Abstract
INTRODUCTION
The surgically induced fetal lamb model is the most commonly used large animal model of myelomeningocele (MMC) but is subject to variation due to surgical technique during defect creation.
MATERIAL & METHODS
Thirty-one fetal lambs underwent creation of the MMC defect, followed by defect repair with either an extracellular matrix (ECM) patch (n=10) or ECM seeded with placental mesenchymal stromal cells (n=21). Postnatal hindlimb function was assessed using the Sheep Locomotor Rating (SLR) scale. Postmortem magnetic resonance imaging of the lumbar spine was used to measure the level and degree of spinal angulation, as well as cross-sectional area of remaining vertebral bone.
RESULTS
Median level of angulation was between the 2nd and 3rd lumbar vertebrae, with a median angle of 24.3 degrees (interquartile range 16.2-35.3). There was a negative correlation between angulation degree and SLR (r = −0.44, p = 0.013). Degree of angulation also negatively correlated with the normalized cross-sectional area of remaining vertebral bone (r = −0.75, p < 0.0001).
DISCUSSION
Surgical creation of fetal MMC leads to varying severity of spinal angulation in the ovine model, which affects postnatal functional outcomes. Postnatal assessment of spinal angulation aids in standardization of the surgical model of fetal MMC repair.
Keywords: animal model, sheep, fetal surgery, myelomeningocele, spina bifida, magnetic resonance imaging, spine curvatures, stem cells, placenta
1. INTRODUCTION
Myelomeningocele (MMC), commonly known as spina bifida, is the result of failed neurulation during the fourth week after fertilization [1]. Exposure of the spinal cord results in spinal cord injury due to neurotoxicity of the amniotic fluid, as well as ongoing intrauterine trauma, commonly known as the “two-hit hypothesis” [2]. Small and large animal models confirmed that prenatal repair could prevent and possibly even reverse some of the spinal cord damage sustained, thereby improving postnatal outcomes [3, 4]. Subsequently, the multicenter Management of Myelomeningocele Study demonstrated that in utero surgical repair improves lower limb motor function for children with MMC [5]. However, only 42% of children treated prenatally were capable of independent ambulation at 30 months of age, suggesting that further improvement may be possible [4]. Translational studies using stem cells to augment prenatal repair are ongoing [6].
The fetal lamb model of MMC, first described by Meuli et al. [7], is the most commonly used large animal model of MMC for fetal repair. This is due to the long gestational period, large fetal size, and relative tolerance of the ovine uterus to instrumentation. As with any surgically created model, deviations in surgical technique can result in variability within the model, which may have unaccounted for impact on measured outcomes. Our laboratory has developed several scoring systems to increase the rigor and reproducibility of the ovine model. The Sheep Locomotor Rating (SLR) scale is a validated, ovine-specific assessment of hindlimb function. It is based in part on the Basso, Beattie, Bresnahan score, a commonly used measure of hindlimb function following spinal cord injury in rodents [8, 9]. Our laboratory also developed a grading system to evaluate the degree of innate fetal healing following MMC defect creation – a variable that may influence the severity of spinal cord injury acquired during gestation [10].
Postoperative angulation of the lumbar spine has previously been anecdotally observed; however, the frequency or impact on functional outcomes has been unreported. Given the neurological deficits that can occur with gibbus deformities (kyphosis resulting from one or more wedge-shaped vertebral bodies) of the human spine, we hypothesized that similar excessive curvature of the ovine spine would negatively impact functional outcomes [11, 12]. In this study, we describe the range of spinal curvature seen following fetal lamb MMC repair and the correlation to postnatal functional outcomes. Additionally, we confirm that our previous findings regarding functional improvement following placental stem cell application in utero are the result of stem cell treatment and not a function of the surgically created defect or any subsequent spinal angulation [13].
2. MATERIALS & METHODS
2.1. Placental Stem Cells
Placental mesenchymal stromal cells (PMSCs) were isolated using a previously described explant culture method from donated early gestation placental chorionic villi [14]. Placental cell culture was not considered as human studies research according to the University of California Davis Institutional Review Board (#301243-3). Briefly, cells were cultured in Dulbecco’s Modified Eagle Medium/high glucose with 5% fetal bovine serum (Hyclone, Thermo Fisher Scientific), 100 U/mL penicillin/100 μg/mL streptomycin (Thermo Fisher Scientific), 20 ng/mL recombinant human basic fibroblast growth factor (R&D Systems), and 20 ng/mL recombinant human epidermal growth factor (R&D Systems). PMSCs were transduced with green fluorescent protein-containing lentiviral vector (University of California Davis, Institute for Regenerative Cures, Sacramento, CA, USA) at passage 4 with a multiplicity of infection of 5. Twenty-four hours prior to the scheduled MMC repair, PMSCs at passage 6 were seeded onto a sheet of 4-ply small intestine submucosa-derived extracellular matrix (ECM) (Biodesign® Dural Graft, Cook Medical, West Lafayette, IN, USA) at densities ranging from 42 to 300 K cells/cm2. Seeded ECMs were incubated in culture medium overnight in a humidified 37 °C, 5% CO2 incubator. Control ECMs without cells were incubated in culture medium under the same conditions. On the morning prior to MMC repair, ECMs seeded with green fluorescent protein-tagged cells were viewed by fluorescent microscopy and imaged to confirm adherence of PMSCs to the ECM, as previously described [15].
2.2. Defect Creation and Repair
MMC defect creation and repair were performed as described previously [16]. Briefly, defect creation was performed at mean gestational age (GA) 77 ± 3 days and consisted of a maternal survival laparotomy and hysterotomy, followed by surgical removal of a section of fetal lumbar skin, the paraspinal muscles, lamina of the 6 lumbar vertebrae, and dura overlying the spinal cord. The hysterotomy and laparotomy incisions were closed, and the ewe allowed to recover.
Defect repair was then performed at mean GA 102 ± 4 days, during which the ewe underwent a second maternal survival laparotomy and hysterotomy. The previously created MMC defect was identified, and any fibrinous exudate overlying the fetal spinal cord was removed. The defect was then repaired with ECM, followed by primary closure of the fetal skin over the patch [13]. Ten animals underwent repair with ECM alone, while 21 were repaired with ECM seeded with PMSCs at varying densities (PMSC-ECM) [14]. Treatment groups were randomly assigned prior to defect repair. The maternal hysterotomy and laparotomy incisions were closed, and the ewe recovered. Fetuses were delivered at term, either by spontaneous vaginal delivery or terminal cesarean section.
Three lambs did not undergo hysterotomy, defect creation, or repair to serve as reference for normal ambulation, spinal angulation, vertebral body composition, and spinal cord cross-sectional measurements.
The University of California Davis Institutional Animal Care and Use Committee (IACUC) approved all animal protocols, and all animal care was in compliance with the Guide for the Care and Use of Laboratory Animals. All facilities used during the study period were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
2.3. Assessment of Hindlimb Motor Function
After delivery, lamb hindlimb motor function was evaluated using the validated SLR scale [8]. Lambs received a score of 0-15 based on hindlimb joint movement, weight support, ambulation, coordination, and ability to clear an obstacle. A score of 0 represented complete hindlimb paralysis, while a score of 15 represented coordinated spontaneous ambulation and clearance of an obstacle. Motor function was video recorded and analyzed at 2 and 24-48 h after delivery by trained examiners. The best performance by each lamb was used in all subsequent analyses.
2.4. Lumbar Spine Imaging
Lambs were euthanized after the second motor function evaluation and immediately perfused with 1 L of 1x phosphate-buffered saline and 2 L of 10% formalin. A block of tissue encompassing the entire region of the repaired surgical defect, the spinal cord, and the lumbar vertebral bodies was dissected. Magnetic resonance imaging (MRI) of this tissue block was then performed.
MRI scans were performed at the Center for Molecular and Genomic Imaging, using a Bruker Biospec 70/30 (7T) preclinical MR scanner (Bruker BioSpin MRI, Ettlingen, Germany) equipped with a 116-mm internal diameter B-GA12S gradient (450 mT/m, 4,500 T/m/s). Images were acquired with a 60 mm ID quadrature coil. A multislice Rapid Acquisition with Repeated Echoes (RARE) sequence was used for coronal image acquisition and was acquired in 2 imaging fields of view (FOV) over 6 min per FOV. The following parameters were used for the RARE acquisition: repetition time = 4750 ms; effective echo time = 24 ms; RARE factor = 4; averages = 2; FOV = 75 × 47.4 × 25.5 mm3 with matrix dimensions = 250 × 158 × 85, resulting in an isotropic resolution of 0.3 mm. The samples were placed in bags to prevent dehydration and were taped to a custom bed to prevent motion during scanning. MR images were processed in Thermo Scientific Amira 3D software (Thermo Fisher Scientific Inc, Berlin, Germany) and the 2 MR scans were manually aligned and merged together.
Using Photoshop® (Adobe Systems Inc, Seattle, WA, USA), lines were drawn through the mid-portion of the lumbar vertebral bodies on mid-sagittal imaging, crossing at the point of maximal angulation. The supplementary angle of the 2 intersecting lines was measured using the Photoshop® Ruler tool and represented the degree of spinal angulation (Fig. 1). The cross-sectional area of the remaining vertebral laminal bone was measured on the transverse view at the point of maximal angulation using ImageJ software (Fig. 2). The cross-sectional area of vertebral lamina was normalized to the average cross-sectional area of vertebral lamina at the corresponding lumbar level of normal lambs (n=3).
Figure 1.

Measurement of Lumbar Spine Angulation. Lines drawn through the mid-portion of the lumbar vertebral bodies on mid-sagittal section of a postmortem MRI. The lines cross at the lumbar level at which maximal angulation occurs, which is between the 2nd and 3rd lumbar vertebrae in this example. The supplementary angle of the intersecting lines represents the degree of spinal angulation, measured here as 32.7 degrees.
Figure 2.

Measurement of Remaining Vertebral Bone Cross-sectional Area. On postmortem MRI, the lumbar vertebral lamina was identified on transverse section at the point of maximal angulation. The cross-sectional area of the remaining lamina was measured using ImageJ software. The cross-sectional area was then normalized to the average cross-sectional lamina area at the corresponding lumbar level of normal lambs.
2.5. Histologic Analysis
The spinal cord of each lamb was then dissected by lumbar segment to allow for histologic assessment of spinal cord compression at the level of maximal angulation. The lumbar segments were dehydrated in 30% sucrose, embedded in Optimal Cutting Temperature compound (Fisher Healthcare Tissue-Plus, Waltham, MA, USA), and frozen. A series of six 20-um cross sections were obtained at the lumbar level associated with maximal angulation on MRI for each animal and stained with Cresyl Violet. The sections were analyzed with ImageJ software to determine the height and width of the spinal cord, which were then averaged for each lamb. The height-to-width ratio, a measure of compression, was normalized by lumbar level to the average value in normal lambs (n=3). A normalized height-to-width ratio value closer to 1 indicated less spinal cord compression.
2.6. Statistical Analysis
Summary statistics are reported as median (25-75% interquartile range [IQR]) as all variables were nonnormally distributed. SLR scores, spinal level, degree of angulation, and normalized percentage of remaining lamina in each treatment group were compared using the Mann-Whitney U test. Treatment groups were defined as repair with ECM alone or ECM seeded with PMSCs at any density. The SLR scores for some of these lambs have been previously published (30/31); however, the effect of spinal angulation on functional outcomes has never been studied [17, 18]. Spearman’s rank-order correlation was used to analyze relationships between variables including SLR scores, level or degree of angulation, percentage of vertebral bone remaining, and normalized spinal cord height-to-width ratio. P values <0.05 were considered statistically significant. All analyses were completed using Prism 7 (GraphPad Software, La Jolla, CA, USA).
3. RESULTS
Thirty-one lambs underwent fetal MMC defect creation and repair. The median SLR score for all animals was 14 (IQR 7.5-15). The median level of angulation was between the 2nd and 3rd lumbar vertebrae (IQR 2-3.5) with a median of 24.3 degrees of angulation (IQR 16.3-34.8; Table 1). The median degree of angulation in normal lambs was 4 (IQR 3.6-7.6). There was no correlation between the spinal level of angulation and SLR score (r = 0.34, p = 0.062; Fig. 3). However, there was a significant negative correlation between the degree of angulation and SLR score for all animals (r = −0.44, p = 0.013; Fig. 4).
Table 1.
Postnatal Functional Outcomes and Spinal Angulation by Treatment
| All Animals (n=31) | ECM only (n=10) | PMSC-ECM (n=21) | p value | |
|---|---|---|---|---|
| SLR Score | 14 (7.5-15) | 7.5 (4.3-12) | 15 (13-15) | 0.026 |
| Lumbar Level of Angulation | 2.5 (2-3.5) | 2 (2-2.5) | 2.5 (2.5-3.5) | 0.025 |
| Degree of Angulation | 24.3 (16.3-34.8) | 29.9 (15.7-49.9) | 24.3 (16.7-33.0) | 0.516 |
| Lamina Cross-sectional Area (% of Normal) | 22.5 (15.2-29.5) | 20.5 (14.6-25.3) | 25.0 (15.9-33.0) | 0.363 |
All values presented as median (IQR). Treatment groups (ECM only and PMSC-ECM) were compared with the Mann-Whitney U test.
ECM, extracellular matrix; PMSC-ECM, ECM seeded with placental mesenchymal stromal cells; SLR, Sheep Locomotor Rating; IQR, interquartile range.
Figure 3.

Correlation Between Spinal Angulation Level and Sheep Locomotor Rating (SLR) Score. The lumbar level at the point of maximal angulation does not correlate with hindlimb motor function (SLR score of 0 represents complete hindlimb paralysis and a score of 15 represents coordinated ambulation and ability to clear an obstacle; r = 0.34, p = 0.062). Similar results were found on subgroup analysis by treatment group.
Figure 4.
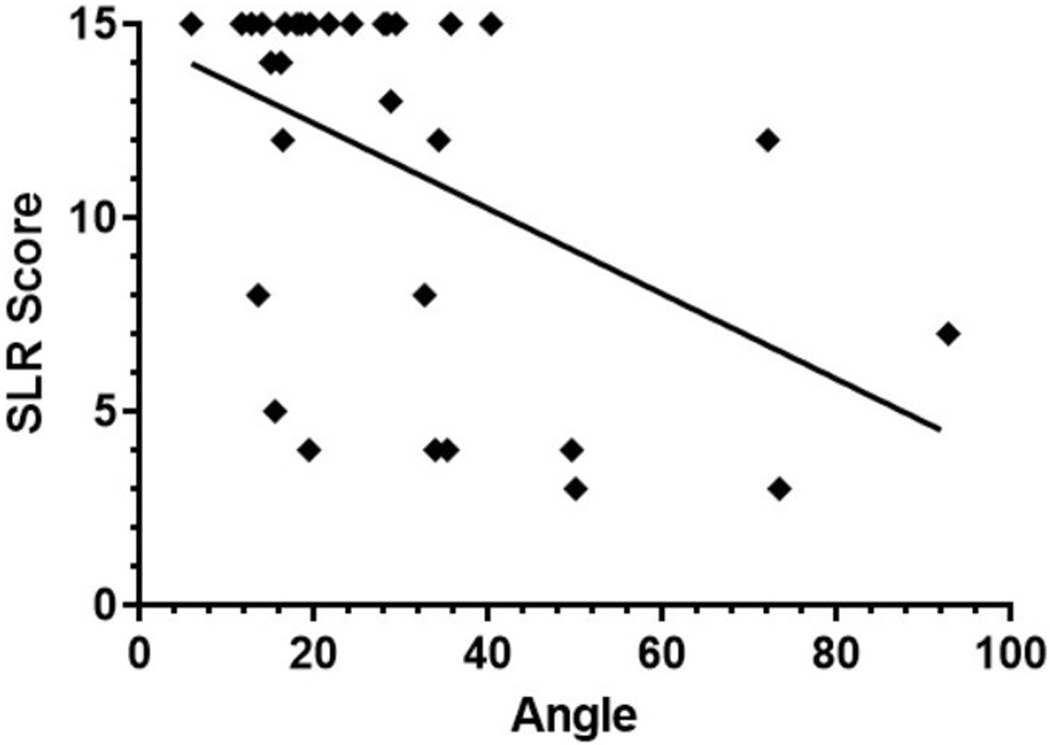
Correlation Between Spinal Angle and Sheep Locomotor Rating (SLR) Score. The degree of the lumbar spine angulation negatively correlates with postnatal hindlimb motor function. Animals with greater spinal angulation had worse hindlimb functional ability (r = −0.44, p = 0.013).
Next, we compared SLR scores, degree of spinal angulation, and lumbar level of angulation by treatment group. There was no difference between lambs treated with PMSC-ECM and those treated with ECM only in the degree of angulation (24.3 vs 29.9 degrees, p = 0.516). However, there were significant differences between treatment groups in SLR scores and lumbar level of angulation. Animals treated with PMSC-ECM had higher SLR scores than those repaired with ECM only (15 vs 7.5, p = 0.026). For animals treated with PMSC-ECM, spinal angulation occurred at lower lumbar levels than animals treated with ECM only (lumbar level 2.5 vs 2.0, p = 0.025; Table 1). Despite a difference in lumbar level between treatment groups, there was still no correlation between lumbar level and SLR score for either treatment group. While there was a negative correlation between degree of spinal angulation and SLR score for the entire cohort, this varied by treatment. The correlation between degree of angulation and SLR score persisted in the PMSC-ECM treatment group (r = −0.55, p = 0.010); however, no correlation was seen for animals treated with ECM alone (r = −0.32, p = 0.358; Fig. 5).
Figure 5.

Effect of Treatment on the Correlation Between Spinal Angle and Hindlimb Motor Function. A significant negative correlation between spinal angle and hindlimb motor function was found for animals treated with placental mesenchymal stromal cells seeded on extracellular matrix (PMSC-ECM) (r = −0.55, p = 0.010). No correlation was seen for animals treated with ECM alone (r = −0.32, p = 0.358). SLR, Sheep Locomotor Rating.
The median percentage of vertebral laminal bone remaining after the standard 6-level laminectomy was 22.5% of normal (IQR 15.2-29.5%). There was no significant difference between the remaining lamina in lambs repaired with ECM only (20.5% of normal [IQR 14.6-25.3%]) compared to those repaired with PMSC-ECM (25.0% of normal [IQR 15.9-33.0%], p = 0.363; Table 1). There was a significant negative association between the lamina remaining and the degree of spinal angulation (r = −0.75, p < 0.001; Fig. 6). This correlation persisted in both the ECM and PMSC-ECM treatment groups (p = 0.006 and <0.0001, respectively).
Figure 6.

Correlation Between Normalized Remaining Vertebral Bone and Spinal Angle. The normalized cross-sectional area of remaining lumbar lamina at the point of maximal angulation negatively correlates with the degree of spinal angulation. Lambs with less remaining vertebral lamina had a greater degree of spinal angulation (r = −0.75, p < 0.0001). Similar results were seen on subgroup analysis by treatment.
Histologic cross sections of the spinal cord from lambs with greater degrees of spinal angulation demonstrated visible compression of the spinal cord compared to those with lesser degrees of angulation (Fig. 7). There was a negative correlation between the degree of spinal angulation and the normalized height-to-width ratio at the point of maximal spinal angulation (r = −0.37, p = 0.043; Fig. 8). The normalized height-to-width ratio of the spinal cord positively correlated with SLR score (r = 0.54, p = 0.002; Fig. 9).
Figure 7.

Histologic Sections of the Spinal Cord Demonstrate Compression at Level of Maximal Angulation. Representative cross sections stained with Cresyl Violet from (A) a lamb with > 60 degree spinal angulation, (B) a lamb with < 60 degree spinal angulation, and (C) a normal lamb. Scale bar = 5 mm. ECM, extracellular matrix.
Figure 8.

Correlation Between Spinal Angle and Normalized Height/Width Ratio of Spinal Cord The normalized height/width ratio negatively correlates with the degree of spinal angulation (r = −0.37, p = 0.043). Lambs with less angulation of the spine also had less spinal cord compression, indicated by a ratio closer to 1.
Figure 9.

Correlation Between Sheep Locomotor Rating (SLR) Score and Normalized Height/Width Ratio of Spinal Cord. The normalized height/width ratio positively correlates with hindlimb motor function (r = 0.54, p = 0.002). Lambs with less compression of the spinal cord, indicated by a ratio closer to 1, demonstrated better hindlimb motor function.
4. DISCUSSION
While normal lambs had minimal to no spinal angulation, some degree of spinal angulation was identified in all lambs that underwent MMC defect creation in utero confirming this is a result of surgical model creation. Most spinal angulation occurred in the upper half of the lumbar spine, with a median level between the second and third lumbar vertebrae. Treatment groups (ECM alone and PMSC-ECM) had significantly different median levels of angulation; however, there was no correlation between spinal level and functional outcomes for the full cohort or in subgroup analysis by treatment group. In contrast, a significant negative correlation was found between the degree of spinal angulation and SLR score for all animals, suggesting that the degree of angulation has more impact than the lumbar level of angulation on postnatal functional outcome.
A negative correlation was also present between the degree of spinal angulation and the proportion of vertebral laminal bone remaining. Lambs with a greater degree of spinal angulation had less vertebral lamina remaining, confirming that the extent of bony resection during defect creation is part of the underlying etiology of the observed spinal angulation. While there was no difference in remaining vertebral bone cross-sectional area between treatment groups, the correlation between degree of angulation and SLR score persisted only for the group that received PMSC-ECM during in utero repair.
A normalized height-to-width ratio was used to evaluate compression of the spinal cord at the point of maximal spinal angulation. A negative correlation was identified between the degree of angulation and the normalized height-to-width ratio of the spinal cord, demonstrating that lambs with greater spinal angulation also had more spinal cord compression. Moreover, the normalized height-to-width ratio positively correlated with motor function score, demonstrating that lambs with more spinal cord compression had worse functional outcomes. These data suggest that spinal cord deformity is part of the mechanism of impaired motor function in lambs with significant spinal angulation.
The technique of MMC defect creation and repair described in this study differs from that originally described by Meuli et al. [7] in the extent of the lumbar laminectomy and removal of the paraspinal muscles but has widely been adopted to prevent tissue regrowth over the defect and more accurately mimic human fetal repair [10, 18–22]. While scoring systems have been developed to characterize the surgically created defect, thereby increasing the rigor and reproducibility of the model, the effect of an in utero multisegment laminectomy on postnatal spinal morphology has not been previously described [10].
Abnormal spinal curvature due to congenital and traumatic etiologies have been described in a variety of quadrupeds, including sheep, horses, and dogs [23–27]. As expected, the resultant clinical characteristics can range from asymptomatic to paralysis depending on the exact location, direction, and degree of deformation. Specifically, kyphotic deformities in animals involving the lumbar spine affect the hindlimbs with clinical findings ranging from back pain to complete paraparesis [24, 28]. Given these clinical examples, it seems logical that spinal angulation in our model could result in motor deficits or deterioration.
For human patients with kyphotic spinal deformities, management decisions are determined by the spinal level involved, degree of angulation, symptom severity, and underlying etiology [12]. Scheuermann disease is the most common cause of kyphotic deformity in children, but is not usually evident until the growth spurt during adolescence. While less common than Scheuermann disease, congenital kyphosis is the result of vertebral body malformations and children are more likely to experience neurologic sequelae due to spinal cord compression. Children with congenital kyphosis involving the low thoracic or lumbar spine often require surgical intervention when angulation reaches 50-60 degrees. Using 60 degrees as a threshold of severe angulation, < 10% of the lambs included in this study (3/31) would qualify as severe.
Wang et al. [13] and Kabagambe et al. [17] have shown that augmentation of prenatal MMC repair with early gestation PMSCs consistently improves functional outcomes in the fetal lamb model. While the exact mechanism of action of these cells has not been fully elucidated, it is widely believed that paracrine activity of secreted neurotrophic factors plays a key role [29–31]. We have demonstrated that the functional improvement observed after repair with PMSC-ECM, as evidenced by higher SLR scores, is not simply the result of the surgical model. While there was a clear correlation between spinal angulation and SLR score, this correlation was not seen for lambs treated with ECM alone. This suggests that PMSCs provide additional protection for the developing spinal cord, beyond the simple mechanical protection of the ECM patch. However, the correlation between spinal angulation and SLR score for lambs treated with PMSC-ECM indicates that, while PMSCs can rescue hindlimb motor function for animals with mild or moderate angulation, they are unable to overcome the additional damage caused by severe angulation and resulting compression of the overlying spinal cord.
Since spinal angulation is an uncommon finding for children with MMC, the angulation observed in these experimental lambs is likely a consequence of the surgical model related to extensive removal of the posterior lumbar vertebral elements and paraspinal musculature. Spinal cord injury resulting from spinal angulation and subsequent compression of the overlying spinal cord could therefore be a confounding factor in studies investigating prenatal MMC repair techniques, particularly those that report functional outcomes. Assessment of postnatal spinal angulation should be performed when evaluating functional outcomes of these animals. Furthermore, lambs with severe deformity, defined as greater than 60 degrees of angulation, can be excluded without compromising results.
There are several limitations of this study. Fetal lambs received different treatments during in utero repair, including varying densities of PMSCs, which may further confound evaluation of postnatal motor function. The underlying mechanism of the spinal angulation is not completely understood but is in part due to bony instability from the extensive laminectomy and lack of the stabilizing paraspinal musculature also removed during defect creation. It is unclear whether the observed angulation will worsen as the lamb ages, but this will be an important consideration for planned longevity studies. Finally, a threshold for surgical intervention in humans was used to guide the designation of severe angulation for the lambs included in this study. While a certain degree of angulation may be clinically significant for a biped human, this may not directly correlate to the quadruped lamb.
In summary, creation of the MMC defect in the fetal lamb model results in varying severity of postnatal spinal angulation and spinal cord compression, which affects functional outcome measures. This appears to be a result of bone removal during creation of the defect. Assessment of postnatal spinal angulation aids in standardization of the surgical fetal lamb model of MMC.
5.1. Acknowledgements:
We thank the staff of the University of California Davis Surgical Bioengineering Laboratory for support with this project; W. Ferrier and L. Talken for assistance in the perioperative care and management of our research animals; and Cook Biotech for their generous donation of the ECM material.
5.4 Funding Sources: This work and authors MV, CP, LG, PK, LL, ZP, BD, AW, and DF were supported by funding from the California Institute of Regenerative Medicine (Grant #PC1-08103), Shriners Hospital for Children (Grant #85120-NCA-16), National Institutes of Health (Grant #5R01NS100761-02), March of Dimes Foundation (Grant #5FY1682), as well as a pilot grant from the University of California-Davis Center for Molecular and Genomic Imaging (https://bme.ucdavis.edu/cmgi/). Author LG was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Statement of Ethics: Animal experiments conform to internationally accepted standards and have been approved by the appropriate institutional review body.
Disclosure Statement: The authors have no conflicts of interest to declare.
References:
- 1.Adzick NS. Fetal myelomeningocele: natural history, pathophysiology, and in-utero intervention. Semin Fetal Neonatal Med. 2010. February;15(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heffez DS, Aryanpur J, Hutchins GM, Freeman JM. The paralysis associated with myelomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery. 1990. June;26(6):987–92. [PubMed] [Google Scholar]
- 3.Heffez DS, Aryanpur J, Rotellini NA, Hutchins GM, Freeman JM. Intrauterine repair of experimental surgically created dysraphism. Neurosurgery. 1993. June;32(6):1005–10. [DOI] [PubMed] [Google Scholar]
- 4.Meuli M, Meuli-Simmen C, Yingling CD, Hutchins GM, Timmel GB, Harrison MR, et al. In utero repair of experimental myelomeningocele saves neurological function at birth. J Pediatr Surg. 1996. March;31(3):397–402. [DOI] [PubMed] [Google Scholar]
- 5.Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, Johnson MP, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011. March;364(11):993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanover M, Pivetti C, Galganksi L, Saadai P, Wang A, Farmer D. Spinal angulation: a limitation of the fetal lamb model of myelomeningocele. Presented at International Fetal Medicine and Surgery Society 36th Annual Meeting; 2017; Jackson Hole, WY. [Google Scholar]
- 7.Meuli M, Meuli-Simmen C, Yingling CD, Hutchins GM, Hoffman KM, Harrison MR, et al. Creation of myelomeningocele in utero: a model of functional damage from spinal cord exposure in fetal sheep. J Pediatr Surg. 1995. July;30(7):1028–32. [DOI] [PubMed] [Google Scholar]
- 8.Brown EG, Keller BA, Pivetti CD, Sitkin NA, Wang A, Farmer DL, et al. Development of a locomotor rating scale for testing motor function in sheep. J Pediatr Surg. 2015. April;50(4):617–21. [DOI] [PubMed] [Google Scholar]
- 9.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. February;12(1):1–21. [DOI] [PubMed] [Google Scholar]
- 10.Brown EG, Keller BA, Pivetti CD, Farmer DL. Innate healing in the fetal sheep model of myelomeningocele: A standardized defect grading system. J Pediatr Surg. 2015. July;50(7):1134–6. [DOI] [PubMed] [Google Scholar]
- 11.Leach JP, Davenport RJ. Neurological Disease In: Walker BR, Colledge NR, Ralston SH, Penman ID, editors. Davidson’s principles and practice of medicine. Edinburgh: Churchill Livingstone/Elsevier; 2014. pp. 1218–22. [Google Scholar]
- 12.Warner WC, Sawyer JR. Scoliosis and kyphosis In: Azar FM, Beaty JH, Canale ST, editors. Campbell’s operative orthopaedics. Volume 2 St Louis (Mo): Elsevier/Mosby; 2017. pp. 2036–58. [Google Scholar]
- 13.Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, et al. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med. 2015. June;4(6):659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lankford L, Chen YJ, Saenz Z, Kumar P, Long C, Farmer D, et al. Manufacture and preparation of human placenta-derived mesenchymal stromal cells for local tissue delivery. Cytotherapy. 2017. June;19(6):680–8. [DOI] [PubMed] [Google Scholar]
- 15.Lankford L, Selby T, Becker J, Ryzhuk V, Long C, Farmer D, et al. Early gestation chorionic villi-derived stromal cells for fetal tissue engineering. World J Stem Cells. 2015. January;7(1):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Koch CS, Compagnone N, Hirose S, Yoder S, Harrison MR, Farmer DL. Myelomeningocele: characterization of a surgically induced sheep model and its central nervous system similarities and differences to the human disease. Am J Obstet Gynecol. 2005. October;193(4):1456–62. [DOI] [PubMed] [Google Scholar]
- 17.Kabagambe S, Keller B, Becker J, Goodman L, Pivetti C, Lankford L, et al. Placental mesenchymal stromal cells seeded on clinical grade extracellular matrix improve ambulation in ovine myelomeningocele. J Pediatr Surg. 2017. October;53(1):178–82. [DOI] [PubMed] [Google Scholar]
- 18.Vanover M, Pivetti C, Lankford L, Kumar P, Galganksi L, Kabagambe S, et al. High density placental mesenchymal stromal cells provide neuronal preservation and improve motor function following in utero treatment of ovine myelomeningocele. J Pediatr Surg. 2019. January;54(1):75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard S, Davey MG, Rintoul NE, Walsh DS, Rorke LB, Adzick NS. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J Pediatr Surg. 2003. March;38(3):451–8. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Li H, Kim AG, Weilerstein A, Radu A, Davey M, et al. Complete tissue coverage achieved by scaffold-based tissue engineering in the fetal sheep model of myelomeningocele. Biomaterials. 2016. January;76:133–43. [DOI] [PubMed] [Google Scholar]
- 21.Papanna R, Mann LK, Snowise S, Morales Y, Prabhu SP, Tseng SC, et al. Neurological outcomes after human umbilical cord patch for in utero spina bifida repair in a sheep model. AJP Rep. 2016. July;6(3):e309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshizawa J, Sbragia L, Paek BW, Sydorak RM, Yamazaki Y, Harrison MR, et al. Fetal surgery for repair of myelomeningocele allows normal development of the rectum in sheep. Pediatr Surg Int. 2003. May;19(3):162–6. [DOI] [PubMed] [Google Scholar]
- 23.Driver A, Henson FM, Kidd JA, Lamas LP, Pilsworth R. Back pathology In: Henson FM, editor. Equinen neck and back pathology: diagnosis and treatment. Hoboken (NJ): John Wiley & Sons Ltd; 2018. pp. 195–238. [Google Scholar]
- 24.Lorenz MD, Coates JR, Kent M. Pelvic limb paresis, paralysis, or ataxia In: Lorenz MD, Coates JR, Kent M, editors. Handbook of veterinary neurology. St Louis (Mo): W.B. Saunders; 2011. pp. 109–61. [Google Scholar]
- 25.Appendix - Congenital, inherited, or breed-associated neurologic and muscular diseases in domestic animals In: Lorenz MD, Coates JR, Kent M, editors. Handbook of veterinary neurology. St Louis (Mo): W.B. Saunders; 2011. pp. 488–524. [Google Scholar]
- 26.Szabo KT, editor. Vertebral column and thorax Congenital malformations in laboratory and farm animals. Academic Press; 1989. pp. 151–60. [Google Scholar]
- 27.Thompson KG, Piripi SA, Dittmer KE. Inherited abnormalities of skeletal development in sheep. Vet J. 2008. September;177(3):324–33. [DOI] [PubMed] [Google Scholar]
- 28.de Heer N, Nout YS. Congenital kyphosis secondary to lumbar vertebral hypoplasia causing paraparesis in a Friesian foal. Equine Vet Educ. 2011;23(5):231–4. [Google Scholar]
- 29.Kumar P, Becker J, Lankford L, Keller B, Farmer DL, Wang A. Role of human placenta-derived mesenchymal stem cells in neuroprotection and neurogenesis. Presented at International Society for Stem Cell Research 14th Annual Meeting; 2016; San Francisco, CA. [Google Scholar]
- 30.Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008. December;17(6):1095–107. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz EM, Prather WR. Cytokines as the major mechanism of mesenchymal stem cell clinical activity: expanding the spectrum of cell therapy. Isr Med Assoc J. 2009. April;11(4):209–11. [PubMed] [Google Scholar]


