Abstract
Background & Aims:
Exposure to hormone contraception has been associated with an increased risk of relapse of inflammatory bowel diseases (IBD). Little is known about the effects of cancer therapies, specifically hormone therapies, on the course of IBD.
Methods:
We conducted a retrospective cohort study, collecting data from 5 medical centers on patients with IBD who received a subsequent diagnosis of breast or prostate cancer from 1997 through 2018. For patients with quiescent IBD at their cancer diagnosis, the primary outcome was relapse of IBD. For patients with active IBD at their cancer diagnosis, the primary outcome was IBD remission.
Results:
Our analysis included 447 patients with IBD (44% with Crohn’s disease, 53% with ulcerative colitis, and 3% with IBD-unclassified) who had either breast (78%) or prostate (22%) cancer. At their cancer diagnosis, 400 patients (90%) had inactive IBD, and 47 (10%) had active IBD. Among patients with inactive IBD, 112 (28%) developed active IBD. Previous exposure to steroids, immunomodulators, or biologics was associated with IBD relapse following a cancer diagnosis (hazard ratio [HR] for steroids, 1.79; 95% CI, 1.18–2.71; HR for immunomodulators, 2.22; 95% CI, 1.38–3.55; HR for biologics, 1.95; 95% CI, 1.01–5.36). Hormone monotherapy (HR, 2.00; 95% CI, 1.21–3.29) and combination cytotoxic and hormone therapy (HR, 1.86; 95% CI, 1.01–3.43) was associated with IBD relapse. Among 34 patients who received only cytotoxic chemotherapy, 75% remained in remission from IBD at 250 months compared with 42% of those who received hormone monotherapy (log rank=0.02). Among patients with active IBD at their cancer diagnosis, 14 (30%) entered remission from IBD, but there were no significant factors of achieving IBD remission.
Conclusions:
In a multicenter retrospective study, we found that patients with IBD and breast or prostate cancer who receive hormone therapy have an increased risk for relapse of IBD and related adverse outcomes.
Keywords: CD, UC, long-term outcome, disease flare
Introduction
Inflammatory bowel diseases (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), are chronic and often progressive inflammatory disorders of the gastrointestinal tract. While there are effective therapies for managing IBD, there is no cure and as a result, more people are living with IBD. As this population ages, patients with IBD are at risk for the same diseases that affect the general aging population, including cancer. Not only are patients with IBD at risk for common cancers such as breast and prostate cancer, but they are at an increased risk of developing colorectal cancer, primarily the result of chronic intestinal inflammation,1–4 and extra-intestinal malignancies such as lymphoma and skin cancers, thought to be a consequence of immunosuppressive therapies and an underlying inflammatory state.2,4–6
Patients with cancer typically require treatment that can include surgery, chemotherapy, radiation, hormone therapy, and/or immunotherapy Patients with IBD may require special consideration as they are often on chronic medical therapies to control inflammation and prevent disease complications. Gastroenterologists and oncologists are increasingly confronted with questions regarding the management of patients with IBD who are diagnosed with cancer. Understanding the effect of cancer treatment on IBD activity may help identify patients at the highest risk for IBD exacerbation during and after specific cancer treatments, and may provide guidance on selection or dosing of cancer therapies, timing of cancer therapies, and continuation or reintroduction of IBD therapies. Collectively, this knowledge would also help manage patient expectations and inform disease monitoring intervals during cancer therapy.
To date, there is a lack of substantial clinical data on the natural course of IBD after a diagnosis of cancer and during cancer treatment. We previously reported in a single-center retrospective cohort study of 84 patients with IBD who were diagnosed with cancer were more likely to remain in remission if they received cytotoxic chemotherapy alone compared to a treatment regimen that included hormone therapy.7 Exposure to hormone therapy represented the greatest risk for flare of IBD.7 Conversely, in a recent study analyzing the outcome of prostate cancer treatment on IBD activity, prostatectomy was more common in patients with IBD and flare in the year prior to cancer was the only predictor of IBD exacerbation in the year following cancer treatment, not type of cancer treatment.8 Both studies were limited by small sample size, limited follow up, and imprecise IBD outcome measures.
In addition, there are few data regarding cancer-specific outcomes, such as chemotherapy tolerance, in patients with IBD. A limited number of small studies have demonstrated conflicting data, with some demonstrating more toxicity and treatment alterations in patients with IBD.9–11 None have examined the specific impact of IBD activity on cancer treatment tolerance.
We therefore aimed to conduct a multicenter retrospective cohort study to analyze the effect of cancer treatment on the course of IBD, with a primary focus on hormone versus cytotoxic therapies for the two most common cancers, breast and prostate cancer. We secondarily aimed to examine the tolerance of cancer therapy for breast and prostate cancer in patients with IBD.
Methods
Study Population and Inclusion Criteria
The electronic medical records of patients from five academic medical centers were queried— including four affiliated with the New York Crohn’s and Colitis Organization (NYCCO) (New York Presbyterian-Columbia University Medical Center, NYU Langone Health, Montefiore Medical Center, Mount Sinai Health) and also the Massachusetts General Hospital. Eligible patients included those with a confirmed diagnosis of IBD, and subsequent diagnosis of breast or prostate cancer from January 1997 to January 2018. A diagnosis of IBD was based on accepted standard criteria including the combination of clinical symptoms, endoscopy, radiology, pathology, and operative reports, while a diagnosis of cancer was based on histopathologic confirmation. All chart review was performed by trained clinicians and data were abstracted according to a shared, uniform data reporting template.
Subjects were excluded if they were not at least 18 years of age at the time of cancer diagnosis, received a total colectomy for UC before the cancer diagnosis, or were currently receiving active radiation or cytotoxic chemotherapy at the time of data collection.
Data Abstraction
Charts were reviewed for basic demographic information, age at IBD diagnosis, IBD subtype and phenotype, concurrent primary sclerosing cholangitis (PSC), smoking status, IBD medical and surgical treatment prior to, during, and after a diagnosis of cancer, age at cancer diagnosis, cancer stage, development of recurrent or second malignancy, cancer treatment regimen, including surgery, chemotherapy, immunotherapy, hormone therapy, or radiation, cancer treatment alterations for toxicity, such as treatment delays, discontinuations, dose modifications, and hospitalizations, IBD activity at, during, and after cancer diagnosis, and duration of gastroenterological follow-up evaluation after cancer treatment.
Given the multiplicity of cancer treatment regimens, type of cancer treatment was classified into cytotoxic chemotherapy, hormone therapy, combination cytotoxic chemotherapy with adjuvant hormonal therapy, or none of these regimens. Cytotoxic chemotherapy encompassed all cytotoxic chemotherapeutic drugs (Supplementary Table 1). Hormone therapies included aromatase inhibitors, selective estrogen receptor modulators, anti-androgens, and gonadotropin releasing hormone agonists. Distinct from cancer treatment modalities such as radiation or cytotoxic chemotherapy which are generally given over short, discrete time periods, adjuvant hormone therapy is often given for years. As such, duration of hormone therapy exposure was also recorded.
Definitions
To differentiate the effect of cancer treatment on IBD activity, patients were categorized into an active and inactive IBD group at their cancer diagnosis. The active group was defined as patients with symptomatic disease and active disease observed on endoscopy up to 60 days before the cancer diagnosis. The inactive IBD group was defined as asymptomatic patients and inactive disease observed on endoscopy up to 60 days before the cancer diagnosis. All patients remained in their respective groups from the time of cancer diagnosis to initiation of cancer treatment.
Outcomes
For patients in the inactive IBD group, our primary outcome was relapse of disease, defined as composite of IBD-related surgery, IBD-related hospital admission, IBD-related disease complication (e.g. fistula, abscess), and/or escalation in IBD therapy. IBD-related hospital admission specifically excluded any indication for hospitalization due to cancer or cancer therapies. For patients in the active IBD group, our primary outcome was remission of IBD, defined as asymptomatic patients and inactive disease observed on endoscopy. Due to the heterogeneity in cancer treatment regimens and durations, patients may meet criteria for the primary outcome directly undergoing cancer treatment or after the completion of cancer treatment. Secondary outcomes included new or recurrent cancer, and cancer treatment tolerance comprising the incidence of cancer treatment alterations or hospitalization due to toxicities. Hospitalization during cancer treatment included any cancer or IBD-related hospitalization during active radiation or cytotoxic chemotherapy.
Follow-up time for all patients was accrued from the time of cancer treatment initiation with patients censored at IBD relapse, loss to follow-up evaluation, end of study period, or death. Patients meeting any component of the composite outcome of IBD relapse qualified for censorship in the analysis.
Statistical Analyses
Continuous variables were summarized using means and standard deviations and compared using the Students t-test, whereas categorical variables were summarized using proportions and compared using Chi-square test. Kaplan–Meier curves were constructed and stratified by chemotherapeutic regimen, no systemic therapy, cytotoxic chemotherapy only, hormone therapy only, or cytotoxic chemotherapy with hormone therapy, and compared with the log-rank test. Cox proportional hazards regression models were constructed to identify independent risk factors of IBD relapse. All tests were considered significant at a 2-sided P-value less than 0.05. SPSS software (IBM) was used to perform all statistical analyses. The study was approved by each institution’s Institutional Review Board.
Results
Characteristics of IBD in the study population
We identified 447 patients with IBD (CD: 44%; UC: 53%; IBD-U: 3%) who had either breast or prostate cancer (Table 1). At their cancer diagnosis, 400 (90%) had inactive IBD and 47 (10%) had active IBD. Overall, 77% were women, and 87% were white. The median age at IBD diagnosis was 41 years (range 4–90) with a median duration of IBD at cancer diagnosis of 20 years (range 0–79 years). Approximately 2% had PSC and almost half were never smokers. Prior to a diagnosis of cancer, 26% were exposed to systemic steroids, 64% to 5-aminosalicylates, 22% to immunomodulators, and 6% to biologics. Nearly 28% of patients had undergone prior IBD surgery. There were no significant differences between those with inactive versus active IBD at cancer diagnosis, except that patients in the active IBD group were more likely to have previous exposure to all IBD therapies and require surgery for their IBD.
Table 1.
Baseline IBD and cancer characteristics stratified by IBD activity at cancer diagnosis.
| Variable | Total | IBD inactive at cancer diagnosis | IBD active at cancer diagnosis |
|---|---|---|---|
| N (%) | 447 (100%) | 400 (89.5%) | 47 (10.5%) |
| IBD Subtype | |||
| Crohn’s disease | 197 (44.1%) | 175 (43.8%) | 22 (46.8%) |
| Ulcerative colitis | 238 (53.2%) | 214 (53.5%) | 24 (51.0%) |
| Indeterminate colitis | 12 (2.7%) | 11 (2.8%) | 1 (2.1%) |
| Sex | |||
| Female | 346 (77.4%) | 315 (78.8%) | 31 (66.0%) |
| Male | 101 (22.6%) | 85 (21.3%) | 16 (34.0%) |
| Race | |||
| White | 388 (86.8%) | 353 (88.3%) | 35 (74.5%) |
| Black | 18 (4.0%) | 15 (3.8%) | 3 (6.4%) |
| Hispanic | 12 (2.7%) | 9 (2.3%) | 3 (6.4%) |
| Asian | 11 (2.5%) | 9 (2.3%) | 2 (4.3%) |
| Unknown | 18 (4.0%) | 14 (3.5%) | 4 (8.6%) |
| Median age at IBD diagnosis (range) | 41 (4–90) | 40 (4–90) | 47 (15–68) |
| Smoking history at cancer diagnosis | |||
| Never | 230 (51.5%) | 207 (51.8%) | 23 (48.9%) |
| Former | 200 (44.7%) | 178 (44.5%) | 22 (46.8%) |
| Current | 17 (3.8%) | 15 (3.8%) | 2 (4.3%) |
| Primary sclerosing cholangitis | 8 (1.8) | 7 (1.8%) | 1 (2.1%) |
| IBD treatment before cancer | |||
| 5-ASA | 227 (50.8%) | 190 (47.5%) | 37 (78.7%) |
| Steroids | 115 (25.7%) | 91 (22.8%) | 24 (51.1%) |
| Immunomodulators | 69 (15.4%) | 55 (13.8%) | 14 (29.8%) |
| Biologics | 26 (5.8%) | 18 (4.5%) | 8 (17.0%) |
| Infliximab | 14 (53.8%) | 10 (55.6%) | 4 (50%) |
| Adalimumab | 9 (34.6%) | 6 (33.3%) | 3 (37.5%) |
| Certolizumab pegol | 3 (11.5%) | 2 (11.1%) | 1 (12.5%) |
| Golimumab | 0 | 0 | 0 |
| Vedolizumab | 0 | 0 | 0 |
| Ustekinumab | 0 | 0 | 0 |
| Previous surgery for IBD | 123 (27.5%) | 107 (26.8%) | 16 (34.0%) |
| Type of Cancer | |||
| Breast | 346 (77.4%) | 315 (78.8%) | 31 (68.1%) |
| Prostate | 101 (22.6%) | 85 (21.3%) | 16 (31.9%) |
| Median age at cancer diagnosis (range) | 58 (23–90) | 57 (28–90) | 62 (38–80) |
| Cancer stage at diagnosis | |||
| I | 160 (25.8%) | 138 (34.5%) | 22 (46.8%) |
| II | 65 (14.5%) | 60 (15.0%) | 5 (10.6%) |
| III | 24 (5.4%) | 21 (5.3%) | 3 (6.4%) |
| IV | 7 (1.6%) | 6 (1.5%) | 1 (2.1%) |
| Unknown | 191 (42.7%) | 175 (43.8%) | 16 (34.0%) |
| Cancer treatment | |||
| Surgery | 384 (85.9%) | 344 (86.0%) | 40 (85.1%) |
| Radiotherapy | 255 (57.0%) | 225 (56.3%) | 30 (63.8%) |
| Immune therapies | 12 (2.7%) | 11 (2.8%) | 1 (2.1%) |
| Cytotoxic chemotherapy only | 34 (7.6%) | 34 (8.5%) | 0 |
| Hormone therapy only | 187 (41.8%) | 164 (41.0%) | 23 (48.9%) |
| Median duration of hormone therapy (range) | 60 (1–149) | 60 (1–149) | 60 (1–120) |
| Cytotoxic chemotherapy and hormone therapy | 73 (16.3%) | 65 (16.3%) | 8 (17.0%) |
| Neither cytotoxic nor hormone therapy | 113 (25.3%) | 100 (25.0%) | 13 (27.7%) |
| Unknown | 40 (8.9%) | 37 (9.3%) | 3 (6.4%) |
Characteristics of cancer in the study population
Overall, 77% of patients had breast cancer and 23% had prostate cancer (Table 1). The median age at cancer diagnosis was 58 years (range 23–90). Of 252 patients with available stage data, the majority were diagnosed in stage I (26%). With respect to cancer treatment, 86% underwent surgery, 57% received radiotherapy, 8% received cytotoxic chemotherapy only, 42% received hormone therapy only, 16% received cytotoxic chemotherapy and hormone therapy, and 25% received none of these agents. Median duration of hormone therapy was 60 months (range 1–169). There were no significant differences in cancer stage, cancer treatment type or duration of hormone therapy between the inactive IBD and active IBD groups.
Course of IBD in patients with cancer
In patients with inactive IBD at their cancer diagnosis, 112 (28%) developed active IBD over a median of 99 months of follow up (range 2–444 months; Table 2). Of 60 patients with inactive IBD on immunomodulators and/or biologics at the time of their cancer diagnosis, 27 (45%) had their IBD therapy discontinued. In patients with active IBD at their cancer diagnosis, 14 (30%) entered remission from IBD over a median of 54 months of follow up (range 1–280 months). Of 14 patients with active IBD on immunomodulators and/or biologics at the time of their cancer diagnosis, 4 (29%) had their IBD therapy discontinued. Patients in the active IBD group were more likely to have continued exposure to all IBD therapies during and after cancer treatment, and require surgery or hospitalization for their IBD after cancer treatment.
Table 2.
Course of IBD and incident cancer stratified by IBD activity at cancer diagnosis.
| Variable | Total | IBD inactive at cancer diagnosis (n=400) | IBD active at cancer diagnosis (n=47) |
|---|---|---|---|
| Development of active IBD* if in remission at cancer diagnosis | - | 112 (28%) | - |
| Remission of IBD if active at cancer diagnosis | - | - | 14 (29.8%) |
| IBD Immunosuppression discontinued for cancer | 31/74 (41.9%) | 27/60 (45.0%) | 4/14 (28.6%) |
| IBD treatment during cancer treatment | |||
| 5-ASA | 188 (42.1%) | 157 (39.3%) | 31 (66.0%) |
| Steroids | 40 (8.9%) | 28 (7.0%) | 12 (25.5%) |
| Immunomodulators | 41 (9.2%) | 33 (8.3%) | 8 (17.0%) |
| Biologics | 24 (5.4%) | 15 (3.8%) | 9 (19.1%) |
| Infliximab | 9 (37.5%) | 6 (40%) | 3 (33.3%) |
| Adalimumab | 9 (37.5%) | 6 (40%) | 3 (33.3%) |
| Certolizumab pegol | 0 | 0 | 0 |
| Golimumab | 1 (4.7%) | 1 (6.7%) | 0 |
| Vedolizumab | 5 (20.8%) | 2 (13.3%) | 3 (33.3%) |
| Ustekinumab | 0 | 0 | 0 |
| IBD treatment after cancer treatment | |||
| 5-ASA | 260 (58.2%) | 225 (56.3%) | 35 (74.5%) |
| Steroids | 94 (21.0%) | 76 (19.0%) | 18 (38.3%) |
| Immunomodulators | 66 (14.8%) | 54 (13.5%) | 12 (25.5%) |
| Biologics | 64 (14.3%) | 50 (12.5%) | 14 (29.8%) |
| Infliximab | 26 (37.7%) | 23 (46%) | 3 (15.8%) |
| Adalimumab | 20 (30%) | 14 (28%) | 6 (31.6%) |
| Certolizumab pegol | 1 (1.4%) | 0 | 1 (5.3%) |
| Golimumab | 2 (2.9%) | 1 (2%) | 1 (5.3%) |
| Vedolizumab | 17 (24.6%) | 10 (5%) | 7 (36.8%) |
| Ustekinumab | 3 (4.3%) | 2 (4%) | 1 (5.3%) |
| Complication of IBD after cancer treatment | |||
| IBD-related hospitalization | 46 (10.3%) | 31 (7.8%) | 15 (31.9%) |
| IBD-related surgery | 29 (6.5%) | 21 (5.3%) | 8 (17.0%) |
| Median duration of follow up, months (range) | 95 (2–444) | 99 (2–444) | 54 (1–280) |
IBD-related surgery, hospital admission, disease complication, steroid prescription, or endoscopic recurrence requiring a change in IBD management
Risk factors for relapse of IBD if inactive IBD at cancer diagnosis
On univariate analysis, previous exposure to systemic steroids, immunomodulators, or biologics was associated with IBD relapse following a cancer diagnosis (Hazard Ratio [HR] 1.79; 95% Confidence Interval [CI] 1.18–2.71, HR 2.22; 95% CI 1.38–3.55, HR 1.95; 95% CI 1.01–5.36, respectively, Table 3). Discontinuation of immunomodulators or biologics did not increase the risk of IBD relapse (HR 0.89; 95% CI 0.39–2.01). Hormone monotherapy and combination cytotoxic and hormone therapy were associated with IBD relapse (HR 2.00; 95% CI 1.21–3.29, HR 1.86; 95% CI 1.01–3.43, respectively) compared to none of these regimens. On multivariable analysis, previous exposure to immunomodulators, hormone monotherapy, and combination cytotoxic and hormone therapy were associated with IBD relapse. Sensitivity analysis according to cancer type and center yielded similar results. Of patients who received only cytotoxic chemotherapy, 75% remained in remission at 250 months compared with 42% of those who received hormone monotherapy (Figure, log rank = 0.02).
Table 3.
Risk factors of IBD relapse if inactive IBD at cancer diagnosis.
| Variable | Bivariate Hazard Ratio (95% CI) | Multivariable Hazard Ratio (95% CI) |
|---|---|---|
| IBD Subtype | ||
| Crohn’s disease | Reference | |
| Ulcerative colitis | 1.18 (0.80–1.73) | |
| Indeterminate colitis | 0.25 (0.34–1.80) | |
| Sex | ||
| Female | Reference | |
| Male | 0.67 (0.41–1.11) | |
| Race/Ethnicity | ||
| White | Reference | |
| Black | 2.07 (0.96–4.47) | |
| Hispanic | 2.69 (1.09–6.65) | 2.15 (0.86–5.38) |
| Asian | 1.85 (0.59–5.87) | |
| Unknown | 0.73 (0.26–2.58) | |
| Age at IBD diagnosis | 1.00 (0.98–1.01) | |
| Smoking history at cancer diagnosis | ||
| Never | Reference | |
| Former | 1.05 (0.72–1.54) | |
| Current | 0.79 (0.25–2.54) | |
| Primary sclerosing cholangitis | 1.05 (0.26–4.26) | |
| IBD treatment before cancer | ||
| 5-ASA | 1.39 (0.92–2.10) | |
| Steroids | 1.79 (1.18–2.71) | 1.32 (0.82–2.11) |
| Immunomodulators | 2.22 (1.38–3.55) | 2.46 (1.41–4.28) |
| Biologics | 1.95 (1.01–5.36) | 1.13 (0.71–7.31) |
| Previous surgery for IBD | 1.24 (0.81–1.88) | |
| Type of Cancer | ||
| Breast | Reference | |
| Prostate | 0.67 (0.41–1.11) | |
| Age at cancer diagnosis | 0.99 (0.98–1.01) | |
| Stage | ||
| I | 0.85 (0.21–3.49) | |
| II | 0.93 (0.22–3.96) | |
| III | 0.77 (0.16–3.82) | |
| IV | Reference | |
| Unknown | 0.27 (0.06–1.14) | |
| Immunomodulators or biologics discontinued for cancer | 0.89 (0.39–2.01) | |
| Cancer treatment | ||
| Surgery | 0.89 (0.45–1.76) | |
| Radiotherapy | 1.37 (0.88–2.16) | |
| Cytotoxic chemotherapy only | 0.91 (0.34–2.42) | |
| Hormone therapy only | 2.00 (1.21–3.29) | 2.40 (1.42–4.06) |
| Duration of hormone therapy | 0.99 (0.97–1.01) | |
| Cytotoxic chemotherapy and hormone therapy | 1.86 (1.01–3.43) | 2.35 (1.25–4.42) |
| Neither cytotoxic nor hormone therapy | Reference |
Figure.
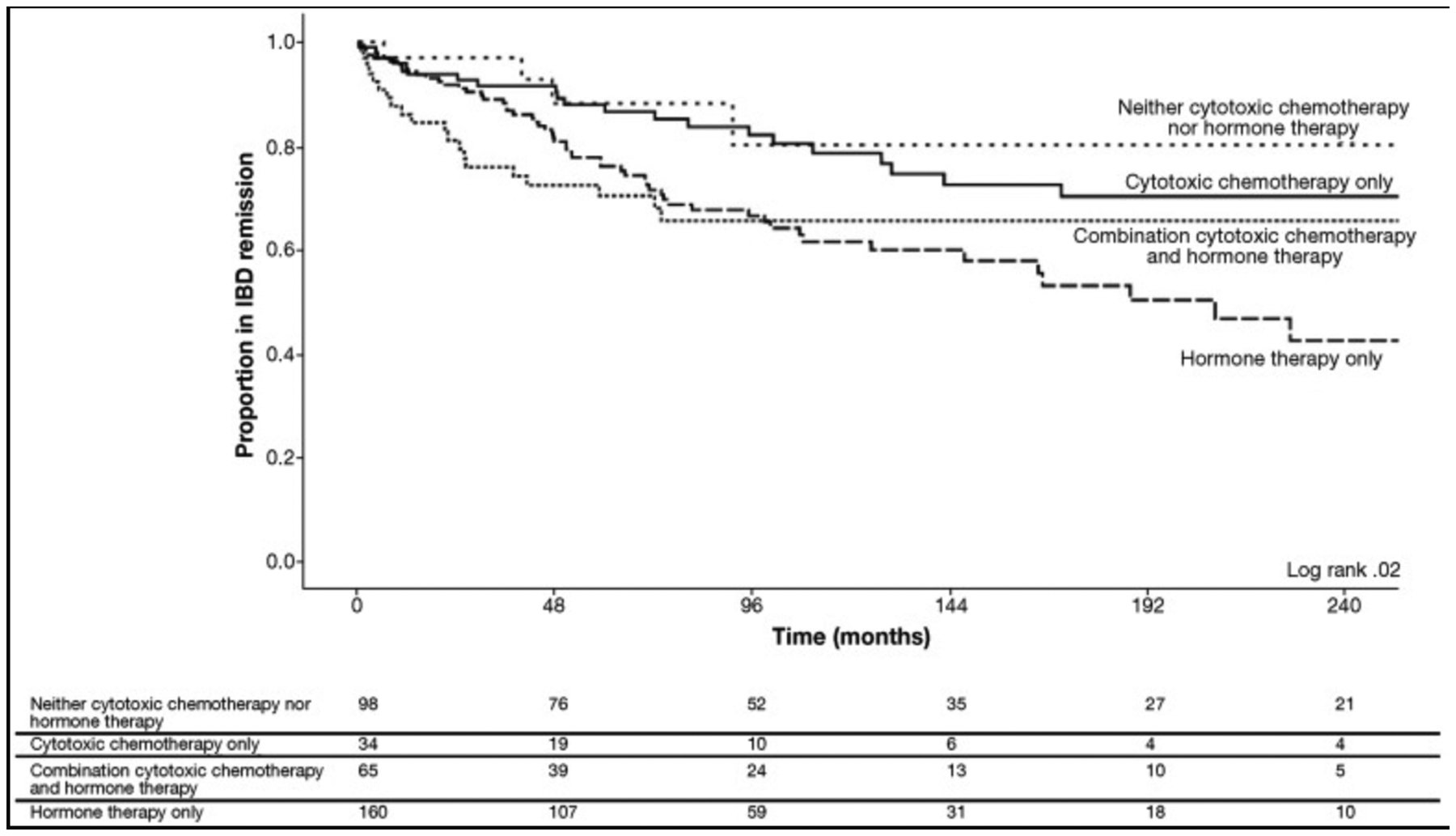
Time to IBD-related surgery, hospital admission, disease complication, steroid prescription, or endoscopic recurrence requiring a change in IBD management stratified by chemotherapeutic regimen.
Risk factors of IBD remission if active IBD at cancer diagnosis
On univariate analysis, there were no statistically significant independent risk factors of achieving IBD remission following a cancer diagnosis (Table 4).
Table 4.
Risk factors for IBD remission if active IBD at cancer diagnosis.
| Variable | Bivariate Hazard Ratio (95% CI) |
|---|---|
| IBD Subtype | |
| Crohn’s disease | Reference |
| Ulcerative colitis | 1.58 (0.55–4.60) |
| Indeterminate colitis | - |
| Sex | |
| Female | Reference |
| Male | 0.55 (0.15–1.97) |
| Race/Ethnicity | |
| White | Reference |
| Black | 2.30 (0.51–10.4) |
| Hispanic | - |
| Asian | 3.11 (0.39–25.1) |
| Unknown | - |
| Age at IBD diagnosis | 1.04 (0.99–1.09) |
| Smoking history at cancer diagnosis | |
| Never | Reference |
| Former | 1.43 (0.48–4.26) |
| Current | 6.14 (0.65–58.0) |
| Primary sclerosing cholangitis | - |
| IBD treatment before cancer | |
| 5-ASA | 0.81 (0.22–2.93) |
| Steroids | 0.91 (0.32–2.59) |
| Immunomodulators | 0.62 (0.17–2.21) |
| Biologics | 0.04 (0.00–14.5) |
| Previous surgery for IBD | 0.58 (0.18–1.86) |
| Type of Cancer | |
| Breast | Reference |
| Prostate | 0.34 (0.08–1.52) |
| Age at cancer diagnosis | 1.04 (0.98–1.10) |
| Stage | |
| I | 1.39 (0.41–4.74) |
| II | 1.92 (0.35–10.5) |
| III | 1.13 (0.13–10.2) |
| IV | Reference |
| Unknown | - |
| Immunomodulators or biologics discontinued for cancer | 0.98 (0.09–10.90) |
| Cancer treatment | |
| Surgery | 26.5 (0.54–1290) |
| Radiotherapy | 1.53 (0.43–5.50) |
| Cytotoxic chemotherapy only | - |
| Hormone therapy only | 1.98 (0.42–9.34) |
| Cytotoxic chemotherapy and hormone therapy | 2.09 (0.35–12.5) |
| Neither cytotoxic nor hormone therapy | Reference |
Cancer Treatment Tolerance
Among patients in whom information was available regarding cancer treatment alteration during active radiation or cytotoxic chemotherapy, patients with active IBD at their cancer diagnosis were significantly more likely to require hospitalization for a complication of IBD or cancer treatment compared to patients with inactive IBD (41% vs 18%, p = 0.001, Table 5). There were no differences in requirement for cancer treatment dose modifications, delays, or discontinuations, based on IBD activity at cancer diagnosis. The most frequently cited reason for cancer treatment alteration was related to gastrointestinal side effects, specifically diarrhea, which was more common in patents with active IBD at their cancer diagnosis.
Table 5.
Cancer treatment alterations and complications stratified by IBD activity at cancer diagnosis.
| Variable | IBD inactive at cancer diagnosis (n=400, 89.5%) | IBD active at cancer diagnosis (n=47, 10.5%) | p-value |
|---|---|---|---|
| Patients with data for cancer treatment alteration | 176 (44.0%) | 15 (31.9%) | - |
| Any treatment alteration | 22 (12.5%) | 2 (13.3%) | 0.720 |
| Dose modification | 10 (5.7%) | 1 (6.7%) | 0.957 |
| Treatment delay | 10 (5.7%) | 2 (13.3%) | 0.401 |
| Treatment discontinuation | 19 (25.0%) | 2 (13.3%) | 0.717 |
| Patients with data for hospitalization during cancer treatment | 334 (83.5%) | 42 (89.4%) | - |
| Hospitalization during cancer treatment | 59 (17.7%) | 17 (40.5%) | 0.001 |
New and recurrent cancer
In terms of incident cancer, 103 (23%) developed a new (57, 13%) and/or recurrent (55, 12%) cancer over 3526 person-years of follow up. Breast cancer recurred in 50 (15%) patients and prostate cancer recurred in 5 (5%) patients. New cancers favored nonmelanoma skin and gastrointestinal malignancies.
Discussion
In this multicenter retrospective cohort study of patients with IBD and either breast or prostate cancer, cancer treatment, and, more specifically hormone therapy, significantly affected the course of IBD. Quiescent IBD was more likely to relapse among patients who received hormone therapies, either alone or in combination with cytotoxic therapies. While few patients received cytotoxic therapies in the present study, there was a trend toward protection from IBD relapse in patients who received cancer treatment monotherapy with cytotoxic agents. Patients with IBD tolerated their cancer treatment regardless of IBD disease activity; however, active IBD resulted in more hospitalizations during cancer treatment. This is the largest study to date to describe the effect of cancer therapies on IBD.
These data are consistent with limited previous reports demonstrating an influence of cancer diagnosis and associated treatment on the course of IBD and subsequent IBD therapeutic management.4,7–9,12,13 Specifically, these data provide further support for our emerging understanding of the relationship between sex hormones and hormone therapies and IBD.
In observational studies, current use of combined hormonal contraception in women has been associated with an increased risk in the development of CD and an equivocal risk in the development of UC.14–18 Postmenopausal use of hormone replacement therapy in women has also been associated with an increased risk in the development of UC.19 In a pooled analysis of population-based studies, age at IBD onset varied with sex suggesting a role for sex hormones in IBD pathogenesis.20 In patients with existing IBD, several studies have demonstrated an impact of hormonal contraception and cyclical hormone fluctuation on the course of IBD.21–23 In a nationwide analysis, long-term use of hormonal contraception, particularly the combination type, was associated with increased risk of surgery among women with established CD.24 However, in the present study, we did not find major differences in outcomes by IBD subtype. Women with IBD reported changes in symptom severity during times of hormone fluctuation including menses, pregnancy, and in the postpartum period.22
In animal models, data have implicated sex hormones, specifically estrogen, in the pathogenesis of IBD, including alteration in intestinal permeability and homeostasis via dysregulation in estrogen-receptors, loss of estrogen-mediated immune protection, and hormone-mediated gut microbiome dysbiosis.25–36 These observational and experimental data confirm biologic plausibility of our results.
This study had several limitations. As a retrospective study, the information contained in the electronic medical record was highly provider dependent and subject to unmeasured confounders. The observed associations between IBD and cancer treatment could be due to the presence of other factors that contribute to cancer, IBD, and to individualized decision-making in respective disease management. As type of cancer determined cancer treatment, we were not able to exclude the possibility that the observed effects of cancer treatment on IBD activity were due to malignancy rather than the agents themselves. Given the specificity of the research question, examining cancer treatment based on either cytotoxic or hormone therapies may not fully represent the unique and heterogeneous action of individual agents within these broad classes. We were underpowered to examine the impact of specific chemotherapy or hormone therapy regimens and associated dosing protocols. Despite the heterogeneity in cancer therapies, we noted very little overall variation in therapy over the data collection period with the exception of the introduction of cancer immune therapies for a small minority of patients, and as such we did not statistically account for major cancer therapy variation over time or calendar year.
In addition, our institutions are major referral centers and our data may not be generalizable to other clinical settings. We broadly defined active or inactive IBD through physician review of charts because standardized disease activity scores were not available within the medical record. As IBD flare or complication of disease often resulted in IBD therapy escalation or hospitalization, meeting any component of the composite outcome qualified for censorship and a detailed distribution of the discrete outcome events was not available for analysis. Furthermore, our finding that withholding immunosuppression for IBD at a cancer diagnosis was not associated with relapse of IBD likely reflects our inability to adjust for IBD severity and prescribing patterns, and perhaps underestimates the potential negative effect of this IBD management strategy at a cancer diagnosis. For cancer treatment tolerance during radiation or cytotoxic chemotherapy, we were unable to fully differentiate IBD versus cancer-related hospitalization. Other comorbidities and individual factors that may influence both cancer and the natural course of IBD also were not considered. However, we attempted to address most known factors predictive of disease relapse.
Overall, the present study confirms an influential role of cancer treatment on the course of IBD, specifically hormone therapies for breast and prostate cancer. Importantly, patients generally tolerated cancer treatment regardless of IBD activity. The present study may provide guidance for developing treatment plans and anticipating disease activity in patients undergoing cancer treatment, especially involving hormone therapies. As breast and prostate cancer are the most common cancers in general population with hormone therapy a mainstay of treatment, these patients should be considered for closer monitoring of IBD activity including objective markers of inflammation with a low threshold to escalate IBD therapy. Additional studies are required to verify and further characterize the results of the present study.
Supplementary Material
Grant Support:
Funded in part by a grant from The Chemotherapy Foundation (SI)
Footnotes
Disclosures:
Jordan E. Axelrad: No relevant conflicts of interest; Ahmad Bazarbashi: No relevant conflicts of interest; James Zhou: No relevant conflicts of interest; Daniel Castañeda: No relevant conflicts of interest; Amandeep Gujral: No relevant conflicts of interest; Dylan Sperling: No relevant conflicts of interest; Jason Glass: No relevant conflicts of interest; Manasi Agrawal: No relevant conflicts of interest; Simon Hong: No relevant conflicts of interest; Garrett Lawlor: Speaking fees from Abbvie,Pfizer, Merck; David Hudesman: Research grants from Pfizer; receiving consulting fees from Takeda, Pfizer, Abbvie, Janssen, Salix; Shannon Chang: No relevant conflicts of interest; Shailja Shah: No relevant conflicts of interest; Vijay Yajnik: Employed by Takeda; Ashwin Ananthakrishnan: Research grants from Pfizer, receiving consulting fees from Janssen, Takeda, Gilead, and Merck; Hamed Khalili: Research grants from Pfizer, Takeda, receiving consulting fees from Abbvie; Jean-Frederic Colombel: Research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Eli Lilly, Medimmune,Merck, Novartis, Pfizer, Protagonist Therapeutics, Sandoz, Second Genome, Seres Therapeutics, Shire, Takeda, Theradiag and Theravance Biopharma; and hold stock options in Intestinal Biotech Development and Genfit.; Steven Itzkowitz: No relevant conflicts of interest
References
- 1.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807–1816 [DOI] [PubMed] [Google Scholar]
- 2.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N. Engl. J. Med 2015;372:1441–1452. [DOI] [PubMed] [Google Scholar]
- 3.Choi C-HR, Al Bakir I, Ding N-SJ, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol 2016;22:4794–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen N, Duricova D, Elkjaer M, et al. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am. J. Gastroenterol 2010;105:1480–1487. [DOI] [PubMed] [Google Scholar]
- 6.Burns JA, Weiner AB, Catalona WJ, et al. Inflammatory bowel disease and the risk of prostate cancer. Eur. Urol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axelrad JE, Fowler SA, Friedman S, et al. Effects of cancer treatment on inflammatory bowel disease remission and reactivation. Clin. Gastroenterol. Hepatol 2012;10:1021–7.e1. [DOI] [PubMed] [Google Scholar]
- 8.Kirk PS, Govani S, Borza T, et al. Implications of prostate cancer treatment in men with inflammatory bowel disease. Urology 2017;104:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axelrad J, Kriplani A, Ozbek U, et al. Chemotherapy tolerance and oncologic outcomes in patients with colorectal cancer with and without inflammatory bowel disease. Clin Colorectal Cancer 2017;16:e205–e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito A, Mizushima T, Takeyama H, et al. Feasibility of Chemotherapy in Patients with Inflammatory Bowel Disease-Related Gastrointestinal Cancer. Hepatogastroenterology 2014;61:942–946. [PubMed] [Google Scholar]
- 11.Tiersten A, Saltz LB. Influence of inflammatory bowel disease on the ability of patients to tolerate systemic fluorouracil-based chemotherapy. J. Clin. Oncol 1996;14:2043–2046. [DOI] [PubMed] [Google Scholar]
- 12.Rajca S, Seksik P, Bourrier A, et al. Impact of the diagnosis and treatment of cancer on the course of inflammatory bowel disease. J. Crohns Colitis 2014;8:819–824. [DOI] [PubMed] [Google Scholar]
- 13.Koc ÖM, Kampen RJW van, Bodegraven AA van. Cancer-Associated Chemotherapy Induces Less IBD Exacerbations and a Reduction of IBD Medication Afterwards. Inflamm. Bowel Dis. 2018;24:1606–1611. [DOI] [PubMed] [Google Scholar]
- 14.Ng SC, Woodrow S, Patel N, et al. Role of genetic and environmental factors in British twins with inflammatory bowel disease. Inflamm. Bowel Dis 2012;18:725–736. [DOI] [PubMed] [Google Scholar]
- 15.Cornish JA, Tan E, Simillis C, et al. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am. J. Gastroenterol 2008;103:2394–2400. [DOI] [PubMed] [Google Scholar]
- 16.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 2013;62:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortizo R, Lee SY, Nguyen ET, et al. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol 2017;29:1064–1070. [DOI] [PubMed] [Google Scholar]
- 18.Halfvarson J, Jess T, Magnuson A, et al. Environmental factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm. Bowel Dis 2006;12:925–933. [DOI] [PubMed] [Google Scholar]
- 19.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Hormone therapy increases risk of ulcerative colitis but not Crohn’s disease. Gastroenterology 2012;143:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-Based Differences in Incidence of Inflammatory Bowel Diseases-Pooled Analysis of Population-Based Studies From Western Countries. Gastroenterology 2018;155:1079–1089.e3. [DOI] [PubMed] [Google Scholar]
- 21.Gawron LM, Goldberger A, Gawron AJ, et al. The impact of hormonal contraception on disease-related cyclical symptoms in women with inflammatory bowel diseases. Inflamm. Bowel Dis 2014;20:1729–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolston VS, Boroujerdi L, Long MD, et al. The Influence of Hormonal Fluctuation on Inflammatory Bowel Disease Symptom Severity-A Cross-Sectional Cohort Study. Inflamm. Bowel Dis 2018;24:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalili H Risk of Inflammatory Bowel Disease with Oral Contraceptives and Menopausal Hormone Therapy: Current Evidence and Future Directions. Drug Saf 2016;39:193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalili H, Granath F, Smedby KE, et al. Association Between Long-term Oral Contraceptive Use and Risk of Crohn’s Disease Complications in a Nationwide Study. Gastroenterology 2016;150:1561–1567.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffl MW, Lange IG, Meyer HHD. The gastrointestinal tract as target of steroid hormone action: Quantification of steroid receptor mRNA expression (AR, ERα, ERβ and PR) in 10 bovine gastrointestinal tract compartments by kinetic RT-PCR. J. Steroid Biochem. Mol. Biol 2003;84:159–166. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl MW, Lange IG, Daxenberger A, et al. Tissue-specific expression pattern of estrogen receptors (ER): Quantification of ERalpha and ERbeta mRNA with real-time RT-PCRNote. APMIS 2001;109:345–355. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW, Lange I, Daxenberger A, et al. Influence of an estrogen treatment on the tissue specific expression pattern of estrogen receptors (ER): Quantification of ER-alpha and ER-beta mRNA with real-time …. Apmis 2001. [DOI] [PubMed] [Google Scholar]
- 28.Campbell-Thompson ML. Estrogen receptor alpha and beta expression in upper gastrointestinal tract with regulation of trefoil factor family 2 mRNA levels in ovariectomized rats. Biochem. Biophys. Res. Commun 1997;240:478–483. [DOI] [PubMed] [Google Scholar]
- 29.Wada-Hiraike O, Imamov O, Hiraike H, et al. Role of estrogen receptor beta in colonic epithelium. Proc. Natl. Acad. Sci. USA 2006;103:2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman WA, Garg RR, Reuter BK, et al. Loss of estrogen-mediated immunoprotection underlies female gender bias in experimental Crohn’s-like ileitis. Mucosal Immunol. 2014;7:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markle JGM, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013;339:1084–1088. [DOI] [PubMed] [Google Scholar]
- 32.Org E, Mehrabian M, Parks BW, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016;7:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez A, Luckey D, Taneja V. The gut microbiome in autoimmunity: Sex matters. Clin. Immunol 2015;159:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 35.Geuking MB, Köller Y, Rupp S, et al. The interplay between the gut microbiota and the immune system. Gut Microbes 2014;5:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


