Abstract
Background
Osteoarthritis (OA) is one of the most prevalent and degenerative diseases with complicated pathology including articular cartilage degradation, subchondral sclerosis and synovitis. Chondrocytes play a crucial role in maintaining cartilage integrity.
Methods
Primary chondrocytes were treated with 10 ng/mL IL-1β alone, or pre-treated with 20 μM baicalin for 5 h followed by co-treatment with 20 μM baicalin and 10 ng/mL IL-1β. CCK-8 assay was used to assess cell viability, and cell apoptosis was analyzed by both PI/FITC-Annexin V staining and quantitating apoptosis-related Bcl-2, Bax and cleaved-caspase-3 expression at both protein and mRNA level by Western blotting and qRT-PCR, respectively. Chondrocytes were transfected with miRNA-766-3p mimic and autophagy flux was examined by LC3, Beclin and p62 Western blotting and by Cyto-ID assay to quantify autophagic vacuoles.
Results
Baicalin treatment decreased the apoptosis rate and the expressions of pro-apoptotic proteins induced by IL-1β, up-regulated anti-apoptotic Bcl-2 expression, and inhibited the degradation of ECM. Baicalin increased autophagy through up-regulating the autophagy markers Beclin-1 expression and LC3 Ⅱ/LC3 Ⅰ ratio and promoting autophagic flux. Contrarily, autophagy inhibition partially alleviated the beneficial effects of baicalin on ECM synthesis and anti-apoptosis in the chondrocytes treated with L-1β. Furthermore, the differential expressional profiles of miR-766-3p and apoptosis-inducing factor mitochondria-associated 1 (AIFM1) were determined in IL-1β and IL-1β + baicalin-treated chondrocytes, and we confirmed AIFM1 was a target of miR-766-3p. MiR-766-3p overexpression suppressed apoptosis and facilitated autophagy and ECM synthesis in the chondrocytes through decreasing AIFM1. Contrarily, silencing of miR-766-3p inhibited chondrocyte autophagy and promoted apoptosis, and this effect could be reversed by AIFM1 silence.
Conclusion
Baicalin protects human OA chondrocytes against IL-1β-induced apoptosis and the degradation of ECM through activating autophagy via miR-766-3p/AIFM1 axis and serves as a potential therapeutic candidate for OA treatment.
Keywords: osteoarthritis, apoptosis-inducing factor mitochondria-associated 1, baicalin, apoptosis
Introduction
Osteoarthritis (OA), a degenerative joint disease with high morbidity and disability, is characterized by consistent cartilage degradation, subchondral sclerosis and synovitis. OA has severe impacts on the health and life quality of the patients and brings heavy socioeconomic burden around the world. Chondrocytes, the sole cell type in cartilage, play a role in both biosynthesis and extracellular matrix (ECM) turnover.1,2 Thus, maintaining chondrocytes in a homeostatic condition is of great importance to maintain cartilage integrity.3 Autophagy is a cellular homeostasis mechanism through degrading and recycling of cytoplasmic components and organelles under the detrimental stress such as hypoxia, injury and nutrient starvation.4–6 Autophagy is involved in the development of multiple disorders such as hepatic fibrosis,7 human neurodegenerative diseases,8,9 muscle atrophy10 and cancers.11,12 Autophagy is also indispensable to maintain the structural and functional integrity of articular cartilage. Deletion of autophagy suppressor mTOR in cartilage promotes chondrocyte autophagy and alleviates aging-induced OA in mice.13 Similarly, autophagy activator rapamycin protects chondrocytes from glucocorticoid-induced apoptosis in OA cartilage.14
MicroRNAs (miRNAs), a family of multifunctional small non-coding RNAs, regulate gene expression by targeting the 3′ untranslated region of mRNAs. MiRNAs play pivotal roles in multiple biological and pathological processes including cancer development, inflammatory diseases and neurological diseases. Several studies have indicated that some microRNA pathways were associated with OA progression via promoting or suppressing autophagy of chondrocytes. For example, miR-155 was found to decrease autophagy in primary human articular chondrocytes.15 MiR-128a was reported to suppress chondrocyte autophagy to aggravate OA progression via inducing Atg2 deregulation.16 Conversely, miR-27a expression level decreased in both OA cartilage and IL-1β-stimulated articular chondrocytes, and IL-1β-induced apoptosis of articular chondrocytes could be rescued by miR-27a inhibition.17 MiR-766-3p has been reported to function as an inhibitor of tumor progression18 and to suppress cancer cell metastasis.19 This miRNA also promoted tumor cell proliferation.20,21 In addition, miR-766-3p exerts anti-inflammatory function in human rheumatoid arthritis (RA) fibroblast-like synoviocytes,22 however, the effect of miR-766-3p in OA development has not been reported.
Baicalin, a principal flavonoid mainly extracted from roots of Scutellaria baicalensis Georgi, has widely biological effects such as anti-inflammation,23 anti-oxidant,24 anti-cancer,25,26 and anti-viral activities.27 Moreover, several literatures demonstrated that baicalin also played a role in ameliorating OA progression.28,29
In the current study, we therefore aimed to investigate the effects of baicalin on articular chondrocyte biological behaviors including autophagy and OA development. The regulation of baicalin on miR-766-3p was also evaluated. The mechanism of this process has guiding significance in providing experimental basis for clinical application of baicalin in the treatment of OA and contributing to the exploitation and utilization of baicalin.
Materials and Methods
Cell Isolation, Cultivation and Treatment
Primary culture of human chondrocytes was obtained from the articular cartilages of 12 adult OA patients (5 men and 7 women; age ranged 55–72 years) who underwent total knee arthroplasty in The First Affiliated Hospital of Nanjing Medical University. This study was approved by the institutional ethics committee of The First Affiliated Hospital of Nanjing Medical University and all participants signed the informed consents. The use of the human tissues in this study was in accordance with the Helsinki Declaration of 1975 and methods involved in this study were performed according to the approved guidelines and regulations.
For chondrocytes isolation and cultivation, human articular cartilages were minced and digested with collagenase II (0.2%) in Dulbecco Modified Eagle Medium (DMEM) at 37°C for 6 h. The cells were filtered through a 40-µm filter. After centrifugation, chondrocytes were suspended and maintained in DMEM containing 10% FBS.
The chondrocytes were treated with 10 ng/mL IL-1β for 12 h, or pretreated with 20 μM baicalin or 100 nM rapamycin for 4 h and then co-treated with 10 ng/mL IL-1β and 20 μM baicalin or 100 nM rapamycin for 12 h.
Cell Transfection
The miRNA-766-3p mimic, negative control oligonucleotides (mimic NC), miRNA-766-3p inhibitor was purchased from RiboBio (Guangzhou, China), and AIFM1 pcDNA3.1 vector (AIFM1) or empty vector (vector), small interfering RNA of AIFM1 (si AIFM1) and scramble siRNA of AIFM1 (siRNA NC) were provided by GenePharma (Shanghai, China). Second-generation human OA chondrocytes were incubated for 24 h, and transfection was performed according to manufacturer’s instruction.
Cell Viability Analysis
Cell viability was determined by using cell-counting kit-8 assay (CCK-8) following manufacture’s instruction. Briefly, after cultured in 96-well plate (5 × 103 cells/well) for 24 h, human OA chondrocytes were stimulated with 10 ng/mL IL-1β alone for 12 h, or pretreated with 20 μM baicalin or 100 nM rapamycin for 4 h and then co-treated with 10 ng/mL IL-1β and 20 μM baicalin or 100 nM rapamycin for 12 h. CCK-8 solution was then added for 1 h at 37 °C. The absorbance was read by a Microplate Reader.
Cell Apoptosis Analysis
To determine cell apoptosis, the cells were stained with PI/FITC-Annexin V (Sigma, St Louis, MO) for 1 h at room temperature in the dark condition after different treatments. Flow cytometry analysis was conducted by using a FACScan (Beckman Coulter, USA).
Autophagy Flux Detection
Cyto-ID assay (Enzo Life Sciences, USA) was used to detect autophagic vacuoles. Briefly, OA chondrocytes in DMEM were plated in six-well plates and then incubated with fluorescent dye Cyto-ID and Hoechst 33342 at 37 °C for 30 min. After incubation, fluorescence was measured and relative Cyto-ID intensity was calculated.
Western Blotting
Total protein was extracted from cultured cells using radioimmunoprecipitation (RIPA) lysis solution and protein concentration was assessed. Equal amount of proteins were separated by SDS-PAGE, and then transferred onto PVDF membranes. After being blocked by 5% non-fat milk in 0.1%TBST (Tris-buffered saline), the membranes were incubated with primary antibodies overnight at 4 °C followed by incubation with HRP-conjugated secondary antibodies for 1 h. Protein signal was detected using an enhanced chemiluminescence (ECL) kit. GAPDH was used as an internal control to normalize protein levels. The primary antibodies used in this study include Bcl-2 (Abcam, Cambridge, MA, USA), BAX (Abcam), Pro-caspase-3 (Abcam), cleaved-caspase-3 (Abcam), ACAN (Santa Cruz, USA), COL2A (Santa Cruz), MMP13 (Santa Cruz), LC3 (Proteintech, Chicago, IL, USA), Beclin-1 (Proteintech), p-62 (Proteintech) and GAPDH (Santa Cruz).
qRT-PCR
Total RNA from cultured chondrocytes was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) and concentration was measured. mRNA was reversely transcribed to cDNA using retroviruses PrimerScriptTM kit. A miRNA-specific TaqManTM MicroRNA kit was used for miRNA detection. Quantitative PCR was performed using FastStart Universal SYBR Green master mix. U6 and GAPDH were used as internal references to normalize miRNA and mRNA levels, respectively. Target gene expressions were calculated using the 2−ΔΔCt method.
Luciferase Reporter Assay
The chondrocytes were co-transfected with pmiR-RB-REPORT vector (RiboBio) containing mutant or wild type 3′-UTR of AIFM1 mRNA, and miR-766-3p mimic or negative control by Lipofectamine 3000 (Invitrogen). The luciferase activity was assessed by a dual-luciferase reporter assay system (Promega, Madison, WI).
Statistical Analysis
All data are displayed as the mean ± S.D., and statistical analysis was performed by using SPSS 22.0. Student’s t-test was used to analyze the differences between two groups, and one-way ANOVA, combined with Bonferroni post hoc test, were used to evaluate the differences among multiple groups. Differences with P < 0.05 were considered as statistical significance.
Results
Baicalin Promotes Cell Viability and Suppressed Cell Apoptosis Under IL-1β Treatment in Human Primary Chondrocytes
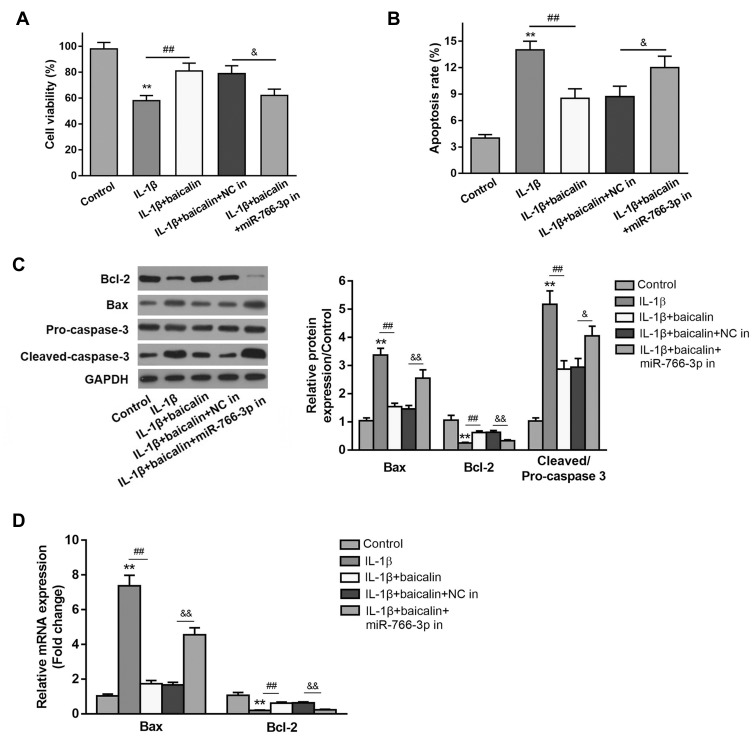
To determine the protective effect of baicalin in IL-β-induced chondrocytes, the chondrocytes were treated with 10 ng/mL IL-1β alone for 12 h, or pre-treated with 20 μM baicalin for 5 h followed by co-treatment with 20 μM baicalin and 10 ng/mL IL-1β for 12 h. Cell viability was detected using the CCK-8 assay. IL-1β treatment reduced chondrocytes viability compared with control group, and 20 μM baicalin significantly increased the viability of OA chondrocytes compared with those treated with IL-1β alone (Figure 1A).
Figure 1.
Baicalin reversed IL-1β-induced viability inhibition and pro-apoptosis of primary human OA chondrocytes. Primary chondrocytes were cultured for 24 h in a 96-well-plate and then treated with 10 ng/mL IL-1β alone for 12 h, or pretreated with 20 μM baicalin or 100 nM rapamycin for 4 h followed by co-treatment with 10 ng/mL IL-1β and 20 μM baicalin or 100 nM rapamycin for 12 h. (A) Cell viability was measured by CCK-8 assay. (B) Cell apoptosis was detected by flow cytometry assay. (C) The expressions of apoptosis-related proteins were determined by qRT-PCR (C) and Western blot (D) analysis. **p < 0.01 vs Control group; ##p<0.01 vs IL-β alone treatment; &p<0.05, &&p<0.01 vs IL-β+baicalin+NC in group.
Based on the above data, we speculated that baicalin may suppress the apoptosis of chondrocytes. Thus, flow cytometry analysis was performed to evaluate the effect of baicalin on chondrocyte apoptosis. IL-1β treatment notably increased the number of apoptotic cells compared to the control cells. While baicalin significantly alleviated IL-1β-induced cell apoptosis (Figure 1B). In addition, the expressions of apoptosis-related proteins such Bcl-2, Bax and cleaved-caspase-3 were examined by RT-qPCR and Western blot. IL-1β stimulation increased the expression of Bax and decreased Bcl-2 expression both at mRNA and protein level, and up-regulated cleaved-caspase-3 expression at protein level. On the contrary, baicalin significantly suppressed the expressions of Bax and cleaved-caspase-3 and promoted Bcl-2 expression. These data suggest that baicalin exerts anti-apoptotic activity in the chondrocytes (Figure 1C and D).
Baicalin Increases the Glycosaminoglycan (GAG) Content and Relieves ECM Degradation in Human Primary Chondrocytes
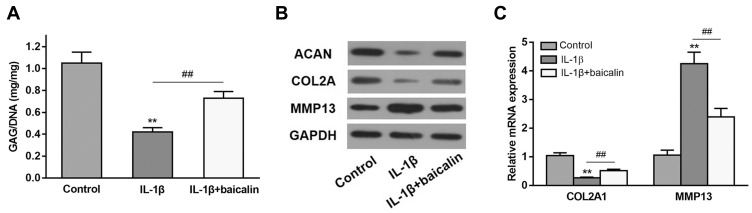
To explore the role of baicalin on GAG content, human OA chondrocytes were treated with 10 ng/mL IL-1β alone for 12 h, or pretreated with 20 μM baicalin for 5 h followed by co-treatment with 10 ng/mL IL-1β and 20 μM baicalin for 12 h, and then the GAG content was determined. As shown in Figure 2A, the GAG content was significantly lower in IL-1β-induced chondrocytes than that in control chondrocytes. However, the GAG content in the OA chondrocytes pretreated with baicalin was markedly elevated compared to that in IL-β-treated alone group.
Figure 2.
Baicalin alleviates IL-1β-induced GAGs content reduction and ECM degradation in chondrocytes. (A) The effect of baicalin and IL-β on intracellular GAG content in human OA chondrocytes was measured using a GAG assay. (B and C) The effect of baicalin and IL-1β on collagen II, aggrecan, and MMP13 expression by qRT-PCR and Western blot. **p < 0.01 compared with control group, ##p < 0.01 compared with IL-1β treated alone.
To evaluate the effect of baicalin on the degradation of ECM, the expressions of collagen II (COL2A), aggrecan (ACAN) and MMP13 were determined. IL-1β treatment notably reduced ACAN and COL2A level but enhanced the expression of MMP13, and baicalin pre-treatment alleviated the above negative effects caused by IL-1β (Figure 2B). Moreover, qRT-PCR of collagen II and MMP13 confirmed the Western blot results (Figure 2C). Collectively, these data suggest that baicalin exerts protective influence on curbing ECM degradation.
Baicalin Enhances Autophagic Activity in Human Primary Chondrocytes
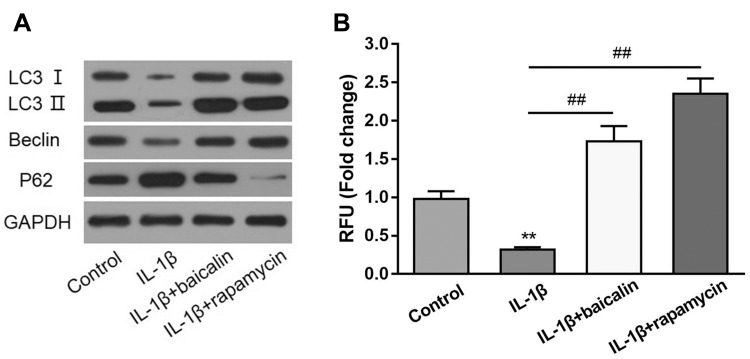
To evaluate whether baicalin could promote autophagy in chondrocytes, human OA chondrocytes were treated with 10 ng/mL IL-1β alone for 24 h, or pre-treated with 20 μM baicalin or 100 nM rapamycin for 5 h and then co-treated with 10 ng/mL IL-1β and 20 μM baicalin or 100 nM rapamycin for 24h. Rapamycin was employed to stimulate autophagy as a positive control. Autophagic markers Beclin-1 and p62, and autophagic flux marker LC3II/LC3I ratio were measured by Western blot. IL-1β treatment downregulated the LC3II/LC3I ratio and the expression of Beclin-1 and up-regulated the expression of p62 compared with the controls. On the contrary, baicalin and rapamycin treatment both reversed IL-1β-induced autophagy inhibition, characterized by elevated LC3II/LC3I ratio and expression of Beclin-1, and decreased expression of p62 (Figure 3A). In addition, IL-1β treatment reduced the number of autophagic vacuoles, whereas, baicalin and rapamycin treatment both restored autophagy activity inhibited by IL-1β (Figure 3B).Therefore, these data indicate that baicalin can reverse detrimental outcomes induced by IL-1β.
Figure 3.
Baicalin activates autophagy and enhances autophagic flux in chondrocytes. (A) The expression levels of autophagic markers LC-3, Belin-1 and p62 were assessed by Western blot. (B) The autophagy activation of chondrocytes after treatment with IL-1β alone, IL-1β + baicalin or IL-1β + rapamycin was measured by Cyto-ID staining assay. **p < 0.01, ## p<0.01.
Baicalin Protects Human OA Chondrocytes Against IL-1β-Induced Apoptosis and ECM Degradation via Activating Autophagy
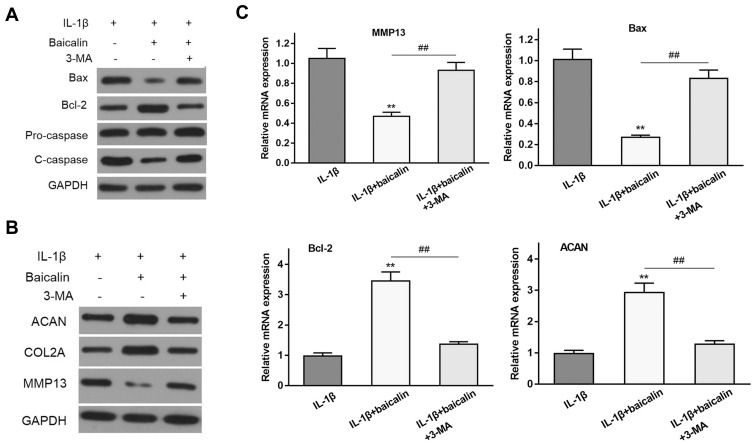
The activation of autophagy contributes to cells anti-apoptosis and balancing anabolism and catabolism of ECM. To confirm whether the positive effects of baicalin on chondrocyte survival and ECM synthesis are mediated via autophagy, autophagy inhibitors 3-methyladenine (3-MA) was used to inhibit autophagy. 3-MA treatment notably up-regulated the level of pro-apoptotic molecules cleaved-caspase-3 and Bax and down-regulated Bcl-2 expression in the chondrocytes (Figure 4A). In addition, the supplement of 3-MA significantly reduced ACAN and CLO2A and increased the expression of MMP13 compared with the baicalin treatment group, suggesting that the degradation of ECM is promoted after autophagy inhibition (Figure 4B). Consistent with Western blot analysis, the result of RT-qPCR showed that 3-MA treatment increased Bax and MMP13 expression, and decreased Bcl-2 and ACAN expression compared to baicalin treatment (Figure 4C). Taken together, these data suggest that autophagy activation is required for baicalin-induced anti-apoptosis and ECM synthesis augmenting in human chondrocytes.
Figure 4.
Baicalin protects human OA chondrocytes against IL-1β-induced apoptosis and ECM degradation depending on autophagy activity. (A) The effects of baicalin and IL-1β on the expression of apoptosis-related proteins with or without autophagy inhibitor 3-methyladenine (3-MA) were examined by Western blot. (B) The effects of baicalin and IL-1β on the expression of ECM components with or without autophagy inhibitor 3-methyladenine (3-MA) were examined by Western blot. (C) The effects of baicalin and IL-1β on the expression of apoptosis-related proteins and ECM components and metalloproteinase with or without autophagy inhibitor 3-methyladenine (3-MA) were also assessed by qRT-PCR. **p < 0.01 compared with IL-1β alone treated group, ##P<0.01 compared with IL-1β+baicalin group.
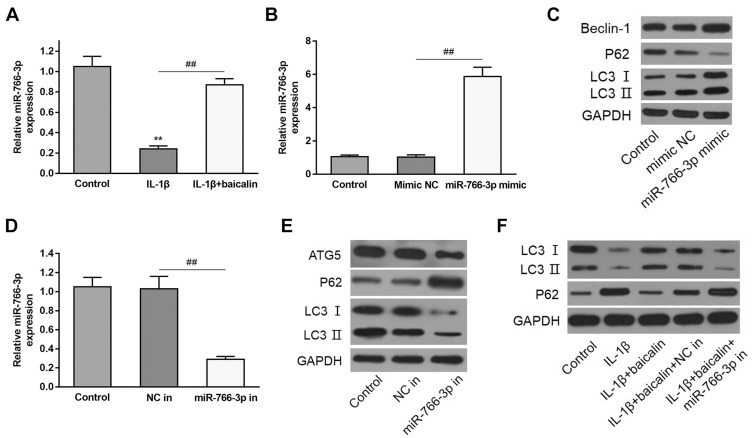
Baicalin Activate Autophagy by the Up-Regulation of MiR-766-3p in the Chondrocytes
To explore the potential mechanism underlying the regulation of baicalin on autophagy, the expression of miR-766-3p in baicalin+IL-1β treated chondrocytes was tested. RT-qPCR analysis demonstrated that IL-1β stimulation pronouncedly reduced the miR-766-3p expression compared with controls, whereas baicalin pretreatment notably increased expression level of miR-766-3p relative to IL-1β alone treated group (Figure 5A), which suggested that baicalin pretreatment play a positive role in up-regulating miR-766-3p expression in IL-1β-induced chondrocytes. To investigate whether miR-766-3p can induce autophagy activation in chondrocytes, we transfected human OA chondrocytes with miR-766-3p mimic or inhibitor, respectively. The expression of miR-766-3p was markedly increased by miR-766-3p mimic transfection, suggesting that miR-766-3p was successfully overexpressed in chondrocytes (Figure 5B). Besides, Western blot analysis for autophagic markers revealed that miR-766-3p overexpression significantly increased the LC3II/LC3I ratio and the expression of Beclin-1 and reduced the expression of p62 compared with compared with those of respective negative control chondrocytes (Figure 5C). On the other hand, the expression of miR-766-3p was notably suppressed by miR-766-3p inhibitor in transfection indicating that miR-766-3p was successfully silenced (Figure 5D). Western blot analysis for autophagic markers showed that miR-766-3p inhibitor markedly reduced the LC3II/LC3I ratio and the expression of Beclin-1 and elevated expression of p62 compared with those of respective negative control chondrocytes (Figure 5E), suggesting that miR-766-3p can promote autophagy in human OA chondrocytes. In view of above results, we hypothesized that miR-766-3p might contribute to baicalin-induced autophagy augmenting; therefore, we further evaluated the effects of miR-766-3p inhibitor (miR-766-3p in) in IL-1β + baicalin-treated chondrocytes. The following results revealed that miR-766-3p inhibitor remarkably reversed baicalin pretreatment triggered beneficial effects on cell viability (Figure 1A), cell apoptosis (Figure 1B–D) and autophagy (Figure 5F). Combing these results, we conclude that baicalin treatment reverses IL-1β-induced apoptosis and autophagy suppression by up-regulating miR-766-3p expression.
Figure 5.
Baicalin pretreatment relieved IL-1β-triggered autophagy inhibition by the up-regulation of miR-766-3p. The sequences of miR-766-3p inhibitor and NC in were respectively transfected into IL-1β + baicalin-treated human OA chondrocytes. (A) qRT-PCR was carried out for assessing miR-766-3p expression in IL-1β alone and IL-1β + baicalin-treated human OA chondrocytes. (B) qRT-PCR was carried out for determining the level of miR-766-3p in NC mimic and miR-766-3p mimic transfected cells. (C) Western blot was performed for illustrating the expressions of autophagy markerBeclin-1, p62 and LC3 in miR-766-3p transfected cells. (D) qRT-PCR was carried out for determining the level of miR-766-3p in NC inhibitor (in) and miR-766-3p inhibitor (in) transfected cells. (E) Western blot was performed for determining the expressions of autophagy marker Beclin-1, p62 and LC3 in NC in and miR-766-3p in transfected cells. (F) The expression of autophagy marker p62 and LC3 in indicated cells. **p < 0.01, ## p<0.01.
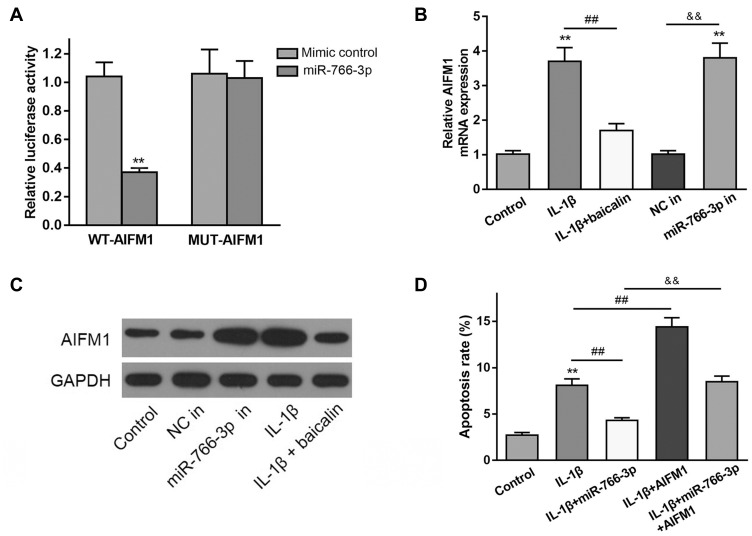
MiR-766-3p Targets AIFM1 to Regulate Autophagy and Cell Apoptosis in Chondrocytes
Using TargetScan bioinformatics software, the 3′‐UTR of AIFM1 mRNA was identified as a potential target of miR‐766‐3p. Then, the direct interaction between miR‐766‐3p and AIFM1 was confirmed by luciferase reporter assay. As shown in Figure 6A, miR‐766‐3p mimic transfection significantly suppress the luciferase activity of wide‐type AIFM1 mRNA 3′‐UTR (AIFM1 3′‐UTR‐wt) in controls, but not mutated AIFM1 mRNA 3′‐UTR (AIFM1 3′‐UTR‐mut). Besides, we noticed that the mRNA and protein expression levels of AIFM1 in IL-1β alone treated group were observable increased than that in the controls, and baicalin treatment significantly decreased AIFM1 mRNA and protein expression induced by IL-1β (Figure 6B and C). These data suggested that AIFM1 was a direct target of miR‐766‐3p and baicalin significantly suppress the expression of AIFM1 protein.
Figure 6.
MiR-766-3p targets AIFM1 to regulate autophagy and cell apoptosis in chondrocytes. (A) Luciferase activity of a dual-luciferase reporter vector containing wild-type 3ʹUTR-AIFM1 or a mutant 3ʹUTR-AIFM1. (B and C) AIFM1 expression at mRNA and protein was determined by qRT-PCR (B) and Western blot (C) in IL-1β alone treated, IL-1β + baicalin treated, NC in, miR-766-3p in transfected human OA chondrocytes. (D) After miR-766-3p or AIFM1 transfection, chondrocytes were treated with IL-1β for 24 h. The apoptosis rate was measured by flow cytometry analysis. **p < 0.01, && p<0.01, ## p<0.01.
To further determine the effects of AIFM1 on the apoptosis and autophagy chondrocytes, the expressions of AIFM1 and miR-766-3p were silenced and overexpressed. As shown in Figure 6D, flow cytometry analysis showed that the overexpression of AIFM1 promoted cell apoptosis than the NC mimic + vector group. Furthermore, no effect was found on cell apoptosis after co-transfection with miR-766-3p and AIFM1 compared to the untreated cells, suggesting overexpression of miR-766-3p reversed the influences induced by AIFM1 overexpression. Contrarily, after transfected with miR-766-3p inhibitor, the cell apoptosis was significantly enhanced in the chondrocytes and knockdown of AIFM1 inhibited this effect. Collectively, these data suggested that miR-766-3p protects chondrocytes from IL-1β-induced apoptosis and autophagy inhibition by targeting AIFM1.
Discussion
Massive cell death of chondrocytes and excessive degradation of ECM are found in OA. Previous studies indicated that chemical and genetic regulations for ECM synthesis and apoptosis played positive roles in OA progression in cell or animal models.30,31 However, only a few studies are found to focus on the therapeutic potential of baicalin in OA.29,32,33 In this study, we evaluated the effect and molecular mechanisms of baicalin in IL-1β-induced injury of chondrocytes and found that administration of baicalin alleviated IL-1β-induced apoptosis and ECM degradation in the chondrocytes. In addition, the results revealed that the molecular mechanism of baicalin is associated with activating autophagy and restoring autophagic flux in human OA chondrocytes.
A growing body of evidence suggests that IL-1β is associated with OA onset and development34–36 by promoting ECM degradation and chondrocytes apoptosis, inhibiting cell proliferation. Herein, we used IL-1β to induce inflammatory injury in human OA chondrocytes in order to mimic OA cell model. In line with expectations, the administration of IL-1β dramatically increased cell apoptosis and the expression of matrix metalloproteinases, and markedly reduced chondrocytes viability and ECM synthesis. Moreover, baicalin treatment reversed the detrimental effects induced by IL-1β, implying that baicalin may play a protective role against L-1β to induce inflammatory injury in human OA chondrocytes.
Autophagy is a cellular homeostatic mechanism in various pathological events.37 And the moderate autophagy is reported to effectively suppress cell apoptosis.38 Reduced autophagy activity is recognized as a feature of articular cartilage in the patients with OA.39 Besides, the formation of autophagosome is reduced in chondrocytes during aging and OA development.15 Our data showed that the expression autophagic markers Beclin-1 were markedly decreased and p62 increased following the application of IL-1β, and LC3 Ⅱ/LC3 Ⅰ ration, a marker of autophagic flux, indicating that IL-1β has a negative role in regulating autophagy in chondrocytes. Baicalin treatment elevates autophagosome protein expression and promotes autophagic flux. Furthermore, the beneficial effects of baicalin could be blocked after the inhibition of autophagic flux by 3-MA, suggesting that baicalin exerts protective effects through promoting autophagic flux.
MiRNAs are recognized as therapeutic targets or diagnostic markers in OA due to their effects in regulating the biological behavior of the chondrocytes. Recent studies demonstrated that the aberrant expression of miRNAs was associated with the onset and progression of OA.40,41 MiR-766-3p has also been previously reported to increase due to aging42 and to exert anti-inflammatory function in human rheumatoid arthritis (RA) fibroblast-like synoviocytes. Here we for the first time demonstrated that baicalin protected human OA chondrocytes against IL-1β-induced cell apoptosis and autophagy inhibition possibly via up-regulation of miR-766-3p. Previous literatures have shown that miR-766-3p could target different genes, such as Wnt3a, SF2, MTA3, NR3C2, and FOSL2, which are associated with the progression of multiple disorders. We predicted that miR-766-3p may target the 3ʹ-UTR of AIFM1 mRNA with bioinformatics analysis. Dual-luciferase reporter assay, combined with qRT-PCR and Western blot, indicated that miR-766-3p mimics reduced mRNA level and protein expression of AIFM1. AIFM1 can promote the apoptosis of hepatoma cell,43 and AIFM1 silencing significantly reversed LPS-induced apoptosis of renal cells,44 and Mycoplasma synoviae induced chicken chondrocytes apoptosis by up-regulating AIFM1 expression.45 Here, the current study for the first time to describe a pro-apoptotic role for AIFM1 in human OA chondrocytes.
In conclusion, we suggested that baicalin protected human OA chondrocytes against IL-1β-induced apoptosis and autophagy suppression. The protective effects might be associated with up-regulation of miR-766-3p, and thereby decreased expression of AIFM1. The findings of the current study indicated that baicalin presents a potential clinic value for OA treatment.
Funding Statement
No funding was received for this work.
Data Sharing Statement
All data generated or analyzed during the present study are included in this published article.
Ethics Approval and Consent to Participate
The present study was performed with the approval of the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University.
Patient Consent for Publication
All participants provided written informed consent.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Musumeci G, Castrogiovanni P, Trovato FM, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16(9):20560–20575. doi: 10.3390/ijms160920560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Rosa M, Szychlinska MA, Tibullo D, Malaguarnera L, Musumeci G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur J Histochem. 2014;58(3):2423. doi: 10.4081/ejh.2014.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. doi: 10.1016/j.joca.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Hu J, Pan Y, et al. miR-140-5p/miR-149 affects chondrocyte proliferation, apoptosis, and autophagy by targeting FUT1 in osteoarthritis. Inflammation. 2018;41(3):959–971. doi: 10.1007/s10753-018-0750-6 [DOI] [PubMed] [Google Scholar]
- 5.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. doi: 10.1126/science.1193497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li YS, Zhang FJ, Zeng C, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83(2):143–148. doi: 10.1016/j.jbspin.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 7.Hidvegi T, Ewing M, Hale P, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329(5988):229–232. doi: 10.1126/science.1190354 [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Yu WH, Kumar A, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141(7):1146–1158. doi: 10.1016/j.cell.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4(5):590–599. doi: 10.4161/auto.6259 [DOI] [PubMed] [Google Scholar]
- 10.Masiero E, Agatea L, Mammucari C, et al. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10(6):507–515. doi: 10.1016/j.cmet.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 11.Mathew R, Kongara S, Beaudoin B, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21(11):1367–1381. doi: 10.1101/gad.1545107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathew R, Karp CM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–1075. doi: 10.1016/j.cell.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Vasheghani F, Li YH, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74(7):1432–1440. doi: 10.1136/annrheumdis-2013-204599 [DOI] [PubMed] [Google Scholar]
- 14.Shen C, Cai GQ, Peng JP, Chen XD. Autophagy protects chondrocytes from glucocorticoids-induced apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthritis Cartilage. 2015;23(12):2279–2287. doi: 10.1016/j.joca.2015.06.020 [DOI] [PubMed] [Google Scholar]
- 15.D’Adamo S, Alvarez-Garcia O, Muramatsu Y, Flamigni F, Lotz MK. MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthritis Cartilage. 2016;24(6):1082–1091. doi: 10.1016/j.joca.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lian WS, Ko JY, Wu RW, et al. MicroRNA-128a represses chondrocyte autophagy and exacerbates knee osteoarthritis by disrupting Atg12. Cell Death Dis. 2018;9(9):919. doi: 10.1038/s41419-018-0994-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai C, Min S, Yan B, et al. MiR-27a promotes the autophagy and apoptosis of IL-1β treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging. 2019;11(16):6371–6384. doi: 10.18632/aging.102194 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.You Y, Que K, Zhou Y, et al. MicroRNA-766-3p inhibits tumour progression by targeting Wnt3a in hepatocellular carcinoma. Mol Cells. 2018;41(9):830–841. doi: 10.14348/molcells.2018.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh K, Lee DS. In vivo validation of metastasis-regulating microRNA-766 in human triple-negative breast cancer cells. Lab Anim Res. 2017;33(3):256–263. doi: 10.5625/lar.2017.33.3.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li YC, Li CF, Chen LB, et al. MicroRNA-766 targeting regulation of SOX6 expression promoted cell proliferation of human colorectal cancer. Onco Targets Ther. 2015;8:2981–2988. doi: 10.2147/OTT.S89459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C, Xue S, Zhang J, et al. DNA-methylation-mediated repression of miR-766-3p promotes cell proliferation via targeting SF2 expression in renal cell carcinoma. Int J Cancer. 2017;141(9):1867–1878. doi: 10.1002/ijc.30853 [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa K, Kawasaki M, Hirai T, et al. MicroRNA-766-3p contributes to anti-inflammatory responses through the indirect inhibition of NF-κB signaling. Int J Mol Sci. 2019;20(4):809. doi: 10.3390/ijms20040809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W, Ku SK, Bae JS. Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation. 2015;38(1):110–125. doi: 10.1007/s10753-014-0013-0 [DOI] [PubMed] [Google Scholar]
- 24.Wang C-Z, Mehendale SR, Yuan C-S. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35(4):543–558. doi: 10.1142/S0192415X07005053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan X, Guo G, Pei X, et al. baicalin inhibits cell viability, migration and invasion in breast cancer by regulating mir-338-3p and morc4. Onco Targets Ther. 2019;12:11183–11193. doi: 10.2147/OTT.S217101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Y, Chen L, Guo S, Li Y. Baicalin induced colon cancer cells apoptosis through miR-217/DKK1-mediated inhibition of Wnt signaling pathway. Mol Biol Rep. 2019;46(2):1693–1700. doi: 10.1007/s11033-019-04618-9 [DOI] [PubMed] [Google Scholar]
- 27.Moghaddam E, Teoh BT, Sam SS, et al. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep. 2014;4:5452. doi: 10.1038/srep05452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Y, Chen D, Lu Q, Liu L, Li X, Li Z. Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Mol Med Rep. 2017;16(3):2985–2991. doi: 10.3892/mmr.2017.6904 [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Baicalin alleviates IL-1β-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed Pharmacother. 2018;99:184–190. doi: 10.1016/j.biopha.2018.01.041 [DOI] [PubMed] [Google Scholar]
- 30.Khan NM, Ahmad I, Haqqi TM. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic Biol Med. 2018;116:159–171. doi: 10.1016/j.freeradbiomed.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Wu Y, Jiang K, et al. Mangiferin prevents TBHP-induced apoptosis and ECM degradation in mouse osteoarthritic chondrocytes via restoring autophagy and ameliorates murine osteoarthritis. Oxid Med Cell Longev. 2019;2019:8783197. doi: 10.1155/2019/8783197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Zhang C, Cai L, et al. Baicalin suppresses IL-1β-induced expression of inflammatory cytokines via blocking NF-κB in human osteoarthritis chondrocytes and shows protective effect in mice osteoarthritis models. Int Immunopharmacol. 2017;52:218–226. doi: 10.1016/j.intimp.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 33.Chen D-S, Cao J-G, Zhu B, Wang Z-L, Wang T-F, Tang -J-J. Baicalin attenuates joint pain and muscle dysfunction by inhibiting muscular oxidative stress in an experimental osteoarthritis rat model. Arch Immunol Ther Exp. 2018;66(6):453–461. doi: 10.1007/s00005-018-0518-6 [DOI] [PubMed] [Google Scholar]
- 34.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier J-P, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 35.Daheshia M, Yao JQ. The interleukin 1β pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35(12):2306–2312. doi: 10.3899/jrheum.080346 [DOI] [PubMed] [Google Scholar]
- 36.Hussein MR, Fathi NA, El-Din AME, et al. Alterations of the CD4+, CD8+ T cell subsets, Interleukins-1β, IL-10, IL-17, tumor necrosis Factor-α and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: preliminary observations. Pathol Oncol Res. 2008;14(3):321–328. doi: 10.1007/s12253-008-9016-1 [DOI] [PubMed] [Google Scholar]
- 37.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3 [DOI] [PubMed] [Google Scholar]
- 38.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801. doi: 10.1002/art.27305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saberi Hosnijeh F, Bierma-Zeinstra SM, Bay-Jensen AC. Osteoarthritis year in review 2018: biomarkers (biochemical markers). Osteoarthr Cartil. 2019;27(3):412–423. doi: 10.1016/j.joca.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 40.Le LT, Swingler TE, Clark IM. Review: the role of microRNAs in osteoarthritis and chondrogenesis. Arthritis Rheum. 2013;65(8):1963–1974. doi: 10.1002/art.37990 [DOI] [PubMed] [Google Scholar]
- 41.Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3(11):e3740. doi: 10.1371/journal.pone.0003740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma A, Diecke S, Zhang WY, et al. The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem. 2013;288(25):18439–18447. doi: 10.1074/jbc.M112.405928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80(1):29–40. doi: 10.1038/ki.2011.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang P, Yi L, Qu S, et al. The biomarker TCONS_00016233 drives septic AKI by targeting the miR-22-3p/AIFM1 signaling axis. Mol Ther Nucleic Acids. 2020;19:1027–1042. doi: 10.1016/j.omtn.2019.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dusanic D, Bencina D, Oven I, Cizelj I, Bencina M, Narat M. Mycoplasma synoviae induces upregulation of apoptotic genes, secretion of nitric oxide and appearance of an apoptotic phenotype in infected chicken chondrocytes. Vet Res. 2012;43:7. doi: 10.1186/1297-9716-43-7 [DOI] [PMC free article] [PubMed] [Google Scholar]