Sir,
Coronavirus disease 2019 (COVID-19) pandemic is associated with a high mortality, especially in those with severe pneumonia. Patients with COVID requiring intensive care unit (ICU) admission have higher cytokine levels compared to those who do not need ICU care.[1] Even among patients admitted to ICU, those discharged from hospital had lower cytokine levels compared to those who died.[2] An immunomodulator may thus be of potential benefit in managing these critically ill COVID patients. The Global Research Collaboration for Infectious Disease Preparedness (GLOPID-R) and the World Health Organization have identified adjuvant therapy as one of the key areas of research to save lives of patients infected with COVID-19.[3] A heat-killed Mycobacterium w (Mw), originally developed as an immunomodulator for leprosy, which acts through the toll-like receptors (TLRs) pathway and enhances the host-T cell responses.[4] We have previously shown the benefit of Mw in patients with severe sepsis.[5] Herein, we describe the safety of Mw in four cases of severe COVID treated with this immunomodulator [Table 1].
Table 1.
Details of patients who received Mycobacterium w vaccine along with standard medical care
| Days in hospital | Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 7 | 1 | 2 | 3 | 7 | |
| Hemoglobin (g/dL) | 13.6 | 13.2 | 12.4 | 12 | 11.6 | 11.6 | 11.8 | 12.7 | 12.8 | 13 | 13.3 | 14.5 | 14.8 | 12.4 | 11.8 | 11.3 | 10.9 | 13.3 | 12.9 | 13.3 | 13.4 | 13 |
| TLC (cells/µL) | 15,200 | 14,800 | 13,800 | 14,900 | 12,900 | 12,600 | 8500 | 14,000 | 11,500 | 11,700 | 12,400 | 10,700 | 8600 | 7000 | 7500 | 8900 | 10,000 | 6400 | 4300 | 6400 | 5600 | 4300 |
| Neutrophils (%) | 96.6 | 94.1 | 95.5 | 94.9 | 91.4 | 91.9 | 76 | 90 | 87.4 | 80.2 | 78 | 79 | 74 | 80.6 | 82.7 | 82 | 82 | 84 | 80 | 84.9 | 81 | 66 |
| Lymphocytes (%) | 2.6 | 3.7 | 3.5 | 3.1 | 4.3 | 4.3 | 13 | 4 | 6.2 | 7.1 | 7.6 | 9.7 | 12 | 14.6 | 11.7 | 14 | 10.8 | 10 | 13.7 | 10.1 | 13 | 24 |
| Eosinophils (%) | 0.4 | 0.3 | 0.2 | 0 | 2.2 | 1.6 | 3.2 | 0.1 | 1.2 | 4.7 | 6.4 | 5.3 | 5 | 0.3 | 0.9 | 1.4 | 2.7 | 4 | 0.7 | 0.7 | 1.6 | 2 |
| Platelet count, cells/µL (×109) | 510 | 507 | 513 | 528 | 507 | 513 | 532 | 243 | 289 | 381 | 411 | 454 | 537 | 112 | 299 | 247 | 348 | 495 | 189 | 204 | 221 | 289 |
| CRP (mg/L) | 251 | 254 | 277 | 176 | 127 | 103 | 29 | 244 | 108 | 77 | 17.4 | 10.9 | 100 | 176 | 155 | 107 | 18 | 32 | 52 | 55 | 33 | |
| Procalcitonin (ng/mL) | 0.02 | 0.03 | - | - | - | - | 0.01 | 0.09 | 0.07 | 0.2 | - | - | 0.04 | 0.08 | 0.08 | - | - | - | - | 0.03 | - | 0.03 |
| FiO2 | 0.6 (RBM) | 0.5 | 0.3 | 0.28 | 0.24 | 0.24 | 0.21 | 0.36 | 0.36 | 0.28 | 0.24 | 0.21 | 0.21 | 0.5 | 0.4 | 0.28 | 0.21 | 0.28 | 0.24 | 0.24 | 0.21 | |
| SpO2 | 89 | 94 | 94 | 94 | 95 | 98 | 98 | 93 | 94 | 96 | 94 | 94 | 93 | 85 | 94 | 94 | 94 | 95 | 94 | 94 | 97 | 98 |
| PaO2:FiO2 ratio | 170 | 200 | 250 | 250 | 280 | 300 | 300 | 220 | 280 | 290 | 300 | 330 | 273 | 200 | 220 | 221 | 250 | 300 | 221 | 250 | 263 | 300 |
| RR | 40 | 35 | 35 | 35 | 30 | 20 | 18 | 35 | 30 | 22 | 20 | 19 | 25 | 35 | 25 | 20 | 20 | 20 | 32 | 25 | 25 | 20 |
| D-dimer | 3619 | 1799 | 1795 | 2033 | 1586 | - | 1081 | 428 | 422 | 333 | 309 | 690 | 710 | 289 | 249 | 317 | 339 | 126 | 127 | 172 | 550 | 590 |
| CKMB | 35 | 65 | 47 | 71 | 44 | 50 | ||||||||||||||||
| AST (U/L) | 207 | 225 | 92 | 110 | 111 | 71 | 76 | 32 | 28 | 27 | 24 | 29 | 36 | 34 | 34 | 43 | 51 | 57 | 43 | 49 | 49 | |
| ALT (U/L) | 236 | 259 | 156 | 156 | 156 | 131 | 125 | 32 | 39 | 34 | 32 | 39 | 26 | 30 | 30 | 36 | 69 | 88 | 54 | 55 | 56 | |
| SAP (U/L) | 97 | 102 | 86 | 100 | 123 | 122 | 115 | 114 | 107 | 96 | 86 | 83 | 82 | 89 | 105 | 125 | 147 | 130 | 43 | 39 | 40 | |
| S. cr (mg/dL) | 1.1 | 1.2 | 0.7 | 0.7 | 0.7 | 0.7 | 0.8 | 0.7 | 0.8 | 0.8 | 0.8 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | 1.2 | 0.8 | 0.9 | 0.9 | ||
| Albumin (g/dL) | 3.2 | 3.1 | 2.6 | 2.6 | 2.6 | 2.7 | 2.7 | 3.2 | 3.1 | 3 | 2.9 | 3.22 | 3.4 | 3.4 | 3.3 | 3 | 3.3 | 4 | 3.8 | 3.6 | 3.4 | |
| Adverse event due to Mw | Mild erythema at injection site | None | Mild erythema at injection site | None | ||||||||||||||||||
ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, CKMB: Creatine kinase–myocardial band, RBM: rebreathing mask, RR: Respiratory rate, SAP: Serum alkaline phosphatase, S. cr: Serum creatinine, SPO2: Peripheral capillary oxygen saturation by pulse oximeter, FiO2: Fraction of oxygen in inspired air, PaO2: Partial pressure of oxygen in arterial blood, TLC: Total leukocyte count, Mw: Mycobacterium w, CRP: C-reactive protein
All the four patients presented with a history of fever, myalgia, and dyspnea and had a history of contact with a patient of COVID-19. At presentation, patients had tachypnea [Table 1] and were hypoxemic at room air. Complete blood count showed neutrophil predominant leukocytosis and lymphocytopenia [Table 1]. The serum D-dimer levels were elevated in all patients at presentation and were >500 ng/mL in one patient. C-reactive protein (CRP) levels were also elevated in all patients, suggesting a hyperinflammatory state. Creatine kinase–myocardial band was elevated in all patients; however, transthoracic echocardiography did not reveal any abnormality. As per our institutional protocol, all patients received standard medical care comprising oral paracetamol (for fever), oral proton-pump inhibitor for stress ulcer prophylaxis (pantoprazole 40 mg/day), and low-molecular-weight heparin for deep venous thrombosis prophylaxis (enoxaparin 1 mg/kg, once daily). Therapeutic anticoagulation (enoxaparin 1 mg/kg, twice daily) was given in patients who had D-dimer levels >500 ng/mL. We used antibiotics (azithromycin or ceftriaxone) if patients had a total leukocyte count of >11,000 cell/μL, procalcitonin >0.5 ng/mL, or if they had hypotension (mean arterial blood pressure <65 mmHg). We did not use hydroxychloroquine in any of these patients. We also used intradermal Mw (0.3 mL/day [0.1 mL contains 0.5 × 10(9) heat-killed Mw] for 3 consecutive days, Immuvac, Cadila Pharmaceuticals, Ahmedabad, India) in addition to standard medical care.
The treatment protocol resulted in clinical and radiological improvement in all the cases [Figure 1]. The CRP levels improved gradually [Figure 2], and all the patients could be successfully managed without the need for mechanical ventilation. Importantly, Mw did not cause any adverse events, similar to our previous experience in patients with severe sepsis.[5]
Figure 1.
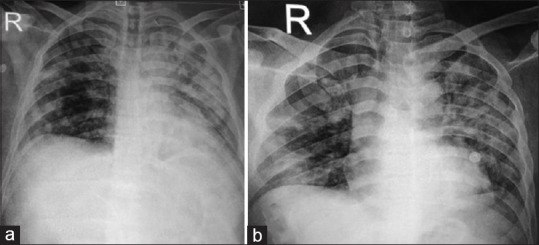
Chest radiograph anteroposterior view of one of our patients demonstrating consolidation and infiltrates in the left upper zone at baseline (a) that improved at day 7 of admission to hospital (b)
Figure 2.
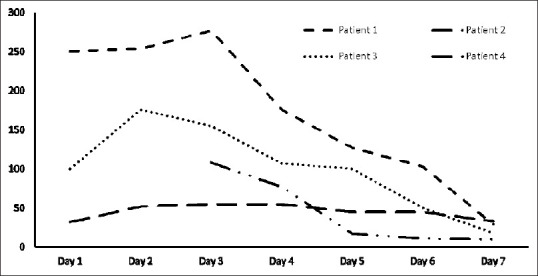
Trend of C-reactive protein during hospital stay
Based on our preliminary experience, we believe that adjunctive Mw is safe in patients with severe COVID-19 infection. However, the efficacy needs to be evaluated in a future randomized controlled trial (clinicaltrials.gov: NCT04347174).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. doi:101007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID 19 Public Health Emergency of International Concern Global Research and Innovation Forum: Towards a Research Roadmap. 2020. [Last accessed on 2020 Mar 23]. Available from: https://wwwwhoint/blueprint/priority-diseases/key-action/Global_Research_Forum_FINAL_VERSION_for_web_14_feb_2020pdfua=1 .
- 4.Desai NM, Khamar BM. Immunotherapy for tuberculous pericarditis. N Engl J Med. 2014;371:2533–4. doi: 10.1056/NEJMc1413185. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal IS, Agarwal R, Aggarwal AN, Jindal SK. A randomized trial of Mycobacterium w in severe sepsis. J Crit Care. 2015;30:85–9. doi: 10.1016/j.jcrc.2014.08.012. [DOI] [PubMed] [Google Scholar]


