Abstract
Background & Aims:
Previous or current infection with Helicobacter pylori (exposure) has been reported to protect against eosinophilic esophagitis (EoE), perhaps due to H pylori-induced immunomodulation. However, findings vary. We performed a systematic review and meta-analysis of comparative studies to more clearly define the association between H pylori exposure and EoE.
Methods:
We searched 4 large databases to identify comparative clinical studies that included sufficient detail to determine the odds or risk of EoE (primary outcome) or esophageal eosinophilia (secondary outcome) among individuals exposed to H pylori (exposed) vs individuals who were tested and found to be unexposed. Estimates were pooled using a random-effects model. Meta-regression and sensitivity analyses were planned a priori. Studies were evaluated for quality, risk of bias, publication bias, and heterogeneity.
Results:
We analyzed 11 observational studies comprising data on 377,795 individuals worldwide. H pylori exposure vs non-exposure was associated with a 37% reduction in odds of EoE (odds ratio [OR], 0.63; 95% CI, 0.51–0.78) and a 38% reduction in odds of esophageal eosinophilia (OR, 0.62; 95% CI, 0.52–0.76). Fewer prospective studies found a significant association between H pylori exposure and EoE (P=.06) than retrospective studies. Effect estimates were not affected by study location, whether the studies were performed in pediatric or adult populations, time period (before vs after 2007), or prevalence of H pylori in the study population.
Conclusions:
In a comprehensive meta-analysis, we found evidence for a significant association between H pylori exposure and reduced odds of EoE. Studies are needed to determine the mechanisms of this association.
Keywords: bacteria, digestive system, allergy, immune system diseases
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic, often times progressive, immune- and allergen-mediated condition defined by a threshold level of mucosal eosinophilic infiltration (≥15 eosinophils per high-power field (Eos/hpf)) in the esophagus along with symptoms of esophageal dysfunction, such as dysphagia or food impaction. While the first cases of EoE were described over a half-century ago1,2, formal recognition of EoE as a disease entity only occurred in the mid-1990s. Over the past several decades, substantial progress has been made in our understanding of the pathophysiology of EoE; however, less is understood about predisposing and protective determinants for the disease. Indeed, both the incidence and prevalence of EoE are rapidly increasing and there is no disputing that the rate well exceeds that attributable merely to increased case-finding and detection bias.3–5 The rate of increase over a short period of time strongly implicates environmental factors and gene-environment interactions, as opposed to genetic factors alone. Thus, now more than ever, investigation into disease determinants is needed, particularly those which are modifiable.
Helicobacter pylori remains the most common chronic bacterial infection worldwide, with marked regional and ethnic variations in prevalence. The formal discovery of H pylori in the 1980s and the subsequent discovery that H pylori causes gastric adenocarcinoma in a small minority of infected individuals, led to eradication campaigns in some areas where gastric cancer is endemic. Eradication efforts coupled with industrialization, improved sanitation, and water conditions heralded a decreasing prevalence of H pylori, which corresponds temporally and geographically to a rising incidence of EoE and other immune-mediated diseases.3,6–10 Accumulating experimental and epidemiologic data generally support an inverse relationship between H pylori and inflammatory bowel disease (IBD), asthma, and food allergy.12–15 H pylori infection often occurs in early childhood and this early immunoregulation and enhanced immunotolerance may plausibly protect against the later development of aberrant Th2-mediated immune responses driving immune-mediated diseases in a susceptible host.13,15–20 Unfortunately, direct experimental evidence clarifying the role, if any, of H pylori on the development of EoE is lacking. However, indirect evidence from observational studies variably supports an inverse relationship between H pylori and EoE. Most recently, a prospective, multicenter case-control study by Molina-Infante and colleagues found a statistically nonsignificant inverse association between H pylori infection and EoE, thus calling into question the relationship.22
Our primary objective was to systematically review the literature for comparative studies detailing the risk of EoE in H pylori-exposed compared to non-exposed individuals. Because of anticipated differences in case definition over time, we also secondarily evaluated studies which included patients with esophageal eosinophilia (EE) in the absence of a formal diagnosis of EoE per se.
METHODS:
The current study follows the methodology stipulated in the Cochrane Handbook24, the PRISMA guidelines25, and MOOSE guidelines.26
Data Sources and Searches
We systematically searched four databases—PubMed, EMBASE, CINAHL, and Web of Science—for relevant literature in conjunction with a certified biomedical librarian at Vanderbilt University (initial search: October 19, 2018). No restrictions were applied based on language, publication date, or peer-reviewed publication type, although we acknowledge that the ability to critically appraise the quality of abstracts and conference abstracts, for example, is limited. Key words searched included: “helicobacter pylori”, “campylobacter pylori”, “helicobacter infections”, “eosinophilic esophagitis”, “esophageal eosinophilia”. The full search strings for each database can be found in the Supplemental material. We also hand-searched references from included studies, as well as relevant review articles and published abstracts.
Inclusion/Exclusion Criteria
All clinical trials (randomized or nonrandomized), cohort (prospective, retrospective), case-control, and cross-sectional studies were considered eligible if they met the following additional criteria: (1) clear definition for eosinophilic esophagitis or esophageal eosinophilia (even if study-specific); (2) comparative study design with distinct comparison between patients with EoE or EE and patients without EoE or EE who had undergone endoscopic evaluation and esophageal biopsies; (3) H pylori testing in both case and comparator groups, along with test results; (4) clear description of demographic details including pediatric versus adult populations; (5) sufficient detail to calculate effect estimates.
Data Extraction
Eligibility assessment and data extraction were carried out independently by S.C.S. and A.T. with discrepancies resolved by a third investigator, N.N. A data collection form was designed by S.C.S. and included the following elements: first author’s last name; publication date; study design; country of origin; study time period; number of patients; study demographics (age, sex, pediatric/adult/both, smoking history, racial/ethnic background); study setting (community-based, hospital-based, multicenter, single-center) and study-specific inclusion/exclusion criteria; H pylori related details (number infected; current vs. former vs. never infection; diagnostic method (e.g. histology, rapid urease test, stool antigen test, etc.); duration of infection; treatment details including antibiotic regimen and eradication success or failure); EoE and EE-related details (age of diagnosis, criteria for diagnosis, clinical symptoms, esophageal findings, presence of other atopic diseases, proton-pump inhibitor (PPI) use and timing of use related to diagnosis, other EoE therapy and response to therapy); endoscopy-related details (indication, number of biopsies, location of biopsies; gross findings) and histologic findings including Eos/hpf. Data extraction was performed in duplicate by S.C.S and A.T.
Primary exposure and outcomes
The primary exposure was H pylori infection (documented as current, former, or current versus former not specified). The primary outcome was EoE. EE was analyzed as a secondary outcome. Study-specific definitions of H pylori exposure, EoE diagnosis, and EE diagnosis were recorded as noted above. Abidance to the strict definition of EoE as ≥15 Eos/hpf on esophageal mucosal biopsies along with esophageal symptoms was documented separately. As detailed below, separate analyses were performed to analyze only those studies adhering to the currently accepted definition of EoE, understanding that there remains controversy regarding the positioning of PPI-responsive EE (PPI-REE).27
Quality and Risk of Bias Assessment
Quality assessment was performed independently by S.C.S and A.T. using the Newcastle-Ottawa Scale (NOS)28 for nonrandomized studies and the Cochrane Risk of Bias tool for randomized trials (although there were no studies fitting the latter category). Discrepancies were resolved by consensus with N.N. For the NOS, studies scored as >/= 7 (of maximum score 9) were considered high-quality, consistent with the literature.28 Separate NOS rubrics were used for case-control, cohort, and cross-sectional studies (modified NOS cohort rubric).29
Qualitative Synthesis and Quantitative Statistical Analysis
Details of each study were summarized. The overall prevalence of H pylori in the study population was recorded. No studies identified reported relative risk. The odds of EoE (primary outcome) or EoE/EE in H pylori exposed versus non-exposed was calculated and reported as odds ratios (ORs) for each individual study. Individual study ORs were combined into a pooled OR (pOR) by using a random effects model. Egger test30 and a funnel plot were used to assess publication bias for the primary outcome. Heterogeneity was estimated with chi-squared and I2 test statistics. The chi-squared test suggests heterogeneity between studies when the P-value is less than 0.15. We further used I2 cut-offs of <30%, 30–59%, 60–75%, and >75% to for low, moderate, substantial, and considerable heterogeneity, respectively.31 Based on the availability of appropriate studies and data, the follow meta-regression analyses were planned a priori to adjust for: study type (cohort versus case-control studies, and prospective versus retrospective studies), study geography (based on literature suggesting geographic differences in the association between H pylori and other esophageal symptoms, namely GERD23), time period (pre-2007 versus post-2007), pediatric only versus adult only populations, prevalence of H pylori (low prevalence, defined as <20% versus moderate-high prevalence, defined as ≥ 20%), former versus current H pylori infection, PPI therapy versus no PPI therapy, serologic response to H pylori specific virulence factors (e.g. VacA, CagA), and duration of H pylori infection prior to EoE diagnosis (or matched time point in comparator group).
All analyses were performed with RevMan 5.1 (Review Manager Version 5.1, Copenhagen, Denmark) and Comprehensive MetaAnalysis (version 2.0; Biostat, Englewood, NJ).
RESULTS
After removal of duplicates, our search yielded 291 results: EMBASE (123), Web of Science (97), PubMed (64), CINAHL (7). A total of 259 articles were excluded for irrelevance based on title and abstract screening. The full texts of the remaining 32 articles were reviewed for eligibility, along with full reference lists. Only one relevant abstract was identified, but these same data were subsequently published as full text and thus only the latter was included32; no brief reports or other alternate publication types were identified. From 32 full-text articles reviewed, 21 were excluded for the reasons detailed in the flow diagram (Figure 1). One additional article was identified from search of reference lists and the full text was reviewed; however, this study was excluded because of lack of H pylori testing in the comparator group.33 Thus, a total of 11 articles were eligible for analyses.
Figure 1:
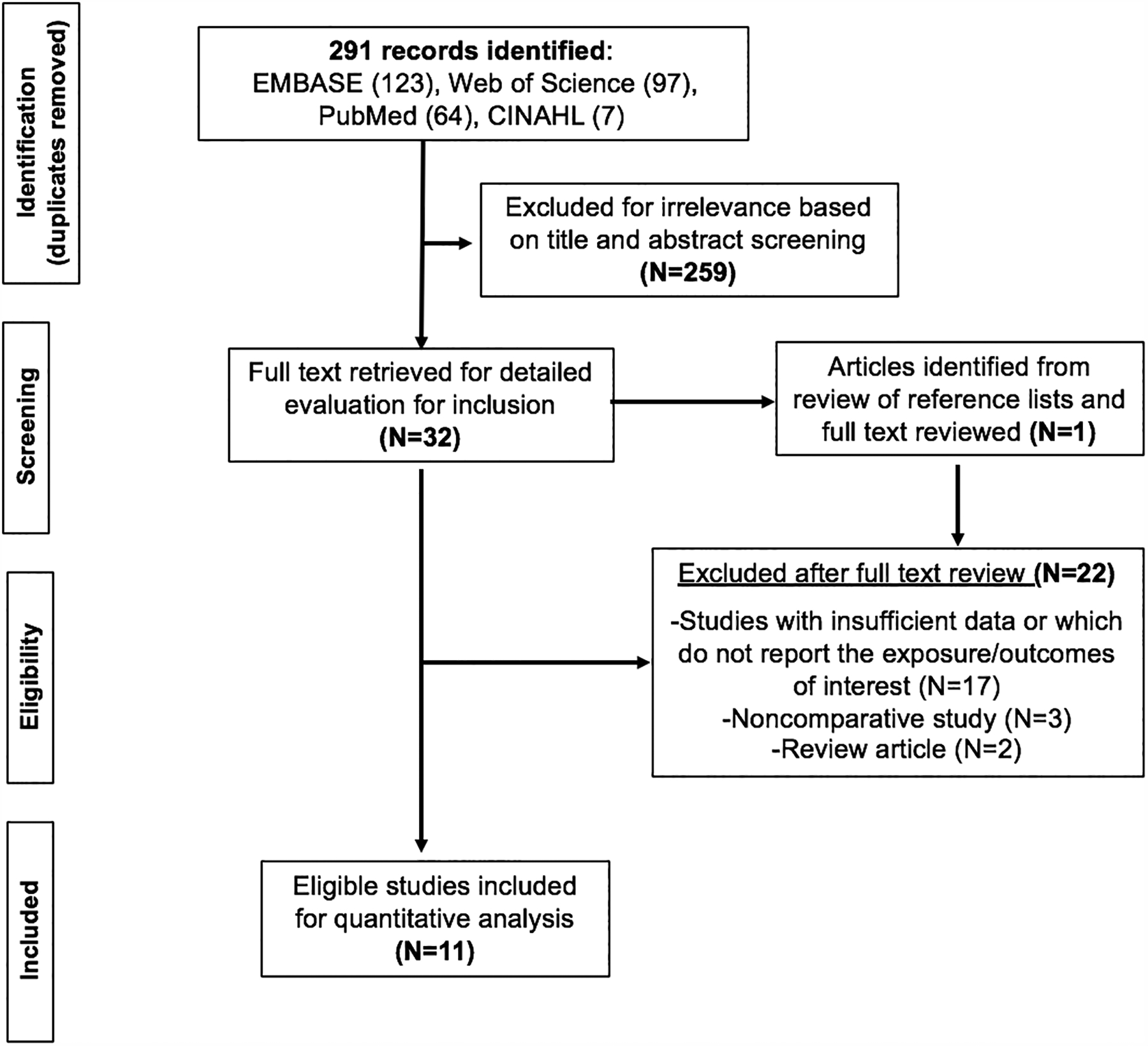
PRISMA diagram of study selection
Characteristics of included studies (Table 1)
Table 1:
Study Characteristics, Diagnostic Terminology, and Endoscopic Protocols
| Author, Year | Study Design | Country or Region | Study Period | Definition of Cases | Definition of Comparators | Endoscopic Protocol | EoE Therapya | Primary Study Objective |
|---|---|---|---|---|---|---|---|---|
| CROSS-SECTIONAL AND COHORT STUDIES | ||||||||
| Cheung, 2003 | Retrospective cohort; Single-Center | Melbourne, Australia | 1989–2000 | EoE: dysphagia and >20 Eos/hpf | Dysphagia and ≤5 Eos/hpf | All had biopsies of lower esophagus, gastric body, antrum, and the third part of the duodenum. Most had biopsies from upper and middle esophagus as well | Unknown | To characterize EoE by comparison with those who presented with dysphagia, but had little or no eosinophilic infiltration in the esophagus |
| Norder, 2018 | Prospective cohort; Single-Center | Sweden | 2009–2014 | EoE: history of esophageal dysfunction and ≥15 Eos/hpf | GERD: typical symptoms, had endoscopic and/or histopathologic esophagitis and <15 Eos/hpf | Diagnostic biopsies, and 2 bacterial samples 2cm above the Z-line, biopsies repeated at the proximal esophagus 5 cm below the upper esophageal sphincter, as well as brush samples and two biopsies from the buccal mucosa | 2 weeks off of PPI prior to biopsies | To compare the microbiome of the esophagus in subjects with GERD and EoE |
| Ronkainen, 2006 | Prospective; Cross-Sectional; Single-Center | Sweden | 1998 | EE: >0 Eos/hpf | 0 Eos/hpf | At least two biopsy samples were taken from the following locations in the esophagus: 2 cm above the Z-line, at the Z-line, and any abnormal areas | Unknown | To assess the prevalence of EoE and the presence of eosinophils in the distal esophagus, and determine the association with upper GI symptoms |
| Sealock, 2013 | Prospective; Cross-Sectional; Single-Center | United States | Not stated | EE: >15 Eos/hpf; EoE (definite): EE + esophageal symptoms + acid suppression meds EoE (probable): EE + either esophageal symptoms OR acid suppression meds | ≤15 eos/hpf | ≥1 esophageal 2–3 cm above the normal SCJ, and biopsies from suspected Barrett’s esophagus and multiple gastric biopsies | Variable | To determine the prevalence and risk factors of EE with or without EoE |
| Ma, 2015 | Prospective; Cross-sectional (Population-based) | China | Not stated | EE: >0–15 Eos/hpf | 0 Eos/hpf | At least 4 esophageal biopsies from 0.5cm above the Z-line and any abnormal areas | Unknown | To describe features of esophageal eosinophilia and eosinophilic esophagitis in a representative sample of adults in Shanghai, China |
| CASE-CONTROL STUDIES | ||||||||
| Dellon, 2011 | Retrospective; Case-control; Multicenter (pathology database) | United States | 2008–2010 | EE: ≥15 Eos/hpf; EoE: EE + clinical suspicion for EoE and no reflux or BE | <15 Eos/hpf | Esophageal and gastric biopsies | Unknown | To investigate the association between EE and H. pylori infection |
| Furuta, 2013 | Retrospective, Case-Control; Single center | Japan | 2010–2011 | EoE: history of EoE (defined by esophageal dysfunction and ≥15 Eos/hpf) | Age and gender matched, normal patients, without history of EoE, presenting for annual check-up. | Not stated | Unknown | To investigate the effect of H. pylori infection on EoE and eosinophilic gastroenteritis in Japanese patients |
| Elitsur, 2014 | Retrospective, (nested) case-control; Single center | United States | 2007–2012 | EoE: ≥15 Eos/hpf (presence of esophageal symptoms not specifically stated) | <15 Eos/hpf | Esophageal biopsies (distal – 3; mid – 3 when EoE was suspected); stomach (antrum – 4 for histology, two for rapid urease test, body – 2); 2 from the duodenal bulb, and 2 from the 2nd part of duodenum | All had failed PPI therapy | To investigate the association between H. pylori infection and EoE in children |
| von Arnim, 2016 | Retrospective, Case-Control; Single Center | Germany | Not stated | EoE: >15 Eos/hpf + Esophageal symptoms | Normal controls: age and sex matched controls without EoE, presenting to the ER 2009–2010. | 2 biopsies from distal (3–5 cm above GEJ) and 2 from the proximal esophagus | Unknown | To assess if H. pylori infection is associated with a reduced risk of developing EoE |
| Sonnenberg2017 | Retrospective, Case-Control; Multicenter (pathology database) | United States | 2008–2015 | 3 definitions, variable certainty:
|
No histologic abnormalities | Esophageal and gastric biopsies | Unknown | To investigate the influence of H. pylori on the ethnic variation of esophageal eosinophilia |
| Molina-Infante, 2018 | Prospective, Case-Control; Multicenter | Spain, Italy, France, Colombia | 2014–2017 | EoE: Esophageal symptoms and ≥15 Eos/hpf | Esophageal symptoms and <5 Eos/hpf (age-, sex-matched) | ≥6 biopsies each from distal and proximal esophagus | Naïve | To determine the association of H. pylori and EoE |
Abbreviations: BE, Barrett’s esophagus; ER, emergency room; Eos, eosinophils; EoE, eosinophilic esophagitis; EE, esophageal eosinophilia; EGD, esophagogastroduodenoscopy; GEJ, gastroesophageal junction; GERD, gastroesophageal reflux disease; hpf, high-power field; IBD, inflammatory bowel disease; PPI, proton-pump inhibitor
Acid suppression, topical steroids, other if listed
All included studies were observational; no interventional studies were identified. Two studies were cohort34,35, 3 studies were cross-sectional36–38, and 6 studies were case-control22,32,39–42. Only one study encompassed a time period prior to 2000 (1989–2000)35; otherwise, all studies included data from 2000 onwards when explicitly stated. One cross-sectional38 and one case-control study42 included East Asian populations, while four studies were from the US, two from Sweden, one each from Germany and Australia, and one multicenter study that included centers from Spain, Italy, France, and Colombia.22
Four studies were population-based, including 2 from a nationwide pathology database.32,36,38,40 We confirmed that the Dellon et al.32 and Sonnenberg et al.40 studies were conducted using the same pathology database; the different names in the respective publications (Caris Life Sciences and Mirica Life Sciences) reflect the change in ownership. Because it is impossible to determine the degree of overlap between the two study populations, we performed a sensitivity analysis removing the Dellon et al. study, since the study time period (2008–2010) was included within the Sonnenberg et al. study (2008–2015).
Additional details of the included study populations are detailed in Table 1.
H pylori-related details
The overall prevalence of H pylori in the pooled study population (i.e. all tested individuals) from cohort and cross-sectional studies was 8.9% (N=5534/62,035), and ranged from a low of 7.0% in the individual studies by Dellon et al. (US)32 to a high of 71.8% in the study by Ma, et al. (China).38 H pylori prevalence was 7.0% (N=179/2543) versus 9.0% (N=5355/59492) in EoE cases versus non EoE comparators. Current versus former H pylori infection was not readily discernible in the studies, as variable methods of testing for H pylori were used and the testing modality with respect to EoE or EE diagnosis was not consistently explicitly stated (thus precluding meta-regression according to current versus former infection). Other details that were not available in any study included duration of H pylori infection prior to EoE/EE diagnosis or matched time point in controls and H pylori-specific virulence factors (e.g. CagA, VacA). Studies variably reported eradication therapy for H pylori and no studies reported eradication success or failure, nor the effect of H pylori therapy on EoE/EE diagnosis or related outcomes. Thus, meta-regression and subanalyses for these categories were not possible.
Eosinophilic Esophagitis and Esophageal Eosinophilia-related details
Nine of the 11 studies defined EoE as ≥15 Eos/hpf (one study >20 Eos/hpf) and esophageal symptoms (see Table 1). Acid-suppressive therapy with PPI was variably reported, and only one study stated failure of PPI therapy.39 One study’s protocol explicitly stated that biopsies were obtained with patients off of PPI therapy for 2 weeks prior to endoscopy.34 Details regarding other EoE therapy were not consistently provided in studies, but these data were abstracted whenever available (Table 2). Endoscopic protocols, when described, were also variable in terms of the number and location of biopsies (Table 2).
Table 2:
H. pylori prevalence in patients with eosinophilic esophagitis or esophageal eosinophilia versus comparator group
| Author, Year | Study Population, N | Age; Pediatric vs. Adult vs. Both | Casesa, N [EoE, unless stated as EE] | Comparator, N | H. pylori-positive | H. pylori-negative | Naïve to H. pylori therapy (yes, no, unknown) | Method(s) of H. pylori diagnosis | Current vs. Former Infection | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, N(%) | Comp., N (%) | Overall, N(%) | Cases, N(%) | Comp., N (%) | Overall, N(%) | ||||||||
| CROSS-SECTIONAL AND COHORT STUDIES | |||||||||||||
| Cheung, 2003 | 42 | Pediatric | 21 | 21 | 1/21 (4.8%) | 2/21 (9.5%) | 3/42 (7.1%) | 20/21 (9.5%) | 19/21 (90.5%) | 39/42 (92.9%) | Unknown | Unknown | Unknown |
| Norder, 2018 | 27f | Adult | 9 | 14 | 0/9 (0.0%) | 2/14 (14.3%) | 2/23 (8.7%) | 9/9 (88.9%) | 12/14 (85.7%) | 20/23 (87.0%) | Unknown | A Campylobacter-like organism (CLO) test (rapid urease test for CLOs, Ballard Medical Products, Draper, UT) was performed on gastric biopsy samples. | Unknown |
| Ronkainen, 2006 | 1000 | Adult | EE: 48 | 952 | 8/48 (1.7%) | 331/952 (34.8%) | 339/1000 (33.9%) | 40/48 (83.3%) | 621/952 (65.2%) | 661/1000 (66.1%) | Unknown | Histology of gastric biopsy with the Warthin-Starry silver stain as well as culture. | Unknown |
| Dellon, 2011 | 165017 | Both (Majority adult; 2.1% <18yo) | EEa:5767 | 56301 | 326/5767 (5.7%) | 4048/56301 (7.2%) | 4374/62068 (7.0%) | 5441/5767 (94.3%) | 52253/56301 (92.8%) | 57694/62068 (93.0%) | Unknown | Histologic antibody staining (gastric biopsy) with concomitant chronic and/or active inflammation in the gastric mucosa | Unknown |
| EoE: 2367 | 56301 | 121/2367 (5.1%) | 4048/56301 (7.2%) | 4169/58668 (7.1%) | 2246/2367 (94.9%) | 52253/56301 (92.8%) | 54499/58668 (92.9%) | ||||||
| Sealock, 2013 | 1357b | Adult | EE: 33c | 1324 | 3/31 (9.7%) | 285/1250 (22.8%) | 288/1281 (22.5%) | 28/31 (90.3%) | 965/1250 (77.2%) | 993/1281 (77.5%) | Variable | Histological diagnosis (gastric biopsies), or if review of the medical record showed a previous positive biopsy, presence of serum antibodies, or treatment received | Unknown |
| Ma, 2015 | 1021 | Adult | EE: 67 | 954 | 46/67 (68.7%) | 687/954 (72.0%) | 733/1021 (71.8%) | 21/67 (31.3%) | 267/954 (28.0%) | 288/1021 (28.2%) | Unknown | Serum H. pylori antibodies were determined using IgG enzyme linked immunosorbent assay (ELISA) | Unknown |
| CASE-CONTROL STUDIES | |||||||||||||
| Furuta, 2013 | 160 | Not stated | 18 | 54 | 4/18 (22.2%) | 30/54 (55.6%) | 34/72 (47.2%) | 14/18 (77.7%) | 24/54 (44.4%) | 38/72 (52.8%) | Unknown | Serum anti-H. pylori antibody were measured using EIA | Unknown |
| Elitsur, 2014 | 966 | Pediatric | 62 | 904 | 1/62 (1.6%) | 30/904 (3.3%) | 31/966 (3.2%) | 61/62 (98.4%) | 874/904 (96.7%) | 935/966 (96.8%) | Unknown | Diagnosed with positivity of both histology (H&E and Giemsa staining), and rapid urease test (CLO). | Unknown |
| von Arnim, 2016 | 174 | Adult | 58 | 116 | 8/58d (13.8%) | 44/116 (37.9%) | 52/174 (29.9%) | 50/58 (86.2%) | 72/116 (62.0%) | 122/174 (70.1%) | Variabled | H. Pylori serology | Both |
| Sonnenberg, 2017 | 596479 | Both | 25969e | 284552 | 1156/25969 (4.5%) | 20683/284552 (7.3%) | 21839/310521 (7.0%) | 24813/25969 (95.5%) | 263869/284552 (92.7%) | 288682/310521 (93.0%) | Unknown | Histologic H. pylori staining as well as signs of chronic and/or active inflammation in gastric mucosa | Unknown |
| Molina-Infante, 2018 | 808 | Both | 404 | 404 | 151/404 (37.4%) | 161/404 (39.9%) | 312/808 (38%) | 253/404 (62.6%) | 243/404 (60.1%) | 496/808 (62%) | Yes | 13C-urea breath test (UBT), monoclonal stool antigen test, or rapid urease test or histology collected by endoscopy | Unknown |
See definitions in Table 1 for each study
76 subjects not tested for H. pylori, thus denominator for calculations is 1281
Case defined by EE. Number of H. pylori positive patients could not be determined for EoE (probable or definite).
8 total H. pylori positive subjects included 5 subjects with prior infection and eradication.
Includes all patients with at least dysphagia and ≥15 Eos/hpf (of these, 11,915 and 6,708 patients met the stricter criteria for group 2 and group 3 categorization as defined in Table 1)
4 subjects did not have H. pylori testing, thus denominator for calculations is 23
H pylori status and Risk of Eosinophilic Esophagitis (primary outcome) or Esophageal Eosinophilia (secondary outcome)
H pylori exposure was associated with a 37% lower odds of EoE (OR 0.63, 95% CI: 0.51–0.78), based on 8 studies (Figure 2). Although 9 of the 11 studies explicitly defined EoE as ≥15 Eos/hpf (one study >20 Eos/hpf) and esophageal symptoms, Sealock et al. reported H pylori information only according to EE status; thus, only 8 of 11 studies were included in the analysis of H pylori and risk of EoE according to a strict definition. Removing this study from the primary analysis did not alter the effect estimate. Based on meta-regression, there was no significant difference in effect estimates between cohort studies and case-control studies (Supplemental Figure 4a, P=0.63). The magnitude and direction of the protective effect estimate of H pylori exposure was unchanged (OR 0.62, 95%CI: 0.52–0.76) when we included the three studies36–38 that reported risk of EE only, as opposed to EoE by strict diagnostic criteria (Figure 3).
Figure 2:
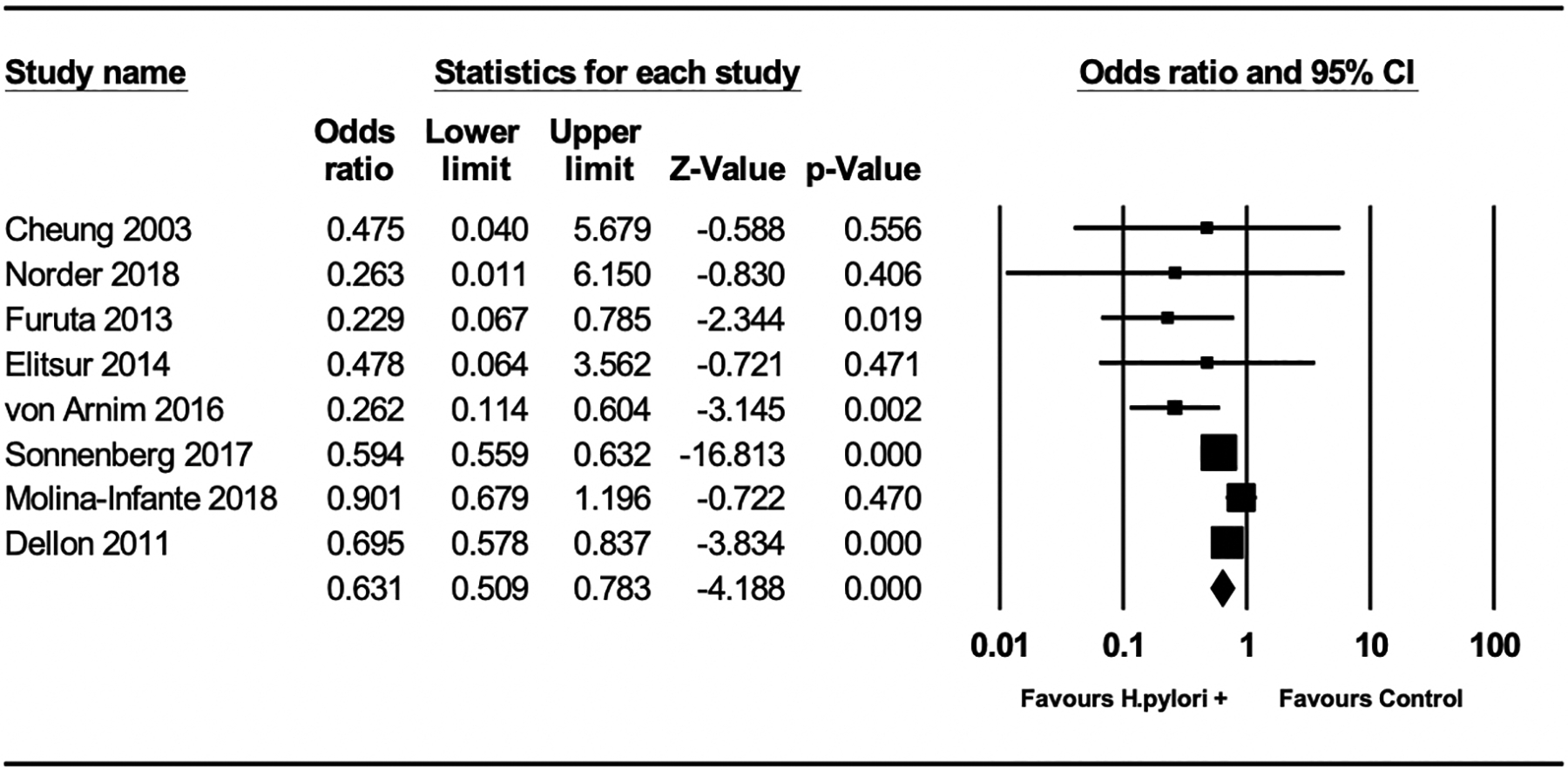
Odds of eosinophilic esophagitis in H pylori exposed versus non-exposed individuals
Figure 3:

Odds of eosinophilic esophagitis or esophageal eosinophilia in H pylori exposed versus non-exposed individuals, pooled odds ratio
Notably, performing sensitivity analyses by removing the Dellon et al. study from both the primary and secondary analyses (as well as meta-regression analyses where appropriate) did not significantly affect the outcomes, other than slightly increasing the magnitude of protective benefit. H pylori exposure was associated with a 43% lower odds of EoE (OR 0.57, 95% CI: 0.40–0.80) or a 42% (OR 0.58, 95% CI: 0.44–0.76) lower odds of EoE/EE compared to non-exposed individuals. (Supplemental Figures 1 and 2).
Additional analyses (meta-regression analyses)
Meta-regression analyses revealed that study geography (US-based studies versus non-US studies, P=0.26), pediatric only versus adult population (P=0.93), time period of patient recruitment (pre-2007 versus post-2007, P=0.18), and H pylori prevalence of the study population (low versus moderate-high prevalence, P=0.58) did not influence the effect estimates (see Supplemental Figures 4b–4e); that is, the magnitude of the protective effect of H pylori exposure on subsequent odds of EoE (or EoE/EE) was stable in each meta-regression analysis. Meta-regression analysis was also performed to adjust for studies done prospectively versus retrospectively. A statistically nonsignificant trend was seen in the effect estimates between these study types, with prospective studies less likely to show a significant association between H pylori exposure and EoE (P=0.06) (Figure 4). As noted above, data were either not available or there were too few studies to perform additional subanalyses. For example, while there were two studies performed in East Asian populations, meta-regression for Eastern versus Western geography could not be performed since one was a cross-sectional study38 and the other a case-control study.42
Figure 4:

Meta-regression analysis showing the difference in effect estimates between prospective and retrospective study designs, with prospective studies less likely to show an association between H pylori exposure and eosinophilic esophagitis
Publication bias and heterogeneity
A funnel plot was generated to assess for publication bias. The symmetric distribution of this plot suggested no publication bias (Supplemental Figure 3). Egger’s test confirmed no publication bias (P-value=0.77). Statistical tests of heterogeneity demonstrated moderate heterogeneity for our primary outcome analysis (I2 = 57.9%, chi-squared test P=0.02) and substantial heterogeneity for the secondary outcome analysis (I2 = 69.3%, chi-squared test P<0.001). Removing the Dellon et al. study from the analyses improved the heterogeneity estimates for the secondary analysis (I2 =50.6%), which was downgraded from substantial to moderate heterogeneity, but did not substantially affect the primary analysis estimate (I2 = 58.5%).
Risk of Bias Assessment according to the Newcastle Ottawa Scale (NOS)
All case-control and cross-sectional studies were rated as high-quality. The two cohort studies34,35 were rated fair-quality, mainly driven by insufficient data for adequate assessment of the comparability parameter. (Supplemental Table 1a and 1b) Removing these two studies and performing a sensitivity analysis limited to only high-quality studies based on NOS score ≥6 did not alter our findings. (Supplemental Figure 5).
DISCUSSION
In this meta-analysis of nearly 378,000 people tested for H pylori, we found that H pylori exposure was associated with a lower likelihood of EoE (and EoE/EE). The directionality of association was consistently observed across pediatric only versus adult populations, US versus non-US geography, low versus moderate-high H pylori prevalence areas, and time period of patient recruitment. Notably, the magnitude and directionality of the protective association between H pylori and EoE was preserved independent of cohort versus case-control study design, although there was a trend towards prospective studies being less likely to demonstrate this association. We acknowledge heterogeneity with respect to some definitions, H pylori testing modality, treatment details, and endoscopic protocols; however, these would expectedly result in nondifferential misclassification and bias towards a null association between H pylori and EoE.
EoE represents a major health burden both in terms of patient morbidity and cost to the healthcare system, with a recent population-based analysis estimating that EoE accounts for $1.4 billion of healthcare spending annually.43 Rates of EoE, among other immune-mediated diseases, continue to escalate not only in Western industrialized countries but also in geographic locations such as East Asia and countries with recently developed economies, where the disease was previously nonexistent.3 The sharp trajectory suggests that a relatively abrupt change in environmental exposures is most responsible for the observed trend, as opposed to a shift in genetic predisposition; this is further supported by twin and familial studies showing that shared environmental exposures more so than genetic factors explain the heritability of EoE.44 Elucidating environmental protective and predisposing determinants potentially offers mechanistic clues that, ideally, can be leveraged for prevention and treatment. Substantive epidemiologic data, including the present comprehensive analysis, and indirect evidence in experimental models of other immune- and allergic-mediated disease implicate H pylori as a protective factor. H pylori has co-evolved with humans for over 100,000 years45,46 and some protective benefit is evolutionarily expected. Two separate population-based analyses from the US47 and the Netherlands48 place the time period for the change in environmental exposure about 40–50 years ago, which corresponds to decreasing H pylori rates as a result of active eradication efforts and industrialization. Operating under the ‘hygiene hypothesis’, factors such as improved socioeconomic status, sanitation efforts, cleaner water sources, and industrialization are cited as leading factors underlying the rise in immune-mediated and allergic diseases, with H pylori argued to be merely a surrogate of these factors. While certainly contributory, these factors are unlikely to fully account for the inverse association between H pylori and EoE, as this association was preserved among Western industrialized countries, post-2007, and irrespective of baseline H pylori prevalence in our study, even after performing a sensitivity analysis removing the Dellon et al. study to account for the potential of overlapping cases with the Sonnenberg et al. study. However, our conclusions do differ from the recent prospective, multicenter case-control study by Molina-Infante and colleagues, which found a null association between H pylori and EoE (OR 0.97, 95% CI: 0.73–1.30) based on 808 patients. As the first prospective case-control study on this topic, their study had other strengths in addition to the prospective study design and multicenter recruitment, such as inclusion of only patients with EoE who were naïve to therapy (e.g. PPI), a standardized endoscopic and biopsy protocol, and testing for H pylori off PPI therapy. That said, it is plausible that bias might at least partially account for the null findings, as control patients were selected based on esophageal symptoms, and many also more commonly had esophageal pathology, such as reflux esophagitis. This is relevant because H pylori has been inversely associated with GERD and presence and severity of erosive esophagitis.49,50 Ultimately, it remains inconclusive as to whether infection with H pylori itself biologically protects against EoE. Our meta-analysis findings that support a protective benefit of H pylori exposure against EoE and thus oppose the findings of the only prospective case-control study highlight the need not only for additional large, prospective investigations, but also for investigations to define mechanisms underlying these observations.
Indeed, experimental data support the protective immunoregulatory phenotype of H pylori.13,16–20 This protection occurs through H pylori-induced alterations in signaling pathways that are similarly implicated in IBD, asthma, and allergy, such as attenuation of inflammatory Th1 and Th17 signaling pathways with or without upregulation of Th2 or T-regulatory pathways.13,17,20 Preliminary data further implicate specific H pylori proteins, including CagA, VacA and gamma-glutamyl transpeptidase.15,51–54 Unfortunately, no included studies specifically reported serologic responses to H pylori specific proteins. Timing of H pylori exposure might also be an important mediator of protection. At least in experimental models of allergy, neonatal H pylori exposure conferred the highest immunotolerance.17,55 No studies commented on the duration of H pylori infection, so we were unable to evaluate the impact of timing of H pylori exposure and magnitude of protection against EoE. We do acknowledge that temporal relationship cannot be definitively established from our study. That said, primary H pylori infection most often occurs during early childhood, particularly in endemic areas, with incidence rates of infection as an adult estimated to be <1% per year.56–58 While adjustment for studies with pediatric only versus adult only populations did not alter our effect estimate significantly, insufficient power is a consideration since only two studies were performed exclusively in pediatric populations (0.3% of our study population, N=1008/377,975). A prospective study detailing timing of H pylori infection and cumulative exposure on risk of subsequent EoE, while the ideal study, is of course logistically limited by time, resources, and the rarity of EoE as an outcome (and potentially an even rarer outcome with H pylori exposure).
The present study has several strengths, including a large sample size across multiple geographies, comprehensive search strategy, and several relevant meta-regression analyses and sensitivity analyses, with consistent results overall and moderate heterogeneity. By including only patients who had undergone H pylori testing, we limited indication bias. Apart from inherent limitations of meta-analyses, our study has the following additional limitations. The lack of information on medication, and specifically PPI use at the time of endoscopy is a potential confounder for the two studies that reported data based on a US population-based pathology database32,40. Between 2008–2015, the time period of enrollment for these studies, many US practitioners prescribed PPIs, which could suppress detection of H pylori59, as first-line therapy for suspected EoE and which potentially could also increase the falsely negative diagnoses of EoE. Differences in biopsy protocol might also contribute to differences in sensitivity of H pylori detection and false negative diagnoses. Secondly, we were unable to account for potential confounders including socioeconomic status and early life exposures, although we would expect any unmeasured confounders to bias towards a null association between H pylori and EoE. Interestingly, we did not see a difference in our effect estimates between low and moderate-high prevalence H pylori populations, populations which presumably would have different environmental exposures. Of note, several studies found a very low prevalence of H pylori overall, which was universally lower in patients with versus without EoE (or EE) (Table 1). The two population-based studies from a large US pathology database reported an overall H pylori prevalence of approximately 7%, which is significantly lower than estimates for the US based on a recent comprehensive meta-analysis of global H pylori prevalence (pooled prevalence for general US population 35.6%, 95% CI: 30.0% – 41.1%).11 The reasons for this discrepancy are unclear, but might reflect the combination of lack of data on PPI use, variability in biopsy protocol and sensitivity of H pylori detection, perhaps on the background of decreasing H pylori prevalence, as has been described for developed countries.22,60–62 Thirdly, we are unable to determine the effect of active versus former infection or the effect of H pylori treatment on risk of EoE (which relates to the timing of infection and duration of cumulative H pylori exposure). Nine of the included studies did not specifically state if H pylori eradication therapy had been previously prescribed, but three of these studies did include H pylori serologic analysis, which would detect previous H pylori exposure and thus potentially a more immunotolerant phenotype and lower risk of EoE.
In conclusion, by performing a meta-analysis of 11 observational studies, we found that H pylori might be associated with decreased odds of EoE, an observation which was preserved across geographies, H pylori prevalence, pediatric versus adult populations, patient recruitment time period and primary study design. The limitations we have noted are difficult to overcome with observational study designs, particularly those constructed retrospectively. While robust prospective trials are ideal, these are logistically limited. Studies in experimental models of EoE would further clarify the putative role of H pylori in EoE pathophysiology by defining mechanisms active in the early phase of disease and protective pathways that, in the future, might be leveraged for clinical benefit.
Supplementary Material
Supplemental Figure 1: Odds of eosinophilic esophagitis in H pylori exposed versus non-exposed individuals (sensitivity analysis with Dellon et al. 2011 study removed)
Supplemental Figure 2: Odds of eosinophilic esophagitis or esophageal eosinophilia in H pylori exposed versus non-exposed individuals (sensitivity analysis with Dellon et al. 2011 study removed)
Supplemental Figure 3: Funnel plot of standard error by log odds ratio showing no publication bias based on symmetric distribution
Supplemental Figures 4a-4e: Meta-regression analyses, a. Study Type (Cohort / cross-sectional vs. Case-control), b. Geography (US vs. Non-US), c. Adult vs Pediatric Only Study Population, d. H pylori Prevalence (Low vs. Moderate-high), e. Time period of Patient Recruitment (Pre-2007 vs. Post-2007)
Supplemental Figure 5–: Odds of eosinophilic esophagitis or esophageal eosinophilia in H pylori exposed versus non-exposed individuals, sensitivity analysis limited to high-quality studies only (based on risk of bias assessment according to the Newcastle Ottawa Scale ≥6)
Grant Support:
none
Abbreviations:
- CI
confidence interval
- Eos
eosinophils
- EoE
eosinophilic esophagitis
- EE
esophageal eosinophilia
- GERD
gastroesophageal reflux disease
- Hpf
high power field
- H pylori
Helicobacter pylori
- IBD
inflammatory bowel disease
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- PPI
proton-pump inhibitor
- US
United States
Footnotes
Disclosures/conflict of interest statement: The authors have no potential conflicts (financial, professional, nor personal) that are relevant to this manuscript.
Writing assistance: No additional writing assistance was used for this manuscript
References
- 1.Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978; 74: 1298–301. [PubMed] [Google Scholar]
- 2.Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 1977; 72: 1312–6. [PubMed] [Google Scholar]
- 3.Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018; 154: 319–332.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBrosse CW, Collins MH, Buckmeier Butz BK, et al. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol 2010; 126: 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131: 788–92. [DOI] [PubMed] [Google Scholar]
- 6.Kreiner E, Waage J, Standl M, et al. Shared genetic variants suggest common pathways in allergy and autoimmune diseases. J Allergy Clin Immunol 2017; 140: 771–81. [DOI] [PubMed] [Google Scholar]
- 7.Agmon-Levin N, Lian Z, Shoenfeld Y. Explosion of autoimmune diseases and the mosaic of old and novel factors. Cell Mol Immunol 2011; 8: 189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada H, Kuhn C, Feillet H, Bach JF. The “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010; 160: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuo T, Kamm MA, Colombel J-F, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2018; 15: 440–52. [DOI] [PubMed] [Google Scholar]
- 10.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet 2018; 390: 2769–78. [DOI] [PubMed] [Google Scholar]
- 11.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017; 153: 420–9. [DOI] [PubMed] [Google Scholar]
- 12.Rokkas T, Gisbert JP, Niv Y, O’Morain C. The association between Helicobacter pylori infection and inflammatory bowel disease based on meta-analysis. United European Gastroenterol J 2015; 3: 539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold IC, Hitzler I, Müller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol 2012; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 2007; 167: 821–7. [DOI] [PubMed] [Google Scholar]
- 15.Kyburz A, Urban S, Altobelli A, et al. Helicobacter pylori and its secreted immunomodulator VacA protect against anaphylaxis in experimental models of food allergy. Clin Exp Allergy 2017; 47: 1331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N, Murata-Kamiya N, Yanagiya K, et al. Mutual reinforcement of inflammation and carcinogenesis by the Helicobacter pylori CagA oncoprotein. Sci Rep 2015; 5: 10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest 2011; 121: 3088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owyang SY, Luther J, Owyang CC, Zhang M, Kao JY. Helicobacter pylori DNA’s anti-inflammatory effect on experimental colitis. Gut Microbes 2012; 3: 168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engler DB, Leonardi I, Hartung ML, et al. Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm Bowel Dis 2015; 21: 854–61. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y-Z, Tan G, Wu F, Zhi F-C. H. pylori attenuates TNBS-induced colitis via increasing mucosal Th2 cells in mice. Oncotarget 2017; 8: 73810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabin RL, Levinson AI. The nexus between atopic disease and autoimmunity: a review of the epidemiological and mechanistic literature. Clin Exp Immunol 2008; 153: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina-Infante J, Gutierrez-Junquera C, Savarino E, et al. Helicobacter pylori infection does not protect against eosinophilic esophagitis: results from a large multicenter case-control study. Am J Gastroenterol 2018; 113: 972–9. [DOI] [PubMed] [Google Scholar]
- 23.Raghunath A, Hungin APS, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ 2003; 326: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (version 5.0.1) London: The Cochrane Collaboration, 2011. [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 27.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology 2018; 155: 1022–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed Nov 7, 2018).
- 29.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013; 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141: 1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo MK, Park J-J, Kim S-H, et al. Prevalence and endoscopic features of eosinophilic esophagitis in patients with esophageal or upper gastrointestinal symptoms. J Dig Dis 2012; 13: 296–303. [DOI] [PubMed] [Google Scholar]
- 34.Norder Grusell E, Dahlén G, Ruth M, Bergquist H, Bove M. The cultivable bacterial flora of the esophagus in subjects with esophagitis. Scand J Gastroenterol 2018; 53: 650–6. [DOI] [PubMed] [Google Scholar]
- 35.Cheung KM, Oliver MR, Cameron DJS, Catto-Smith AG, Chow CW. Esophageal eosinophilia in children with dysphagia. J Pediatr Gastroenterol Nutr 2003; 37: 498–503. [DOI] [PubMed] [Google Scholar]
- 36.Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56: 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sealock RJ, Kramer JR, Verstovsek G, et al. The prevalence of oesophageal eosinophilia and eosinophilic oesophagitis: a prospective study in unselected patients presenting to endoscopy. Aliment Pharmacol Ther 2013; 37: 825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma X, Xu Q, Zheng Y, et al. Prevalence of esophageal eosinophilia and eosinophilic esophagitis in adults: a population-based endoscopic study in Shanghai, China. Dig Dis Sci 2015; 60: 1716–23. [DOI] [PubMed] [Google Scholar]
- 39.Elitsur Y, Alrazzak BA, Preston D, Demetieva Y. Does Helicobacter pylori protect against eosinophilic esophagitis in children? Helicobacter 2014; 19: 367–71. [DOI] [PubMed] [Google Scholar]
- 40.Sonnenberg A, Dellon ES, Turner KO, Genta RM. The influence of Helicobacter pylori on the ethnic distribution of esophageal eosinophilia. Helicobacter 2017; 22 DOI: 10.1111/hel.12370. [DOI] [PubMed] [Google Scholar]
- 41.von Arnim U, Wex T, Link A, et al. Helicobacter pylori infection is associated with a reduced risk of developing eosinophilic oesophagitis. Aliment Pharmacol Ther 2016; 43: 825–30. [DOI] [PubMed] [Google Scholar]
- 42.Furuta K, Adachi K, Aimi M, et al. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr 2013; 53: 60–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol 2015; 110: 626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander ES, Martin LJ, Collins MH, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol 2014; 134: 1084–1092.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breurec S, Raymond J, Thiberge J-M, et al. Impact of human migrations on diversity of Helicobacter pylori in Cambodia and New Caledonia. Helicobacter 2013; 18: 249–61. [DOI] [PubMed] [Google Scholar]
- 46.Kodaman N, Pazos A, Schneider BG, et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc Natl Acad Sci U S A 2014; 111: 1455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014; 12: 589–96.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Rhijn BD, Verheij J, Smout AJPM, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil 2013; 25: 47–52.e5. [DOI] [PubMed] [Google Scholar]
- 49.Chourasia D, Misra A, Tripathi S, Krishnani N, Ghoshal UC. Patients with Helicobacter pylori infection have less severe gastroesophageal reflux disease: a study using endoscopy, 24-hour gastric and esophageal pH metry. Indian J Gastroenterol 2011; 30: 12–21. [DOI] [PubMed] [Google Scholar]
- 50.Richter JE, Falk GW, Vaezi MF. Helicobacter pylori and gastroesophageal reflux disease: the bug may not be all bad. Am J Gastroenterol 1998; 93: 1800–2. [DOI] [PubMed] [Google Scholar]
- 51.Oertli M, Noben M, Engler DB, et al. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A 2013; 110: 3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricci V, Giannouli M, Romano M, Zarrilli R. Helicobacter pylori gamma-glutamyl transpeptidase and its pathogenic role. World J Gastroenterol 2014; 20: 630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wüstner S, Anderl F, Wanisch A, et al. Helicobacter pylori γ-glutamyl transferase contributes to colonization and differential recruitment of T cells during persistence. Sci Rep 2017; 7: 13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lina TT, Pinchuk IV, House J, et al. CagA-dependent downregulation of B7-H2 expression on gastric mucosa and inhibition of Th17 responses during Helicobacter pylori infection. J Immunol 2013; 191: 3838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnold IC, Lee JY, Amieva MR, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 2011; 140: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cullen DJ, Collins BJ, Christiansen KJ, et al. When is Helicobacter pylori infection acquired? Gut 1993; 34: 1681–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valle J, Kekki M, Sipponen P, Ihamäki T, Siurala M. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand J Gastroenterol 1996; 31: 546–50. [DOI] [PubMed] [Google Scholar]
- 58.Imrie C, Rowland M, Bourke B, Drumm B. Is Helicobacter pylori infection in childhood a risk factor for gastric cancer? Pediatrics 2001; 107: 373–80. [DOI] [PubMed] [Google Scholar]
- 59.Genta RM, Lash RH. Helicobacter pylori-negative gastritis: seek, yet ye shall not always find. Am J Surg Pathol 2010; 34: e25–34. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen T, Ramsey D, Graham D, et al. The Prevalence of Helicobacter pylori Remains High in African American and Hispanic Veterans. Helicobacter 2015; 20: 305–15. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe M, Ito H, Hosono S, et al. Declining trends in prevalence of Helicobacter pylori infection by birth-year in a Japanese population. Cancer Sci 2015; 106: 1738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agréus L, Hellström PM, Talley NJ, et al. Towards a healthy stomach? Helicobacter pylori prevalence has dramatically decreased over 23 years in adults in a Swedish community. United European Gastroenterol J 2016; 4: 686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Odds of eosinophilic esophagitis in H pylori exposed versus non-exposed individuals (sensitivity analysis with Dellon et al. 2011 study removed)
Supplemental Figure 2: Odds of eosinophilic esophagitis or esophageal eosinophilia in H pylori exposed versus non-exposed individuals (sensitivity analysis with Dellon et al. 2011 study removed)
Supplemental Figure 3: Funnel plot of standard error by log odds ratio showing no publication bias based on symmetric distribution
Supplemental Figures 4a-4e: Meta-regression analyses, a. Study Type (Cohort / cross-sectional vs. Case-control), b. Geography (US vs. Non-US), c. Adult vs Pediatric Only Study Population, d. H pylori Prevalence (Low vs. Moderate-high), e. Time period of Patient Recruitment (Pre-2007 vs. Post-2007)
Supplemental Figure 5–: Odds of eosinophilic esophagitis or esophageal eosinophilia in H pylori exposed versus non-exposed individuals, sensitivity analysis limited to high-quality studies only (based on risk of bias assessment according to the Newcastle Ottawa Scale ≥6)


