ABSTRACT
A 40-year-old man developed acute brainstem dysfunction 3 days after hospital admission with symptoms of the novel SARS-CoV-2 infection (COVID-19). Magnetic resonance imaging showed changes in keeping with inflammation of the brainstem and the upper cervical cord, leading to a diagnosis of rhombencephalitis. No other cause explained the patient's abnormal neurological findings. He was managed conservatively with rapid spontaneous improvement in some of his neurological signs and was discharged home with continued neurology follow up.
KEYWORDS: COVID-19, rhombencephalitis, brainstem encephalitis, MRI, hepatitis
Case presentation
A 40-year-old never-smoker with minimum alcohol intake, originally from Nigeria and now settled in the UK with his family after moving here 7 years ago, attended the emergency department reporting a 10-day history of persistent fever and progressive dyspnoea on exertion while self-isolating at home during the COVID-19 crisis. He was on long-term treatment with ramipril and amlodipine for hypertension and on dorzolamide (a carbonic anhydrase inhibitor) with timolol maleate eye drops for closed angle glaucoma. He reported malaise, a new cough with yellow sputum and diarrhoea (non-bloody) over 3 days. There was no recent foreign travel or family history of medical conditions but he shared the concern that his wife was currently pregnant.
On presentation, temperature was 38.4°C, heart rate regular at 86 beats/minute, blood pressure 129/83 mmHg; oxygen saturation 93% on room air as he was tachypnoeic at 32 breaths/minute. On auscultation, heart sounds were normal but there were bi-basal crackles. There was no gross focal neurological deficit. Initial 12-lead electrocardiography showed sinus tachycardia and chest X-ray showed a right lower zone consolidation. Arterial blood gas on room air revealed hypoxia (PaO2 8.77 kPa) with pH 7.432, PaCO2 4.21 kPa, HCO3– 20.6 mmol/L, base excess 2.6 mmol/L, lactate 0.98 mmol/L. Haemoglobin was 139 g/L, white cell count 7.0 × 109/L (lymphocytes 1.2 × 109/L) and C-reactive protein (CRP) marginally raised at 50 mg/L, and similar slight increases were seen in serum gamma glutamyl transferase (GGT) 107 U/L (range 0–75) and alanine aminotransferase (ALT) 88 U/L (range 0–45), with other liver tests and urinary electrolytes within the normal ranges.
Diagnosis
Working diagnosis was COVID-19 pneumonia.
Initial management and prognosis
The patient continued with oral amoxicillin 500 mg three times per day and oral paracetamol 1 g four times per day. He was isolated and an upper respiratory swab (nose/throat) detected SARS-CoV-2 ribonucleic acid (RNA) by PCR.
Case progression and outcome
Although initially well, on the third day of admission the patient complained of an unsteady gait. Over the next 24 hours he developed diplopia, oscillopsia, limb ataxia, altered sensation in right arm, hiccups and dribbling when eating or drinking. Clinical examination revealed mild bilateral facial weakness, reduced tongue movements to both sides and slight deviation to the right on protrusion. Palate movement was normal. The motor and sensory function of trigeminal nerve was intact. Range of eye movement was normal but with an upbeat nystagmus on all directions of movement. There was upper (mild) and lower (moderate) limb ataxia, more prominent on the right. Peripheral reflexes were intact and plantar responses were flexor. Proprioception and joint position sense were intact. Upper and lower limb muscle power was normal.
Clinical differential included a Miller–Fisher variant of Guillain–Barré as a potential post-infectious complication of the COVID-19 illness, but an urgently organised magnetic resonance imaging of the brain and cervical spine suggested an inflammatory rhombencephalitis/myelitis; Fig 1 highlights the increased signal lesion in the right inferior cerebellar peduncle, extending to involve a small portion of the upper cord. The lesion measured 13 mm in maximum cross-sectional area and 28 mm in longitudinal extent. There was swelling at the affected tissue and associated micro-haemorrhage. The supratentorial region of the brain was normal.
Fig 1.
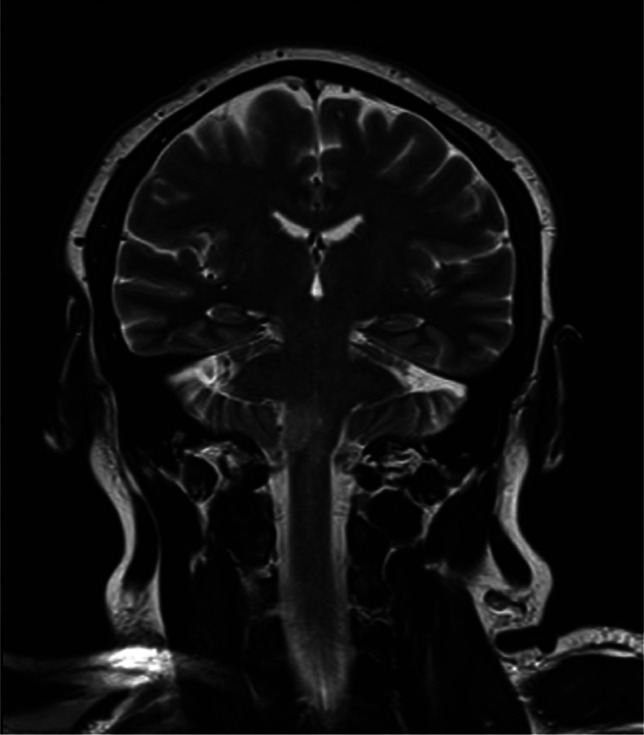
T2 coronal MRI.
Cerebrospinal fluid (CSF) showed a normal protein (423 mg/L) with no rise in white cells and negative bacterial culture. Unfortunately only a low volume sample could be obtained and PCR for SARS-CoV-2 RNA was not possible. Myelin oligodendrocyte glycoprotein antibody (MOG-IgG) and aquaporin 4 antibody results were requested.
The patient acutely developed derangement of liver enzymes with GGT 628 U/L, ALT 542 U/L, bilirubin 28 μmol/L (range 0–21) and a rise in alkaline phosphatase from initial 70 U/L to 132 U/L (upper range 130 U/L). Liver ultrasound suggested an inflammatory diffusely hypoechoic liver with a raised periportal and pericholecystic echogenicity. Liver infection screen including hepatitis A, B, C, HIV 1 and 2, and syphilis antibody screen were negative.
The patient's neurological symptoms and liver enzymes improved steadily and he was discharged home after 11 days with neurology follow-up. At discharge he was prescribed gabapentin 300 mg twice per day orally with some resulting improvement in his hiccups and nystagmus but oscillopsia and ataxia persisted.
Discussion
The world is currently focusing on the COVID-19 pandemic, with the majority of admissions and complications being related to pulmonary dysfunction. This case is a rare presentation of COVID-19 infection presenting a wider inflammatory process involving respiratory and hepatic systems but progressing to cause neurological dysfunction.
The term rhombencephalitis has previously been used interchangeably with brain stem encephalitis even though they are anatomically slightly different; here, it refers to inflammatory disease affecting the hindbrain. Previous literature has reported autoimmune, infective and paraneoplastic causes. Moragas et al in 2011 reported a series of 97 patients where 32% had an unknown cause, followed by multiple sclerosis, Behçet disease, listeria monocytogenes infection, paraneoplastic syndrome, Epstein–Barr virus and tuberculosis.1 Listeria monocytogenes has been recognised as the most common infectious cause but is unlikely here given the lack of associated CSF protein rise or pleocytosis. Another case series in 2016 explored patients with MOG-IgG positive optic neuritis and myelitis, with the authors identifying a subgroup with brainstem involvement and recognised cases with post-infective onset.2 Anti-MOG antibodies are found in up to half of patients with an acquired demyelinating syndrome.
A recent case report by Moriquchi et al from Wuhan, China reported a case of meningitis/encephalitis associated with COVID-19 presenting with symptoms of meningism and a raised CSF white cell count.3 Hung et al reported a case of severe acute respiratory syndrome coronavirus (SARS-CoV) associated encephalitis; the CSF cell count, protein and glucose were normal, but SARS-CoV-2 RNA was detected in the CSF.4 Another case report from China described acute myelitis as a complication of COVID-19 presenting with flaccid lower limb weakness and persistent fever; a lumbar puncture was not performed but the authors felt the likely cause of myelitis was a post-viral inflammatory process.5
As illustrated with this unusual case of rhombencephalitis, with evidence for a wider multi-system inflammatory response and the development of neurological symptoms over time, it is important to be vigilant for late non-respiratory manifestations of COVID-19 infections.
Learning points
Rhombencephalitis is a complication of COVID-19 infection.
CSF should be examined to exclude other infections.
There is currently a lack of guidance on the management of COVID-19 neurological complications, with little information on timelines of improvement and follow up.
References
- 1.Moragas M, Martínez-Yélamos S, Majós C, et al. Rhombencephalitis: a series of 97 patients. Medicine (Baltimore) 2011;90:256–61. [DOI] [PubMed] [Google Scholar]
- 2.Jarius S, Kleiter I, Ruprecht K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 3: Brainstem involvement – frequency, presentation and outcome. J Neuroinflammation 2016;13:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020;94:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung EC, Chim SS, Chan PK, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem 2003;49:2108–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao K, Huang J, Dai D, et al. Acute myelitis after SARS-CoV-2 infection: a case report. MedRxiv 2020.03.16.20035105 (10.1101/2020.03.16.20035105). [Google Scholar]


