sample. Then, samples were measured and rotated automatically according to the sample tray position. The content of total chlorine analyzed will be listed as its percentage.
2.8. Oxidation of the Fats Portion
A portion of the 20 mg oil sample was measured and diluted in 100 mL of isooctane. An absorbance value of the specific extinction values of 232 nm and 268 nm was measured as described in the AOCS Official Method, Ch. 5-91.
2.9. Aclyglycerol Composition
The FFA and monoacylglycerol (MAG) to DAG ratio as well as the 1,3-DAG to 1,2-DAG ratio were analyzed according to the AOCS Official Method Cd 11-96 using an HPLC system (Alliance model Waters 22.695 Separation Modules, Wilmslow, UK) equipped with an ELSD detector (Alliance model Waters 2424 ELS Detector, Wilmslow, UK).
The test sample was diluted in a mixture of n-hexane and isopropanol (ratio 9:1). The diluted sample was filtered through a PTFE membrane filter and injected into a normal phase silica column (150 mm × 4.6 mm, LiChrospher ® 60 Si (10 µm), Hibar, Merck, Selangor, Malaysia). The injection volume was 20 µL. Eluent A (n-hexane) and eluent B (n-hexane:ethyl acetate:isopropanol at an 8:1:1 ratio) were used to establish a gradient program. The gradient started with 98% of eluent A and 2% of eluent B and reached 65% eluent A and 35% of eluent B at 8 min; then, 2% of eluent A and 98% of eluent B at 8.5 min, held for 15 min; and lastly, 98% of eluent A and 2% of eluent B at 15.1 min, held until 19 min. Total flow rate of the eluent was 2 mL/min. The column oven temperature was 40 °C, while the drift tube and temperature of the detector was 60 and 36 °C, respectively. Nitrogen gas flow was constant at 40 psi.
2.10. MCPD Esters and GE Analysis
Determination of the 2-MCPD ester, 3-MCPD ester and GE was carried out by using an indirect quantification method according to the AOCS Official Method Cd 29a-13. All the sample preparation procedures were carried out as described in the manual. A derivatized sample was injected into a gas chromatograph with a triple quadrupole mass spectrometer detector (Shimadzu, GCMS-TQ 8040, Kyoto, Japan). The sample injection amount was 1 µL. Separation was performed using a capillary column Equity-1MS (Supelco, 30 m length, 0.25 mm i.d., and 1.0 μm film thickness, Taufkirchen, Germany). The transfer line temperature was 300 °C, followed by an increasing temperature program (80 °C at the start, held for 1 min; 10 °C/min until 170 °C; 3 °C/min until 200 °C; and then 15 °C/min until 300 °C, held for 15 min). Multiple reaction monitoring (MRM) was used to detect the presence of MCPD esters and GE. Each compound was identified and quantified based on the targeted ion and reference ion. Targeted ion pairs of 3-MCPD-d5, 3-MCPD and 2-MCPD were m/z 150.00 > 93.10, 147 > 91.10 and 196 > 104.10, respectively. Meanwhile, targeted ion pairs of 3-MBPD-d5 and 3-MBPD (GE) were 150 > 93.10 and 242.00 > 147.10, respectively [30].
2.11. Statistical Analysis
Data are presented as the mean value with the standard deviation. Calculation of the mean values, standard deviations and statistical analyses were computed using MINITAB 17 software (Minitab Inc., State College, PA, USA). Significance among the mean values of the data was tested by one-way analysis of variance (ANOVA) with Tukey’s test at a confidence level of 95% (α = 0.05).
3. Results and Discussion
3.1. Effects of Different Antioxidants on the Changes of MCPD Esters and GE
The concentration of the raw samples (without antioxidants) were checked prior to the experiments. The 3-MCPD ester content of palm olein and soft stearin was between 3.15 and 4.19 mg/kg and 2.38 and 2.59 mg/kg, respectively. The content of 2-MCPD esters of palm olein and soft stearin was about half of the 3-MCPD ester, which was recorded for palm olein and soft stearin sample as between 1.77 and 2.11 mg/kg and 1.29 and 1.40 mg/kg, respectively. Lastly, the GE content of the palm olein sample was between 5.82 and 7.05 mg/kg, while a soft stearin sample contained GE at a concentration between 2.82 and 3.12 mg/kg. These findings are in agreement with previous findings in palm olein samples for frying [31], margarine samples from the market [30] and a market survey on palm oil samples from different countries [32]. GE is commonly higher than the content of 3-MCPD esters, as stated in the EFSA journal in 2016 [8]. The experiment was continued by analyzing the effects of an antioxidant at a fixed concentration (200 mg/kg) on the changes of MCPD esters and GE content after baking at 160 °C for 20 min using palm olein or soft stearin as the shortening. From Table 1, the content of the 2-MCPD ester found in palm olein fortified with 200 mg/kg rosemary extract and tocopherol was significantly lower compared to BHA. In addition, in the soft stearin sample, the rosemary extract-fortified sample yielded a lower GE content at 0.607 mg/kg. Overall, the content of 2- and 3-MCPD ester was found slightly lower when rosemary extract and tocopherol were used compared to BHA.
Table 1.
Effects of different antioxidants (200 ppm) on the content of 2-monochloropropandiol (MCPD), 3-MCPD and glycidyl ester (GE) on the fats portion extracted from cake baked at 160 °C for 20 min with different shortenings.
| Sample | Antioxidant | 3-MCPD, mg/kg | 2-MCPD, mg/kg | GE, mg/kg |
|---|---|---|---|---|
| BHA | 3.439 ± 0.029 a | 2.334 ± 0.021 a | 1.987 ± 0.039 a | |
| Palm Olein | Rosemary | 3.431 ± 0.065 a | 2.051 ± 0.010 b | 1.979 ± 0.029 a |
| Tocopherol | 3.527 ± 0.189 a | 2.143 ± 0.105 b | 1.985 ± 0.047 a | |
| BHA | 2.222 ± 0.028 a | 1.534 ± 0.114 a | 0.665 ± 0.001 a | |
| Soft Stearin | Rosemary | 2.176 ± 0.032 a | 1.489 ± 0.040 a | 0.607 ± 0.032 b |
| Tocopherol | 2.172 ± 0.023 a | 1.435 ± 0.012 a | 0.668 ± 0.010 a |
Values are the mean of two determinations from two replicate experiments ± standard deviation (n = 4). Mean values with different superscript letters in the same column are significantly different at p < 0.05.
3.2. Electron Spin Resonance Measurement
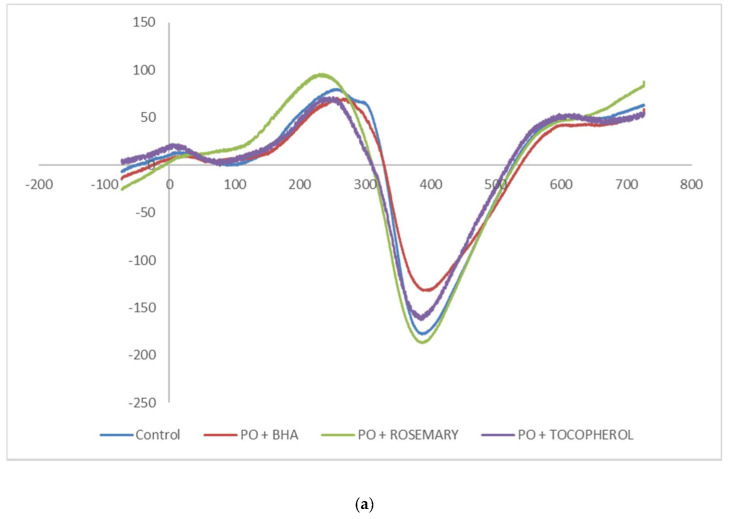
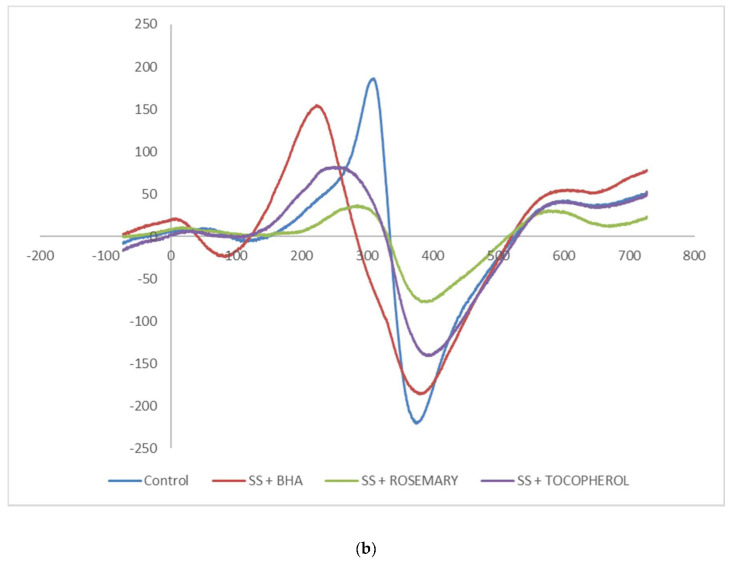
The Electron Spin Resonance (ESR) spectra as shown in Figure 1a,b are palm olein and soft stearin samples, respectively. ESR can measure the chemical species with unpaired electron(s), and they are normally referring to, but not limited to, the free radicals and transition metal ions [33]. From Figure 1a, the results showed similar ESR spectra among the palm olein samples. The signal intensity from the highest to lowest was rosemary ≈ control (without antioxidant added) > tocopherol > BHA. However, adding antioxidants to the soft stearin sample yielded different results. From Figure 1b, the ESR signal intensity was highest in the control sample, indicating significant amounts of radicals were present, followed in the signal intensity sequence by BHA > tocopherol > rosemary.
Figure 1.
(a) The ESR spectra of a palm olein sample fortified with BHA, rosemary extract and tocopherol at 200 mg/kg and the control (no antioxidant); (b) the ESR spectra of a soft stearin sample fortified with BHA, rosemary extract and tocopherol at 200 mg/kg and the control (no antioxidant).
The results clearly showed that addition of antioxidants to the baking recipe was effective to reduce the radical presence in the sample. The reduced signal suggested antioxidant was able to scavenge the free radicals [34] formed during the baking process.
The G-values obtained from the ESR spectrum are listed in Table 2. The G-value is defined as the number of molecules destroyed or products formed per 100 eV of energy absorbed [35]. The G-value of a substance is normally constant, and the G-value of a free radical is 2.00. The G-values of the control samples were 1.98 and 1.96 for palm olein and soft stearin, respectively. A G-value less than 2.00 is considered an electron-poor substance. Therefore, addition of antioxidants to soft stearin was more effective to inhibit the production of radicals. Soft stearin has more saturated fatty acid than palm olein, and the saturated structures are resistant to reactions such as oxidation.
Table 2.
Effects of different antioxidants (200 ppm) on the FFA content, specific extinction value and acylglycerol composition in the fats portion extracted from cake baked at 160 °C for 20 min with different shortenings.
| Sample | Antioxidant | FFA, % | K268, CT | K232, CD | FFA/DAG | MAG/DAG | 1,3/1,2-DAG | G-Value | Total Chlorine, % |
|---|---|---|---|---|---|---|---|---|---|
| Palm Olein | BHA | 0.39 ± 0.14 b | 0.757 ± 0.072 a | 4.393 ± 0.505 b | 0.202 ± 0.006 b | 0.018 ± 0.002 a | 2.599 ± 0.017 a | 2.000 ± 0.004 b | 0.182 ± 0.010 a |
| Rosemary | 0.27 ± 0.05 ab | 0.753 ± 0.111 a | 4.149 ± 0.497 b | 0.172 ± 0.005 c | 0.018 ± 0.004 a | 2.583 ± 0.053 a | 2.120 ± 0.010 a | 0.181 ± 0.005 a | |
| Tocopherol | 0.49 ± 0.03 a | 0.832 ± 0.067 a | 5.076 ± 0.339 a | 0.236 ± 0.010 a | 0.017 ± 0.003 a | 2.499 ± 0.028 b | 2.133 ± 0.032 a | 0.203 ± 0.007 a | |
| Soft Stearin | BHA | 0.32 ± 0.04 a | 0.617 ± 0.012 b | 3.317 ± 0.067 b | 0.224 ± 0.009 b | 0.018 ± 0.002 b | 2.558 ± 0.052 a | 2.329 ± 0.022 a | 0.176 ± 0.017 a |
| Rosemary | 0.24 ± 0.03 b | 0.621 ± 0.001 b | 3.298 ± 0.004 b | 0.176 ± 0.016 c | 0.018 ± 0.001 b | 2.415 ± 0.078 b | 1.980 ± 0.002 b | 0.183 ± 0.001 a | |
| Tocopherol | 0.31 ± 0.02 a | 0.642 ± 0.018 a | 3.845 ± 0.099 a | 0.282 ± 0.018 a | 0.024 ± 0.003 a | 2.565 ± 0.067 a | 1.999 ± 0.003 b | 0.188 ± 0.004 a |
Values are the mean of three determinations from two replicate experiments ± standard deviation (n = 6), except for the mean values of G-value and total chlorine %, which are the mean of two determinations from two replicate experiments ± standard deviation (n = 4). Mean values with different superscript letters in the same column are significantly different at p < 0.05.
3.3. Oxidation and Stability of the Fats Portion with Addition of Single Antioxidant
The fats extracted from the baked sample were further analyzed with the oxidation state (K268 and K232 value), free fatty acid (FFA) content and the ratio of acylglycerol composition to DAG. Table 2 shows that the rosemary extract was able to suppress the formation of FFA in both the palm olein and soft stearin samples during the baking process. The FFA content was between 0.24% and 0.27% of the palmitic acid equivalent.
A previous study on antioxidant addition to frying palm olein showed that rosemary extract was able to perverse the oil quality in terms of oxidation stability [23]. Similarly, rosemary addition to the shortening was able to give a lower FFA-to-DAG ratio compared to BHA or tocopherol. In terms of the FFA-to-DAG ratio, we observed that the rosemary extract was able to inhibit the formation of FFA and/or DAG in the fats portion, followed by BHA and tocopherol. The formation of FFA and DAG was derived from TAG hydrolysis, and results showed that addition of rosemary extract at 200 mg/kg was effective to suppress the hydrolysis process among the samples studied.
The presence of 1,3 DAG is always abundant in palm-based fats and oils because DAG is a more stable isomer than DAG with a fatty acid chain at the 1,2-positions [36]. The ratio of 1,3-DAG to 1,2-DAG among all the samples studied ranged from 2.415 to 2.599. These values were lower than the control sample. The control sample without antioxidant addition showed a 1,3- to 1,2-DAG ratio at 2.583 and 2.898, for palm olein and soft stearin, respectively. This observation showed that the antioxidant addition was not effective to maintain the DAG in its stable form, which is the 1,3-position of DAG. The results obtained from the same laboratory, with the baking system at a higher temperature (180 or 200 °C), further lowered the ratio of 1,3- to 1,2-DAG because hydrolysis of TAG yielded unstable 1,2-DAG instead of stabilized 1,3-DAG [6].
The oxidation state of the samples was checked by specific extinction values. The values as shown in Table 2, for samples fortified with BHA and rosemary, were able to give a close absorbance at K268 and K232. However, tocopherol was not as effective as BHA and/or rosemary to inhibit the formation of conjugated dienes (CDs) and trienes (CTs). Tocopherol had a significantly (p < 0.05) higher value of CT and CD observed in both tocopherol-fortified palm olein and soft stearin.
3.4. Synergistic Effect of Antioxidant on the Changes in MCPD Esters and GE
From the discussion in the above section, MCPD esters and GE changes were not obvious when only a single dose of antioxidant was used. Therefore, the experiment was extended to fortify the shortening in combination with BHA and either rosemary extract or tocopherol. Rosemary or tocopherol was added to the shortening containing 200 mg/kg BHA at 400, 800 and 1200 mg/kg. Data obtained are shown in Figure 2.
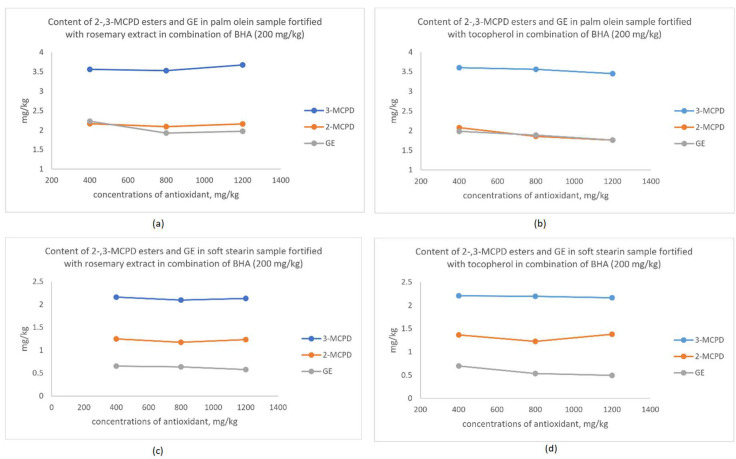
Figure 2.
(a) Changes of 3- and 2-MCPD esters and GE content in a palm olein sample with different concentrations of rosemary extract and BHA (200 mg/kg). (b) Changes in 3- and 2-MCPD esters and GE content in a palm olein sample with different concentrations of tocopherol and BHA (200 mg/kg). (c) Changes of 3- and 2-MCPD esters and GE content in a soft stearin sample with different concentrations of rosemary extract and BHA (200 mg/kg). (d) Changes of 3- and 2-MCPD esters and GE content in a soft stearin sample with different concentrations of tocopherol and BHA (200 mg/kg).
In the palm olein sample (see Figure 2a,b), when the rosemary extract was added at concentrations of 800 and 1200 mg/kg in combination with 200 mg/kg of BHA, the GE content was able to be reduced from 2.230 mg/kg to 1.925 and 1.972 mg/kg. The reduction was statistically significant (p < 0.05). However, addition of tocopherol in combination with BHA was more effective in lowering MCPD esters and GE formation during the baking process. Tocopherol addition at a concentration of 800 mg/kg was sufficient to reduce the formation of 2-MCPD ester (1.854 mg/kg) and GE (1.888 mg/kg). Notably, the content of 2- and 3-MCPD esters and GE were lowered significantly (p < 0.05) to 1.758 mg/kg, 3.453 mg/kg and 1.761 mg/kg, respectively, when the tocopherol concentration was increased to 1200 mg/kg.
In the soft stearin sample (see Figure 2c,d), the 3-MCPD ester content was reduced with 800 mg/kg of rosemary extract in combination with 200 mg/kg BHA. No significant changes occurred in the 2-MCPD ester and GE content. The GE content was reduced significantly (p < 0.05) from 0.696 mg/kg to 0.533 and 0.494 mg/kg when the addition of tocopherol was increased to 800 and 1200 mg/kg.
Overall, the figures showed that tocopherol combined with BHA was more effective in producing a finished product with lower contaminants compared to the rosemary extract in combination with BHA. Since tocopherol alone was able to inhibit the radical formation (Figure 1), its synergistic effects with BHA was expected. Total antioxidant capacities by combining antioxidants was proven to be more effectives in scavenging the radical [37]. Therefore, the radical-induced forming mechanism of the MCPD esters and GE [15] was interrupted by the tocopherol and rosemary extract combined with BHA.
3.5. Synergistic Effect of Antioxidants on the Changes of Oxidation State and Stability
From Table 3, the results showed that the overall changes of the oxidation state and stability of the acylglycerol composition was stable by antioxidant synergistic effects. In terms of oxidation state, rosemary when combined with BHA was more effective in inhibiting the formation of CT. The ratio among FFA/DAG, MAG/DAG and 1,3-/1,2-DAG was very similar for each treatment. The oxidation level of the samples studied was considered at its minimal level according to the synergistic effects of rosemary or tocopherol addition combined with BHA. The FFA % by titration method showed an increased FFA % when the concentration of the antioxidant was higher, but that did not affect the specific extinction value and acylglycerol composition. In short, the data agreed with the previous findings stated that the synergism between the antioxidants works to control lipid oxidation in a Rancimat test [38] and in sunflower oil triacylglycerol [39].
Table 3.
Synergistic effects of the antioxidants on the FFA content, specific extinction values and acylglycerol composition in the fats portion extracted from cake baked at 160 °C for 20 min with different shortenings.
| Sample | Antioxidants | FFA, % | K268, CT | K232, CD | FFA/DAG | MAG/DAG | 1,3/1,2-DAG |
|---|---|---|---|---|---|---|---|
| Palm Olein | Rosemary, mg/kg |
||||||
| 400 | 0.34 ± 0.13 bA | 0.891 ± 0.029 bB | 3.453 ± 0.105 aB | 0.134 ± 0.023 aA | 0.015 ± 0.004 aA | 2.367 ± 0.079 bA | |
| 800 | 0.53 ± 0.04 aB | 0.886 ± 0.010 bB | 3.487 ± 0.073 aB | 0.123 ± 0.020 aB | 0.016 ± 0.004 aB | 2.564 ± 0.045 aA | |
| 1200 | 0.42 ± 0.08 abA | 0.903 ± 0.001 aB | 3.541 ± 0.054 aB | 0.108 ± 0.014 aB | 0.018 ± 0.005 aA | 2.416 ± 0.096 aA | |
| Tocopherol, mg/kg |
|||||||
| 400 | 0.39 ± 0.04 bA | 1.008 ± 0.028 bA | 4.112 ± 0.087 cA | 0.148 ± 0.011 bA | 0.018 ± 0.003 aA | 2.346 ± 0.064 bA | |
| 800 | 0.63 ± 0.05 aA | 0.961 ± 0.001 cA | 4.536 ± 0.025 bA | 0.223 ± 0.001 aA | 0.024 ± 0.005 aA | 2.472 ± 0.063 aA | |
| 1200 | 0.53 ± 0.07 aA | 1.085 ± 0.002 aA | 4.811 ± 0.008 aA | 0.217 ± 0.006 aA | 0.019 ± 0.005 aA | 2.323 ± 0.050 bA | |
| Soft Stearin | Rosemary, mg/kg |
||||||
| 400 | 0.39 ± 0.06 bA | 0.728 ± 0.065 bB | 3.076 ± 0.045 bB | 0.160 ± 0.004 cB | 0.019 ± 0.004 aA | 2.477 ± 0.077 aA | |
| 800 | 0.55 ± 0.07 aA | 0.740 ± 0.008 bB | 2.899 ± 0.059 cB | 0.183 ± 0.005 bB | 0.028 ± 0.009 aA | 2.588 ± 0.034 aA | |
| 1200 | 0.41 ± 0.06 aA | 0.879 ± 0.006 aA | 4.171 ± 0.013 aA | 0.269 ± 0.011 aA | 0.019 ± 0.006 aA | 2.420 ± 0.121 aA | |
| Tocopherol, mg/kg |
|||||||
| 400 | 0.39 ± 0.03 abA | 0.822 ± 0.009 aA | 3.712 ± 0.034 sA | 0.230 ± 0.032 bA | 0.020 ± 0.004 aA | 2.512 ± 0.121 aA | |
| 800 | 0.43 ± 0.04 aB | 0.781 ± 0.002 bA | 3.678 ± 0.002 aA | 0.282 ± 0.013 aA | 0.024 ± 0.005 aA | 2.523 ± 0.126 aA | |
| 1200 | 0.35 ± 0.03 bA | 0.757 ± 0.003 cB | 2.993 ± 0.018 aB | 0.280 ± 0.010 aA | 0.025 ± 0.001 aA | 2.403 ± 0.059 aA |
Values are the mean of three determinations from two replicate experiments ± standard deviation (n = 6). Mean values with different superscript letters in the same column are significantly different at p < 0.05 by ANOVA and the Tukey test. Mean AB values of the same column for each parameter are significantly different at p < 0.05 (comparison between rosemary and tocopherol addition in each sample).
3.6. MCPD Esters and GE Formation with the Presence of Potential Precursors
MCPD esters formation pathways are well discussed in the available literature. MCPD esters can be formed via a direct substitution of the chlorine anion by a glycerol carbon atom (replacing either the OH group or a fatty acid ester group), and via an intermediate epoxide ring or acyloxonium group before a nucleophilic attack by a chlorine anion [14]. In addition, a more recent study also suggested a chlorine radical or chlorine compound attack on a cyclic acyloxonium free radical intermediate, through the free radical mechanism [24,40].
By looking at these possible formation mechanisms described in the available literature, the baking system is a favorable system to encourage the formation of MCPD esters and/or GE. In addition, chlorine, as one of the important precursors for 3-MCPD ester formation, can be introduced through various sources of ingredients, such as flour [41]. The relationship between chlorine content and the 3-MCPD ester has proven to be a strong positive one based on a previous study [42]. However, the recipe used during the experiments was controlled and therefore no significant change in the total chlorine percentage was observed throughout the experiment (see Table 2). Therefore, it is known that the formation or inhibition of the MCPD esters by antioxidants in this study was not affected by the factor of chlorine amount. The MCPD esters are formed through multiple reaction pathways, and different pathways can occur simultaneously. Moreover, the formation and decomposition of the MCPD esters should occur simultaneously as well. For instance, the scavenging activity exhibited by the rosemary extract was positive in the soft stearin sample (ESR measurements), but the content of the 2- and 3-MCPD esters was not reduced significantly. Similarly, the presence of tocopherol in combination with BHA was able to give a finished product with lower MCPD esters, but the ability to inhibit oxidation was weaker. The observations showed that there was no direct relationship between the presence of radical as a potential precursor to the MCPD esters formation. However, due to the abundance of potential precursors (TAG, DAG and chlorine compounds), MCPD esters could still be formed via substitution of chlorine and acyloxonium intermediate reactions. There is controversial discussion about the relationship of GE and MCPD esters formation, and therefore the use of antioxidants in reducing GE is still unclear [10]. However, a recent study, concerning the addition of antioxidants to a frying system, had clearly shown that antioxidants were effective in controlling and inhibiting the formation of 3-MCPD ester and GE at the same time [23]. The results showed that GE content was lowered during a series of tests of a continuous frying system with the presence of antioxidants.
In this study, our results showed that antioxidants delay the oxidation of the lipid portion and were able to scavenge the radicals by donating electrons in the baking system either at a single dosage or in combinations with two antioxidants. A synergistic effect by combining the natural (tocopherol or rosemary extract) and synthetic (BHA) antioxidants was more effective to control the MCPD esters and GE formation, especially when a higher concentration of natural antioxidant was used (800 and/or 1200 mg/kg).
4. Conclusions
The addition of antioxidants into a baking recipe using palm olein and soft stearin as shortening inhibited the formation of radicals observed from the ESR spectrometry measurements. The fats portion from the finished product was considered stable among all the treatments with antioxidants. At a single dosage, rosemary extract was more effective than either tocopherol or BHA to produce lower 2-MCPD ester, 3-MCPD ester and GE in both the studied samples. In addition, a single dose of antioxidants was sufficient to improve the stability of the finished products in terms of oxidation status. A combination of rosemary extract or tocopherol with 200 mg/kg BHA was more effective in controlling the formation of 2-MCPD ester, 3-MCPD ester and GE when the concentration of the natural antioxidant was higher (800 and 1200 mg/kg).
Acknowledgments
The authors want to thank Shimadzu (Malaysia) Sdn. Bhd in providing the GCMSMS system and EDX system.
Author Contributions
Conceptualization, C.P.T. and Y.W.; methodology, K.M.G., Y.H.W.; software, T.B.T. and M.M.Y.; validation, K.M.G., F.A. and O.M.L.; formal analysis, K.M.G. and Y.H.W.; investigation, K.M.G. and C.P.T.; resources, C.P.T. and Y.W.; data curation, K.M.G.; writing—original draft preparation, K.M.G.; writing—review and editing, C.P.T.; visualization, T.B.T. and M.M.Y.; supervision, C.P.T., A.F. and O.M.L.; project administration, C.P.T. and I.A.N.; funding acquisition, C.P.T. and I.A.N. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Putra Grant, Universiti Putra Malaysia (Project number UPM/700-2/1/GPB/2018/9629500). The authors would like to extend their gratitude to the King Saud University (Riyadh, Saudi Arabia) for the funding of this research through Researchers Supporting Project number (RSP-2019/80).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dian N.L., Hamid R.A., Kanagaratnam S., Isa W.R., Hassim N.A., Ismail N.H., Omar Z., Sahri M.M. Palm oil and palm kernel oil: Versatile Ingredients for Food Applications. J. Oil Palm Res. 2018;29:487–511. doi: 10.21894/jopr.2017.00014. [DOI] [Google Scholar]
- 2.Clemens R., Hayes A.W., Sundram K., Pressman P. Palm oil and threats to a critically important food source. Toxicol. Res. Appl. 2017;1:239784731769984. doi: 10.1177/2397847317699844. [DOI] [Google Scholar]
- 3.Nor Aini I., Miskandar M.S. Utilization of palm oil and palm products in shortenings and margarines. Eur. J. Lipid Sci. Technol. 2007;109:422–432. doi: 10.1002/ejlt.200600232. [DOI] [Google Scholar]
- 4.Gesteiro E., Guijarro L., Sanchez-Muniz F.J., Vidal-Carou M.D.C., Troncoso A., Venanci L., Jimeno V., Quilez J., Anadon A., Gonzalez-Gross M. Palm Oil on the Edge. Nutrients. 2019;11:2008. doi: 10.3390/nu11092008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Z., Stipkovits L., Zheng H., Serventi L., Brennan C.S. Bovine Milk Fats and Their Replacers in Baked Goods: A Review. Foods. 2019;8:383. doi: 10.3390/foods8090383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goh K.M., Wong Y.H., Abas F., Lai O.M., Cheong L.Z., Wang Y., Wang Y., Tan C.P. Effects of shortening and baking temperature on quality, MCPD ester and glycidyl ester content of conventional baked cake. LWT. 2019;116:108553. doi: 10.1016/j.lwt.2019.108553. [DOI] [Google Scholar]
- 7.Lakum R., Sonwai S. Production of trans-free margarine fat by enzymatic interesterification of soy bean oil, palm stearin and coconut stearin blend. Int. J. Food Sci. Technol. 2018;53:2761–2769. doi: 10.1111/ijfs.13888. [DOI] [Google Scholar]
- 8.Wallace H., Jan A., Barregård L., Bignami M., Ceccatelli S., Cottrill B., Dinovi M., Edler L., Grasl-Kraupp B., Hogstrand C., et al. Risks for human health related to the presence of 3-and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2016;14:4426. [Google Scholar]
- 9.Bakhiya N., Abraham K., Gurtler R., Appel K.E., Lampen A. Toxicological assessment of 3-chloropropane-1,2-diol and glycidol fatty acid esters in food. Mol. Nutr. Food Res. 2011;55:509–521. doi: 10.1002/mnfr.201000550. [DOI] [PubMed] [Google Scholar]
- 10.Cheng W.W., Liu G.Q., Wang L.Q., Liu Z.S. Glycidyl fatty acid esters in refined edible oils: A review on formation, occurrence, analysis, and elimination methods. Compr. Rev. Food Sci. Food Saf. 2017;16:263–281. doi: 10.1111/1541-4337.12251. [DOI] [PubMed] [Google Scholar]
- 11.Hamlet C.G., Sadd P.A., Gray D.A. Influence of composition, moisture, pH and temperature on the formation and decay kinetics of monochloropropanediols in wheat flour dough. Eur. Food Res. Technol. 2003;216:122–128. doi: 10.1007/s00217-002-0621-z. [DOI] [Google Scholar]
- 12.Hamlet C.G., Sadd P.A., Gray D.A. Generation of monochloropropanediols (MCPDs) in model dough systems. 1. Leavened doughs. J. Agric. Food Chem. 2004;52:2059–2066. doi: 10.1021/jf035077w. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu M., Vosmann K., Matthäus B. Generation of 3-monochloro-1,2-propanediol and related materials from tri-, di-, and monoolein at deodorization temperature. Eur. J. Lipid Sci. Technol. 2012;114:1268–1273. doi: 10.1002/ejlt.201200078. [DOI] [Google Scholar]
- 14.Rahn A.K.K., Yaylayan V.A. What do we know about the molecular mechanism of 3-MCPD ester formation? Eur. J. Lipid Sci. Technol. 2011;113:323–329. doi: 10.1002/ejlt.201000310. [DOI] [Google Scholar]
- 15.Zhang H., Jin P., Zhang M., Cheong L.Z., Hu P., Zhao Y., Yu L., Wang Y., Jiang Y., Xu X. Mitigation of 3-Monochloro-1,2-propanediol Ester Formation by Radical Scavengers. J. Agric. Food Chem. 2016;64:5887–5892. doi: 10.1021/acs.jafc.6b02016. [DOI] [PubMed] [Google Scholar]
- 16.Tabakaeva O.V., Piekoszewski W., Kalenik T.K., Maximova S.N., Tabakaev A.V., Poleshyk D.V., Proniewicz L. Antiradical Activity of Hydrolysates and Extracts from Mollusk A. broughtonii and Practical Application to the Stabilization of Lipids. Foods. 2020;9:304. doi: 10.3390/foods9030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel E. Antioxidants in Food and Biology. The Oily Press LD.; Dundee, UK: 2007. [Google Scholar]
- 18.Aladedunye F., Przybylski R., Matthaus B. Performance of antioxidative compounds under frying conditions: A review. Crit. Rev. Food Sci. Nutr. 2015;57:1539–1561. doi: 10.1080/10408398.2013.777686. [DOI] [PubMed] [Google Scholar]
- 19.Wan Yahaya W.A., Abu Yazid N., Mohd Azman N.A., Almajano M.P. Antioxidant Activities and Total Phenolic Content of Malaysian Herbs as Components of Active Packaging Film in Beef Patties. Antioxidants. 2019;8:204. doi: 10.3390/antiox8070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H.W., Choi Y.S., Choi J.H., Kim H.Y., Hwang K.E., Song D.H., Lee S.Y., Lee M.A., Kim C.J. Antioxidant effects of soy sauce on color stability and lipid oxidation of raw beef patties during cold storage. Meat Sci. 2013;95:641–646. doi: 10.1016/j.meatsci.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes P.A.R., Ferreira S.S., Bastos R., Ferreira I., Cruz M.T., Pinto A., Coelho E., Passos C.P., Coimbra M.A., Cardoso S.M., et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants. 2019;8:189. doi: 10.3390/antiox8060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rico D., Penas E., Garcia M.D.C., Martinez-Villaluenga C., Rai D.K., Birsan R.I., Frias J., Martin-Diana A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods. 2020;9:296. doi: 10.3390/foods9030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong Y.H., Goh K.M., Nyam K.L., Nehdi I.A., Sbihi H.M., Tan C.P. Effects of natural and synthetic antioxidants on changes in 3-MCPD esters and glycidyl ester in palm olein during deep-fat frying. Food Control. 2019;96:488–493. doi: 10.1016/j.foodcont.2018.10.006. [DOI] [Google Scholar]
- 24.Zhang X., Gao B., Qin F., Shi H., Jiang Y., Xu X., Yu L.L. Free radical mediated formation of 3-monochloropropanediol (3-MCPD) fatty acid diesters. J. Agric. Food Chem. 2013;61:2548–2555. doi: 10.1021/jf305252q. [DOI] [PubMed] [Google Scholar]
- 25.Bonwick G.A., Birch C.S. European Regulation of Process Contaminants in Food. Royal Society of Chemistry; London, UK: 2019. [Google Scholar]
- 26.Knutsen H.K., Alexander J., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., Grasl-Kraupp B., et al. Update of the risk assessment on 3-monochloropropane diol and its fatty acid esters. EFSA J. 2018:16. doi: 10.2903/j.efsa.2018.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arifin N., Cheong L.-Z., Koh S.-P., Long K., Tan C.-P., Yusoff M.S.A., Nor Aini I., Lo S.-K., Lai O.-M. Physicochemical Properties and Sensory Attributes of Medium- and Long-Chain Triacylglycerols (MLCT)-Enriched Bakery Shortening. Food Bioprocess Technol. 2009;4:587–596. doi: 10.1007/s11947-009-0204-0. [DOI] [Google Scholar]
- 28.Chen C.W., Chong C.L., Ghazali H.M., Lai O.M. Interpretation of triacylglycerol profiles of palm oil, palm kernel oil and their binary blends. Food Chem. 2007;100:178–191. doi: 10.1016/j.foodchem.2005.09.044. [DOI] [Google Scholar]
- 29.AOCS . Official Method Ca 5a-40: Free Fatty Acids. The American Oil Chemists’ Society; Champaign, IL, USA: 2012. [Google Scholar]
- 30.Goh K.M., Wong Y.H., Ang M.Y., Yeo S.C.M., Abas F., Lai O.M., Tan C.P. Comparison assessment between SIM and MRM mode in the analysis of 3-MCPD ester, 2-MCPD ester and glycidyl ester. Food Res. Int. 2019;121:553–560. doi: 10.1016/j.foodres.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Wong Y.H., Muhamad H., Abas F., Lai O.M., Nyam K.L., Tan C.P. Effects of temperature and NaCl on the formation of 3-MCPD esters and glycidyl esters in refined, bleached and deodorized palm olein during deep-fat frying of potato chips. Food Chem. 2017;219:126–130. doi: 10.1016/j.foodchem.2016.09.130. [DOI] [PubMed] [Google Scholar]
- 32.Abd Razak R.A., Ahmad Tarmizi A.H., Kuntom A. Survey on Commercial Palm Olein and Oil Extracted from Snack Products in Selected Asian Countries—Part 2: Quantification of 3-monochloropropane-1, 2-diol Esters (3-MCPDE) Palm Oil Dev. 2017;66:14–17. [Google Scholar]
- 33.Yu L.L., Cheng Z. Application of electron spin resonance (ESR) spectrometry in nutraceutical and food research. Mol. Nutr. Food Res. 2008;52:62–78. doi: 10.1002/mnfr.200700395. [DOI] [PubMed] [Google Scholar]
- 34.Bhat R., Sridhar K.R., Bhushan B. Free radicals in velvet bean seeds (Mucuna pruriens L. DC.) and their status after γ-irradiation and conventional processing. LWT—Food Sci. Technol. 2007;40:1570–1577. doi: 10.1016/j.lwt.2006.12.002. [DOI] [Google Scholar]
- 35.Bhushan B., Bhat R., Sharma A. Status of free radicals in indian monsooned coffee beans γ-irradiated for disinfestation. J. Agric. Food Chem. 2003;51:4960–4964. doi: 10.1021/jf030187o. [DOI] [PubMed] [Google Scholar]
- 36.Qi J.F., Wang X.Y., Shin J.A., Lee Y.H., Jang Y.S., Lee J.H., Hong S.T., Lee K.T. Relative oxidative stability of diacylglycerol and triacylglycerol oils. J. Food Sci. 2015;80:C510–C514. doi: 10.1111/1750-3841.12792. [DOI] [PubMed] [Google Scholar]
- 37.Wang S., Meckling K.A., Marcone M.F., Kakuda Y., Tsao R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011;59:960–968. doi: 10.1021/jf1040977. [DOI] [PubMed] [Google Scholar]
- 38.Judde A., Villeneuve P., Rossignol-Castera A., Le Guillou A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J. Am. Oil Chem. Soc. 2003;80:1209–1215. doi: 10.1007/s11746-003-0844-4. [DOI] [Google Scholar]
- 39.Marinova E., Toneva A., Yanishlieva N. Synergistic antioxidant effect of α-tocopherol and myricetin on the autoxidation of triacylglycerols of sunflower oil. Food Chem. 2008;106:628–633. doi: 10.1016/j.foodchem.2007.06.022. [DOI] [Google Scholar]
- 40.Zhang Z., Gao B., Zhang X., Jiang Y., Xu X., Yu L.L. Formation of 3-monochloro-1,2-propanediol (3-MCPD) di- and monoesters from tristearoylglycerol (TSG) and the potential catalytic effect of Fe(2)(+) and Fe(3)(+) J. Agric. Food Chem. 2015;63:1839–1848. doi: 10.1021/jf5061216. [DOI] [PubMed] [Google Scholar]
- 41.Bosmans G.M., Peene L.J., Van Haesendonck I., Brijs K., Delcour J.A. Impact of chlorine treatment on properties of wheat flour and its components in the presence of sucrose. Food Chem. 2019;274:434–443. doi: 10.1016/j.foodchem.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Tiong S.H., Saparin N., Teh H.F., Ng T.L.M., Md Zain M.Z.B., Neoh B.K., Md Noor A., Tan C.P., Lai O.M., Appleton D.R. Natural Organochlorines as Precursors of 3-Monochloropropanediol Esters in Vegetable Oils. J. Agric. Food Chem. 2018;66:999–1007. doi: 10.1021/acs.jafc.7b04995. [DOI] [PubMed] [Google Scholar]