Abstract
Heme oxygenase-1 (HO-1) is highly induced in various human disease states, including cancer, indicating that HO-1 is an emerging target of cancer therapy. In this study, we investigated that the mechanisms of hemin-induced HO-1 expression and its signaling pathways in human breast cancer cell. We used MCF-7 cells, a human breast cancer cell line. Hemin increased HO-1 expression in MCF-7 cells in a dose- and time-dependent manner. Hemin enhanced HO-1 expression through the activation of c-Jun N-terminal kinases (JNK) signaling pathway. Hemin also induced activation of Nrf2, a major transcription factor of HO-1 expression. These responses in MCF-7 cells were completely blocked by pretreatment with brazilin, a HO-1 regulator. These results indicated that brazilin inhibits hemin-induced HO-1 expressions through inactivation of JNK/Nrf2 in MCF-7 cells. Thus, our findings suggest that HO-1 is an important anticancer-target of brazilin in human breast cancer.
Keywords: heme oxygenase-1, JNK, Nrf2, breast cancer, MCF-7, hemin, brazilin
1. Introduction
Heme oxygenase-1 (HO-1) was identified by Maines [1], as a liver microsomal protein with degradation activity of heme to bilirubin. HO-1 catalyzes the degradation of heme to form the open-chain tetrapyrrole biliverdin-Ixα, carbon monoxide (CO), and free iron, which play crucial roles in the adaptation to defense against oxidative stress and cellular stress [2,3]. HO-1 is induced in cells by cellular stress including oxidants [4], hypoxia condition [5], cytokine [6,7], and ultraviolet light irradiation [5,8]. These findings suggest that HO-1 has cytoprotective actions. Therefore, studies focused on HO-1 to find potential managements for various diseases, including cancer [9,10], inflammatory diseases [11], respiratory disease [12], and circulation system [13,14]. It is known that expression of high level of HO-1 occurs in various tumors including in renal cancer [15], acute T cell leukemia [16], prostate cancer [17], lung [18], and squamous cell carcinoma in head and neck [19]. Many studies suggested the HO-1 were implicated in tumorigenesis and cancer prognosis like chemotherapeutic sensitivity [20], cancer invasion [21,22], aggressiveness [23,24] and survival rate prediction [25]. Taken together, these findings indicate that the investigating of the role of HO-1 and its targets with molecular mechanism for cancer therapy seem to be important [26].
Breast cancer (BRCA) is most common types of cancer in woman worldwide, and one of the most important causes of cancer-related mortality for women [27,28]. MCF-7 cells were obtained at the Michigan Cancer Foundation (MCF) in 1973 and most commonly used xenograft model of breast cancer [28]. In gene expression profiling analysis, the MCF-7 were possess characteristics that estrogen receptor 1 (ESR1)-positive, progesterone receptor (PGR)-negative, Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2/HER2)-negative and epidermal growth factor receptor (EGFR)-negative [29]. Whereas heme oxygenase (HOs), CCAAT/enhance binding protein (C/EBP) homolog protein (CHOP), glucocorticoid-inducible kinase 1 (SGK-1), prostate apoptosis response (Par-4), Caveolin-1 (Cav-1) mRNA expressions affects to cellular survivals [27]. Therefore, there are requires studies how heme oxygenase affects cancer progression/regulation in MCF-7 cells, the molecular mechanism of playing role in the search of anti-cancer technology.
Caesalpinia sappan (C. sappan) has been used as an Asian traditional medicine for acute/chronic human diseases [30]. The major component of C. sappan, 7,11b-dihydrobenz[b]indeno [1,2-d] pyran-3,6a,9,10(6H)-tetrol (brazilin) [30,31], exhibits various biological effects, including anti-inflammatory [32], and anti-hepatotoxicity activity [33], and inhibition of protein kinase C [34]. Recent studies have reported that brazilin has anticancer activities such as anti-mitotic and apoptotic activity [35,36]. In this study, we examined whether brazilin induces expression of HO-1 using a human breast cancer line.
2. Materials and Methods
2.1. Cells and Materials
A breast cancer cell line, MCF-7, was purchased from American Type Culture Collection (ATCC; VA. USA). The cells were maintained in high glucose containing DMEM (Dulbecco’s modified Eagle’s medium)–10% fetal bovine serum (FBS, v/v)–antibiotics/antimycotics (Gibco, Gaithersburg, MD, USA) at CO2 incubator. Brazilin was obtained from MP Biomedicals LLC (Irvine, CA, USA). Hemin, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DMEM, and anti-β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibodies related to phospho-JNK (p-JNK, Cat #4668), phospho-p38 (p-p38, Cat #9216) and phospho-ERK (p-ERK, Cat #4695) were purchased from Cell Signaling Technology (Beverly, MA, USA). HO-1 antibody was from Enzo Life Sciences (Cat #ADI-OSA-111-D, NY, USA). Nrf2 (Cat # sc-13032), PCNA (Cat # sc-9857), and secondary antibodies were from Santa Cruz Biotechnology (Paso Robles, CA, USA). Specific inhibitors against MAPK, SP600125 (JNK inhibitor), SB203580 (p38 inhibitor) and PD98059 (ERK inhibitor) were purchased from MilliporeSigma (Burlington, MA, USA). [α-32P]dCTP was purchased from Amersham plc (Buckinghamshire, UK). Culture medium, supplements and cell culture tested PBS were obtained from Gibco (ME, USA).
2.2. Cell Viability Assay
The cell viability (and/or growth rate) of MCF-7 was assayed using an MTT assay. Briefly, cells were inoculated in a microtiter plate 96 well (3 × 103 cells/well, confluent less than 60%), and then incubated at CO2 incubator for 12 h. Cells were either untreated or treated with 2.5, 5, 10, 20, 50 μM brazilin and 25, 50, 100 μM hemin at 37 °C and further incubated for 24 h. The cells were washed with PBS prior to the addition of 0.5 mg/mL MTT (100 μL per well), and then incubated at 37 °C for 30 min. Insoluble formazan were solubilized with DMSO (100 μL per well) and measured at 570 nm using a microplate reader (Model 3550, Bio-Rad, Hercules, CA, USA).
2.3. Western Blot Analysis
MCF-7 cells (7.0 × 105 cells/sample) were treated with 2.5, 5, 10, 20 μM brazilin for 60 min, and then incubated with 50 μM hemin at 37 °C for 24 h. Cells were lysed with commercial ice-cold lysis reagents (M-PER Mammalian Protein Extraction Reagent, Pierce Biotechnology, Rockford, IL, USA). Protein extracts (10 μg per well) were separated by SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes (GE Healthcare Life Sciences, Pittsburgh, PA, USA). Membrane was blocked for 1 to 2 h with 5% skim milk or 3% immunoglobulin-free bovine serum albumin (BSA), and then incubated 15 h at 4 °C with 1.0 μg/mL of primary antibody (1:1000 to 2000). Horseradish peroxidase-conjugated anti-IgG monoclonal antibody (1:1000 to 2000) was used as the secondary antibody. Specific band were analyzed with LAS-1000 (Fuji Film, Tokyo, Japan). The images were quantified using a computer image analysis software, Image Reader Pro (Fuji Film, Tokyo, Japan) and ImageJ (National Institutes of Health, Bethesda, MD, USA).
2.4. Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated using the commercial kits (FastPureTM RNA Kit, Takara Bio Inc., Kusatsu, Shiga Japan). The RNA concentration and purities were determined by absorbance at 260/280 nm. cDNA was synthesized from 1 μg total RNA using a PrimeScriptTM RT reagent Kit (Takara, Japan). HO-1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression were determined by real-time qPCR using the sequence detection system (ABI PRISM 7900, Applied Biosystems, Waltham, MA, USA), and SYBR Green. The primers were used to amplify cDNA of HO-1 (NM002133) (sense: 5′-GCC AGC AAC AAA GTG CAA G-3′; antisense: 5′-GGC ATA AAG CCC TAC AGC AA-3′) and GAPDH (NM002046) (sense: 5′-ATG GAA ATC CCA TCA CCA TCT T; antisense: CGC CCC ACT TGA TTT TGG-3′) (Genotech, Daejeon, Korea). The variation in mRNA concentration of all genes were normalized to the GAPDH housekeeping gene. Relative quantitation was performed using the comparative Ct method according to the manufacturer’s instructions. Data were presented the mean ± S.E.M of three independent experiments.
2.5. Isolation Extracts of Nuclear Fraction
MCF-7 cells (2 × 106 cells/sample) were treated with brazilin in the presence or absence of hemin (50 μM) for 4 h. Cells were washed twice and carefully detached by scrapping into 1.5 mL of ice-cold PBS (pH 7.5) and then pelleted (4000× g at 4 °C for 5 min). Cytoplasmic and nuclear fraction were extracted from cells using commercial kit (NE-PER)(Pierce Biotechnology, IL, USA).
2.6. Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extract of cells was prepared as described above. An oligonucleotide containing the Nrf2 (5′-TGG GGA ACC TGT GCT GAG TCA CTG GAG-3′) were purchased from Genotech (Daejeon, Korea). Electrophoretic mobility shift assay was performed as described in Hellman and Fried [37]. Specific binding was controlled by competition with a 50-fold excess of cold Nrf2 oligonucleotide.
2.7. Statistical Analysis
All tests were performed at least in three independent experiments. To compare means within groups we performed student t-test. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Endogenous Cytotoxicity of Brazilin and Hemin on MCF-7 Cells
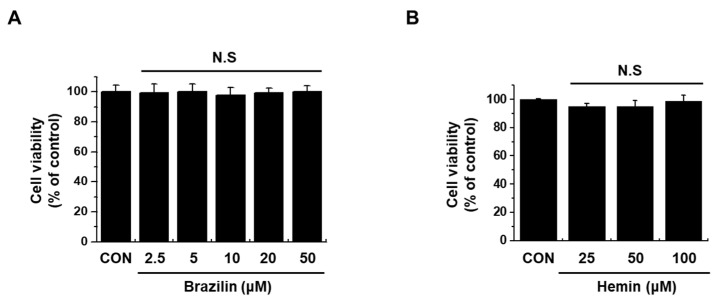
To examine the endogenous cytotoxicity of brazilin (Figure 1A) and hemin (Figure 1B) on MCF-7 cells, we treated cells with brazilin (0–50 μM) and hemin (0–100 μM) for 24 h. The influence of brazilin and hemin on MCF-7 cellular toxicity was then analyzed using MTT assay. Treatment of MCF-7 cells with indicated dosage of brazilin and hemin shown no significant change in cell viability (Figure 2A,B). Therefore, we performed further experiments in a non-toxic dose of brazilin and hemin.
Figure 1.
Chemical structure of (A) brazilin (CAS number: 474-07-7, C16H14O5, MW: 286.28) and (B) hemin (CAS number: 16009-13-5, C34H32ClFeN4O4, MW: 651.94).
Figure 2.
Effect of brazilin and hemin on the viability of MCF-7 cells. (A) Brazilin and (B) Hemin was treated with indicated concentration for 24 h. An established MTT assay was used to detect viability of cells. Data are presented as Means ± S.E.M of three independent experiments. N.S stands for not statistically significant.
3.2. Effect of Hemin on HO-1 Expression in MCF-7 Cells
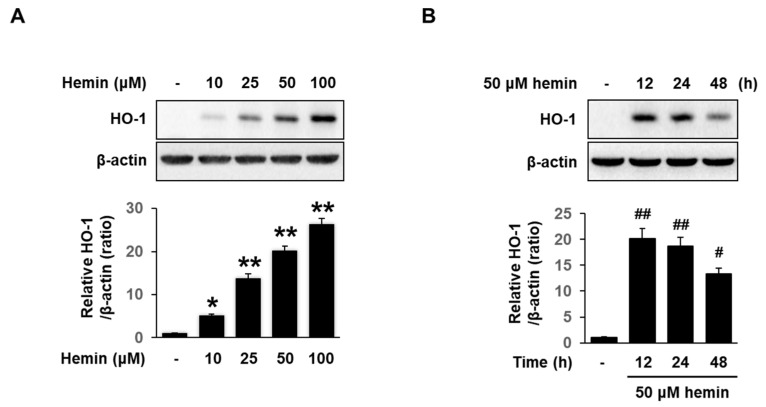
To investigate the effect of hemin on HO-1 expression in MCF-7 cells, we performed Western blotting. Western blot analysis revealed that hemin increased HO-1 expression in a dose- and time-dependent manner (Figure 3A,B).
Figure 3.
Hemin increase expression of HO-1 in MCF-7 cells. HO-1 protein expression were analyzed by Western blotting. (A) MCF-7 cells were treated with 10, 25, 50, and 100 μM hemin for 24 h. (B) Cells were treated with 50 μM hemin for 12, 24, and 48 h. Lower panels: Quantification of intensity in three different experiments using a ImageJ software. Data are presented as Means ± S.E.M of three independent experiments. * p < 0.05, ** p < 0.001, # p < 0.01 and ## p < 0.001 vs. control.
3.3. Effect of Brazilin on Hemin-Induced HO-1 Expression in MCF-7 Cells
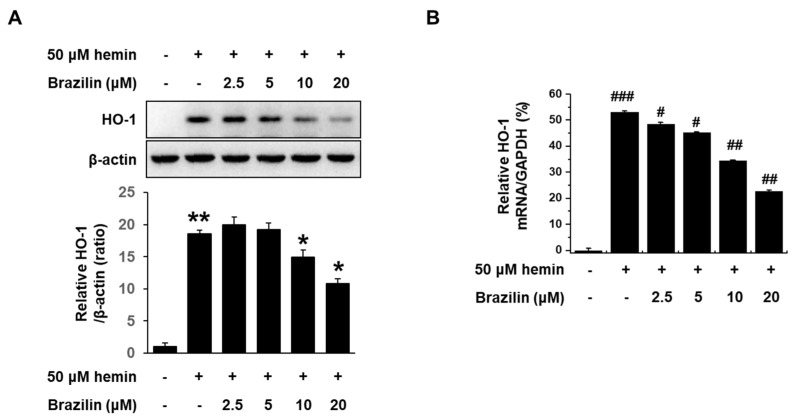
To investigate the effect of brazilin on hemin-induced HO-1 expression in MCF-7 cells, we performed Western blotting and real-time PCR. Western blot analysis and real-time PCR revealed that brazilin-suppressed hemin-induced HO-1 expression in a dose-dependent manner (Figure 4A,B).
Figure 4.
Brazilin regulates increase of hemin-induced HO-1 expression in MCF-7 cells. Regulatory effects of brazilin on (A) hemin-induced HO-1 protein expression and (B) levels of HO-1 mRNA. Lower panels: Quantification of intensity in three different experiments using a ImageJ software. Cells were treated with brazilin for 1 h, and then incubated with 50 μM hemin for 24 h. HO-1 mRNA levels were analyzed by real-time PCR, and GAPDH was used as an internal control. Data are presented as Means ± S.E.M of three independent experiments. * p < 0.05 vs. hemin and ** p < 0.001 vs. control. # p < 0.05 and ## p < 0.01 vs. hemin. ### p < 0.001 vs. control.
3.4. Effects of Hemin on MAPK Activation in MCF-7 Cells
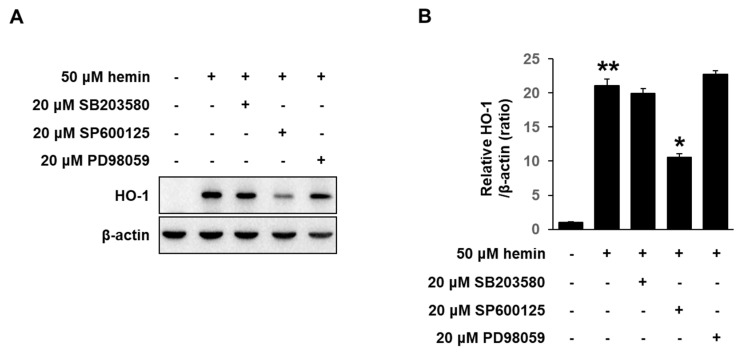
The effect of hemin on JNK/ERK/p38 activities was investigated using Western blotting. MCF-7 cells pretreated with inhibitors of ERK (PD98059), JNK (SP600125), and p38 (SB203580) for 1 h were further incubated with hemin (50 μM) for 24 h. As shown in Figure 5A,B, inhibition of JNK only blocked hemin-induced HO-1 protein expression in MCF-7 cells. These results suggest that hemin-induced HO-1 expression were mediated by JNK signal pathway.
Figure 5.
Hemin-induced HO-1 activation through JNK/Nrf2 signaling pathway in MCF-7 cells. (A) MCF-7 cells were treated with inhibitors of p38 (20 μM SB203580), JNK (20 μM SP600125) and ERK (20 μM PD98059) for 1 h, and then incubated with 50 μM of hemin for 24 h. The HO-1 protein expression was analyzed using Western blotting. (B) Quantification of intensity using a ImageJ software. Data are presented as Means ± S.E.M of three independent experiments. * p < 0.01 vs. hemin and ** p < 0.001 vs. control.
3.5. Effects of Brazilin on Hemin-Induced JNK Activation in MCF-7 Cells
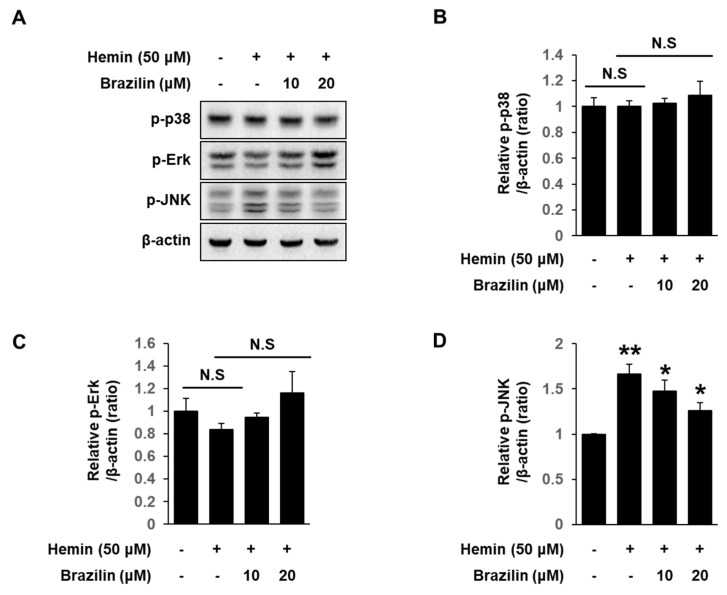
To investigate the effect of brazilin on hemin-induced JNK activation, we performed Western blotting. As shown in Figure 6A–D, hemin significantly induced phosphorylation of JNK only. Interestingly, brazilin also only significantly blocked phosphorylation of JNK by hemin in MCF-7 cells (Figure 6A,D).
Figure 6.
Brazilin regulates hemin-induced HO-1 activation through phosphorylation of Erk and dephosphorylation of JNK. (A) Western blot analysis of phosphorylated proteins of p38, Erk and JNK on hemin-induced HO-1 activation. Cells were pretreated with brazilin and then incubated with 50 μM hemin for 1 h. Levels of phosphorylated p38 (B), ERK (C) and JNK (D) were quantified by ImageJ software. Data are presented as Means ± S.E.M of three independent experiments. * p < 0.05 vs. hemin and ** p < 0.001 vs. control. N.S stands for not statistically significant.
3.6. Effect of Brazilin on Hemin-Induced Nrf2 Activation in MCF-7 Cells
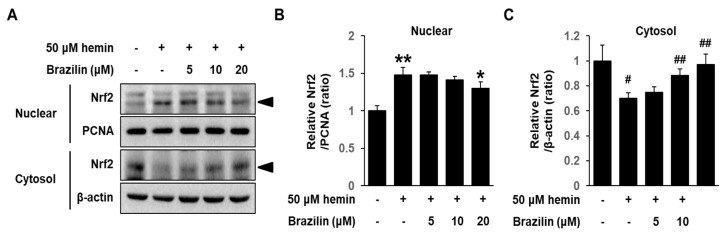
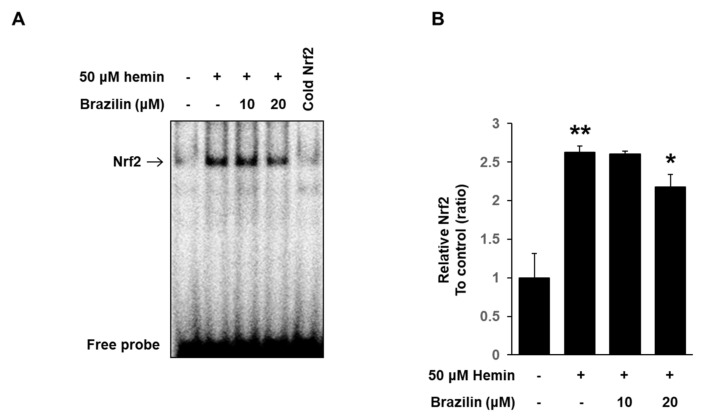
To further understand the inhibitory mechanism of brazilin on HO-1 transcriptional regulation, we determined whether brazilin inhibits Nrf2 activation in MCF-7 cell after stimulation with hemin using Western blotting and EMSA. Brazilin caused inhibitory actions on nuclear translocation of Nrf2 (Figure 7A–C). Furthermore, DNA binding activity of hemin-induced Nrf2 was markedly inhibited by treatment of brazilin in a dose-dependent manner (Figure 8A,B). These results indicate that Nrf2 nuclear translocation is an important mechanism on hemin-induced HO-1 expression and that brazilin regulates Nrf2 translocation.
Figure 7.
Brazilin regulates hemin-induced Nrf2 translocation into nuclear region. (A) Translocation of Nrf2 to the nucleus. Cells were treated with brazilin in the presence of hemin. Following 4 h incubation, nuclear and cytoplasm extracts were prepared. Protein levels were determined by Western blotting. PCNA for nuclear protein and β-actin for cytosol protein were used as loading controls. (B,C) Quantification of Nrf2 level in nucleus and cytosol fraction, respectively. Intensity of bands on Western blots was analyzed using a ImageJ software. Data are presented as Means ± S.E.M of three independent experiments. * p < 0.05 vs. hemin and ** p < 0.005 vs. control. # p < 0.01 vs. control and ## p < 0.005 vs. hemin. Arrow indicates Nrf2 molecule specific bands.
Figure 8.
Effect of brazilin on Nrf2 DNA binding activity to murine HO-1 promoter oligonucleotide. (A) Effects of brazilin on Nrf2 DNA binding activity on MCF-7 cells. Brazilin were treated with cells in the presence of hemin. Levels of Nrf2 DNA binding was analyzed by EMSA following Materials and Methods. (B) Quantification of Nrf2 complex on EMSA data was measured using ImageJ software. Data are presented as Means ± S.E.M of three independent experiments. * p < 0.05 vs. hemin and ** p < 0.001 vs. control.
4. Discussion
This study provides evidence that brazilin, a major component of Caesalpinia sappan, blocks hemin-induced HO-1 expression in MCF-7 cells and JNK/Nrf2-mediated HO-1 expression. Thus, HO-1 may be an important target in the screening of novel pharmaceuticals to treatment of breast cancer. In human cells, two isoforms of HO are present. HO-1 is a stress-inducible enzyme, while HO-2 is a constitutive enzyme as genetically and enzymatically [38]. Hemin induces the expression of HO-1 in a variety of cell types including endothelium [39], aortic tissues [40], embryonic fibroblast and adipocytes [41].
In this study, hemin increased HO-1 expression in MCF-7 cells in a dose- and time-dependent manner, and brazilin substantially inhibited hemin-induced HO-1 expression in human breast cancer cells. It has been known that MAPK and ROS control HO-1 expression through Nrf2 activation in various cells such as pulmonary aortic endothelial cells (PAEC) [42], hepatic and hematopoietic system [43], and hepatic carcinoma cells [44]. Indeed, increases of HO-1 expression were supported anti-apoptotic effects in monocyte [45], tumor necrosis [46], then many trials were suggested with previously reported inhibitors [45]. Therefore, this study reveals that an increase in the level of hemin-induced HO-1 markedly reduces the signaling mechanism of MCH-7 cells by brazilin.
In this study, hemin increased HO-1 expression (protein and mRNA level) in MCF-7 cells in a dose- and time-dependent manner. Among MAPK signaling molecules, hemin induced only JNK activation in MCF-7 cells. Our results also showed that hemin induces HO-1 expression through Nrf2 activation in MCF-7 cells. Interestingly, these responses were completely blocked by pretreatment with brazilin, indicating that brazilin inhibits hemin-induced HO-1 expressions through inactivation of JNK/Nrf2 in MCF-7 cells. In cancer cells, elevation of HO-1 expression increased in various tumors [15,16,17,18,19] and hemin, an iron-containing porphyrin with chlorine, is available HO-1 inducer in various cell types including normal and cancer cells [47,48]. In this study, we observed hemin-induced increases of HO-1 expression in MCF-7 cells and brazilin is potent regulator through HO-1 expression. On the other hand, we investigated whether brazilin-induced HO-1 expression, but it was not observed (data not shown). Moreover, novel HO-1 inhibition tools were reported such as arylethanolimidazole derivatives in cancer [49], caffeic acid phenethyl ester in diabetes [50] and fumarate hydratase as target genes in cancer [51].
Taken together, these data and previous publications indicates that hemin induced HO-1expression and JNK/Nrf2 signaling pathway plays a regulatory role in MCF-7 cells indicates that cytoprotective role in MCF cells. Therefore, blockage effects of brazilin against hemin-induced HO-1 expression in MCF-7 cells suggest useful therapeutic/prevention implication. However, the brazilin growth-inhibitory effect on human breast cancer cells still need to be studied.
This study was concluded that HO-1 is an important anti-cancer target of brazilin in human breast cancer via inhibit hemin-induced HO-1 expression through inactivation of JNK/Nrf2 in MCF-7 cells [52]. Therefore, brazilin or other HO-1 regulators may be of use in the treatment breast cancer and required further studies.
Acknowledgments
The authors thank Mie-Jae Im for critical reading of the manuscript.
Author Contributions
Conceptualization J.-S.K.; methodology and investigation, H.-Y.J. and O.-Y.H.; writing—original draft preparation, H.-Y.J. and J.-S.K.; writing—review and editing, E.-Y.C., K.-H.P. and J.-S.K.; supervision, E.-Y.C., K.-H.P. and J.-S.K.; project administration, J.-S.K.; funding acquisition, J.-S.K. and K.-H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2019R1A2C1003454 to J.-S.K. and 2019R1H1A2039731 to K.-H.P.). K.-H.P. is recipient of research supportive fund from Nambu University (2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Maines M.D. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. doi: 10.1096/fasebj.2.10.3290025. [DOI] [PubMed] [Google Scholar]
- 2.Maines M.D. The heme oxygenase system: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 3.Florczyk U.M., Jozkowicz A., Dulak J. Biliverdin reductase: New features of an old enzyme and its potential therapeutic significance. Pharmacol. Rep. 2008;60:38–48. [PMC free article] [PubMed] [Google Scholar]
- 4.Clark J.E., Foresti R., Green C.J., Motterlini R. Dynamics of heme oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem. J. 2000;348:615–619. doi: 10.1042/bj3480615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keyse S., A Applegate L., Tromvoukis Y., Tyrrell R.M. Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol. Cell. Boil. 1990;10:4967–4969. doi: 10.1128/MCB.10.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzardini M., Terao M., Falciani F., Cantoni L. Cytokine induction of heme oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the heme oxygenase gene. Biochem. J. 1993;290:343–347. doi: 10.1042/bj2900343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantoni L., Rossi C., Rizzardini M., Gadina M., Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic heme oxygenase. Feedback regulation by glucocorticoids. Biochem. J. 1991;279:891–894. doi: 10.1042/bj2790891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee P.J., Jiang B.H., Chin B.Y. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997;272:5375–5381. doi: 10.1074/jbc.272.9.5375. [DOI] [PubMed] [Google Scholar]
- 9.Becker J.C., Fukui H., Imai Y., Sekikawa A., Kimura T., Yamagishi H., Yoshitake N., Pohle T., Domschke W., Fujimori T. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand. J. Gastroenterol. 2007;42:852–858. doi: 10.1080/00365520701192383. [DOI] [PubMed] [Google Scholar]
- 10.Busserolles J., Megías J., Terencio M.C., Alcaraz M.J. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. Int. J. Biochem. Cell Boil. 2006;38:1510–1517. doi: 10.1016/j.biocel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Willis D., Moore A.R., Frederick R., Willoughby D. Heme oxygenase: A novel target for the modulation of inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 12.Lee P.J., Alam J., Wiegand G.W., Choi A.M. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. USA. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y.-M., Wu B.J., Witting P.K., Stocker R. Probucol Protects Against Smooth Muscle Cell Proliferation by Upregulating Heme Oxygenase-1. Circulation. 2004;110:1855–1860. doi: 10.1161/01.CIR.0000142610.10530.25. [DOI] [PubMed] [Google Scholar]
- 14.Brouard S., Otterbein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M., Soares M. Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman A.I., Choudhury M., Da Silva J.-L., Schwartzman M.L., Abraham N.G. Overexpression of the Heme Oxygenase Gene in Renal Cell Carcinoma. Exp. Boil. Med. 1997;214:54–75. doi: 10.3181/00379727-214-44069. [DOI] [PubMed] [Google Scholar]
- 16.Choi B.-M., Pae H.-O., Jeong Y.-R., Oh G.-S., Jun C.-D., Kim B.-R., Kim Y.-M., Chung H.-T. Overexpression of heme oxygenase (HO)-1 renders jurkat T cells resistant to Fas-mediated apoptosis: Involvement of iron released by HO-1. Free Radic. Boil. Med. 2004;36:858–871. doi: 10.1016/j.freeradbiomed.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Maines M.D., Abrahamsson P.-A. Expression of heme oxygenase-1 (HSP32) in human prostate: Normal, hyperplastic, and tumor tissue distribution. Urology. 1996;47:727–733. doi: 10.1016/S0090-4295(96)00010-6. [DOI] [PubMed] [Google Scholar]
- 18.Degese M.S., Mendizabal J.E., Gandini N.A., Gutkind J.S., Molinolo A., Hewitt S.M., Curino A.C., Coso O.A., Facchinetti M.M. Expression of heme oxygenase-1 in non-small cell lung cancer (NSCLC) and its correlation with clinical data. Lung Cancer. 2012;77:168–175. doi: 10.1016/j.lungcan.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandini N.A., Fermento M.E., Salomón D.G., Blasco J., Patel V., Gutkind J.S., Molinolo A.A., Facchinetti M.M., Curino A.C. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp. Mol. Pathol. 2012;93:237–245. doi: 10.1016/j.yexmp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Yin H., Fang J., Liao L., Maeda H., Su Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. BMC Cancer. 2014;14:436. doi: 10.1186/1471-2407-14-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai J.-R., Wang H.-M., Liu P.-L., Chen Y.-H., Yang M.-C., Chou S.-H., Cheng Y.-J., Yin W.-H., Chong I.-W. High expression of heme oxygenase-1 is associated with tumor invasiveness and poor clinical outcome in non-small cell lung cancer patients. Cell. Oncol. 2012;35:461–471. doi: 10.1007/s13402-012-0105-5. [DOI] [PubMed] [Google Scholar]
- 22.Yin Y., Liu Q., Wang B., Chen G., Xu L., Zhou H. Expression and function of heme oxygenase-1 in human gastric cancer. Exp. Boil. Med. 2012;237:362–371. doi: 10.1258/ebm.2011.011193. [DOI] [PubMed] [Google Scholar]
- 23.Miyata Y., Kanda S., Mitsunari K., Asai A., Sakai H. Heme oxygenase-1 expression is associated with tumor aggressiveness and outcomes in patients with bladder cancer: A correlation with smoking intensity. Transl. Res. 2014;164:468–476. doi: 10.1016/j.trsl.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Noh S.J., Bae J.S., Jamiyandorj U., Park H.S., Kwon K., Jung S.H., Youn H.J., Lee H., Park B.-H., Chung M.J., et al. Expression of nerve growth factor and heme oxygenase-1 predict poor survival of breast carcinoma patients. BMC Cancer. 2013;13:516. doi: 10.1186/1471-2407-13-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandini N.A., Fermento M.E., Salomón D.G., Obiol D.J., Andrés N.C., Zenklusen J.C., Arévalo J., Blasco J., Romero A.L., Facchinetti M.M., et al. Heme oxygenase-1 expression in human gliomas and its correlation with poor prognosis in patients with astrocytoma. Tumor Boil. 2013;35:2803–2815. doi: 10.1007/s13277-013-1373-z. [DOI] [PubMed] [Google Scholar]
- 26.Chau L.-Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015;22:22. doi: 10.1186/s12929-015-0128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salis O., Bedir A., Ozdemir T., Okuyucu A., Alacam H. The relationship between anticancer effect of metformin and the transcriptional regulation of certain genes (CHOP, CAV-1, HO-1, SGK-1 and Par-4) on MCF-7 cell line. Eur. Rev. Med. Pharmacol. Sci. 2014;18:1602–1609. [PubMed] [Google Scholar]
- 28.Levenson A.S., Jordan V.C. MCF-7: The first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–3078. [PubMed] [Google Scholar]
- 29.Kao J., Salari K., Bocanegra M., Choi Y.-L., Girard L., Gandhi J., Kwei K.A., Hernandez-Boussard T., Wang P., Gazdar A.F., et al. Molecular Profiling of Breast Cancer Cell Lines Defines Relevant Tumor Models and Provides a Resource for Cancer Gene Discovery. PLoS ONE. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim M.-Y., Jeon J.-H., Jeong E.-Y., Lee C.-H., Lee H.-S. Antimicrobial activity of 5-hydroxy-1,4-naphthoquinone isolated from Caesalpinia sappan toward intestinal bacteria. Food Chem. 2007;100:1254–1258. doi: 10.1016/j.foodchem.2005.12.009. [DOI] [Google Scholar]
- 31.Hikino H., Taguchi T., Fujimura H., Hiramatsu Y. Antiinflammatory principles of Caesalpinia sappan wood and of Haematoxylon campechianum wood. Planta Med. 1977;31:214–220. doi: 10.1055/s-0028-1097516. [DOI] [PubMed] [Google Scholar]
- 32.Bae I.-K., Min H.-Y., Han A.-R., Seo E.-K., Lee S.K. Suppression of lipopolysaccharide-induced expression of inducible nitric oxide synthase by brazilin in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2005;513:237–242. doi: 10.1016/j.ejphar.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Moon C.-K., Park K.-S., Kim S.-G., Won H.-S., Chung J.H. Brazilin protects cultured rat hepatocytes from BrCCl3-induced toxicity. Drug Chem. Toxicol. 1992;15:81–91. doi: 10.3109/01480549209035174. [DOI] [PubMed] [Google Scholar]
- 34.Kim S.-G., Kim Y.-M., Khil L.-Y., Jeon S.-D., So D.-S., Moon C.-H., Moon C.-K. Brazilin inhibits activities of protein kinase C and insulin receptor serine kinase in rat liver. Arch. Pharmacal. Res. 1998;21:140–146. doi: 10.1007/bf02974018. [DOI] [PubMed] [Google Scholar]
- 35.Kim S.-H., Lyu H.-N., Kim Y.S., Jeon Y.H., Kim W., Kim S., Lim J.-K., Lee H.W., Baek N.-I., Choi K.-Y., et al. Brazilin Isolated from Caesalpinia sappan Suppresses Nuclear Envelope Reassembly by Inhibiting Barrier-to-Autointegration Factor Phosphorylation. J. Pharmacol. Exp. Ther. 2014;352:175–184. doi: 10.1124/jpet.114.218792. [DOI] [PubMed] [Google Scholar]
- 36.Lee D.-Y., Lee M.-K., Kim G.-S., Noh H.-J., Lee M.-H. Brazilin Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells. Molecules. 2013;18:2449–2457. doi: 10.3390/molecules18022449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellman L., Fried M. Electrophoretic mobility shift assay (EMSA) for detecting protein–nucleic acid interactions. Nat. Protoc. 2007;2:1849–1861. doi: 10.1038/nprot.2007.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foresti R., Hoque M., Bains S., Green C.J., Motterlini R. Heme and nitric oxide: Synergism in the modulation of the endothelial heme oxygenase-1 pathway. Biochem. J. 2003;372:381–390. doi: 10.1042/bj20021516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ndisang J.F., Wu L., Zhao W., Wang R. Induction of heme oxygenase-1 and stimulation of cGMP production by hemin in aortic tissues from hypertensive rats. Blood. 2003;101:3893–3900. doi: 10.1182/blood-2002-08-2608. [DOI] [PubMed] [Google Scholar]
- 40.Abate A., Yang G., Wong R.J., Schroder H., Stevenson D.K., Dennery P.A. Apigenin decreases hemin-mediated heme oxygenase-1 induction. Free Radic. Biol. Med. 2005;39:711–718. doi: 10.1016/j.freeradbiomed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Ryter S.W., Xi S., Hartsfield C.L., Choi A.M.K. Mitogen Activated Protein Kinase (MAPK) Pathway Regulates Heme Oxygenase-1 Gene Expression by Hypoxia in Vascular Cells. Antioxid. Redox Signal. 2002;4:587–592. doi: 10.1089/15230860260220085. [DOI] [PubMed] [Google Scholar]
- 42.Lin C.-Y., Hsiao W.-C., Huang C.-J., Kao C.-F., Hsu G.-S.W. Heme oxygenase-1 induction by the ROS–JNK pathway plays a role in aluminum-induced anemia. J. Inorg. Biochem. 2013;128:221–228. doi: 10.1016/j.jinorgbio.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Gong P., Stewart D., Hu B., Li N., Cook J., Nel A., Alam J. Activation of the Mouse Heme Oxygenase-1 Gene by 15-Deoxy-Δ12,14-Prostaglandin J2 Is Mediated by the Stress Response Elements and Transcription Factor Nrf2. Antioxid. Redox Signal. 2002;4:249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- 44.Lang D., Reuter S., Buzescu T., August C., Heidenreich S. Heme-induced heme oxygenase-1 (HO-1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation. Int. Immunol. 2004;17:155–165. doi: 10.1093/intimm/dxh196. [DOI] [PubMed] [Google Scholar]
- 45.Chen G.G., Liu Z., Vlantis A., Tse G., Leung B., Van Hasselt C. Heme oxygenase-1 protects against apoptosis induced by tumor necrosis factor-α and cycloheximide in papillary thyroid carcinoma cells. J. Cell. Biochem. 2004;92:1246–1256. doi: 10.1002/jcb.20157. [DOI] [PubMed] [Google Scholar]
- 46.Chiang S.-K., Chen S.-E., Chang L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018;20:39. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzales S., A Erario M., Tomaro M.L. Heme oxygenase-1 induction and dependent increase in ferritin. A protective antioxidant stratagem in hemin-treated rat brain. Dev. Neurosci. 2002;24:61–168. doi: 10.1159/000065686. [DOI] [PubMed] [Google Scholar]
- 48.Ciaffaglione V., Intagliata S., Pittalà V., Marrazzo A., Sorrenti V., Vanella L., Rescifina A., Floresta G., Sultan A., Greish K., et al. New Arylethanolimidazole Derivatives as HO-1 Inhibitors with Cytotoxicity against MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2020;21:1923. doi: 10.3390/ijms21061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorrenti V., Raffaele M., Vanella L., Acquaviva R., Salerno L., Pittalà V., Intagliata S., Di Giacomo C. Protective Effects of Caffeic Acid Phenethyl Ester (CAPE) and Novel Cape Analogue as Inducers of Heme Oxygenase-1 in Streptozotocin-Induced Type 1 Diabetic Rats. Int. J. Mol. Sci. 2019;20:2441. doi: 10.3390/ijms20102441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Podkalicka P., Mucha O., Kruczek S., Biela A., Andrysiak K., Stępniewski J., Mikulski M., Gałęzowski M., Sitarz K., Brzózka K., et al. Synthetically Lethal Interactions of Heme Oxygenase-1 and Fumarate Hydratase Genes. Biomolecules. 2020;10:143. doi: 10.3390/biom10010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muñoz-Sánchez J., Chánez-Cárdenas M.E. A Review on Hemeoxygenase-2: Focus on Cellular Protection and Oxygen Response. Oxidative Med. Cell. Longev. 2014;2014:1–16. doi: 10.1155/2014/604981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang H.Y. Master’s Thesis. Graduated School Chonbuk National University; Jeonju, Korea: Feb 22, 2017. Brazilin Has Anticancer Activity through HO-1 Pathway in MCF-7 Human Breast Cancer Cells. [Google Scholar]