Abstract
Background:
Although muscle dysfunction is a major contributor to morbidity in chronic obstructive pulmonary disease (COPD), assessment of skeletal muscle, and diaphragm function is not routinely performed in COPD patients.
Objectives:
(1) The aim is to assess muscle dysfunction in COPD by measuring the zone of apposition of diaphragm, diaphragm excursion, thickness of diaphragm, and rectus femoris cross-sectional area (RFCSA) with ultrasonography. (2) To correlate the above assessments with spirometric parameters; notably forced expiratory volume in 1 s (FEV1).
Methods:
Twenty-four consecutive stable COPD patients and 18 controls were included after obtaining written informed consent. Demographic and clinical data, spirometric values, 6-min walk distance, and sonographic parameters mentioned above were compiled for the analysis.
Results:
All included participants were male with a mean age of 62.5 ± 8.4 years. The mean FEV1 in cases was 1.12 ± 0.4 L versus 2.41 ± 0.5 L in controls. The diaphragm thickness (1.8 ± 0.5 mm vs. 2.2 ± 0.6 mm; P = 0.005) and RFCSA was significantly lower in COPD patients (4.8 ± 1.3 cm2 vs. 6.12 ± 1.2 cm2; P = 0.02). However, diaphragm excursion (5.35 ± 2.8 cm vs. 7 ± 2.6 cm) although lower in COPD patients, was not significantly different between the groups. Correlation between FEV1 and ultrasound diaphragm measurements and RFCSA by Spearman's Rho correlation was poor (ρ = 0.2).
Conclusion:
Ultrasonographic assessment of the diaphragm and rectus femoris can be used as markers to assess skeletal muscle dysfunction in COPD as diaphragmatic function and RFCSA were lower in COPD patients.
KEY WORDS: Chronic obstructive pulmonary disease, diaphragm excursion and thickness, rectus femoris cross-sectional area, ultrasound, zone of apposition
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), with a global prevalence of 11.7%, is one of the leading causes of death in the world.[1] Although spirometry is the gold standard test for the diagnosis of COPD, it performs poorly as a comprehensive tool for the assessment of symptoms and their impact on health-related quality of life of participants with COPD.[2] A vital factor such as a change in peripheral muscle composition, is related to impaired health status in COPD.[3] Reduced muscle strength secondary to muscle wasting is one of the major determinants of mortality in COPD[4] apart from other factors such as smoking, body mass index, and poor lung function.[5,6]
The respiratory muscles are distinctive among skeletal muscles since they must work relentlessly throughout life. The diaphragm is the principal inspiratory pump muscle, and it is more resistant to developing fatigue than limb muscles.
In patients with COPD, the diaphragm and the chest wall are in a disadvantageous position in the length-tension relationship of the respiratory muscles.[7] The flattening of diaphragm results in an increased radius of curvature, which persists even at residual volume (RV).[8]
Furthermore, because of the horizontal orientation of the diaphragm and the loss of the zone of apposition (ZOA) between the diaphragm and the chest wall, the force vector on the lower rib cage becomes inward rather than cephalad, and hence, lower rib cage motion during inspiration becomes paradoxical, moving inward on inspiration instead of outward, a movement referred to as the “Hoover sign.”[9]
Assessment of diaphragm function in participants with COPD is a challenging task. Various methods have been used in gauging the diaphragm muscle morphometry and function, including spirometry, maximal inspiratory and expiratory pressures, chest X-ray, fluoroscopy, and computed tomography (CT) scan. A specific test of diaphragm function, used in research settings, is the measurement of maximal transdiaphragmatic pressure which is invasive and not routinely available in daily practice.[10] Noninvasive methods that specifically assess diaphragm function, such as ultrasound measurement of ZOA of the diaphragm and diaphragm excursion, have been initiated in clinical use only in recent times.[11]
The thickness and excursion of the diaphragm can be evaluated in B- or M-mode ultrasonographicaly.[12,13] Although the diaphragm excursion has been studied widely in the critical care settings,[14] its utility in COPD patients is yet to emerge. The thickness of the diaphragm has been the bone of contention, where some authors have suggested its correlation with the lung function and others have refuted the same.[8,14]
Skeletal muscle dysfunction is a frequent and clinically relevant systemic manifestation of COPD that predicts morbidity and mortality independently from the severity of lung function impairment as judged by forced expiratory volume in 1 s (FEV1).[12] Even in noncachectic patients with COPD, quadriceps strength is typically reduced by up to 30% compared with healthy elderly participants. Quadriceps strength independently predicts increased health-care utilization and mortality in COPD.[7,15] Similarly, the mid-thigh cross-sectional area measured by CT is a reliable test, which can predict mortality. While CT and magnetic resonance imaging (MRI) of the quadriceps have been studied in COPD, ultrasound use to assess limb muscle size has recently emerged as a newer, comparable, and noninvasive modality.[4,16,17]
It is being increasingly appreciated that ultrasonography (USG) is revolutionizing pulmonary practice, and many diagnostic and inventions are based on ultrasound imaging. Ultrasound is also radiation-free and inexpensive. The use of ultrasound to evaluate diaphragm function is very well established in critical care setup at the bedside. The literature on the clinical utility of USG in participants with COPD is lacking. We hypothesize that ultrasound can be useful in evaluating diaphragm function (ZOA, diaphragm excursion, thickness, rectus femoris cross-sectional area [RFCSA]) and quadriceps muscle size in patients with COPD and healthy participants. The study explores how muscle size and function measured in this manner correlates with FEV1.
METHODS
The study was initiated after institutional ethics board approval and data were procured after obtaining written informed consent from each participant. Twenty-four consecutive stable COPD patients aged between 40 and 80 years and using only inhaled medication were taken up for the study. Patients with diaphragmatic palsy or other neuromuscular disorders, history of significant renal, hepatic, or endocrine disease or those with the use of oral steroids in the preceding 4 weeks were excluded from the study. Eighteen age- and sex-matched healthy controls with modified Medical Research Council (mMRC) dyspnea score of 0 or 1, no spirometric evidence of airflow obstruction and no organ dysfunction or comorbidity affecting the legs were included as controls. The sample size was calculated based on previous data[13] where 20 patients in each group were required to detect correlation between ZOA and FEV1 with 80% power and at a 5% significance level.
Data regarding the demographics, smoking history, and exacerbations in the previous year, comorbid illness were collected. Spirometry (Medical Equipment Europe GmbH, smart PFT), 6-min walk test (as per ATS guideline), COPD assessment test score and ultrasound assessment of diaphragm by ZOA, diaphragm excursion, and diaphragm thickness were collected for the participants with COPD. Controls also completed the above examinations.
Ultrasonographic assessment of muscle function
All the ultrasound parameters were measured by DS, who is a certified radiologist. Ultrasound measurements were made with participants comfortably reclined at 45ο. The linear ultrasound probe (Sonosite S-ICU) was positioned against the skin in the right 5th intercostal space (or at a lower intercoastal space where the diaphragm was visualized) in the mid-axillary line, and the participant was instructed to breathe normally. The subject was asked to breathe to total lung capacity (TLC) (maximum inspiration with glottis open) and the diaphragm insertion was noted in the costal recess; the length of the diaphragm in the apposition to the coastal region was measured [TLC LZOA; Figure 1]. The diaphragm position and length were also measured, as the subject breathed quietly over 5–10 breaths, at the average end-inspiratory and end-expiratory position (functional residual capacity [FRC] LZOA). The subject was then instructed to exhale completely to RV and the diaphragm position and the length of the diaphragm in apposition to the coastal region were measured (RV LZOA). The difference in the length of the diaphragm at the ZOA marked at TLC and at RV is the excursion of the diaphragm [RV LZOA - TLC LZOA; Figure 2].
Figure 1.
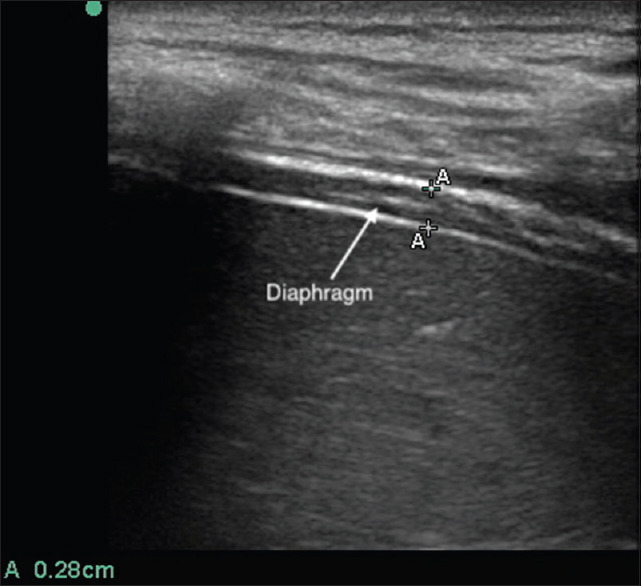
Diaphragm at zone of apposition in B mode
Figure 2.
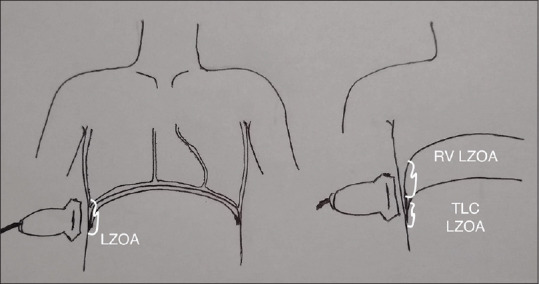
Diaphragm excursion (residual volume LZOA - total lung capacity LZOA)
Each breath associated with ultrasound measurement was captured by video recording and measurements were confirmed from the frozen image in the recording. An average value of five maneuvers were taken for each participant.
Diaphragm thickness was measured in the M-mode, by estimating the maximum thickness in expiration [Figure 3].
Figure 3.
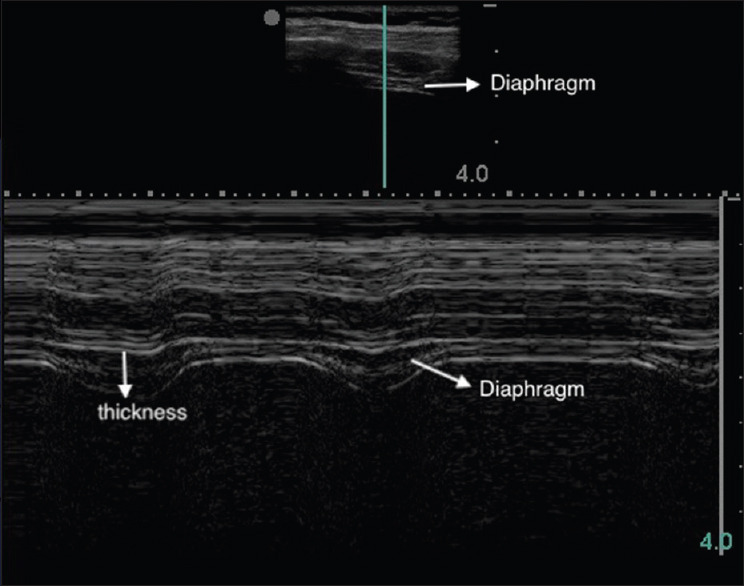
Diaphragm thickness in M mode
The RFCSA was measured by B-mode USG using an 8–16 MHz 6 cm linear array transducer placed perpendicular to the long axis of the thigh on its superior aspect, three-fifths of the distance from the anterior superior iliac spine to the superior patellar border, with the subject in supine position and the rested right leg held in passive extension. RFCSA was calculated by a planimetric technique after the inner echogenic line of the rectus femoris is outlined by a movable cursor on a frozen image [Figure 4].
Figure 4.
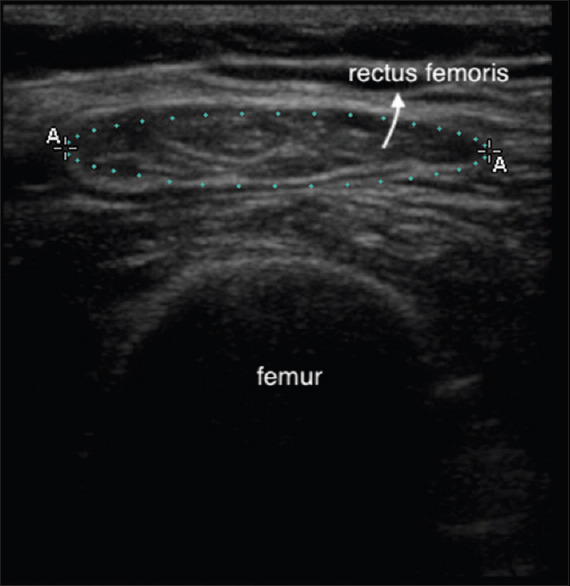
Rectus femoris cross-sectional area by planimetry
Statistical analysis
Descriptive statistics and Student's t-test were used for demographic analysis and comparison of means; Chi-square test or Fisher's exact test was used for comparison of proportions. Statistical significance was accepted at a two-sided P ≤ 0.05. Correlation between ZOA, diaphragm excursion, diaphragm thickness, RFCSA, and FEV1 was done with Spearman's Rho Correlation. Data collected were analyzed with SPSS version 17, SPSS Inc, Chicago, IL, USA.
RESULTS
The clinical characteristics of the COPD patients and the controls are depicted in Table 1. All patients with COPD were smokers, of whom 11 were current smokers. In comparison, 13 (72.22%) of the controls were smokers; eight patients were classified as very severe COPD and the remaining 16 were severe COPD by GOLD criteria; 11 patients had a history of hospital admissions in the previous year.
Table 1.
Clinical characteristics of subjects
| Parameter | Case (n=24) | Control (n=18) | P |
|---|---|---|---|
| Age (years) | 61.5±8.4 | 61.9±7.8 | 0.097 |
| Height (cm) | 161.5±7.9 | 168.1±5.5 | 0.115 |
| Waist/hip ratio | 0.94±0.05 | 0.93±0.03 | 0.761 |
| BMI (kg/m2) | 20.6±3.5 | 24.4±3.4 | 0.805 |
| Smoking (n) | 24 | 13 | 0.088 |
| Hoover’s sign positive (n) | 17 | 0 | 0.025 |
BMI: Body mass index
The results of the functional assessment of the participants are given in Table 2. The values of the parameters measured by ultrasound for the right diaphragm and rectus femoris are given in Table 3. The ZOA measurement was also attempted on the left diaphragm. However, the left diaphragm was visualized in only four of the 24 patients, and in the rest of the patients, the lung movement obscuring the diaphragm, evident as the curtain sign, was observed. Hence, left diaphragm measurements were not included for the study.
Table 2.
Functional assessment parameters
| Parameter (mean±SD) | Case (n=24) | Control (n=18) | P |
|---|---|---|---|
| 6 min walk distance (m) | 387.5±92.8 | 465.6±132.5 | 0.04 |
| CAT score | 13.1±6.2 | 6.3±4.8 | 0.001 |
| FEV1 (l) | 1.12±0.4 | 2.41±0.5 | 0.001 |
| FEV1/FVC ratio | 63.2±8.4 | 83.9±7.1 | 0.001 |
SD: Standard deviation, COPD: Chronic obstructive pulmonary disease, CAT score: COPD assessment test, FEV1: Forced expiratory volume in 1 s, FVC: Forced vital capacity
Table 3.
Ultrasound Measurements of the diaphragm at total lung capacity, functional residual capacity, and residual volume and rectus femoris cross-sectional area
| Parameter (mean±SD) | Case (n=24) | Control (n=18) | P | 95% CI |
|---|---|---|---|---|
| Diaphragm thicknes (mm) | 1.7±0.5 | 2.3±0.6 | 0.005 | −0.98-−0.18 |
| TLC LZOA (cm) | 1.9±1.2* | 2.1±1.0 | 0.38 | −1.09-0.43 |
| FRC LZOA (cm) | 4.1±1.6 | 4.7±1.7 | 0.28 | −0.51-1.69 |
| RV LZOA (cm) | 7.8±2.4 | 9.8±1.6 | 0.01 | −3.50-−0.43 |
| Diaphragm excursion | 5.35±2.8 | 7±2.6 | 0.07 | −3.4-0.18 |
| RFCSA (cm2) | 4.7±0.3 | 6.07±1.2 | 0.003 | −2.23-−0.49 |
*Four COPD patients had a TLC LZOA in the range of 0–0.2 cm. Hence not included in this calculation. TLC: Total lung capacity, LZOA: Length of the zone of apposition, FRC:, RV: Residual volume, RFCSA: Rectus femoris cross-sectional area, COPD: Chronic obstructive pulmonary disease, CI: Confidence interval, SD: Standard deviation
The ZOA of the diaphragm at different lung volumes were lower in COPD patients [Table 3], significantly lower at RV (P = 0.01). An important aspect to be noted was that four of the COPD patients had ZOA at maximal inspiration in the range of 0–0.2 cm, and hence were not included in the comparison of means for ZOA. The thickness of the diaphragm was noted to be significantly reduced in COPD patients as compared to controls in the study (1.7 ± 0.5 mm vs. 2.3 ± 0.6 mm). However, the correlation between FEV1 and the diaphragm thickness was modest in our study (ρ =0.3). Similarly, there was no significant correlation between FEV1 and RFCSA by Spearman's Rho correlation (ρ =0.2).
DISCUSSION
Respiratory and nonrespiratory muscle dysfunction, especially lower limb muscle dysfunction, is an important contributor to morbidity and poor quality of life in patients with COPD. Multi-modal assessment of the muscles by testing the muscle mass (anatomy), muscle strength, and endurance (dynamometer) and metabolic function by muscle biopsy and fiber typing has been reported in many previous studies.[18] However, ultrasound has been used only sparingly in clinical practice as an assessment tool in patients with COPD.
Ultrasound measurement of Respiratory muscle assessment
Zone of apposition of the diaphragm and excursion of the diaphragm
The altered geometry and mechanics of the diaphragm and chest wall, leading to pulmonary hyperinflation, contribute to muscle dysfunction in COPD. ZOA of diaphragm is described as the area of muscle fibers vertically oriented, in contact with the chest wall. The ZOA, in patients with severe COPD, was approximately 50% of that in control subjects, when measured in various lung volumes (RV and FRC) by ultrasound.[13]
In severe COPD or during an acute exacerbation, the patient might have insufficient effort to perform spirometry. Measurement of ZOA by ultrasound does not require any forced expiratory effort on the patient's part and is easy to perform. The difference between the ZOA at RV and ZOA at TLC can be also used as a measure of excursion of diaphragm. Once the correlation between the spirometry derived FEV1 values and ZOA/excursion of diaphragm is established, ZOA/excursion of the diaphragm can be taken as a surrogate marker for the assessment of the severity of muscle dysfunction and exacerbation risk of COPD.
Gorman et al. have demonstrated reduced coronal length of the diaphragm by measuring ZOA, rib cage diameters with magnetometers, pneumotachometer to measure the airflow and to use complex formulae to calculate the length of diaphragm and volume displaced during the respiratory maneuvers.[13] The study was carried out in a simple manner using an ultrasound machine, thus obviating the need for complex equipment and calculations.
Various methods have been described to evaluate diaphragm excursion by ultrasound, although there is no consensus as to the best method to assess the diaphragm excursion. Some of these measurement methods include:
diaphragm thickness and kinetics in the ZOA during relaxation and respiratory efforts, with the patient in the sitting position, using 7.5 MHz ultrasound linear probe in B mode at the space between the anterior to mid-axillary lines[19,20]
downward movement of the left branch of the portal vein during inspiration, with the patient in the supine position, in B mode using a 3.5-MHz convex transducer placed in the right subcostal region[21]
craniocaudal movement of the diaphragm, with the patient in the supine position, measured in M mode with the curvilinear probe placed sub-coastally and anteriorly with the liver (right side) or the spleen (left side) used as ultrasound windows[15]
downward movement of the lung silhouette (curtain movement) as a surrogate marker of the downward movement of both hemidiaphragm, with the patient in sitting position, evaluated by B mode with 3.5MHz transducer in the mid scapular line.[22]
Our study uses the difference measured between the length of the diaphragm at the ZOA marked at TLC and RV as the excursion of the diaphragm. The diaphragm excursion was calculated as RV LZOA - TLC LZOA. This novel method has not been described in literature thus far.
In our study, using the above-mentioned method of diaphragm assessment, we found that diaphragm excursion was lower in COPD patients as compared to matched controls, tending towards significance. However, with respect to the correlation between diaphragm excursion and lung function, the literature review shows conflicting evidence, wherein Scheibe et al. have established that diaphragm excursion on ultrasound correlates with FEV1;[22] while Smargiassi et al. found no relationship of diaphragm excursion and lung function.[11]
Other mechanisms are purported to downplay the contribution of diaphragm dysfunction to impaired lung function in COPD patients. There is evidence that neural drive increases by three-fold, along with adaptations of the type of muscle fibers to preserve endurance and strength of muscles as well as a change in the shape of the chest to accommodate the increased lung volume. In fact, the Hoovers sign is thought to be a sign of increased neural drive as opposed to the radial contraction of the diaphragm.[7] This is probably the reason for the discrepancy in our study between diaphragm dysfunction and lung function (FEV1) as 17 of the 24 COPD patients had Hoover's sign. Since our study included a limited number of COPD patients, a significant correlation between diaphragm function and FEV1 could not be established.
Further studies, encompassing a larger number of COPD patients, are needed to prove the relationship of diaphragm excursion and lung function, especially FEV1. Despite the above-mentioned limitations of sample size and co-existent increased neural drive, our study confirms the premise that ZOA and diaphragm excursion are considerably reduced in COPD patients.
Thickness of the diaphragm
The thickness of the diaphragm was noted to be significantly reduced in COPD patients in our study. Smargiassi et al. studied 32 COPD patients and have determined that thickness of the diaphragm at different lung volumes was closely related to inspiratory capacity, vital capacity and TLC.[11] On the other hand, Baria et al. have demonstrated that a significant difference in diaphragm thickness or thickening fraction does not exist between COPD patients and healthy controls.[23] According to a study by Eryüksel et al., diaphragmatic thickness fraction assessment in COPD does not help to predict exacerbations or identify subjects who are at high risk of symptoms.[24]
Nevertheless, ultrasound measurements of the diaphragm function are already being used as an important assessment tool in critical care. Estimation of diaphragm thickening ratio,[23] and diaphragm thickening index have been described as predictors of successful weaning in ventilated patients.[15,25] The evaluation of the role of these additional assessments would probably result in a more comprehensive estimation of diaphragm dysfunction in COPD subjects.
Ultrasound measurement of nonrespiratory muscle assessment
The overall muscle mass, including the size and type of its muscle fibers, is a predictor of the muscle force. Muscle mass has been assessed by multiple methods-from simple measurements of four-site skinfold thickness, to complex measurements such as bioelectrical impedance, creatinine height index (to estimate total skeletal muscle mass by biochemistry) and noninvasive methods such as measures of regional limb skeletal muscle mass by dual-energy X-ray absorptiometry scanning, MRI, CT and USG.[4,17]
Age-related muscle loss has been demonstrated in all the component four muscles of the quadriceps group.[26] Based on this premise, the most easily accessible muscle, the rectus femoris was measured as a surrogate in our study.
In a previous study, quadriceps strength shared a linear relationship with RFCSA in both healthy controls (r = 0.80; P < 0.001) and patients with COPD (r = 0.78; P < 0.001).[16] Preliminary observations confirm a good relationship between RFCSA and quadriceps strength and CT mid-thigh cross sectional area (CSA).
The rectus femoris border was visualized and area calculated with ease in both COPD patients and controls. Prior literature has suggested that the visualization of the rectus femoris border may be challenging in obese patients and consequently, the RFCSA may be unmeasurable. Since none of our participants were obese, the estimation of RFCSA was hassle-free.
The RFCSA was significantly reduced in COPD patients in comparison to control subjects [Figure 5; P = 0.02]. Therefore, RFCSA can be used as a marker for the presence and severity of skeletal muscle dysfunction in COPD patients.
Figure 5.
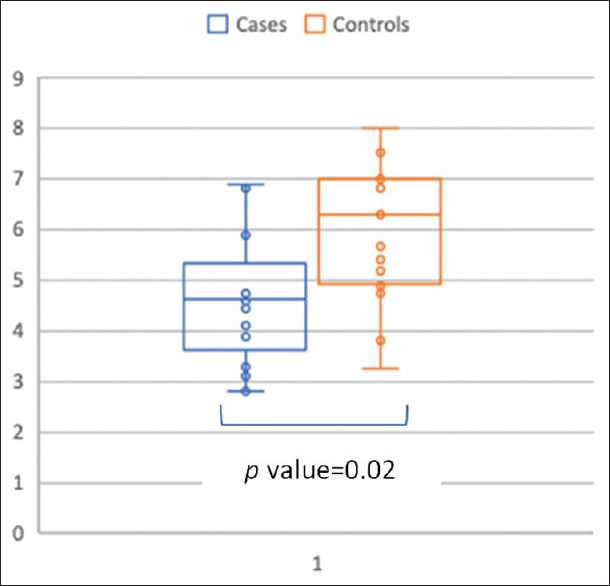
Box whisker plot-rectus femoris cross-sectional area in cm2 in COPD patients (blue) and healthy controls (red)
Aerobic and resistance training of the muscles has been shown to increase the muscle mass by 8.6% and 21%, respectively, as measured by RFCSA.[27,28] Similarly, RFCSA by ultrasound measurement has been used to monitor muscle mass after neuromuscular electrical stimulation and a significant increase of 19.7% was noted. Hence, RFCSA can be a valuable tool to assess not only the severity of muscle dysfunction but also to determine the improvement in muscle mass after pulmonary rehabilitation with exercise-based interventions. Compliance to a regular exercise regimen may improve when patients are shown the change in muscle mass as visual feedback.
Limitations of the study
Participants with COPD were in a severe and very severe category. Thus, the effect on muscle mass and function in mild and moderate COPD cannot be extrapolated from this study. Although the diaphragm excursion and ZOA were lower in COPD patients compared to controls, statistical significance could not be achieved. The inclusion of a higher number of subjects in both arms could have benefitted the study.
Prior experiments on measuring ZOA used complex formulae and equipment for the assessment which are difficult to translate in day-to-day practice at the patient's bedside. Ultrasound assessment methods deployed in our study are simple, can be used at the bedside, and in future be used as a marker for muscle dysfunction in COPD.
CONCLUSION
Ultrasound assessment is minimally invasive, cost-effective and diagnostic as a marker for identifying clinically significant muscle disease in COPD. Ultrasound measurement of diaphragm thickness, diaphragm excursion, and RFCSA can be used to assess muscle dysfunction in COPD. These assessments, if validated across varying severity of COPD, may help to predict the severity and prognosis of muscle dysfunction in COPD patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buist S. Gold 2018 Glob Initiat Chronic Obstr Lung Dis. 2018. [Last accessed on 2018 Feb 04]. Available from: http://wwwwhoint/respiratory/copd/GOLD_WR_06pdf .
- 3.Montes de Oca M, Torres SH, Gonzalez Y, Romero E, Hernández N, Mata A, et al. Peripheral muscle composition and health status in patients with COPD. Respir Med. 2006;100:1800–6. doi: 10.1016/j.rmed.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–13. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 5.Boutou AK, Shrikrishna D, Tanner RJ, Smith C, Kelly JL, Ward SP, et al. Lung function indices for predicting mortality in COPD. Eur Respir J. 2013;42:616–25. doi: 10.1183/09031936.00146012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: Findings from the NHANES III follow-up study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–48. doi: 10.2147/copd.s5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenzie DK, Butler JE, Gandevia SC. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2009;107:621–9. doi: 10.1152/japplphysiol.00163.2009. [DOI] [PubMed] [Google Scholar]
- 8.Troyer AD, Wilson TA. Action of the diaphragm on the rib cage. J Appl Physiol (1985) 2016;121:391–400. doi: 10.1152/japplphysiol.00268.2016. [DOI] [PubMed] [Google Scholar]
- 9.Wilson TA. Compartmental models of the chest wall and the origin of Hoover's sign. Respir Physiol Neurobiol. 2015;210:23–9. doi: 10.1016/j.resp.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Sassoon CS, Gruer SE, Sieck GC. Temporal relationships of ventilatory failure, pump failure, and diaphragm fatigue. J Appl Physiol. 1996;81:238–45. doi: 10.1152/jappl.1996.81.1.238. [DOI] [PubMed] [Google Scholar]
- 11.Smargiassi A, Inchingolo R, Tagliaboschi L, Di Marco Berardino A, Valente S, Corbo GM. Ultrasonographic assessment of the diaphragm in chronic obstructive pulmonary disease patients: Relationships with pulmonary function and the influence of body composition – A pilot study. Respiration. 2014;87:364–71. doi: 10.1159/000358564. [DOI] [PubMed] [Google Scholar]
- 12.Steiner MC, Roubenoff R, Tal-Singer R, Polkey MI. Prospects for the development of effective pharmacotherapy targeted at the skeletal muscles in chronic obstructive pulmonary disease: A translational review. Thorax. 2012;67:1102–9. doi: 10.1136/thoraxjnl-2012-201765. [DOI] [PubMed] [Google Scholar]
- 13.Gorman RB, McKenzie DK, Pride NB, Tolman JF, Gandevia SC. Diaphragm length during tidal breathing in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1461–9. doi: 10.1164/rccm.200111-087OC. [DOI] [PubMed] [Google Scholar]
- 14.Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39:801–10. doi: 10.1007/s00134-013-2823-1. [DOI] [PubMed] [Google Scholar]
- 15.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 16.Seymour JM, Ward K, Sidhu PS, Puthucheary Z, Steier J, Jolley CJ, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64:418–23. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 17.Mathur S, Takai KP, Macintyre DL, Reid D. Estimation of thigh muscle mass with magnetic resonance imaging in older adults and people with chronic obstructive pulmonary disease. Phys Ther. 2008;88:219–30. doi: 10.2522/ptj.20070052. [DOI] [PubMed] [Google Scholar]
- 18.Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159:S1–40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 19.Ueki J, De Bruin PF, Pride NB. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax. 1995;50:1157–61. doi: 10.1136/thx.50.11.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abd El Aziz AA, Elwahsh RA, Abdelaal GA, Abdullah MS, Saad RA. Diaphragmatic assessment in COPD patients by different modalities. [Last accessed on 2019 Jan 24];Egypt J Chest Dis Tuberc. 2017 66:247–50. Available from: https://wwwsciencedirectcom/science/article/pii/S0422763817300298 . [Google Scholar]
- 21.Paulin E, Yamaguti WP, Chammas MC, Shibao S, Stelmach R, Cukier A, et al. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir Med. 2007;101:2113–8. doi: 10.1016/j.rmed.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Scheibe N, Sosnowski N, Pinkhasik A, Vonderbank S, Bastian A. Sonographic evaluation of diaphragmatic dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:1925–30. doi: 10.2147/COPD.S85659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baria MR, Shahgholi L, Sorenson EJ, Harper CJ, Lim KG, Strommen JA, et al. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest. 2014;146:680–5. doi: 10.1378/chest.13-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eryüksel E, Cimşit C, Bekir M, Cimsit Ç, Karakurt S. Diaphragmatic thickness fraction in subjects at high-risk for COPD Exacerbations. Respir Care. 2017;62:1565–70. doi: 10.4187/respcare.05646. [DOI] [PubMed] [Google Scholar]
- 25.Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: Influence on weaning from mechanical ventilation. Crit Care Med. 2011;39:2627–30. doi: 10.1097/CCM.0b013e3182266408. [DOI] [PubMed] [Google Scholar]
- 26.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol. 2001;90:2070–4. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 27.De Brandt J, Spruit MA, Hansen D, Franssen FM, Derave W, Sillen MJ, et al. Changes in lower limb muscle function and muscle mass following exercise-based interventions in patients with chronic obstructive pulmonary disease: A review of the English-language literature. Chron Respir Dis. 2018;15:182–219. doi: 10.1177/1479972317709642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon MK, Houchen L, Harrison S, Singh SJ, Morgan MD, Steiner MC. Ultrasound assessment of lower limb muscle mass in response to resistance training in COPD. Respir Res. 2012;13:119. doi: 10.1186/1465-9921-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]


