Abstract
Background & Aims:
Patients with inflammatory bowel diseases (IBD) who have post-inflammatory polyps (PIPs) have an increased risk of colorectal neoplasia (CRN). European guidelines propose that patients with PIPs receive more frequent surveillance colonoscopies, despite limited evidence of this increased risk. We aimed to define the risk of CRN and colectomy in patients with IBD and PIPs.
Methods:
We conducted a multicenter retrospective cohort study of patients with IBD who underwent colonoscopic surveillance for CRN, from January 1997 through January 2017, at 5 academic hospitals and 2 large non-academic hospitals in New York or the Netherlands. Eligible patients had confirmed colonic disease with duration of 8 years or more (or any duration, if they also have primary sclerosing cholangitis) and no prior history of advanced CRN (high-grade dysplasia or colorectal cancer) or colectomy. The primary outcome was occurrence of advanced CRN according to PIP status; secondary outcomes were occurrence of CRN (inclusive of low-grade dysplasia) and colectomy.
Results:
Among 1582 eligible patients, 462 patients (29.2%) had PIPs. PIPs were associated with more severe inflammation (adjusted odds ratio [aOR], 1.32; 95% CI, 1.13–1.55), greater disease extent (aOR 1.92; 95% CI, 1.34–2.74), and lower likelihood of primary sclerosing cholangitis (aOR 0.38; 95% CI, 0.26–0.55). During a median follow-up period of 4.8 years, the time until development of advanced CRN did not differ significantly between patients with vs without PIPs. PIPs did not independently increase risk of advanced CRN (adjusted hazard ratio, 1.17; 95% CI, 0.59–2.31). The colectomy rate was significantly higher in patients with PIPs (P=0.01).
Conclusions:
In a retrospective analysis of data from 2 large independent surveillance cohorts, PIPs were associated with greater severity and extent of colon inflammation and higher rates of colectomy, but were not associated with development of any degree of CRN. Therefore, intervals for surveillance should not be shortened solely based on the presence of PIPs.
Keywords: PSC, Ulcerative Colitis, Crohn’s Colitis, Crohn’s Disease, Quality Improvement, Endoscopy
Introduction
Patients with longstanding inflammatory bowel disease (IBD) colitis are at increased risk of developing colorectal dysplasia and colorectal cancer (CRC).1,2 Current guidelines recommend performing surveillance colonoscopies at regular intervals to screen for colorectal neoplasia (CRN, dysplasia or carcinoma).3–6 Leading European guidelines stratify patients with IBD colitis into groups with low, intermediate or high-risk of CRC based on several risk factors, including the presence of post-inflammatory polyps (PIPs).3,5,6 Commonly referred to as “pseudopolyps”, PIPs are encountered in 20–45% of patients with IBD and colonic involvement.7–10 Older case-control studies reported a 1.9- to 2.5-fold increased risk of CRC in patients with PIPs.8,9,11 More recently, however, in a large retrospective cohort study of patients with ulcerative colitis (UC) undergoing CRN surveillance, PIPs did not independently predict CRN or predict progression from low-grade dysplasia (LGD) to advanced CRN (ACRN; defined as high-grade dysplasia (HGD) or CRC).10,12
Theoretically, the risk of CRN could be increased in patients with PIPs if their presence indicates prior severe inflammation. Alternatively, PIPs may obscure otherwise visible and resectable dysplastic lesions during surveillance. Direct malignant transformation of PIPs is generally considered unlikely.13 Regardless of the mechanism, there is a gap in the literature as to whether PIPs are independent predictors of ACRN. Clarifying this risk has far-reaching implications with respect to the burden of surveillance colonoscopies in patients with IBD and PIPs. If possible, safe lengthening of surveillance intervals would impact quality of life and promote cost containment and resource stewardship. Using a large multicenter cohort of patients with confirmed colonic IBD undergoing colonoscopic surveillance, we primarily aimed to determine whether PIPs are associated with increased risk of ACRN, and secondarily with CRN or colectomy. We also aimed to delineate predisposing or protective factors for PIPs and to define the prevalence of CRN in biopsied PIPs.
Methods
Study design and population
This retrospective cohort study identified patients with confirmed colitis undergoing colonoscopic surveillance for CRN between January 1997- January 2017 from two large IBD cohorts: the Mount Sinai Hospital (MSH, New York, USA) cohort and a Dutch cohort coordinated by the Utrecht University Medical Center (UMCU, Utrecht, The Netherlands), comprising 5 academic hospitals and 2 large non-academic hospitals. The search strategy has been described in detail previously.14 Inclusion criteria were: 1) diagnosis of IBD (UC, Crohn’s disease (CD), IBD-unclassified (IBD-U)); 2) confirmed colonic disease by endoscopy and histology of at least 8 years, or of any duration if concomitant primary sclerosing cholangitis (PSC; confirmed by ERCP, MRCP, or liver biopsy); 3) enrollment in a dysplasia surveillance program; 4) ≥ 2 surveillance colonoscopies with available colonoscopy and pathology reports, or ≥ 1 surveillance colonoscopy if interval ACRN was diagnosed on pathology obtained by another method; 5) at least left-sided disease extent (UC), involvement of >30% of the colonic surface (CD or IBD-U), or any extent if concomitant PSC; and, after meeting these inclusion criteria, 6) no history of ACRN or colectomy prior to (or within the three months following) the first surveillance colonoscopy within the predefined study period (i.e. “index colonoscopy”).
Data collection
The following baseline and clinical data were collected from the electronic health record (EHR) documentation using the same data collection format and definitions for both cohorts: date of birth, sex, age at IBD diagnosis, IBD type (UC, CD, or IBD-U), family history of CRC, diagnosis of PSC (confirmed by histology or endoscopic/radiologic cholangiography) and prior history of colonic dysplasia (defined as indefinite for dysplasia (IND) or LGD at or before the index colonoscopy). Maximum extent of colonic disease was determined based on prior history as documented in the EHR and maximal disease extent during colonoscopic surveillance according to either endoscopic and/or histologic findings. Any documented exposure to medication was collected before and during follow-up, including 5-aminosalicylates (5-ASA), immunomodulators (azathioprine or 6-mercaptopurine), methotrexate and biologicals (including infliximab, adalimumab, certolizumab, golimumab, ustekinumab, natalizumab and vedolizumab). Surveillance procedures were defined as colonoscopies in which either segmental random biopsies or chromoendoscopy were employed. Data from these procedures were collected from colonoscopy and pathology reports. In addition, data from any procedure (e.g. colectomy) leading to a diagnosis of ACRN were recorded. Colonoscopies that did not meet these criteria were excluded. Endoscopic inflammation (1 - Normal/inactive; 2 – Mild; 3 – Moderate; 4 – Severe) and histologic inflammation (1- Normal; 2 – Inactive; 3 - Mild; 4 - Moderate; 5 – Severe) were scored per segment. A mean inflammation score was calculated by averaging the scores of the most severely inflamed segment of all recorded surveillance colonoscopies.
For each endoscopic (or surgical) procedure, the following data were collected: date of procedure, presence of PIPs, quality of bowel preparation (adequate [excellent or good] or inadequate [fair or poor]), extent of intubation and endoscopic/histologic inflammation. Quality measures were reported relative to the number of surveillance procedures performed during follow-up (i.e. percentage of procedures with adequate bowel preparation or cecal intubation). For the USA cohort only, if the endoscopy report described PIPs as “many”, “limiting visibility” or “fields” patients were subclassified as having “many PIPs”. In the absence of these descriptors, patients were subclassified as having “few PIPs”. Furthermore, colonic location of PIPs, number of PIPs biopsied (including any lesion that was reported to be a PIP in the endoscopy or pathology report), and presence and grade of dysplasia in aforementioned lesions were extracted. These data were not available in the Dutch cohort.
Histologic diagnosis and highest grade of CRN (defined as LGD, HGD, CRC) or IND were recorded per segment. At all participating institutions, specimens with suspected CRN are routinely reviewed by at least two pathologists. No samples were re-reviewed and no alterations to the finalized reports were made for the purposes of this study.
Colectomy was defined as either subtotal colectomy or total proctocolectomy. Colectomy date and indication (medically refractory disease (MRD), stricture, dysplasia (CRN of any degree, suspected or confirmed) or multiple (combination of the former)) were documented. Histologic findings from colectomy specimens (e.g. dysplasia, cancer) were recorded. For colectomies, only the highest grade of CRN was recorded for the purposes of this study. Thus, an outcome of IND, for example, implies that there was no synchronous diagnosis of LGD, HGD or CRC.
The date of the index colonoscopy was set as the start of follow-up and the time-at-risk. The total duration of follow-up was defined as the interval between the index colonoscopy (t0) and time tx, which was the first occurrence of any of the following events: the primary outcome, any censoring event, or the predefined end of the study period (January 31, 2017). Patients were censored at colectomy, a diagnosis of ACRN, or last follow-up before the end of the study period.
Outcomes of Interest
The primary outcome of the study was the rate of occurrence of ACRN. Secondary outcomes were the rate of occurrence of CRN and colectomy. Furthermore, factors associated with presence or absence of PIPs, and factors predictive of or protective against ACRN and CRN were explored.
Statistical analyses
Descriptive statistics and comparative test statistics were reported according to the distribution of the data. Missing data were interpreted as absence of a characteristic for categorical parameters and excluded for continuous parameters. Time-to-event analyses were conducted for ACRN, CRN (defined as LGD, HGD, or CRC) and colectomy. For analyses of CRN, patients with “prior dysplasia” (defined as IND or LGD diagnosed at or before the index colonoscopy) were excluded. There were no missing data for the primary analyses of (A)CRN. Survival analysis was performed using Kaplan-Meier curves with log-rank test for significance. Patients were censored as defined above. Cox regression analysis was used to identify predictors for ACRN and CRN (hazard rates; HR), both for the joint cohort and stratified by cohort geography (USA versus Dutch cohort). Logistic regression was used instead of Cox regression to identify factors associated with PIPs (odds ratios; OR) since the majority of patients with PIPs had presented with PIPs at the index colonoscopy (i.e. “prevalent cases” instead of “incident cases”). As the primary exposure of interest, PIPs were included a priori in all multivariable analyses. PSC was also included a priori in all models, as it is an established strong predictor of ACRN.14–17 In addition, covariates with P<0.10 on univariable analyses were included in the multivariable models. Interactions between covariates included in the multivariable models and the presence of PIPs were tested by comparing the log-likelihood ratios of the models that included the interaction term with the models that included these covariates as independent variables; no significant interactions were identified. We additionally performed the following time-trend analyses for our primary and secondary outcomes: 1) stratified analysis according to date of index colonoscopy; 2) sensitivity analysis excluding patients with colonoscopies prior to 01/01/2000; and 3) multivariable Cox regression analysis with year of the index colonoscopy included as an independent variable.
Reported HRs or ORs indicate risk or odds, respectively, per unit increase of corresponding parameters (e.g. per 1 year for disease duration). Mean endoscopic and histologic inflammation were collinear; the latter was preferred and included in the regression models.10 In order to limit the risk of immortal time bias for incident cases of PIPs, PIPs were included in the Cox regression models as a time-changing covariate.18
Statistical significance was set at a two-tailed P-value <0.05. The Bonferroni method was used to correct for multiple testing in independent subgroup analyses where appropriate. All analyses were performed using SPSS version 24 (Armonk, NY: IBM Corp.).
Study oversight
This study was reviewed and approved by the Institutional Review Board (IRB) at MSH. In the Netherlands, this study received exempt status from the IRB as it is exempt from the law of human-bound research.
Results
Patient characteristics
Our search yielded 1582 eligible patients: 429 patients in the USA cohort and 1153 in the Dutch cohort (Figure 1). The accrual of the cohort is depicted in Figure 2. The median follow-up time was 4.8 (IQR: 2.8 – 6.7) years, providing 8182 patient-years of follow-up. Characteristics of the USA and the Dutch cohorts are compared in Supplementary table 1.
Figure 1:
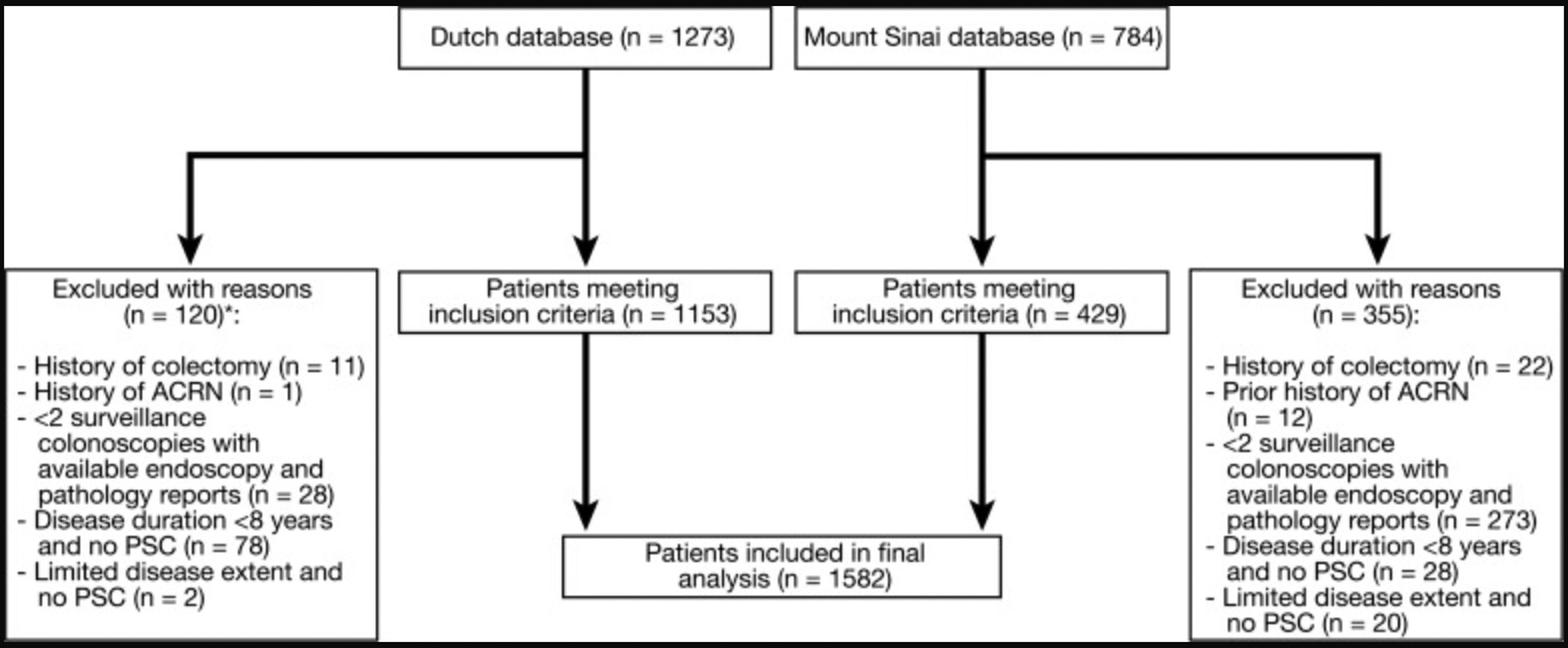
Flowchart of patient selection from databases. *Exclusion rate in the Dutch cohort is lower than in the USA cohort, because the majority of ineligible patients were excluded prior to data entry.
Figure 2:
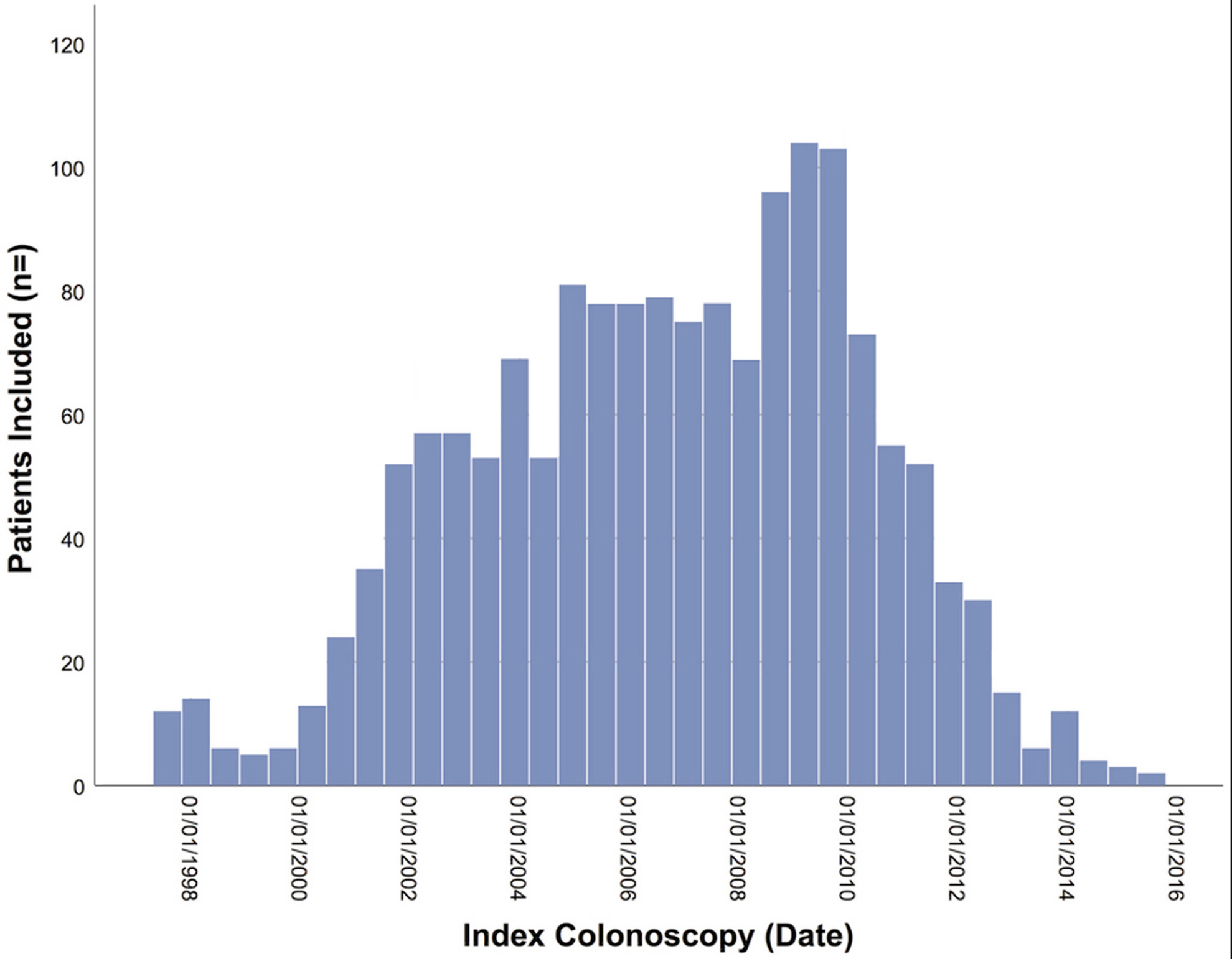
Accrual of the cohort.
Factors associated with PIPs
PIPs were present in 462 (29.2%) patients. Characteristics of patients with versus without PIPs are compared in (Table 1). PIPs were prevalent in 300 (19.0%) patients, and incident in 162 (10.2%) patients during follow-up. Among patients with PIPs, 273 (59.1%) had PIPs reported on multiple procedures. Out of 140 patients in the USA cohort with PIPs, 94 (67.1%) were categorized as “few”, while the remaining one-third was categorized as “many”. On multivariable logistic regression analysis histologic inflammation, extensive disease and cohort geography (USA versus Dutch cohort) were each independently associated with presence of PIPs. PSC was independently associated with absence of PIPs (Table 2).
Table 1:
Patient characteristics and follow-up data stratified by presence of PIPs
| PIPs n=462 |
No PIPs n=1120 |
P value* | |
|---|---|---|---|
| Baseline and disease-related characteristics | |||
| Age at index colonoscopy (years), median (IQR) | 45 (36 – 56) | 45.5 (35 – 54) | 0.43 |
| Sex, n (%) | 0.42 | ||
| Male | 238 (51.1) | 597 (53.3) | |
| Female | 227 (48.9) | 523 (46.7) | |
| IBD type, n (%) | 0.81 | ||
| Ulcerative colitis | 279 (60.4) | 230 (53.6) | |
| Crohn’s disease | 170 (36.8) | 181 (42.2) | |
| IBD-unclassified | 13 (2.8) | 18 (4.2) | |
| Incident PIPs, n (%) | 162 (35.1) | - | - |
| Follow-up before first diagnosis of PIPs (years), median (IQR) | 2.9 (2.0 – 4.7) | ||
| Family history of colorectal cancer, n (%) | 29 (6.3) | 64 (5.7) | 0.67 |
| Disease duration at index colonoscopy (years), median (IQR) | 14 (10 – 22) | 14 (10 – 22) | 0.40 |
| Dysplasia# at/before index colonoscopy, n (%) | 70 (15.2) | 163 (14.6) | 0.41 |
| Low-grade dysplasia | 34 (7.4) | 91 (8.1) | |
| Indefinite for dysplasia | 18 (3.9) | 27 (2.4) | |
| Unspecified | 17 (3.7) | 45 (4.0) | |
| Extensive disease, n (%) | 396 (88) | 879 (83) | 0.01 |
|
Primary sclerosing
cholangitis, n (%) |
38 (8.2) | 196 (17.5) | <0.0005 |
| Exposure to medication | |||
| 5-Aminosalicylates | 393 (85.1) | 893 (79.7) | 0.01 |
| Thiopurines | 265 (57.4) | 475 (42.4) | <0.0005 |
| Methotrexate | 30 (6.5) | 60 (5.4) | 0.38 |
| Biologicals | 125 (27.1) | 196 (17.5) | <0.0005 |
| Colonoscopic Surveillance Details | |||
| Number of procedures/year, median (IQR) | 0.7 (0.6 – 1.0) | 0.7 (0.6 – 1.0) | 0.49 |
| Mean inflammation score | |||
| Endoscopic | 1.50 (1.00 – 2.00) | 1.41 (1.00 – 1.80) | 0.001 |
| Histologic | 2.60 (2.00 – 3.00) | 2.50 (2.00 – 3.00) | <0.0005 |
| Cecum intubated, mean (SD) % of procedures | 86.0 (22.3) | 87.4 (22.3) | 0.21 |
| Adequate bowel preparation, mean (SD) % of procedures | 97.6 (10.5) | 98.1 (8.5) | 0.09 |
| Duration of follow-up (years), median (IQR) | 5.4 (3.3 – 7.6) | 4.5 (2.7 – 6.6) | <0.0005 |
Classification of PIPs in this table includes both prevalent and incident PIPs.
Significant at P <0.05 level. PIPs: Post-inflammatory polyps.
Patients with HGD at/before the index colonoscopy were excluded
Table 2:
Factors associated with presence of PIPs, logistic regression analysis
| Univariable | Multivariable** | ||||||
|---|---|---|---|---|---|---|---|
| Variable | PIPs (%) | OR | 95% CI | P value* | aOR | 95% | P value* |
| Patients with PIPs, n(%) | 462 (100) | ||||||
| Age at IBD diagnosis | - | 1.00 | 0.99 – 1.01 | 0.96 | |||
| Male sex | 238 (51) | 1.09 | 0.88 – 1.36 | 0.42 | |||
| Extensive disease | 396 (88) | 1.51 | 1.09 – 2.08 | 0.01 | 1.92 | 1.34 – 2.74 | <0.0005 |
| USA cohort# | 140 (30) | 1.25 | 0.98 – 1.59 | 0.06 | 1.40 | 1.04 – 1.88 | 0.03 |
| Mean histologic inflammation^ | - | 1.39 | 1.21 – 1.60 | <0.0005 | 1.32 | 1.13 – 1.55 | 0.001 |
| Primary sclerosing cholangitis | 38 (8.2) | 0.42 | 0.29 – 0.61 | <0.0005 | 0.38 | 0.26 – 0.55 | <0.0005 |
| Crohn’s disease## | 170 (37) | 1.06 | 0.84 – 1.32 | 0.64 | |||
| Disease duration at time of index colonoscopy | - | 1.01 | 1.00 – 1.02 | 0.13 | |||
Significant at P <0.05 level. PIPs: Post-inflammatory polyps.
Reference category: Dutch cohort;
Prior to the first reported PIP;
Reference category: ulcerative colitis/IBD-unclassified.
Note: 77 patients (15 with PIPs) were excluded due to missing data.
Neoplastic outcomes according to PIP status
Rate of occurrence of ACRN (primary outcome)
During follow-up, 17 patients (3.7%) with PIPs developed ACRN, compared to 24 (2.0%) without PIPs. There was no significant difference in occurrence of ACRN among patients with versus without PIPs (Figure 3a, P=0.41), with a median time to ACRN of 3.8 (IQR: 2.1 – 6.3) vs. 4.2 (IQR: 3.0 – 5.3) years, respectively. There was no difference in the rate of ACRN according to the density of PIPs (few versus many, USA cohort only) (Figure S1, P=0.36); or according to multiple reporting of PIPs ( ≥ 2 procedures) versus single reporting (1 procedure, P=0.41). Statistical non-significance in rates of ACRN between patients with versus without PIPs remained in the following subgroups: UC/IBD-U patients, CD patients, the Dutch cohort, USA cohort (Figure 3c–f), patients with/without PSC and patients with/without prior dysplasia (data not shown; each P>0.10).
Figure 3:
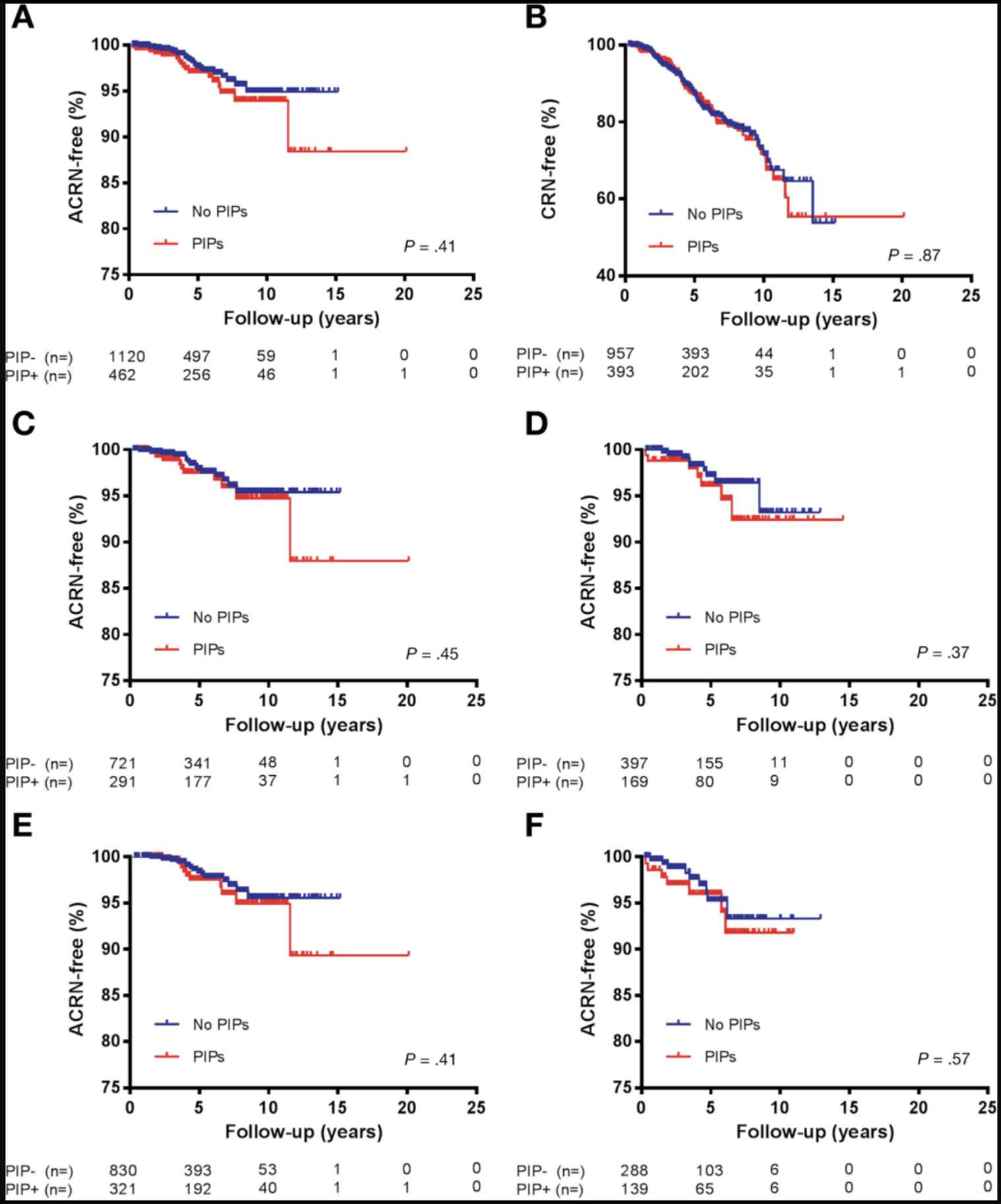
Kaplan-Meier curves, ACRN-free survival and CRN-free survival.
Predictors of ACRN
On multivariable Cox regression analysis, PIPs were not predictive of ACRN (Table 3). PSC, disease duration, prior dysplasia and mean histologic inflammation were independent positive predictors of ACRN occurrence, while cecal intubation was protective against ACRN. On stratified analysis by geographic cohort (USA vs Dutch cohort) and date of index colonoscopy (before versus after 01/01/2005), PIPs similarly did not independently predict ACRN. Furthermore, exposure to thiopurines was a significant, independent predictor of ACRN in the USA cohort only (aHR 0.29; 95%CI 0.09 – 1.00), but not in the combined study cohort. Finally, in a subgroup analysis of patients without prior dysplasia, a diagnosis of LGD during follow-up increased the risk of subsequent ACRN by over 5-fold (aHR 5.04; 95%CI: 2.67–9.52, P<0.0005) as compared to patients without incident LGD.
Table 3:
Predictors of ACRN, Cox regression analysis
| Univariable | Multivariable** | ||||||
|---|---|---|---|---|---|---|---|
| Variable | ACRN (%) | HR | 95% CI | P value* | aHR | 95% CI | P value* |
| Patients with ACRN, n (%) | 41 (100) | ||||||
| Age at index colonoscopy | - | 1.02 | 0.99 – 1.04 | 0.17 | |||
| Male sex | 27 (65.9) | 1.77 | 0.93 – 3.38 | 0.08 | 1.96 | 0.99 – 3.88 | 0.06 |
| USA cohort# | 16 (39.0) | 2.41 | 1.28 – 4.55 | 0.01 | 1.39 | 0.66 – 2.91 | 0.39 |
| Presence of PIPs^ | 17 (41.5) | 1.56 | 0.82 – 2.96 | 0.17 | 1.17 | 0.59 – 2.31 | 0.65 |
| Primary sclerosing cholangitis | 9 (22.0) | 1.70 | 0.81 – 3.57 | 0.16 | 2.30 | 1.05 – 5.06 | 0.04 |
| Dysplasia at/before index colonoscopy## | 19 (46.3) | 5.92 | 3.06 – 11.42 | <0.0005 | 4.89 | 2.60 – 9.22 | <0.0005 |
| Mean histologic inflammation | - | 2.40 | 1.63 – 3.53 | <0.0005 | 2.11 | 1.34 – 3.34 | <0.001 |
| Disease duration at index colonoscopy | - | 1.05 | 1.02 – 1.08 | 0.003 | 1.04 | 1.01 – 1.08 | 0.005 |
| Cecum reached | - | 0.11 | 0.01 – 0.85 | 0.03 | 0.09 | 0.01 – 0.68 | 0.02 |
| Family history of Colorectal Cancer | 5 (12.2) | 2.32 | 0.91 – 5.91 | 0.08 | 1.94 | 0.73 – 5.15 | 0.18 |
| Exposure to 5-Aminosalicylates | 38 (92.7) | 2.42 | 0.75 – 7.86 | 0.14 | |||
| Crohn’s Disease^^ | 16 (39.0) | 1.38 | 0.74 – 2.60 | 0.31 | |||
| Adequate Bowel Preparation | - | 1.25 | 0.27 – 5.69 | 0.78 | |||
| Exposure to biologicals | 7 (17.1) | 1.05 | 0.46 – 2.37 | 0.91 | |||
| Number of surveillance colonoscopies | - | 0.92 | 0.78 – 1.10 | 0.36 | |||
| Exposure to thiopurines | - | 0.70 | 0.37 – 1.33 | 0.27 | |||
| Extensive disease | 33 (80.5) | 0.56 | 0.26 – 1.22 | 0.15 | |||
Significant at P <0.05 level. ACRN: Advanced colorectal neoplasia. PIPs: Post-inflammatory polyps.
Reference category: Dutch cohort.
Analyzed as time-changing covariate.
Indefinite for dysplasia or low-grade dysplasia (patients with HGD excluded from outset).
Reference category: Ulcerative colitis/IBD-unclassified.
Note:38 patients (1 ACRN) were excluded due to missing data.
Rate of occurrence of CRN (secondary outcome)
The analyses for CRN were restricted to patients without prior dysplasia (n=1350). As defined previously, CRN is inclusive of LGD, HGD and CRC. During follow-up, 188 patients (13.9%) were diagnosed with CRN, 64 (16.3%) with PIPs and 124 (13.0%) without PIPs. There was no significant difference in the rate of CRN occurrence between patients with PIPs versus without PIPs (Figure 3b). Similar to ACRN, time-to-CRN was not significantly different in patients with PIPs reported on multiple procedures ( ≥ 2) versus on one procedure only (P=0.84). Statistical non-significance remained when comparing time-to-CRN in patients with versus without PIPs on subgroup analyses, including: USA cohort, Dutch cohort (Supplementary Figure S2a–b), UC/IBD-U patients, CD patients, and patients with versus without PSC (data not shown; all P>0.30). PIPs did not independently predict CRN (aHR 1.25; 95%CI: 0.88 – 1.77). Rather, male sex, increasing age, PSC and disease duration were significant positive independent predictors of CRN. Increasing number of surveillance colonoscopies was protective (Supplementary Table 2). Similar to ACRN, stratified analyses based on geographic cohort and date of index colonoscopy confirmed that PIPs were not independently associated with CRN. Furthermore, biologicals were independently protective against CRN in the post-2005 subgroup (aHR 0.50; 95% CI: 0.28 – 0.91). No other predictors of CRN were identified by additional time-trend analyses, as described in our methods.
Presence of CRN in biopsied PIPs (descriptive, USA cohort only):
Within the USA cohort, 104 patients (74.2% of patients with PIPs in the USA cohort) had lesions biopsied or resected that were suspected or confirmed PIPs, yielding 360 biopsy jars with histologic data on PIPs. CRN was never detected in a histologically confirmed PIP. In PIPs identified by endoscopy, LGD was found in 3 patients (2.8%) and HGD in 1 (1%), but none of these lesions was histologically confirmed to be a PIP. Additionally, 9 (8.7%) patients were diagnosed with IND in a PIP identified by the endoscopist, of which 6 (66.7%) were histologically confirmed PIPs.
Rate of occurrence of colectomy according to PIP status (secondary outcome)
A total of 83 (5.3%) patients underwent colectomy during follow-up. Patients with PIPs more frequently underwent colectomy compared to those without PIPs (8.4% vs. 3.9%) and had a significantly shorter time to colectomy, 3.9 (IQR: 2.6 – 6.3) vs. 4.1 (IQR: 2.5 – 5.1) years, respectively (Figure 4a, P=0.01). Prior to colectomy, ACRN or CRN had occurred in 26 and 18 patients, respectively. In 39 patients (19 with PIPs and 20 without PIPs; 2.5% of the entire cohort), colectomy was performed before a CRN-related outcome was reached. These patients were censored for the analyses of (A)CRN after a median of 4.2 years of follow-up. We further explored colectomy as an outcome on stratified analysis according to presence versus absence of PIPs and cohort geography (Figure 4b–c), and by comparing patients with versus without PIPs among 8 different subgroups (Dutch and USA cohort, CD and UC/IBD-U, patients with and without PSC, index colonoscopy pre- and post-2005). The Bonferroni correction for multiple testing was applied, resulting in a threshold for significance of P<0.006 for comparing patients with versus without PIPs in 8 independent subgroups. Only in the subgroup of CD patients did patients with PIPs versus without PIPs have a significantly higher risk of colectomy (data not shown, P=0.005), but not in the USA cohort (Figure 4b, P=0.54), in patients with UC/IBD-U (P=0.30), with concomitant PSC (P=0.02) or without PSC (P=0.01), nor among patients included pre-2005 (P=0.03) or post-2005 (data not shown, P=0.10). Notably, in the subgroup of Dutch patients, the rate of colectomy was higher in patients with PIPs versus without PIPs (Figure 4b, P=0.008), but this was statistically nonsignificant after correction for multiple testing. However, when comparing indications for colectomy stratified by PIP status and cohort geography, there was a significant difference in colectomies performed for “medically refractory disease” (MRD) between the groups in Figure 4c (P=0.004), and specifically between Dutch patients with versus without PIPs (P=0.001). No other indications for colectomy were significantly different between the groups.
Figure 4:
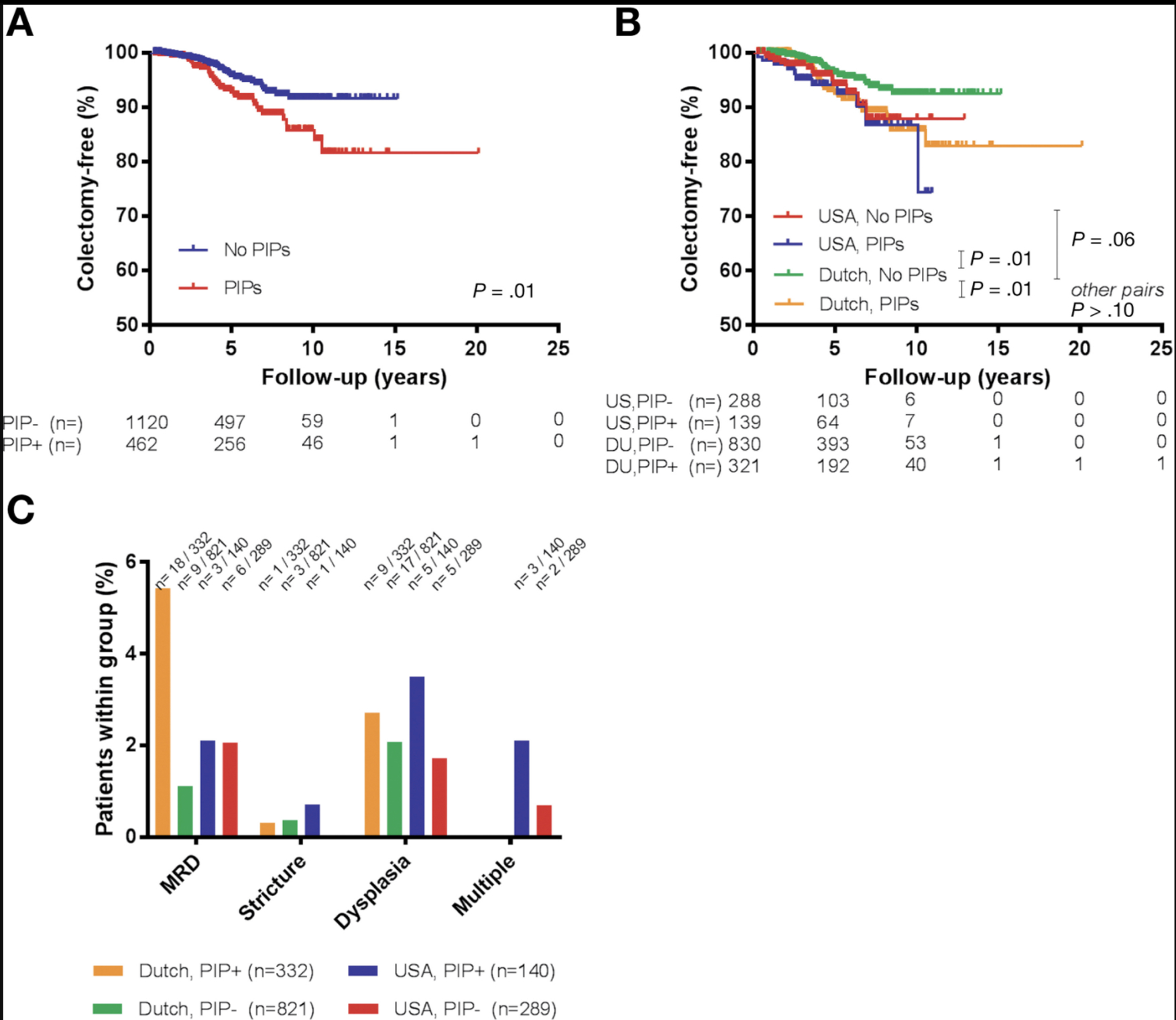
Kaplan-Meier curves and reasons for colectomy.
Discussion
In this multinational retrospective cohort study of nearly 1600 patients with confirmed colonic IBD undergoing colonoscopic CRN surveillance, PIPs were not a significant independent predictor of dysplasia or CRC. We did find, however, that patients with PIPs had more severe histologic inflammation, more often had extensive colitis, and were significantly more likely to undergo colectomy. Our findings suggest that PIPs are related to the inflammatory burden, but are not themselves a dominant risk factor for CRN.
In contrast, previous studies broadly examining predictors of CRC in IBD reported a significant, independent association between PIPs and CRC.8,9,11 Limitations of these older case-control studies include selection bias by comparing CRC-patients with low-risk controls, inadequate control for inflammation and less sophisticated endoscopic techniques. Conversely, in this study we utilized a cohort design restricted to patients with confirmed colonic IBD undergoing CRN surveillance and distinctly controlled for histologic inflammation, a well-established predictor of ACRN.7,10,19,20 Indeed, mean inflammation scores were highly predictive of both ACRN and PIPs in our cohort. Similar to our findings, a recent cohort study of 987 UC patients undergoing CRN surveillance also found that PIPs did not independently predict CRN risk after controlling for cumulative inflammatory burden.10 In that study, patients with CD or IBD-U were excluded, and only 42 patients with PSC were enrolled. Further, PIPs were not the primary variable of interest in that study. In our study, we comprehensively evaluate PIPs and utilize sophisticated analytics to address biases relevant to PIPs and CRN. We confirmed that no independent association between PIPs and ACRN exists in a broader population inclusive of patients with CD or PSC. In this context, a novel finding is that PSC was associated with a significantly lower likelihood of PIPs. This underscores the prevailing hypothesis that the phenotype of PSC-IBD colitis is distinct from non-PSC associated IBD colitis, including clinically quiescent disease.21 Regarding PIPs in Crohn’s colitis, data are scarce.9 By enrolling a substantial number of patients with Crohn’s colitis, we provided evidence that PIPs do not independently predict (A)CRN in this group. Because IBD phenotype was not a predictor of (A)CRN we suggest that surveillance intervals should be independent of IBD phenotype.
While PIPs were not predictive of CRN, patients with PIPs did have significantly higher rates of colectomy. A key strength of our study is that all included patients were undergoing surveillance because of either at least 8 years of colonic disease duration or a concomitant diagnosis of PSC. Thus, even though patients with PIPs underwent colectomy more frequently than patient without PIPs, our cohort was universally at-risk for ACRN at inclusion. Furthermore, very few patients underwent colectomy before a CRN-related outcome was reached, and the median follow-up in these patients was only slightly reduced as compared to the entire cohort (4.2 versus 4.8 years, respectively). That said, we concede that early colectomy in patients with PIPs might obscure an increased risk of CRC. Clinically, though, the competing risk of uncontrolled inflammation necessitating colectomy likely outweighs the risk of CRC in such patients. Indeed, in our cohort, patients with PIPs underwent significantly more colectomies indicated for MRD, but not for dysplasia. Moreover, this was found solely in the Dutch cohort. The reasons for this difference between the two geographic cohorts are unclear, but possibly reflect differences in clinical management and threshold for colectomy. While it is certainly possible that those undergoing colectomy for MRD were more at risk for ACRN in the long term, this risk is likely not driven by PIPs themselves, but by the well-established risk factor of colonic inflammation, confirmed also by our findings.7,19,20,10
There are some limitations to our study, beyond those that are inherent to retrospective research. Standardized scores were not employed, but there was collinearity between endoscopic and histologic inflammation scores and an association with ACRN, as expected. While we are unable to provide absolute numbers on how often a dysplasia diagnosis was confirmed by a second expert pathologist, this is standard practice at each included institution. Indeed, confirmation of LGD by a pathology expert panel better predicts ACRN.22 A second limitation is that reporting of PIPs by endoscopists was not standardized. Consequently, PIPs might be disregarded in the context of other pathologic findings (although, anecdotally, we expect such an occurrence to be exceedingly rare in our cohort, particularly on colonoscopies indicated specifically for surveillance). We improved the accuracy of identifying PIPs by including histologic evidence of PIPs where available. Notably, rates of (A)CRN did not differ according to how often PIPs were reported in colonoscopy reports. Because underreporting of PIPs might underestimate time-at-risk in patients with PIPs, we analyzed PIPs as a fixed parameter in survival analysis, which has the countereffect of overestimating time-at-risk for patients with PIPs. We also analyzed PIPs as a time-changing covariate to account for incident PIPs after the index colonoscopy and minimize the risk of immortal time bias.18 In both analyses, PIPs still were not independent predictors of (A)CRN. Notably, though, in these same models, histologic inflammation independently predicted ACRN, and increasing number of surveillance colonoscopies was protective against CRN, both findings that are consistent with literature and support the internal validity of our findings.7,10,19,20,23 All told, substantial misclassification of PIPs seems unlikely, as endoscopists have good interobserver agreement for identifying PIPs based on endoscopic assessment.24 Regarding density of PIPs and ACRN risk, our study is unfortunately underpowered to draw conclusions regarding this issue. With this caveat, our data do suggest that even extensive PIPs in and of themselves might not grossly increase the risk of ACRN, but certainly inadequate visualization of the colonic mucosa and higher inflammatory burden in this setting are important considerations. Prospective, adequately powered studies are needed to better inform clinical decision-making in this setting.
We further acknowledge some baseline differences between the two national cohorts, including more severe inflammation and higher use of biologicals in the USA cohort. The treatment approach concerning biologicals may be different between the USA and the Netherlands, particularly during the time period of this study when data were still emerging regarding the (cost-)efficacy of biologicals. Alternatively, this difference might also indicate a more severe patient population given that the USA cohort represents a tertiary IBD referral center. In our cohort, exposure to biologicals was protective against CRN and not ACRN, but only in the subgroup analysis of patients included after 2005 (presumably due to the more routine use in this time period). Although this is compelling, our study was not designed to extensively assess the chemoprotective effect of medications, and the literature remains inconclusive regarding the potential chemoprotective effect of biologicals.25,26 Regardless of these baseline differences between the two geographic cohorts, comprehensive subgroup analyses by country of origin, stratified Cox regression modelling and including country of origin as an independent covariate in the multivariable models showed no modifying or interacting effect on the null association between PIPs and (A)CRN.
Our study has several strengths. One key strength is the large size of our surveillance cohort, with nearly 1600 patients who are well-characterized with respect to clinical, endoscopic, and histologic follow-up data. This large sample size would have allowed us to a detect a clinically relevant hazard rate for both CRN and ACRN. Sample size is of pivotal importance, as ACRN is a rare outcome (incidence of 5.01/1000 patient-years in our surveillance cohort, and also comparable to a recent UC surveillance cohort).10 Our analyses were robust with no missing data for our primary outcome. We controlled for several relevant covariates including histologic inflammation, as well as evaluated PIPs as a fixed and also a time-changing covariate to account for underreporting of PIPs and immortal time bias, respectively. That we found already established predictive factors (e.g. inflammation, disease duration, PSC, prior dysplasia) to be independently associated with ACRN supports the internal validity of our study. Furthermore, our findings were essentially validated in two independent surveillance cohorts since neither stratification by geography nor inclusion of geography as a covariate modified the null association between PIPs and our primary and secondary (A)CRN outcomes. It should be highlighted that our cohort reflects a particularly high-risk population for ACRN, with a 14–16% prevalence of PSC and the majority enrolled from tertiary IBD referral centers. Despite this enrichment of potential outcomes, we still did not find an independent association of PIPs with (A)CRN. The lower incidence of ACRN in recent compared to historical IBD cohorts might reflect improved management of patients with high inflammatory potential in our era of “treat-to-target” and “top-down” treatment paradigms. This is highly relevant to our study, as our findings indicate that PIPs are related to more severe and extensive inflammation. With a decreasing incidence of ACRN in most IBD patients, the need for evidence-based risk factors to accurately identify high-risk patients only increases. Utilizing a risk stratification model to guide surveillance intervals is less costly and equally effective as a program without risk stratification.27
In conclusion, the current practice of surveillance for CRN is resource-intensive, costly, time-consuming, inconvenient, and likely has a negative impact on the quality of life for patients with IBD. Appropriate categorization of IBD patients according to their risk of CRC as part of an integrated surveillance program with intervals determined by an evidenced-based composite risk score should reduce costs, optimize resource utilization, and maximize patients’ quality of life. PIPs have had a reputation of being an ominous risk factor for developing CRN. Our findings should provide some degree of reassurance for clinicians and patients that PIPs are not, in themselves, the worrisome lesions they once were considered. Our data suggest that PIPs are not independently associated with increased risk of any degree of CRN on intermediate-term follow-up, an observation that should be considered in developing future IBD colonoscopic surveillance guidelines.
Authors names in bold designate shared co-first authorship.
Supplementary Material
Supplementary Figure 1. Kaplan-Meier curves, ACRN-free survival according to density of PIPs, USA cohort. fPIP: few PIPs. mPIP: many PIPs.
Supplementary Figure 2. Kaplan-Meier curves, CRN-free survival in patients without prior dysplasia, USA and Dutch Cohorts.
Disclosures:
RM, SCS, JRH, EM, DC, JG, JE, AK, JA and SHI have no disclosures.
J. Torres has served as a consultant for Takeda, and received speaker fees from Takeda, Abbvie and Ferring. T. Ullman has served as consultant for Salix/Valeant and for Janssen. J-F. Colombel has served as consultant, advisory board member or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Lilly, Medimmune, Merck & Co., Pfizer, PPM Services, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag and Theravance Biopharma, has stock options in Intestinal Biotech Development, Genfit and has received research grants from AbbVie, Takeda, Janssen and Janssen. B. Oldenburg has served as consultant for MSD, Takeda, Abbvie, Ferring, Cablon, Janssen and received unrestricted grants from Abbvie, Ferring, dr. Falk, MSD, Takeda, Janssen and speakers’ fees from Ferring and MSD.
Abbreviations:
- ACRN
Advanced colorectal neoplasia
- aHR
Adjusted hazard ratio
- aOR
Adjusted odds ratio
- ASA
Aminosalicylate
- CD
Crohn’s Disease
- CRC
Colorectal cancer
- CRN
Colorectal neoplasia
- EHR
Electronic health record
- HGD
High-grade dysplasia
- HR
Hazard ratio
- IBD
Inflammatory bowel disease
- IBD-U
Inflammatory bowel disease – unclassified
- IND
Indefinite dysplasia
- IQR
Interquartile range
- LGD
Low-grade dysplasia
- MRD
Medically refractory disease
- MSH
Mount Sinai Hospital
- OR
Odds ratio
- PIP
Post-inflammatory polyp
- PSC
Primary sclerosing cholangitis
- SD
Standard deviation
- UC
Ulcerative colitis
References
- 1.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10(6):639–645. [DOI] [PubMed] [Google Scholar]
- 2.Lutgens MWMD, van Oijen MGH, van der Heijden GJMG, et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19(4):789–799. [DOI] [PubMed] [Google Scholar]
- 3.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59(5):666–689. [DOI] [PubMed] [Google Scholar]
- 4.Farraye FA, Odze RD, Eaden J, et al. AGA Medical Position Statement on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. Gastroenterology. 2010;138(2):738–745. [DOI] [PubMed] [Google Scholar]
- 5.Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11(6):649–670. [DOI] [PubMed] [Google Scholar]
- 6.Annese V, Daperno M, Rutter MD, Eliakim R, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohn’s Colitis. 2013;7(12):982–1018. [DOI] [PubMed] [Google Scholar]
- 7.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126(2):451–459. [DOI] [PubMed] [Google Scholar]
- 8.Velayos FS, Loftus EVJ, Jess T, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130(7):1941–1949. [DOI] [PubMed] [Google Scholar]
- 9.Baars JE, Looman CWN, Steyerberg EW, et al. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011;106(2):319–328. [DOI] [PubMed] [Google Scholar]
- 10.Choi C-HR, Al Bakir I, Ding N-S (John), et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2017: Published Online First. doi: 10.1136/gutjnl-2017-314190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutter MD, Saunders BP, Wilkinson KH, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53(12):1813–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi CR, Ignjatovic-Wilson A, Askari A, et al. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol. 2015;110(10):1461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Politis DS, Katsanos KH, Tsianos EV, et al. Pseudopolyps in inflammatory bowel diseases: Have we learned enough? World J Gastroenterol. 2017;23(9):1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah SC, Ten Hove JR, Castaneda D, et al. High Risk of Advanced Colorectal Neoplasia in Patients With Primary Sclerosing Cholangitis Associated With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2018: Published online first. doi: 10.1016/j.cgh.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Claessen MMH, Vleggaar FP, Tytgat KMAJ, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50(1):158–164. [DOI] [PubMed] [Google Scholar]
- 16.Zheng H-H, Jiang X-L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28(4):383–390. [DOI] [PubMed] [Google Scholar]
- 17.Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56(1):48–54. [DOI] [PubMed] [Google Scholar]
- 18.Levesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 19.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133(4):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathy C, Schneider K, Chen Y-Y, et al. Gross versus microscopic pancolitis and the occurrence of neoplasia in ulcerative colitis. Inflamm Bowel Dis. 2003;9(6):351–355. [DOI] [PubMed] [Google Scholar]
- 21.de Vries AB, Janse M, Blokzijl H, et al. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21(6):1956–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Schaik FDM, ten Kate FJW, Offerhaus GJA, et al. Misclassification of dysplasia in patients with inflammatory bowel disease: consequences for progression rates to advanced neoplasia. Inflamm Bowel Dis. 2011;17(5):1108–1116. [DOI] [PubMed] [Google Scholar]
- 23.Bye WA, Nguyen TM, Parker CE, et al. Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane database Syst Rev. 2017;9:CD000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thia KT, Loftus EVJ, Pardi DS, et al. Measurement of disease activity in ulcerative colitis: interobserver agreement and predictors of severity. Inflamm Bowel Dis. 2011;17(6):1257–1264. [DOI] [PubMed] [Google Scholar]
- 25.Yadav S, Singh S, Harmsen WS, et al. Effect of Medications on Risk of Cancer in Patients With Inflammatory Bowel Diseases: A Population-Based Cohort Study from Olmsted County, Minnesota. Mayo Clin Proc. 2015;90(6):738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyboe Andersen N, Pasternak B, Basit S, et al. Association between tumor necrosis factor-alpha antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. 2014;311(23):2406–2413. [DOI] [PubMed] [Google Scholar]
- 27.Lutgens M, van Oijen M, Mooiweer E, et al. A risk-profiling approach for surveillance of inflammatory bowel disease-colorectal carcinoma is more cost-effective: a comparative cost-effectiveness analysis between international guidelines. Gastrointest Endosc. 2014;80(5):842–848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Kaplan-Meier curves, ACRN-free survival according to density of PIPs, USA cohort. fPIP: few PIPs. mPIP: many PIPs.
Supplementary Figure 2. Kaplan-Meier curves, CRN-free survival in patients without prior dysplasia, USA and Dutch Cohorts.


