Abstract
Background and aims
Little is known about the clinical significance of indefinite dysplasia (IND) in patients with inflammatory bowel diseases (IBD) undergoing colonoscopic surveillance for colorectal neoplasia.
Methods
We conducted a retrospective cohort analysis of 492 patients with colonic IBD for 8 or more years or concomitant primary sclerosing cholangitis, with no history of advanced colorectal neoplasia (high-grade dysplasia or colorectal cancer) or colectomy, undergoing colorectal neoplasia surveillance at tertiary IBD referral center from 2001 through 2017. Subjects received consistent histopathologic grading of dysplasia. We collected data on time to development of (advanced) colorectal neoplasia or colectomy using Kaplan Meier methods. We identified factors independently associated with (advanced) colorectal neoplasia with multivariable Cox regression analysis.
Results
After 2149 person-years of follow-up, 53 patients (10.8%) received a diagnosis of IND without prior or synchronous low-grade dysplasia (LGD). Compared to patients without dysplasia, patients with IND had a significantly higher risk of advanced colorectal neoplasia (adjusted hazard ratio, 6.85; 95% CI, 1.78–26.4) and colorectal neoplasia (adjusted hazard ratio, 3.25; 95% CI, 1.50–7.05), but not colectomy (P=.78). Compared to IND, LGD was associated with a significantly higher risk of advanced colorectal neoplasia (P=.05). Following a diagnosis of no dysplasia, IND only, or LGD, the incidence rates of advanced colorectal neoplasia were 0.4% per patient-year, 3.1% per patient-year, and 8.4% per patient-year, respectively.
Conclusions
In a retrospective analysis of patients with IBD undergoing colorectal neoplasia surveillance with consistent histopathologic grading of dysplasia, IND was independently associated with a significant increase in risk of advanced colorectal neoplasia. These findings require validation and if confirmed, a reappraisal of the colorectal neoplasia surveillance guidelines.
Keywords: ulcerative colitis, Crohn’s disease, carcinogenesis, neoplasm
Introduction
Patients with longstanding colitis due to inflammatory bowel disease (IBD) are at increased risk of colorectal cancer (CRC).1,2 Surveillance with early detection and management of colorectal neoplasia (CRN; defined as low-grade or high-grade dysplasia [LGD, HGD] or CRC) is universally recommended by major gastroenterological societies.3–5 There is a large body of evidence establishing the risk of advanced CRN (ACRN: defined as HGD or CRC) following a diagnosis of LGD.6–8 In contrast, the clinical significance and course of indefinite dysplasia (IND) is less well defined.
A few studies have compared the natural history of IND to no dysplasia (NoD) among IBD patients.9–13 However, the data are inconclusive due to small sample sizes and unaddressed confounders such as primary sclerosing cholangitis (PSC) and severity of inflammation.14–17 In the modern era characterized by a vast expansion in medical options to control inflammation, and endoscopic advancements to enhance mucosal visualization, defining the natural history of IND is fundamental to optimizing evidenced-based clinical algorithms for surveillance in IBD.
Our primary objective was to conduct a retrospective cohort analysis of patients with IBD colitis participating in a CRN surveillance program to estimate the risk of ACRN among patients diagnosed with IND, in the absence of prior or synchronous LGD or ACRN, as compared to patients with NoD. Secondary objectives were to estimate the risk of CRN or colectomy among patients with IND, and to compare the risk of ACRN or colectomy between patients with a diagnosis of LGD versus IND.
Methods
Population
We conducted a retrospective cohort analysis of patients with IBD undergoing colonoscopic surveillance between January 2001 - December 2017 at a tertiary IBD referral center (The Mount Sinai Hospital (MSH), New York, NY, United States of America). Eligible patients were identified as described previously.16,18 Inclusion criteria were: an endoscopically and histologically confirmed diagnosis of IBD (Crohn’s disease [CD], ulcerative colitis [UC] or IBD-unclassified [IBD-U]); at least left-sided colonic involvement (UC patients, Montreal classification E2 or E3) or ≥30% involvement of colonic mucosa (CD, IBD-U); disease duration of ≥ 8 years, or any disease extent or duration in patients with concomitant PSC; an “index” surveillance colonoscopy (defined below) that was followed at least 3 months later by a procedure that allowed for colonic histologic assessment (i.e. at least one subsequent surveillance colonoscopy or colectomy specimen, or any type of procedure yielding a diagnosis of IND or CRN) and; histology analyzed by specialized IBD pathologists at MSH. Patients were excluded if ACRN or colectomy occurred prior to or within 3 months of the index colonoscopy. Patients with a history of IND or LGD prior to the index colonoscopy (henceforth referred to as “prior dysplasia”) were not excluded.
Definitions and Classifications
Data were abstracted from the electronic health record (EHR), along with endoscopy and pathology reports according to the definitions described below and in Supplementary Methods. No reports or pathology specimens were re-reviewed for the purposes of this study.
Surveillance colonoscopies were defined as procedures with segmental random biopsies or utilization of chromoendoscopy. The “index colonoscopy” was defined as the first surveillance colonoscopy which met the study criteria. The index colonoscopy date was set as the start of follow-up (T0). Surveillance colonoscopies that additionally had adequate quality metrics (good bowel preparation and cecal intubation) were termed “adequate surveillance colonoscopies” and were included as covariate in regression models. The term “progression” indicates the occurrence of more advanced neoplasia over time in a patient, e.g. IND to LGD or LGD to ACRN, and encompasses both progression of individual lesions and occurrence of additional, more advanced lesions at other anatomically distinct locations.
Patients were classified as NoD, IND or LGD according to the criteria in Supplementary Table 1. We considered all procedures chronologically (prior history, index colonoscopy and individual follow-up procedures). Only the highest dysplasia grade was considered for each procedure. Once IND or LGD was diagnosed, a patient remained in that category for the rest of the analysis regardless of subsequent findings or absence of findings. If no IND or LGD was diagnosed prior to censoring, a patient was classified as NoD. Thus, “IND” indicates no prior or synchronous LGD. “Prevalent dysplasia” was defined as IND or LGD detected at, or prior to, the index colonoscopy. “Incident dysplasia” was defined as IND or LGD detected at a follow-up procedure in a patient without prior dysplasia or dysplasia at the index colonoscopy.
Histologic assessment
Histologic inflammation was scored on a 5-point scale (1 – normal; 2 – inactive; 3 – mild; 4 – moderate; 5 – severe) modified from the Mount Sinai Division of GI Pathology Histological Activity Index (MSHAI), which has high interobserver agreement.19–21 Mean histologic inflammation was the mean score of the most inflamed colonic segment from the included surveillance colonoscopies. Histopathological grading of dysplasia was according to the Riddell classification.22 At MSH, all slides with suspected dysplasia are routinely reviewed by a panel of expert gastrointestinal (GI) pathologists, supervised by one senior GI pathologist (NH).
Outcomes
The primary outcome was ACRN incidence. Secondary outcomes were the CRN or colectomy incidence. We also compared the incidence of ACRN and colectomy among patients with IND versus LGD (secondary analysis).
tatistical analysis
Categorical and continuous variables were compared using the χ2 or Fisher’s exact test and Student t or Mann-Whitney U test, respectively. Kaplan Meier curves were generated with log rank tests. Time at risk for progression was initiated at the diagnosis of IND or LGD for patients with incident dysplasia, and at the index colonoscopy for patients with NoD or prevalent dysplasia. Patients were censored at the outcome of interest (ACRN for primary analysis, CRN or colectomy for secondary analyses), or last available surveillance colonoscopy or colectomy. Patients with IND or LGD diagnosed at the last available procedure were excluded from the Kaplan Meier analysis, unless this was the outcome of interest.
Cox regression modeling was used to identify independent predictors of (A)CRN. Log-log plots were used to assess the proportional hazards assumption of time-static covariates. Dysplasia was entered as a time-changing covariate that could change from NoD to either IND or LGD over time, but not from IND to LGD. Previously established predictors of ACRN (PSC, histologic inflammation, disease extent, disease duration, number of adequate surveillance colonoscopies and prior dysplasia) and variables with p<0.10 on the univariable analysis were included in the multivariable model. Interactions between dysplasia and other covariates were tested by comparing log-likelihood ratios of the multivariable models with, versus without, the interaction term. Patients with missing data for covariates included in the multivariable model were excluded (<5% of patients). Subgroup analyses were performed for PSC status and for IND patients with, versus without, dysplasia at the second procedure (i.e. the first surveillance colonoscopy after the diagnosis of IND), and sensitivity analyses were performed excluding patients with prior dysplasia and patients with ≤6 months of follow-up. Patients with prevalent LGD were excluded from the analysis of CRN. The Bonferroni method was used to correct for multiple testing in subgroup and sensitivity analyses.
Statistical analyses were performed using SPSS Statistics 25.0 (IBM Corp., Armonk, N.Y., USA).
Study oversight
This study was approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board.
Results
Of 1562 patients with IBD in the MSH surveillance database, 492 patients met the eligibility criteria for analysis (Supplementary Figure 1). During 2149 patient-years of follow-up, 32 (6.5%) patients developed ACRN. Fifty-three (10.8%) were categorized as IND, 80 (16.3%) as LGD and 359 (73.0%) as NoD. Among the 53 patients with IND, 15 (28.3%) had IND diagnosed prior to the index colonoscopy, 13 (24.5%) at the index colonoscopy, 25 (47.1%) during follow-up after a median of 3.5 (IQR: 1.9 – 5.4) years. Seven (13.2%) patients were diagnosed with IND at the last available follow-up and excluded from the Kaplan Meier analyses. The proportion of patients classified as IND did not change significantly over time during the study period (Data not shown, p=0.20).
Comparison Between IND versus NoD
Patient characteristics
As noted in the Methods section, “IND” refers to IND in the absence of prior or synchronous LGD. Compared to patients with NoD, patients with IND more often had extensive colitis and PSC (Table 1). The two groups were similar with respect to mean age, sex, IBD type, disease duration, family history of CRC, and medication exposure. Colonic surveillance was more intensive in patients with IND compared to NoD, as evidenced by more adequate surveillance colonoscopies, more biopsies per procedure, shorter intervals between procedures, increased utilization of chromoendosocopy and slightly longer follow-up (all p<0.05). The IND group also had more severe histological inflammation.
Table 1:
Patient characteristics (NoD versus IND).
| No dysplasia (n=359) |
Indefinite dysplasia (n=53) |
p value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age (y), mean (SD) | 40.5 (14.9) | 44.5 (14.9) | 0.07 |
| Male Sex, n (%) | 181 (50.4) | 32 (60.4) | 0.18 |
| IBD-type, n (%) | |||
| UC | 185 (51.5) | 30 (56.6) | |
| CD | 161 (44.8) | 19 (35.8) | |
| IBDU | 13 (3.6) | 4 (7.5) | 0.25 |
| PSC, n (%) | 73 (20.3) | 17 (32.1) | 0.053 |
| Disease duration (y), median (IQR) | 11.6 (8.5 – 20.4) | 12.6 (8.7 – 21.1) | 0.46 |
| Extensive colitis, n (%) | 180 (50.1) | 39 (73.6) | 0.001* |
| Family history of CRC, n (%) | 14 (3.9) | 3 (5.7) | 0.47 |
| Medication exposure, n (%) |
|||
| Biologicals | 167 (46.5) | 22 (41.5) | 0.50 |
| Immunomodulators | 217 (60.4) | 35 (66.0) | 0.43 |
| 5-Aminosalicylates | 317 (88.3) | 45 (84.9) | 0.48 |
| Colonoscopic Surveillance Details | |||
| Duration of follow-up (y), mean (SD) | 4.2 (2.5) | 5.8 (3.4) | <0.0005* |
| Number of adequate surveillance colonoscopies, median (IQR) | 2.0 (2.0 – 3.0) | 3.0 (2.0 – 6.0) | 0.006* |
| Average number of biopsy jars per procedure, median (IQR) | 7.3 (5.5 – 8.6) | 8.1 (7.3 – 9.4) | <0.0005* |
| Interval between surveillance colonoscopies (y), median (IQR) | 1.24 (0.82 – 1.61) | 0.94 (0.68 – 1.27) | 0.03* |
| Procedures with chromoendoscopy, n (% of total number of procedures per group) | 29 (2.5) | 20 (6.4) | <0.0005* |
| Mean Histologic inflammation, mean (SD) | 2.9 (0.9) | 3.4 (1.0) | <0.001* |
Significant at p<0.05.
Incidence of ACRN
Compared to patients with NoD, patients with IND had a significantly higher rate of progression to ACRN (Figure 1a; p<0.0005). The incidence rate of ACRN was 3.1% per person-year in the IND group and 0.4% per person-year in the NoD group. ACRN developed in 7 (13.2%) patients with IND at a median of 4.0 (IQR 3.0 – 4.8) years after the diagnosis of IND, compared with only 6 (1.7%) patients with NoD at a median of 5.5 (IQR 4.4 – 8.2) years after index colonoscopy.
Figure 1.

Progression to ACRN. *significant
The more rapid progression to ACRN among patients with IND compared to NoD remained significant when sensitivity analyses were performed for only patients with >6 months of follow-up (Figure 1b; p<0.0005) and only patients without prior dysplasia (Figure 1c; p<0.0005). When analyzing patients with PSC, those with IND (vs. NoD) had a higher risk of progression to ACRN (Figure 1d; p<0.0005), but this pattern was not seen in patients without PSC (Figure 1e; p=0.37).
In those patients who were diagnosed with IND, a subsequent surveillance procedure was performed in 43 patients after a median of 1.1 (IQR 0.81 – 1.57) years. Importantly, if dysplasia was not confirmed on the second procedure – versus confirmation of IND or a higher grade lesion - the risk of ACRN was significantly lower (Figure 1f; 0.5% versus 9.9% per patient-year; p<0.0005). Of note, in 10 out of 16 (63%) patients with IND or CRN on the second colonoscopy, IND was again the highest grade lesion. Such patients with “persistent IND” had an equal risk of ACRN as those with a higher grade lesion on the second procedure (data not shown, p = 0.77).
Independent predictors of ACRN
Compared to NoD, IND was associated with a 6.85-fold higher risk of ACRN (aHR 6.85; 95% CI 1.78 – 26.4; Table 2). Other significant, independent positive predictors of progression to ACRN were LGD (aHR 21.5; 95% CI 5.93 – 77.8) and histologic inflammation (aHR 1.70; 95% CI 1.03 – 2.79), whereas adequate surveillance colonoscopies were protective (aHR 0.49; 95% CI 0.36 – 0.66). There was a trend towards a statistically significant interaction between PSC and the number of surveillance colonoscopies (p=0.053). PSC was an independent predictor of ACRN only when the number of surveillance procedures was omitted from the multivariable model (aHR 2.87; 95%CI 1.14 – 7.20). There were otherwise no significant interactions.
Table 2.
Predictors of ACRN, Cox regression analysis
| Univariable | Multivariable4 | ||||||
|---|---|---|---|---|---|---|---|
| Events n (%) |
HR | 95% CI | p value | aHR | 95% CI | p value | |
| ACRN, n (%) | 32 (100) | ||||||
| Age | - | 1.03 | 1.01 – 1.05 | 0.02* | 1.03 | 1.00 – 1.07 | 0.07 |
| Male Sex | 18 (56) | 1.16 | 0.58 – 2.33 | 0.68 | - | - | - |
| Dysplasia1 | - | - | <0.0005* | - | - | <0.0005* | |
| IND | 7 (22) | 8.63 | 2.79 – 26.7 | <0.0005* | 6.85 | 1.78 – 26.4 | 0.005* |
| LGD | 19 (59) | 24.4 | 9.73 – 61.3 | <0.0005* | 21.5 | 5.93 – 77.8 | <0.0005* |
| Prior dysplasia | 18 (56) | 10.9 | 5.41 – 22.1 | <0.0005* | 2.05 | 0.75 – 5.60 | 0.16 |
| PSC2 | 12 (38) | 2.29 | 1.12 – 4.68 | 0.02* | 1.14 | 0.36 – 3.63 | 0.83 |
| Histologic Inflammation | - | 1.77 | 1.19 – 2.64 | 0.005* | 1.70 | 1.03 – 2.79 | 0.04* |
| Disease duration | - | 1.05 | 1.02 – 1.08 | 0.001* | 1.00 | 0.96 – 1.03 | 0.81 |
| Crohn’s Disease3 | 10 (31) | 0.67 | 0.32 – 1.41 | 0.29 | - | - | - |
| Number of adequate surveillance colonoscopies | - | 0.55 | 0.43 – 0.71 | <0.0005* | 0.49 | 0.36 – 0.66 | <0.0005* |
| Extensive colitis | 23 (72) | 1.94 | 0.89 – 4.21 | 0.10 | 1.82 | 0.74 – 4.44 | 0.19 |
| Family history of CRC | 2 (6) | 1.48 | 0.35 – 6.18 | 0.59 | - | - | - |
| Exposure to biologicals | 10 (31) | 0.67 | 0.32 – 1.43 | 0.30 | - | - | - |
| Exposure to immunomodulators | 13 (41) | 0.51 | 0.25 – 1.04 | 0.06 | 0.88 | 0.37 – 2.09 | 0.78 |
| Exposure to 5-ASA | 25 (78) | 0.44 | 0.19 – 1.03 | 0.06 | 0.99 | 0.34 – 2.93 | 0.99 |
1) Entered as time-changing covariate 2) interaction between PSC and number of adequate surveillance colonoscopies (p=0.053), aHR 2.87;95%CI 1.14 – 7.70 for PSC when number of surveillance colonoscopies is omitted from the model. 3) Reference category: UC/IBD-U 3) 16 patients excluded due to missing data.
Significant at p<0.05.
Incidence of CRN and Independent Predictors
Patients with IND had a significantly higher rate of progression to CRN (LGD, HGD or CRC) compared to patients with NoD (Figure 2a; p<0.0005). Fourteen (30.4%) patients with IND developed CRN after a median of 2.75 (IQR: 0.79 – 5.05) years, whereas only 35 (9.0%) patients with NoD developed CRN even after a median of 4.2 (IQR 2.3 – 6.1) years. The incidence of CRN following a diagnosis of IND was 7.0% per patient-year, compared to 2.2% per patient-year in patients with NoD.
Figure 2.
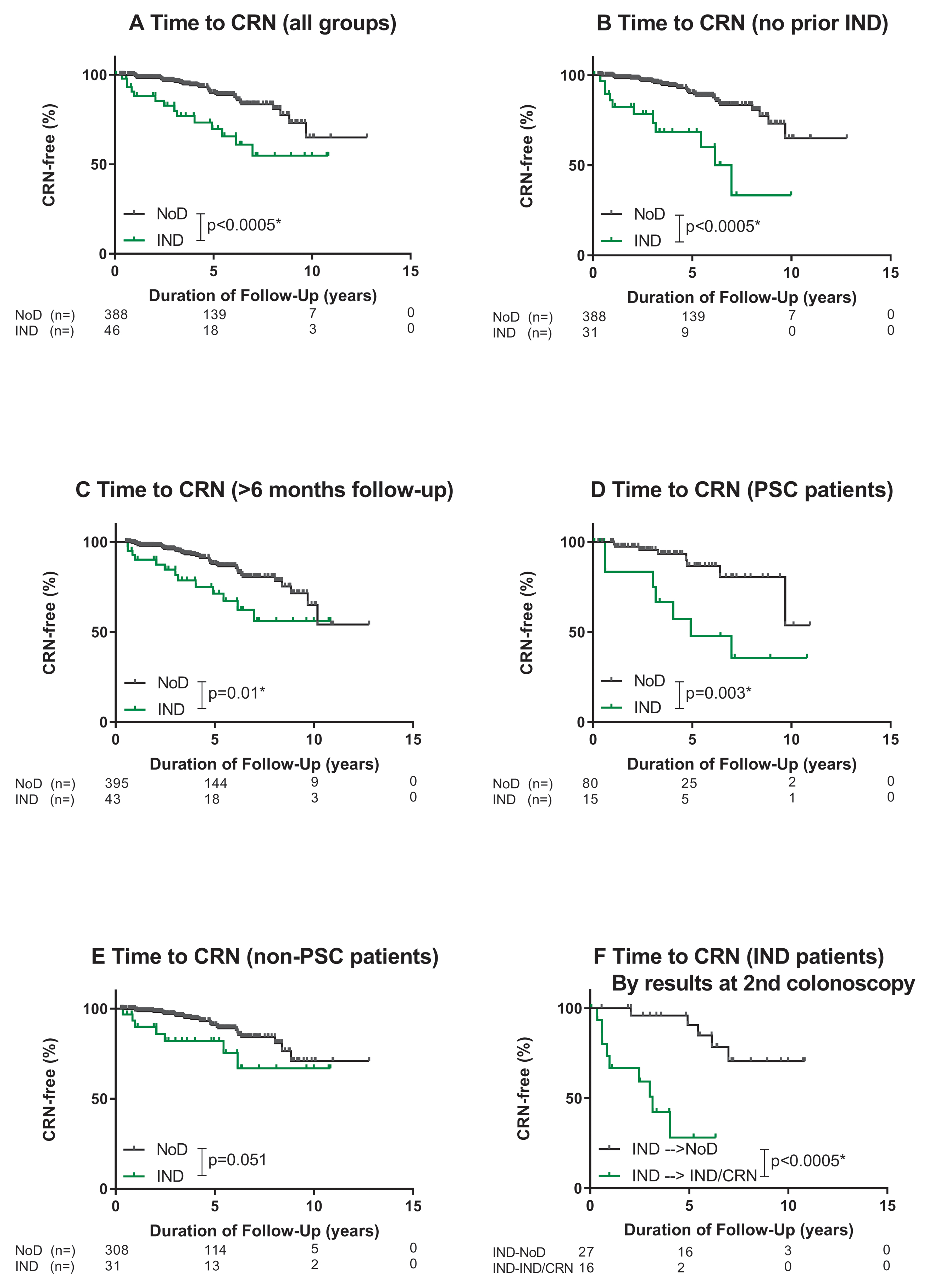
Progression to CRN. *significant
The more rapid progression to CRN among patients with IND compared to NoD remained significant when sensitivity analyses were performed for patients without IND prior to the index colonoscopy (Figure 2b, p<0.0005), and those with >6 months of follow-up (Figure 2c, p=0.01). In the subgroup of patients with PSC, patients with IND had significantly higher rates of progression to CRN compared to patients with NoD, (Figure 2d, p=0.003). This was not the case, however, for those without PSC (Figure 2e, p=0.051). The Bonferroni-corrected threshold for significance in these analyses was p<0.0125.
IND patients without dysplasia on the subsequent surveillance colonoscopy – compared to those in whom IND or a higher grade lesion was confirmed – had a significantly lower risk of CRN (Figure 2f; 3.1% versus 23.3% per patient-year; p <0.0005).
On multivariable analysis, patients with IND had a 3.25-fold (95%CI: 1.50 – 7.05) higher adjusted risk of developing CRN compared to patients with NoD (Table 3). No other significant independent predictors of CRN were identified. There were no significant interactions.
Table 3.
Predictors of CRN, Cox regression analysis
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Events n (%) |
HR | 95% CI | p-value | aHR | 95% CI | p-value | |
| CRN, n (%) | 46 (100) | ||||||
| Age | - | 1.03 | 1.01 – 1.05 | 0.003* | 1.02 | 0.99 – 1.04 | 0.18 |
| Male Sex | 23 (50) | 1.07 | 0.61 – 1.88 | 0.81 | - | - | - |
| IND1 | 14 (30) | 2.57 | 1.33 – 4.95 | 0.005* | 3.25 | 1.50 – 7.05 | 0.003* |
| Prior IND | 3 (7) | 0.89 | 0.27 – 2.92 | 0.85 | 0.64 | 0.18 – 2.22 | 0.48 |
| PSC | 15 (33) | 1.64 | 0.89 – 3.02 | 0.11 | 1.69 | 0.81 – 3.50 | 0.16 |
| Mean Histologic Inflammation | - | 1.09 | 0.79 – 1.51 | 0.59 | 1.13 | 0.77 – 1.65 | 0.53 |
| Disease duration | - | 1.02 | 1.00 – 1.05 | 0.07 | 1.02 | 0.99 – 1.06 | 0.21 |
| Crohn’s Disease2 | 16 (35) | 0.73 | 0.40 – 1.32 | 0.29 | - | - | - |
| Number of adequate surveillance colonoscopies | - | 1.11 | 0.97 – 1.27 | 0.12 | 1.08 | 0.93 – 1.24 | 0.33 |
| Extensive colitis | 35 (76) | 1.67 | 0.90 – 3.12 | 0.11 | 1.26 | 0.61 – 2.61 | 0.53 |
| Family history of CRC | 1 (2) | 0.46 | 0.06 – 3.38 | 0.45 | - | - | - |
| Exposure to biologicals | 16 (35) | 0.67 | 0.37 – 1.22 | 0.19 | - | - | - |
| Exposure to immunomodulators | 22 (48) | 0.55 | 0.31 – 0.97 | 0.04 | 0.64 | 0.34 – 1.21 | 0.17 |
| Exposure to 5-ASA | 39 (85) | 0.43 | 0.21 – 0.86 | 0.02 | 0.56 | 0.25 – 1.25 | 0.16 |
1) Entered as time-changing covariate 2) Reference category: UC/IBD-U 3) 7 patients were excluded due to missing values.
Significant at p<0.05.
Comparison Between IND versus LGD
Patient characteristics
Compared to patients with IND as the highest grade lesion, patients with LGD more often had longer disease duration, less extensive colitis, less use of immunomodulators, and less severe histologic inflammation (Supplementary Table 2). Duration of follow-up was shorter in patients with LGD, but there were otherwise no differences in colonoscopic surveillance details between the groups. IND was significantly less frequently visible, and less frequently polypoid than LGD (Supplementary Table 3).23 IND trended toward more often being unifocal compared to LGD (p=0.052).
Incidence of ACRN
As expected, compared to patients with IND only, patients with LGD had a significantly higher rate of progression to ACRN (Figure 1a; p=0.05). ACRN occurred in 19 patients with LGD (23.8%) at a median of 0.81 (IQR 0.36 – 2.0) years following a diagnosis of LGD, at a rate of 8.4% per person-year. On multivariable analysis, LGD significantly and independently predicted ACRN compared to IND (aHR 3.14; 95%CI 1.02–9.62, based upon the model in Table 2 with IND instead of NoD as the reference category). However, after correcting for multiple testing (threshold p<0.0125), there was no significant difference in progression to ACRN between patients with LGD versus IND on subgroup or sensitivity analyses (Figure 1b–e).
Colectomy incidence and indications
In the IND group, 4 (8.9%) patients underwent colectomy at a rate of 1.9% per patient-year, whereas in the NoD group, 25 (7.0%) patients underwent colectomy at a rate of 1.6% per patient-year. Compared to patients with NoD, patients with IND did not have a significantly higher risk of colectomy (Figure 3; p=0.78). While the incidence rate of colectomy was higher among patients with LGD versus IND (7.6% versus 1.9% per patient-year, p=0.02; Figure 3), the proportion who had “dysplasia” as the indication for colectomy was 75% in both groups (Supplementary Table 4).
Figure 3.

Occurrence of Colectomy. *significant at p<0.05.
Discussion
In this retrospective analysis of nearly 500 patients with IBD undergoing colonoscopic surveillance, we report that patients with IND had a significant, and independent, increased risk of ACRN compared to patients without dysplasia. Compared to NoD, IND was associated with a 2.7% per patient-year higher rate of incident ACRN. Furthermore, IND was a significant independent predictor of CRN, but not of colectomy. This further establishes IND as a clinically relevant, independent risk factor for neoplasia in patients with IBD.
Prior studies reported rates of IND progression to (A)CRN ranging anywhere from 1.0–7.3% per patient-year.9–13,24,25 In addition to the low incidence of (A)CRN, this wide range may be explained by various limitations, several of which are overcome in the present study. First, unlike the present study, there was variable effort in controlling for established confounders of ACRN, including disease extent and duration,1 PSC,16,17 and histologic inflammation.14,15 Active inflammation and reactive epithelial atypia can be difficult to discriminate from dysplasia, partly explaining why the interobserver agreement among pathologists in the diagnosis of IND and LGD in IBD is poor.26–29 This is underscored by our finding that patients with IND had more extensive and severe inflammation compared to patients with either NoD or LGD. A critical strength of our study is that we established that IND remained predictive of ACRN even after adjusting for histologic inflammation and disease extent as critically relevant confounders. A second limitation of prior studies which is overcome here is that the quality of the histopathological diagnosis of dysplasia differed within, and between, previous studies on IND.9–13,24,25 In our study, dysplasia was diagnosed by specialized GI pathologists according to the Riddell classification, and confirmed in a peer review setting overseen by an expert pathologist in IBD-associated dysplasia (NH) in order to establish a consensus diagnosis.22 This confirmatory approach is in line with guidelines for routine clinical practice whenever a diagnosis of dysplasia is considered.5 Notably, our external validity is supported by results from a large IBD cohort with similar grading of dysplasia, reporting similar progression rates from IND to CRN (6.1% per patient-year versus 7.0% in our study).13
Our rigorous analytic approach and attention to relevant sources of bias are other important strengths of this study. The neoplastic risk of IND versus NoD can be overestimated due to immortal time bias, because the start of follow-up differs between the groups (the first colonoscopy versus the diagnosis of incident IND).30 No prior studies have utilized time-changing covariates to account for this bias.9,10,12,13 Additionally, we corrected for the frequency and quality of colonoscopic surveillance by adjusting for the number of adequately performed surveillance procedures. Since patients were censored at the last available surveillance colonoscopy or colectomy, absence of dysplasia was reliably confirmed in patients who did not reach the outcome of (A)CRN. Regardless, patients with IND still had a near 7-fold higher risk of ACRN per year compared to NoD.
Given the clinical implications, our results demonstrating that following a diagnosis of IND, the findings on the subsequent colonoscopy predict the risk of ACRN, warrant emphasis as well as external validation. Patients with IND whose subsequent surveillance colonoscopy showed no IND or CRN, had similar risk of ACRN as patients with NoD from the outset (0.5% per patient-year). By contrast, patients with IND whose subsequent surveillance colonoscopy confirmed IND or CRN, had a risk of ACRN similar to LGD patients (9.9% per patient-year). Based on these and our study’s main findings that IND is independently associated with incident ACRN, it seems reasonable to advocate that IND surveillance intervals should be similar to LGD initially with lengthening of the interval if subsequent colonoscopy shows no dysplasia.
Other novel findings of our study include that IND was significantly less often visible, less often polypoid, and trended towards being more often unifocal compared to LGD. Patients with IND had a significantly lower risk of ACRN compared to LGD on multivariable analysis. These findings are in line with most,9,12 but not all,13 prior studies. Unfortunately, insufficient power limited robust sensitivity and subgroup analyses. Notably, among patients who progressed to ACRN, progression occurred more rapidly following a diagnosis of LGD versus IND (median 0.8 versus 4.0 years, respectively).
Our study is not without limitations, most of which are inherent to any retrospective cohort analysis. Patients with a prior history of IND or LGD were not excluded so, in cases where the diagnosis was made outside of MSH, the quality of the histopathological diagnosis could not be guaranteed. However, sensitivity analyses showed that IND predicted (A)CRN independent of prior dysplasia. The data were insufficient to assess the impact of endoscopic resection of lesions. Our cohort, selected from a tertiary IBD referral center, is likely at higher risk for neoplastic progression. The prevalence of PSC was particularly high (especially in patients with IND) and PSC was predictive of ACRN. Although we adjusted for concomitant PSC and established that IND independently predicted (A)CRN, we were not able to confirm specifically that IND predicted (A)CRN in patients without PSC. This is most likely explained by the low incidences of IND and (A)CRN in non-PSC patients, but this remains to be confirmed. While there was no significant difference in colectomy rates between the NoD (7%, 25/359) and IND (8.9%, 4/53) groups, this likely reflects insufficient power. More clinically relevant is that the indication for colectomy did differ: the indication for colectomy was significantly more often dysplasia/CRC for patients with IND versus NoD and less often inflammation and/or stricture, albeit neither of the latter were statistically significant. We cannot rule out that some patients who underwent colectomy for non-dysplastic reasons might otherwise have developed (A)CRN on longer follow-up. Because the proportion of patients in the IND and NoD groups undergoing colectomies was similar, this is unlikely to influence the overall conclusions. Lastly, our per-group sample size and low incidence of (A)CRN resulted in wide confidence intervals for the magnitude of the effect of IND on progression to (A)CRN. Future investigations confirming and externally validating our findings are needed. In addition, defining biomarkers for progression from IND to ACRN, such as aneuploidy and p53 overexpression, might be important adjuncts for risk stratification.25,24,31
In conclusion, based on a large cohort of patients with IBD undergoing colonoscopic surveillance with consistent grading of all dysplasia, we have established that the diagnosis of IND in itself is an important, independent risk factor for ACRN. We look forward to prospective validation studies since the clinical significance of a diagnosis of IND was heretofore poorly defined. As such, no clinical guidelines have provided clear recommendations for the management of IND.3–5 In the future, IND should be considered in evidence-based risk-stratification models to guide optimal CRN surveillance and management among patients with IBD.3,5 Such models would allow for effective surveillance, and thereby limit the physical and psychological burden on patients, as well as societal healthcare costs.
Supplementary Material
Acknowledgments
Grant support: SCS is partly funded by K12 HS026395–01.
Abbreviations:
- ACRN
advanced colorectal neoplasia
- aHR
adjusted hazard rate
- CD
Crohn’s disease
- CI
confidence interval
- CRC
colorectal cancer
- CRN
colorectal neoplasia
- EHR
electronic health record
- GI
gastrointestinal
- HGD
high-grade dysplasia
- HR
hazard rate
- IBD
inflammatory bowel disease
- IBD-U
IBD-unclassified
- IND
indefinite dysplasia
- IQR
interquartile range
- LGD
low-grade dysplasia
- MSH
Mount Sinai Hospital
- NoD
no dysplasia
- PSC
primary sclerosing cholangitis
- UC
ulcerative colitis
Footnotes
Disclosures: RM, SCS, DC, JG, JE, AK, JA and SHI have no disclosures. J. Torres has served as a consultant for Takeda, and received speaker fees from Takeda, Abbvie and Ferring. T. Ullman has served as consultant for Salix/Valeant and for Janssen. J-F. Colombel has served as consultant, advisory board member or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Lilly, Medimmune, Merck & Co., Pfizer, PPM Services, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag and Theravance Biopharma, has stock options in Intestinal Biotech Development, Genfit and has received research grants from AbbVie, Takeda, Janssen and Janssen. Noam Harpaz has served as a consultant for Celgene, Abbvie and Lilly USA. B. Oldenburg has served as consultant for MSD, Takeda, Abbvie, Ferring, Cablon, Janssen and received unrestricted grants from Abbvie, Ferring, dr. Falk, MSD, Takeda, Janssen and speakers’ fees from Ferring and MSD.
Writing Assistance: not applicable.
REFERENCES
- 1.Beaugerie L, Svrcek M, Seksik P, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology.2013;145(1):166–175. [DOI] [PubMed] [Google Scholar]
- 2.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol.2012;10(6):639–645. [DOI] [PubMed] [Google Scholar]
- 3.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut.2010;59(5):666–689. [DOI] [PubMed] [Google Scholar]
- 4.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology.2010;138(2):738–745. [DOI] [PubMed] [Google Scholar]
- 5.Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis.2017;11(6):649–670. [DOI] [PubMed] [Google Scholar]
- 6.Choi CR, Ignjatovic-Wilson A, Askari A, et al. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol.2015;110(10):1461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas T, Abrams KA, Robinson RJ, et al. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther.2007;25(6):657–668. [DOI] [PubMed] [Google Scholar]
- 8.Ullman T, Croog V, Harpaz N, et al. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology.2003;125(5):1311–1319. [DOI] [PubMed] [Google Scholar]
- 9.van Schaik FDM, ten Kate FJW, Offerhaus GJA, et al. Misclassification of dysplasia in patients with inflammatory bowel disease: consequences for progression rates to advanced neoplasia. Inflamm Bowel Dis.2011;17(5):1108–1116. [DOI] [PubMed] [Google Scholar]
- 10.Pekow JR, Hetzel JT, Rothe JA, et al. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm Bowel Dis.2010;16(8):1352–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai KK, Horvath B, Xie H, et al. Risk for colorectal neoplasia in patients with inflammatory bowel disease and mucosa indefinite for dysplasia. Inflamm Bowel Dis.2015;21(2):378–384. [DOI] [PubMed] [Google Scholar]
- 12.Ullman T, Croog V, Harpaz N, et al. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clin Gastroenterol Hepatol.2008;6(11):1225–30. [DOI] [PubMed] [Google Scholar]
- 13.Choi C-HR, Rutter MD, Askari A, et al. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am J Gastroenterol.2015;110:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology.2004;126(2):451–459. [DOI] [PubMed] [Google Scholar]
- 15.Choi C-HR, Al Bakir I, Ding N-S, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut.2019;68(3)414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah SC, Ten Hove JR, Castaneda D, et al. High Risk of Advanced Colorectal Neoplasia in Patients With Primary Sclerosing Cholangitis Associated With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol.2018;16(7):1106–1113. [DOI] [PubMed] [Google Scholar]
- 17.Claessen MMH, Vleggaar FP, Tytgat KMAJ, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol.2009;50(1):158–164. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud R, Shah SC, Ten Hove JR, et al. No Association Between Pseudopolyps and Colorectal Neoplasia in Patients With Inflammatory Bowel Diseases. Gastroenterology.2019;156:1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiel M, Qin L, Suriawinata A, et al. Histological grading of disease activity in chronic IBD: inter- and intra-observer variation among pathologists with different levels of experience. Mod Pathol.2003. [Google Scholar]
- 20.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology.2007;133(4):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hefti MM, Chessin DB, Harpaz NH, et al. Severity of inflammation as a predictor of colectomy in patients with chronic ulcerative colitis. Dis Colon Rectum.2009;52(2):193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol.1983;14(11):931–968. [DOI] [PubMed] [Google Scholar]
- 23.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology.2015;148(3):639–651. [DOI] [PubMed] [Google Scholar]
- 24.Wen KW, Rabinovitch PS, Wang D, et al. Utility of DNA Flow Cytometric Analysis of Paraffin-Embedded Tissue in the Risk Stratification and Management of “Indefinite for Dysplasia” in Patients with Inflammatory Bowel Disease. J Crohns Colitis.2019;30(4):472–481. [DOI] [PubMed] [Google Scholar]
- 25.Choi W-T, Rabinovitch PS, Wang D, et al. Outcome of “indefinite for dysplasia” in inflammatory bowel disease: correlation with DNA flow cytometry and other risk factors of colorectal cancer. Hum Pathol.2015;46(7):939–947. [DOI] [PubMed] [Google Scholar]
- 26.Eaden J, Abrams K, McKay H, et al. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol.2001;194(2):152–157. [DOI] [PubMed] [Google Scholar]
- 27.Melville DM, Jass JR, Morson BC, et al. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum Pathol.1989;20(10):1008–1014. [DOI] [PubMed] [Google Scholar]
- 28.Dixon MF, Brown LJ, Gilmour HM, et al. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology.1988;13(4):385–397. [DOI] [PubMed] [Google Scholar]
- 29.Odze RD, Goldblum J, Noffsinger A, et al. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod Pathol.2002;15(4):379–386. [DOI] [PubMed] [Google Scholar]
- 30.Levesque LE, Hanley JA, Kezouh A, e al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ.2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 31.Horvath B, Liu G, Wu X, et al. Overexpression of p53 predicts colorectal neoplasia risk in patients with inflammatory bowel disease and mucosa changes indefinite for dysplasia. Gastroenterol Rep.2015;3(4):344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


