Abstract
Interactions along the neuro-cardiac axis are being explored with regard to their involvement in cardiac diseases, including catecholaminergic polymorphic ventricular tachycardia, hypertension, atrial fibrillation, long QT syndrome and sudden death in epilepsy. Interrogation of the pathophysiology and pathogenesis of neuro-cardiac diseases in animal models present challenges resulting from species differences, phenotypic variation, developmental effects and limited availability of data relevant at both the tissue and cellular level. By contrast, tissue-engineered models containing cardiomyocytes and peripheral sympathetic and parasympathetic neurons afford characterization of cellular- and tissue-level behaviours while maintaining precise control over developmental conditions, cellular genotype and phenotype. Such approaches are uniquely suited to long-term, high-throughput characterization using optical recording techniques with the potential for increased translational benefit compared to more established techniques. Furthermore, tissue-engineered constructs provide an intermediary between whole animal/tissue experiments and in silico models. This paper reviews the advantages of tissue engineering methods of multiple cell types and optical imaging techniques for the characterization of neuro-cardiac diseases.
Keywords: neuro-cardiac, co-culture, optical mapping, sympathetic, arrhythmia
1. Introduction
Neuro-cardiac disease describes the pathophysiological interaction between the nervous system (NS) and cardiovascular system (CVS), with pathological activity in one system resulting in pathological behaviour in the other [1]. Hypertension, catecholaminergic polymorphic ventricular tachycardia (CPVT), long QT syndrome (LQTS), as well as other syncope and seizure disorders have all been proposed as neuro-cardiac diseases [2–6]. CPVT [2], atrial fibrillation [7], LQTS, ventricular tachycardia [7] and atrio-ventricular (AV) block [8] are coincident with seizure presentation in clinical populations [2,4,7,9]. Seizures have also been reported in up to 50% of CPVT patients earlier in life before the presentation of CVPT-related symptoms [2,10]. This observation raises questions about our fundamental understanding of these diseases. For example, in seizures or cardiac arrhythmias in CPVT patients, is the actual triggering event neuronal or cardiac? This is important to know because it could contribute in the effectiveness of current therapeutic strategies including pharmacological approaches and surgical interventions [11]. Therefore, a greater understanding of the mechanisms underlying the pathophysiology of neuro-cardiac diseases is required to improve the diagnosis and treatment of such disorders.
The CVS is under homeostatic control via the autonomic nervous system (ANS). Aberrant sympathetic and parasympathetic NS activity, hyperinnervation and disorganized neuro-cardiac interfaces have been correlated with cardiac arrhythmia [12,13]. Additionally, plasticity in sympathetic and parasympathetic neuronal networks of the heart and developmental interactions may influence neuro-cardiac behaviours [5,14,15]. Furthermore, cell surface characteristics and secretion of growth factors can shape the development, maturation and ultimately the function of cardiomyocytes and neurons [6,16–18]. Pathogenic interactions between the NS and CVS can operate in either direction. For example, NS to CVS pathogenesis was confirmed in a rodent model by Larsen et al. for hypertension phenotypes [6], while the potential for CVS to NS pathogenesis has been highlighted by the observation that premature ventricular contractions (PVCs) have the capacity to excite a population of neurons independent of cardiac pacing and haemodynamic forces [19].
Both neurons and cardiomyocytes are excitatory cells expressing similar ion channels and rhythmic behaviours. Mutations that affect ion channels expressed in cardiac cells have the potential to affect the same channels when expressed in neurons [2,20,21]. For instance, genetic autopsies have revealed gain-of-function mutations in sodium channels (Nav1.5) and type 2 ryanodine receptors (RyR2) as well as loss of function in potassium channels (Kv7.1, hERG), which are common to both sudden unexplained death in epilepsy (SUDEP) and cardiac arrhythmias [4,14,21–23]. Dysregulation of plasma membrane potentials and intracellular calcium regulation can prolong depolarization, resulting in concomitant seizure and cardiac arrhythmia phenotypes [7,21].
Neuro-cardiac diseases act at all levels from the cellular to whole and multi-organ, and a detailed understanding is required across these scales. Currently, neuro-cardiac interactions are most commonly studied using whole animal models [14,19,24] and tissue-engineered constructs [6,16]. However, the intricate communication between neural and cardiac tissue in the heart could lead to confounding results in whole animal studies of neuro-cardiac diseases [25]. In comparison, tissue culture models enable experimenter control over the complexity of the system [26] and can help to bridge the gap in understanding the mechanisms of pathophysiology between the cellular and organ levels. Furthermore, the planar surface geometry of tissue culture models is uniquely suited to optical actuation and characterization [27–29]. Advances in optical actuation and characterization will enable long-term, high-throughput characterization of neuro-cardiac diseases [30,31].
In this mini review, we will focus on the recent advances in optical imaging of engineered tissue culture methods that allow the study of synergistic effects of neural and cardiac tissue in neuro-cardiac diseases. To illustrate the importance of such methods, we will first discuss how common cellular mechanisms, such as Ca2+ or K+ regulation through transmembrane channels, can affect both neural and cardiac tissue in CPVT and LQTS, as examples of diseases that were initially considered solely cardiac.
2. Neuro-cardiac physiology and disease
Calcium plays an integral role in neuro-cardiac transmission affecting both cardiomyocyte contractility and neuronal excitability. Calcium dysregulation underlies many cardiovascular pathologies such as hypertrophy [32,33], hypertension [34,35] and arrhythmias [36–38]. In the central nervous system (CNS) and peripheral nervous system (PNS), calcium is involved in neurotransmitter release, generation of excitatory or inhibitory postsynaptic potentials and the induction of different forms of synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD) in the hippocampus [39,40] or in the stellate and superior cervical sympathetic ganglia [41,42]. Neuronal calcium dysregulation, therefore, has the potential to influence the functional connectivity within neuronal networks of both the CNS and ANS along the neuro-cardiac axis. Taken together, mutations affecting proteins linked to calcium signalling (such as RyR2) provide a substrate for interplay between the NS and the CVS that can lead to both cardiovascular and neurological pathologies.
CPVT is a lethal genetic disease associated with arrhythmogenesis caused by mishandling of intracellular calcium leading to inappropriate calcium release events from the sarcoplasmic reticulum (SR) and aberrant muscle contractions in the heart [36,43–47]. With symptoms including syncope and sudden cardiac death, CPVT is normally first diagnosed in early childhood to young adulthood with a mortality rate reaching 50% by the age of 20 years [48,49]. Aberrant calcium release in cardiomyocytes can lead to abnormal diastolic membrane depolarization that can ultimately impair the cardiac beating rate and cause arrhythmias. The most frequent form of CPVT, CPVT1, is caused by mutations to the cardiac ryanodine receptor RyR2, for which more than 150 mutations have been reported [36,45]. RyR2 is the main calcium release channel involved in calcium-induced calcium release during excitation–contraction (EC) coupling in cardiac cells [50,51]. In 2006, work by Liu et al. [52] elegantly demonstrated that delayed afterdepolarizations (DADs), a major risk factor for arrhythmogenesis, may be linked to RyR2 with the CPVT-related mutation R4496C.
CPVT was originally described as an exclusively cardiovascular disease whereby stress or exercise induces elevation of catecholamines that can lead to ventricular tachycardia [48]. Recent research in humans [53] and mouse models carrying RyR2 mutations associated with CPVT [2,14] shows coincidence of CPVT, seizures and SUDEP, suggesting a possible connection between the induction of cardiac arrhythmias and irregular CNS activity. In fact, pharmacologically induced seizures and brainstem spreading depolarization led to fatal cardiac arrhythmias in mice carrying the gain-of-function mutation RyR2-R176Q [14]. These data suggest that neurological events such as epileptic seizures could be linked to long-term effects on the heart. Nevertheless, CPVT can also be manifested as a neuro-cardiac disease in the absence of abrupt CNS activity. Recent work suggests that reciprocal pathogenesis in the PNS and CVS could underlie the generation of arrhythmias in animal models of cardiac disease [19] and in CPVT patients. For example, cardiac pacing alone is insufficient to induce arrhythmic events in CPVT patients, but when combined with sympathetic stimulation may result in arrhythmia [24]. These results were corroborated in mice expressing the RyR2 mutation R2474S, which exhibit an exercise-induced ventricular tachycardia phenotype with greater sensitivity to β-adrenergic receptor stimulation than cardiac pacing [24].
LQTS is an inherited channelopathy associated with syncope and sudden death in the young, with the two most common LQTS subtypes, LQT1 and LQT2 caused by mutations in genes encoding the potassium channels KCNQ1 (responsible for the slow delayed rectifier current IKs) and KCNH2 (responsible for the rapid delayed rectifier current IKr), respectively [47]. LQT1, in particular, is associated with sympathetically triggered cardiac events, specifically physical activity and water immersion [47]. During sympathetic stimulation, L-type calcium channel (LTCC) activity increases, resulting in a net influx of calcium ions and an increased propensity for early afterdepolarizations (EADs). In vitro, adrenergic stimulation provoked EADs in cardiomyocytes with KCNQ1 mutations derived from induced pluripotent stem cells (iPSCs), while β-blockers effectively prevented EADs [54]. EADs are typically caused by reactivation of LTCC during the plateau phase of the action potential, with reactivation facilitated by bradycardia, a hallmark of LQT1 from fetal life onwards [55].
Historically, children with severe LQTS, such as those with double LQT1 mutations, have often been misdiagnosed as epileptics [56], sometimes with tragic consequences as most antiepileptic drugs are contraindicated in LQTS. However, more recent studies have shown that the issue might be more complex, as a clinical seizure phenotype was found in 22% of genotype-ascertained LQT1 patients [4]. Moreover, dominant LQT1 mutations expressed in the heart and brain of mice, and corresponding to those linked with the genetic LQTS disorders Romano–Ward syndrome and Jervell and Lange-Nielsen syndrome in humans, resulted in both cardiac arrhythmias and epileptic seizures, revealing a potential dual neuro-cardiac arrhythmogenic role for KCNQ1 mutations [3].
The synergy between both the CVS and the PNS in both CPVT and LQTS is further exemplified by the common preventive neuromodulator therapeutic strategies employed for the treatment of these disorders, which includes surgical cardiac sympathetic denervation [57–59]. Taken together, these observations highlight the need for more elaborate studies to elucidate the intricate mechanisms underlying neuro-cardiac transmission. Such studies will also have relevance for other diseases affecting the neuro-cardiac axis. Takotsubo syndrome (TTS), for example, a transient but severe form of acute heart failure [60], has been linked to sudden emotional or stressful events resulting in elevation in sympathetic activity [61–63]. Although the pathophysiology of TTS is not currently well understood [63], direct measurement in patients with TTS has shown an increase in sympathetic nerve activity associated with a decrease in spontaneous baroreflex control of sympathetic activity [64]. Studies that enable investigation of the modulation of cardiac tissue by sympathetic neurons at the cellular level are likely to be highly relevant for gaining a better understanding of the mechanisms that underlie such diseases.
3. In vitro approaches to examine neuro-cardiac interactions
While the need for more elaborate research on the pathophysiology of neuro-cardiac diseases is increasingly acknowledged [65,66], the complexity of neuro-cardiac interactions is difficult to investigate using whole animal models. For example, cross-talk between different centres of the afferent and efferent systems involved in cardiac neurotransmission (figure 1; more complex in-depth schemes are available such as [67]) can produce compensatory responses to pathological insults or drug effects, masking the underlying mechanisms that may be specific to either the neuronal or the cardiac tissue. Studies using engineered tissue culture models facilitate tighter control over the composition of the cell types (neuronal or cardiac) allowing for the investigation of tissue-specific responses induced by pathological factors (such as genetic mutations) [6] or drugs. As a result, it is possible to characterize the degree of influence of factors that are normally inaccessible using traditional ex vivo and in vivo techniques. When combined with advances in optical actuation and characterization, monolayer cultures are well suited for long-term, high-throughput investigation [28–31] of neuro-cardiac diseases. Here, we will discuss in vitro tissue culture approaches and recent advances in optical imaging of engineered tissue that could be applied to advance our current understanding of neuro-cardiac physiology and for the pathophysiology of neuro-cardiac diseases, such as CPVT.
Figure 1.
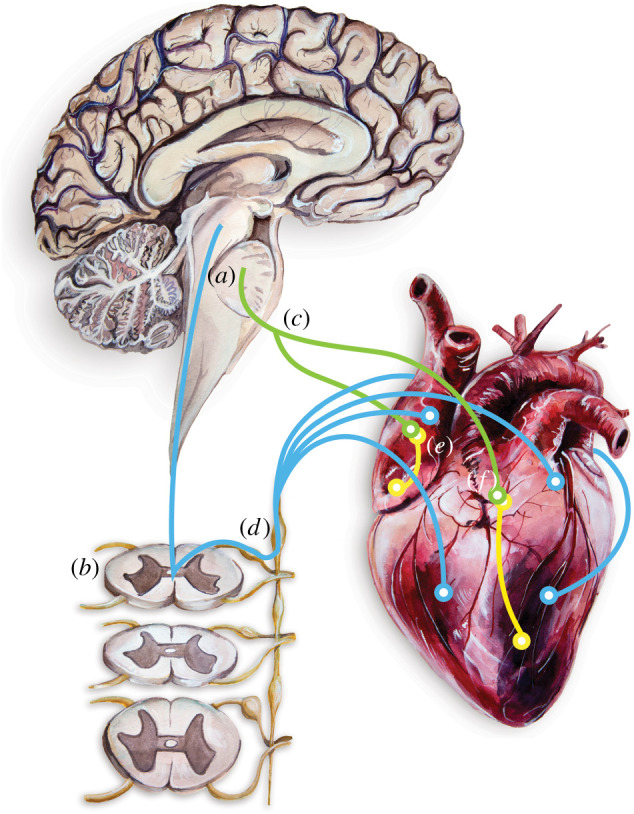
Simplified illustration of the centres of cardiac neurotransmission, where blue and green lines indicate potentially bidirectional pathways of both efferent and afferent communication. The brainstem (a) comprises the main autonomic centres of the CNS, parts of which may be stimulated through spreading depolarization during an epileptic seizure [14]. Neurons in these centres project via the spinal cord (b) or bilateral vagus nerves (c) to cardiac postganglionic neurons in the ganglia of the sympathetic chain (d) or the intrinsic ganglionated plexi on the heart (e), respectively (reviewed by Ashton et al. [15]). Ganglionated plexi neurons are situated predominately in fat deposits on the surface of the heart (basal pale regions (f)), and they innervate the vasculature and tissues of the specialized conduction system and working myocardium. Painting and art work by M. Cremer.
3.1. Tissue engineering
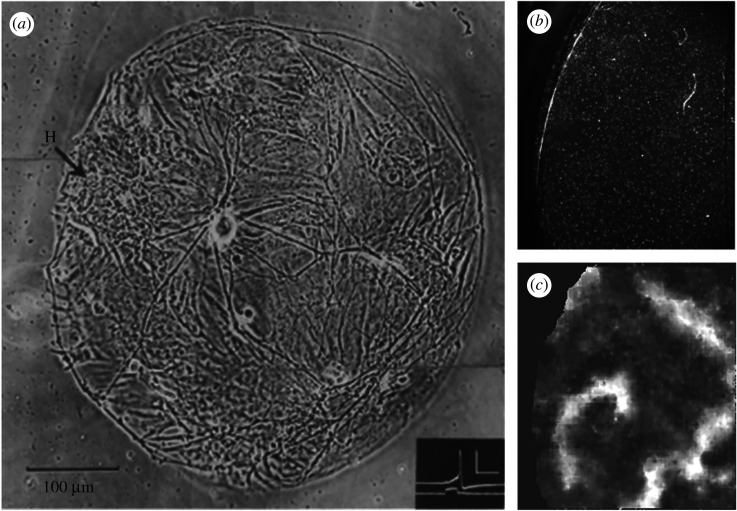
Tissue engineering has proved to be beneficial in understanding neuro-cardiac pathophysiology. Co-culture techniques of neurons and myocytes, as demonstrated in the 1970s by Furshpan et al., allow for cell-level access, providing an intermediate model between mono-culture and the multicellular complexity of whole organ experimentation (figure 2a) [68]. One method for preserving the extracellular matrix architecture and multicellular composition of native tissue is through human organotypic cultured cardiac slices as described by Kang et al. [26]. In mono-culture and co-culture systems, however, cell population composition, density and environment can be determined, thereby controlling factors out of reach in ex vivo and in vivo experiments. Altering the density of cell growth—sparse or confluent cultures—regulates the degree of cellular coupling by gap junctions [69]. For example, a confluent mono-culture of cardiomyocytes supports propagation of excitation waves [27,70] (figure 2b,c).
Figure 2.
(a) Micro co-culture containing a solitary rat sympathetic neuron innervating cardiomyocyte clusters. A single cardiomyocyte cluster is indicated by the arrow at H. The neuron is at 19 days in culture. Inset shows a corresponding action potential recorded from this neuron in response to current injection. Inset scale represents 50 mV (y-axis) and 20 ms (x-axis). (b,c) Reconstruction of the wavefront in a cardiac monolayer. Representative raw image (b) and following background subtraction to reveal waves, a spiral pattern of excitation wavefront is observed (c) from a cardiac monolayer culture using dye-free optical imaging. (a) Reproduced from [68] with permission from Dr Peter R MacLeish, Morehouse School of Medicine, Atlanta, GA, USA, and Dr Paul O'Lague, Department of Molecular, Cell and Developmental Biology, University of California, Los Angeles, USA. (b,c) Adapted from [27] with permission from Dr Rebecca Burton and Prof. Gil Bub, University of Oxford.
Alternatively, the sparse cardiomyocyte and sympathetic neuron cultures used by Furshpan et al. [68] reduced synaptic density, refining the connectivity between cultured cells (figure 2a, [68]). Tissue culture systems also confer precise control of the cellular environment through controlling the media composition and the culture surface. By culturing neurons in media conditioned by the growth of cardiomyocytes, Lockhart et al. [17] verified the importance of cell surface characteristics and hetero-cellular contact on neuron and cardiomyocyte differentiation [17]. The structural anisotropy of native cardiac tissues can be recapitulated through patterning of the culture surface, as shown by several studies [71–73]. Pattern analysis and spatial statistics have demonstrated that traditional neuronal cultures deviate from complete spatial randomness [74]. Patterned culture surfaces change the spatial organization of the somata and dendrites of cultured neurons enabling control of network morphology [74]. Structured patterning of myocytes and neurons offers a new model to address complex questions relating to neuro-cardiac behaviour in models of physiology and pathophysiology where tissue properties are directionally dependent.
3.2. Human-induced pluripotent stem cell tissue engineering approaches
Engineered tissue constructs support complex behaviours and recapitulate structure–function relationships enabling precise measurement of macroscopic behaviour (such as spiral re-entrant waves, figure 2c) [27,70,75–77]. The translational capacity of tissue culture models has improved with the design of anisotropic tissues as well as the use of human-induced pluripotent stem cells (hiPSCs) [54,72,73,78,79]. Species differences between rodents and humans can result in poor translation from in vivo experimental studies to human patients. Moretti et al. [54] and Itzhaki et al. [79] have demonstrated very elegantly the power and utility of induced pluripotent stem cells in recapitulating disease phenotype in LQTS [54,79]. However, the immaturity of the hiPSC phenotype presents challenges to their use for pathophysiological modelling and drug testing that should be taken into consideration in such studies [80–84]. For example, Jonsson et al. [80] have illustrated a lack of functional IK1 potassium current and a shift in the activation threshold for sodium channel (INa) activation in human embryonic stem cell-derived cardiomyocytes which inhibited their ability to model arrhythmia in response to proarrhythmic drugs. The lack of functional IK1 in hiPSCs should be of particular concern for studies aimed at modelling CPVT as mutations in KCNJ2, which encodes the pore-forming subunit of Kir2.1, have been linked to this disease [85]. The dynamic clamp [86] and optical dynamic clamp techniques [84] have, however, been used to simulate IK1 behaviours in hiPSC, thus recapitulating human adult ventricular cardiomyocyte phenotypes and improving their utility for high-throughput screens. Robust functional coupling of hiPSC-derived neurons with target tissues is essential to study intercellular physiology, which has proven difficult to achieve. However, in 2016, Oh et al. [87] successfully demonstrated functional synapses between co-cultured hiPSC-derived sympathetic neurons and rat neonatal ventricular myocytes. While developing consistent hiPSC cardiac cultures with adult phenotypes is challenging [88,89], human tissue culture models of neuro-cardiac diseases provide the potential for high-throughput characterization, improving the translational success of drug research. Challenges remain in developing all human iPSC model systems with accurate adult phenotypes. Some of the problems to solve include the excessive variability observed in differentiated cells which includes discrepancies in genetic, epigenetic and transcriptional features and cardiomyocyte functionality [89].
3.3. Optical techniques in tissue culture
The planar surface topology of tissue culture is well suited to minimally invasive optical techniques, which allow for precision-controlled characterization of neuro-cardiac interactions. The curving surface, degree of movement, tissue thickness and inhomogeneity of in vivo and ex vivo cardiac tissues are challenging for optical characterization techniques [76]. Optical experimental techniques can be divided into two categories: optical actuation and optical characterization.
With optical actuation, light modulates cellular behaviour using techniques such as optogenetics and optically caged compounds [30,31,84,90–94]. Bruegmann et al. [93,94] have illustrated optogenetic actuation of in vitro and in vivo mouse cardiac tissues including defibrillation of ventricular tachycardia. Prando et al. used the spatio-temporal specificity of optogenetic actuation to characterize the dynamics of neuro-cardiac interactions in vivo as well as in vitro [66]. In culture, optogenetic actuation has been used to recover the IK1 current in hiPSC [84] as well as induce and reverse spiral waves [27]. Since the kinetic and pharmacological characteristics of opsins are known to influence cellular behaviour (reviewed by O'Shea et al. [95]), researchers can control for these effects through tandem-cell-unit [28,96] and spatially controlled delivery patterns [97]. In the tandem-cell-unit method, opsins are expressed in non-excitable cells electrically coupled to cardiomyocytes leaving the electrophysiological properties of cardiomyocytes relatively intact [28,96]. Similarly, cell type-specific expression of opsins in sympathetic or parasympathetic neurons allows optical actuation of cardiac tissue by influencing the release of physiologically relevant mediators, such as norepinephrine or acetylcholine [98,99]. Transgene patterning has improved quantitative assessment of cell behaviour by tracking and specifically measuring transduced cells [97].
Optically caged compounds enable reversible localized release of biologically active molecules circumnavigating temporal delays and reducing the diffusion effects associated with washing-in a drug or compound [100]. Caged compounds are biologically active molecules made inert by a photoactive protecting group [91]. The spatial–temporal resolution of caged compounds is determined by the speed of a light pulse and kinetics of caging [91,95]. Typically, caged compounds enable instantaneous modification of intracellular signalling molecules.
Optical characterization techniques, such as fluorescent voltage- and calcium-sensitive dyes as well as Förster resonance energy transfer (FRET), are powerful tools for optically mapping cardiomyocyte physiology. High-resolution FRET imaging quantitatively assesses cellular processes at the resolution of microdomains through fluorescence shifts of a tagged biologically active compound [101]. The reversibility of these optical characterization techniques affords visualization of oscillatory cellular behaviours. For example, cAMP FRET sensors in co-culture have illuminated the structural importance of neuro-cardiac junctional domains [66], as well as potential pathogenesis of hypertension via the dominant role of sympathetic stellate neurons in driving β-adrenergic responses in an animal model of hypertension [6].
Optical mapping of conduction velocity in both whole hearts and engineered cardiac tissues is a useful tool for the study of cardiac arrhythmias, providing the ability to visualize in near real time the conduction of electrical signals. In culture, contact fluorescence imaging uses hexagonally packed optical fibres contacting the glass bottom of a culture dish to record electrically induced and electrically terminated arrhythmias [102]. An all-optical approach to actuation and characterization is possible with appropriate spectral spacing [28,96]. In 2016, Klimas et al. demonstrated an all-optical actuation and high-throughput characterization system called OptoDyCE [28]. However, phototoxicity and bleaching limit the functionality of fluorescence measurements for long-term characterization.
By contrast, dye-free optical imaging (figure 2b,c) circumvents the damaging heat and irradiance effects of fluorescent imaging by recording the rhythmic contractions of cardiomyocytes grown in monolayers using interference patterns generated by the interaction of light from an off-axis, partially coherent light source [27]. Non-invasive phase contrast macro-optics has been shown to help in visualizing the contractile motion of cardiac cells [75]. In 2004, Hwang et al. recorded complex re-entrant arrhythmias in cardiomyocyte mono-cultures by using propagation-induced changes in light intensity in phase contrast images [75]. Once the phase contrast data are processed for intensity variations with time, characteristic images of arrhythmic spiral waves emerge (figure 2c and also in [27,75,103]). Combining optical actuation with dye-free imaging, Burton et al. [27] demonstrated optical control over the chirality, induction and termination of spiral waves with patterned light. Dye-free imaging with optical actuation shows promise as a high-throughput method for co-culture studies and affords long-term monitoring (as opposed to fluorescence) and characterization data that is inaccessible with more traditional fluorescent imaging techniques. Traditional tools such as planar patch clamp systems and microelectrode arrays rely on contact-based interrogation [29]. As demonstrated by the dye-free, optical mapping studies aforementioned, all-optical approaches allow non-contact actuation with the precision needed to control wave patterns, which current electrical and pharmacological methods for wave modulation lack. These methods offer the opportunity to actively interrogate and manipulate cell dynamics in space and time (discussed in this review by Entcheva & Bub [104]). The data generated by this method can be used for analysing simple wave dynamics (for example, to calculate conduction velocity of planar waves). The interpretation of complex spiral waves or wavelet patterns may be hindered by similarities in the optical signals from excitation and relaxation waves (Supplementary fig. 1 in [27]).
4. Discussion and future directions
The complex pathophysiology of neuro-cardiac diseases is dependent upon an array of secretory factors, cell surface characteristics, rhythmic behaviours, connectivity and communication across multiple cell types including cardiomyocytes and neurons. Using techniques such as hetero-cellular tissue culture and micropatterned surfaces, experiments characterizing multisystem physiology can be designed with unprecedented reproducibility, specificity and cellular access. Tissue culture models provide experimental access to model the intricate connections between the PNS and CVS at the cellular level, a degree of access that is not possible using more traditional in vivo and ex vivo techniques. Cardiac tissue monolayers are excitable media that serve as an intermediary between in silico and in vivo experimentation [77,105,106]. Combining engineered tissue models with cellular electrophysiology, optical actuation and optical characterization further enhances the experimental precision accessible to characterize neuro-cardiac physiology and pathophysiology. Advances in combined optical actuation and characterization techniques present an opportunity for long-term, contact-free investigation of neuro-cardiac diseases. Further validation of tissue culture models and optical techniques present an opportunity for low-cost, high-throughput testing of therapeutics for complex neuro-cardiac diseases.
Supplementary Material
Acknowledgements
We thank Prof. Neil Herring, University of Oxford for comments and suggestions relating to figure 1.
Data accessibility
This article has no additional data.
Authors' contributions
R.A.B.B. initiated the review topic. R.A.B.B., C.S. and M.C. designed the review. M.C. painted the art work in figure 1. All authors contributed to the writing, editing and approval of the manuscript.
Competing interests
The authors have nothing to declare.
Funding
This work was supported by a Colin Pillinger Royal Society International Exchange Award (IE160164). R.A.B.B. is funded by a Sir Henry Dale Wellcome Trust and Royal Society Fellowship (grant no. 109371/Z/15/Z) and acknowledges support from The Returning Carers’ Fund, Medical Sciences Division, University of Oxford. R.A.B.B. is a Senior Research Fellow at Linacre College. S.J.B. is funded by a British Heart Foundation Project Grant (PG/18/4/33521). C.S. is funded by the Blaschko Trust (Department of Pharmacology and Linacre College, University of Oxford). C.S. is a Junior Research Fellow at Linacre College. J.M.M. acknowledges support from the Freemasons Foundation, Marsden Fund (18-UoA-100), and Auckland Medical Research Foundation (1118003). A.W. is funded by the Hugh Green Foundation, Cure Kids and the Auckland Medical Research Foundation. G.B. acknowledges support from the Canadian Heart and Stroke Foundation.
References
- 1.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. 2015. Cardiac innervation and sudden cardiac death. Circ. Res. 116, 2005–2019. ( 10.1161/CIRCRESAHA.116.304679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehnart SE, et al. 2008. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J. Clin. Invest. 118, 2230–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. 2009. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci. Transl. Med. 1, 1–9. ( 10.1126/scitranslmed.3000289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AAM, Ackerman MJ. 2009. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 72, 224–231. ( 10.1212/01.wnl.0000335760.02995.ca) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Sirenko S, Juhaszova M, Sollott SJ, Shukla S, Yaniv Y, Lakatta EG. 2014. Age-associated abnormalities of intrinsic automaticity of sinoatrial nodal cells are linked to deficient cAMP-PKA-Ca 2+ signaling. Am. J. Physiol. Circ. Physiol. 306, H1385–H1397. ( 10.1152/ajpheart.00088.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen HE, Lefkimmiatis K, Paterson DJ. 2016. Sympathetic neurons are a powerful driver of myocyte function in cardiovascular disease. Sci. Rep. 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nei M, Ho RT, Sperling MR. 2000. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 41, 542–548. ( 10.1111/j.1528-1157.2000.tb00207.x) [DOI] [PubMed] [Google Scholar]
- 8.Tigaran S, Mølgaard H, Dam M. 2002. Atrio-ventricular block: a possible explanation of sudden unexpected death in epilepsy. Acta Neurol. Scand. 106, 229–233. ( 10.1034/j.1600-0404.2002.02017.x) [DOI] [PubMed] [Google Scholar]
- 9.Leutmezer F, Schernthaner C, Lurger S, Pötzelberger K, Baumgartner C. 2003. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia 44, 348–354. ( 10.1046/j.1528-1157.2003.34702.x) [DOI] [PubMed] [Google Scholar]
- 10.Duan H, Lu Y, Yan S, Qiao L, Hua Y, Li Y, Zhou K, Wang C. 2018. A delayed diagnosis of catecholaminergic polymorphic ventricular tachycardia with a mutant of RYR2 at c.7580T>G for 6 years in a 9-year-old child. Medicine (United States) 97, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ. 2014. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat. Rev. Cardiol. 11, 346–353. ( 10.1038/nrcardio.2014.19) [DOI] [PubMed] [Google Scholar]
- 12.Cao JM, et al. 2000. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101, 1960–1969. ( 10.1161/01.CIR.101.16.1960) [DOI] [PubMed] [Google Scholar]
- 13.Shcherbakova OG, Hurt CM, Xiang Y, Dell'Acqua ML, Zhang Q, Tsien RW, Kobilka BK. 2007. Organization of β-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J. Cell Biol. 176, 521–533. ( 10.1083/jcb.200604167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiba I, Wehrens XHT, Noebels JL. 2016. Leaky RyR2 channels unleash a brainstem spreading depolarization mechanism of sudden cardiac death. Proc. Natl Acad. Sci. USA 113, E4895–E4903. ( 10.1073/pnas.1605216113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashton JL, Burton RAB, Bub G, Smaill BH, Montgomery JM. 2018. Synaptic plasticity in cardiac innervation and its potential role in atrial fibrillation. Front. Physiol. 9, 1–9. ( 10.3389/fphys.2018.00240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa S, Barnett JV, Sen L, Galper JB, Smith TW, Marsh JOD. 1992. Direct contact between sympathetic neurons and rat cardiac myocytes in vitro increases expression of functional calcium channels. J. Clin. Invest. 89, 1085–1093. ( 10.1172/JCI115688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart ST, Mead JN, Pisano JM, Slonimsky JD, Birren SJ. 2000. Nerve growth factor collaborates with myocyte-derived factors to promote development of presynaptic sites in cultured sympathetic neurons. J. Neurobiol. 42, 460–476. () [DOI] [PubMed] [Google Scholar]
- 18.Mias C, et al. 2013. Cardiac fibroblasts regulate sympathetic nerve sprouting and neurocardiac synapse stability. PLoS ONE 8, e79068 ( 10.1371/journal.pone.0079068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamon D, et al. 2017. Premature ventricular contraction coupling interval variability destabilizes cardiac neuronal and electrophysiological control. Circ. Arrhythmia Electrophysiol. 10, 1–12. ( 10.1161/CIRCEP.116.004937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loaiza R, Benkusky NA, Powers PP, Hacker T, Noujaim S, Ackerman MJ, Jalife J, Valdivia HH. 2013. Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 112, 298–308. ( 10.1161/CIRCRESAHA.112.274803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biet M, Morin N, Lessard-Beaudoin M, Graham RK, Duss S, Gagné J, Sanon NT, Carmant L, Dumaine R. 2015. Prolongation of action potential duration and QT interval during epilepsy linked to increased contribution of neuronal sodium channels to cardiac late Na+ current. Circ. Arrhythmia Electrophysiol. 8, 912–920. ( 10.1161/CIRCEP.114.002693) [DOI] [PubMed] [Google Scholar]
- 22.Anderson JH, Bos JM, Cascino GD, Ackerman MJ. 2014. Prevalence and spectrum of electroencephalogram-identified epileptiform activity among patients with long QT syndrome. Heart Rhythm 11, 53–57. ( 10.1016/j.hrthm.2013.10.010) [DOI] [PubMed] [Google Scholar]
- 23.Bagnall RD, et al. 2016. Exome-based analysis of cardiac arrhythmia, respiratory control, epilepsy genes in sudden unexpected death in epilepsy. Ann. Neurol. 79, 522–534. ( 10.1002/ana.24596) [DOI] [PubMed] [Google Scholar]
- 24.Danielsen TK, et al. 2018. Arrhythmia initiation in catecholaminergic polymorphic ventricular tachycardia type 1 depends on both heart rate and sympathetic stimulation. PLoS ONE 13, e0207100 ( 10.1371/journal.pone.0207100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young CN, Davisson RL. 2011. In vivo assessment of neurocardiovascular regulation in the mouse: principals, progress, and prospects. Am. J. Physiol. Heart Circ. Physiol. 301, H654–H662. ( 10.1152/ajpheart.00355.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang C, Qiao Y, Li G, Baechle K, Camelliti P, Rentschler S, Efimov IR. 2016. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials. Sci. Rep. 6, 1–13. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton RAB, Klimas A, Ambrosi CM, Tomek J, Corbett A, Entcheva E, Bub G. 2015. Optical control of excitation waves in cardiac tissue. Nat. Photonics 9, 813–816. ( 10.1038/nphoton.2015.196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klimas A, Ambrosi CM, Yu J, Williams JC, Bien H, Entcheva E. 2016. OptoDyCE as an automated system for high-throughput all-optical dynamic cardiac electrophysiology. Nat. Commun. 7, 1–12. ( 10.1038/ncomms11542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimas A, Ortiz G, Boggess SC, Miller EW, Entcheva E. 2019. Multimodal on-axis platform for all-optical electrophysiology with near-infrared probes in human stem-cell-derived cardiomyocytes. Prog. Biophys. Mol. Biol. 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karathanos TV, Boyle PM, Trayanova NA. 2016. Light-based approaches to cardiac arrhythmia research: from basic science to translational applications. Clin. Med. Insights Cardiol. 10s1, 47–60. ( 10.4137/CMC.S39711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasse P, Funken M, Beiert T, Bruegmann T. 2019. Optogenetic termination of cardiac arrhythmia: mechanistic enlightenment and therapeutic application? Front. Physiol. 10, 675 ( 10.3389/fphys.2019.00675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balke C, Shorofsky SR. 1998. Alterations in calcium handling in cardiac hypertrophy and heart failure. Cardiovasc. Res. 37, 290–299. ( 10.1016/S0008-6363(97)00272-1) [DOI] [PubMed] [Google Scholar]
- 33.Heineke J, Molkentin JD. 2006. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 7, 589–600. ( 10.1038/nrm1983) [DOI] [PubMed] [Google Scholar]
- 34.Xiao YF, McArdle JJ. 1994. Elevated density and altered pharmacologic properties of myocardial calcium current of the spontaneously hypertensive rat. J. Hypertens. 12, 783–790. [PubMed] [Google Scholar]
- 35.Heaton DA, Lei M, Li D, Golding S, Dawson TA, Mohan RM, Paterson DJ. 2006. Remodeling of the cardiac pacemaker L-type calcium current and its β-adrenergic responsiveness in hypertension after neuronal NO synthase gene transfer. Hypertension 48, 443–452. ( 10.1161/01.HYP.0000233383.04280.3c) [DOI] [PubMed] [Google Scholar]
- 36.Priori SG, Chen SRW. 2011. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 108, 871–883. ( 10.1161/CIRCRESAHA.110.226845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Matthews GDK, Lei M, Huang CLH. 2013. Abnormal Ca2+ homeostasis, atrial arrhythmogenesis, and sinus node dysfunction in murine hearts modeling RyR2 modification. Front. Physiol. 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin G, Hassan F, Haroun AR, Murphy LL, Crotti L, Schwartz PJ, George AL, Satin J. 2014. Arrhythmogenic calmodulin mutations disrupt intracellular cardiomyocyte Ca2+ regulation by distinct mechanisms. J. Am. Heart Assoc. 3, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bliss TVP, Lomo T. 1973. long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. ( 10.1113/jphysiol.1973.sp010273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudek SM, Bear MF. 1992. Homosynaptic long-term depression in area CAl of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Neurobiology 89, 4363–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alkadhi KA, Alzoubi KH, Aleisa AM. 2005. Plasticity of synaptic transmission in autonomic ganglia. Prog. Neurobiol. 75, 83–108. ( 10.1016/j.pneurobio.2005.02.002) [DOI] [PubMed] [Google Scholar]
- 42.Alkadhi KA, Al-Hijailan RS, Alzoubi KH. 2008. Long-term depression in the superior cervical ganglion of the rat. Brain Res. 1234, 25–31. ( 10.1016/j.brainres.2008.07.112) [DOI] [PubMed] [Google Scholar]
- 43.Lahat H, et al. 2001. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Ann. Med. 69, 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. 2001. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200. ( 10.1161/01.CIR.103.2.196) [DOI] [PubMed] [Google Scholar]
- 45.Leenhardt A, Denjoy I, Guicheney P. 2012. Catecholaminergic polymorphic ventricular tachycardia. Circ. Arrhythmia Electrophysiol. 5, 1044–1052. ( 10.1161/CIRCEP.111.962027) [DOI] [PubMed] [Google Scholar]
- 46.Sumitomo N. 2016. Current topics in catecholaminergic polymorphic ventricular tachycardia. J. Arrhythmia 32, 344–351. ( 10.1016/j.joa.2015.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skinner JR, Winbo A, Abrams D, Vohra J, Wilde AA. 2019. Channelopathies that lead to sudden cardiac death: clinical and genetic aspects. Heart Lung Circ. 28, 22–30. ( 10.1016/j.hlc.2018.09.007) [DOI] [PubMed] [Google Scholar]
- 48.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. 1995. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow-up of 21 patients. Circulation 91, 1512–1519. ( 10.1161/01.CIR.91.5.1512) [DOI] [PubMed] [Google Scholar]
- 49.Swan H, et al. 1999. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J. Am. Coll. Cardiol. 34, 2035–2042. ( 10.1016/S0735-1097(99)00461-1) [DOI] [PubMed] [Google Scholar]
- 50.Bers DM. 2002. Cardiac excitation-contraction coupling. Nature 415, 198–205. ( 10.1038/415198a) [DOI] [PubMed] [Google Scholar]
- 51.Peng W, et al. 2016. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 354, aah5324 ( 10.1126/science.aah5324) [DOI] [PubMed] [Google Scholar]
- 52.Liu N, et al. 2006. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ. Res. 99, 292–298. ( 10.1161/01.RES.0000235869.50747.e1) [DOI] [PubMed] [Google Scholar]
- 53.Yap SM, Smyth S. 2019. Ryanodine receptor 2 (RYR2) mutation: a potentially novel neurocardiac calcium channelopathy manifesting as primary generalised epilepsy. Seizure 67, 11–14. ( 10.1016/j.seizure.2019.02.017) [DOI] [PubMed] [Google Scholar]
- 54.Moretti A, et al. 2010. Patient-specific induced pluripotent stem-cell models for Long-QT syndrome. N. Engl. J. Med. 363, 1397–1409. ( 10.1056/NEJMoa0908679) [DOI] [PubMed] [Google Scholar]
- 55.Winbo A, Fosdal I, Lindh M, Diamant UB, Persson J, Wettrell G, Rydberg A. 2015. Third trimester fetal heart rate predicts phenotype and mutation burden in the type 1 long qt syndrome. Circ. Arrhythmia Electrophysiol. 8, 806–814. ( 10.1161/CIRCEP.114.002552) [DOI] [PubMed] [Google Scholar]
- 56.Winbo A, Stattin EL, Diamant UB, Persson J, Jensen SM, Rydberg A. 2012. Prevalence, mutation spectrum, cardiac phenotype of the Jervell and Lange-Nielsen syndrome in Sweden. Europace 14, 1799–1806. ( 10.1093/europace/eus111) [DOI] [PubMed] [Google Scholar]
- 57.Wilde AAM, et al. 2008. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N. Engl. J. Med. 358, 2024–2029. ( 10.1056/NEJMoa0708006) [DOI] [PubMed] [Google Scholar]
- 58.Collura CA, Johnson JN, Moir C, Ackerman MJ. 2009. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm 6, 752–759. ( 10.1016/j.hrthm.2009.03.024) [DOI] [PubMed] [Google Scholar]
- 59.De Ferrari GM, et al. 2015. Clinical management of catecholaminergic polymorphic ventricular tachycardia the role of left cardiac sympathetic denervation. Circulation 131, 2185–2193. ( 10.1161/CIRCULATIONAHA.115.015731) [DOI] [PubMed] [Google Scholar]
- 60.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. 2008. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 118, 2754–2762. ( 10.1161/CIRCULATIONAHA.108.767012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akashi YJ, Nef HM, Mollmann H, Ueyama T. 2010. Stress cardiomyopathy. Annu. Rev. Med. 61, 271–286. ( 10.1146/annurev.med.041908.191750) [DOI] [PubMed] [Google Scholar]
- 62.Paur H, et al. 2012. High levels of circulating epinehrine trigger apical cardiodepression in a beta(2)-adrenergic receptor/G(i)-dependent manner a new model of Takotsubo cardiomyopathy. Circulation 126(6): 697–706. ( 10.1161/CIRCULATIONAHA.112.111591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Land S, et al. 2014. Computational modeling of Takotsubo cardiomyopathy: effect of spatially varying beta-adrenergic stimulation in the rat left ventricle. Am. J. Physiol. Heart Circ. Physiol. 307, H1487–H1496. ( 10.1152/ajpheart.00443.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaccaro A, et al. 2014. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS ONE 9, e93278 ( 10.1371/journal.pone.0093278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Habecker BA, et al. 2016. Molecular and cellular neurocardiology: development, cellular and molecular adaptations to heart disease. J. Physiol. 594, 3853–3875. ( 10.1113/JP271840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prando V, et al. 2018. Dynamics of neuroeffector coupling at cardiac sympathetic synapses. J. Physiol. 596, 2055–2075. ( 10.1113/JP275693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herring N, Kalla M, Paterson DJ. 2019. The autonomic nervous system and cardiac arrhythmias: current concepts and emerging therapies. Nat. Rev. Cardiol. 16, 707–726. ( 10.1038/s41569-019-0221-2) [DOI] [PubMed] [Google Scholar]
- 68.Furshpan EJ, MacLeish PR, O'Lague PH, Potter DD. 1976. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, dual-function neurons. Proc. Natl Acad. Sci. USA 73, 4225–4229. ( 10.1073/pnas.73.11.4225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. 2007. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys. J. 92, 4121–4132. ( 10.1529/biophysj.106.101410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang SM, Kim TY, Lee KJ. 2005. Complex-periodic spiral waves in confluent cardiac cell cultures induced by localized inhomogeneities. Proc. Natl Acad. Sci. USA 102, 10 363–10 368. ( 10.1073/pnas.0501539102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bursac N, Parker KK, Iravanian S, Tung L. 2002. Cardiomyocyte cultures with controlled macroscopic anisotropy. Circ. Res. 91, 1–9. ( 10.1161/01.RES.0000047530.88338.EB) [DOI] [PubMed] [Google Scholar]
- 72.Camelliti P, Gallagher JO, Kohl P, McCulloch AD. 2006. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat. Protoc. 1, 1379–1391. ( 10.1038/nprot.2006.203) [DOI] [PubMed] [Google Scholar]
- 73.Badie N, Scull JA, Klinger RY, Krol A, Bursac N. 2012. Conduction block in micropatterned cardiomyocyte cultures replicating the structure of ventricular cross-sections. Cardiovasc. Res. 93, 263–271. ( 10.1093/cvr/cvr304) [DOI] [PubMed] [Google Scholar]
- 74.Millet LJ, Collens MB, Perry GLW, Bashir R. 2011. Pattern analysis and spatial distribution of neurons in culture. Integr. Biol. 3, 1167–1178. ( 10.1039/c1ib00054c) [DOI] [PubMed] [Google Scholar]
- 75.Hwang S, Yea K, Lee KJ. 2004. Regular and alternant spiral waves of contractile motion on rat ventricle cell cultures. Phys. Rev. Lett. 92, 1–4. ( 10.1103/PhysRevLett.92.198103) [DOI] [PubMed] [Google Scholar]
- 76.Tung L, Zhang Y. 2006. Optical imaging of arrhythmias in tissue culture. J. Electrocardiol. 39, 2–6. ( 10.1016/j.jelectrocard.2006.04.010) [DOI] [PubMed] [Google Scholar]
- 77.Bub G, Burton RAB. 2015. Macro-micro imaging of cardiac-neural circuits in co-cultures from normal and diseased hearts. J. Physiol. 593, 3047–3053. ( 10.1113/jphysiol.2014.285460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badie N, Bursac N. 2009. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys. J. 96, 3873–3885. ( 10.1016/j.bpj.2009.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Itzhaki I, et al. 2011. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471, 225–230. ( 10.1038/nature09747) [DOI] [PubMed] [Google Scholar]
- 80.Jonsson MKB, Vos MA, Mirams GR, Duker G, Sartipy P, de Boer TP, van Veen TAB. 2012. Application of human stem cell-derived cardiomyocytes in safety pharmacology requires caution beyond hERG. J. Mol. Cell. Cardiol. 52, 998–1008. ( 10.1016/j.yjmcc.2012.02.002) [DOI] [PubMed] [Google Scholar]
- 81.Gintant G, Fermini B, Stockbridge N, Strauss D. 2017. The evolving roles of human iPSC-derived cardiomyocytes in drug safety and discovery. Cell Stem Cell 21, 14–17. ( 10.1016/j.stem.2017.06.005) [DOI] [PubMed] [Google Scholar]
- 82.Sala L, Bellin M, Mummery CL. 2017. Integrating cardiomyocytes from human pluripotent stem cells in safety pharmacology: has the time come? Br. J. Pharmacol. 174, 3749–3765. ( 10.1111/bph.13577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goversen B, van der Heyden MAG, van Veen TAB, de Boer TP (2018). The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: special focus on IK1. Pharmacol. Ther. 183, 127–136. ( 10.1016/j.pharmthera.2017.10.001) [DOI] [PubMed] [Google Scholar]
- 84.Quach B, Krogh-Madsen T, Entcheva E, Christini DJ. 2018. Light-activated dynamic clamp using iPSC-derived cardiomyocytes. Biophys. J. 115, 2206–2217. ( 10.1016/j.bpj.2018.10.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vega AL, Tester DJ, Ackerman MJ, Makielski JC. 2009. Protein kinase A-dependent biophysical phenotype for V227F-KCNJ2 mutation in catecholaminergic polymorphic ventricular tachycardia. Circ. Arrythm Eectrophysiol. 2, 540–547. ( 10.1161/CIRCEP.109.872309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goversen B, Becker N, Stoelzle-Feix S, Obergrussberger A, Vos MA, Veen TAB, van Fertig N, Boer TP. 2018. A hybrid model for safety pharmacology on an automated patch clamp platform: using dynamic clamp to join iPSC-derived cardiomyocytes and simulations of Ik1 ion channels in real-time. Front. Physiol. 8, 1–10. ( 10.3389/fphys.2017.01094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh Y, Cho G-S, Li Z, Dong X, Kwon C, Lee G. 2016. Functional coupling with cardiac muscle promotes maturation of hPSC-derived sympathetic neurons. Cell Stem Cell 19, 95–106. ( 10.1016/j.stem.2016.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tu C, Chao BS, Wu JC. 2018. Strategies for improving the maturity of human induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 123, 512–514. ( 10.1161/CIRCRESAHA.118.313472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biendarra-Tiegs SM, Secreto FJ, Nelson TJ. 2020. Addressing variability and heterogeneity of induced pluripotent stem cell-derived cardiomyocytes. In Advances in experimental medicine and biology (ed. Turksen K.), pp 1–29. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 90.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. 2005. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268. ( 10.1038/nn1525) [DOI] [PubMed] [Google Scholar]
- 91.Ellis-Davies GCR. 2007. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods 4, 619–628. ( 10.1038/nmeth1072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miesenböck G. 2009. The optogenetic catechism. Science 326, 395–399. ( 10.1126/science.1174520) [DOI] [PubMed] [Google Scholar]
- 93.Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, Sasse P. 2010. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 7, 897–900. ( 10.1038/nmeth.1512) [DOI] [PubMed] [Google Scholar]
- 94.Bruegmann T, Boyle PM, Vogt CC, Karathanos TV, Arevalo HJ, Fleischmann BK, Trayanova NA, Sasse P. 2016. Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations. J. Clin. Invest. 126, 3894–3904. ( 10.1172/JCI88950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Shea C, et al. 2019. Cardiac optogenetics and optical mapping—overcoming spectral congestion in all-optical cardiac electrophysiology. Front. Physiol. 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jia Z, et al. 2011. Stimulating cardiac muscle by light cardiac optogenetics by cell delivery. Circ. Arrhythmia Electrophysiol. 4, 753–760. ( 10.1161/CIRCEP.111.964247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ambrosi CM, Boyle PM, Chen K, Trayanova NA, Entcheva E. 2015. Optogenetics-enabled assessment of viral gene and cell therapy for restoration of cardiac excitability. Sci. Rep. 5, 1–16. ( 10.1038/srep17350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wengrowski AM, Wang X, Tapa S, Posnack NG, Mendelowitz D, Kay MW. 2015. Optogenetic release of norepinephrine from cardiac sympathetic neurons alters mechanical and electrical function. Cardiovasc. Res. 105, 143–150. ( 10.1093/cvr/cvu258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moreno A, Endicott K, Skancke M, Dwyer MK, Brennan J, Efimov IR, Trachiotis G, Mendelowitz D, Kay MW. 2019. Sudden heart rate reduction upon optogenetic release of acetylcholine from cardiac parasympathetic neurons in perfused hearts. Front. Physiol. 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filevich O, Salierno M, Etchenique R. 2010. A caged nicotine with nanosecond range kinetics and visible light sensitivity. J. Inorg. Biochem. 104, 1248–1251. ( 10.1016/j.jinorgbio.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 101.Zaccolo M, de Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. 2000. A genetically encoded, flourescent indicator for cyclic AMP in living cells. Nat. Cell Biol. 2, 25–29. ( 10.1038/71345) [DOI] [PubMed] [Google Scholar]
- 102.Entcheva E, Lu SN, Troppman RH, Sharma V, Tung L. 2000. Contact fluorescence imaging of reentry in monolayers of cultured neonatal rat ventricular myocytes. J. Cardiovasc. Electrophysiol. 11, 665–676. ( 10.1111/j.1540-8167.2000.tb00029.x) [DOI] [PubMed] [Google Scholar]
- 103.Tomek J, Burton RAB, Bub G. 2016. Ccoffinn: automated wave tracking in cultured cardiac monolayers. Biophys. J. 111, 1595–1599. ( 10.1016/j.bpj.2016.08.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Entcheva E, Bub G. 2016. All-optical control of cardiac excitation: combined high-resolution optogenetic actuation and optical mapping. J. Physiol. 594, 2503–2510. ( 10.1113/JP271559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hayakawa T, Kunihiro T, Ando T, Kobayashi S, Matsui E, Yada H, Kanda Y, Kurokawa J, Furukawa T. 2014. Image-based evaluation of contraction-relaxation kinetics of human-induced pluripotent stem cell-derived cardiomyocytes: correlation and complementarity with extracellular electrophysiology. J. Mol. Cell. Cardiol. 77, 178–191. ( 10.1016/j.yjmcc.2014.09.010) [DOI] [PubMed] [Google Scholar]
- 106.Paci M, Pölönen RP, Cori D, Penttinen K, Aalto-Setälä K, Severi S, Hyttinen J. 2018. Automatic optimization of an in silico model of human iPSC derived cardiomyocytes recapitulating calcium handling abnormalities. Front. Physiol. 9, 1–14. ( 10.3389/fphys.2018.00709) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.