Abstract
Mass spectrometry-based phosphoproteomics is becoming an essential methodology for the study of global cellular signaling. Numerous bioinformatics resources are available to facilitate the translation of phosphopeptide identification and quantification results into novel biological and clinical insights, a critical step in phosphoproteomics data analysis. These resources include knowledge bases of kinases and phosphatases, phosphorylation sites, kinase inhibitors, and sequence variants affecting kinase function, and bioinformatics tools that can predict phosphorylation sites in addition to the kinase that phosphorylates them, infer kinase activity, and predict the effect of mutations on kinase signaling. However, these resources exist in silos and it is challenging to select among multiple resources with similar functions. Therefore, we put together a comprehensive collection of resources related to phosphoproteomics data interpretation, compared the use of tools with similar functions, and assessed the usability from the standpoint of typical biologists or clinicians. Overall, tools could be improved by standardization of enzyme names, flexibility of data input and output format, consistent maintenance, and detailed manuals.
Keywords: Phosphoproteomics, Bioinformatics tools, Kinase, Phosphorylation, Signaling
Background
Kinase signaling, the reversible enzymatic addition of a phosphate group to a substrate, is an essential part of cellular activity. Because its dysregulation contributes to many diseases, numerous clinical trials have been performed with kinase inhibitors resulting in over 50 FDA-approved small molecules and targeted antibodies [1, 2]. Therefore, detailed knowledge of the kinase signaling process is essential for the understanding of diseases and the development of new therapies.
While kinase signaling has been studied for over 100 years using a variety of experimental methods, the recent generation of mass spectrometry-based phosphoproteomic profiling allows for an unprecedented global exploration of phosphorylation. Phosphoproteomics data analysis involves two major steps. The first step includes the identification, phosphosite localization, and quantification of phosphopeptides. The second step aims to translate phosphopeptide identification and quantification results into novel biological and clinical insights. Although analyses in the first step are typically performed by the proteomics cores using standardized computational tools, those in the second step require and can benefit from active involvement of biologists and clinicians.
A vast array of resources and tools are available to facilitate the interpretation of phosphopeptide identification and quantification results. However, each of these tools exists as a silo without connection to tools with complementary functions. In addition, many tools have overlapping functions but differ in underlying knowledge bases, algorithms, input and output format of data, accessibility, advantages, limitations, and maintenance. Although newly developed tools are usually compared to similar, previously published tools, comparisons often do not include real-world, biological use-cases. For example, inference of kinase activity based on the observed phosphorylation of its substrates is a powerful application of phosphoproteomics profiling, and multiple methods have been developed to address this need [3, 4]. However, there has been little validation of the methods and only one benchmarking study comparing a few of the methods has been published [3].
Biological and clinical scientists are in the best position to extract biologically and clinically relevant findings from phosphoproteomics data, however, they are rarely consulted for tool design input or requested to test the final product. Furthermore, there is no comprehensive list of tools to aid those using phosphoproteomic data in their research. Therefore, this article aims to provide a comprehensive collection of resources that can be used to gain insights from phosphoproteomic data, including knowledge bases of kinases and phosphatases, phosphorylation sites, kinase inhibitors, and sequence variants affecting kinase function, and bioinformatics tools that can predict phosphorylation sites in addition to the kinase that phosphorylates them, infer kinase activity, and predict the effect of mutations on kinase signaling. We perform some benchmarking comparisons to determine the best tool available and assess usability of the tools from the standpoint of typical biologists or clinicians.
Main text
Collection of knowledge bases and tools
The OMICtools resource (https://omictools.com) is a manually curated collection of bioinformatics tools [5]. This site was searched in July 2019 for tools using the words ‘kinase’, ‘phosphorylation’, ‘phospho’, or ‘phosphatase’. In addition, several more tools were collected from the literature. Only tools that were freely available, still accessible, and non-obsolete were included, and tools specific for organisms other than human were discarded. The year of last update was assumed to be the year of publication unless otherwise noted on the website. These tools may be accessed by a downloadable, locally-run tool (Tool) or by a website (Web) that may have downloadable (DL) results or database information. The website URLs for all resources can be found in Additional file 1: Table S1. Each website was accessed in July 2019 and data statistics were collected for human proteins from downloadable files where possible and from websites or manuscripts for online-only resources.
Knowledge bases of kinases and phosphatases
General information about the components involved in kinase signaling is required throughout the analysis and interpretation of phosphoproteomics data. Knowledge bases for kinase signaling can be separated into those collecting information on the enzymes, and those collecting experimentally validated phosphorylation sites. Of the 16 different resources that collect information specifically on protein kinases and phosphatases, 13 provide data on kinases, while 5 provide data on phosphatases (Table 1). Only two resources, the Eukaryotic Protein Kinase & Protein Phosphatase Database (EKPD) and its updated version iEKPD contain information on both types of enzymes [6]. Most databases are only available as online websites, but some provide an option for downloading data (Table 1).
Table 1.
Knowledge bases of human kinases and phosphatases
| Name | Last update | Method of access | Version | Enzyme | Human enzyme number | References |
|---|---|---|---|---|---|---|
| KinWeb | 2005 | Web | Protein Kinases | 519 | [11] | |
| Kinomer | 2008 | Web|DL | 1 | Protein Kinases | 505 | [8] |
| MOKCa | 2008 | Web | Protein Kinases | 423 | [14] | |
| HuPho | 2012 | Web|DL | Phosphatases | 313 | [18] | |
| KIDFamMap | 2012 | Web | Protein Kinases | 399 | [17] | |
| EKPD | 2013 | Web | 1.1 | Protein Kinases and Phosphatases | 676 | [6] |
| KinBase | 2014 | Web | Protein Kinases | 538 | [7] | |
| KinMutBase | 2015 | Web|DL | 4 | Protein Kinases | 31 | [10] |
| DEPOD | 2016 | Web|DL | 1.1 | Phosphatases | 239 | [19] |
| KinaseNET | 2017 | Web | Protein Kinases | >530 | ||
| Kin-Driver | 2017 | Web|DL | 82 | Protein Kinases | 518 | [15] |
| Phosphatome | 2017 | Web | 3 | Phosphatases | 189 | [20] |
| iEKPD | 2018 | Web|DL | 2.0 | Protein kinases and Phosphatases | 695 | [105] |
| KLIFS | 2018 | Web|DL | 2.4 | Protein Kinases | 292 | [16, 106] |
| KinG | 2019 | Web|DL | Protein Kinases | 1502 | [9] | |
| ProKinO | 2019 | Web | 2 | Protein Kinases | 538 | [12] |
The kinase knowledge bases can be further separated into two different types: those that include comprehensive data on all known protein kinases, and those that were developed for a specific purpose, such as collecting driver mutations in kinases (Kin-Driver). Notably, no kinase resource collects data on non-protein kinases. KinBase, which was developed by Gerard Manning, contains 538 protein kinases and is considered the primary source of human protein kinases and their classification [7]. Many other resources base their kinase list on KinBase.
Kinomer and KinG are general kinase sequence databases that provide very little other information [8, 9]. KinMutBase, a collection of disease-causing mutations in protein kinase domains, is outdated, contains data on only 31 kinases, and primarily consists of broken links [10]. KinWeb and EKPD provide gene and protein identifiers, classification, description, and sequence information, but these data can also be found in other resources. However, KinWeb does have prediction of the disulfide bonding state of cysteines in the protein, as well as prediction of alpha helices, and EKPD presents data in an easy-to-read format [6, 11].
Use of the remaining general resources depends on which data one wants to access. KinaseNET, ProKinO, and iEKPD contain the most comprehensive data on protein kinases, but KinaseNET and ProKinO are only available as online resources [12, 13]. They include protein sequences, links to the kinases in other databases (e.g., UniProt, Ensembl, Entrez), information on the kinase domains, expression in tissue, and disease associations. ProKinO specifically contains pathway information, mutations and their disease associations, chromosomal location of the kinase, and links to published manuscripts. KinaseNET includes PTMs, known binding partners, inhibitors, upstream kinases, downstream substrates, and information about regulation. KinaseNET provides all data on a single page, ProKinO requires more than 10 clicks on separate tabs and pages to obtain all information on a kinase, and iEKPD contains links for 13 additional annotations.
For disease studies, MOKCa and Kin-Driver specifically have data on protein kinase mutations [14, 15]. MOKCa has tissue specificity of mutations while Kin-Driver focuses on driver mutations and reports whether the mutation is activating or inactivating. KLIFS provides structural information for approximately half of the protein kinases bound to various ligands [16]. Finally, KIDFamMap combines structural data with known kinase inhibitors and diseases [17].
Because phosphatases are less well studied than kinases, there are fewer resources dedicated to their collection. EKPD and iEKPD provide the same information for phosphatases as they do for kinases. HuPho, however, was the first comprehensive collection of phosphatases and the database includes pathway and substrate data, as well as siRNA phenotype data and links to orthologs in other species [18]. DEPOD also includes pathways, substrates, and links to orthologs in addition to interacting partners and upstream kinases [19]. Finally, Phosphatome.Net is the phosphatase version of KinBase [20]. The website contains basic classification and sequence information.
Knowledge bases of phosphorylation sites
Besides information about specific kinases and phosphatases, data on phosphorylation sites are important for studying the signaling process. Phosphorylation site databases collect information on the location of phosphorylated residues in proteins from experimental data. These experiments can be low-throughput or high-throughput. High-throughput phosphorylation site identifications are assigned by probability unlike the more stringent experimental validation in low-throughput experiments, but some databases combine sites from both types of experiments without identifying the source experiment type.
In addition to phosphorylation site information, 16 of the 27 (60%) resources collect interactions between kinases or phosphatases and their substrates (Table 2). These often do not include the exact phosphorylation site, but instead provide interactions between an enzyme and its substrate at the gene level.
Table 2.
Databases of phosphorylation sites
| Name | Last update | Method of access | Version | Sites | Proteins | Kinases | Phosphatases | Data Type | References |
|---|---|---|---|---|---|---|---|---|---|
| PhosphoPep | 2007 | Web|DL | 2.0 | 3980 | MS | [30, 31] | |||
| HPRD | 2010 | Web|DL | 9 | 78,005 | 11,807 | 291 | 42 | UNSP | [21, 107, 108] |
| Phospho.ELM | 2010 | Web|DL | 9.0 | 26,651 | 5374 | 250 | HT, LT | [24, 109, 110] | |
| Phospho3D | 2010 | Web|DL | 2.0 | 1770 | 59 | HT, LT | [44] | ||
| PHOSIDA | 2011 | Web|DL | 3.24 | 24,262 | 8283 | MS | [26, 111] | ||
| HuPho | 2012 | Web|DL | 190 | 121 | 55 | UNSP | [18] | ||
| PTMfunc | 2012 | Web | 31,165 | MS | [32] | ||||
| ANIA | 2013 | Web|DL | 305 | 220 | LT | [35, 112] | |||
| PhosphoNetworks | 2013 | Web|DL | 1140 | 255 | UNSP | [36] | |||
| RegPhos | 2013 | Web|DL | 2.0 | 66,301 | 10,849 | 380 | UNSP | [42, 113] | |
| Kinome NetworkX | 2014 | DL | 173,460 | 18,610 | 357 | UNSP | [41] | ||
| ProteomeScout | 2014 | Web|DL | 2 | 290,007 | 23,387 | MS | [39, 114] | ||
| LymPHOS | 2015 | Web|DL | 2 | 15,566 | 4937 | MS | [27, 115] | ||
| PhosphoDB | 2015 | Web | 25,864 | 6222 | MS | [29] | |||
| dbPAF | 2016 | Web|DL | 1.0 | 244,034 | 18,773 | UNSP | [38] | ||
| DEPOD | 2016 | Web|DL | 1.1 | 253 | 210 | 88 | UNSP | [19] | |
| KANPHOS | 2016 | Web | β | 73 | MS | [25] | |||
| PhosphoAtlas | 2016 | DL | 2595 | 1284 | 501 | UNSP | [1] | ||
| Phosphopedia | 2016 | Web | 1.0 | 109,611 | 11,428 | MS | [28] | ||
| Phosphatome | 2017 | Web | 3 | 6008 | 2000 | 319 | 106 | UNSP | [20] |
| PhosphoNET | 2017 | Web | 966,817a | 22,698 | 488 | UNSP + pred | [43] | ||
| PTMD | 2018 | Web|DL | 1.0 | 690 | 434 | UNSP | [37] | ||
| qPhos | 2018 | Web|DL | 199,071 | 18,402 | MS | [33] | |||
| dbPTM | 2019 | Web|DL | v2019 | 257,527 | 19,713 | 25 | UNSP | [40, 64, 116, 117] | |
| PhosphoSitePlus | 2019 | Web|DL | Aug-19 | 239,664 | 20,115 | 372 | HT, LT | [23] | |
| Signor | 2019 | Web|DL | May-19 | 3593 | 1285 | 336 | 70 | UNSP | [34, 118] |
| Swiss-Prot | 2019 | Web|DL | Jun-19 | 40,135 | 7965 | 352 | UNSP | [22, 119] |
The number of unique kinases and phosphatases reported to phosphorylate sites in the database is included. For some databases, these numbers include enzyme groups in addition to individual enzymes. Data type indicates whether the data are from mass spectrometry (MS) experiments, separated high-throughput (HT) and low-throughput (LT) experiments, or whether the database combines data from both HT and LT experiments without specifying (UNSP)
aIndicates inclusion of predicted phosphorylation sites (pred)
The four main resources for phosphorylation sites curated data manually from the literature (Fig. 1). HPRD and Swiss-Prot are general databases of all proteins [21, 22]. The remaining two, PhosphoSitePlus and Phospho.ELM, specifically contain phosphorylation site information [23, 24]. Both PhosphoSitePlus and Swiss-Prot are frequently updated, while HPRD and Phospho.ELM were last updated in 2010. All four of these databases also include kinase information for sites if known.
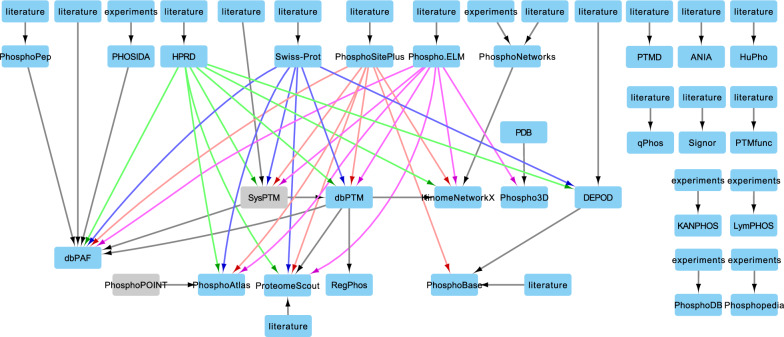
Fig. 1.
Network of phosphorylation site and kinase-substrate interaction databases. Gray nodes indicate databases that are no longer accessible. Arrows point from the knowledge source to the collecting database. Arrows originating from the four most highly used databases are colored by source (green = HPRD, blue = Swiss-Prot, red = PhosphoSitePlus, pink = Phospho.ELM)
Other smaller databases were generated through manual curation or publication of a laboratory’s own phosphorylation site data. KANPHOS collects phosphorylation sites in neural signaling identified by high-throughput experiments [25]. LymPHOS, PhosphoDB, Phosphopedia, and PHOSIDA are collections of data that were primarily produced in cell lines [26–29]. PhosphoPEP integrates mass spectrometry experiments from Cell Signaling Technology and their own laboratory [30, 31]. PTMfunc and qPhos both collect mass spectrometry experiments and add functional predictions and kinase activity from various tools [32, 33]. Signor extracts high quality signaling interactions from the literature [34]. Finally, ANIA, PTMD, and PhosphoNetworks curate the literature for a specific purpose. ANIA collects phosphorylation sites that serve as binding sites for 14-3-3 proteins, while PhosphoNetworks creates a kinase-substrate network curated from the literature and a protein microarray experiment, and PTMD collects disease-related phosphorylation sites [35–37].
The remaining resources integrate phosphorylation sites and kinase information from other databases (Fig. 1). The database dbPAF collects phosphorylation sites from several databases [38]. ProteomeScout also collects phosphorylation sites from other databases along with literature-curated experiments and provides a tool for analyzing a user’s data [39]. The database dbPTM collects all PTMs and the responsible enzyme from several sources [40]. Kinome NetworkX, RegPhos, and PhosphoAtlas curate and integrate data specifically to create kinase-substrate networks [1, 41, 42]. PhosphoNET is an online-only tool that includes predicted phosphorylation sites in addition to those with experimental evidence [43]. Finally, Phospho3D specifically collects phosphorylation sites with 3D structures [44].
Five databases collect information on phosphatase-substrate interactions. As mentioned, DEPOD, HuPho, and Phosphatome.Net all curate enzyme interactions from the literature. HPRD and Signor also collect some site-specific phosphatase information.
Each database contains a different number of phosphorylation sites and enzyme–substrate relationships depending on the source and method of collection (Table 2). ProteomeScout, PhosphoSitePlus, dbPTM, and dbPAF contain the most experimentally validated, downloadable sites. The site numbers for these four databases include specific protein isoforms, as do several other resources. PhosphoAtlas contains substrates for the largest number of individual kinases. Signor, Swiss-Prot, RegPhos, Phospho3D, dbPTM, and Phospho.ELM have substrates for individual kinases and kinase families. Finally, PhosphoSitePlus has substrates for some specific kinase isoforms.
Errors in substrate databases
Based on our examination, PhosphoSitePlus is the preferred resource for experimentally-identified phosphorylation sites and kinases for phosphorylation sites. PhosphoSitePlus is frequently updated, well-curated, and distinguishes between low and high-throughput identified sites. The downstream integrating databases suffer from ID mapping errors. For example, in PhosphoAtlas there is an entry for PEG (paternally expressed gene 3) phosphorylating CDC25B. PEG is not a known kinase, but pEg3 kinase (also known as maternal embryonic leucine zipper kinase, MELK) is known to phosphorylate CDC25B [45]. Many of the downstream databases also have issues with PDPK1 and PDK1. The gene PDPK1, 3-phosphoinositide-dependent protein kinase 1, produces a protein known to the biological community as PDK1. However, there is an additional kinase, pyruvate dehydrogenase kinase, that is produced by the gene PDK1. Databases that try to integrate sites frequently attribute the substrates of PDPK1 to PDK1. Finally, integrating databases propagate errors from the original databases. For example, HPRD contains an entry for PTPN11 phosphorylating PTK2B although PTPN11 is a known phosphatase and not a kinase. The original manuscript connected to this entry confirmed that PTPN11 is a phosphatase and that it just binds to PTK2B at that particular site [46]. Databases that collect information from HPRD, such as RegPhos and PhosphoAtlas, include this incorrect entry for PTPN11.
Known substrates of kinases and phosphatases
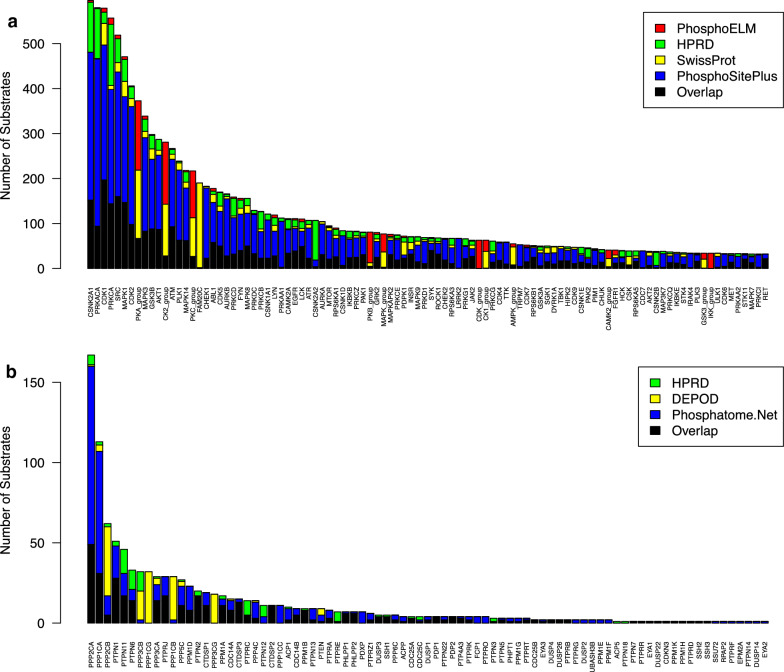
The four main databases of kinases together produce 485 substrate sets of individual kinases and kinase families (Fig. 2a). PhosphoSitePlus contains the most unique sites, while other databases contribute only a few additional sites per kinase. CSNK2A1 has the most substrates (596), while over half of the sets contain fewer than 10 substrates.
Fig. 2.
Number of substrates per kinase and phosphatase. a Number of substrates for the top 100 kinases in four databases. Substrates present in more than one database are colored black while the remaining sites are unique to each database. b Number of substrates for each phosphatase in DEPOD (yellow), HPRD (green), Phosphatome.Net (blue), or in more than one database (black)
For substrates of phosphatases, DEPOD, HPRD, and Phosphatome.Net combined produce sets for 83 phosphatases. The most unique information comes from DEPOD and Phosphatome.Net. The number of known sites for each phosphatase is far fewer than that for kinases. PPP2CA has the most substrates (167), while 70% of the phosphatases have fewer than 10 substrates (Fig. 2b).
Phosphorylation site prediction tools
Despite decades of research, very few phosphorylation sites have known kinases or phosphatases. Of the sites in PhosphoSitePlus, only about 3% have an experimentally validated human kinase. Therefore, numerous tools have been developed to predict which sites in a protein can be phosphorylated and which kinases phosphorylate that given site.
These prediction tools were developed using a variety of features and methods and have been reviewed elsewhere [47, 48]. The early versions of phosphorylation site predictors were motif-based. They generated the frequency of amino acids surrounding a site and searched for that pattern in protein sequences. Later tools used more sophisticated methods such as support vector machines (SVM), random forest, Bayesian probability, position specific scoring matrices (PSSM), and deep neural networks [49–53]. Besides amino acid sequence, tools included a vast array of features such as the 3D structure of the phosphorylation site, disorder score, cell cycle data, and co-expression of kinases and substrates [54–56]. Others, like NetworKIN and iGPS, used protein–protein interaction data to filter predictions [57, 58]. Table 3 provides an overview of all currently available tools to predict phosphorylation sites or kinases for phosphorylation sites. While a few tools have been developed to predict sites for phosphatases, only Ptpset, NetPhorest, and NetworKIN are still accessible [49, 58].
Table 3.
Available phosphorylation site and kinase-substrate prediction tools
| Tool | Last update | Version | Prediction type | Method | Kinases/phosphatases | Type | References |
|---|---|---|---|---|---|---|---|
| DISPHOS | 2004 | 1.3 | Phosphorylation sites | Bagged logistic regression | 0 | Web | [54] |
| PPSP | 2006 | 1.06 | Phosphorylation sites of kinases | Bayesian decision theory | 68 | Web | [52] |
| KinasePhos2.0 | 2007 | 2.0 | Phosphorylation sites of kinases | SVM | 58 | Web | [51] |
| pkaPS | 2007 | Phosphorylation sites of PKA | Scoring function | 1 | Web|DL | [72] | |
| PhoScan | 2008 | Phosphorylation sites of kinases | Scoring function | 48 | Web|Tool | [60] | |
| Phos3D | 2009 | Phosphorylation sites and some kinase specificity | SVM | 5 | Web | [55] | |
| Musite | 2010 | 1 | Phosphorylation sites and some kinase specificity | SVM | 13 | Web|DL | [62] |
| PHOSIDA Predictor | 2011 | 3.24 | Phosphorylation S and T sites | SVM | 0 | Web | [26] |
| Predikin | 2011 | Phosphorylation sites of kinases | PSSM | any | Web|DL | [53] | |
| GPS-Polo | 2012 | 1.0 | Phosphorylation sites of Plk | Group-based scoring function PSSM | 1 | Web|Tool | [120] |
| iGPS | 2012 | 1.0.1 | Phosphorylation sites of kinases in vivo | GPS with PPI | 407 | Tool | [57] |
| CEASAR | 2013 | Kinases for known phosphorylation sites | Naïve Bayes | 289 | DL | [56] | |
| HMMpTM | 2013 | Phosphorylation sites of kinases and topology | HMM | 9 | Web|DL | [121] | |
| PKIS | 2013 | Phosphorylation sites of kinases | SVM | 56 | Web | [122] | |
| GPS | 2014 | 5.0 | Phosphorylation sites of kinases | Group-based scoring function PSSM | 464 | Web|DL|Tool | [61] |
| NetPhorest | 2014 | 2.1 | Phosphorylation sites of kinases | ANN and PSSM | 244 | Web|DL|Tool | [58, 65] |
| NetworKIN | 2014 | 3.0 | Phosphorylation sites of kinases in vivo | Naïve Bayes with PPI | 123 | Web|DL|Tool | [58, 66] |
| phos_pred | 2014 | Predicts phosphorylation sites for kinases | Random forest | 54 | Toola | [49] | |
| PhosphoSVM | 2014 | Phosphorylation sites | SVM | 0 | Web | [123] | |
| Ptpset | 2014 | Dephosphorylation sites of phosphatases | KNN | 3 | Web | [124] | |
| jEcho | 2015 | 1.0 | Phosphorylation sites of kinases | Weight vector | 12 | Tool | [68] |
| KSP-PUEL | 2015 | Phosphorylation sites of kinases | SVM ensemble | 2a | Tool | [63] | |
| Scansite | 2015 | 4 | Kinase motifs in proteins | PSSM | 70 | Web|DL | [125] |
| DAPPLE | 2016 | 2 | Phosphorylation sites | BLAST | 0 | Web|DL | [126] |
| iPhos-PseEn | 2016 | Phosphorylation sites | Random forest ensemble | 0 | Web | [127] | |
| PhosphoPICK | 2016 | Phosphorylation sites of kinases | Bayesian network | 107 | Web|DL | [70] | |
| PhosD | 2016 | Kinase-substrate relationships | Probabilistic model | 399 | DL | [128] | |
| MusiteDeep | 2017 | Phosphorylation sites and some kinase specificity | DNN | 5 | Toola | [50] | |
| NetPhos | 2017 | 3.1 | Phosphorylation sites and some kinase specificity | ANN | 17 | Web|Toola | [59, 71] |
| PhosphoNET | 2017 | Phosphorylation sites of kinases | PSSM | 488 | Web | [43] | |
| PhosPred-RF | 2017 | Phosphorylation sites | Random forest | 0 | Web | [129] | |
| AKID | 2018 | Phosphorylation sites of kinases | DNN | 496 | Web|DL|Toola | [69] | |
| CapsNet_PTM | 2018 | Phosphorylation sites and some kinase specificity | DNN | any | Tool | [130] | |
| PTM-ssMP | 2018 | Phosphorylation sites and some kinase specificity | SVM | 9 | Web|DL | [131] | |
| Quokka | 2018 | Phosphorylation sites of kinase families | Logistic regression | 65 | Web|DL | [132] | |
| TyrPred | 2018 | Phosphorylation sites of Tyr kinases | Elastic net | 15 | Web | [133] | |
| CoPhosK | 2019 | Kinases of phosphorylation sites | Naïve Bayes | 101 | Web|Tool | [134] | |
| DeepPhos | 2019 | Phosphorylation sites and some kinase specificity | DNN | 20 | Tool | [67] |
aIndicates number of trained kinases, but tool can be trained with others
SVM support vector machine, PSSM position specific scoring matrix, GSEA gene set enrichment analysis, ANN artificial neural network, DNN deep neural network, HMM hidden Markov model, PPI protein–protein interaction, KNN K-nearest neighbor
aIndicates tool is not available for all three main operating systems (Linux, Mac, Windows)
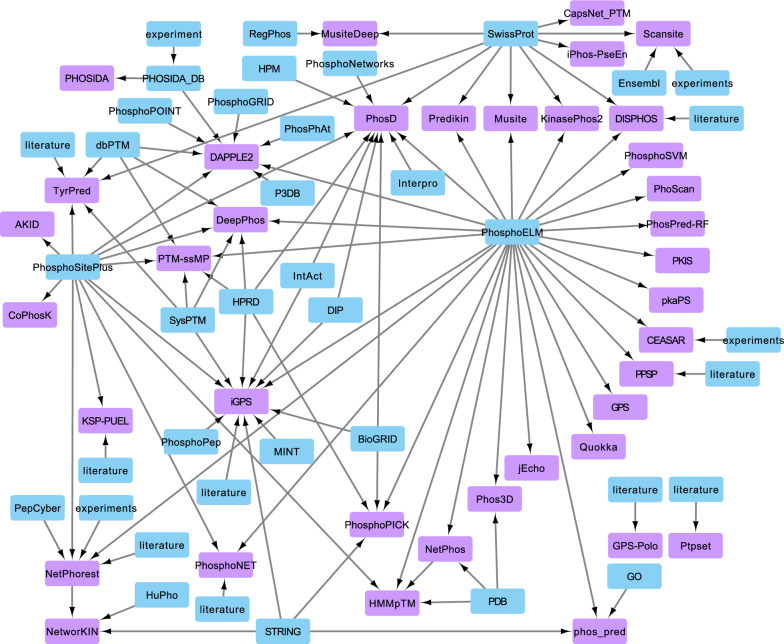
Figure 3 shows phosphorylation site predictor tools and the resources they used to make predictions. Almost all phosphorylation site predictors were trained using data from Phospho.ELM. Swiss-Prot and PhosphoSitePlus were also heavily used resources. Notably, almost all tools were developed using experimentally verified substrate data as the training set. Therefore, the tools are only able to predict the responsible kinase if there is sufficient data for substrates of that kinase.
Fig. 3.
Network of phosphorylation site predictor tools and the resources used to make predictions. Tools are colored purple while the databases used by the tools are colored blue
A researcher may utilize these prediction tools to identify kinases phosphorylating single substrates of interest, for which web-based tools would suffice. However, the limit on the number of sequences submitted for prediction and the lack of downloadable results prevent these same tools for being useful in large-scale phosphoproteomic studies. Unfortunately, many tools appropriate for large-scale studies have multiple issues limiting their use. First, tools can be difficult to install, platform-specific, and lack manuals on use. For example, NetPhos [59] is downloadable but can only be run on Linux, whereas PhoScan [60] can only be run on Windows machines. Other tools require commercial software such as MATLAB or even require understanding a programming language to modify hard-coded variables. Finally, tools like GPS [61] and phos_pred [49] provide pre-defined cutoffs for prediction, while others like musite [62] and KSP-PUEL [63] allow users to define their own thresholds or to train the models using their own data.
Testing kinase-substrate relationship prediction tools
For large-scale kinase-substrate prediction, 14 pre-trained tools were available that provide downloadable results. The best, unbiased way to test these tools is to use validated sites that were not used for the training of any tool. Unfortunately, most tools do not report the actual sites used for training and finding a set of sites to fit these criteria is nearly impossible. Therefore, we evaluated all 14 tools using gold-standard positive and negative human phosphorylation sites downloaded from dbPTM [64] for four serine/threonine kinases (CDK1, CK2, MAPK1, and PKA). Positive sites were serines and threonines experimentally validated to be phosphorylated by a particular kinase. Negative sites were serines and threonines not known to be phosphorylated on the same proteins. The outcomes might be biased in favor of newer tools and those that used some of these sites in their training.
Tools predicting kinases for phosphorylation sites (Table 3) were accessed through local tool installation or through the tool’s website. PhoScan [60] and phos_pred [49] were run locally on a Windows laptop, while NetPhorest [65], NetworKIN [66], iGPS [57], GPS [61], DeepPhos [67], jEcho [68], and MusiteDeep [50] were run locally on a Mac laptop. AKID [69], PhosphoPICK [70], NetPhos [71], Musite [62], and pkaPS [72] were accessed via their websites. Tools were set with the lowest threshold if they did not have an option to return scores for all sites. For each site, the maximum score was retained if the tool predicted for more than kinase isoform (e.g., the maximum score of PKCalpha and PKCbeta on the same site). If a tool did not return a score for a site, the lowest possible score was given to the site. The receiver operating characteristic (ROC) curve and area under the ROC curve (AUROC) were calculated for the results from each tool using the R package ROCR [73].
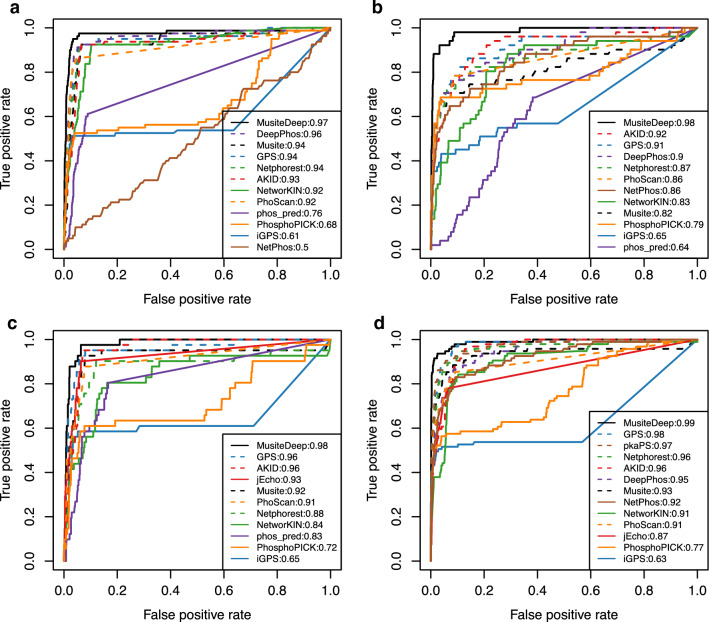
ROC curves for four kinases (CDK1, CK2, MAPK1, and PKA) are shown in Fig. 4. Notably, musite was unable to predict for a few random protein sequences in each submission. DeepPhos and phos_pred both required manual edits of hard-coded variables. MusiteDeep and GPS had the highest area under the curve (AUC) for all kinases tested. The PKA-specific tool pkaPS also performed well. Performance for most tools, however, varied across kinases.
Fig. 4.
ROC curves for substrate prediction of four kinases. The false positive and true positive rates of substrate prediction for a CDK1, b CK2, c MAPK1, and d PKA. The AUC for each tool is listed next to the tool name
Comparison of kinase activity tools
The known or predicted kinases for phosphorylation sites can be used to infer kinase activity from global phosphoproteomic data. Tools and methods have been developed to predict kinase activity, but there has been little effort spent towards comparing these tools or determining the most biologically-relevant set of parameters. The available tools (PHOSIDA, KEA2, KSEA App, PHOXTRACK, INKA, and IKAP) each use a different algorithm to infer activity (Table 4). The PHOSIDA de novo motif finder uses a simple method of bootstrapping to determine enrichment of sequence motifs in a set of phosphorylated peptides and then matches those to known kinase motifs [26]. Kinase Enrichment Analysis 2 (KEA2) uses over-representation analysis to determine enrichment of kinase substrates in a condition [74]. Similarly, the KSEA App uses mean phosphorylation of substrates of kinases as a proxy for activity [4]. PHOXTRACK modified pre-ranked gene set enrichment analysis (GSEA) to determine enrichment of known kinase targets [75]. IKAP extended these methods using a cost function to infer the relative contributions of multiple kinases acting on the same site [76]. Finally, INKA combines the GSEA method with activating phosphorylation on kinases [77].
Table 4.
Kinase activity prediction and phosphoproteomic dataset analysis tools
| Tool | Last update | Prediction type | Method | Input | Type | References |
|---|---|---|---|---|---|---|
| PHOSIDA Motif Finder | 2011 | Sequence motifs | Bootstrap | Phosphosite 13mer | Web | [26] |
| CellNOpt | 2012 | Time-course analysis | Logic formalisms | Interactions and phosphoproteomic data | Tool | [85] |
| KEA2 | 2012 | Kinase activity | Fisher’s exact test | Gene symbols and phosphosite | Web|DL|Tool | [74] |
| Sorad | 2013 | Time-course analysis | Ordinary differential equations | Phosphoproteomic data | Tool | [86] |
| PHOXTRACK | 2014 | Kinase activity | GSEA | Phosphosite 13mer and log2 expression | Web|DL | [75] |
| PhosFox | 2015 | Phosphorylation site comparison between groups | Comparison | Phosphoproteomic data | Tool | [84] |
| SELPHI | 2015 | Phosphoproteomic data analysis | Multiple functions | Phosphoproteomic data | Web|DL | [83] |
| DynaPho | 2016 | Phosphoproteomic analysis for multiple conditions | Activity modules | phosphoproteomic data | Web|DL | [87] |
| IKAP | 2016 | Kinase activity | Cost function | Phosphoproteomic data | Tool | [76] |
| KinasePA | 2016 | Kinase perturbation in multiple treatments | Directional hypothesis testing framework | Phosphoproteomic data | Web|Tool | [88] |
| KSEA | 2017 | Kinase activity | Z score | Phosphoproteomic data | Web|DL|Tool | [4] |
| CLUE | 2018 | Time-course kinase activity | k-Means clustering | Phosphoproteomic data | Tool | [41] |
| INKA | 2019 | Kinase activity | GSEA and phosphorylation | Phosphoproteomic data | Web|DL | [77] |
GSEA gene set enrichment analysis
We used a phosphoproteomic dataset from a cell line experiment with 20 kinase inhibitors [78] to test four kinase activity prediction tools. Because PHOSIDA is only available online without downloadable results, we excluded this tool from further analysis. INKA was also excluded as it requires MaxQuant search result files. The R programming environment was used to create files in the input format for each tool. Significantly downregulated sites for each inhibitor were submitted to KEA2 and significantly inhibited kinases were defined as those with false discovery rate (FDR) < 0.05 and at least 3 overlapping substrates [74]. The log2 fold change for each thirteenmer phosphorylation site (± 6 amino acids surrounding the phosphorylated site) was submitted to PHOXTRACK (1000 permutations, minimum number of substrates = 3, weighted statistics) [75]. Significantly inhibited kinases were defined as those with FDR < 0.05 and normalized enrichment value < 0. The fold change for each site with each inhibitor was submitted to the KSEA app website and significantly inhibited kinases were defined as those with FDR < 0.05, at least 3 substrates in the dataset, and a z score < 0 [4]. The substrates of kinases from PhosphoSitePlus (version July 2017) and Signor (version October 2017) were used for IKAP [23, 34, 76]. IKAP was run locally on a Mac laptop with the bounds between -11 and 11 and 50 iterations. The 5 kinases with the lowest activity scores for each experiment were chosen. The positive set were kinases known to be inhibited by each drug (as reported in supplementary table in Ref. [78]); all other kinases predicted by the tools were considered to be negative. The significant kinases for each tool were counted for presence in the positive and negative sets.
Comparison of these tools is challenging because they use different input and underlying databases. KEA2 requires a set of sites in the format of HGNC symbol and phosphorylated amino acid residue position separated by an underscore. It contains sets for 250 different kinases. KSEA App requires a strictly formatted comma-delimited file with the HGNC symbol, phosphorylated position, and non-log-transformed fold change. Users can choose between known sets from the July 2016 release of PhosphoSitePlus or the known + predicted site sets from PhosphoSitePlus and NetworKIN. PHOXTRACK requires a two-column file with a thirteenmer peptide and log-transformed fold change. It can use substrate sets from the four main databases or a user-supplied database. Finally, IKAP required tabular data entered into MATLAB, manual modification of MATLAB code to change parameters, and allowed a user to upload their own set of substrates. Because one thirteenmer might match multiple proteins and phosphorylated positions, the actual substrate list presented to each tool may differ slightly.
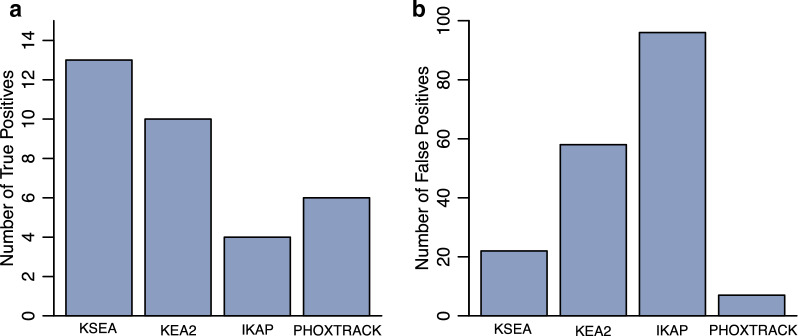
To determine how well each tool covered the known targets of kinases, we counted the number of significantly downregulated known kinases of each inhibitor and the significantly downregulated kinases of each inhibitor that were not known targets of that inhibitor. The KSEA App made the most true positive predictions across all experiments, while IKAP made the fewest true positive predictions (Fig. 5a). PHOXTRACK made the fewest false positive predictions (Fig. 5b).
Fig. 5.
True and false positive predictions for kinase activity prediction tools. a For all 20 inhibitors, the number of known targets predicted to be significantly downregulated by each tool. b For all inhibitors, the number of all significantly downregulated kinases that do not match known inhibitor targets
Besides upstream kinase activity, phosphoproteomics data could additionally be used to explore altered downstream pathways. While standard tools and methods such as GSEA are typically used for this analysis, all are limited to using overall gene-level phosphorylation [79]. Unfortunately the functional contribution of individual sites to pathway signaling is poorly annotated in gene set databases, although PTMsigDB has some limited pathway sets [80]. Until new tools are built to handle individual sites in pathway analysis, a user might combine the results from kinase activity prediction to assemble altered kinases into pathways using tools such as String, RegPhos2, or Wikipathways [42, 81, 82].
Differential and clustering analysis of phosphoproteomics data
Besides activity prediction, phosphoproteomic data can be used for other analyses. SELPHI is a good tool to first explore the data as it allows biologists to quickly and easily analyze phosphoproteomic data with clustering analyses, kinase-substrate correlation, and pathway enrichment [83]. PhosFox then compares phosphorylated peptides between conditions [84]. Finally, a set of tools (CellNOpt, Sorad, CLUE, DynaPho, and KinasePA) were developed specifically for phosphoproteomic time-course or multiple condition analyses (Table 4) [41, 85–88].
Prediction of mutation effect
Analysis and interpretation of phosphoproteomic data can be enhanced with other multi-omics data types. For example, sequence variants can affect kinase function or presence of a phosphorylation site. The databases PhosSNP [89] and ActiveDriverDB [90] collect gene polymorphisms and somatic mutations, respectively, near phosphorylation sites and categorize them based on suspected effect (Table 5). ActiveDriverDB also includes predictions from Mutations Impact on Phosphorylation (MIMP), which uses Bayesian statistics to predict whether mutations around a phosphorylation site will change which kinase binds to that site [91]. It can predict rewiring for 124 kinases using experimentally validated data, or it can be extended to predict for 322 kinases using predicted kinase-substrate relationships. ReKINect also predicts rewiring from mutations, but it further predicts the destruction or creation of phosphorylation sites and inactivation or constitutive activation of kinases [92]. PhosphoPICK-SNP is also similar to MIMP. It predicts the kinase responsible for phosphorylating a site, and whether a mutation affects its ability to phosphorylate the site [93]. While all of the tools are easy to use, the databases are better for individual searches and the three prediction tools are better for analysis of a user’s mutation data.
Table 5.
Resources for studying the effect of mutations on kinases and phosphorylation sites
| Tool | Last update | Version | Prediction type | Method | Kinases | Method of access | References |
|---|---|---|---|---|---|---|---|
| PhosSNP | 2009 | 1.0 | SNVs that might influence phosphorylation status | Rules | Tool | [89] | |
| MIMP | 2015 | Missense SNV impact on kinase-substrate | Bayesian model | 322 | Web|DL|Tool | [91] | |
| ReKINect | 2015 | Effect of SNV on signaling network | PSSM | Web|DL | [92] | ||
| PhosphoPICK-SNP | 2016 | Effect of SNV on phosphorylation level | Bayesian models | 107 | Web|DL | [93] | |
| ActiveDriverDB | 2017 | Somatic variants affecting phosphorylation | Rules | 322 | Web|DL | [90] |
SNV single nucleotide variation, PSSM position specific scoring matrix
Resources for kinase inhibitors
After discovering altered kinases from phosphoproteomic data to use as therapeutic targets, identifying inhibitors is essential. Most available resources connect known drugs to their known kinase targets (Table 6). DrugKiNET shows the known inhibitors for kinases, and the kinases that a compound inhibits. It also predicts which kinases a drug can inhibit. K-Map extends these interactions to suggest the best compound to inhibit a set of kinases [94]. Finally, KinomeSelector groups kinases by sequence similarity and similarity of drug response. It then allows a user to choose a subset of kinases to target that cover the kinome [58].
Table 6.
Kinase-inhibitor relationship resources
| Tool | Last update | Description | Kinases | Inhibitors | Method of access | References |
|---|---|---|---|---|---|---|
| K-Map | 2013 | Best inhibitor for a set of kinases | 300 or 442 | 178 or 72 | Web|DL | [94] |
| KinomeSelector | 2014 | Minimal set of kinases to inhibit | > 500 | NA | Web|DL | [58] |
| DrugKiNET | 2017 | Known and predicted drug activity on kinases | > 800 | Web|DL |
K-Map has two different databases—one with 178 drugs inhibiting 300 kinases and one with 72 drugs inhibiting 442 kinases
Other kinase signaling tools
The final set of bioinformatics tools, summarized in Table 7, enhance phosphoproteomic analysis and cover visualization, data retrieval, and prediction tools. Additional kinases from a genome can be predicted by Kinannote [95] and KinConform can predict whether those kinases are active in structure files [96]. KinMap [97] is used to visualize the entire kinome tree and PhosphoLogo [98] is used to generate sequence logos of kinases. On the other side, RLIMS-P and eFIP are both tools that extract data on phosphorylation interactions from the literature [99, 100]. Then CPhos identifies phosphorylation sites of interest that are conserved across species [101]. PyTMs [102] is a tool to visualize 3D structures of phosphorylation sites and ultimately RegPhos2.0 [42] can be used to visualize signaling networks. RegPhos2.0 also provides heatmaps for kinase and substrate mRNA expression in cancer. Finally, 14-3-3-Pred predicts phosphorylation sites in protein sequences that might bind to 14-3-3 proteins, further adding to the phosphorylation-related signaling network [103].
Table 7.
Visualization, data retrieval, and prediction tools
| Tool | Last update | Version | Type | Input | Output | Method of access | References |
|---|---|---|---|---|---|---|---|
| CPhos | 2012 | 1.3 | Phosphorylation site conservation | Phosphopeptides | Conservation scores | Tool | [101] |
| PhosphoLogo | 2012 | Visualization of sequence motifs | Sequence motifs | Motif visualization | Tool | [98] | |
| RegPhos2.0 | 2013 | 2.0 | Visualization of kinase data | Gene names | Network visualization or cancer gene expression | Web|DL | [42, 113] |
| eFIP | 2014 | Returns publications involving phosphorylation | Gene names or words | Publications matching those words | Web | [100] | |
| RLIMS-P | 2014 | 2.0 | returns protein phosphorylation information from literature | PMIDs or keywords | kinase, substrate, and site | Web|DL | [99] |
| PyTMs | 2015 | 1.2 | pyMOL plugin to add PTMs to protein models | Protein models, PTMs | PTMs integrated in protein models | Tool | [102] |
| 14-3-3-Pred | 2015 | Predicts 14-3-3 binding phosphosite | Protein sequences | Predicted 14-3-3 binding sites | Web|DL | [103] | |
| KinMap | 2016 | Kinome tree visualization | Kinases | Tree with highlighted branches | Web|DL | [97] | |
| KinConform | 2017 | Determines which structures are kinases | Structures | Active or inactive kinase chains | Tool | [96] | |
| Kinannote | 2017 | 1.0 | Classifies sequences as kinases | Protein sequences | Kinase annotation | Tool | [95] |
Discussion
The available databases and tools for studying kinase signaling cover diverse functions and include information on enzymes and their substrates, inhibitors, activity, and mutations. Together these knowledge bases, prediction tools, and analysis tools comprise the current best standard for studying kinase signaling and many can be used without extensive computational knowledge. Overall, these tools allow a researcher to discover vast amounts of information from their phosphoproteomic data and some tools can even perform entire sets of analyses with a single button click [83].
Despite the work that has been done, there is room for advancement to fully utilize phosphoproteomic data for use in the clinic. First, the majority of tools focus almost exclusively on the study of protein kinases. However, phosphatases are critical components of the kinase signaling cascade and are frequently dysregulated in cancer. Understanding the role of the interplay between kinases and phosphatases on the net phosphorylation seen in global phosphoproteomic data is essential to identifying abnormal cell signaling in disease. Furthermore, while the current tools and research are aimed at studying dysregulated protein phosphorylation, non-protein phosphorylation is also often altered in disease. For example, hexokinases, which phosphorylate glucose, drive glucose metabolism and contribute to tumor initiation in mouse models of lung and breast cancers [104]. The development of resources and tools to study non-protein kinases and phosphatases could advance research in a variety of fields.
While the current tools provide critical functions, their error rate and accuracy could be improved. Errors are frequently propagated or amplified when tools collect data from a variety of resources. However, the impact of these errors on downstream analyses and biological inferences remains to be determined.
For all tools, usability can be an issue, both for bioinformaticians and biologists with no computational experience. Tools are frequently platform-dependent, do not allow downloadable results, and are not well annotated. Furthermore, tools are difficult to compare or to use more than one during analysis. The input and output formats are not standardized and use a variety of protein naming conventions.
The largest challenge was deciphering input limitations and understanding results. For example, submitting a sequence with a large number of phosphorylatable residues to GPS caused the software to stall without an error message and no documentation mentioned a size limit. Musite did not provide results for a sequence or two each run without explanation. Furthermore, downloadable result files for many tools had no column headers so the column contents were unknown. For example, the downloadable file from musite has no column titles, so you have to check the table on the website to understand the results. Additionally, scores are usually presented without explanation. Only careful reading of the manuscript or the manual elucidates what value signifies a “good” response. For example, in Scansite, the score 0 is the best, with scores closest to 0 indicating the best match. But in PhosphoPICK, the score indicates the probability of being phosphorylated by a kinase at that site so a score closer to 1 is better. Experts in machine learning might understand the score without explanation, but naïve users likely will not.
One way to fix this challenge is to have a detailed, easy-to-find manual. The manual should include ways to run the tool, the underlying mechanism of the method, and detailed description of the results. The description of the results should also be available where results are visualized. Furthermore, sample input is helpful for a new user to test the tool and determine whether the results will be useful for their experiment before preparing their own data files.
Conclusions
There are many tools and resources that can be used to study kinase signaling and these tools will become even more essential with the continued production of phosphoproteomic data. It is essential for the biological community to research under-studied enzymes and to validate specific substrates of kinases and phosphatases. Furthermore, bioinformaticians should consider creating tools that utilize information from both sides of the enzymatic phosphorylation reaction. Finally, resources should be carefully planned, easy to use, and well maintained and the community should work to standardize the use of enzyme IDs and phosphorylation site location.
Supplementary information
Additional file 1. List of URLs for all resources.
Acknowledgements
The authors thank Bo Wen for his help in installation and execution of the deep learning tools.
Abbreviations
- ANN
Artificial neural network
- AUC
Area under the curve
- AUROC
Area under the ROC curve
- DL
Downloadable
- FDR
False discovery rate
- GSEA
Gene set enrichment analysis
- HMM
Hidden Markov model
- HT
High-throughput
- LT
Low-throughput
- MELK
Maternal embryonic leucine zipper kinase
- PDK1
Pyruvate dehydrogenase kinase 1
- PDPK1
3-Phosphoinositide-dependent protein kinase 1
- PEG
Paternally expressed gene 3
- PPI
Protein-protein interaction
- PSSM
Position specific scoring matrix
- PTM
Post-translational modification
- ROC
Receiver operating characteristic
- SVM
Support vector machine
- Web
Website
Authors’ contributions
SRS designed, analyzed, and drafted the review. BZ guided the study and contributed to the revision of the review. Both authors read and approved the final manuscript.
Funding
This work was supported by National Institutes of Health Grants T15-LM007450 and U24CA210954, by Grant CPRIT RR160027 from the Cancer Prevention & Research Institutes of Texas (CPRIT), and by funding from the McNair Medical Institute at The Robert and Janice McNair Foundation. BZ is a CPRIT Scholar in Cancer Research and a McNair Scholar.
Availability of data and materials
The data used for comparing kinase activity inference tools can be found in PubMed with PMID: 28674151.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
The original online version of this article was revised: In the section heading “Knowledge bases of kinases and phosphatases”, 6th paragraph, the 3rd sentence that reads as “DEPOD used data from HuPho as a starting point and therefore contains much of the same information [19]” should have read as “DEPOD also includes pathways, substrates, and links to orthologs in addition to interacting partners and upstream kinases [19].”
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/7/2024
A Correction to this paper has been published: 10.1186/s12014-024-09473-w
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12014-020-09290-x.
References
- 1.Olow A, Chen Z, Niedner RH, Wolf DM, Yau C, Pankov A, et al. An atlas of the human kinome reveals the mutational landscape underlying dysregulated phosphorylation cascades in cancer. Cancer Res. 2016;76(7):1733–1745. doi: 10.1158/0008-5472.CAN-15-2325-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhullar KS, Lagarón NO, McGowan EM, Parmar I, Jha A, Hubbard BP, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17:1–20. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Armenta C, Ochoa D, Gonçalves E, Saez-Rodriguez J, Beltrao P. Benchmarking substrate-based kinase activity inference using phosphoproteomic data. Bioinformatics. 2017;33(12):1845–1851. doi: 10.1093/bioinformatics/btx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiredja DD, Koyutürk M, Chance MR. The KSEA App: a web-based tool for kinase activity inference from quantitative phosphoproteomics. Bioinformatics. 2017;33:3489–3491. doi: 10.1093/bioinformatics/btx415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry VJ, Bandrowski AE, Pepin A-S, Gonzalez BJ, Desfeux A. OMICtools: an informative directory for multi-omic data analysis. Database. 2014;2014:bau069. doi: 10.1093/database/bau069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Liu Z, Cheng H, Gao T, Pan Z, Yang Q, et al. EKPD: a hierarchical database of eukaryotic protein kinases and protein phosphatases. Nucleic Acids Res. 2014;42(Database issue):D496–D502. doi: 10.1093/nar/gkt1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The Protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 8.Martin DMA, Miranda-Saavedra D, Barton GJ. Kinomer v 1.0: a database of systematically classified eukaryotic protein kinases. Nucleic Acids Res. 2009;37(Database issue):D244–D250. doi: 10.1093/nar/gkn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupa A, Abhinandan KR, Srinivasan N. KinG: a database of protein kinases in genomes. Nucleic Acids Res. 2004;32(Database issue):D153–D155. doi: 10.1093/nar/gkh019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortutay C, Väliaho J, Stenberg K, Vihinen M. KinMutBase: a registry of disease-causing mutations in protein kinase domains. Hum Mutat. 2005;25(5):435–442. doi: 10.1002/humu.20166. [DOI] [PubMed] [Google Scholar]
- 11.Milanesi L, Petrillo M, Sepe L, Boccia A, D’Agostino N, Passamano M, et al. Systematic analysis of human kinase genes: a large number of genes and alternative splicing events result in functional and structural diversity. BMC Bioinform. 2005;6(4):S20. doi: 10.1186/1471-2105-6-S4-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McSkimming DI, Dastgheib S, Talevich E, Narayanan A, Katiyar S, Taylor SS, et al. ProKinO: a unified resource for mining the cancer kinome. Hum Mutat. 2015;36(2):175. doi: 10.1002/humu.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Peng D, Zhou J, Lin S, Wang C, Ning W, et al. iEKPD 2.0: an update with rich annotations for eukaryotic protein kinases, protein phosphatases and proteins containing phosphoprotein-binding domains. Nucleic Acids Res. 2018;47:D344–D350. doi: 10.1093/nar/gky1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson CJ, Gao Q, Mitsopoulous C, Zvelebil M, Pearl LH, Pearl FMG. MoKCa database–mutations of kinases in cancer. Nucleic Acids Res. 2009;37(Database issue):D824–D831. doi: 10.1093/nar/gkn832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonetti FL, Tornador C, Nabau-Moretó N, Molina-Vila MA, Marino-Buslje C. Kin-Driver: a database of driver mutations in protein kinases. Database. 2014;2014:bau104. doi: 10.1093/database/bau104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Linden OPJ, Kooistra AJ, Leurs R, de Esch IJP, de Graaf C. KLIFS: a knowledge-based structural database to navigate kinase-ligand interaction space. J Med Chem. 2014;57(2):249–277. doi: 10.1021/jm400378w. [DOI] [PubMed] [Google Scholar]
- 17.Chiu Y-Y, Lin C-T, Huang J-W, Hsu K-C, Tseng J-H, You S-R, et al. KIDFamMap: a database of kinase-inhibitor-disease family maps for kinase inhibitor selectivity and binding mechanisms. Nucleic Acids Res. 2013;41(Database issue):D430–D440. doi: 10.1093/nar/gks1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberti S, Sacco F, Calderone A, Perfetto L, Iannuccelli M, Panni S, et al. HuPho: the human phosphatase portal. FEBS J. 2013;280(2):379–387. doi: 10.1111/j.1742-4658.2012.08712.x. [DOI] [PubMed] [Google Scholar]
- 19.Duan G, Li X, Köhn M. The human DEPhOsphorylation database DEPOD: a 2015 update. Nucleic Acids Res. 2015;43(Database issue):D531–D535. doi: 10.1093/nar/gku1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen MJ, Dixon JE, Manning G. Genomics and evolution of protein phosphatases. Sci Signal. 2017;10(474):eaag1796. doi: 10.1126/scisignal.aag1796. [DOI] [PubMed] [Google Scholar]
- 21.Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, et al. Human Protein Reference Database–2009 update. Nucleic Acids Res. 2009;37(Database issue):D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45(D1):D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43(Database issue):D512–D520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinkel H, Chica C, Via A, Gould CM, Jensen LJ, Gibson TJ, et al. Phospho.ELM: a database of phosphorylation sites—update 2011. Nucleic Acids Res. 2011;39(Database issue):D261–D267. doi: 10.1093/nar/gkq1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai T, Yoshimoto J, Kannon T, Kuroda K, Kaibuchi K. Phosphorylation signals in striatal medium spiny neurons. Trends Pharmacol Sci. 2016;37(10):858–871. doi: 10.1016/j.tips.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Gnad F, Gunawardena J, Mann M. PHOSIDA 2011: the posttranslational modification database. Nucleic Acids Res. 2011;39(Database issue):D253–D260. doi: 10.1093/nar/gkq1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TD, Vidal-Cortes O, Gallardo O, Abian J, Carrascal M. LymPHOS 2.0: an update of a phosphosite database of primary human T cells. Database. 2015;2015:bav115. doi: 10.1093/database/bav115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence RT, Searle BC, Llovet A, Villén J. “Plug-and-play” investigation of the human phosphoproteome by targeted high-resolution mass spectrometry. Nat Methods. 2016;13(5):431–434. doi: 10.1038/nmeth.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giansanti P, Aye TT, van den Toorn H, Peng M, van Breukelen B, Heck AJR. An augmented multiple-protease-based human phosphopeptide atlas. Cell Reports. 2015;11(11):1834–1843. doi: 10.1016/j.celrep.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Bodenmiller B, Malmstrom J, Gerrits B, Campbell D, Lam H, Schmidt A, et al. PhosphoPep—a phosphoproteome resource for systems biology research in Drosophila Kc167 cells. Mol Syst Biol. 2007;3:139. doi: 10.1038/msb4100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodenmiller B, Campbell D, Gerrits B, Lam H, Jovanovic M, Picotti P, et al. PhosphoPep—a database of protein phosphorylation sites in model organisms. Nat Biotechnol. 2008;26(12):1339–1340. doi: 10.1038/nbt1208-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltrao P, Albanèse V, Kenner LR, Swaney DL, Burlingame A, Villén J, et al. Systematic functional prioritization of protein posttranslational modifications. Cell. 2012;150(2):413–425. doi: 10.1016/j.cell.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu K, Zhang Q, Liu Z, Zhao Q, Zhang X, Wang Y, et al. qPhos: a database of protein phosphorylation dynamics in humans. Nucleic Acids Res. 2018;8:D451–D458. doi: 10.1093/nar/gky1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perfetto L, Briganti L, Calderone A, Cerquone Perpetuini A, Iannuccelli M, Langone F, et al. SIGNOR: a database of causal relationships between biological entities. Nucleic Acids Res. 2016;44(D1):D548–D554. doi: 10.1093/nar/gkv1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinti M, Madeira F, Murugesan G, Hoxhaj G, Toth R, Mackintosh C. ANIA: annotation and integrated analysis of the 14-3-3 interactome. Database. 2014;2014:085. doi: 10.1093/database/bat085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J, Rho H-S, Newman RH, Zhang J, Zhu H, Qian J. PhosphoNetworks: a database for human phosphorylation networks. Bioinformatics. 2014;30(1):141–142. doi: 10.1093/bioinformatics/btt627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Wang Y, Lin S, Deng W, Peng D, Cui Q, et al. PTMD: a database of human disease-associated post-translational modifications. Genomics Proteomics Bioinform. 2018;16(4):244–251. doi: 10.1016/j.gpb.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ullah S, Lin S, Xu Y, Deng W, Ma L, Zhang Y, et al. dbPAF: an integrative database of protein phosphorylation in animals and fungi. Sci Rep. 2016;6:srep23534. doi: 10.1038/srep23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matlock MK, Holehouse AS, Naegle KM. ProteomeScout: a repository and analysis resource for post-translational modifications and proteins. Nucleic Acids Res. 2015;43(Database issue):D521–D530. doi: 10.1093/nar/gku1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang K-Y, Su M-G, Kao H-J, Hsieh Y-C, Jhong J-H, Cheng K-H, et al. dbPTM 2016: 10-year anniversary of a resource for post-translational modification of proteins. Nucleic Acids Res. 2016;44(D1):D435–D446. doi: 10.1093/nar/gkv1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng F, Jia P, Wang Q, Zhao Z. Quantitative network mapping of the human kinome interactome reveals new clues for rational kinase inhibitor discovery and individualized cancer therapy. Oncotarget. 2014;5(11):3697–3710. doi: 10.18632/oncotarget.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang K-Y, Wu H-Y, Chen Y-J, Lu C-T, Su M-G, Hsieh Y-C, et al. RegPhos 2.0: an updated resource to explore protein kinase-substrate phosphorylation networks in mammals. Database. 2014;2014:bau34. doi: 10.1093/database/bau034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safaei J, Maňuch J, Gupta A, Stacho L, Pelech S. Prediction of 492 human protein kinase substrate specificities. Proteome Sci. 2011;9(Suppl 1):S6. doi: 10.1186/1477-5956-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanzoni A, Carbajo D, Diella F, Gherardini PF, Tramontano A, Helmer-Citterich M, et al. Phospho3D 2.0: an enhanced database of three-dimensional structures of phosphorylation sites. Nucleic Acids Res. 2011;39(1):D268–D271. doi: 10.1093/nar/gkq936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davezac N, Baldin V, Blot J, Ducommun B, Tassan J-P. Human pEg3 kinase associates with and phosphorylates CDC25B phosphatase: a potential role for pEg3 in cell cycle regulation. Oncogene. 2002;21(50):7630–7641. doi: 10.1038/sj.onc.1205870. [DOI] [PubMed] [Google Scholar]
- 46.Chauhan D, Pandey P, Hideshima T, Treon S, Raje N, Davies FE, et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275(36):27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 47.Trost B, Kusalik A. Computational prediction of eukaryotic phosphorylation sites. Bioinformatics. 2011;27(21):2927–2935. doi: 10.1093/bioinformatics/btr525. [DOI] [PubMed] [Google Scholar]
- 48.Miller ML, Blom N. Kinase-specific prediction of protein phosphorylation sites. Methods Mol Biol. 2009;527(299–310):x. doi: 10.1007/978-1-60327-834-8_22. [DOI] [PubMed] [Google Scholar]
- 49.Fan W, Xu X, Shen Y, Feng H, Li A, Wang M. Prediction of protein kinase-specific phosphorylation sites in hierarchical structure using functional information and random forest. Amino Acids. 2014;46(4):1069–1078. doi: 10.1007/s00726-014-1669-3. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Zeng S, Xu C, Qiu W, Liang Y, Joshi T, et al. MusiteDeep: a deep-learning framework for general and kinase-specific phosphorylation site prediction. Bioinformatics. 2017;33(24):3909–3916. doi: 10.1093/bioinformatics/btx496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong Y-H, Lee T-Y, Liang H-K, Huang C-M, Wang T-Y, Yang Y-H, et al. KinasePhos 2.0: a web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 2007;35(1):W588–W594. doi: 10.1093/nar/gkm322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue Y, Li A, Wang L, Feng H, Yao X. PPSP: prediction of PK-specific phosphorylation site with Bayesian decision theory. BMC Bioinform. 2006;20(7):163. doi: 10.1186/1471-2105-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saunders NFW, Brinkworth RI, Huber T, Kemp BE, Kobe B. Predikin and PredikinDB: a computational framework for the prediction of protein kinase peptide specificity and an associated database of phosphorylation sites. BMC Bioinform. 2008;26(9):245. doi: 10.1186/1471-2105-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iakoucheva LM, Radivojac P, Brown CJ, O’Connor TR, Sikes JG, Obradovic Z, et al. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32(3):1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durek P, Schudoma C, Weckwerth W, Selbig J, Walther D. Detection and characterization of 3D-signature phosphorylation site motifs and their contribution towards improved phosphorylation site prediction in proteins. BMC Bioinform. 2009;21(10):117. doi: 10.1186/1471-2105-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newman RH, Hu J, Rho H-S, Xie Z, Woodard C, Neiswinger J, et al. Construction of human activity-based phosphorylation networks. Mol Syst Biol. 2013;9:655. doi: 10.1038/msb.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song C, Ye M, Liu Z, Cheng H, Jiang X, Han G, et al. Systematic analysis of protein phosphorylation networks from phosphoproteomic data. Mol Cell Proteomics. 2012;11(10):1070–1083. doi: 10.1074/mcp.M111.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horn H, Schoof EM, Kim J, Robin X, Miller ML, Diella F, et al. KinomeXplorer: an integrated platform for kinome biology studies. Nat Methods. 2014;11(6):603–604. doi: 10.1038/nmeth.2968. [DOI] [PubMed] [Google Scholar]
- 59.Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4(6):1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- 60.Li T, Li F, Zhang X. Prediction of kinase-specific phosphorylation sites with sequence features by a log-odds ratio approach. Proteins. 2008;70(2):404–414. doi: 10.1002/prot.21563. [DOI] [PubMed] [Google Scholar]
- 61.Xue Y, Liu Z, Cao J, Ma Q, Gao X, Wang Q, et al. GPS 2.1: enhanced prediction of kinase-specific phosphorylation sites with an algorithm of motif length selection. Protein Eng Des Sel. 2011;24(3):255–260. doi: 10.1093/protein/gzq094. [DOI] [PubMed] [Google Scholar]
- 62.Gao J, Thelen JJ, Dunker AK, Xu D. Musite, a tool for global prediction of general and kinase-specific phosphorylation sites. Mol Cell Proteomics. 2010;9(12):2586. doi: 10.1074/mcp.M110.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang P, Humphrey SJ, James DE, Yang YH, Jothi R. Positive-unlabeled ensemble learning for kinase substrate prediction from dynamic phosphoproteomics data. Bioinformatics. 2016;32(2):252–259. doi: 10.1093/bioinformatics/btv550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang K-Y, Lee T-Y, Kao H-J, Ma C-T, Lee C-C, Lin T-H, et al. dbPTM in 2019: exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. 2019;47(D1):D298–D308. doi: 10.1093/nar/gky1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller ML, Jensen LJ, Diella F, Jørgensen C, Tinti M, Li L, et al. Linear motif atlas for phosphorylation-dependent signaling. Sci Signal. 2008;1(35):ra2. doi: 10.1126/scisignal.1159433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linding R, Jensen LJ, Ostheimer GJ, van Vugt MATM, Jørgensen C, Miron IM, et al. Systematic discovery of in vivo phosphorylation networks. Cell. 2007;129(7):1415–1426. doi: 10.1016/j.cell.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo F, Wang M, Liu Y, Zhao X-M, Li A. DeepPhos: prediction of protein phosphorylation sites with deep learning. Bioinformatics. 2019;35:2766–2773. doi: 10.1093/bioinformatics/bty1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao M, Zhang Z, Mai G, Luo Y, Zhou F. jEcho: an evolved weight vector to CHaracterize the protein’s posttranslational modification mOtifs. Interdiscip Sci. 2015;7(2):194–199. doi: 10.1007/s12539-015-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parca L, Ariano B, Cabibbo A, Paoletti M, Tamburrini A, Palmeri A, et al. Kinome-wide identification of phosphorylation networks in eukaryotic proteomes. Bioinformatics. 2019;35(3):372–379. doi: 10.1093/bioinformatics/bty545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patrick R, Lê Cao K-A, Kobe B, Bodén M. PhosphoPICK: modelling cellular context to map kinase-substrate phosphorylation events. Bioinformatics. 2015;31(3):382–389. doi: 10.1093/bioinformatics/btu663. [DOI] [PubMed] [Google Scholar]
- 71.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294(5):1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 72.Neuberger G, Schneider G, Eisenhaber F. pkaPS: prediction of protein kinase A phosphorylation sites with the simplified kinase-substrate binding model. Biol Direct. 2007;12(2):1. doi: 10.1186/1745-6150-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21(20):3940–3941. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 74.Lachmann A, Ma’ayan A. KEA: kinase enrichment analysis. Bioinformatics. 2009;25(5):684–686. doi: 10.1093/bioinformatics/btp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weidner C, Fischer C, Sauer S. PHOXTRACK-a tool for interpreting comprehensive datasets of post-translational modifications of proteins. Bioinformatics. 2014;30(23):3410–3411. doi: 10.1093/bioinformatics/btu572. [DOI] [PubMed] [Google Scholar]
- 76.Mischnik M, Sacco F, Cox J, Schneider H-C, Schäfer M, Hendlich M, et al. IKAP: a heuristic framework for inference of kinase activities from Phosphoproteomics data. Bioinformatics. 2016;32(3):424–431. doi: 10.1093/bioinformatics/btv699. [DOI] [PubMed] [Google Scholar]
- 77.Beekhof R, van Alphen C, Henneman AA, Knol JC, Pham TV, Rolfs F, et al. INKA, an integrative data analysis pipeline for phosphoproteomic inference of active kinases. Mol Syst Biol. 2019;15(4):e8250. doi: 10.15252/msb.20188250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilkes EH, Terfve C, Gribben JG, Saez-Rodriguez J, Cutillas PR. Empirical inference of circuitry and plasticity in a kinase signaling network. Proc Natl Acad Sci USA. 2015;112(25):7719–7724. doi: 10.1073/pnas.1423344112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krug K, Mertins P, Zhang B, Hornbeck P, Raju R, Ahmad R, et al. A curated resource for phosphosite-specific signature analysis. Mol Cell Proteomics. 2019;18(3):576–593. doi: 10.1074/mcp.TIR118.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018;46(Database issue):D661–D667. doi: 10.1093/nar/gkx1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petsalaki E, Helbig AO, Gopal A, Pasculescu A, Roth FP, Pawson T. SELPHI: correlation-based identification of kinase-associated networks from global phospho-proteomics data sets. Nucleic Acids Res. 2015;43(W1):W276–W282. doi: 10.1093/nar/gkv459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Söderholm S, Hintsanen P, Öhman T, Aittokallio T, Nyman TA. PhosFox: a bioinformatics tool for peptide-level processing of LC-MS/MS-based phosphoproteomic data. Proteome Sci. 2014;12:36. doi: 10.1186/1477-5956-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Terfve C, Cokelaer T, Henriques D, MacNamara A, Goncalves E, Morris MK, et al. CellNOptR: a flexible toolkit to train protein signaling networks to data using multiple logic formalisms. BMC Syst Biol. 2012;18(6):133. doi: 10.1186/1752-0509-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Äijö T, Granberg K, Lähdesmäki H. Sorad: a systems biology approach to predict and modulate dynamic signaling pathway response from phosphoproteome time-course measurements. Bioinformatics. 2013;29(10):1283–1291. doi: 10.1093/bioinformatics/btt130. [DOI] [PubMed] [Google Scholar]
- 87.Hsu C-L, Wang J-K, Lu P-C, Huang H-C, Juan H-F. DynaPho: a web platform for inferring the dynamics of time-series phosphoproteomics. Bioinformatics. 2017;33:3664–3666. doi: 10.1093/bioinformatics/btx443. [DOI] [PubMed] [Google Scholar]
- 88.Yang P, Patrick E, Humphrey SJ, Ghazanfar S, James DE, Jothi R, et al. KinasePA: phosphoproteomics data annotation using hypothesis driven kinase perturbation analysis. Proteomics. 2016;16(13):1868–1871. doi: 10.1002/pmic.201600068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ren J, Jiang C, Gao X, Liu Z, Yuan Z, Jin C, et al. PhosSNP for systematic analysis of genetic polymorphisms that influence protein phosphorylation. Mol Cell Proteomics. 2010;9(4):623. doi: 10.1074/mcp.M900273-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krassowski M, Paczkowska M, Cullion K, Huang T, Dzneladze I, Ouellette BFF, et al. ActiveDriverDB: human disease mutations and genome variation in post-translational modification sites of proteins. Nucleic Acids Res. 2018;46(D1):D901–D910. doi: 10.1093/nar/gkx973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wagih O, Reimand J, Bader GD. MIMP: predicting the impact of mutations on kinase-substrate phosphorylation. Nat Methods. 2015;12(6):531–533. doi: 10.1038/nmeth.3396. [DOI] [PubMed] [Google Scholar]
- 92.Creixell P, Schoof EM, Simpson CD, Longden J, Miller CJ, Lou HJ, et al. Kinome-wide decoding of network-attacking mutations rewiring cancer signaling. Cell. 2015;163(1):202–217. doi: 10.1016/j.cell.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patrick R, Kobe B, Lê Cao K-A, Bodén M. PhosphoPICK-SNP: quantifying the effect of amino acid variants on protein phosphorylation. Bioinformatics. 2017;33(12):1773–1781. doi: 10.1093/bioinformatics/btx072. [DOI] [PubMed] [Google Scholar]
- 94.Kim J, Yoo M, Kang J, Tan AC. K-Map: connecting kinases with therapeutics for drug repurposing and development. Hum Genomics. 2013;23(7):20. doi: 10.1186/1479-7364-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldberg JM, Griggs AD, Smith JL, Haas BJ, Wortman JR, Zeng Q. Kinannote, a computer program to identify and classify members of the eukaryotic protein kinase superfamily. Bioinformatics. 2013;29(19):2387–2394. doi: 10.1093/bioinformatics/btt419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McSkimming DI, Rasheed K, Kannan N. Classifying kinase conformations using a machine learning approach. BMC Bioinform. 2017;18(1):86. doi: 10.1186/s12859-017-1506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eid S, Turk S, Volkamer A, Rippmann F, Fulle S. KinMap: a web-based tool for interactive navigation through human kinome data. BMC Bioinform. 2017;18(1):16. doi: 10.1186/s12859-016-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Douglass J, Gunaratne R, Bradford D, Saeed F, Hoffert JD, Steinbach PJ, et al. Identifying protein kinase target preferences using mass spectrometry. Am J Physiol Cell Physiol. 2012;303(7):C715–C727. doi: 10.1152/ajpcell.00166.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torii M, Li G, Li Z, Oughtred R, Diella F, Celen I, et al. RLIMS-P: an online text-mining tool for literature-based extraction of protein phosphorylation information. Database. 2014;2014:bau081. doi: 10.1093/database/bau081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arighi CN, Siu AY, Tudor CO, Nchoutmboube JA, Wu CH, Shanker VK. eFIP: a tool for mining functional impact of phosphorylation from literature. Methods Mol Biol. 2011;694:63–75. doi: 10.1007/978-1-60761-977-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao B, Pisitkun T, Hoffert JD, Knepper MA, Saeed F. CPhos: a program to calculate and visualize evolutionarily conserved functional phosphorylation sites. Proteomics. 2012;12(22):3299–3303. doi: 10.1002/pmic.201200189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Warnecke A, Sandalova T, Achour A, Harris RA. PyTMs: a useful PyMOL plugin for modeling common post-translational modifications. BMC Bioinform. 2014;28(15):370. doi: 10.1186/s12859-014-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Madeira F, Tinti M, Murugesan G, Berrett E, Stafford M, Toth R, et al. 14-3-3-Pred: improved methods to predict 14-3-3-binding phosphopeptides. Bioinformatics. 2015;31(14):2276–2283. doi: 10.1093/bioinformatics/btv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo Y, Peng D, Zhou J, Lin S, Wang C, Ning W, et al. iEKPD 2.0: an update with rich annotations for eukaryotic protein kinases, protein phosphatases and proteins containing phosphoprotein-binding domains. Nucleic Acids Res. 2019;47(D1):D344–D350. doi: 10.1093/nar/gky1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kooistra AJ, Kanev GK, van Linden OPJ, Leurs R, de Esch IJP, de Graaf C. KLIFS: a structural kinase-ligand interaction database. Nucleic Acids Res. 2016;44(D1):D365–D371. doi: 10.1093/nar/gkv1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peri S, Navarro JD, Amanchy R, Kristiansen TZ, Jonnalagadda CK, Surendranath V, et al. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 2003;13(10):2363–2371. doi: 10.1101/gr.1680803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mishra GR, Suresh M, Kumaran K, Kannabiran N, Suresh S, Bala P, et al. Human protein reference database—2006 update. Nucleic Acids Res. 2006;34(1):D411–D414. doi: 10.1093/nar/gkj141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diella F, Cameron S, Gemünd C, Linding R, Via A, Kuster B, et al. Phospho.ELM: a database of experimentally verified phosphorylation sites in eukaryotic proteins. BMC Bioinform. 2004;5:79. doi: 10.1186/1471-2105-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diella F, Gould CM, Chica C, Via A, Gibson TJ. Phospho.ELM: a database of phosphorylation sites—update 2008. Nucleic Acids Res. 2008;36(Database issue):D240–D244. doi: 10.1093/nar/gkm772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gnad F, Ren S, Cox J, Olsen JV, Macek B, Oroshi M, et al. PHOSIDA (phosphorylation site database): management, structural and evolutionary investigation, and prediction of phosphosites. Genome Biol. 2007;8(11):R250. doi: 10.1186/gb-2007-8-11-r250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tinti M, Johnson C, Toth R, Ferrier DEK, Mackintosh C. Evolution of signal multiplexing by 14-3-3-binding 2R-ohnologue protein families in the vertebrates. Open Biol. 2012;2(7):120103. doi: 10.1098/rsob.120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee T-Y, Bo-Kai Hsu J, Chang W-C, Huang H-D. RegPhos: a system to explore the protein kinase-substrate phosphorylation network in humans. Nucleic Acids Res. 2011;39(Database issue):D777–D787. doi: 10.1093/nar/gkq970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naegle KM, Welsch RE, Yaffe MB, White FM, Lauffenburger DA. MCAM: multiple clustering analysis methodology for deriving hypotheses and insights from high-throughput proteomic datasets. PLoS Comput Biol. 2011;7(7):e1002119. doi: 10.1371/journal.pcbi.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ovelleiro D, Carrascal M, Casas V, Abian J. LymPHOS: design of a phosphosite database of primary human T cells. Proteomics. 2009;9(14):3741–3751. doi: 10.1002/pmic.200800701. [DOI] [PubMed] [Google Scholar]
- 116.Lee T-Y, Huang H-D, Hung J-H, Huang H-Y, Yang Y-S, Wang T-H. dbPTM: an information repository of protein post-translational modification. Nucleic Acids Res. 2006;34(Database issue):D622–D627. doi: 10.1093/nar/gkj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu C-T, Huang K-Y, Su M-G, Lee T-Y, Bretaña NA, Chang W-C, et al. DbPTM 3.0: an informative resource for investigating substrate site specificity and functional association of protein post-translational modifications. Nucleic Acids Res. 2013;41(Database issue):D295–D305. doi: 10.1093/nar/gks1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lo Surdo P, Calderone A, Cesareni G, Perfetto L. SIGNOR: a database of causal relationships between biological entities-a short guide to searching and browsing. Curr Protoc Bioinform. 2017;58:8–23. doi: 10.1002/cpbi.28. [DOI] [PubMed] [Google Scholar]
- 119.Quintaje SB, Orchard S. The annotation of both human and mouse kinomes in UniProtKB/Swiss-Prot: one small step in manual annotation, one giant leap for full comprehension of genomes. Mol Cell Proteomics. 2008;7(8):1409. doi: 10.1074/mcp.R700001-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Z, Ren J, Cao J, He J, Yao X, Jin C, et al. Systematic analysis of the Plk-mediated phosphoregulation in eukaryotes. Brief Bioinform. 2013;14(3):344–360. doi: 10.1093/bib/bbs041. [DOI] [PubMed] [Google Scholar]
- 121.Tsaousis GN, Bagos PG, Hamodrakas SJ. HMMpTM: improving transmembrane protein topology prediction using phosphorylation and glycosylation site prediction. Biochim Biophys Acta. 2014;1844(2):316–322. doi: 10.1016/j.bbapap.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 122.Zou L, Wang M, Shen Y, Liao J, Li A, Wang M. PKIS: computational identification of protein kinases for experimentally discovered protein phosphorylation sites. BMC Bioinform. 2013;13(14):247. doi: 10.1186/1471-2105-14-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dou Y, Yao B, Zhang C. PhosphoSVM: prediction of phosphorylation sites by integrating various protein sequence attributes with a support vector machine. Amino Acids. 2014;46(6):1459–1469. doi: 10.1007/s00726-014-1711-5. [DOI] [PubMed] [Google Scholar]
- 124.Wu Z, Lu M, Li T. Prediction of substrate sites for protein phosphatases 1B, SHP-1, and SHP-2 based on sequence features. Amino Acids. 2014;46(8):1919–1928. doi: 10.1007/s00726-014-1739-6. [DOI] [PubMed] [Google Scholar]
- 125.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31(13):3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trost B, Maleki F, Kusalik A, Napper S. DAPPLE 2: a tool for the homology-based prediction of post-translational modification sites. J Proteome Res. 2016;15(8):2760–2767. doi: 10.1021/acs.jproteome.6b00304. [DOI] [PubMed] [Google Scholar]
- 127.Qiu W-R, Xiao X, Xu Z-C, Chou K-C. iPhos-PseEn: identifying phosphorylation sites in proteins by fusing different pseudo components into an ensemble classifier. Oncotarget. 2016;7(32):51270–51283. doi: 10.18632/oncotarget.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qin G-M, Li R-Y, Zhao X-M. PhosD: inferring kinase-substrate interactions based on protein domains. Bioinformatics. 2017;33(8):1197–1204. doi: 10.1093/bioinformatics/btw792. [DOI] [PubMed] [Google Scholar]
- 129.Wei L, Xing P, Tang J, Zou Q. PhosPred-RF: a novel sequence-based predictor for phosphorylation sites using sequential information only. IEEE Trans Nanobioscience. 2017;16(4):240–247. doi: 10.1109/TNB.2017.2661756. [DOI] [PubMed] [Google Scholar]
- 130.Wang D, Liang Y, Xu D. Capsule network for protein post-translational modification site prediction. Bioinformatics. 2019;35(14):2386–2394. doi: 10.1093/bioinformatics/bty977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu Y, Wang M, Xi J, Luo F, Li A. PTM-ssMP: a web server for predicting different types of post-translational modification sites using novel site-specific modification profile. Int J Biol Sci. 2018;14(8):946–956. doi: 10.7150/ijbs.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li F, Li C, Marquez-Lago TT, Leier A, Akutsu T, Purcell AW, et al. Quokka: a comprehensive tool for rapid and accurate prediction of kinase family-specific phosphorylation sites in the human proteome. Bioinformatics. 2018;34:4223–4231. doi: 10.1093/bioinformatics/bty522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao M, Chen G, Wang L, Wen P, Shi S. Computational prediction and analysis for tyrosine post-translational modifications via elastic Net. J Chem Inf Model. 2018;58(6):1272–1281. doi: 10.1021/acs.jcim.7b00688. [DOI] [PubMed] [Google Scholar]
- 134.Ayati M, Wiredja D, Schlatzer D, Maxwell S, Li M, Koyutürk M, et al. CoPhosK: a method for comprehensive kinase substrate annotation using co-phosphorylation analysis. PLoS Comput Biol. 2019;15(2):e1006678. doi: 10.1371/journal.pcbi.1006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of URLs for all resources.
Data Availability Statement
The data used for comparing kinase activity inference tools can be found in PubMed with PMID: 28674151.