Abstract
Purpose
To systematically estimate the patient-reported outcomes (PROs) and economic burden of sickle cell disease (SCD) among adults in the United States (US).
Patients and Methods
Two systematic literature reviews (SLRs), one each for the PROs and economic topics, were performed using MEDLINE and Embase to identify observational studies of adults with SCD. Included studies were published between 2007 and 2018 and evaluated health-related quality of life (HRQL), function, healthcare resource utilization (HCRU), or costs. Given the high degree of clinical and methodological heterogeneity, findings were summarized qualitatively.
Results
The SLRs identified 7 studies evaluating the PROs and 15 studies evaluating the economic burden meeting the pre-specified selection criteria. The PRO evidence showed the prevalence of depression and anxiety to be 21–33% and 7–36%, respectively, in adults with SCD. The mean SF-36 physical summary scores ranged from 33.6 to 59.0 and from 46.3 to 61.5 for the mental summary scores. Overall HRQL for adults with SCD was poor and significantly worse in those with opioid use. Adult SCD patients were found to have varying rates of emergency department (ED) utilization (0.3–3.5 annual ED visits), hospitalizations (0.5–27.9 per patient per year), and/or readmission (12–41%). Key factors associated with significant HCRU were age, dental infection, and SCD-related complications. SCD specialized care settings and SCD intensive management strategy were reported to significantly decrease the number of hospitalizations.
Conclusion
This systematic evidence synthesis found that disease burden measured by PROs and economic burden of SCD on adults in the US are substantial despite the availability of approved SCD treatments during 2007–2018. The use of hydroxyurea, optimal management with opioids, and employing intensive treatment strategies may help decrease the overall burden to patients and healthcare systems. Published data on costs associated with SCD are limited and highlight the need for more economic studies to characterize the full burden of the disease.
Keywords: sickle cell disease, patient-reported outcomes, health-related quality of life, economic burden
Introduction
Sickle cell disease (SCD) is a lifelong genetic disease that affects hemoglobin synthesis, causing erythrocytes to become rigid and form a sickle-like shape upon deoxygenation.1 It is also characterized by recurrent vaso-occlusion that involves multicellular adhesions between multiple blood cells, as well as progressive vascular and organ damage.2–4 In the United States (US), SCD is the most common inherited blood disorder, affecting approximately 100,000 patients, and an additional 3.5 million have the sickle cell trait.1 The population of adult patients with SCD is growing due to early diagnosis of the disease and improved quality of patient care. Most children with sickle cell anemia (94%) and nearly all children with milder forms of SCD (98%) survive to adulthood.5
The majority of patients with SCD experience sickle cell pain crises, also known as vaso-occlusive crises (VOC), and as many as 30% of patients experience chronic pain.6,7 VOCs, the hallmark of SCD, are severe painful events that result in frequent emergency department (ED) visits and hospitalizations.8 They are also associated with increased mortality and diminished health-related quality of life (HRQL).9 In addition, other complications of SCD, such as acute chest syndrome (ACS), hepatic/splenic sequestration, stroke, and long-term end-organ damage, which require intensive healthcare resource utilization (HCRU), are thought to contribute to the decrease in quality of life.10,11
The only known cure for SCD is bone marrow transplant which only few patients qualify for.12 As a result, the mainstay of treatment for SCD over the last 30 years has been erythrocyte transfusions and hydroxyurea. More recently, the US Food and Drug Administration (FDA) approved L-glutamine oral powder in 2017, and crizanlizumab and voxelotor in November 2019 as additional treatment options for patients with SCD.13–15 There are several ongoing gene therapy trials, none of which is approved for use yet. Therefore, the majority of patients have relied on limited available therapies to manage SCD and related complications over their lifetime.12
Two systematic literature reviews (SLRs) were recently conducted among patients with SCD.16,17 One of these summarized the details of factors influencing hospital utilization while the second reported psychometric properties of various patient-reported outcome (PRO) instruments that may be suitable for use in future clinical trials of patients with SCD. Neither review reported on PRO results or costs associated with the disease or its treatment. While there are many studies evaluating the burden in SCD, to our knowledge, this is the first study synthesizing the literature to understand the key drivers of the burden. We conducted two separate SLRs to identify and synthesize the recent literature on the PRO results and economic burden of SCD among adults in the US. In addition, with the advent of novel/emerging therapies after more than two decades, it is important to identify areas of unmet need in SCD where these therapies may help mitigate the burden.
Patients and Methods
Search Strategy
A systematic literature search of Embase, Embase In-process, MEDLINE, and MEDLINE In-Process was conducted for each of the topics of interest using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses18 (PRISMA) and Cochrane Collaboration19 guidelines. The searches were conducted in September 2018 and limited to studies of humans published in English between 1997 and 2018. No grey literature or conference proceedings were considered. A manual search of bibliographies of identified SLRs and/or meta-analyses was performed to identify any additional relevant studies. For details of the search strategy, see the S1 and S2 Tables. A full review protocol is available upon request.
Study Selection and Data Extraction
The approach for identifying the evidence for both topics was similar; two independent reviewers completed study selection using pre-defined inclusion and exclusion criteria detailed in S3 Table. The full-text screening consisted of a two-step approach where the latter step involved the application of additional selection criteria to prioritize the most relevant studies for the outcomes of interest. At the abstract and full-text screening, all records were reviewed by one researcher and 50% of records were validated by a second researcher. A third reviewer was consulted to resolve discrepancies. For each included study, data were extracted by one researcher into a pre-designed standardized data extraction template and validated for correctness and accuracy by a second researcher. Any discrepancies were resolved by a third reviewer. Studies published in multiple articles were extracted as one study. The following information was obtained from each study (where applicable): author, year of publication, patient characteristics, disease characteristics, population size, and study type. For the purpose of this review, we defined outcomes for PROs broadly to include HRQL, work productivity, and other function (e.g., coping strategy and pain) to understand the impact of SCD on patients’ day-to-day lives and also society. Outcomes for economic burden included: HCRU, direct medical cost, and indirect non-medical costs. The risk of bias was not assessed using a formal tool; instead, aspects of quality for observational studies were taken into consideration during evidence synthesis (e.g., results from prospective studies were highlighted over retrospective studies).
Results
Results are presented separately for each SLR; PRO results and economic burden. For each SLR, the comparability of studies was assessed whether their results could be synthesized quantitatively. However, the high degree of clinical and methodological heterogeneity observed in included studies for both SLRs precluded the ability to conduct direct meta-analyses; thus, the findings in the following sections are summarized qualitatively.
PRO Results of SCD in Adults
Overview of Search Results
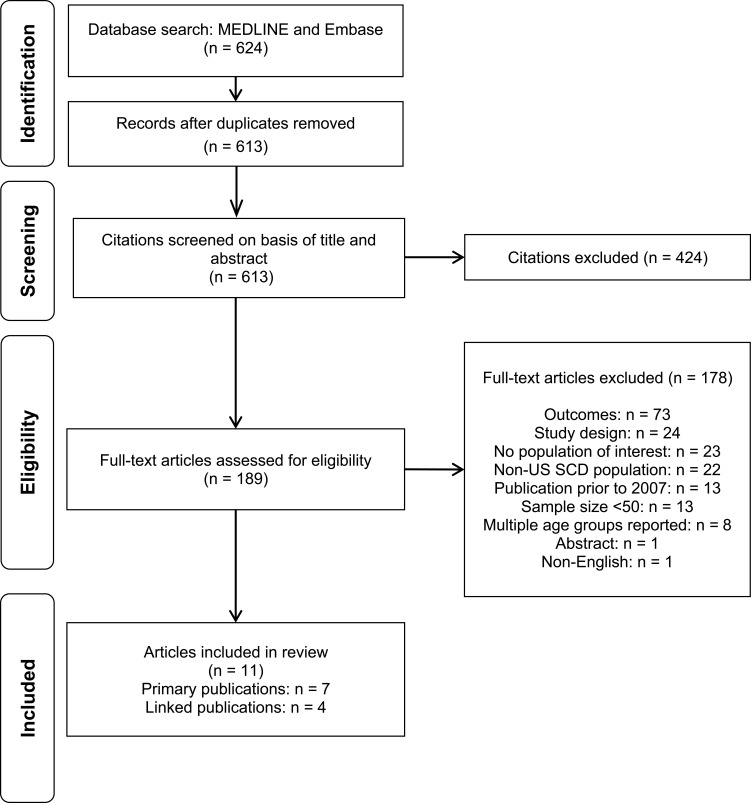
The electronic database search yielded 613 unique records for the title and abstract screening. Of these, 189 citations were considered relevant for full-text review. After the full-text screening, 11 publications (representing seven studies) met the eligibility criteria for the review of PROs. The PRISMA diagram is presented in Figure 1.
Figure 1.
PRISMA flow diagram for Patient-Reported Outcomes.
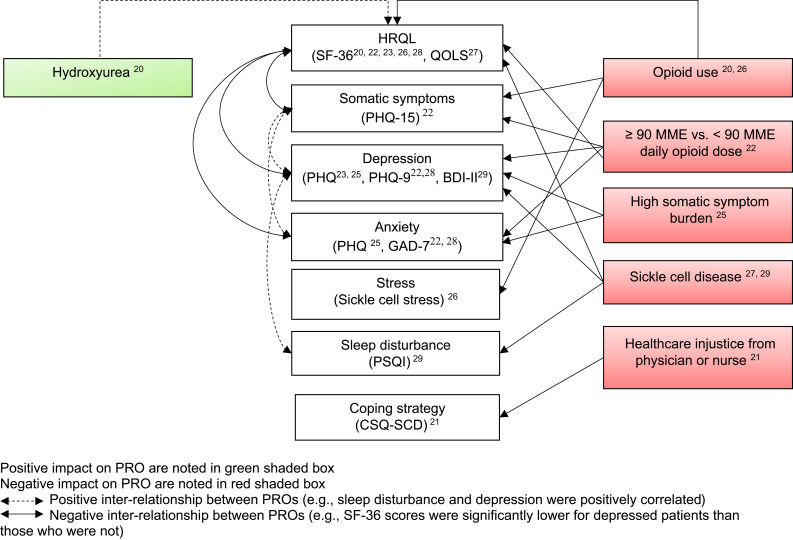
The key characteristics of the seven studies6,20-29 are presented in Table 1 and key results can be found in Figure 2. Six studies were cross-sectional20–22,27–29 and one was a prospective observational study (with four related publications).6,23-26 Some studies reported multiple outcomes, with five studies evaluating patients’ HRQL,20,22,23,27,28 four assessing depression and/or anxiety,22,23,28,29 two studies evaluating coping style, and one study each assessing sleep quality,29 pain, stress, social support, and social interaction.23 A description of the PRO instruments identified in this review is provided in Table 2
Table 1.
Primary Studies for the Patient-Reported Outcomes in Adults with SCD
| Author (Year) | Study Design and Setting | N | Age (years) Mean (SD) Male | Outcome | Methods of Elicitation | Summary of Results |
|---|---|---|---|---|---|---|
| Adam (2010)20 | Cross-sectional study at a hospital in NC | 185 | NR 50% |
HRQL | SF-36^ |
|
| Ezenwa (2017)21 | Cross-sectional study at an outpatient clinic in IL | 52 | 34 (11) 21% |
Coping strategy | CSQ-SCD^ |
|
| Karafin (2018)22 | Cross-sectional study at a hospital in WI | 99 | 30 (25–37)* 35% |
HRQL Somatic symptoms, Depression, Anxiety |
SF-36^ PHQ-15¥PHQ-9¥GAD-7 ¥ |
|
| Levenson (2008);23 Levenson (2007);24 Smith (2008);6 Sogutlu (2011);25 Smith (2015)26 | Prospective observational study at academic and community practices in VA | 232 | 34.4 (11.4) 38% |
HRQL Depression/anxiety, Pain, Coping styles, Stress, Social support, Social interaction |
SF-36^ PHQ¥ CSQ^ Sickle cell stress¥ MSPSS^ TENSE¥ |
|
| Mann-Jiles (2009)27 | Cross-sectional study at an outpatient clinic in OH | 62 | NR 40% |
HRQL | QOLS^ |
|
| Treadwell (2015)28 | Cross-sectional study at an urban comprehensive sickle cell center in CA | 77 | 31.6 (13.1)40% | HRQL Depression and Anxiety |
SF-36^ PHQ-9¥ GAD-7¥ |
|
| Wallen (2014)29 | Cross-sectional study at a National Institute of Health Clinical Center in MD | 328 | 34 (27–46)*49% | Depression and Sleep quality | BDI-II¥ PSQI¥ |
|
Notes: Where significant is mentioned, it indicates statistical significance at the 5% level. Details about the identified PRO instruments can be found in Table 2. ^ Higher scores indicate positive outcomes (such as greater functioning) or higher frequency of using particular pain-coping strategy. ¥ Higher scores indicate negative outcomes (such as greater pain, more somatic or depression symptoms). * Reported as median age (IQR)
Abbreviations: BDI-II, Beck Depression Inventory II; CA, California; CSQ, Coping Strategy Questionnaire; GAD, generalized anxiety disorder; HRQL, health-related quality of life; IL, Illinois; MCS, mental component summary; MD, Maryland; MSPSS, Multidimensional Scale of Perceived Social Support; MME, morphine milligram equivalents; NC, North Carolina; NR, not reported; OH, Ohio; PCS, physical component summary; PHQ, Patient Health Questionnaire; PSQI, Pittsburgh Sleep Quality Index; QoL, quality of life; QOLS, Quality of Life Scale; SCD, sickle cell disease; SD, standard deviation; SF-36, Short Form Health Survey; TENSE, Test of Negative Social Exchange; VA, Virginia; WI, Wisconsin.
Figure 2.
Patient-reported outcomes among adults with SCD.
Abbreviations: BDI-II, Beck Depression Inventory II; CSQ, Coping Strategy Questionnaire; GAD, Generalized Anxiety Disorder; HRQL, health-related quality of life; MME, morphine milligram equivalents; PHQ, Patient Health Questionnaire; PSQI, Pittsburgh Sleep Quality Index; QOLS, Quality of Life Scale; SCD, sickle cell disease; SF-36, Short Form Health Survey.
Table 2.
Description of Patient-Reported Outcome Instruments
| Instrument | Outcome | Description | Range | Interpretation |
|---|---|---|---|---|
| Short-form-36 (SF-36) | HRQL | Multidimensional HRQL instrument of 36-item tool dividing into 8 subscales of health status: consisting of four physical health scales (physical functioning, role limitations due to physical problems, bodily pain, and general health perceptions) and four mental health scales (vitality, social functioning, role limitations due to emotional problems, and mental health). These eight scales can be aggregated into two summary measures: PCS and MCS scores | Each scale is directly transformed into a 0–100 scale on the assumption that each question carries equal weight | Higher scores represent a higher level of functioning |
| Coping Strategies Questionnaire-Sickle Cell Disease (CSQ-SCD) | Coping strategy | An 80-item questionnaire that measures the various ways patients with SCD cope with pain | The adapted CSQ includes 80 items, rated on a 7-point Likert scale (0 = “never performed during pain” to 6 = “always performed during pain”) | Higher scores indicate greater use of the coping style |
| Patient Health Questionnaire-15(PHQ-15) | Somatic symptoms | A 15-item questionnaire that measures the somatic symptom burden regarding the 15 most common somatic symptoms (≤ 4 = normal, 5–9 = low, 10–14 = moderate, > 14 = high) | Total scores range from 0 to 30, with 15 representing the threshold for severe somatic symptomatic burden | Higher scores indicate greater somatic symptom burden |
| Patient Health Questionnaire-9 (PHQ-9) | Depression | A 9-item survey that measures the frequency by which a subject experiences symptoms of depression (≤ 4 = low symptom burden, 5–9 = mild depression, 10–14 = moderate depression, > 15 = moderately severe and severe depression) | Total scores range from 0 to 27, with 20 representing the threshold for severe depressive symptomatic burden | Higher scores indicate greater depressive symptom |
| Generalized Anxiety Disorder (GAD-7) | Anxiety | A 7-item questionnaire that measures the severity of generalized anxiety disorder. It asks about symptoms including feeling nervous, anxious, or on edge over the past two weeks. Respondents indicate how difficult the symptoms make it for them to engage in daily activities from 0 (“not difficult at all”) to 3 (“extremely difficult”) | Total scores range from 0 to 21. Cut-off points for total scores across the seven items of 5, 10, and 15 represent mild, moderate, and severe levels of anxiety symptoms, respectively | Higher scores indicated greater anxiety symptom |
| Coping Strategies Questionnaire (CSQ) | Coping styles | Originally developed to measure cognitive and behavioral coping styles in chronic low back pain, but was later revised for patients with SCD with the addition of new subscales for strategies particularly relevant to SCD | The adapted CSQ includes 80 items, rated on a 7-point Likert scale (0 = “never performed during pain” to 6 = “always performed during pain”) | Higher scores indicate greater use of the coping style |
| Sickle cell stress | Stress | A 10-item instrument developed to measure sickle cell-related stress | Not reported | Higher scores indicate greater stress |
| Multidimensional Scale of Perceived Social Support (MSPSS) | Social support | A self-report measure to subjectively assess social support on three subscales: family, friends, and significant other | Respondents rate how they feel about statements related to social support on a 7-point Likert-like scale ranging from 1 (“very strongly disagree”) to 7 (“very strongly agree”) | Higher scores indicate greater perceived social support |
| Test of Negative Social Exchange (TENSE) | Social interaction | A 45-item instrument measuring negative social exchange | Participants rate how often people in their lives engage in a list of 45 behaviors, including hostility, rejection, conflict, ridicule, insensitivity, and criticism, over the past month on a scale of 0 (“not at all”) to 4 (“about every day”) | Higher scores reflect greater perceived negative social exchanges. |
| Burckhardt and Anderson’s 16-item Self-report Quality of Life Scale (QOLS) | HRQL | A 16-item instrument measuring quality of life in the following five domains: material and physical well-being; relationships with other people; social, community, and civic activities; personal development and fulfillment; and recreation | Items are scored on a 7-point Likert-type scale. Scores range from 16 to 112. Scores can be interpreted relative to the average QOLS score for a healthy population (90) | Higher scores indicate a higher level of functioning |
| Beck Depression Inventory II (BDI-II) | Depression | A 21-item instrument measuring the presence and severity of depression in adults | Individual items are rated on a 4-point intensity scale rather than on a frequency dimension. A rating of 3 indicates most severe intensity, while 0 indicates the absence of a problem. Cut-off scores for depression vary by study with scores between 14 and 17 indicating mild depression and scores ≥ 20 indicating severe depression | A higher score indicates greater depression |
| Pittsburgh Sleep Quality Index (PSQI) | Sleep quality | 19 self-rated questions and five questions rated by the partner. The 19 self-rated questions assess a wide variety of factors relating to sleep quality, sleep latency, duration, habitual sleep efficiency, sleep disturbances, use of sleep medications and daytime dysfunction | Score ranges from 0 to 21. A PSQI score of 6 or greater indicates some degree of sleep disturbance | A higher score indicates worse sleep quality |
Abbreviations: BDI-II, Beck Depression Inventory II; CSQ, Coping Strategy Questionnaire; GAD, generalized anxiety disorder; HRQL, health-related quality of life; MCS, mental component summary; MSPSS, Multidimensional Scale of Perceived Social Support; PCS, physical component summary; PHQ, Patient Health Questionnaire; PSQI, Pittsburgh Sleep Quality Index; QoL, quality of life; QOLS, Quality of Life Scale; SCD, sickle cell disease; SF-36, Short Form Health Survey; TENSE, Test of Negative Social Exchange.
The Impact of SCD on HRQL
Five studies (four cross-sectional20,22,27,28 and one prospective observational23) reported on HRQL in adult patients with SCD. HRQL was assessed by the 36-item Short-Form Survey (SF-36) in four studies20,22,23,28 while one study used the Burckhardt and Anderson’s 16-item Self-report Quality of Life Scale (QOLS)27 (Table 1). The overall HRQL for adult patients with SCD appeared to be poor. The mean SF-36 scores ranged from 33.6 to 59.0 for the physical component summary (PCS), and from 46.3 to 61.5 for the mental component summary (MCS), both summary scores were lower than the general population.20,22,23,28 Similarly, one cross-sectional study reported a mean QOLS score of 83.6 (SD: 13.2), indicating that patients had a lower quality of life than the general population (QOLS score of 90).27
Factors Affecting HRQL
Patient and Disease Characteristics
The association between emotional distress and HRQL was evaluated in two separate analyses of the Pain in Sickle Cell Epidemiology Study (PiSCES). These analyses reported that lower HRQL (physical and mental) was significantly correlated with greater depression and/or anxiety symptoms (p < 0.001) and SCD patients with high (as opposed to low) somatic symptom burden had significantly lower scores on all of the eight SF-36 subscale scores (p < 0.001).23,25 However, there were no significant differences found in any of the SF-36 subscales between alcohol users and non-users. Alcohol users were defined as any of the following happening more than once in the last 6 months: drinking even though a physician suggesting stopping due to health problem; drinking, or being hungover while going to school, working, or taking care of children or other responsibilities; missing or being late for work, school, or other activities; having a problem getting along with other people while drinking; or driving a car while intoxicated. Surprisingly, alcohol users reported significantly higher PCS scores as compared to non-users (37.3 vs 34.2; p = 0.032).24 When the relationship between HRQL and sex and genotype was evaluated among SCD patients attending an outpatient clinic in North Carolina, there were no significant differences found in both the PCS and MCS scores between different SCD genotypes and gender.20
Management Approach for SCD
One cross-sectional study evaluated HRQL in outpatient adults with SCD using either opioids, hydroxyurea, both, or neither.20 The study reported that SF-36 global, PCS, and MCS scores were significantly higher (p < 0.05) in patients treated with either no medications (60.16, 59.01, and 61.30, respectively) or hydroxyurea alone (59.17, 56.85, and 61.48, respectively) compared to those treated with opioids only (45.56, 44.82, and 46.3, respectively) or with both opioids and hydroxyurea (48.54, 45.35, and 51.73, respectively). Patients treated with hydroxyurea alone reported significantly higher scores for all subscales compared with patients receiving opioids only (p < 0.05), except the role-physical subscale, where HRQL was numerically higher, but not significantly (p-value not reported) different between the two groups of patients.
Three studies focused specifically on evaluating the association between opioid use and HRQL among adults with SCD.20,22,26 Similar to the results reported above,20 the PiSCES investigators reported that patients using opioids had significantly lower HRQL than patients not using opioids (mean PCS 33.6 vs 42.8; p < 0.0001 and mean MCS 46.6 vs 51.4; p = 0.025).26 Furthermore, when the association between opioid dose and HRQL was evaluated, higher daily opioid dose was associated with significantly lower HRQL than lower daily opioid dose (median PCS 30 vs 35; p = 0.02 and median MCS 46 vs 54; p = 0.03).22
Other Patient-Reported Outcomes
Several studies identified in this SLR evaluated the impact of SCD on other PROs (eg, pain, depression, anxiety, and sleep disturbances) that were often described as common symptoms and comorbid conditions of adult patients with SCD. More specifically, three studies evaluated patient symptoms that were reported alongside HRQL including depression, anxiety, pain, and stress, as well as coping styles, and social support and interaction, and also the association between other humanistic outcomes and HRQL.22,23,28 Two studies assessed the impact of SCD on patient’s coping strategy, depression, and sleep quality without reporting on HRQL.21,29
Pain
The PiSCES study prospectively evaluated pain using daily diaries in adults with SCD.6,23-26 The study reported that 54% of SCD patients had pain on more than half of total patient-days, confirming considerable levels of pain persist in a substantial proportion of patients with SCD.6 Furthermore, 29% of patients had pain every day (>95% days), whereas only 14% had a pain-free daily life (≤5% days). When total diary days (n = 31,017) were categorized by pain severity (pain with healthcare visit, pain with crisis, pain without healthcare visit or crisis, and no pain), more than half of total patient-days were classified as either pain with healthcare visit, crisis, and pain without healthcare visit or crisis (4%, 13%, and 38% of total patient-days, respectively). Additionally, it was found that while patients with SCD frequently had pain, more than half of patients managed pain (including crises) at home or without healthcare utilization.
Depression and long-acting opioid use were found to be significantly associated with more proportional days in pain and higher frequent pain intensity, respectively.23,26 However, when the effect of depression on crisis pain was evaluated, depressed patients with SCD reported a similar proportion of days in crises than non-depressed patients with SCD (16.7% of days vs 14.7%; p = 0.57).23 Alcohol use or degree of somatic symptom burden did not have a significant impact on pain.24,25
Depression and/or Anxiety
Four studies evaluated depression and/or anxiety.22,23,28,29 At least one in five adult patients with SCD (21–33%) seemed to have depressive symptoms as measured by the Patient Health Questionnaire (PHQ),23 PHQ-9,22,28 or the Beck Depression Inventory II (BDI-II). Studies measuring anxiety with the PHQ or Generalized Anxiety Disorder (GAD-7) reported that 7–36% of adult patients with SCD had symptoms of anxiety.22,23,28 Adult patients with SCD who used high doses of daily opioids, or who reported high somatic symptom burden and sleep disturbance were significantly more likely to have depressive and/or anxiety symptoms than those not using high-dose opioids or without high somatic symptom burden.22,25,29 Furthermore, depression and anxiety were correlated with pain.
Coping Strategies
Evidence from three studies showed that adult patients with SCD who used opioids or alcohol or who perceived healthcare injustice appeared to employ different pain coping strategies than those who did not, as measured using the Coping Strategy Questionnaire (CSQ) or the CSQ-SCD.21,24,26 The PiSCES study reported that there were no significant differences in the use of active or positive coping styles (p = 0.059 and p = 0.312, respectively) between opioid and non-opioid users. There were, however, significant differences in using negative coping styles, with more opioid users adopting this style compared to those who did not use opioids (p = 0.008). Patients who used alcohol were more likely to cope with SCD pain by diverting attention (p = 0.004), ignoring pain (p = 0.03), using anger self-statements (p = 0.006), calming self-statements (p = 0.007), or fear self-statements (p = 0.016) than those who did not.24 Patients who perceived healthcare injustice more frequently used catastrophizing (3.5 vs 2.5; p = 0.016) and isolation (3.9 vs 2.8; p = 0.028) as measured using the CSQ-SCD.21
Sleep Disturbance, Stress, and Social Support and Interaction
The PiSCES study also evaluated a subgroup of patients who reported pain at home and found that the majority of patients (86%) used opioids for pain management.26 The study also evaluated the relationship between opioid use and psychosocial variables (ie, somatic symptoms, sickle cell stress, and social support and interaction). Adults with SCD who used opioids for pain relief reported significantly higher sickle cell-related stress (mean: 2.06 [SD: 0.94] vs 1.54 [SD: 1.02]; p = 0.005) and somatic symptom burden (mean: 7.37 [SD: 3.70] vs 5.87 [SD: 4.24]; p = 0.042) compared to those who did not. However, no significant difference was found for perceived social support (mean: 5.59 [SD: 1.34] vs 5.96 [SD: 1.15]; p = 0.144) and negative social interaction (mean: 0.98 vs 0.77; p = 0.078) between patients who used opioids and those who did not as measured by the Multidimensional Scale of Perceived Social Support and the Test of Negative Social Exchange, respectively.
Patients with SCD appear to suffer from poor sleep quality due to pain. One study reported the prevalence of sleep disturbance among adults with SCD as measured by the Pittsburgh Sleep Quality Index as 71%.29 The study found that poor sleep quality was significantly correlated with more days of pain (p = 0.003) and more frequent severe acute pain episodes required emergency room visits or hospitalizations (p < 0.001).
Economic Burden of SCD in Adults
Overview of Search Results
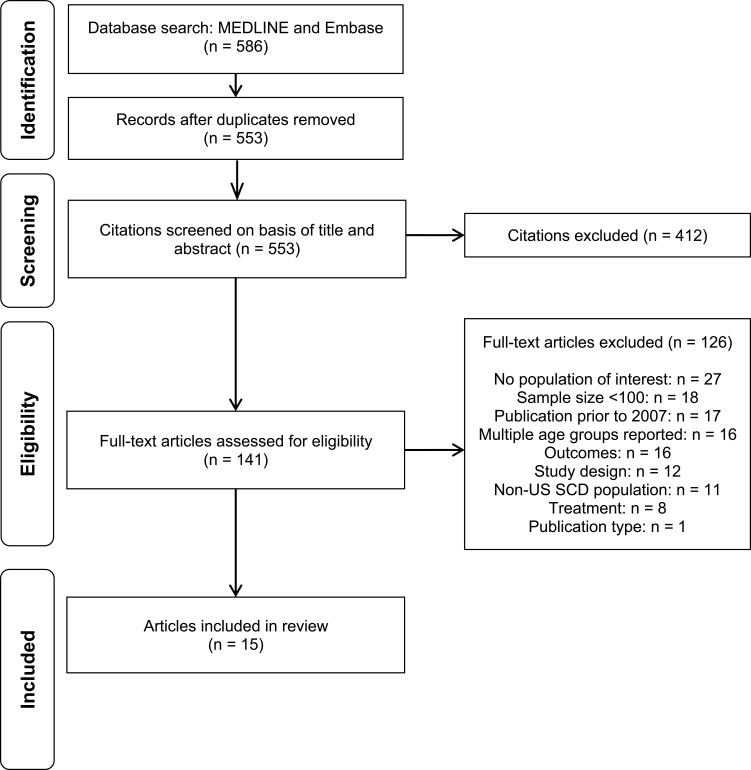
The electronic database search yielded 586 unique records from the title and abstract screening, of which 141 citations were considered relevant for full-text review. After the full-text screening, 15 publications met the eligibility criteria for the economic burden review. The PRISMA diagram is presented in Figure 3.
Figure 3.
PRISMA flow diagram for economic burden.
The key characteristics of 15 included studies are presented in Table 3.30–44 Five retrospective studies evaluated electronic medical records (EMRs)33–35,38,42 and nine studies were retrospective database analyses.30–32,36,37,39,40,43,44 One study used data from the Sickle Cell Data Collection Program, a registry collecting data from Georgia and California.41 Fourteen studies reported on HCRU,30,32-44 while one reported direct medical costs.31
Table 3.
Primary Studies for the Economic Outcomes in Adults with SCD
| Author (Year) | Study Design | Region | Population | N | Age (Years), Mean ± SD Male (%) | Hydroxyurea Use | Economic Outcome(s) Assessed |
|---|---|---|---|---|---|---|---|
| Allareddy (2014)30 | Retrospective database analysis | US | Adults with SCD hospitalized with ACS (ages >21 years) | 24,699 hospitalizations | 33.2 (0.19) 49 |
NR | LOS |
| Blinder (2013)31* | Retrospective database analysis | FL, NJ, MO, KS, IA | Adults with SCD (ages ≥18 years) | NR | NR | Mixed, proportion NR | ED visits; hospitalizations; outpatients visits; healthcare costs |
| Brousseau (2010)32* | Retrospective database analysis | AZ, CA, FL, MA, MO, NY, SC, TN | Adults with SCD (ages ≥18 years) | 13,256 | NR | NR | Rate of SCD-related acute care utilization (treat-and-release ED visits and hospitalization) and rehospitalizations |
| Curtis (2015)33 | Retrospective medical record study at a hospital | NY | Adults with SCD(ages 18–87) | 432 | NR 46 |
38% | ED visits |
| Inoue (2016)34* | Retrospective medical record study at a hospital | MI | Adults with SCD (ages ≥18 years) | 2007–2008: 43.5† 2011–2012: 36.5† 2013: 38† |
NR | NR | ED visits; ED admission |
| Koch (2015)35 |
Retrospective medical record study at a hospital | WI | Adults with SCD(ages NR) | 115 | 28 (16)‡34 | Mixed, proportion NR | Rate of ED/hospital admission; 30-day re-admission |
| Laurence (2013)36 | Cross-sectional study | US | Adults with SCD (ages ≥18 years) | Dental infection:1572 visits No dental infection:549,045 visits |
32.1 (0.6) and 31.9 (0.2)36.1 and 43.8 | NR | Admission during ED visit |
| Leschke (2012)37* | Retrospective database analysis | WI | Adults with SCD (ages >19 years) | 222 | NR | NR | Rehospitalizations (14 and 30 days) |
| Molokie (2018)38 | Retrospective medical record study at a hospital | IL | Adults with SCD (ages ≥18 years) | 148 | 35.1 (11.9)35 | NR | Admission from ED and SCD acute care unit; LOS |
| Ogunbayo (2017)39 | Retrospective database analysis | US | Adults with AMI ± SCA (ages ≥18 years) | SCD: 495 Controls: 495 |
47.21 (22)47.1 | NR | LOS |
| Okam (2014)40 | Retrospective database analysis | US | Black adults with SCD (ages ≥18 years) | 1998: 54,4902008: 55,042 | NR | NR | SCD-related hospitalization rate; LOS |
| Paulukonis(2017)41* | Retrospective registry study | CA | Individuals with SCD (ages ≥20 years) |
3407 | NR | NR | Proportion of individuals with at least 1 treat-and-release ED visit; mean annual ED visit |
| Ter-Minassian (2018)42 | Retrospective medical record study at 2 SCD programs | MD | Adults with SCD (ages ≥21 years) | 454 | 35 (21–75)‡41 | 26% | ED visits; Inpatient visit; Ambulatory visit; urgent care visit; hematology visit |
| Wolfson (2012)43* | Retrospective database analysis | CA | Adults with SCD (ages ≥21 years) | 2087 | NR | NR | ED visits |
| Zhou (2018)44* | Retrospective database analysis | US | Individuals with SCD related inpatient and acute encounters (ages ≥18 years) | 14,890 | NR | 21% | 30-day all-cause readmission; 30-day all-cause acute care encounters |
Notes: *Study included both pediatric and adult populations and reported results separately for each cohort. Only adult data are presented in the table. † Reported as patients per year. ‡ Reported as median age (IQR)
Abbreviations: ACS, acute chest syndrome; AMI, acute myocardial infarction; AZ, Arizona; LOS, lengths of stay; CA, California; ED, emergency department; FL, Florida; IA, Iowa; IL, Illinois; KS, Kansas; MA, Massachusetts; MD, Maryland; MI, Michigan; MO, Missouri; NA, not applicable; NJ, New Jersey; NR, not reported; NY, New York; SC, South Carolina; SCA, sickle cell anemia; SCD, sickle cell disease; SD, standard deviation; TN, Tennessee; US, United States; WI, Wisconsin.
The Impact of SCD on HCRU
Patients with SCD reported to use considerable healthcare resources, often driven by hospitalization. The SLR identified 10 studies that reported hospitalization and/or rehospitalization as an HCRU-related outcome (Table 3).31,32,34-38,40,42,44 Hospitalization and/or rehospitalization were defined as SCD-related visits in three studies,32,40,44 and pain-related due to SCD/VOC visits in two studies.34,38 Five studies did not report reason(s) for hospitalizations and/or rehospitalization.31,35-37,42 The mean number of hospitalizations varied greatly from 0.5 to 27.9 per patient per year and the 30-day re-hospitalization rate ranged from 11.9% to 41.1%.32,35,37,42 Four studies reported hospitalizations either as the percent admitted from ED or acute care unit visits,34,38 the number of hospitalizations per 100,000 US population,40 or mean quarterly number of inpatient visits.31 The large variation in reporting of hospitalizations made it difficult to compare between studies, which are thus summarized qualitatively. No temporal association was observed for SCD-related hospitalizations and hospitalization rate.34,40
The mean length of hospitalization in adults with SCD ranged from 5.6 to 9.3 days,30,38-40 with a similar length of stay (LOS) reported for ED vs acute care unit (8.7 days vs 9.3 days; p = not reported).38 In addition, there was minimal change in average LOS between 1998 and 2008 (5.8 days vs 5.6 days; p = 0.29), in a study that evaluated hospitalization trends in the 11 years following FDA approval of hydroxyurea.40
ED visits were reported as being either SCD-related or pain-related (due to SCD) in three studies;32,34,44 five studies did not report reasons for ED visits.31,33,41-43 Adults with SCD were found to have varying rates of ED visits ranging from 0.3 to 3.5 per annum.32,41,42 Five studies reported ED visits either as the total number of ED visits over the study period, mean quarterly number of ED visits, or a proportion of patients with varying frequency of ED visits.31,33,34,43,44 Variations in reporting of the ED utilization limited the comparability of findings across these studies. One EMR study evaluated ED utilization in a NY hospital and reported that more than 50% of patients with SCD had two or more ED visits in one follow-up year and 22% of patients had six or more visits accounting for 77% of the total visits.33 Of note, when ED utilization pattern was evaluated for SCD patients who presented to ED with VOC pain over three time periods, no discernible pattern was found (2007–2008; 264.5 visits per year for 43.5 patients per year, 2011–2012; 448 visits per year for 36.5 patients per year, and January to December 2013; 382 visits per year for 38 patients per year).34
Potential Factors Affecting HCRU
Patient and Disease Characteristics
Age and Insurance
Across four studies identified in the economic SLR, HCRU was reported to be highest among younger adult patients with SCD. The first retrospective database study using the 2005 and 2006 HCUP data found that patients aged 18–30 years had the highest number of ED visits (1.59 per patient per year) compared to older age groups (0.33 to 1.29 per patient per year),32 with the second retrospective database study also reporting highest utilization among patients aged 21–30 years (7.5 ED visits per patient) compared to patients aged 31 years or older (4.6 to 6.7 visits per patient).43 Using data over a 10-year time period (2005–2014), the third registry study confirmed that the rate of ED visits was highest among young adults in their 30s, ranging from 0.5 to 3.4 mean annual ED visits.41 The hospitalization and re-hospitalization rate was also the highest for patients aged 18–30 years compared to other older age groups (2.02 vs 0.72 to 1.65 per patient year and 41.1% vs 11.9% to 38.8%, respectively).32
When the association of age, sex, insurance, genotype and HCRU was evaluated using regression analysis performed by study authors, older age was associated with decreased HCRU (ED, inpatient, urgent care visits; p < 0.001, p < 0.001, and p < 0.003). The study also found that SCD patients on public insurance had significantly higher ED visits (incidence rate ratio [IRR] = 3.79 [2.45–5.85], p < 0.001), higher inpatient visits (IRR = 2.62 [1.71–4.03], p < 0.001), and higher urgent care visits (IRR = 2.44 [1.64–3.63], p < 0.001) than SCD patients on commercial insurance.42
Comorbidities and Complications of SCD
A cross-sectional study of EDs across the US found that upon presentation to an ED, SCD patients with a diagnosis of dental infections were significantly more likely to be admitted to hospital than were those without dental infections (prevalence ratio: 1.65 [95% CI: 1.51–1.80]; p < 0.001).36 When stratified by VOC status, among patients with a VOC diagnosis, patients with dental infection were more likely to be admitted to the hospital than those with no dental infection (prevalence ratio: 1.72 [95% CI: 1.58–1.87]; p < 0.001). However, no significant association was found among patients without a VOC diagnosis.
SCD-related complications, such as ACS, may be associated with increased HCRU. One retrospective database analysis of SCD adults hospitalized with ACS reported that the mean LOS was 7.8 days (SE: 0.13).30 Younger age, being female, having used mechanical ventilation for 96 or more consecutive hours and not been admitted as an emergent case in the hospital were all independently significantly associated with longer hospital stay (p < 0.001).
Management Approach for SCD
Hydroxyurea
There is conflicting evidence on the use of hydroxyurea and HCRU reported by three identified studies showing inconsistent results.33,35,44 One EMR study of adults with SCD observed a significant positive relationship between the number of ED visits and hydroxyurea use: 23.0% of patients with 0–1 ED visits used hydroxyurea, 40.3% of patients with 2–5 visits used hydroxyurea, and 65.3% of patients with six or more ED visits used hydroxyurea (p < 0.001),33 while the second retrospective database study found that hydroxyurea use was associated with lower 30-day all-cause readmission among adults treated with either ≥1 g or 0.5–1 g compared to adults treated with <0.5 g (OR = 0.72 [95% CI 0.52–0.99]; p = 0.045 and OR = 0.73 [95% CI 0.57–0.93]; p = 0.012, respectively).44 Of note, in the first study, the average first dose (mg/kg) did not significantly differ between the three groups (16.6, 17.8, 19.7 in the 0–1, 2–5 and ≥6 groups, respectively; p = 0.40). The third study found ED/hospital admissions and 30-day readmission significantly decreased after the introduction of the intensive management strategy (ie, the introduction of hydroxyurea, prophylactic transfusion therapy for the prevention of VOC, pain management, and referrals for subspecialty care) in a subset of patients with 12 or more visits (27.9 to 13.5 per patient-year, p < 0.001 and 13.5 to 1.8 per patient-year, p < 0.001).35 However, the reduction in ED/hospital admission was not demonstrated in the entire cohort (7.1 to 6.1 per patient-year, p = 0.84).
Iron Chelation Therapies
One retrospective database analysis using Medicaid claims data from five states reported the differences in HCRU between patients receiving iron chelation therapies and those not receiving such therapy.31 Among chronically transfused patients (defined as ≥10 transfusions), receiving iron chelation therapies was associated with significantly lower ED visits (2.09 vs 3.06; p = 0.007) and number of hospitalized days (3.37 vs 6.13; p < 0.001) per quarter than patients who did not receive iron chelation therapies. However, no significant differences were found in the mean number of outpatient days per quarter (4.24 vs 4.29; p = 0.898).
Dedicated SCD Treatment Settings
One retrospective study, which analyzed EMRs of adults with SCD experiencing acute pain episodes in Illinois between two settings (ED vs acute care unit for SCD), reported that there were differences in hospital admission but not for LOS.38 Of ED visits, 70% were admitted to the hospital as compared to 37% of the SCD acute care unit visits resulting in hospital admissions. The study authors noted the main difference between the two settings was hourly opioid dose, with SCD acute care unit more consistently applying higher opioid dose for VOC in line with the published guideline.
Medical Costs
Medical costs were only reported in one retrospective database analysis among patients receiving iron chelation therapy and those who did not.31 Among chronically transfused patients (defined as ≥10 transfusions), receiving iron chelation therapy was associated with significantly higher pharmacy costs but lower inpatient and ED costs than those without ($3096 vs $1540 p < 0.001; $6438 vs $9719; p = 0.002 and $233 vs $592; p = 0.002, respectively), leading to similar total costs ($14,511 vs $12,966; p = 0.249).
Discussion
The aim of the present systematic evidence synthesis was to identify and report on the economic burden and disease burden captured by PROs among adults with SCD in the US. Overall, the findings of the identified studies consistently demonstrated that the disease burden for adults with SCD is high, including impaired HRQL, more depressive and/or anxiety symptoms, and poor sleep quality. Similarly, our findings confirmed that SCD imposes a substantial HCRU impact on patients and healthcare systems.
Acute VOC is a key characteristic of SCD and is often unpredictable and extremely painful. Unsurprisingly, there is a high prevalence of adults reporting depressive symptoms and/or anxiety, seen in 21–33% and 7–36% of patients.22,23,28,29 The rate is considerably higher than what is reported for the general US population: 6.7% for depression and 3.1% for generalized anxiety disorder. The presence of depression and/or anxiety can impose significant disease burden and ultimately lead to poor HRQL. Our findings suggested that opioid use was associated with worse HRQL than non-opioid use, meaning it may not provide adequate relief for adults.20,26 In addition, the daily use of high-dose opioids was associated with significantly higher somatic symptom burden, depression, anxiety, and lower HRQL.22 However, due to the cross-sectional nature of the study design, a causal relationship could not be established between worse HRQL, depression, and anxiety and the use of higher opioid use or chronic pain as experienced by these patients. As pain control, specifically opioid use, for adults remains a key outcome of treatment, the question remains unanswered on how to best mitigate the disease burden of patients with SCD with acute or chronic pain.
While the efficacy of hydroxyurea has been well demonstrated in randomized controlled trials, its impact on general well-being in the real world is not well understood, with one study reporting a positive impact on social functioning, pain, and general health perception in a subset of patients with moderate-to-severe disease.45 Based on one cross-sectional study, the limited evidence from our findings indicated that patients given hydroxyurea experienced improved HRQL when compared to those given opioids, but showed comparable HRQL to patients on neither treatment.20
For the economic burden, three studies evaluated the impact of hydroxyurea among adults with SCD.33,35,44 The present systematic evidence synthesis identified conflicting evidence on the impact of hydroxyurea on HCRU. For example, one retrospective study found that there was a significant positive relationship between the number of ED visits and hydroxyurea use.33 However, as the authors noted, due to the cross-sectional study design, it is not clear whether ED visits would have been higher if the patients were not being treated with hydroxyurea. The two other studies demonstrated the benefit of using hydroxyurea and adherence to hydroxyurea in reducing the rate of ED, hospital admission, and 30-day readmission.35,44 Though the volume of literature on hydroxyurea use among adults with SCD was sparse, findings indicated considerable ED utilization among hydroxyurea users, and gaps remained in providing treatment options that prevent acute complications that lead to HCRU. Of note, the finding supported the use of hydroxyurea for adults with SCD in line with the National Heart, Lung, and Blood Institute guideline and underscored the importance of adherence and proper dosing.12
Most of the identified economic studies focused on HCRU, while only one reported on healthcare costs. Adults with SCD were found to have varying rates of ED utilization (0.3–3.5 annual ED visits), hospitalizations (0.5–27.9 hospitalizations per patient per year), and/or readmission (12–41%). The economic burden of SCD varied based on numerous factors. Some subgroups such as young adults in their 20s and 30s, those with dental infection, and those with ACS were identified as having higher rates of healthcare utilization similar to what was previously reported.16,46 Conversely, evidence was found that SCD acute care units reduce admission rates, though LOS was similar regardless of whether patients were first treated in SCD acute care units or EDs.38 Additionally, intensive management strategy consisting of SCD interventions, pain management, and referrals for subspecialty care were found to reduce the ED, hospital admission, and 30-day readmission rates.35
An obvious data gap related to the impact of SCD was academic or work performance and costs (both direct and indirect), which can help quantify the full economic burden of SCD. Ballas provided a helpful perspective on the cost of healthcare for patients with SCD based on studies published in the 1990s and 2000s.47 In this paper, the author estimated the average annual costs for the healthcare of an adult in Pennsylvania would be $231,050 in 2009. In the same year, another paper by Kauf et al reported that the average lifetime cost of care would be $460,151 per patient with SCD, much lower than the estimation by Ballas.48 A recent cohort simulation modeling study by Lubeck et al (published outside of the time span of our SLR) evaluated the association between SCD and lifetime income and found a lost income of $695,000 for an individual with SCD compared to a matched individual without SCD.49 Considering the variability of evaluated outcomes across the above-mentioned studies and that most cost data are now outdated, future studies should be designed to estimate both direct and indirect costs and evaluate the cost of treating SCD-related complications in light of recent approved treatments. Since SCD is associated with significant morbidity and can directly affect work productivity, evaluating the size of income loss and recent direct costs associated with SCD would better quantify the economic burden from a societal perspective.50 In addition, such data can be useful as input for evaluating the cost-effectiveness of new or emerging therapies and allocate appropriate resources for patients with SCD.
Strengths and Limitations
To the best of our knowledge, this is the first systematic evidence synthesis that provides a comprehensive view of the overall disease burden of SCD from an economic and psychosocial perspective in the US. In addition, the review provides the most recent and relevant literature pertaining to the PROs and economic burden of adults with SCD since 2007. Our study has several limitations. First, like all systematic evidence syntheses, it is limited by publication bias. For example, studies that identified a significant burden of the disease are more likely to be published. Second, included articles were limited to the English language, were restricted to a US population and only included articles published since 2007. This was done to ensure that the most relevant articles were included as clinical practice may vary over time, but external validity may be limited outside the US. Third, included studies were observational in nature; thus, causality could not be identified, a limitation not uncommon in systematic reviews. In addition, most studies were retrospective and did not account for the risk of confounding bias. For example, varying degrees of disease severity and transfusion status may have influenced reported outcomes. Differences in included studies in how data were collected and summarized and how outcomes (eg, hospitalizations) were reported precluded us from conducting a meta-analysis. Fourth, there were limitations inherent to observational studies (eg, database analysis, EMR study), including errors of omission and commission, lack of/sparse information on patient characteristics (including disease severity), unobserved confounders, and treatment adherence. Finally, the review considered studies that included adults defined by study authors, and one study included patients aged 16 and older.
Conclusion
From 2007 to 2018, disease burden measured by PROs and economic burden of SCD in adults in the US was substantial despite the availability of approved treatments for the disease. The use of hydroxyurea, optimal management of opioids, and employing intensive treatment strategies may help limit the overall burden to patients and the healthcare system in the US. Published data on direct and indirect cost are limited and highlight the need for more economic studies to characterize the full burden of the disease.
Funding Statement
Novartis provided the funding for the study.
Abbreviations
ACS, acute chest syndrome; BDI-II, Beck Depression Inventory II; CSQ, Coping Strategy Questionnaire; ED, Emergency department; FDA, Food and Drug Administration; GAD, Generalized Anxiety Disorder; HCRU, healthcare resource utilization; HRQL, health-related quality of life; LOS, lengths of stay; MCS, Mental Component Summary; MME, Morphine milligram equivalents; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PRO, patient-reported outcome; QOL, quality of life; SCA, sickle cell anemia; SCD, sickle cell disease; SLR, systematic literature review; US, United States; VOC, vaso-occlusive crises.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
SL and MB are employed by Novartis. DV and GS are employed by Evidera, which provides consulting and other research services to pharmaceutical, medical device, and related organizations. DR and SA were employees of Evidera at the time this study was executed. In their salaried positions, they work with a variety of companies and organizations, and are precluded from receiving payment or honoraria directly from these organizations for services rendered. MB reports grants from Novartis, during the conduct of the study; personal fees from Amgen, outside the submitted work. DR reports grants from Novartis, during the conduct of the study; personal fees from Amgen, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517 [DOI] [PubMed] [Google Scholar]
- 2.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84(9):618–625. doi: 10.1002/ajh.21475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801–809. doi: 10.1182/blood-2015-09-618538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccin A, Murphy C, Eakins E, et al. Insight into the complex pathophysiology of sickle cell anaemia and possible treatment. Eur J Haematol. 2019;102(4):319–330. doi: 10.1111/ejh.13212 [DOI] [PubMed] [Google Scholar]
- 5.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease daily pain in sickle cell disease. Ann Intern Med. 2008;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004 [DOI] [PubMed] [Google Scholar]
- 7.Jordan L, Adams-Graves P, Kanter-Washko J, et al. Multicenter COMPACT study of COMplications in patients with sickle cell disease and utilization of iron chelation therapy. Curr Med Res Opin. 2015;31(3):513–523. doi: 10.1185/03007995.2014.998815 [DOI] [PubMed] [Google Scholar]
- 8.Bhor M, Xie L, Paulose J, Yuce H, Shah N Rate of vaso-occlusive crisis episodes among sickle cell disease patients in the us medicaid population. Presented at the 46th Sickle Cell Disease Association of America (SCDAA) 2018. [Google Scholar]
- 9.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647–3656. doi: 10.1182/blood-2012-04-383430 [DOI] [PubMed] [Google Scholar]
- 10.Schatz J, Puffer ES. Neuropsychological aspects of sickle cell disease In: Brown RT, editor. Comprehensive Handbook of Childhood Cancer and Sickle Cell Disease: A Biopsychosocial Approach. New York, NY: Oxford University Press; 2006:449–470. [Google Scholar]
- 11.Meier ER, Miller JL. Sickle cell disease in children. Drugs. 2012;72(7):895–906. doi: 10.2165/11632890-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report. Washington DC: USA NHLBI; 2014. [Google Scholar]
- 13.Endari [Package Insert]. Torrance, CA: Emmaus Medical Inc; 2017. [Google Scholar]
- 14.Adakveo [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2019. [Google Scholar]
- 15.Oxbryta [Full Prescribing Information]. South San Francisco, CA: Global Blood Therapeutics, Inc; 2019. [Google Scholar]
- 16.Benenson I, Jadotte Y, Echevarria M. Factors influencing utilization of hospital services by adult sickle cell disease patients: a systematic review. JBI Database Syst Rev Implement Rep. 2017;15(3):765–808. doi: 10.11124/JBISRIR-2016-002983 [DOI] [PubMed] [Google Scholar]
- 17.Sarri G, Bhor M, Abogunrin S, et al. Systematic literature review and assessment of patient-reported outcome instruments in sickle cell disease. Health Qual Life Outcomes. 2018;16(1):99. doi: 10.1186/s12955-018-0930-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochrane Haematological Malignancies Group. How to develop a search strategy for a Cochrane Review; 2007. Available from: https://chmg.cochrane.org/sites/chmg.cochrane.org/files/uploads/How%20to%20develop%20a%20search%20strategy-support-manual.pdf.
- 20.Adam SS, Telen MJ, Jonassaint CR, De Castro LM, Jonassaint JC. The relationship of opioid analgesia to quality of life in an adult sickle cell population. Health Outcomes Res Med. 2010;1(1):e29–e37. doi: 10.1016/j.ehrm.2010.04.002 [DOI] [Google Scholar]
- 21.Ezenwa MO, Yao Y, Molokie RE, et al. Coping with pain in the face of healthcare injustice in patients with sickle cell disease. J Immigrant Minority Health. 2017;19(6):1449–1456. doi: 10.1007/s10903-016-0432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karafin MS, Singavi A, Hussain J, et al. Predictive factors of daily opioid use and quality of life in adults with sickle cell disease. Hematology. 2018;23:1–8. [DOI] [PubMed] [Google Scholar]
- 23.Levenson JL, McClish DK, Dahman BA, et al. Depression and anxiety in adults with sickle cell disease: the PiSCES project. Psychosom Med. 2008;70(2):192–196. doi: 10.1097/PSY.0b013e31815ff5c5 [DOI] [PubMed] [Google Scholar]
- 24.Levenson JL, McClish DK, Dahman BA, et al. Alcohol abuse in sickle cell disease: the Pisces Project. Am j Addict. 2007;16(5):383–388. doi: 10.1080/10550490701525434 [DOI] [PubMed] [Google Scholar]
- 25.Sogutlu A, Levenson JL, McClish DK, Rosef SD, Smith WR. Somatic symptom burden in adults with sickle cell disease predicts pain, depression, anxiety, health care utilization, and quality of life: the PiSCES project. Psychosomatics. 2011;52(3):272–279. doi: 10.1016/j.psym.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 26.Smith WR, McClish DK, Dahman BA, et al. Daily home opioid use in adults with sickle cell disease: the PiSCES project. J Opioid Manag. 2015;11(3):243–253. doi: 10.5055/jom.2015.0273 [DOI] [PubMed] [Google Scholar]
- 27.Mann‐Jiles V, Morris DL. Quality of life of adult patients with sickle cell disease. J Am Assoc Nurse Pract. 2009;21(6):340–349. doi: 10.1111/j.1745-7599.2009.00416.x [DOI] [PubMed] [Google Scholar]
- 28.Treadwell M, Barreda F, Kaur K, Gildengorin G. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manage. 2015;22(1):10–20. [Google Scholar]
- 29.Wallen GR, Minniti CP, Krumlauf M, et al. Sleep disturbance, depression and pain in adults with sickle cell disease. BMC Psychiatry. 2014;14(1):207. doi: 10.1186/1471-244X-14-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allareddy V, Roy A, Lee MK, et al. Outcomes of acute chest syndrome in adult patients with sickle cell disease: predictors of mortality. PLoS One. 2014;9(4):e94387. doi: 10.1371/journal.pone.0094387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blinder MA, Vekeman F, Sasane M, Trahey A, Paley C, Duh MS. Age‐related treatment patterns in sickle cell disease patients and the associated sickle cell complications and healthcare costs. Pediatr Blood Cancer. 2013;60(5):828–835. doi: 10.1002/pbc.24459 [DOI] [PubMed] [Google Scholar]
- 32.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–1294. doi: 10.1001/jama.2010.378 [DOI] [PubMed] [Google Scholar]
- 33.Curtis SA, Danda N, Etzion Z, Cohen HW, Billett HH. Elevated steady state WBC and platelet counts are associated with frequent emergency room use in adults with sickle cell anemia. PLoS One. 2015;10(8):e0133116. doi: 10.1371/journal.pone.0133116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue S, Mushtaq R, Sanikommu SR, Mbeumo C, LaChance J, Roebuck M. Pain management trend of vaso-occulsive crisis (VOC) at a community hospital emergency department (ED) for patients with sickle cell disease. Ann Hematol. 2016;95(2):221–225. doi: 10.1007/s00277-015-2558-x [DOI] [PubMed] [Google Scholar]
- 35.Koch KL, Karafin MS, Simpson P, Field JJ. Intensive management of high‐utilizing adults with sickle cell disease lowers admissions. Am J Hematol. 2015;90(3):215–219. doi: 10.1002/ajh.23912 [DOI] [PubMed] [Google Scholar]
- 36.Laurence B, Haywood C Jr, Lanzkron S. Dental infections increase the likelihood of hospital admissions among adult patients with sickle cell disease. Community Dent Health. 2013;30(3):168–172. [PMC free article] [PubMed] [Google Scholar]
- 37.Leschke J, Panepinto JA, Nimmer M, Hoffmann RG, Yan K, Brousseau DC. Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409. doi: 10.1002/pbc.23140 [DOI] [PubMed] [Google Scholar]
- 38.Molokie RE, Montminy C, Dionisio C, et al. Opioid doses and acute care utilization outcomes for adults with sickle cell disease: ED versus acute care unit. Am J Emerg Med. 2018;36(1):88–92. doi: 10.1016/j.ajem.2017.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogunbayo GO, Misumida N, Olorunfemi O, et al. Comparison of outcomes in patients having acute myocardial infarction with versus without sickle-cell anemia. Am J Cardiol. 2017;120(10):1768–1771. doi: 10.1016/j.amjcard.2017.07.108 [DOI] [PubMed] [Google Scholar]
- 40.Okam MM, Shaykevich S, Ebert BL, Zaslavsky AM, Ayanian JZ. National trends in hospitalizations for sickle cell disease in the United States following the FDA approval of hydroxyurea, 1998 to 2008. Med Care. 2014;52(7):612. doi: 10.1097/MLR.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulukonis ST, Feuchtbaum LB, Coates TD, et al. Emergency department utilization by Californians with sickle cell disease, 2005–2014. Pediatr Blood Cancer. 2017;64(6):e26390. doi: 10.1002/pbc.26390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ter-Minassian M, Lanzkron S, Derus A, Brown E, Horberg MA. Quality metrics and health care utilization for adult patients with sickle cell disease. J Natl Med Assoc. 2018;111(1):54–61. [DOI] [PubMed] [Google Scholar]
- 43.Wolfson JA, Schrager SM, Khanna R, Coates TD, Kipke MD. Sickle cell disease in California: sociodemographic predictors of emergency department utilization. Pediatr Blood Cancer. 2012;58(1):66–73. doi: 10.1002/pbc.22979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou J, Han J, Nutescu EA, Gordeuk VR, Saraf SL, Calip GS. Hydroxycarbamide adherence and cumulative dose associated with hospital readmission in sickle cell disease: a 6-year population-based cohort study. Br J Haematol. 2018;182(2):259–270. doi: 10.1111/bjh.15396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballas SK, Barton FB, Waclawiw MA, et al. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual Life Outcomes. 2006;4:59. doi: 10.1186/1477-7525-4-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piccin A, Fleming P, Eakins E, McGovern E, Smith OP, McMahon C. Sickle cell disease and dental treatment. J Ir Dent Assoc. 2008;54(2):75–79. [PubMed] [Google Scholar]
- 47.Ballas SK. The cost of health care for patients with sickle cell disease. Am J Hematol. 2009;84(6):320–322. doi: 10.1002/ajh.21443 [DOI] [PubMed] [Google Scholar]
- 48.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84(6):323–327. doi: 10.1002/ajh.21408 [DOI] [PubMed] [Google Scholar]
- 49.Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2(11):e1915374. doi: 10.1001/jamanetworkopen.2019.15374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29(6):1533–1547. doi: 10.1007/s11136-019-02412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cochrane Haematological Malignancies Group. How to develop a search strategy for a Cochrane Review; 2007. Available from: https://chmg.cochrane.org/sites/chmg.cochrane.org/files/uploads/How%20to%20develop%20a%20search%20strategy-support-manual.pdf.