Abstract
Background
Expert guidelines discourage use of antipseudomonal β-lactams and fluoroquinolones in lower-risk patients with community-acquired complicated intra-abdominal infection (CA cIAI). Compliance with these recommendations across US hospitals is unclear. This study sought to determine treatment patterns and associated outcomes among adult hospitalized lower-risk patients with CA cIAI.
Methods
A study using data from the Premier Healthcare Database (10/2015–12/2017) was performed. Inclusion criteria: age ≥18 years; hospitalized; had a cIAI at admission; and received antibiotics within the first 4 hospital days. Patients were excluded if they were high risk, were transferred from another health care facility, had a recent hospital admission, or received dialysis within 30 days of admission. Empiric antibiotic treatment patterns and associated outcomes were quantified.
Results
Overall, 46 722 (66%) patients with cIAIs met the lower-risk CA IAI study criteria. Among lower-risk CA IAI patients, the mean (SD) age was 53.4 (18.2) years, and 71% had a Charlson Comorbidity Index score of 0. The most common diagnosis was acute appendicitis with peritonitis (59.7%). Among lower-risk CA IAI patients, 54% received piperacillin/tazobactam, 20% received a fluoroquinolone (FQ), 11% received ceftriaxone, and 7% received ampicillin/sulbactam. Overall, the median hospital length of stay was 4 days and median costs were $12 345 USD. Nearly 90% of patients were discharged home, and <1% died. Outcomes were similar across all empiric treatments received.
Conclusions
Overuse of antipseudomonal β-lactams and fluoroquinolones was commonplace among lower-risk CA IAI patients. These findings can serve as the basis for an antimicrobial stewardship initiative in hospitals aspiring to reduce the use of broad-spectrum antibiotics.
Keywords: cIAI, infection, outcomes, treatment
Antimicrobial therapy plays an integral role in the management of patients with complicated intra-abdominal infections (cIAIs). Empiric treatment selection is based on patients’ location before cIAI and background medical conditions, severity of infection, anatomical site of infection, and antibiotic resistance rates at the local health care institution [1, 2]. Broadly, treatment selection is based on whether the cIAI is community-acquired (CA) or health care–associated (HA) and if the patient is characterized as having a lower or higher risk for treatment failure or death. Most patients with cIAI meet the CA and lower-risk classifications [2]. Among patients with lower-risk CA cIAI, expert guidelines recommend narrower-spectrum antimicrobial agents with activity against the “common gram-negative Enterobacteriaceae, aerobic streptococci, and obligate anaerobic microorganisms.” Per the Surgical Infection Society Revised Guidelines on the Management of IAI [2], recommended empiric antimicrobial regimens for patients with lower-risk CA cIAI include ertapenem, moxifloxacin, cefotaxime, or ceftriaxone plus metronidazole, and in penicillin-allergic patients, ciprofloxacin (levofloxacin in formulary fluoroquinolone) plus metronidazole. Carbapenems (except etrapenem) and piperacillin/tazobactam (TZP) are discouraged in adult lower-risk patients with CA cIAI to avoid excessive use and potential promotion of resistance [1, 2]. Fluoroquinolones are also not recommended for use in institutions with high rates of fluoroquinolone resistance among common gram-negative Enterobacteriaceae. There are also growing safety concerns with use of fluroquinolones. The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have updated the labeling of all fluoroquinolones, advising of the serious risk of multiple disabling and potentially irreversible adverse reactions associated with their use [3, 4]. Most recently, the FDA and EMA updated the labeling of all fluoroquinolones to include the increased risk of aortic aneurysm associated with their use and recommending prescription of fluoroquinolones to patients only when no other treatment options are available [3, 4].
While treatment recommendations are well delineated in the guidelines, there are scant data on how well US hospitals have adopted these recommendations [5]. More importantly, it is not clear how often carbapenems, TZP, cefepime, and fluoroquinolones are used in clinical practice among lower-risk patients with CA cIAIs. There are also limited real-world data on the outcomes associated with the antibiotics used in the treatment of adult hospitalized lower-risk patients with CA cIAI. Given these gaps in the literature, the intent of this descriptive study was to examine antibiotic treatment patterns and associated outcomes among adult hospitalized lower-risk patients with CA cIAI across US hospitals. Emphasis was placed on quantifying use of antipseudomonal carbapenems, TZP, cefepime, and fluoroquinolones among adult hospitalized lower-risk patients with CA cIAI.
METHODS
Study Design
A retrospective observational study was conducted to examine treatment patterns and associated outcomes of lower-risk adult patients with CA cIAI across US hospitals. Data for the study are from the Premier Healthcare Database, which currently contains data from >730 million patient encounters [6]. The Premier Healthcare Database contains data from standard hospital discharge files, including a patient’s demographic and disease states. In addition to the data elements available in most of the standard hospital discharge files, the Premier Healthcare Database also contains a date-stamped log of billed items, including procedures, medications, and laboratory, diagnostic, and therapeutic services at the individual patient level. Information on hospital characteristics, including geographic location, bed size, and teaching status, is also available. However, no clinical laboratory values are available in the database. Preliminary comparisons between patient and hospital characteristics for the hospitals that submit data to Premier Healthcare Database and those of the probability sample of hospitals and patients selected for the National Hospital Discharge Survey (NHDS) suggest that the patient populations are similar with regard to patient age, gender, length of stay, mortality, primary discharge diagnosis, and primary procedure groups. The database was fully de-identified and compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA); as such, no special permission was required to review patient records and extract the data. Given the de-identified and retrospective nature of the data, as well as the observational study design, written patient consent was neither required nor sought.
Study Population
This study included all inpatient discharges from patients aged 18 years or older with evidence of a cIAI between 10/2015 and 12/2017 [7, 8]. The first qualifying cIAI discharge during the study period was defined as the index cIAI admission.
Inclusion Criteria
An inpatient hospitalization for patients aged 18 years or older, discharged between 10/2015 and 12/2017.
The first qualifying hospitalization with evidence of a cIAI was flagged as the index hospitalization.
For patients with multiple cIAI admissions, only the first cIAI was considered.
Evidence of cIAI defined by algorithms based on diagnosis or procedure codes (Appendix A).
Primary cIAI diagnosis and a cIAI surgical procedure or a secondary cIAI diagnosis and cIAI surgical procedure within 5 days of admission.
Received an antibiotic within the first 4 hospital days.
Exclusion Criteria
Met criteria for high-risk patient (sepsis, severe sepsis, septic shock; ≥3 components of sepsis; or ≥2 physiologic risk factors) at admission (Appendix B) [2].
Had a hospital admission in past 30 days.
Health care facility point of origin (transferred from another hospital or health care facility).
Died or was discharged alive on the index date.
Received any dialysis within 30 days before index admission.
Treatment Classification
Patients were assigned into non–mutually exclusive cohorts based on antibiotics received during the first 4 days of hospitalization. Empiric treatment regimens received during the first 4 days of hospitalization were determined. We also calculated the number of patients who received 1 of the following antibiotics during the first 4 days of hospitalization: TZP, meropenem, cefepime, and fluoroquinolones.
Baseline Covariates
Patients’ demographic and clinical characteristics were based on available information during the qualifying admission period. Patient-level covariates included in the analysis were demographics, comorbidities, Charlson Comorbidity Index (CCI) scores [9], cIAI infection designation, and antibiotics received during qualifying admission. Hospital-level covariates included region, population served, teaching status, and hospital size. Outcome measures included duration of antibiotic therapy, hospital length of stay (LOS), hospital costs, in-hospital mortality, discharge destination (eg, home, long-term care facility, skilled nursing facility, hospice), and 30-day readmissions postdischarge.
Statistical Methods
Unadjusted descriptive statistics were used to characterize the patient population. Patient demographics, clinical conditions, hospital characteristics, and outcomes were examined, and summary statistics were reported. Data measured on a continuous scale were expressed as median and interquartile range (IQR). Dichotomous and categorical data were expressed as counts and percentages of patients in the categories. Outcomes were stratified by empiric treatment received, but no inferential statistics were performed as this was a descriptive study. Analyses were conducted using SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
During study period, 77 663 patients had a qualifying hospitalization with evidence of a cIAI, representing ~2% of all admissions during study time frame. Among the 77 663 patients, 30 941 did not meet the study criteria, resulting in a final study population of 46 722 lower-risk patients with CA cIAIs. Baseline hospital- and patient-level characteristics of the lower-risk cIAI study population are shown in Table 1. Most lower-risk CA cIAI patients received care in hospitals classified as urban (88.4%) and nonteaching (62.7%). The largest category of hospital size was >500 beds (29.2%), and the South was the region with the most patients (41.7%). The mean (SD) age was 54 (18) years, and 52% were male. Most patients were white (76.3%), the median (IQR) CCI score was 0 (0–1), and 71% of patients had a CCI score of 0. Chronic lung diseases (12.9%) and diabetes without complications (13.3%) were the only comorbidities present in >10% of the study population. Acute appendicitis with peritonitis was the most common diagnosis (59.7%), followed by peritoneal abscess (9.9%), non-trauma-related perforation of intestine (8.9%), and fistula of intestine (5.1%).
Table 1.
Baseline Characteristics of Included Patients (n = 46 722)
| Age, mean (SD), y | 54 (18) |
|---|---|
| Sex, male | 24 657 (52) |
| Race | |
| White | 35 646 (74.3) |
| Black or African American | 5746 (12.3) |
| Other | 5330 (11.4) |
| Charlson Comorbidity Index, median (IQR) | 0 (0–1) |
| Myocardial infarction | 1510 (3.2) |
| Congestive heart failure | 2384 (5.1) |
| Peripheral vascular disease | 618 (1.3) |
| Dementia | 874 (1.9) |
| Cerebrovascular disease | 489 (1.0) |
| Chronic lung disease | 6042 (12.9) |
| Connective tissue disease | 827 (1.8) |
| Ulcer | 3685 (7.9) |
| Chronic liver disease | 2353 (5.0) |
| Hemiplegia | 205 (0.4) |
| Moderate or severe kidney disease | 2838 (6.1) |
| Diabetes without complications | 6232 (13.2) |
| Diabetes with complications | 1433 (3.1) |
| Tumor | 2849 6.1) |
| Malignant tumor, metastasis | 1287 (2.8) |
| AIDS | 97 (0.2) |
| Complicated intra-abdominal infection diagnosis | |
| Acute appendicitis with peritonitis | 28 004 (59.7) |
| Peritoneal abscess | 4624 (9.9) |
| Perforated intestine | 4142 (8.9) |
| Fistula of intestine | 2406 (5.1) |
| Peritonitis (unspecified) | 1901 (5.1) |
| Hospital-level covariates | |
| Hospital region | |
| South | 19 481 (41.7) |
| West | 10 670 (22.8) |
| Midwest | 9072 (19.4) |
| Northeast | 7499 (16.1) |
| Population served | |
| Urban | 41 304 (88.4) |
| Rural | 5418 (11.6) |
| Teaching hospital | 29 315 (62.7) |
| Nonteaching hospital | 17 407 (37.3) |
| No. of hospital beds | |
| 000–099 | 3092 (6.6) |
| 100–199 | 7428 (15.9) |
| 200–299 | 9228 (19.8) |
| 300–399 | 8060 (17.3) |
| 400–499 | 5263 (11.3) |
| 500+ | 13 651 (29.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
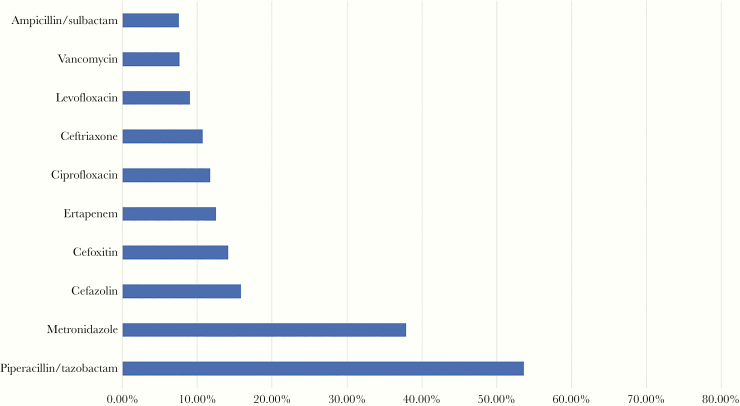
The most frequently used antibiotics during the first 4 days of hospitalization are shown in Figure 1. Piperacillin/tazobactam (54%) was the most commonly used antibiotic. Fluroquinolone-containing regimens (ciprofloxacin and levofloxacin) were used in 20% of the study population. Of the 9339 patients who received a fluoroquinolone, 8066 (86.3%) also received metronidazole. Approximately 11% of the study population received ceftriaxone, and 7.5% received ampicillin/sulbactam. Of the 5006 who received ceftriaxone, 3959 (79.1%) also received metronidazole. Cefepime and meropenem were prescribed less frequently (each prescribed in 3% of patients). In total, 8% received ≥2 of the following agents during the first 4 days of hospitalization: TZP, meropenem, cefepime, and fluoroquinolone.
Figure 1.
Most common empiric antibiotics received among patients with lower-risk complicated intra-abdominal infections.
Overall, the median (IQR) LOS was 4 (2–8) days, and the median (IQR) total hospital cost was $12 345 ($8447–$20 399) US dollars. Less than 1% of patients died during their hospitalization, and most were discharged home (89.0%) or transferred to another hospital (9.5%). Approximately 10% of patients had a hospital re-admission within 30 days of discharge. Outcomes of patients who received a TZP-, meropenem-, cefepime-, or fluroquinolone-containing regimen are shown in Table 2. Although this study was not designed to compare outcomes across regimens received and no inferential statistics were performed, hospital LOS, total costs, discharge destination, and 30-day readmission rates were largely similar across regimens, with the exception of those who received meropenem or cefepime. Closer examination of these patients indicated that they were typically older, had more comorbid conditions, and had higher CCI scores (data not shown). Outcomes were also alike among lower-risk CA cIAI patients who received 0, 1, or ≥2 of the following antibiotics: TZP, meropenem, cefepime, and fluoroquinolone(s) (Table 3).
Table 2.
Outcomes by Empiric Therapy Received
| Overall | TZP | FQ | CR0 | SAM | MEM | FEP | |
|---|---|---|---|---|---|---|---|
| No. of patients (%) | 46 722 (100) | 25 070 (54.7) | 9339a (20.0) | 5006b (10.7) | 3492 (7.4) | 1308 (2.8) | 1331 (2.8) |
| LOT, median (IQR), d | 5 (3–8) | 4 (3–6) | 3 (1–5) | 2 (1–5) | 3 (1–4) | 5 (2–8) | 3 (1–5) |
| LOS, median (IQR), d | 4 (2–8) | 5 (3–8) | 5 (3–8) | 5 (3–9) | 4 (2–7) | 7 (4–12) | 7(4–12) |
| Hospital costs, median (IQR), $ | 12 345 (8447–20 399) | 12 377 (8591–19 748) | 12 823 (8829–20 836) | 13 995 (9336–22 470) | 11 723 (8409–18 509) | 18 256 (11 680–30 724) | 18 388 (11 047–31 101) |
| 30-d hospital re-admission, % | 9.9 | 9.7 | 9.4 | 9.9 | 8.9 | 12.8 | 12.2 |
| Hospital discharge location, % | |||||||
| Home | 88.9 | 89.5 | 87.1 | 85.0 | 91.7 | 78.0 | 76.5 |
| Hospital | 9.5 | 8.9 | 11.3 | 12.9 | 6.9 | 18.5 | 19.6 |
| Death | 0.9 | 0.9 | 0.9 | 1.1 | 0.8 | 2.1 | 2.4 |
| Hospice | 0.6 | 0.6 | 0.7 | 1.0 | 0.5 | 1.38 | 1.4 |
Abbreviations: CR0, ceftriaxone; FEP, cefepime; FQ, fluoroquinolones; IQR, interquartile range; LOS, hospital length of stay; LOT, length of therapy; MEM, meropenem; SAM, ampillin/sulbactam; TZP, piperacillin/tazobactam.
a8066 also received metronidazole.
b3959 also received metronidazole.
Table 3.
Outcomes by Receipt of 1, 2, and ≥2 of the Following Antibiotics: Piperacillin/Tazobactam, Meropenem, Cefepime, Ciprofloxacin, and Levofloxacin
| None | 1 | ≥2 | |
|---|---|---|---|
| No. of patients | 7028 | 23 827 | 15 867 |
| LOS, median (IQR), d | 4 (2–8) | 4 (2–7) | 5 (3–9) |
| Hospital costs, median (IQR), $ | 11 440 (7723–21 159) | 11 781 (8211–19 079) | 13 664 (9295–22 052) |
| 30-d hospital re-admission, % | 10.9 | 9.8 | 9.7 |
| Hospital discharge location, % | |||
| Home | 89.4 | 90.1 | 86.8 |
| Hospital | 8.9 | 8.5 | 11.3 |
| Death | 1.0 | 0.8 | 1.1 |
| Hospice | 0.7 | 0.5 | 0.8 |
Abbreviations: IQR, interquartile range; LOS, length of stay.
DISCUSSION
Overall, the findings from this study suggest that use of non-guideline-concordant therapies for patients with lower-risk CA cIAIs is commonplace. Empiric therapy with TZP or fluoroquinolones occurred in >75% of patients meeting the criteria for lower-risk CA cIAI employed in this study. The rationale for discouraging the empiric use of these agents in low-risk CA cIAI relates to their spectrum of activity [1, 2]. The concerns with fluoroquinolones stem from a lack of antibacterial activity against common gram-negative pathogens associated with cIAI. Rates of fluoroquinolone resistance among Escherichia coli, the most common cIAI pathogen, now exceed 25% in most regions in the United States, limiting their utility as an effective first-line empiric option [10]. In contrast to fluoroquinolones, TZP is an overly broad-spectrum antipseudomonal β-lactam therapy for lower-risk CA cIAI patients. One of the primary reasons to use TZP is for patients with suspected or documented P. aeruginosa infections. Among lower-risk patients with CA cIAI, P. aeruginosa represented <5% of culture-positive cIAI patients in a recently published multicenter observational study [11]. It is important to note that cultures are not often collected in patients with lower-risk CA cIAIs, and the point estimate of 5% is likely conservative. Furthermore, the need to even treat P. aeruginosa in cIAI patients without high acuity of illness is questionable, as studies have shown successful outcomes in patients who did not receive an antipseudomonal agent [1, 2, 12–14].
Despite being overly broad, the extensive use of TZP in lower-risk CA cIAI patients suggests that its use is not considered problematic by many clinicians. While certain antibiotics like carbapenems, third- and fourth-generation cephalosporins, and fluoroquinolones are generally considered the agents that pose the greatest risks, use of all broad-spectrum antibiotics, including TZP, increases the risk of CDI and antibiotic resistance [15, 16]. In a recent case–control study of surgical trauma patients, CDI cases were more likely to have received TZP relative to CDI-negative controls (odds ratio [OR], 2.4; 95% CI, 1.3–4.5) [17]. Similarly, in a study involving 64 US academic medical centers, use of β-lactam/β-lactamase inhibitor combinations (of which 80% was due to TZP use) was associated with an increased odds of acquiring an HA-CDI (OR, 1.49; 95% CI, 1.36–1.64) [18]. Interestingly, the odds of acquiring an HA-CDI were found to be relatively comparable among patients who received third- or fourth-generation cephalosporins (OR, 1.75; 95% CI, 1.62–1.89), carbapenems (OR, 1.60; 95% CI, 1.44–1.79), and β-lactam/β-lactamase inhibitor combinations (OR, 1.49; 95% CI, 1.36–1.64). Clinical observational studies also suggest that prior exposure to TZP, albeit to a lesser extent than carbapenems, increases the risk of acquiring a carbapenem-resistant gram-negative infection [19–21]. In support of these clinical observations, a recent study of ICU patients indicated that TZP contributes to microbiota disruption and leads to increased carbapenem-resistant P. aeruginosa acquisition [22]. There are also data that correlate TZP use with the increased rates of TZP resistance in E. coli and Klebsiella sp. [23]. Additionally, Teshome and colleagues demonstrated that each additional day of treatment with TZP was associated with an 8% risk of new resistance development among patients with severe sepsis or septic shock [24].
Although this study was not designed to compare outcomes by treatment received and no inferential statistics were performed, another interesting observation from this study was that outcomes were largely similar across the varying empiric treatment regimens used in lower-risk cIAI patients. This observation needs to be interpreted cautiously, as no inferential statistics were performed, but it potentially highlights that there may be an opportunity to use more narrow-spectrum antibiotic therapies and limit the use of fluoroquinolones and antibiotics with antipseudomonal activity. Studies demonstrate that shifts in antibiotic usage patterns, regardless of patient population, are best accomplished through the development of interdisciplinary guidelines and staff education [25–28]. Site-of-care clinical pathways, especially those built into electronic medical record support systems, empower antibiotic stewardship programs to ensure that the appropriate therapies are empirically initiated and provide an avenue for them to affect therapy changes when performing perspective audit and feedback if guidelines are not followed upfront [25].
There are several things to consider when interpreting the findings of this study. This was a descriptive study, and its intent was simply to highlight the proportion of adult hospitalized lower-risk patients with cIAI who received a non-guideline-concordant therapy. This study was not designed to compare outcomes by treatment received, nor does it control for the multitude of factors that influence diverse outcomes like readmissions or LOS. To properly compare outcomes across treatment groups, additional analyses that account for baseline differences between groups would have been needed. Laboratory or other electronic medical record information was not available in the Premier Healthcare Database. We therefore had to use International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), diagnosis codes to derive the cIAI cohort and the disease severity and comorbidity measures used in this study. It is quite possible that not all clinical conditions were coded and some higher risk patients and HA cIAIs were misclassified as lower risk and CA cIAIs, respectively. To minimize major “risk” classification errors (ie, classifying lower-risk patients as higher-risk), we developed comprehensive algorithms to fully capture patients with sepsis and physiologic risk factors. We also implemented several safeguards to ensure that HA cIAIs were not classified as CA. In addition to laboratory and electronic medical data, microbiologic data were not included in this study. It is quite possible that patients classified as lower risk CA cIAI received a board-spectrum antibiotic to treat a suspected or documented antibiotic-resistant pathogen like an extended spectrum β-lactamase (ESBL)–producing Enterobacteriaceae recovered on culture. Of note, the SIS Surgical Infection Society guidelines do not recommend routinely obtaining peritoneal fluid cultures in lower-risk patients with CA IAI for the purposes of guiding antimicrobial therapy, as studies suggest that cultures rarely, if ever, provide information useful to the clinician [2, 29]. We also did not have allergy information, and it is possible that some of the patients who received a fluoroquinolone were intolerant to β-lactams. Conservatively, even if the approach employed in this study misclassified 30%–50% of patients, the findings would still indicate that the majority of lower-risk patients with CA received non-guideline-concordant therapy. Of note, sepsis, severe sepsis, and septic shock are well-defined codes in the ICD-10-CM, and it unlikely that many patients with any of these conditions would have been missed as their codes are critical for reimbursement [30]. In support of this, the mortality rates and hospital LOS among lower-risk patients with CA cIAIs were very low, suggesting that the algorithm for classifying patients was fairly accurate. However, the algorithms used in this study were not designed or intended to be treatment prediction tools to define therapy in adult patients who presented to the hospital with a cIAI. In order for this to serve as a prediction tool, it will need to be prospectively validated across multiple institutions using data from the medical record.
In conclusion, overuse of non-guideline-concordant broad-spectrum antipseudomonal antibiotics, namely TPZ and fluoroquinolones, was found to be commonplace among lower-risk patients with CA cIAIs. Similar outcomes across varying empiric treatment regimens suggest that there is an opportunity to use narrow-spectrum antibiotic therapies and limit the use of antibiotics with antipseudomonal activity. This process of decreasing use of inappropriate broad-spectrum antibiotics is consistent with the CDC’s core elements for antibiotic stewardship [28] and could potentially help to decrease the incidence of developing CDI and subsequent antibiotic-resistant infections. As with all studies of this nature, the findings need to be confirmed in prospective studies. For now, these findings can serve as the basis for an antimicrobial stewardship initiative to assess the use of broad-spectrum antibiotics among lower-risk patients with CA cIAI.
Acknowledgments
Financial support. This work was supported by Tetraphase Pharmaceuticals.
Potential conflicts of interest. Mr. Izmailyan and Drs. Olesky and Lawrence are current or former employees of and have stock in Tetraphase. Dr. Lodise has served as a consultant, scientific advisor, and speakers’ bureau participant for Tetraphase. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Author contributions. All authors participated in the study design, implementation of the study protocol, analysis and interpretation of the data, and drafting of the report. All authors were responsible for data interpretation and drafting of the report. All authors provided critical reviews and final approval of the manuscript. The approval of the manuscript and decision to submit the manuscript for publication were the responsibility of the coauthors, led by T.L.
Data availability. Data are not publicly available.
APPENDIX A. CRITERIA FOR DIAGNOSIS OF A COMPLICATED INTRA-ABDOMINAL INFECTION ≥1 ICD-10 DIAGNOSIS CODE FROM GROUP A AND ≥1 ICD-10 PROCEDURE CODE FROM GROUP B
GROUP A (ICD-10 DIAGNOSIS CODE)
K57.12, K57.13, K57.32, K57.33, K63.2, K63.3, K63.1 K25.1, K56.60, K25.2, K56.60, K25.5, K56.60, K25.6, K26.1, K26.2, K26.5, K26.6, K27.1, K2.72, K27.5, K27.6, K27.6, K28.1, K28.2, K28.5, K28.6, K35.2, K35.3, K37, K36, K67, K65.8, K65.0, K65.1, K65.2, K65.0, K68.12, K68.19, K68.9, K6.53, K65.4, K65.8, K65.9, K63.0, K75.0, K75.1, K72.90, K72.91, K76.6, K76.7, K72.10, K72.90, K82.2 plus (K80.00, K80.01, K80.42, K80.43, K80.62, K80.63, K80.66, or K80.67), K81.0, K83.0
GROUP B (ICD-10 PROCEDURE)
0DB40ZZ, 0DB43ZZ, 0DB44ZZ, 0DB47ZZ, 0DT40ZZ, 0DT44ZZ, 0DT47ZZ, 0DT48ZZ, 0DB60ZZ, 0DB63ZZ, 0DB67ZZ, 0DT70ZZ, 0DT74ZZ, 0DT77ZZ, 0DT78ZZ, 0D160ZA, 0D164ZA, 0D168ZA, 0DB60ZZ, 0DB63ZZ, 0DB64ZZ, 0DB67ZZ, 0DB68ZZ, 0D160ZA, 0D164ZA, 0D168ZA, 0DB60ZZ, 0DB63ZZ, 0DB64ZZ, 0DB67ZZ, 0DB68ZZ, 0DB64Z3, 0DB60ZZ, 0DB63ZZ, 0DB67ZZ, 0D13079, 0D1307A, 0D1307B, 0DT60ZZ, 0DT64ZZ, 0DT67ZZ, 0DT68ZZ, 0DT60ZZ, 0DT64ZZ, 0DT67ZZ, 0DT68ZZ, 0DQ60ZZ, 0DQ63ZZ, 0DQ64ZZ, 0DQ67ZZ, 0DQ68ZZ, 0DQ90ZZ, 0DQ93ZZ, 0DQ94ZZ, 0DQ97ZZ, 0DQ98ZZ, 0DQ60ZZ, 0DQ63ZZ, 0DQ64ZZ, 0DQ67ZZ, 0DQ68ZZ, 0DQ90ZZ, 0DQ93ZZ, 0DQ94ZZ, 0DQ97ZZ, 0DQ98ZZ, 0DQ60ZZ, 0DQ63ZZ, 0DQ64ZZ, 0DQ67ZZ, 0DQ68ZZ, 0DB80ZZ, 0DB83ZZ, 0DB84ZZ, 0DB87ZZ, 0DB88ZZ, 0DT90ZZ, 0DT94ZZ, 0DT97ZZ, 0DT98ZZ, 0DTA0ZZ, 0DTA4ZZ, 0DTA7ZZ, 0DTA8ZZ, 0DTB0ZZ, 0DTB4ZZ, 0DTB7ZZ, 0DTB8ZZ, 0DT80ZZ, 0DT84ZZ, 0DT87ZZ, 0DT88ZZ, 0D1H0Z4, 0D1H4Z4, 0D1H8Z4, 0D1K0Z4, 0D1K4Z4, 0D1K8Z4, 0D1L0Z4, 0D1L4Z4, 0D1L8Z4, 0D1N0Z4, 0D1N4Z4, 0D1N8Z4, 0D1B0Z4, 0D1B4Z4, 0D1B8Z4, 0D1B0Z4, 0D1B4Z4, 0D1B8Z4, 0D1B0Z4, 0D1B4Z4, 0D1B8Z4, 0D1B0Z4, 0D1B4Z4, 0D1B8Z4, 0DQ90ZZ, 0DQ93ZZ, 0DQ94ZZ, 0DQ97ZZ, 0DQ98ZZ, 0DQ90ZZ, 0DQ93ZZ, 0DQ94ZZ, 0DQ97ZZ, 0DQ98ZZ, 0DQ80ZZ, 0DQ83ZZ, 0DQ84ZZ, 0DQ87ZZ, 0DQ88ZZ, 0DQA0ZZ, 0DQA3ZZ, 0DQA4ZZ, 0DQA7ZZ, 0DQA8ZZ, 0DQB0ZZ, 0DQB3ZZ, 0DQB4ZZ, 0DQB7ZZ, 0DQB8ZZ, 0DQ80ZZ, 0DQ80ZZ, 0DQ83ZZ, 0DQ83ZZ, 0DQ84ZZ, 0DQ84ZZ, 0DQ87ZZ, 0DQ87ZZ, 0DQ88ZZ, 0DQ88ZZ, 0DQA0ZZ, 0DQA3ZZ, 0DQA4ZZ, 0DQA7ZZ, 0DQA8ZZ, 0DQB0ZZ, 0DQB0ZZ, 0DQB3ZZ, 0DQB3ZZ, 0DQB4ZZ, 0DQB4ZZ, 0DQB7ZZ, 0DQB7ZZ, 0DQB8ZZ, 0DQB8ZZ, 0DQE0ZZ, 0DQE3ZZ, 0DQE4ZZ, 0DQE7ZZ, 0DQE8ZZ, 0DQN0ZZ, 0DQN3ZZ, 0DQN4ZZ, 0DQN7ZZ, 0DQN8ZZ, 0DQP0ZZ, 0DQP3ZZ, 0DQP4ZZ, 0DQP7ZZ, 0DQP8ZZ, 0HQ6XZZ, 0HQ7XZZ, 0DQE0ZZ, 0DQE3ZZ, 0DQE4ZZ, 0DQE7ZZ, 0DQE8ZZ, 0DQH0ZZ, 0DQH3ZZ, 0DQH4ZZ, 0DQH7ZZ, 0DQH8ZZ, 0DQK0ZZ, 0DQK3ZZ, 0DQK4ZZ, 0DQK7ZZ, 0DQK8ZZ, 0DQN0ZZ, 0DQN3ZZ, 0DQN4ZZ, 0DQN7ZZ, 0DQN8ZZ, 0DQE0ZZ, 0DQE3ZZ, 0DQE4ZZ, 0DQE7ZZ, 0DQE8ZZ, 0DQH0ZZ, 0DQH3ZZ, 0DQH4ZZ, 0DQH7ZZ, 0DQH8ZZ, 0DQN0ZZ, 0DQN0ZZ, 0DQN3ZZ, 0DQN3ZZ, 0DQN4ZZ, 0DQN4ZZ, 0DQN7ZZ, 0DQN7ZZ, 0DQN8ZZ, 0DQN8ZZ, 0HQ9XZZ, 0DQ90ZZ, 0DQ93ZZ, 0DQ94ZZ, 0DQ97ZZ, 0DQ98ZZ, 0DQE0ZZ, 0DQE3ZZ, 0DQE4ZZ, 0DQE7ZZ, 0DQE8ZZ, 0DS90ZZ, 0DS94ZZ, 0DS97ZZ, 0DS98ZZ, 0DSA0ZZ, 0DSA4ZZ, 0DSA7ZZ, 0DSA8ZZ, 0DSB0ZZ, 0DSB4ZZ, 0DSB7ZZ, 0DSB8ZZ, 0DSH0ZZ, 0DSH4ZZ, 0DSH7ZZ, 0DSH8ZZ, 0DSK0ZZ, 0DSK4ZZ, 0DSK7ZZ, 0DSK8ZZ, 0DSL0ZZ, 0DSL4ZZ, 0DSL7ZZ, 0DSL8ZZ, 0DSM0ZZ, 0DSM4ZZ, 0DSM7ZZ, 0DSM8ZZ, 0DSN0ZZ, 0DSN4ZZ, 0DSN7ZZ, 0DSN8ZZ, 0DS90ZZ, 0DS94ZZ, 0DS97ZZ, 0DS98ZZ, 0DSA0ZZ, 0DSA4ZZ, 0DSA7ZZ, 0DSA8ZZ, 0DSB0ZZ, 0DSB4ZZ, 0DSB7ZZ, 0DSB8ZZ, 0DSH0ZZ, 0DSH4ZZ, 0DSH7ZZ, 0DSH8ZZ, 0DSK0ZZ, 0DSK4ZZ, 0DSK7ZZ, 0DSK8ZZ, 0DSL0ZZ, 0DSL4ZZ, 0DSL7ZZ, 0DSL8ZZ, 0DSM0ZZ, 0DSM4ZZ, 0DSM7ZZ, 0DSM8ZZ, 0DSN0ZZ, 0DSN4ZZ, 0DSN7ZZ, 0DSN8ZZ, 0D7N0ZZ, 0D7N3ZZ, 0D7N4ZZ, 0D780ZZ, 0D783ZZ, 0D784ZZ, 0D7E0ZZ, 0D7E3ZZ, 0D7E4ZZ, 0DQ80ZZ, 0DQ83ZZ, 0DQ84ZZ, 0DQ87ZZ, 0DQ88ZZ, 0DQA0ZZ, 0DQA3ZZ, 0DQA4ZZ, 0DQA7ZZ, 0DQA8ZZ, 0DQB0ZZ, 0DQB3ZZ, 0DQB4ZZ, 0DQB7ZZ, 0DQB8ZZ, 0DQE0ZZ, 0DQE3ZZ, 0DQE4ZZ, 0DQE7ZZ, 0DQE8ZZ, 0DQ83ZZ, 0DQ84ZZ, 0DQ87ZZ, 0DQ88ZZ, 0DQE0ZZ, 0DQE3ZZ, 0DQE4ZZ, 0DQE7ZZ, 0DQE8ZZ, 0DTJ4ZZ, 0DTJ0ZZ, 0DTJ7ZZ, 0DTJ8ZZ, 0DTJ4ZZ, 0DTJ0ZZ, 0DTJ7ZZ, 0DTJ8ZZ, 0D9J00Z, 0D9J0ZZ, 0D9J30Z, 0D9J3ZZ, 0D9J40Z, 0D9J4ZZ, 0D9J70Z, 0D9J7ZZ, 0D9J80Z, 0D9J8ZZ, 0D9J00Z, 0D9J0ZZ, 0D9J30Z, 0D9J3ZZ, 0D9J40Z, 0D9J4ZZ, 0D9J70Z, 0D9J7ZZ, 0D9J80Z, 0D9J8ZZ, 0DQJ0ZZ, 0DQJ0ZZ, 0DQJ3ZZ, 0DQJ3ZZ, 0DQJ4ZZ, 0DQJ4ZZ, 0DQJ7ZZ, 0DQJ7ZZ, 0DQJ8ZZ, 0DQJ8ZZ, 0HQ6XZZ, 0HQ7XZZ, 0DQJ0ZZ, 0DQJ3ZZ, 0DQJ4ZZ, 0DQJ7ZZ, 0DQJ8ZZ
0F9000Z, 0F900ZZ, 0FC00ZZ, 0FC03ZZ, 0FC04ZZ, 0F900ZX, 0FB00ZX, 0FB03ZX, 0FB04ZX, 0FJ03ZZ, 0F900ZZ, 0F903ZZ, 0F904ZZ, 0FB00ZZ, 0FB03ZZ, 0FB04ZZ, 0F500ZZ, 0F503ZZ, 0F504ZZ, 0F500ZZ, 0F500ZZ, 0F503ZZ, 0F504ZZ, 0FT10ZZ, 0FT14ZZ, 0FT20ZZ, 0FT24ZZ, 0FT00ZZ, 0FT04ZZ, 0FY00Z0, 0FY00Z1, 0FY00Z2, 0FY00Z0, 0FY00Z1, 0FY00Z2, 0FQ00ZZ, 0FQ03ZZ, 0FQ04ZZ, 0FQ00ZZ, 0FQ03ZZ, 0FQ04ZZ, 0FS00ZZ, 0FS04ZZ, 0F9430Z, 0F940ZZ, 0F9400Z, 0FC40ZZ, 0FC43ZZ, 0FC44ZZ, 0FF40ZZ, 0FF43ZZ, 0FF44ZZ, 0FF47ZZ, 0F940ZX, 0F950ZX, 0F960ZX, 0F980ZX, 0F990ZX, 0F9C0ZX, 0F9D0ZX, 0FB40ZX, 0FB50ZX, 0FB60ZX, 0FB80ZX, 0FB90ZX, 0FBC0ZX, 0FB40ZZ, 0FB43ZZ, 0FT40ZZ, 0FT44ZZ, 0FB44ZZ, 0F140D5, 0F140D6, 0F140D7, 0F140Z5, 0F140Z6, 0F140Z7, 0F144D5, 0F144D6, 0F144D7, 0F144Z5, 0F144Z6, 0F144Z7, 0F140D3, 0F140DB, 0F140Z3, 0F140ZB, 0F144D3, 0F144DB, 0F144Z3, 0F144ZB, 0F140D4, 0F140Z4, 0F144D4, 0F144Z4, 0F140D8, 0F140D9, 0F140Z8, 0F140Z9, 0F144D8, 0F144D9, 0F144Z8, 0F144Z9, 0F190D3, 0F190Z3, 0F194D3, 0F194Z3, 0F150D3, 0F150DB, 0F150Z3, 0F150ZB, 0F154D3, 0F154DB, 0F154Z3, 0F154ZB, 0F160D3, 0F160DB, 0F160Z3, 0F160ZB, 0F164D3, 0F164DB, 0F164Z3, 0F164ZB, 0F180D3, 0F180DB, 0F180Z3, 0F180ZB, 0F184D3, 0F184DB, 0F184Z3, 0F184ZB, 0F190DB, 0F190ZB, 0F194DB, 0F194ZB, 0F150D5, 0F150D6, 0F150D7, 0F150D8, 0F150D9, 0F150Z5, 0F150Z6, 0F150Z7, 0F150Z8, 0F150Z9, 0F154D5, 0F154D6, 0F154D7, 0F154D8, 0F154D9, 0F154Z5, 0F154Z6, 0F154Z7, 0F154Z8, 0F154Z9, 0F160D5, 0F160D6, 0F160D7, 0F160D8, 0F160D9, 0F160Z5, 0F160Z6, 0F160Z7, 0F160Z8, 0F160Z9, 0F164D5, 0F164D6, 0F164D7, 0F164D8, 0F164D9, 0F164Z5, 0F164Z6, 0F164Z7, 0F164Z8, 0F164Z9, 0F180D4, 0F180D5, 0F180D6, 0F180D7, 0F180D8, 0F180D9, 0F180Z4, 0F180Z5, 0F180Z6, 0F180Z7, 0F180Z8, 0F180Z9, 0F184D4, 0F184D5, 0F184D6, 0F184D7, 0F184D8, 0F184D9, 0F184Z4, 0F184Z5, 0F184Z6, 0F184Z7, 0F184Z8, 0F184Z9, 0F190D4, 0F190D5, 0F190D6, 0F190D7, 0F190D8, 0F190D9, 0F190Z4, 0F190Z5, 0F190Z6, 0F190Z7, 0F190Z8, 0F190Z9, 0F194D4, 0F194D5, 0F194D6, 0F194D7, 0F194D8, 0F194D9, 0F194Z4, 0F194Z5, 0F194Z6, 0F194Z7, 0F194Z8, 0F194Z9, 0FC90ZZ, 0FC90ZZ, 0F9970Z, 0FC50ZZ, 0FC60ZZ, 0FC80ZZ, 0FF50ZZ, 0FF53ZZ, 0FF54ZZ, 0FF57ZZ, 0FF60ZZ, 0FF63ZZ, 0FF64ZZ, 0FF67ZZ, 0FF80ZZ, 0FF83ZZ, 0FF84ZZ, 0FF87ZZ, 0FF90ZZ, 0FF93ZZ, 0FF94ZZ, 0FF97ZZ, 0FFC0ZZ, 0FFC3ZZ, 0FFC4ZZ, 0FFC7ZZ, 0F9900Z, 0F990ZZ, 0F9930Z, 0F9940Z, 0FJB0ZZ, 0FJB3ZZ, 0FJB4ZZ, 0FJB7ZZ, 0FJB8ZZ, 0F9500Z, 0F950ZZ, 0F9530Z, 0F953ZZ, 0F9540Z, 0F954ZZ, 0F9570Z, 0F957ZZ, 0F9580Z, 0F958ZZ, 0F9600Z, 0F960ZZ, 0F9630Z, 0F963ZZ, 0F9640Z, 0F964ZZ, 0F9670Z, 0F967ZZ, 0F9680Z, 0F968ZZ, 0F9800Z, 0F980ZZ, 0F9830Z, 0F983ZZ, 0F9840Z, 0F984ZZ, 0F9870Z, 0F987ZZ, 0F9880Z, 0F988ZZ, 0FHB0DZ, 0FHB3DZ, 0FHB7DZ, 0FJB0ZZ, 0FB80ZZ, 0FB83ZZ, 0FB87ZZ, 0FBC0ZZ, 0FBC3ZZ, 0FBC7ZZ, 0FTC0ZZ, 0FTC4ZZ, 0FTC7ZZ, 0FTC8ZZ, 0FB90ZZ, 0FB93ZZ, 0FB97ZZ, 0FT90ZZ, 0FT94ZZ, 0FT97ZZ, 0FT98ZZ
0F550ZZ, 0F553ZZ, 0F557ZZ, 0F560ZZ, 0F563ZZ, 0F567ZZ, 0F580ZZ, 0F583ZZ, 0F587ZZ, 0FB50ZZ, 0FB53ZZ, 0FB57ZZ, 0FB60ZZ, 0FB63ZZ, 0FB67ZZ, 0FB80ZZ, 0FB83ZZ, 0FB87ZZ, 0FT50ZZ, 0FT54ZZ, 0FT57ZZ, 0FT58ZZ, 0FT60ZZ, 0FT64ZZ, 0FT67ZZ, 0FT68ZZ, 0FT80ZZ, 0FT84ZZ, 0FT87ZZ, 0FT88ZZ
0FQ90ZZ, 0FQ93ZZ, 0FQ94ZZ, 0FQ97ZZ, 0FQ98ZZ, 0FQ90ZZ, 0FQ93ZZ, 0FQ94ZZ, 0FQ97ZZ, 0FQ98ZZ, 0FQ50ZZ, 0FQ53ZZ, 0FQ54ZZ, 0FQ57ZZ, 0FQ58ZZ, 0FQ60ZZ, 0FQ63ZZ, 0FQ64ZZ, 0FQ67ZZ, 0FQ68ZZ, 0FQ80ZZ, 0FQ83ZZ, 0FQ84ZZ, 0FQ87ZZ, 0FQ88ZZ, 0F7C0DZ, 0F7C0ZZ, 0F7C3DZ, 0F7C3ZZ, 0F7C4DZ, 0F7C4ZZ, 0F7C7DZ, 0F7C7ZZ, 0F8G0ZZ, 0F8G3ZZ, 0FCC0ZZ, 0FQC0ZZ, 0FQC3ZZ, 0FQC4ZZ, 0FQC7ZZ, 0FQC8ZZ, 0FQC0ZZ, 0FQC3ZZ, 0FQC4ZZ, 0FQC7ZZ, 0FQC8ZZ, 0FQ40ZZ, 0FQ43ZZ, 0FQ44ZZ, 0FQ40ZZ, 0FQ43ZZ, 0FQ44ZZ, 0WQFXZ2, 0DQ60ZZ, 0DQ60ZZ, 0DQ63ZZ, 0DQ63ZZ, 0DQ64ZZ, 0DQ64ZZ, 0DQ67ZZ, 0DQ67ZZ, 0DQ68ZZ, 0DQ68ZZ, 0DQ80ZZ, 0DQ80ZZ, 0DQ83ZZ, 0DQ83ZZ, 0DQ84ZZ, 0DQ84ZZ, 0DQ87ZZ, 0DQ87ZZ, 0DQ88ZZ, 0DQ88ZZ, 0DQ90ZZ, 0DQ93ZZ, 0DQ94ZZ, 0DQ97ZZ, 0DQ98ZZ, 0DQA0ZZ, 0DQA3ZZ, 0DQA4ZZ, 0DQA7ZZ, 0DQA8ZZ, 0DQE0ZZ, 0DQE3ZZ, 0DQE4ZZ, 0DQE7ZZ, 0DQE8ZZ, 0FQ40ZZ, 0FQ40ZZ, 0FQ40ZZ, 0FQ43ZZ, 0FQ43ZZ, 0FQ43ZZ, 0FQ44ZZ, 0FQ44ZZ, 0FQ44ZZ, 0FQ50ZZ, 0FQ53ZZ, 0FQ54ZZ, 0FQ57ZZ, 0FQ58ZZ, 0FQ60ZZ, 0FQ63ZZ, 0FQ64ZZ, 0FQ67ZZ, 0FQ68ZZ, 0FQ80ZZ, 0FQ83ZZ, 0FQ84ZZ, 0FQ87ZZ, 0FQ88ZZ, 0FQ90ZZ, 0FQ93ZZ, 0FQ94ZZ, 0FQ97ZZ, 0FQ98ZZ, 0FP40DZ, 0FP43DZ, 0FP44DZ, 0FR50JZ, 0FR54JZ, 0FR60JZ, 0FR64JZ, 0FR80JZ, 0FR84JZ, 0FR90JZ, 0FR94JZ, 0FS40ZZ, 0FS44ZZ, 0F9D00Z, 0F9D30Z, 0F9D40Z, 0F9D70Z, 0F9G00Z, 0F9G30Z, 0F9G40Z, 0F9D0ZZ, 0F9D3ZZ, 0F9D4ZZ, 0F9D7ZZ, 0F9D8ZZ, 0F9G0ZZ, 0F9G3ZZ, 0F9G4ZZ, 0FCD0ZZ, 0FCD7ZZ, 0FCG0ZZ, 0FCG3ZZ, 0FCG4ZZ, 0FFD0ZZ, 0FFD3ZZ, 0FFD4ZZ, 0FFD7ZZ, 0FFD8ZZ, 0F9G0ZX, 0FBG0ZX, 0F5D0ZZ, 0F5D3ZZ, 0F5D7ZZ, 0F5G0ZZ, 0F5G3ZZ, 0FBD0ZZ, 0FBD3ZZ, 0FBD7ZZ, 0FBG0ZZ, 0FBG3ZZ, 0FTD0ZZ, 0FTD7ZZ, 0F9G3ZZ, 0F9G4ZZ, 0F1D0D3, 0F1D0DB, 0F1D0Z3, 0F1D0ZB, 0F1D4D3, 0F1D4DB, 0F1D4Z3, 0F1D4ZB, DB90ZZ, 0DB93ZZ, 0DB94ZZ, 0DB97ZZ, 0DB98ZZ, 0FBG0ZZ, 0FBG0ZZ, 0FBG3ZZ, 0FBG3ZZ, 0FBG4ZZ, 0FBG4ZZ, 0FBG0ZZ, 0FBG3ZZ, 0FBG4ZZ, 0FBG0ZZ, 0FBG3ZZ, 0FBG4ZZ, 0FBG0ZZ, 0FBG3ZZ, 0FBG4ZZ, 0DT90ZZ, 0DT94ZZ, 0DT97ZZ, 0DT98ZZ, 0FTG0ZZ, 0FTG4ZZ, 0D1607A, 0D160JA, 0D160KA, 0D160ZA, 0DT90ZZ, 0DT90ZZ, 0F190Z3, 0F1G0ZC, 0FTG0ZZ, 0FTG0ZZ, 0FYG0Z0, 0FYG0Z1, 0FYG0Z2, 0FSG0ZZ, 0FSG4ZZ, 0FYG0Z0, 0FYG0Z1, 0FYG0Z2, 0F7D0DZ, 0F7D3DZ, 0F7D7DZ, 0FHD0DZ, 0FHD3DZ, 0FHD7DZ, 0FUD37Z, 0FUD47Z, 0FQG0ZZ, 0FQG3ZZ, 0FQG4ZZ, 0F1D0D3, 0F1D0DB, 0F1D0Z3, 0F1D0ZB, 0F1D4D3, 0F1D4DB, 0F1D4Z3, 0F1D4ZB, 0F1G0D3, 0F1G0DB, 0F1G0Z3, 0F1G0ZB, 0F1G4D3, 0F1G4DB, 0F1G4Z3, 0F1G4ZB, 0F7D3ZZ, 0FQD0ZZ, 0FQD3ZZ, 0FQD4ZZ, 0FQD7ZZ, 0FQD8ZZ, 0DJ00ZZ, 0DJ60ZZ, 0DJD0ZZ, 0DJU0ZZ, 0DJW0ZZ, 0WJG0ZZ, 0WJJ0ZZ, 0WJP0ZZ, 0WJR0ZZ, 0WJF4ZZ, 0WJG4ZZ, 0WJJ4ZZ, 0WJP4ZZ, 0WJR4ZZ, 0D5S0ZZ, 0D5S3ZZ, 0D5S4ZZ, 0D5T0ZZ, 0D5T3ZZ, 0D5T4ZZ, 0D5V0ZZ, 0D5V3ZZ, 0D5V4ZZ, 0D5W0ZZ, 0D5W3ZZ, 0D5W4ZZ, 0DBS0ZZ, 0DBS3ZZ, 0DBS4ZZ, 0DBT0ZZ, 0DBT3ZZ, 0DBT4ZZ, 0DBV0ZZ, 0DBV3ZZ, 0DBV4ZZ, 0DBW0ZZ, 0DBW3ZZ, 0DBW4ZZ, 0DTS0ZZ, 0DTS4ZZ, 0DTT0ZZ, 0DTT4ZZ, 0DN84ZZ, 0DNE4ZZ, 0DNJ4ZZ, 0DNS4ZZ, 0DNT4ZZ, 0DNV4ZZ, 0DNW4ZZ, 0FN04ZZ, 0FN44ZZ, 0FN54ZZ, 0FN64ZZ, 0FN84ZZ, 0FN94ZZ, 0FNG4ZZ, 0DNE0ZZ, 0DNE3ZZ, 0DNJ0ZZ, 0DNJ3ZZ, 0DNS0ZZ, 0DNS3ZZ, 0DNT0ZZ, 0DNT3ZZ, 0DNV0ZZ, 0DNV3ZZ, 0DNW0ZZ, 0DNW3ZZ, 0FN00ZZ, 0FN03ZZ, 0FN40ZZ, 0FN43ZZ, 0FN50ZZ, 0FN53ZZ, 0FN57ZZ, 0FN58ZZ, 0FN60ZZ, 0FN63ZZ, 0FN67ZZ, 0FN68ZZ, 0FN80ZZ, 0FN83ZZ, 0FN87ZZ, 0FN88ZZ, 0FN90ZZ, 0FN93ZZ, 0FN97ZZ, 0FN98ZZ, 0FNG0ZZ, 0FNG3ZZ, 0DCS0ZZ, 0DCS3ZZ, 0DCS4ZZ, 0DCT0ZZ, 0DCT3ZZ, 0DCT4ZZ, 0DCV0ZZ, 0DCV3ZZ, 0DCV4ZZ, 0DCW0ZZ, 0DCW3ZZ, 0DCW4ZZ, 0WCG0ZZ, 0WCG3ZZ, 0WCG4ZZ, 0W1G0J4, 0W1G3J4, 0W1G4J4, 0W1G0JY, 0W1G4JY, 0W9J00Z, 0W9J0ZZ, 0W9J40Z, 0W9J4ZZ, 0WWG00Z, 0WWG0JZ, 0WWG30Z, 0WWG3JZ, 0WWG40Z, 0WWG4JZ
APPENDIX B. CRITERIA FOR HIGH-RISK PATIENTS BASED ON ADMITTING DIAGNOSIS CODES
A patient is considered high risk if they meet any 1 of the following at admission:
Sepsis, severe sepsis, septic shock
At least 3 components of sepsis
At least 2 physiologic risk factors
| Classification and Corresponding ICD-10 Diagnosis Codes [2] |
|---|
| Sepsis, severe sepsis, septic shock |
| A40 Streptococcal sepsis A41 Other sepsis A48.3 Toxic shock syndrome T81.12 Postprocedural septic shock R65.2 Severe sepsis R65.20 Severe sepsis without septic shock R65.21 Severe sepsis with septic shock R65.X SIRS, unspecified |
| Components of sepsis |
| Fever R50 Fever of other and unknown origins Tachycardia R00.0 Tachycardia, unspecified R00.2 Palpitations, tachypnea R06.82 Tachypnea, unspecified R06.02 Shortness of breath, altered mental status R41.82 Altered mental status, unspecified; hypoglycemia E16.1 Other hypoglycemia E16.2 Hypoglycemia, unspecified; hyperglycemia R73.9 Hyperglycemia, unspecified; leukocytosis |
| D72.82 Elevated white blood cell count D72.825 Bandemia D72.828 Other elevated white blood cell count D72.829 Elevated white blood cell count, unspecified; leukopenia D72.81 Decreased white blood cell count; hypotension I95.X Hypotension R03.1 Nonspecific low blood pressure reading, hypoxemia R06.00 Dyspnea, unspecified R09.02 Hypoxemia, oliguria R34.X Oliguria N18.6 End-stage renal disease N17.X Acute kidney failure N19 Unspecified kidney failure N99.0 Postprocedural (acute) (chronic) kidney failure, coagulation abnormalities D65 Disseminated intravascular coagulation [defibrination syndrome] R79.1 Abnormal coagulation profile, thrombocytopenia D69.4 Other primary thrombocytopenia D69.5 Secondary thrombocytopenia D69.6 Thrombocytopenia, unspecified; hyperbilirubinemia E80.7 Disorder of bilirubin metabolism, unspecified; acute lung injury/acute respiratory failure; respiratory failure J96.X Respiratory failure, not elsewhere classified J95.82 Postprocedural respiratory failure J96 Respiratory failure, not elsewhere classified J98.1 Pulmonary collapse J96.0 Acute respiratory failure J96.00 Acute respiratory failure, unspecified whether with hypoxia or hypercapnia |
| J96.01 Acute respiratory failure with hypoxia J96.02 Acute respiratory failure with hypercapnia Z99.11 Dependence on respirator (ventilator) J96.X Respiratory failure, not elsewhere classified |
| Physiologic risk factors |
| Advanced age ≥70 y |
| Malignancy C00, C01, C02, C03, C04, C05, C06, C07, C08, C09, C10, C11, C12, C13, C14, C15, C16, C17, C18, C19, C20, C21, C22, C23, C24, C25, C26, C30, C31, C32, C33, C34, C37, C38, C39, C40, C41, C43, C45, C46, C47, C48, C49, C50, C51, C52, C53, C54, C55, C56, C57, C58, C60, C61, C62, C63, C64, C65, C66, C67, C68, C69, C70, C71, C72, C73, C74, C75, C76, C81, C82, C83, C84, C85, C88, C90, C91, C92, C93, C94, C95, C96, C97 |
| Kidney dysfunction or significant renal disease N18.6 End-stage renal disease N17.X Acute kidney failure N19 Unspecified kidney failure I12.0 Hypertensive chronic kidney disease with stage 5 chronic kidney disease or end-stage renal disease I13.11 Hypertensive heart and chronic kidney disease without heart failure with stage 5 chronic kidney disease or end-stage renal disease N99.0 Postprocedural (acute) (chronic) kidney failure I13.2 Hypertensive heart and chronic kidney disease with heart failure and with stage 5 chronic kidney disease or end-stage renal disease N18.6 End-stage renal disease |
| Hepatic dysfunction/significant liver disease or cirrhosis K91.82 Postprocedural hepatic failure K70 Alcoholic liver disease K71 Toxic liver disease K72 Hepatic failure, not elsewhere classified K74 Fibrosis and cirrhosis of liver K75 Other inflammatory liver diseases K76 Other diseases of liver K77 Liver disorders in diseases classified elsewhere B15-B19 hepatitis P78.81 congenital cirrhosis of liver |
| Hypoalbuminemia R77.0 Abnormality of albumin |
| Significant cardiovascular compromise R94.3 Abnormal results of CV function studies R55 Syncope and collapse |
Derived from Mazuski et al. [2].
Abbreviations: CV, Cardiovascular; ICD-10, International Classification of Diseases, Tenth Revision; SIRS, Systemic inflammatory response syndrome.
References
- 1. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133–64. [DOI] [PubMed] [Google Scholar]
- 2. Mazuski JE, Tessier JM, May AK, et al. The surgical infection society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt) 2017; 18:1–76. [DOI] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration. FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes. July 10, 2018 Available at: www.fda.gov/Drugs/drugsafety/ucm611032.htm. Accessed 20 September 2018.
- 4. FDA Drug Safety Communication. FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics. Accessed 1 March 2020.
- 5. Nguyen MP, Crotty MP, Daniel B, Dominguez E. An evaluation of guideline concordance in the management of intra-abdominal infections. Surg Infect (Larchmt) 2019; 20:650–7. [DOI] [PubMed] [Google Scholar]
- 6.Premier Applied Sciences, Premier Inc. Premier healthcare database white paper: data that informs and performs. Published 18 February 2018. Available at: https://www.premierinc.com/transforming-healthcare/healthcareperformance-improvement/premier-applied-sciences/. Accessed 1 March 2020.
- 7. Lodise TP, Palazzolo C, Reksc K, et al. Comparisons of 30-day admission and 30-day total health care costs between patients who were treated with oritavancin or vancomycin for a skin infection in the outpatient setting. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zilberberg MD, Nathanson BH, Ditch K, et al. Carbapenem treatment and outcomes among patients with culture-positive complicated intra-abdominal infections in US hospitals: a retrospective cohort study. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 10. Hawser S, Hoban DJ, Badal RE, et al. Epidemiology and antimicrobial susceptibility of gram-negative aerobic bacteria causing intra-abdominal infections during 2010-2011. J Chemother 2015; 27:67–73. [DOI] [PubMed] [Google Scholar]
- 11. Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW study. World J Emerg Surg 2014; 9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lan SH, Chang SP, Lai CC, et al. The efficacy and safety of eravacycline in the treatment of complicated intra-abdominal infections: a systemic review and meta-analysis of randomized controlled trials. J Clin Med 2019; 8:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solomkin JS, Gardovskis J, Lawrence K, et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis 2019; 69:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solomkin J, Evans D, Slepavicius A, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 2017; 152:224–32. [DOI] [PubMed] [Google Scholar]
- 15. Gross AE, Johannes RS, Gupta V, et al. The effect of a piperacillin/tazobactam shortage on antimicrobial prescribing and Clostridium difficile risk in 88 US medical centers. Clin Infect Dis 2017; 65:613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah K, Pass LA, Cox M, et al. Evaluating contemporary antibiotics as a risk factor for Clostridium difficile infection in surgical trauma patients. J Trauma Acute Care Surg 2012; 72:691–5. [DOI] [PubMed] [Google Scholar]
- 18. Pakyz AL, Jawahar R, Wang Q, Harpe SE. Medication risk factors associated with healthcare-associated Clostridium difficile infection: a multilevel model case-control study among 64 US academic medical centres. J Antimicrob Chemother 2014; 69:1127–31. [DOI] [PubMed] [Google Scholar]
- 19. Tsao LH, Hsin CY, Liu HY, et al. Risk factors for healthcare-associated infection caused by carbapenem-resistant Pseudomonas aeruginosa. J Microbiol Immunol Infect 2018; 51:359–66. [DOI] [PubMed] [Google Scholar]
- 20. Patel N, Harrington S, Dihmess A, et al. Clinical epidemiology of carbapenem-intermediate or -resistant Enterobacteriaceae. J Antimicrob Chemother 2011; 66:1600–8. [DOI] [PubMed] [Google Scholar]
- 21. Lodise TP Jr, Miller C, Patel N, et al. Identification of patients with Pseudomonas aeruginosa respiratory tract infections at greatest risk of infection with carbapenem-resistant isolates. Infect Control Hosp Epidemiol 2007; 28:959–65. [DOI] [PubMed] [Google Scholar]
- 22. Pettigrew MM, Gent JF, Kong Y, et al. Gastrointestinal microbiota disruption and risk of colonization with carbapenem-resistant Pseudomonas aeruginosa in ICU patients. Clin Infect Dis 2019; 69:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suseno M, Das S, Semel J, Thomson R Jr. Emerging piperacillin/tazobactam resistance in Escherichia coli and Klebsiella sp. Open Forum Infect Dis 2018; 5Suppl 1:S719. [Google Scholar]
- 24. Teshome BF, Vouri SM, Hampton N, et al. Duration of exposure to antipseudomonal beta-lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy 2019; 39:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carratalà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med 2012; 172:922–8. [DOI] [PubMed] [Google Scholar]
- 27. Jenkins TC, Sabel AL, Sarcone EE, et al. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis 2010; 51:895–903. [DOI] [PubMed] [Google Scholar]
- 28.US Centers for Disease Control and Prevention. CDC core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed 1 March 2020.
- 29. Davies HO, Alkhamesi NA, Dawson PM. Peritoneal fluid culture in appendicitis: review in changing times. Int J Surg 2010; 8:426–9. [DOI] [PubMed] [Google Scholar]
- 30. Kulanko J. Small differences in sepsis and SIRS guidelines can result in major differences in reimbursement. Available at: https://www.aapc.com/blog/31689-sepsis-and-sirs-in-icd-10-cm/.