Abstract
Targeted gene ablation studies of the endocrine pancreas have long suffered from suboptimal Cre deleter strains. In many cases, Cre lines purportedly specific for beta cells also displayed expression in other islet endocrine cells or in a subset of neurons in the brain. Several pancreas and endocrine Cre lines have experienced silencing or mosaicism over time. In addition, many Cre transgenic constructs were designed to include the hGH mini-gene, which by itself increases beta-cell replication and decreases beta-cell function. More recently, driver lines with Cre or CreER inserted into the Ins1 locus were generated, with the intent of producing β cell-specific Cre lines with faithful recapitulation of insulin expression. These lines were bred in multiple labs to several different mouse lines harboring various lox alleles. In our hands, the ability of the Ins1-Cre and Ins1-CreER lines to delete target genes varied from that originally reported, with both alleles displaying low levels of expression, increased levels of methylation compared to the wild-type allele, and ultimately inefficient or absent target deletion. Thus, caution is warranted in the interpretation of results obtained with these genetic tools, and Cre expression and activity should be monitored regularly when using these lines.
Keywords: islets, mouse models, beta cells
The pancreas field has long searched for ideal spatially and temporally regulated Cre recombinase mouse lines, both for exocrine pancreatic epithelium and for specific endocrine cell types, a quest which has been hampered by several factors (1): the overlap of gene expression between the brain and the endocrine pancreas conferred by several promoters (1–3); the overlap of gene expression between enteroendocrine cells and the endocrine pancreas, including preproglucagon and somatostatin (3–5); expression of the hGH mini-gene in several pancreas-targeting Cre transgenic lines, which results in increased β-cell proliferation and either decreased (6, 7) or increased insulin secretion (8) in the absence of any other genetic manipulation (4); and mosaicism, aberrant activation, or silencing of several pancreas-specific Cre lines (9).
Many Cre driver lines were derived using standard transgenesis. Transgenic lines can suffer from positional effect variegation, so that integration into a heterochromatic or telomeric region results in diminished expression or silencing (10), and integration into sites that are targets of strong enhancers can cause expression of the transgene in unexpected tissues. In addition, silencing of many transgenic lines occurs over multiple generations so that, with time, the expression pattern and efficiency of Cre can differ from its initial characterization. The inactivation that can occur with time has been attributed to a variety of factors, including the presence of bacterial or viral DNA sequences in the transgene construct, high-copy number (11), and CpG content (12). Silenced transgenes have high levels of CpG methylation (11), a general marker of silenced chromatin (13).
Cre drivers engineered to be expressed in an endogenous locus (so-called knock-ins or, more formally, gene replacement alleles), are thought to have several advantages over transgenic lines. Expression of the Cre will be driven only by endogenous regulatory elements, eliminating positional integration effects. However, disadvantages of the gene replacement strategy also exist. Although gene replacement models can be engineered to maintain expression of the endogenous gene, many gene replacement Cre lines ablate expression of the endogenous gene. This approach can cause problems when the gene in question is haploinsufficient, or when used in a homozygous state, since some of these null alleles will have nonwild-type phenotypes alone or in conjunction with an additional ablated gene, that is, result in a synthetic phenotype.
In 2015, 2 new beta cell-specific Cre lines were introduced, Ins1-Cre and Ins1-CreER, both of which were integrated into the Ins1 locus (14). These new lines drove considerable excitement in the β-cell field with their promised fidelity in expression and likely resistance to mosaicism and silencing. In addition, these lines would be predicted and were reported to have a wild-type phenotype despite the absence of 1 Ins1 allele due to the presence of Ins gene duplication in mice, specifically the presence of the Ins2 gene. However, we present evidence that the Ins1-Cre and Ins1-CreERlines are, in fact, susceptible to silencing by CpG methylation, and that silencing has been observed at multiple institutions.
Methods
Mice
Creb fl (15, 16), Foxm1fl (17, 18), Ins1-Cre (14, 19), Ins1-CreER (14, 20), Pcbp2fl (21, 22), rat insulin 2 promoter (RIP)-Cre (23, 24), Pdx1-Cre (25, 26), MIP-CreER (2, 27), Gt(ROSA)26SorEYFP (28, 29), Gt(ROSA)26Sortm14(CAG-tdTomato)Hze (30, 31), EP4fl/fl (32, 33), and Gcg-CreER (34, 35) mice, as well as genotyping methods, have all been reported previously. The generation of G6pc2fl/fl mice was achieved by purchasing embryonic stem (ES) cells containing a floxed G6pc2 allele from the Knockout Mouse Project (KOMP) Repository (Project #CSD28099 (36)). In the floxed allele, FRT sites surround the LacZ and Neo components of the targeting vector, whereas LoxP sites surround G6pc2 exon 3, which encodes the third transmembrane domain. These ES cells were injected into fertilized eggs by the Vanderbilt Transgenic Mouse/ES Stem Cell Resource.
All mice were maintained on mixed genetic backgrounds, with the exception of the Pcbp2 line, which was maintained on a C57Bl6/N background. Male and female mice between the ages of 8 and 20 weeks were used. Mice were housed in pathogen-free cages with corn cob or paper bedding in a 12-hour light/dark cycle, with ad libitum access to food and water. For all studies, littermate cohorts were used. All mice were maintained and experiments conducted in accordance with protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee or the Vanderbilt University Institutional Animal Care and Use Committee under the supervision of the Office of Animal Welfare.
Tamoxifen administration
Tamoxifen at 25 mg/mL (T5648; Sigma-Aldrich, St. Louis, MO), dissolved in 90% (v/v) sunflower seed oil and 10% (v/v) ethanol, or vehicle was orally administered in the home cage. For C57BL/6 male Ins1CreErt2/+;Pcbp2fl/fl mice, tamoxifen was delivered by oral gavage at 5 weeks of age every other morning for a total of 3 injections in 5 days. For Ins1CreErt2/+;Crebfl/fl or Gcg-CreER mice, tamoxifen was administered interperitoneally at 8 weeks of age for 3 consecutive mornings, as previously described (35). Ins1CreErt2/+;EP4fl/fl mice were injected subcutaneously, as previously described (37). Treatment versus vehicle groups were randomly assigned.
Glucose tolerance tests
Glucose tolerance tests were performed after a 16-hour overnight fast in cages with Alpha-Dri bedding. Mice were injected with 2 g/kg dextrose in 1X phosphate-buffered saline and blood glucose was determined by a handheld glucometer. Injection order was randomly assigned and mice were assessed at specific time points after injections.
Lentiviral transduction of islets
Cre recombinase under the control of the rat insulin 2 promoter were cloned into the CMV pLenti puro lentiviral backbone. 293 T cells (38) were transfected in OptiMEM and lentivirus was collected 2- and 3-days post-transfection in standard Dulbecco’s Modified Eagle’s Medium. Lentivirus was concentrated by ultracentrifugation and viral titers were determined by qRT-PCR. Islets were isolated from 8-week-old mice by collagenase digestion and transduced with lentivirus as described (39). Islets were cultured overnight in serum-free islet medium at a multiplicity of infection (MOI) of 90 and harvested 10 days post-transduction.
Immunohistochemistry, immunofluorescence, and quantification of histological features
For CREB immunolabeling, pancreata were fixed in 4% paraformaldehyde for 4 hours at room temperature, washed in PBS, incubated in 30% sucrose in 1XPBS overnight, and frozen on dry ice in Optimal Cutting Temperature before sectioning. For PCBP2 detection, pancreata were fixed in 4% PFA at 4°C overnight, embedded in paraffin, and sectioned, or islets were collected, dispersed to single cells, and fixed for 10 minutes at room temperature. Fixed cells were attached to slides by cytospin and permeabilized with 0.1% Triton X-100 (10 minutes at room temperature). For Cre immunolabeling, pancreata were fixed in 4% PFA at room temperature, embedded in paraffin, and sectioned. Antigen retrieval was performed by a fast boil for 1 minute followed by slow boil for 7.5 minutes in 1X TEG buffer of pH 9.0.
The following primary antibodies were used: polyclonal guinea pig anti-insulin (1:1000; Dako A0564 (40)), rabbit anti-CREB (1:1000; Cell Signaling 9197 (41)), rabbit anti-PCBP2 (1:1000; a gift from Dr. Stephen Liebhaber, University of Pennsylvania (42)), rat anti-dsRed (1:1000; Chromotek, Planegg, Germany (43)), and rabbit anti-Cre (1:2000; Novagen, Madison, WI (44)). Secondary antibodies were used at 1:400–1:600 and obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) (45–48). Imaging was performed on the BZ-X Series All-in-One Fluorescence Microscope (Keyence, Elmwood Park, NJ), the Nikon EclipseE600 Metamorph Imaging Software (Molecular Devices, Downingtown, PA), or on a Nikon EclipseE80i using iVision imaging software (BioVision Technologies, Milpitas, CA). For quantification of CREB expression in β cells, ~4000 β cells were counted per animal. For PCPB2, ~200 cells were counted per animal. Researchers were blinded to genotype of animals while counting.
RNA and DNA isolation
Islets were collected from 12-week-old (Foxm1fl/fl line), 20-week-old (Crebfl/fl line), or 8-week-old (Pcbp2fl/fl and Gcg-CreER line) by collagenase digestion, followed by RNA and DNA isolation using the All-Prep micro kit (Qiagen, Germantown, MD), or as previously described (49).
Flow cytometry analysis of yellow fluorescent protein expression
Ins1-Cre; Gt(ROSA)26SorEYFP/+, Gt(ROSA)26SorEYFP/+, or Ins1-Cre; Gt(ROSA)26Sor+/+ mice were derived from breeding to the Ins1-Cre;Foxm1fl line. Islets from 8- to 12 week-old mice were isolated by collagenase digestion, cultured overnight, then dispersed using 0.025% Trypsin digestion followed by fixation in 4% PFA. Cells were perforated using saponin, then immunolabeled with rabbit anti-glucagon (1:200; Santa Cruz, Dallas, TX (50)), guinea pig anti-insulin (1:1000; Dako, Now Agilent, Santa Clara, CA (40)) and goat anti-GFP (1:200; Abcam, Cambridge, MA (51)), followed by immunolabeling with fluorescently-labeled secondary antibodies. Cells were then subjected to flow cytometry.
Methylation analysis
Pyrosequencing primers for the Ins1 locus and the Cre or CreER gene replacement alleles were designed manually using Primer3Plus (see Table 1). 50 ng of genomic DNA were bisulfite converted using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA). Target regions were amplified using the PyroMark PCR Kit (Qiagen) using the manufacturer’s protocol. Pyrosequencing and methylation results were read on PyroMark Q96 MD using the PyroMark Gold Q96 CDT Reagents (Qiagen).
Table 1.
Primers used: primers 1–7 were used for pyrosequencing and 8–11 for qRT-PCR.
| Primer Number | Primer Name | Sequence 5’ - 3’ |
|---|---|---|
| 1 | Ins1_Promoter_F | TTTAGTTGATTAATGAGTGGGTT |
| 2 | Ins1_Promoter_R | CCTTAACTCAAATAAAATCCCAA |
| 3 | Ins1_wt_F | TTTTATTTGGTGTGTGGGGA |
| 4 | Ins1_wt_R | ACAACACTAATCCACAATACCA |
| 5 | Ins1_wt_seq | AGTGGAGGATTTATAAGTGGA |
| 6 | Cre/CreER_F and seq | AGTTTTGATTTGAGGTTAGA |
| 7 | Cre/CreER_R | ATCACAAACTTAAAATTCCT |
| 8 | Cre F qPCR Penn | CGGTCTGGCAGTAAAAACTAT |
| 9 | CreR qPCR Penn | CAGGGTGTTATAAGCAATCCC |
| 10 | CreF qPCR Vandy | TACCGGAGATCATGCAAGCTG |
| 11 | CreR qPCR Vandy | TGCCCCTGTTTCACTATCCAG |
| 12 | Foxm1F qPCR | CTCCAAGGCAAAGACAGGAG |
| 13 | Foxm1R qPCR | GCCCGTCAGAACTCATCTTT |
Statistics
Data are presented as means ± SEM. Student’s t-test or one-way or two-way ANOVA was used, as described in the figure legends. P-values < 0.05 were considered significant.
Results
When characterizing a variety of mice with floxed alleles bred to the Ins1-Cre and Ins1-CreER mice, multiple investigators at different institutions (Table 2) noticed the inefficient deletion of a target gene associated with a mild phenotype and accompanied by partial Cre expression, or the complete lack of a phenotype accompanied by absent Cre expression. Below, we detail our experiences implementing these Cre replacement alleles.
Table 2.
Detailed mouse line information.
| Mouse Line | Lab Using Mouse Line | Institution Housing Mice | Original Reference for Cre Allele |
|---|---|---|---|
| Ins1-Cre; Foxm1 fl/fl | Golson | University of Pennsylvania | (13) |
| RIP-Cre; Foxm1 fl/fl | Golson | University of Pennsylvania | (17) |
| Ins1-Cre;G6pc2 | O’Brien | Vanderbilt University Medical Center | (13) |
| Ins1-Cre;EP4 | Gannon | Vanderbilt University Medical Center | (13) |
| Ins1-Cre ER ; Creb fl/fl | Golson | Vanderbilt University Medical Center | (13) |
| Ins1-Cre ER ; Pcbp2 | Stoffers | University of Pennsylvania | (13) |
| Ins1-Cre ER | Gannon | Vanderbilt University Medical Center | (13) |
| MIP-CRE ER | Gannon | Vanderbilt University Medical Center | (2) |
| Pdx1-Cre | Gannon | Vanderbilt University Medical Center | (19) |
| Gcg-CreER | Golson | University of Pennsylvania | (23) |
Ins1-Cre fails to efficiently delete targets
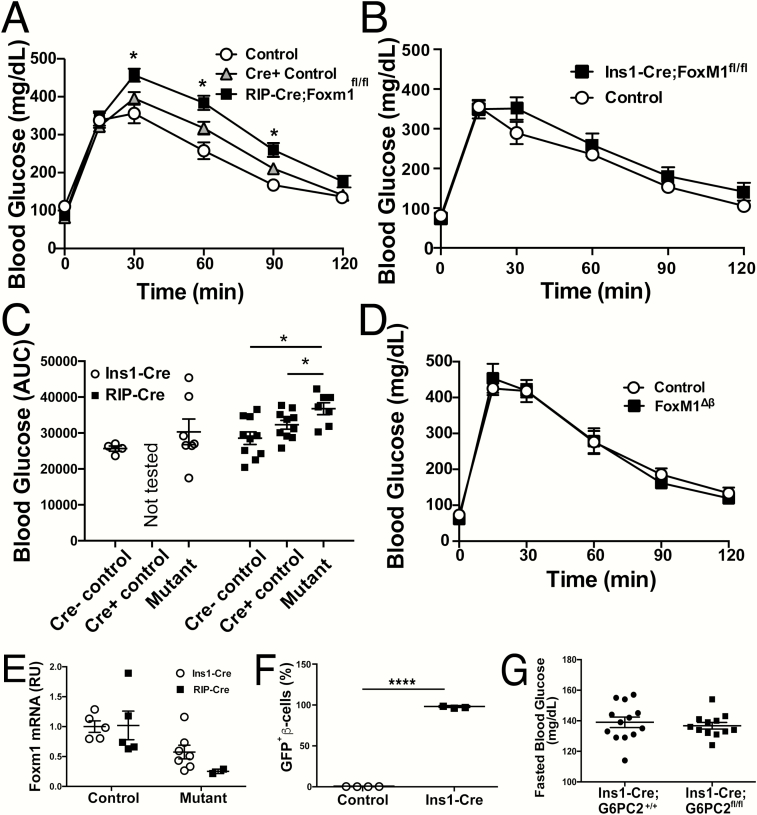
FoxM1 is a transcription factor required for proliferation in a variety of tissues (52). When Foxm1 is ablated in the pancreas using Pdx1-Creearly, a Cre line that does not contain the hGH mini-gene (6, 53), beta-cell replication and elevations in beta-cell mass stall at around 4 weeks of age in both males and females (54). Pdx1-Cre;Foxm1fl/fl male mice display glucose intolerance or diabetes by 9 weeks of age, a phenotype that is replicated when Foxm1 is ablated using RIP-Cre, even when compared to RIP-Cre controls (Fig. 1A and 1C). When we employed Ins1-Cre (acquired from Jackson Laboratories) to delete Foxm1, the impairment in glucose tolerance observed was significantly diminished compared to that observed when Foxm1 was deleted by other Cre drivers (Fig. 1B and 1C and (54)) and was not statistically significantly different from the glucose tolerance of littermate controls. We further noted that Ins1-Cre;Foxm1fl/fl mice had a wide discrepancy in glucose excursion, which is easily appreciated when examining the area under the curve (AUC) (Fig. 1C); in fact, only 2 of 7 mice had elevated glucose compared to their littermate controls.
Figure 1.
Ins1-Cre yields an attenuated glucose-tolerance phenotype when recombining Foxm1 compared to other Cre lines. Glucose tolerance tests for Cre- (n = 4), Cre+ (n = 10), and RIP-Cre;Foxm1fl/fl (n = 7) male mice (A); and controls (n = 4) and Ins1-Cre;Foxm1fl/fl male mice (n = 10) at 9 weeks of age (B). C: Area under the curve for GTTs presented in (A) and (B). D: Glucose tolerance tests in female controls (n = 4) and Ins1-Cre; Foxm1fl/fl (n = 3) mice at GD15.5. E:Foxm1 mRNA expression from whole islets. F: Efficient recombination of the RosaYFP locus despite lack of Foxm1 recombination using the Ins1-Cre deleter strain. G: No difference in blood glucose between Ins1-Cre+/-;G6PC2+/+ and Ins1-Cre+/-;G6PC2fl/fl male mice. For A–B, *P > 0.05 by Two-Way ANOVA. For C–E, *P < 0.05 and ****P < 0.001 by Student’s t-test.
While female Pdx1-Cre;Foxm1fl/fl display the same decreased beta-cell mass as males, their blood glucose homeostasis is normal except when further stressed metabolically, such as during pregnancy (55). However, Ins1-Cre;Foxm1fl/fl female mice exhibit no alteration in glucose tolerance at gestational day (GD) 15.5 compared to controls (Fig. 1D).
We hypothesized that the diminished phenotype in both males and females was due to the inefficient removal of Foxm1. We therefore examined expression of Foxm1 in whole islets. Foxm1 RNA levels in Ins1-Cre;Foxm1fl/fl mice ranged from 25% to 125% of the control average, while those in RIP-Cre mice were around 25% of control (Fig. 1E), confirming less efficient deletion when using Ins1-Cre than when using RIP-Cre. Flow analysis on islets from Ins1-Cre;Foxm1fl;Gt(ROSA)26SorEYFP/+ mice demonstrated that more than 95% of beta cells exhibited yellow fluorescent protein (YFP) expression (Fig. 1F). Although seemingly at odds with the less extensive deletion of Foxm1, these data likely reflect different requirements of Cre protein levels for recombination at different loci (9, 56). Taken together, these data indicate that Ins1-Cre deletion of Foxm1 is variable and in most cases insufficient to result in altered glucose homeostasis.
A second line bred to the Ins1-Cre deleter strain also failed to demonstrate the expected abnormal phenotype. G6PC2 is highly enriched in islets with low expression in other metabolic tissues (57). G6PC2 catalyzes the hydrolysis of glucose-6-phosphate to glucose and inorganic phosphate, thereby opposing the action of glucokinase and creating a futile substrate cycle. G6pc2-null mice have reduced fasting blood glucose (FBG), with no difference in fasting plasma insulin, due to a leftward shift in the sensitivity of GSIS to glucose (57). However, Ins1-Cre;G6pc2fl/fl mice display no difference in FBG compared to controls (Fig. 1G), which was unexpected given the specificity of G6pc2 expression to islets and the phenotype of the null mice.
Ins1-CreER also fails to ablate targets
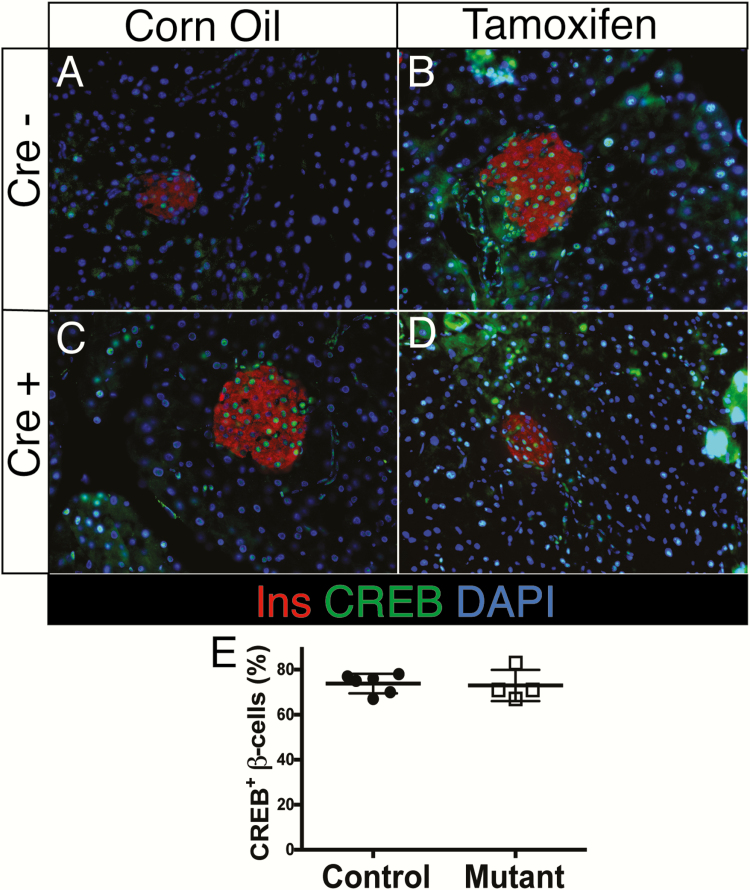
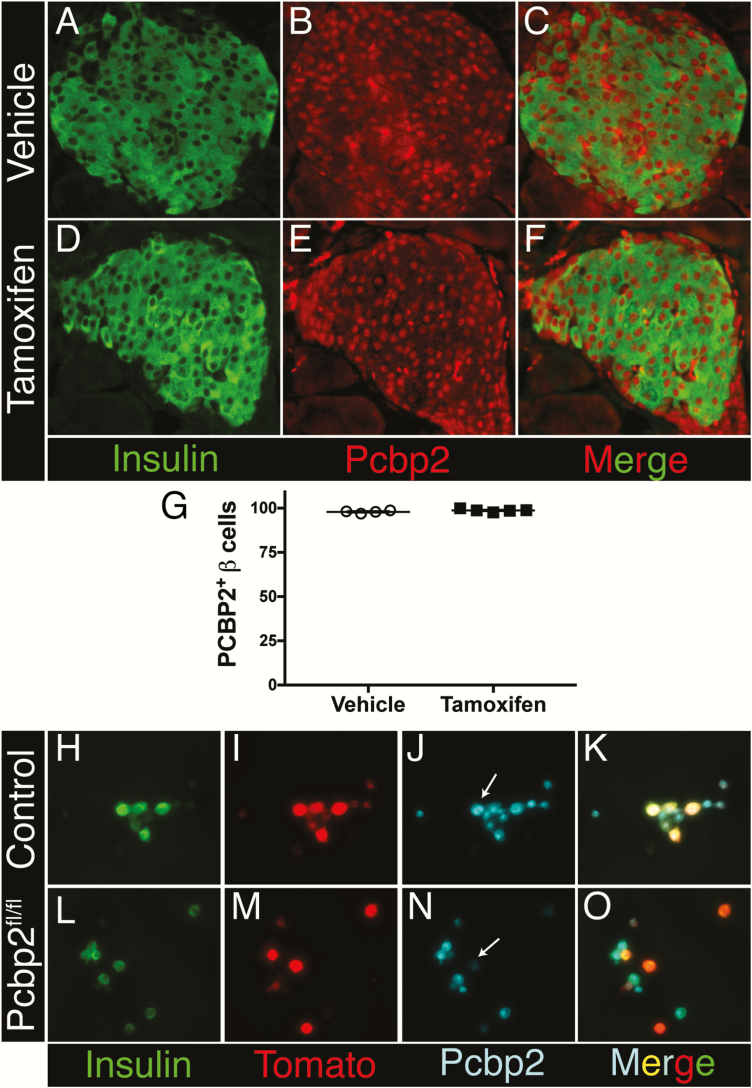
In addition to utilizing Ins1-Cre, we also employed the Ins1-CreER allele to delete multiple targets, including the transcription factor cAMP response element-binding protein (Creb) and the RNA-binding protein Poly-C-binding protein 2 (Pcbp2). Immunolabeling of the CREB protein in tamoxifen-injected Ins1-CreER;Crebfl/fl mice revealed no alteration in the number of CREB+ beta cells (Fig. 2). Creb has previously been deleted in beta cells using Pdx1-CreER, and efficient loss of CREB was demonstrated using the same antiserum employed here (58). Similarly, when using Ins1-CreER to delete Pcbp2, PCBP2 could be detected in all beta cells of Ins1-CreER;Pcbp2fl/fl mice even after tamoxifen injection (Fig. 3A–3F). By contrast, PCBP2 was effectively reduced after ex vivo lentiviral delivery of Cre recombinase (present in 231/231 or 100% of control beta cells, but only 11/198 or 5.6% of Pcbp2fl/fl mice), reflecting both the specificity of the PCBP2 antiserum and the ability of the floxed Pcbp2 locus to undergo Cre-mediated recombination in beta cells (Fig. 3G–3N). Remaining low PCBP2 expression in some beta cells is likely due to protein perdurance.
Figure 2.
Ins1-Cre ER fails to ablate CREB expression in beta cells after IP tamoxifen injection. Immunofluorescence for insulin and CREB in corn-oil (A and C) or tamoxifen-treated (B and D) Cre- (A and B) or Cre+ (C and D) Crebfl/fl male mice. E: Quantification of CREB+ β -cells (n = 4–6, no significant difference by Student’s t-test).
Figure 3.
Ins1-Cre ER fails to ablate Pcbp2 expression in beta cells after tamoxifen gavage. Immunofluorescence for insulin and PCBP2 in oil (A–C) or tamoxifen-treated (D–F) Ins1-CreER;Pcbp2fl/fl mice. G: Quantification of PCPB2 expression in vehicle or tamoxifen-treated Ins1-CreER;Pcbp2fl/fl mice. Immunofluorescence for insulin, tdTomato, and PCBP2 in dispersed islet cells from Pcbp2+/+; Gt(ROSA)26Sortm14(CAG-tdTomato)Hz/+ (H–K) and Pcbp2fl/fl; Gt(ROSA)26Sortm14(CAG-tdTomato)Hz/+ mice (L–O) following ex vivo lentiviral delivery of Cre recombinase. White arrowheads indicate transduced beta cells as determined by the coexpression of insulin and the Cre-inducible tdTomato reporter.
Low to zero Cre expression is observed in multiple independent Ins1-Cre and Ins1-CreER colonies
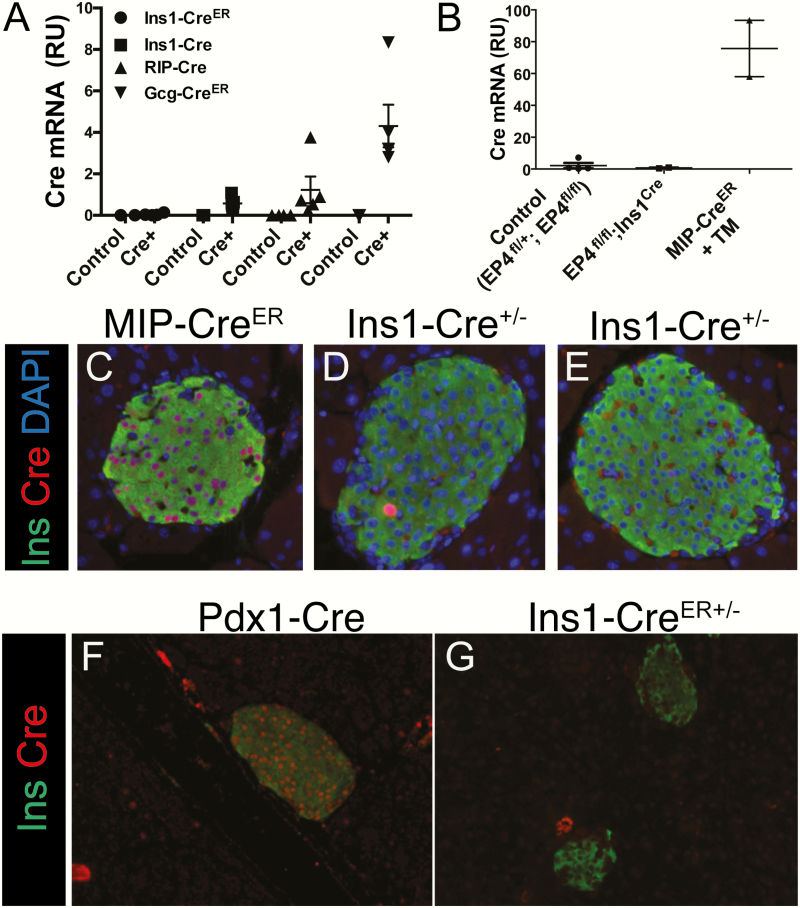
The inability of Ins1-Cre and Ins1-CreER lines to ablate Foxm1, Creb, and Pcbp2, despite successful ablation by other Cre drivers or methods of Cre introduction, suggests silencing of Cre expression. To assess this possibility, we compared whole-islet Cre expression from Ins1-Cre, Ins1-CreER, RIP-Cre, and GCG-CreER lines. Virtually no Cre transcript was detected in the Ins1-CreER islets. The Ins1-Cre mice bred to Foxm1fl displayed Cre expression at an average of one half of that of RIP-Cre mice. Interestingly, although alpha cells make up only around 15% of the mouse islet, Cre expression from the replacement allele Gcg-CreER (57) was around 4 times higher than from the RIP-Cre transgene and 8 times higher than from the Ins1-Cre allele (Fig. 4A).
Figure 4.
Ins1-Cre and Ins1-CreER have low or absent Cre expression. A: Cre expression in Ins1-CreER, Ins1-Cre, RIP-Cre, and Gcg-CreER mice. Legend indicates Cre line while X-axis label indicates genotype (n = 3–5). B:Ins1-Cre;EP4fl/fl mice display no Cre mRNA expression. C–G:Ins1-Cre+/Cre and Ins1-CreER(/Cre) mice (D, E, G) display no Cre protein expression, while it is easily detectable in MIP-CreER mice that received subcutaneous injections of tamoxifen (C) as well as in Pdx1-Cre (F) mice.
We also characterized Cre expression in the islets of Ins1-CreER mice bred to EP4fl/fl mice. No Cre expression was detected in Ins1-CreER mice, although abundant Cre expression was apparent in MIP-CreER mice (Fig. 4B). To further examine whether Cre was expressed in the beta cells of Ins1-Cre and Ins1-CreER mice, immunolabeling of Cre protein was performed (Fig. 4C–4G). No Cre protein was detected in these Ins1-Cre and Ins1-CreER lines; however, the majority of beta cells in Pdx1-Cre and tamoxifen-injected MIP-CreER mice expressed Cre.
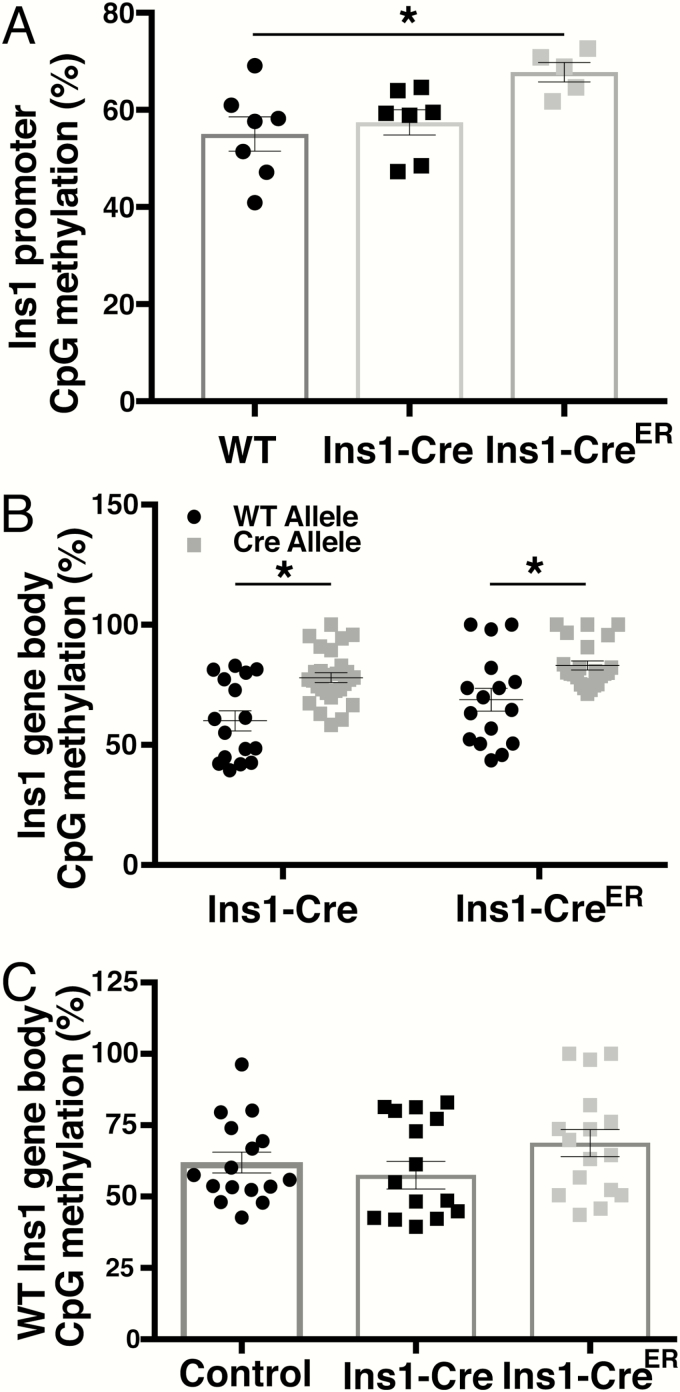
Both Ins1-Cre driver alleles exhibit higher methylation than wild-typealleles
Since both the Ins1-Cre and Ins1-CreER lines failed to delete floxed alleles and lacked Cre mRNA and protein expression, we postulated that the gene replacement alleles had been silenced by cytosine methylation of CpGs within their promoter and gene body. The Ins1 promoter, whether of the wild-type or Cre allele, has 1 CpG, while the gene bodies of the wild-type and Cre alleles contain 4 CpGs each. We used pyrosequencing to determine the percent of methylation of CpGs in both the promoter and gene body of the wild-type, Ins1-Cre, and Ins1-CreER alleles in whole islets. The promoter sequence of Ins1 is identical in both the wild-type and Ins1-CreER alleles, and they cannot, therefore, be distinguished from one another. However, since the gene body is altered in the Ins1-CreER allele, the percent of methylation can be separately determined for the wild-type and exogenous alleles.
Mice with no Cre allele had ~55% CpG methylation in the Ins1 promoter. No difference was observed between control and Ins1-Cre mice. In contrast, in Ins1-CreER mice, the Ins1 promoter displayed close to 70% methylation (Fig. 5A). Since we could discriminate between the wild-type and the 2 Ins1-Cre alleles within the gene body, we compared methylation of the two alleles within Ins1-Cre+/Cre and Ins1-CreER(+/Cre) islets. For both Ins1-Cre and Ins1-CreER mice, the Cre allele was more highly methylated than the wild-type Ins1 (Fig. 5B). Methylation of the wild-type allele in Ins1-Cre and Ins1-CreER mice was not altered compared to control mice (Fig. 5C), indicating that the alteration observed in methylation of the Cre alleles is not due to differences in the percentage of beta cells within the isolated islets. In addition, this result demonstrates that, as expected, the Cre allele does not have an effect on the methylation of the wild-type allele in trans.
Figure 5.
CpG methylation in the promoter and gene body of Ins1 replacement alleles. A: Methylation of single CpG located the in Ins1 promoter of Ins1-Cre+/+, Ins1-Cre+/Cre, and Ins1-CreER+/Cre mice (n = 5–7 mice). B: Methylation of each of 4 CpGs in the wild-type and Cre allele in the Ins1 gene body in Ins1-Cre and Ins1-CreER mice (n = 4–8 mice). C: Methylation of each of 4 CPGs in the wild-type allele of Ins1 in wild-type, Ins1-Cre, and Ins1-CreER mice (n = 4 mice). Note that gene body methylation data for Cre+ mice in (B) and (C) is the same but provided in 2 separate graphs for clarity in comparison to wild-type mice. *P < 0.05 by one-way ANOVA. Both male and female mice are included in all assays.
Discussion
New pancreas-, endocrine-, and beta-cell specific Cre drivers are necessary for the diabetes and pancreas research fields, since the existing lines that have been used for decades are not as tissue-specific as originally described, have become silenced, or contain the hGH mini-gene, which independently drives beta-cell replication. The recently established Ins1-Cre lines were targeted to the Ins1 locus and were therefore predicted to faithfully express Cre in the Ins1 expression domain. In addition, since these lines were not generated by transgenesis, they were not expected to be subject to the position effect variegation and gene silencing that has plagued some transgenic lines.
To our disappointment, both the Ins1-Cre and Ins1-CreER lines demonstrated partial or complete silencing. The Ins1-Cre line demonstrated inefficient deletion of Foxm1, with partial silencing in some labs and complete silencing in others. The Ins1-CreER line was completely silenced for 3 different labs and failed to recombine 2 different targets. All 3 Ins1-CreER lines were separately obtained from Jackson Laboratories; they were also maintained as completely separate stocks after their acquisition. Thus, their inactivation represents independent silencing events. Two labs, one at University of Pennsylvania and one at Vanderbilt, also obtained their Ins1-Cre line individually from Jackson labs; the Vanderbilt lab received the Ins1-Cre line independently by an intrainstitutional transfer. Interestingly, the Ins1-Cre line in the originating lab continues to exhibit effective target deletion while the recipient lab line has silenced, demonstrating that these alleles can become silenced suddenly and may be responsive to changes in the animal housing environment.
It has been reported previously that loxP-flanked alleles differ in the ease with which they can be excised by Cre recombinase (9). Thus, higher levels of Cre protein are required for some loxP targets than for others. This result is not surprising, given that the distance between 2 loxP sites and their localization within chromatin is different for each target. It appears that the “safe harbor” Rosa26 locus, which has been used for multiple Cre activity reporter lines, is particularly accessible, and the loxP-flanked “stop” sequences are often more easily removed than the loxP-flanked gene of interest. The alleles tested here also seem to be more resistant to deletion than the reporter line, as almost all beta cells in Ins1-Cre;YFP+/YFP mice from the Foxm1 line expressed YFP. We were unfortunately not able to able to directly examine beta-cell Foxm1 expression in mice that have the YFP reporter since Foxm1 and the Rosa locus (Thumpd3) are both located on chromosome 6, at a distance of 15 Mb apart, and are in linkage disequilibrium.
Cre expression was detectable in Ins1-Cre mice bred to the Foxm1 line, although not for Ins1-Cre mice bred to EP4fl or G6pc2fl lines. For the Foxm1 lineage, the residual Cre expression, recombination of the YFP reporter, and the 2 Ins1-Cre;Foxm1f/fl mice that do exhibit elevated blood glucose excursion during a glucose tolerance test (GTT) suggest that Cre expression is diminished before it becomes completely silenced; furthermore, we may have been investigating generations during which the silencing event was occurring.
CpG methylation within the gene body of the exogenous Cre alleles was elevated in both the Ins1-Cre and Ins1-CreER lines compared to methylation of the endogenous allele, further evidence of inactivation of these Cre lines. However, while the Ins1-CreER mice had increased methylation of a CpG within the Ins1 promoter, CpG methylation of the Ins1 promoter in Ins1-Cre mice did not differ from controls, which could be an explanation for the differential silencing of the 2 lines.
Silencing of Cre lines is likely influenced by environmental factors. Thus, we recognize that these lines probably have continued utility for researchers in the beta-cell field. We would, however, suggest using an abundance of caution with these lines and ensuring that any gene of interest is efficiently deleted prior to extensive investment in phenotyping.
As mentioned previously, Cre lines historically used by the beta-cell community have a variety of shortcomings. Which, then, should be used by researchers for whom the Thorens Ins1-Cre line silences? Several lines are available, some of which have a proven history and some which have been used only infrequently. The choice of Cre lines should be made based on experimental design, as detailed below.
Around the same time as the Thorens Ins1-Cre alleles were published, an Ins1-Cre transgenic mouse was also developed (59). Then, in 2016, two new beta cell-specific gene replacement allele Cre lines that preserve insulin expression were reported. One was introduced into the Ins2 locus using a self-cleaving peptide sequence separating the Ins1 and Cre coding regions (60). The second incorporates an internal ribosomal entry site upstream of the 3’ UTR of Ins1 (61). These transgenic and gene replacement allele lines all lack brain expression and displayed no difference in glucose tolerance during GTTs. However, none of these models has been extensively utilized, and as with any conditional deletion model study, appropriate controls must be employed to ensure the effects observed are due to the loss of the targeted gene and not due to transgene expression or alteration of expression of other endogenous genes. Furthermore, they are expressed constitutively, rendering them unsuitable for studies requiring temporal specificity.
Fortunately, at least 2 additional inducible models are available. One of these lines is MIP-CreER, which, despite expression of the hGH minigene, only alters glucose homeostasis and beta-cell phenotypes in some situations (7, 37, 62), such as high-fat diet feeding and streptozotocin treatment (7, 62). However, additional considerations should be considered when using the MIP-CreER mice, such as (1) the hGH can mask subtle phenotypes (63); (2) even in the absence tamofixen, MIP-CreER mice have elevated beta-cell mass both when fed either chow or a high-fat diet (37), and the presence of the transgene in conjunction with tamoxifen treatment diminishes streptozotocin-induced beta-cell death (7); (3) tamoxifen alone improves glucose tolerance and limits beta-cell proliferation (37); and (4) tamoxifen can be unsuitable when examining gene ablation in utero, since doses high enough to effect recombination in the embryo can cause abortions and are also detrimental to the health of the dam (64).
A combination of a tissue-specifically driven reverse tetracycline transactivator (rtTA) and a Cre linked to a tetracycline operator (Tet-O) along with doxycycline treatment can also drive Cre expression in a tissue- and temporally specific manner. While the rat insulin promoter is active in the embryo, multiple labs have reported the lack of hypothalamic rtTA activity in adult RIP-rtTA mice treated with doxycycline (65, 66). An additional option is a recently published transgenic Ins1-rtTA mouse with no brain activity and no alteration in glycemia (59). Unlike tamoxifen, doxycycline can be used safely to recombine genes in utero, with a minimal potential side effect of yellowed teeth in the embryo (67). Like tamoxifen, though, the use of doxycycline is not without its drawbacks. Doxyclyine is an antibiotic, affects gut microbiota, and has been reported to alter blood glucose homeostasis (68).
Our study and others studies demonstrating caveats of Cre-reliant recombination in the beta cell highlight the necessity of appropriate controls in these types of experiments. Of utmost importance is the assessment of the conditional ablation of target genes. Additionally, since genetic alterations will behave differently on various mouse backgrounds, mice harboring the Cre transgene should be included as controls in every investigation using Cre lines. Careful selection of a Cre line with appropriate controls will provide the most reproducible, reliable results.
Acknowledgments
We would like to thank Stephen A. Liebhaber (University of Pennsylvania) for gifting the anti-PCPB2 antiserum.
Financial Support: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK110183 to M.L.G., and 1R01DK122039 to D.A.S.), the Department of Veterans Affairs Merit 1 I01 BX003744-01 to M.G., and the National Institute of Health Vanderbilt Molecular Endocrinology Training Program grant 5T32 DK07563 to K.J.B.
Author Contributions: M.L.G. conceptualized the overall studies, designed and performed experiments, and wrote the manuscript. D.A.S., M.G., and R.O. conceptualized the overall studies, designed experiments, and edited the manuscript. E.M., K.O., M.W.H., B.A.C., S.E.T., K.J.B., A.Y., and T.T. designed and performed experiments and edited the manuscript.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Martel G, Dutar P, Epelbaum J, Viollet C. Somatostatinergic systems: an update on brain functions in normal and pathological aging. Front Endocrinol (Lausanne). 2012;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59(12):3090–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song J, Xu Y, Hu X, Choi B, Tong Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis. 2010;48(11):628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261(25):11880–11889. [PubMed] [Google Scholar]
- 5. Krejs GJ. Physiological role of somatostatin in the digestive tract: gastric acid secretion, intestinal absorption, and motility. Scand J Gastroenterol Suppl. 1986;21(119):47–53. [DOI] [PubMed] [Google Scholar]
- 6. Brouwers B, de Faudeur G, Osipovich AB, et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 2014;20(6):979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oropeza D, Jouvet N, Budry L, et al. Phenotypic characterization of MIP-CreERT1Lphi mice with transgene-driven islet expression of human growth hormone. Diabetes. 2015;64(11):3798–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baan M, Kibbe CR, Bushkofsky JR, Harris TW, Sherman DS, Davis DB. Transgenic expression of the human growth hormone minigene promotes pancreatic β-cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2015;309(7):R788–R794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pedram M, Sprung CN, Gao Q, Lo AW, Reynolds GE, Murnane JP. Telomere position effect and silencing of transgenes near telomeres in the mouse. Mol Cell Biol. 2006;26(5):1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garrick D, Fiering S, Martin DI, Whitelaw E. Repeat-induced gene silencing in mammals. Nat Genet. 1998;18(1):56–59. [DOI] [PubMed] [Google Scholar]
- 12. Chevalier-Mariette C, Henry I, Montfort L, et al. CpG content affects gene silencing in mice: evidence from novel transgenes. Genome Biol. 2003;4(9):R53. https://genomebiology.biomedcentral.com/articles/10.1186/gb-2003-4-9-r53#citeas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegfried Z, Cedar H. DNA methylation: a molecular lock. Curr Biol. 1997;7(5):R305–R307. [DOI] [PubMed] [Google Scholar]
- 14. Thorens B, Tarussio D, Maestro MA, Rovira M, Heikkilä E, Ferrer J. Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia. 2015;58(3):558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RRID:IMSR_JAX:025178. https://scicrunch.org/resolver/RRID:IMSR_JAX:025178.
- 16. Lee D, Le Lay J, Kaestner KH. The transcription factor CREB has no non-redundant functions in hepatic glucose metabolism in mice. Diabetologia. 2014;57(6):1242–1248. [DOI] [PubMed] [Google Scholar]
- 17.RRID:IMSR_EM:12633. https://scicrunch.org/resolver/RRID:IMSR_EM:12633.
- 18. Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A. 2002;99(26):16881–16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RRID:IMSR_EM:10468. https://scicrunch.org/resolver/RRID:IMSR_EM:10468.
- 20.RRID:IMSR_JAX:026802. https://scicrunch.org/resolver/RRID:IMSR_JAX:026802.
- 21.RRID:MGI:5909012. https://scicrunch.org/resolver/RRID:MGI:5909012.
- 22. Ghanem LR, Kromer A, Silverman IM, et al. The Poly(C) binding protein Pcbp2 and its retrotransposed derivative Pcbp1 are independently essential to mouse development. Mol Cell Biol. 2016;36(2):304–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RRID:IMS4_JAX:003573. https://scicrunch.org/resolver/RRID:IMS4_JAX:003573.
- 24. Postic C, Shiota M, Niswender KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–315. [DOI] [PubMed] [Google Scholar]
- 25.RRID:IMS4_JAX:024968. https://scicrunch.org/resolver/RRID:IMS4_JAX:024968.
- 26. Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–450. [DOI] [PubMed] [Google Scholar]
- 27.RRID:IMSR_JAX:024709. https://scicrunch.org/resolver/RRID:IMSR_JAX:024709.
- 28.RRID:IMSR_JAX:006148. https://scicrunch.org/resolver/RRID:IMSR_JAX:006148.
- 29. Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;(1):4. https://bmcdevbiol.biomedcentral.com/track/pdf/10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RRID:IMSR_JAX:007914. https://scicrunch.org/resolver/RRID:IMSR_JAX:007914.
- 31. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneider A, Guan Y, Zhang Y, et al. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40(1):7–14. [DOI] [PubMed] [Google Scholar]
- 33.RRID:IMSR_JAX:028102. https://scicrunch.org/resolver/RRID:IMSR_JAX:028102.
- 34.RRID:MMRRC_042277-JAX. https://scicrunch.org/resolver/RRID:MMRRC_042277-JAX.
- 35. Ackermann AM, Zhang J, Heller A, Briker A, Kaestner KH. High-fidelity Glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Mol Metab. 2017;6(3):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.RRID:MMRRC_055836-UCD. https://scicrunch.org/RRID:MMRRC_055836-UCD.
- 37. Carboneau BA, Le TD, Dunn JC, Gannon M. Unexpected effects of the MIP-CreER transgene and tamoxifen on beta-cell growth in C57Bl6/J male mice. Physiol Rep. 2016;4(18):e12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.RRID:CVCL_0063. https://scicrunch.org/resolver/RRID:CVCL_0063.
- 39. Jimenez-Moreno CM, Herrera-Gomez IG, Lopez-Noriega L, et al. A simple high efficiency intra-islet transduction protocol using lentiviral vectors. Curr Gene Ther. 2015;15(4): 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RRID:AB_10013624. https://scicrunch.org/resolver/RRID:AB_10013624.
- 41.RRID:AB_331277. https://scicrunch.org/resolver/RRID:AB_331277.
- 42.RRID:AB_2827812. https://scicrunch.org/resolver/RRID:AB_2827812.
- 43.RRID:AB_2336064. https://scicrunch.org/resolver/RRID:AB_2336064.
- 44.RRID:AB-2314229. https://scicrunch.org/resolver/RRID:AB-2314229.
- 45. scicrunch.org/resolver/RRID:AB_2340467RA.
- 46.RRID:AB_2340606. https://scicrunch.org/resolver/RRID:AB_2340606.
- 47.RRID:AB_2340607. https://scicrunch.org/RRID:AB_2340607.
- 48.RRID:AB_2340667. https://scicrunch.org/resolver/RRID:AB_2340667.
- 49. Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M. Opposing effects of prostaglandin E2 receptors EP3 and EP4 on mouse and human β-cell survival and proliferation. Mol Metab. 2017;6(6):548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.RRID:AB_641026. https://scicrunch.org/resolver/RRID:AB_641026.
- 51.RRID:AB_305643. https://scicrunch.org/resolver/RRID:AB_305643.
- 52. Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398. [DOI] [PubMed] [Google Scholar]
- 53. Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129(10):2447–2457. [DOI] [PubMed] [Google Scholar]
- 54. Zhang H, Ackermann AM, Gusarova GA, et al. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol. 2006;20(8):1853–1866. [DOI] [PubMed] [Google Scholar]
- 55. Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59(1):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bramswig NC, Everett LJ, Schug J, et al. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest. 2013;123(3):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pound LD, Oeser JK, O’Brien TP, et al. G6PC2: a negative regulator of basal glucose-stimulated insulin secretion. Diabetes. 2013;62(5):1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shin S, Le Lay J, Everett LJ, Gupta R, Rafiq K, Kaestner KH. CREB mediates the insulinotropic and anti-apoptotic effects of GLP-1 signaling in adult mouse β-cells. Mol Metab. 2014;3(8):803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheng Y, Su Y, Shan A, et al. Generation and characterization of transgenic mice expressing mouse ins1 promoter for pancreatic β-Cell-Specific Gene Overexpression and Knockout. Endocrinology. 2015;156(7):2724–2731. [DOI] [PubMed] [Google Scholar]
- 60. Li L, Gao L, Wang K, et al. Knockin of Cre Gene at Ins2 Locus Reveals No Cre activity in mouse hypothalamic neurons. Sci Rep. 2016;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hasegawa Y, Hoshino Y, Ibrahim AE, et al. Generation of CRISPR/Cas9-mediated bicistronic knock-in ins1-cre driver mice. Exp Anim. 2016;65(3):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Douros JD, Lewis AG, Smith EP, et al. Enhanced glucose control following vertical sleeve gastrectomy does not require a β-Cell Glucagon-Like Peptide 1 Receptor. Diabetes. 2018;67(8):1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stancill JS, Osipovich AB, Cartailler JP, Magnuson MA. Transgene-associated human growth hormone expression in pancreatic β-cells impairs identification of sex-based gene expression differences. Am J Physiol Endocrinol Metab. 2019;316(2): E196–E209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ved N, Curran A, Ashcroft FM, Sparrow DB. Tamoxifen administration in pregnant mice can be deleterious to both mother and embryo. Lab Anim. 2019;53(6):630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pullen TJ, Sylow L, Sun G, Halestrap AP, Richter EA, Rutter GA. Overexpression of monocarboxylate transporter-1 (SLC16A1) in mouse pancreatic β-cells leads to relative hyperinsulinism during exercise. Diabetes. 2012;61(7):1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Golson ML, Maulis MF, Dunn JC, et al. Activated FoxM1 attenuates streptozotocin-mediated β-cell death. Mol Endocrinol. 2014;28(9):1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cross R, Ling C, Day NP, McGready R, Paris DH. Revisiting doxycycline in pregnancy and early childhood–time to rebuild its reputation? Expert Opin Drug Saf. 2016;15(3):367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang N, Tian X, Chen Y, et al. Low dose doxycycline decreases systemic inflammation and improves glycemic control, lipid profiles, and islet morphology and function in db/db mice. Sci Rep. 2017;7(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]