Abstract
Introduction
Circular RNAs (circRNAs) are deregulated in many types of human cancers, including non-small cell lung cancer (NSCLC). In this study, we aimed to explore the functional role of circMYLK in NSCLC.
Materials and Methods
The expression levels of circMYLK and miR-195-5p in NSCLC tissues and cell lines were detected by RT-qPCR analysis. MTT assay, colony formation assay and transwell assay were performed to investigate the effects of circMYLK and miR-195-5p on the malignant phenotypes of NSCLC cells. The glucose consumption and lactate production of NSCLC cells were detected using commercial kits. The direct binding relation between circMYLK and miR-195-5p in NSCLC was predicted by bioinformatics analysis and validated by dual-luciferase reporter assay.
Results
The results showed that circMYLK was significantly up-regulated in NSCLC tissues and cell lines, and its high expression was closely associated with deleterious clinicopathological characteristics and poor prognosis of NSCLC patients. Knockdown of circMYLK remarkably inhibited the malignant phenotypes of NSCLC cells, including proliferation, migration, invasion, glucose consumption and lactate production. Moreover, circMYLK was identified as a molecule sponge for miR-195-5p, and glucose transporter member 3 (GLUT3) was shown to be a target gene of miR-195-5p in NSCLC. Further rescue experiments revealed that the oncogenic effects of circMYLK on NSCLC cells could be largely abrogated by co-transfection with miR-195-5p mimic.
Conclusion
In summary, our study provides convincing evidence that circMYLK serves as a tumor promoter in NSCLC and can be used as a potential therapeutic target for NSCLC patients.
Keywords: non-small cell lung cancer, circular RNA MYLK, miR-195-5p, glycolysis, glucose transporter member 3
Introduction
Lung cancer is the leading cause of cancer-related deaths around the world, and non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases.1 Recently, great advancements have been made in the diagnostic and therapeutic methods; however, due to high rates of metastasis and recurrence, the clinical outcomes of NSCLC patients remain dismal.2 Accordingly, there is an urgent need to explore the molecular mechanisms of NSCLC and identify potential therapeutic targets.
More than 90% of human genome is made up of non-coding RNA (ncRNAs).3 Circular RNAs (circRNAs), a novel class of ncRNAs that widely exist in almost all eukaryotic cells, are featured by their covalently closed-loop structures with neither 5ʹ to 3ʹ polarity nor polyadenylated tail.4 Many circRNAs have cell-type specific expression and are linked to various pathophysiological processes. In particular, they can either function as oncogenic stimuli or tumor suppressors in cancer.5 As a novel circRNA that has just been identified, circMYLK was reported to be highly expressed and closely associated with tumor progression in bladder cancer,6 prostate cancer7 and hepatocellular carcinoma.8 In this study, we aimed to investigate the functional role of circMYLK and the underlying mechanisms in NSCLC.
Materials and Methods
Patients and Tissue Samples
NSCLC tissues and matched adjacent normal tissues were obtained from 103 patients who underwent surgery at Chongqing University Cancer Hospital (Chongqing, China). All patients did not receive any radiotherapy or chemotherapy before surgery. The fresh samples were immediately frozen in liquid nitrogen and stored at −80°C until further use. This study was approved by the Ethics Committee of Chongqing University Cancer Hospital, and written informed consent was obtained from all subjects.
Cell Culture and Cell Transfection
NSCLC cell lines (H23, A549, H1299 and SPC-A1) and a normal human bronchial epithelial cell line (16HBE) were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). These cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Logan City, UT, USA) supplemented with 10% fetal bovine serum (FBS; HyClone) and 1% penicillin/streptomycin at 37°C in a humidified incubator with 5% CO2.
The small interfering RNAs (siRNAs) targeting circMYLK (si-circMYLK) and GLUT3 (si-GLUT3), the negative control siRNA (si-NC), miR-195-5p mimic and the scrambled mimic control (miR-NC) were designed and synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The synthesized circMYLK or GLUT3 gene fragment was inserted into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) to gain the circMYLK-overexpression or GLUT3-overexpression plasmid. An empty vector was used as a negative control. Cells were cultured to 70–80% confluence, and cell transfection was then performed using Lipofectamine 2000 (Invitrogen).
RT-qPCR Analysis
Total RNA from tissues or cells was extracted using TRIzol reagent (Invitrogen), and cDNAs were synthetized by the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). Thereafter, qPCR analysis was performed using the SYBR Premix Ex Taq II kit (TaKaRa) on an iCycler iQ™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The relative gene expression was calculated using the 2−ΔΔCt method.9 GAPDH or U6 was used as an internal reference.
MTT Assay
Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl-2H-tetrazoliumbromide (MTT) assay. Cells were seeded in 96-well plates at a density of 5×103 cells/well, and cultured for 24–96 h. Then, 20 µL MTT (5 mg/mL; Sigma-Aldrich, St. Louis, MO, USA) solution was added to each well. After incubation for an additional 4 h at 37°C, the insoluble formazan was dissolved in DMSO, and the absorbance of each well was measured at 570 nm using an ELx808 microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Colony Formation Assay
Cells were seeded in each well of a 6-well plate at a density of 500 cells/well. After 14 days of incubation, the colonies were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet.
Transwell Assay
Cells suspended in 200 μL serum-free medium were added into the upper chamber of transwell plates (8 μm pore size; Corning Inc., Corning, NY, USA). The lower chamber was filled with medium containing 10% FBS. After 48 h, the cells on the upper surface were removed using cotton swabs, whereas the cells on the lower membrane surface were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The number of stained cells was counted under a microscope.
Western Blot Analysis
Cells were lysed with RIPA lysis buffer (Beyotime, Shanghai, China). Equal amounts of protein samples were separated by SDS-polyacrylamide gel electrophoresis, and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk, the membranes were incubated with primary antibodies at 4°C overnight, followed by the incubation with HRP-conjugated secondary antibody at room temperature for 2 h. Protein bands were visualized using an enhanced chemiluminescent detection kit (Beyotime). GAPDH served as a loading control.
Measurement of Glucose Consumption and Lactate Production
The levels of glucose and lactate were measured using a glucose assay kit (BioVision, Milpitas, CA, USA) and a lactate assay kit (BioVision), respectively. Glucose consumption and lactate production were calculated based on the standard curve.
Dual-Luciferase Reporter Assay
The wild-type and mutant binding sites of miR-195-5p in circMYLK sequence or GLUT3 mRNA were amplified by PCR and cloned into the psiCHECK-2 vector (Promega, Madison, WI, USA). Cells were placed into a 24-well plate, and then co-transfected with the luciferase reporter vectors and miR-195-5p mimic or miR-NC using Lipofectamine 2000. After 48 h, the luciferase activity was detected using the Dual-Luciferase Reporter Assay System (Promega).
Statistical Analysis
All statistical analysis was performed by GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA, USA) and SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). All continuous variables were presented as the mean ± standard deviation (SD). The differences between groups were analyzed by Student's t-test or one-way ANOVA followed by Tukey's test. Survival curves were generated by Kaplan-Meier analysis and compared using Log-rank test. P values were calculated and those less than 0.05 were considered significant.
Results
circMYLK Is Up-Regulated in NSCLC Tissues and Cell Lines
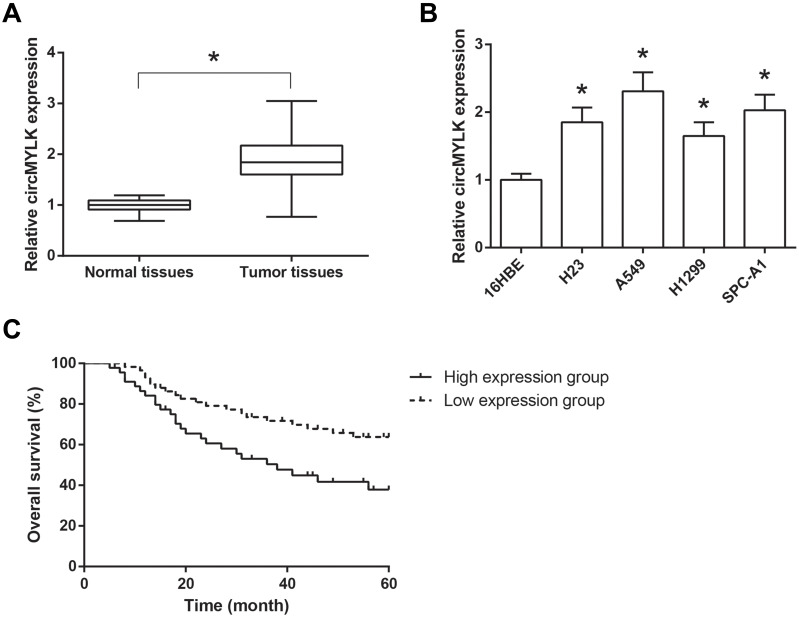
The expression levels of circMYLK were markedly higher in NSCLC tissues compared with those in adjacent normal tissues, as indicated by RT-qPCR analysis (Figure 1A). Consistently, compared to the normal 16HBE cells, circMYLK expression was also notably increased in NSCLC cell lines (H23, A549, H1299 and SPC-A1) (Figure 1B).
Figure 1.
circMYLK is up-regulated in NSCLC tissues and cell lines. (A) The expression levels of circMYLK in 103 pairs of NSCLC tissues and adjacent normal tissues, detected by RT-qPCR analysis. (B) The expression levels of circMYLK in NSCLC cell lines and normal 16HBE cells. *P<0.05 vs normal tissues or 16HBE cells. (C) Association of circMYLK expression with overall survival of NSCLC patients.
We further discussed the clinical significance of circMYLK in NSCLC. According to the median circMYLK expression level, these NSCLC patients were then allocated into two groups, including high expression group (n=45) and low expression group (n=58). As shown in Table 1, higher expression of circMYLK was significantly associated with larger tumor size (P=0.022) and advanced TNM stage (P=0.015) of NSCLC patients. Then, we performed survival analysis and found that NSCLC patients with higher circMYLK expression presented a significant poorer overall survival (Figure 1C).
Table 1.
Relationship Between circMYLK Expression and Clinicopathological Characteristics of NSCLC Patients (n=103)
| Characteristics | Total Number | circMYLK Expression | P value | |
|---|---|---|---|---|
| High (n=45) | Low (n=58) | |||
| Age (years) | 0.313 | |||
| <60 | 40 | 15 | 25 | |
| ≥60 | 63 | 30 | 33 | |
| Gender | 0.395 | |||
| Male | 71 | 33 | 38 | |
| Female | 32 | 12 | 20 | |
| Smoking history | 0.559 | |||
| Yes | 47 | 22 | 25 | |
| No | 56 | 23 | 33 | |
| Histology type | 0.585 | |||
| Adenocarcinoma | 61 | 28 | 33 | |
| Squamous | 42 | 17 | 25 | |
| Tumor size (cm) | 0.022 | |||
| <3 | 61 | 21 | 40 | |
| ≥3 | 42 | 24 | 18 | |
| TNM stage | 0.015 | |||
| I–II | 64 | 22 | 42 | |
| III–IV | 39 | 23 | 16 | |
| Lymph nodes metastasis | 0.143 | |||
| Yes | 58 | 29 | 29 | |
| No | 45 | 16 | 29 | |
circMYLK Promotes Glycolysis and Proliferation of NSCLC Cells
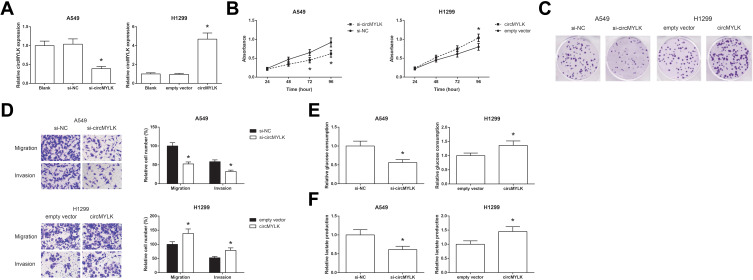
We then investigated the effects of circMYLK on the biological behaviors of NSCLC cells. circMYLK was knocked down in A549 cells and overexpressed in H1299 cells (Figure 2A). Knockdown of circMYLK in A549 cells led to a marked decrease in cell proliferation rate, as indicated by MTT assay, and circMYLK overexpression accelerated the proliferation of H1299 cells (Figure 2B). Similar results were also obtained from colony formation assay (Figure 2C). Moreover, transwell assay demonstrated that circMYLK knockdown notably impaired the migration and invasion abilities of A549 cells, whereas these abilities of H1299 cells were strikingly enhanced by circMYLK overexpression (Figure 2D). Glycolysis is a key characteristic of cancer metabolism, and we further discovered that the rates of glucose consumption and lactate production were remarkably reduced in A549 cells when circMYLK was knocked down, and circMYLK overexpression had the opposite effects on these glycolytic markers in H1299 cells (Figure 2E and F).
Figure 2.
circMYLK promotes glycolysis and proliferation of NSCLC cells. (A) The expression levels of circMYLK in A549 and H1299 cells after transfection. (B) The proliferation of A549 and H1299 cells after transfection, detected by MTT assay. (C) The colony formation ability of A549 and H1299 cells after transfection, detected by colony formation assay. (D) The migration and invasion of A549 and H1299 cells after transfection, detected by transwell assay. (E) The glucose consumption in A549 and H1299 cells after transfection, detected by a commercial kit. (F) The lactate production in A549 and H1299 cells after transfection, detected by a commercial kit. *P<0.05 vs si-NC or empty vector-transfected cells.
circMYLK Directly Binds to miR-195-5p in NSCLC
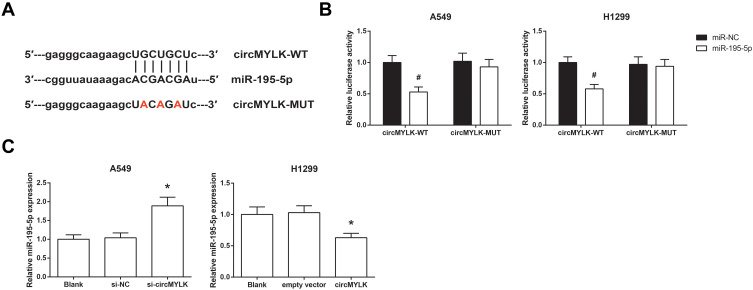
Through the Starbase database (http://starbase.sysu.edu.cn/index.php), it was shown that circMYLK sequence might contain the complementary binding sites of miR-195-5p (Figure 3A). To confirm the prediction, dual-luciferase reporter assay was then performed, and the results showed that co-transfection of miR-195-5p mimic and the circMYLK-WT vector notably reduced the luciferase activity in A549 and H1299 cells, but mutation of the binding sites abolished the effects (Figure 3B). In addition, we also found that miR-195-5p expression was boosted by circMYLK knockdown in A549 cells while inhibited by circMYLK overexpression in H1299 cells (Figure 3C).
Figure 3.
circMYLK directly binds to miR-195-5p in NSCLC. (A) The putative binding sites between circMYLK and miR-195-5p. (B) Relative luciferase activity in A549 and H1299 cells after transfection. (C) The expression levels of miR-195-5p in A549 and H1299 cells after transfection. *P<0.05 vs si-NC or empty vector-transfected cells; #P<0.05 vs miR-NC-transfected cells.
miR-195-5p Is Down-Regulated in NSCLC Tissues and Cell Lines
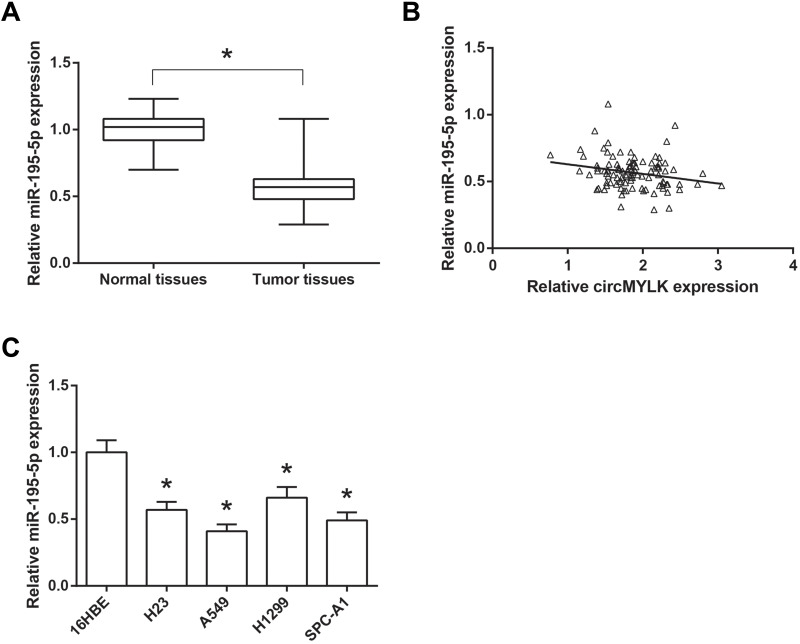
Furthermore, we found that the expression levels of miR-195-5p were enormously decreased in NSCLC tissues, compared with normal tissues (Figure 4A), and a significant inverse correlation between the expression levels of circMYLK and miR-195-5p was also observed in NSCLC tissues (r=−0.224, P=0.023; Figure 4B). Besides, as shown in Figure 4C, compared to the normal 16HBE cells, miR-195-5p expression was also markedly decreased in NSCLC cell lines (H23, A549, H1299 and SPC-A1).
Figure 4.
miR-195-5p is down-regulated in NSCLC tissues and cell lines. (A) The expression levels of miR-195-5p in 103 pairs of NSCLC tissues and adjacent normal tissues. (B) Statistical correlation between the expression levels of circMYLK and miR-195-5p in NSCLC tissues. (C) The expression levels of miR-195-5p in NSCLC cell lines and normal 16HBE cells. *P<0.05 vs normal tissues or 16HBE cells.
miR-195-5p Blocks the Oncogenic Role of circMYLK in NSCLC Cells
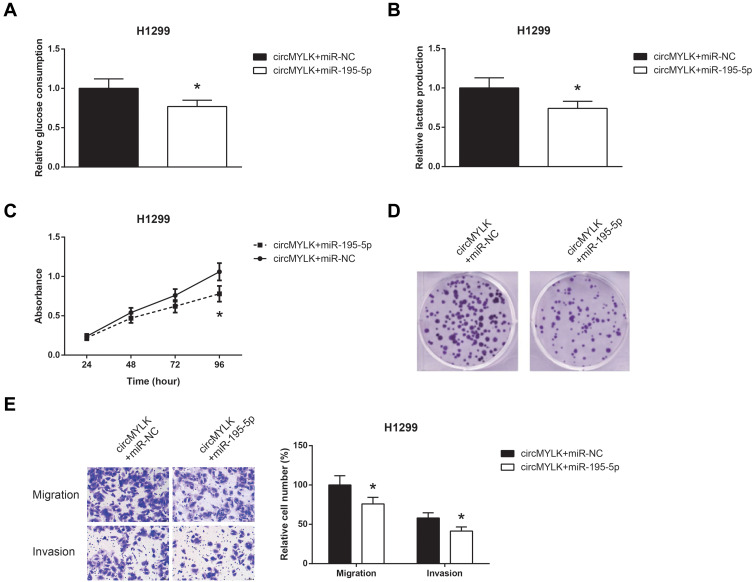
Then, rescue experiments were performed, and as shown in Figure 5A and B, co-transfection with miR-195-5p mimic obviously restrained the increased glucose consumption and lactate production in circMYLK-overexpressing H1299 cells. We further noticed that the promotive effects of circMYLK overexpression on the proliferation, colony formation, migration and invasion abilities of H1299 cells were also blocked by miR-195-5p restoration (Figure 5C–E).
Figure 5.
miR-195-5p blocks the oncogenic role of circMYLK in NSCLC cells. (A) The glucose consumption in H1299 cells after transfection. (B) The lactate production in H1299 cells after transfection. (C) The proliferation of H1299 cells after transfection. (D) The colony formation ability of H1299 cells after transfection. (E) The migration and invasion of H1299 cells after transfection. *P<0.05 vs pcDNA3.1-circMYLK+miR-NC-transfected cells.
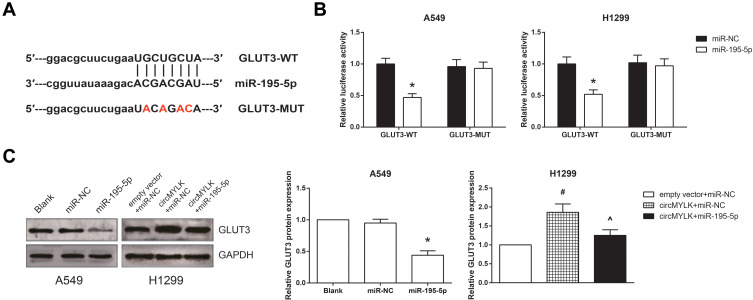
GLUT3 Is a Direct Target Gene of miR-195-5p in NSCLC
We further searched the potential target genes for miR-195-5p using the TargetScan database (http://www.targetscan.org) and selected glucose transporter member 3 (GLUT3) as a candidate (Figure 6A). In addition, co-transfection with GLUT3-WT and miR-195-5p mimic remarkably reduced the luciferase activity in A549 and H1299 cells (Figure 6B). Moreover, Western blot analysis showed that miR-195-5p mimic decreased the GLUT3 protein expression in A549 cells, whereas the GLUT3 protein expression was significantly repressed by miR-195-5p mimic in circMYLK-overexpressing H1299 cells (Figure 6C).
Figure 6.
GLUT3 is a direct target gene of miR-195-5p in NSCLC. (A) The putative binding sites between miR-195-5p and GLUT3 mRNA. (B) Relative luciferase activity in A549 and H1299 cells after transfection. (C) The expression levels of GLUT3 protein in A549 and H1299 cells after transfection. *P<0.05 vs miR-NC-transfected cells; #P<0.05 vs empty vector+miR-NC-transfected cells; ^P<0.05 vs pcDNA3.1-circMYLK+miR-NC-transfected cells.
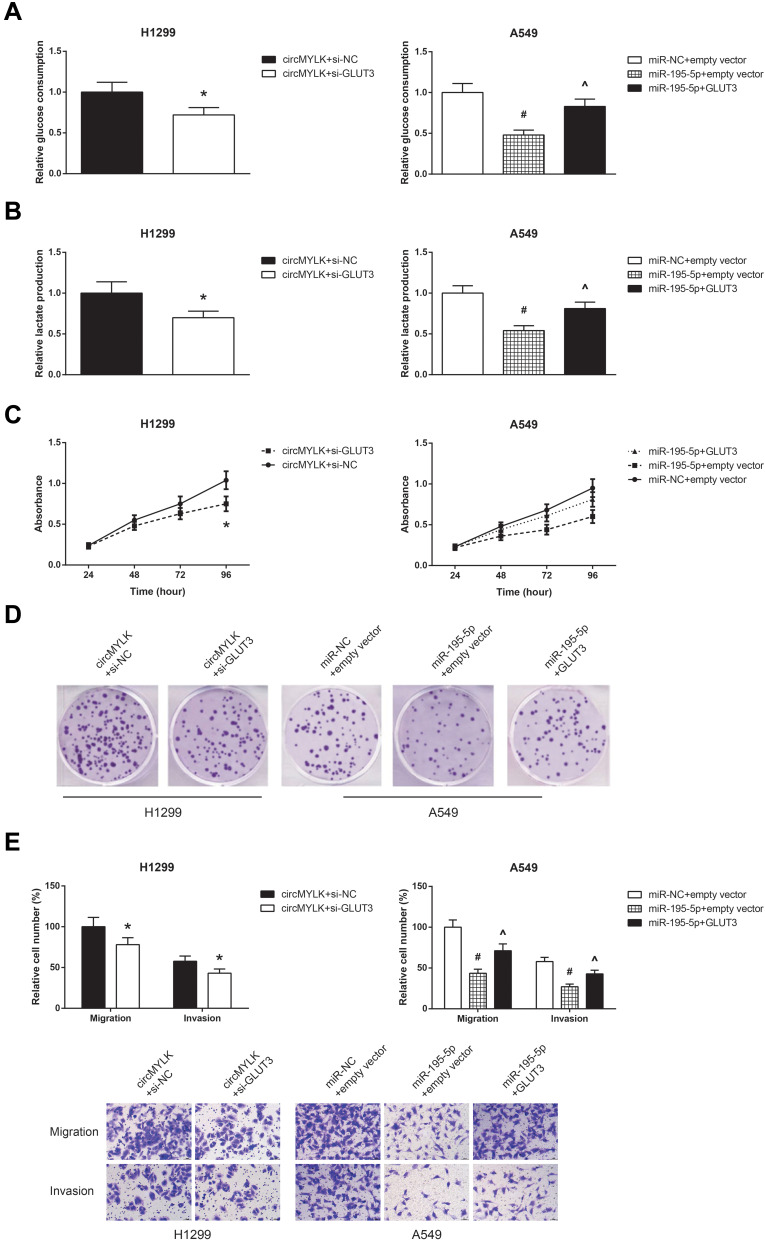
GLUT3 Knockdown Blocks the Oncogenic Role of circMYLK in NSCLC Cells
We further observed that GLUT3 knockdown also restrained the increased glucose consumption and lactate production in circMYLK-overexpressing H1299 cells (Figure 7A and B), accompanied by the impairment of cell proliferation, colony formation ability, migration and invasion (Figure 7C–E). Besides, we also confirmed that the inhibitory effects of miR-195-5p mimic on the glucose consumption, lactate production, proliferation, colony formation, migration and invasion of A549 cells were effectively blocked by GLUT3 restoration.
Figure 7.
GLUT3 knockdown blocks the oncogenic role of circMYLK in NSCLC cells. (A) The glucose consumption in A549 and H1299 cells after transfection. (B) The lactate production in A549 and H1299 cells after transfection. (C) The proliferation of A549 and H1299 cells after transfection. (D) The colony formation ability of A549 and H1299 cells after transfection. (E) The migration and invasion of A549 and H1299 cells after transfection. *P<0.05 vs pcDNA3.1-circMYLK+si-NC-transfected cells; #P<0.05 vs miR-NC+empty vector-transfected cells; ^P<0.05 vs miR-195-5p mimic+empty vector-transfected cells.
Discussion
NSCLC is one of the most common types of malignancies. At present, many circRNAs were identified to play crucial roles in NSCLC progression. These circRNAs also have potential as diagnostic and prognostic biomarkers for this fatal malignancy.10 For example, up-regulated circARHGAP10 predicts an unfavorable prognosis in NSCLC patients.11 By contrast, circSMARCA5 may function as a tumor suppressor in NSCLC.12 In this study, we aimed to explore the functional role of circMYLK in NSCLC.
Our results first confirmed that circMYLK was drastically augmented in NSCLC tissues and cell lines, and its high expression was closely associated with deleterious clinicopathological characteristics and poor prognosis of NSCLC patients. Through a series of functional experiments, we found that circMYLK overexpression promoted, while circMYLK knockdown suppressed the proliferation, migration and invasion of NSCLC cells. Cancer cells use glucose at a high level by glycolysis, producing lactate as an end product. Glycolysis is not only essential for tumor cell growth but also critical for tumor cell migration and invasion.13 In this study, the role of circMYLK in promoting the glycolytic phenotype of NSCLC cells was also highlighted.
In recent years, increasing numbers of reports have found that circRNAs can directly interact with miRNAs through serving as competing endogenous RNAs (ceRNAs) or “RNA sponges”, thereby reducing their inhibitory effects on target mRNAs.14 For example, in laryngeal squamous cell carcinoma, circMYLK serves its oncogenic role partly by sponging miR-195-5p.15 miR-195-5p is widely regarded as a tumor suppressor in NSCLC,16,17 and in this study, we also identified the direct binding relation between circMYLK and miR-195-5p in NSCLC. An inverse correlation between circMYLK and miR-195-5p expression in NSCLC tissues was also observed. More importantly, through rescue experiments, it was further confirmed that miR-195-5p restoration could diminish the oncogenic role of circMYLK in NSCLC cells.
Solute carriers of glucose transporter (GLUT) family regulate the first step of cellular glucose usage. The glucose transporter 3 (GLUT3) is a high-affinity glucose transporter, and its up-regulation has been reported in many cancers.18 It was also reported that GLUT3 contributes to glucose uptake and proliferation of NSCLC cells.19 In this study, GLUT3 was identified as a direct target of miR-195-5p in NSCLC, and this binding relation was also reported in bladder cancer cells.20 We further confirmed that GLUT3 knockdown could also block the oncogenic role of circMYLK in NSCLC cells, partly by reducing glycolysis.
In conclusion, this study identified circMYLK as an oncogenic circRNA that promotes malignant progression and glycolysis of NSCLC partly by sponging miR-195-5p and increasing GLUT3 expression. We believe that circMYLK has the potential to be a prospective therapeutic target for NSCLC patients.
Funding Statement
This work was supported by Chongqing Special Performance Incentive and Guidance Program for Scientific Research Institutions (Grant Nos. cstc2018jxj1130042 and cstc2018jxj1130045).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355(9202):479–485. doi: 10.1016/S0140-6736(00)82038-3 [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet. 2013;382(9893):720–731. doi: 10.1016/S0140-6736(13)61715-8 [DOI] [PubMed] [Google Scholar]
- 3.Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–388. doi: 10.1080/15476286.2015.1020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Huang S. Circular RNA: an emerging non-coding RNA as a regulator and biomarker in cancer. Cancer Lett. 2018;418:41–50. doi: 10.1016/j.canlet.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 6.Zhong Z, Huang M, Lv M, et al. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 7.Dai Y, Li D, Chen X, et al. Circular RNA myosin light chain kinase (MYLK) promotes prostate cancer progression through modulating Mir-29a expression. Med Sci Monit. 2018;24:3462–3471. doi: 10.12659/MSM.908009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Hu Y, Zeng Q, et al. Circular RNA MYLK promotes hepatocellular carcinoma progression by increasing Rab23 expression by sponging miR-362-3p. Cancer Cell Int. 2019;19:211. doi: 10.1186/s12935-019-0926-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 10.Li C, Zhang L, Meng G, et al. Circular RNAs: pivotal molecular regulators and novel diagnostic and prognostic biomarkers in non-small cell lung cancer. J Cancer Res Clin Oncol. 2019;145(12):2875–2889. doi: 10.1007/s00432-019-03045-4 [DOI] [PubMed] [Google Scholar]
- 11.Jin M, Shi C, Yang C, Liu J, Huang G. Upregulated circRNA ARHGAP10 predicts an unfavorable prognosis in NSCLC through regulation of the miR-150-5p/GLUT-1 Axis. Mol Ther Nucleic Acids. 2019;18:219–231. doi: 10.1016/j.omtn.2019.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong S. Circular RNA SMARCA5 may serve as a tumor suppressor in non-small cell lung cancer. J Clin Lab Anal. 2020;34:e23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han T, Kang D, Ji D, et al. How does cancer cell metabolism affect tumor migration and invasion? Cell Adh Migr. 2013;7(5):395–403. Epub 2013/ 10/18. doi: 10.4161/cam.26345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Y, Du Y, Yang X, et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer. 2018;17(1):79. doi: 10.1186/s12943-018-0827-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan X, Shen N, Chen J, Wang J, Zhu Q, Zhai Z. Circular RNA MYLK serves as an oncogene to promote cancer progression via microRNA-195/cyclin D1 axis in laryngeal squamous cell carcinoma. Biosci Rep. 2019;39(9). doi: 10.1042/BSR20190227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J, Xu T, Chen F, Zhang Y. MiRNA-195-5p functions as a tumor suppressor and a predictive of poor prognosis in non-small cell lung cancer by directly targeting CIAPIN1. Pathol Oncol Res. 2019;25(3):1181–1190. doi: 10.1007/s12253-018-0552-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo J, Pan J, Jin Y, Li M, Chen M. MiR-195-5p inhibits proliferation and induces apoptosis of non-small cell lung cancer cells by targeting CEP55. Onco Targets Ther. 2019;12:11465–11474. doi: 10.2147/OTT.S226921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ancey PB, Contat C, Meylan E. Glucose transporters in cancer – from tumor cells to the tumor microenvironment. FEBS J. 2018;285(16):2926–2943. doi: 10.1111/febs.14577 [DOI] [PubMed] [Google Scholar]
- 19.Masin M, Vazquez J, Rossi S, et al. GLUT3 is induced during epithelial-mesenchymal transition and promotes tumor cell proliferation in non-small cell lung cancer. Cancer Metab. 2014;2:11. doi: 10.1186/2049-3002-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fei X, Qi M, Wu B, Song Y, Wang Y, Li T. MicroRNA-195-5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett. 2012;586(4):392–397. doi: 10.1016/j.febslet.2012.01.006 [DOI] [PubMed] [Google Scholar]