Abstract
Background
Exudative pleural effusion (EPE) is a common diagnostic challenge. The utility of medical thoracoscopy (MT) and closed pleural biopsy (CPB) to aid in the diagnosis of EPE has been reported in many published studies. Herein, we perform a systematic review and meta-analysis to compare the diagnostic yield and safety of CPB and MT in EPE.
Methods
Four databases were searched for studies reporting the diagnostic yield of CPB and MT for EPE. The quality of the included studies was evaluated according to the quality assessment of diagnostic accuracy studies (QUADAS) tool. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and complication risks were compared between the two groups.
Results
Ten studies dealing with CPB and twenty-three studies dealing with MT for the diagnosis of EPE were included in this meta-analysis. Pooled sensitivity, specificity, PLR, NLR and DOR of CPB group was 77%, 99%, 32.55, 0.22, 165.71, respectively, while pooled sensitivity, specificity, PLR, NLR and DOR of MT group was 93%, 100%, 10.82, 0.08, 162.81, respectively. The area under the summary receiver operating characteristic (SROC) curve of CPB and MT were both 0.97. The ability of CPB to diagnose non-malignant diseases was like MT (69% vs. 68%), while the ability was lower than that of MT to diagnose malignant diseases (72% vs. 92%). The pooled diagnostic accuracy of CPB and MT for mesothelioma was 26% (95% CI, 14–38%) and 42% (95% CI, 22–62%) (P<0.001), respectively. The rate of complications with CBP was lower than that reported for MT.
Conclusions
CBP is a relatively accurate tool with a lower complication rate compared to MT in the diagnosis of EPE, especially in diagnosing non-malignant diseases. We confirm the utility of MT in the diagnostic workup of malignancy (especially mesothelioma); however, in selected cases, CPB could be used as the first diagnostic approach with a favorable safety profile.
Keywords: Closed pleural biopsy (CPB), medical thoracoscopy (MT), exudative pleural effusion (EPE), diagnosis, meta-analysis
Introduction
Pleural effusion, referring to the abnormal accumulation of fluid in the pleural cavity, is potentially caused due to several reasons. For example, malignancies (either primitive or secondary pleural tumors), infections like tuberculosis or pneumonia, systemic conditions like in the case of congestive heart failure, renal failure, and connective tissue disorders, exposure to drugs and pulmonary embolism (1). When a unilateral pleural effusion is detected, an extensive investigation with non-invasive and invasive procedures must manage the patient correctly (2). At least 15–20 percent of all pleural effusions are still undiagnosed despite extensive clinical work (3). The simplest and least invasive way to establish the diagnosis of undiagnosed exudative pleural effusion (EPE) is thoracocentesis with pleural fluid cytology and microbiological analysis, but this procedure has a high rate of false-negative results, with a sensitivity of around 60% (range, 40–87%) thus especially in patients with a high suspicious of neoplastic or infectious disease further exams have to be considered (4).
Thus, the next step in undiagnosed EPE is to obtain pleural tissue samples for pathological and microbiological examination. Currently, there are two ways to obtain tissue samples of the pleura, closed pleural biopsy (CPB) or medical thoracoscopy (MT). CPB was firstly described in 1955 by De Francis and co-workers (5,6) and it consists of taking a transthoracic biopsy of the pleura and a diagnostic accuracy of slightly less than 60% if the procedure is performed blindly, but this percentage rises if the pleural biopsy is taken image-guided (ultrasound- and CT scan-guided) (7). It can be performed in an outpatient setting, but limitations of the procedure are that multiple biopsies could not be done and abnormalities in the apical or diaphragmatic region are difficult to be biopsied; complications of CPB are a pain, pneumothorax, vasovagal reaction, haemothorax, hematoma, and transient fever. MT has its roots in the early 1900s: with this technique, most of the pleural cavity can be overseen, allowing pleural biopsy under direct visualization and also therapeutic interventions such as talc pleurodesis. MT has a diagnostic yield ranging from 91% to 95% for malignant pleural effusions (MPE) and 100% for tuberculous pleural effusions (TPE) (8), so it is currently considered as the gold standard (9). However, because of the prohibitive cost, need for specialized equipment, and the need for specialized training of physicians and medical staff, thoracoscopy is not widely available in community health centers.
At present, many published studies have described the diagnostic utility of CPB and MT, but with some inconsistent results that make it difficult to draw firm conclusions. Therefore, in order to increase statistical power and reduce uncertainty, we performed a systematic review of CPB and MT in patients with EPE and compared the overall diagnostic yield and safety of CPB and MT in the diagnosis of EPE using a meta-analytic approach.
Methods
Search strategy
The literature search was independently conducted by two investigators (Y Wei and K Shen) who searched four databases (PubMed, Web of Science, Cochrane Library and Embase) for studies that described the diagnostic value and safety of CPB or MT, and also searched for references of the included studies to identify other relevant studies. The following terms were used in the literature search: “closed pleural biopsy”, “CPB”, “medical thoracoscopy”, “MT”, “exudative pleural effusion”, “EPE”, “indeterminate pleural effusion”, “undiagnosed exudative pleural effusion”, “pleural biopsy”, “pleuroscope”, “thoracoscope”, “diagnostic accuracy”, “sensitivity and specificity”. Literature citations within selected studies were also searched to find other potentially relevant studies. The search included studies published from January 2000 to July 2018.
Eligibility criteria
Studies that met the following criteria were included: (I) prospective or retrospective studies that enrolled at least 20 consecutive patients; (II) studies providing the diagnostic yield and complications of CPB and/or MT for the diagnosis of indeterminate pleural effusion; (III) full text available in the English language; (IV) when the results of the same study were reported in different articles, only the latest and complete data were included in this meta-analysis; (V) the search was focused on researches that had been conducted in humans. The following type of studies was excluded: (I) conference abstracts, review articles, case report and letters to journal editors; (II) studies describing CPB and/or MT in <20 patients; and (III) studies that did not provide sufficient data for statistical analysis. Two reviewers (Y Wei and K Shen) independently assessed the eligibility of enrolled study. Any disagreement was resolved by discussion between the reviewers.
Literature review and data extraction
Study retrieval were conducted by two independent reviewers, and disagreements were resolved by consensus. First, a review of the title and abstract was initially conducted to identify potentially relevant articles, then the eligibility by reading the full text of the potentially relevant articles was evaluated; lastly, we also searched for references of the included articles to identify other relevant studies. Two authors (Y Wei and K Shen) independently extracted the data into a standard data extraction form. The extracted items included publication details such as authors, year of publication, and country where the study was conducted, type of study (prospective or retrospective), number of enrolled patients, inclusion criteria, true positive (TP), false positive (FP), true negative (TN), false negative (FN) from each study and complications.
Assessment of study quality
The quality assessment of diagnostic accuracy studies (QUADAS) tool was used (10). The QUADAS test can result in a maximum score of 14, in which a score of 1 is given when a criterion is fulfilled, 0 if a criterion is unclear, and −1 if the criterion is not achieved. Two reviewers (Y Wei and K Shen), working independently, assessed the quality of selected papers according to empirical evidence, expert opinion, and formal consensus (11).
Statistical analysis
Statistical software packages Meta-Disc (Version 1.4, XI Cochrane Colloquium; Barcelona, Spain) and STATA version 12.0 software (Stata Corporation College Station, TX, USA) were used to perform the statistical analysis.
Determination of the pooled effect
Meta-analysis was done by calculating the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR; PLR/NLR) for each study. The sensitivity, specificity, PLR, and NLR were pooled using the DerSimonian-Laird random-effects model, while DOR was pooled using the fixed effects model to derive a pooled estimate with 95% confidence intervals (CI). What would be considered a good diagnostic test would have a PLR >10 and NLR <0.1. Also, a summary receiver operating characteristic curve (SROC) was constructed using the random effects model. The area under the curve (AUC) was computed by the numeric integration of the curve equation by the trapezoidal method. A perfect test will have values of AUC close to 1 while poor test has AUC close to 0.5.
Assessment of Heterogeneity and estimation of publication bias
Heterogeneity for the individual outcomes was assessed by using the I2 test, which measures the extent of inconsistency among the results of the studies. An I2 value of ≥50% indicates significant heterogeneity. Heterogeneity was also assessed by using the Cochran Q statistic. A P value <0.1 was significant. A fixed-effect model (FEM) was used in the analyses for which no significant heterogeneity was found among different studies; otherwise, the random effects model (REM) was applied. Funnel plots and the Deek test were used to investigate the potential presence of publication bias.
Results
Study Selection and characteristics of included studies
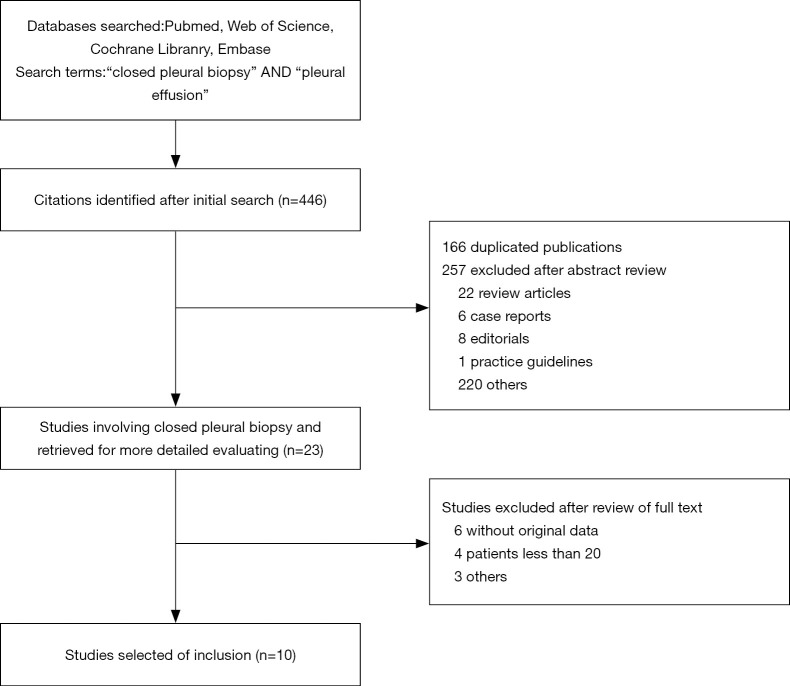
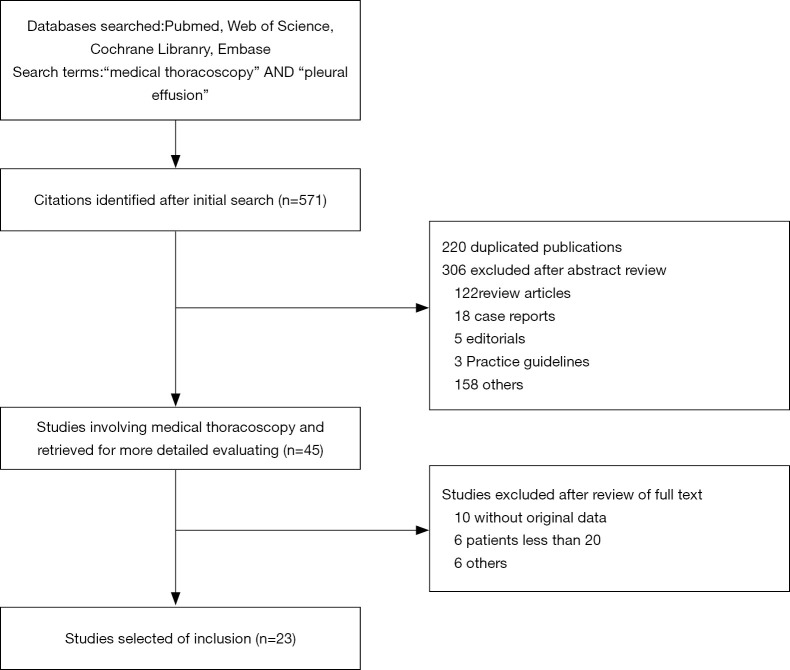
The search and selection flow diagram of studies on CPB and MT are shown in Figures 1 and 2, respectively. For CPB, our initial database search yielded a total of 446 citations (Figure 1) from which 10 studies on CPB ultimately met our inclusion criteria and were selected for meta-analysis (2,7,12-19); for MT, our initial database search yielded a total of 571 citations (Figure 2) from which 23 studies on MT ultimately met our inclusion criteria and were selected for meta-analysis (1,8,20-40). Tables 1 and 2 show the basic features of the studies selected for analysis as well as their QUADAS scores. Among the 10 CPB publications on CPB, 4 studies are retrospective, and 6 are perspective (included one randomized controlled trial). Nine studies were conducted at single centers, and one study was multicentric. Among the 23 published studies on MT, 12 studies are retrospective, and 11 are perspective (included two randomized controlled trials). Eighteen studies were conducted at single centers, and five studies were multicentric.
Figure 1.
Search and selection flow diagram of studies on closed pleural biopsy.
Figure 2.
Search and selection flow diagram of studies on medical thoracoscopy.
Table 1. Characteristics of included studies on closed pleural biopsy.
| Author [year] | Country/region | Number of centres |
Type of study | No. of patients | Inclusion criteria | Q score | TP | FP | FN | TN | No. of complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nusair et al. [2002] | Israel | Unicentric | Retrospective | 44 | Patients with an exudative pleural effusion with no accompanying pleural or subpleural thickening ormass | 5 | 13 | 0 | 10 | 19 | 3 |
| Al-Shimemeri et al. [2003] | Saudi Arabia | Unicentric | Retrospective | 110 | Patients with an exudative pleural effusion | 6 | 54 | 0 | 32 | 24 | 4 |
| Maskell et al. [2003] | UK | Unicentric | RCT | 25 | Patients with cytologically negative suspected malignant pleural effusions | 7 | 15 | 0 | 9 | 1 | 1 |
| Botana-Rial et al. [2013] | Spain | Unicentric | Prospective | 67 | Patients with an exudative pleural effusion | 4 | 48 | 0 | 6 | 13 | 1 |
| Jakubec et al. [2014] | Olomouc | Unicentric | Prospective | 222 | Patients with pleural effusion and cytologic examination of pleural fluid was negative | 7 | 99 | 0 | 58 | 65 | 22 |
| Devkota et al. [2014] | Nepal | Unicentric | Prospective | 47 | Patients with an exudative pleural effusion | 6 | 28 | 0 | 3 | 16 | – |
| Maturu et al. [2015] | India | Unicentric | Retrospective | 84 | Patients with exudative pleural effusions and remain undiagnosed after thoracentesis | 7 | 63 | 0 | 13 | 8 | 7 |
| Báez-Saldaña et al. [2017] | México | Unicentric | Prospective | 863 | Diagnosing malignancy in patients with pleural effusion | 5 | 450 | 5 | 137 | 271 | 38 |
| Zuberi et al. [2016] | Pakistan | Multicentric | Prospective | 94 | Patients with an exudative pleural effusion | 7 | 74 | 0 | 2 | 20 | – |
| Rajawat et al. [2017] | India | Unicentric | Retrospective | 191 | Patients with exudative lymphocytic pleural effusion | 4 | 123 | 0 | 13 | 55 | – |
TP, true positive; FP, false positive; FN, false negative; TN, true negative; RCT, randomized controlled trial.
Table 2. Characteristics of included studies on medical thoracoscopy.
| Author [year] | Country | Number of centres | Type of study | No. of patients | Inclusion criteria | Q score | TP | FP | FN | TN | No. of complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al. [2007] | USA | Unicentric | Prospective | 51 | Patients with indeterminate pleural effusions | 6 | 49 | 0 | 2 | 0 | 13 |
| Munavvar et al. [2007] | UK | Unicentric | Prospective | 55 | Pleural effusions undiagnosed after thoracentesis | 6 | 49 | 0 | 5 | 1 | 0 |
| Wang et al. [2008] | China | Unicentric | Retrospective | 27 | Patients with undiagnosed pleural effusions | 6 | 24 | 0 | 2 | 1 | 4 |
| Ng et al. [2008] | Malaysia | Unicentric | Retrospective | 22 | Patients with undiagnosed pleural effusions | 8 | 14 | 0 | 2 | 6 | 4 |
| Sasada et al. [2009] | Japan | Unicentric | Prospective | 20 | Pleural effusions undiagnosed after one thoracentesis and pleural biopsy | 6 | 12 | 0 | 8 | 0 | 0 |
| Xie et al. [2009] | China | Unicentric | Retrospective | 30 | Pleural effusions undiagnosed after thoracentesis | 5 | 30 | 0 | 0 | 0 | 0 |
| Ishida et al. [2009] | Japan | Unicentric | Prospective | 45 | Pleural effusions undiagnosed after thoracentesis | 5 | 44 | 0 | 1 | 0 | 1 |
| Kannan et al. [2009] | Malaysia | Multicentric | Prospective | 61 | Pleural effusions undiagnosed after thoracentesis | 6 | 55 | 0 | 6 | 0 | 1 |
| Mohan et al. [2010] | UK | Unicentric | Retrospective | 160 | Pleural effusions undiagnosed after thoracentesis | 5 | 143 | 0 | 13 | 4 | 6 |
| Huang et al. [2011] | China | Unicentric | Retrospective | 47 | Patients with pleural effusion and thickening of unknown etiology | 7 | 44 | 0 | 0 | 3 | 5 |
| Davies et al. [2010] | UK | Unicentric | Retrospective | 142 | Patients who underwent medical thoracoscopy | 7 | 98 | 0 | 5 | 39 | / |
| Metintas et al. [2010] | Turkey | Unicentric | RCT | 51 | patients with exudative Pleural effusion that could not be diagnosed by cytologic analysis | 9 | 40 | 0 | 2 | 9 | 25 |
| Khan et al. [2012] | UK | Multicentric | Retrospective | 66 | Patients with unilateral exudative pleural effusions | 8 | 62 | 0 | 4 | 0 | 8 |
| Prabhu et al. [2012] | India | Multicentric | Prospective | 68 | Pleural effusions undiagnosed after thoracentesis | 5 | 65 | 0 | 2 | 1 | 4 |
| Rozman et al. [2013] | Slovenia | Unicentric | Prospective | 41 | Pleural effusions undiagnosed after less invasive means | 7 | 40 | 0 | 1 | 0 | 4 |
| Dhooria et al. [2014] | India | Unicentric | RCT | 82 | Patients with undiagnosed exudative pleural effusions | 7 | 50 | 0 | 21 | 11 | 25 |
| Willendrup et al. [2014] | Denmark | Multicentric | Retrospective | 56 | Patients with unexplained exudative pleural effusion | 9 | 44 | 0 | 11 | 1 | 2 |
| Gao et al. [2014] | China | Multicentric | Retrospective | 215 | Patients with undiagnosed exudative pleural effusion | 6 | 190 | 0 | 2 | 23 | 16 |
| Kiani et al. [2015] | Iran | Unicentric | Prospective | 300 | Patients with undiagnosed pleural effusions | 6 | 261 | 0 | 4 | 35 | 11 |
| Verma et al. [2015] | Singapore | Unicentric | Retrospective | 41 | Patients with symptomatic pleural effusions | 9 | 36 | 0 | 2 | 3 | 6 |
| Nattusamy et al. [2015] | India | Unicentric | Retrospective | 48 | Patients with undiagnosed pleural effusions | 8 | 32 | 0 | 3 | 13 | 11 |
| Patil et al. [2016] | India | Unicentric | Prospective | 129 | Patients with undiagnosed exudative pleural effusions using rigid thoracoscope | 7 | 110 | 0 | 16 | 3 | 17 |
| Kim et al. [2017] | Korea | Unicentric | Retrospective | 26 | Patients who had undergone MT | 8 | 19 | 0 | 2 | 5 | 1 |
TP, true positive; FP, false positive; FN, false negative; TN, true negative; RCT, randomized controlled trial; MT, medical thoracoscopy.
Diagnostic accuracy of studies dealing with CPB and MT
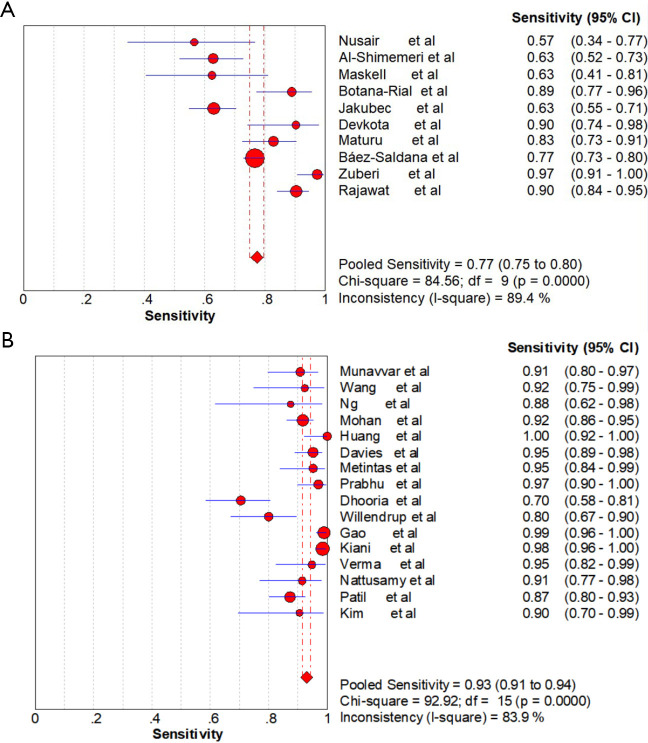
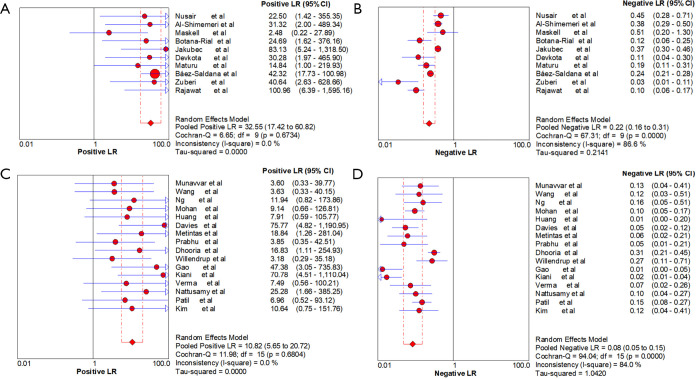
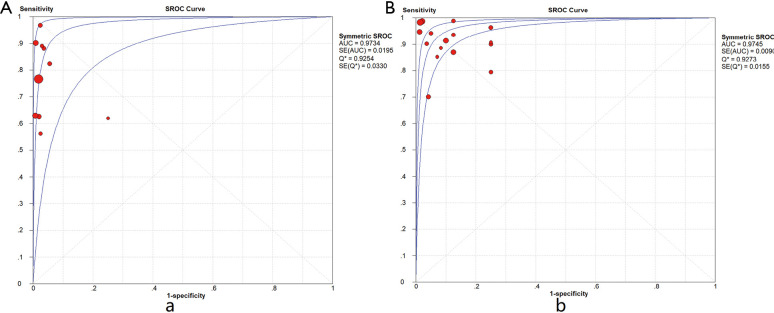
Ten studies (1,747 subjects) described the diagnostic yield of CPB in patients with an EPE. The sensitivity in the diagnosis of undetermined EPE ranged from 57% to 97% (Figure 3A), with the pooled sensitivity being 77% (95% CI, 75–80%). The pooled specificity of the procedure was 99% (Figure S1A). Twenty-three studies (1,783 subjects) described the diagnostic yield of MT in patients with an EPE and Figure 3B depicted the forest plot of sensitivity and showed a pooled sensitivity of 93% (95% CI, 91–94%). The forest plot for specificity was shown in Figure S1B. All the included studies yielded the pooled specificity of MT was 100%. The pooled positive and negative LR of CPB were 32.55 (95% CI, 17.42–60.82) and 0.22 (95% CI, 0.16–0.31) respectively (Figure S2A,S2B), while Figure S2C,S2D illustrated the forest plots of positive and negative LR of MT, pooled positive and negative LR (95% CI) were 10.82 (5.65–20.72) and 0.08 (0.05–0.15), respectively. The diagnostic odds ratio (Figure S3A) of CPB was 165.71 (95% CI, 85.14–322.54) and the pooled diagnostic odds ratio (95% CI) of MT was 162.81 (68.41–387.44) (Figure S3B). Both the area under the SROC curve were 0.97 (Figure S4A,S4B).
Figure 3.
Forest plot of sensitivity analysis. (A) Forest plot of the pooled sensitivity analysis of CPB in undiagnosed exudative pleural effusions. (B) Forest plot of the pooled sensitivity analysis of MT in undiagnosed exudative pleural effusions. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Subgroup analysis
Separate subgroup analyses were conducted in subjects with malignant diseases and non-malignant diseases (tuberculosis, infections and so on), after excluding those studies that do not report the diagnostic accuracy in malignant and non-malignant diseases. The resulted pooled diagnostic accuracy of CPB and MT for non-malignant diseases were 69% (95% CI, 51–88%) and 68% (95% CI, 48–87%) (Figure 4A), respectively, whereas the pooled diagnostic accuracy of CPB and MT for malignant diseases were 72% (95% CI, 62–82%) and 92% (95% CI, 88–95%), respectively (Figure 4B). The ability of CPB to diagnose non-malignant diseases was similar to MT, while the ability was lower than that of MT to diagnose malignant diseases. In addition, another subgroup analysis showed that the pooled diagnostic accuracy of CPB and MT for mesothelioma was 26% (95% CI, 14–38%) and 42% (95% CI, 22–62%) (P<0.001), respectively (Figure 4C).
Figure 4.
Forest plot of subgroup analysis. (A) Forest plot of the pooled diagnostic accuracy of CPB and MT for non-malignant diseases. (B) Forest plot of the pooled diagnostic accuracy of CPB and MT for malignant diseases. (C) Forest plot of the pooled diagnostic accuracy of CPB and MT for mesothelioma. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Heterogeneity and publication bias
Because of significant statistical heterogeneity for the outcomes (sensitivity, NLR), a random effects model was used. No significant statistical heterogeneity for other outcomes (specificity, PLR, DOR) and using a fixed effects model was found. There was no publication bias either on visual inspection of the funnel plot (Figure S5A) or on statistical tests for studies describing CPB (P=0.74). The funnel plots of publication bias for studies describing MT show some asymmetry, and the evaluation of publication bias showed that the Deek test was significant (P=0.00) and (Figure S5B).
Complication rates
The main shortcoming of MT for the undiagnosed EPE was safety. The complication rate of MT in the diagnosis of pleural effusions of undetermined etiology ranged from 2–30% with the pooled rate being 8% (95% CI, 6–11%), which was significantly higher (P<0.001) than that of CPB ranged from 1–10% with the pooled rate being 5% (95% CI, 3–7%) (Figure S6). The most common complication of CPB was pneumothorax, while as for MT, the most common complications were subcutaneous emphysema and fever.
Figure S6.
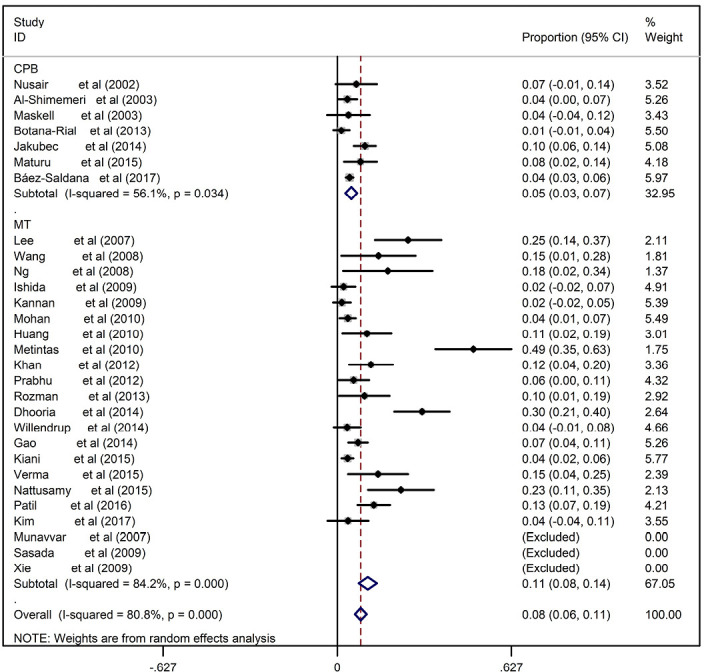
Forest plot of the pooled complication rates of CPB and MT. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Discussion
In this meta-analysis, we compared the overall diagnostic efficacy and safety of CPB and MT in EPE from previously published studies. The results of this meta-analysis (10 studies, 1,747 patients with undiagnosed EPE) suggested that CPB was a relatively accurate tool with pooled sensitivity (77%) and an excellent specificity (99%) in patients with undetermined EPE; the sensitivity was lower than the pooled (23 studies, 1,783 patients) sensitivity of MT (93%). Although the diagnostic accuracy of CPB was not superior to that of MT, the major advantage of CPB over MT was its safety. Our meta-analysis proved that lower pooled complication rates with CPB (5%) compared to MT (8%). Furthermore, in subgroup analysis, the ability of CPB to diagnose non-malignant diseases was like MT (69% vs. 68%), while the ability was lower than that of MT to diagnose malignant diseases (72% vs. 92%). Another subgroup analysis showed that the pooled diagnostic accuracy of CPB and MT for mesothelioma was 26% (95% CI, 14–38%) and 42% (95% CI, 22–62%) (P<0.001), respectively.
Since the early 19th century, thoracentesis (with biochemical, cytological, and microbiological analysis of pleural fluid) has been the standard initial intervention to determine the cause of pleural effusion (41). However, thoracentesis is often non-diagnostic, with the diagnostic yield of pleural fluid cytology varying widely in the published literature, ranging from 40% to 90%. Therefore, to allow for proper treatment of the effusion, other approaches must be considered for those patients with non-diagnostic pleural effusion. In 1955, Defrancis et al. first proposed the use of CPB (without the need for surgery) for the diagnosis of pleural effusion (5). Its gained widespread use for more than 5 decades is due to its ease of operation, low cost, excellent patient tolerance, within a brief period of hospital stay, and low morbidity and mortality. Maturu et al. showed that CPB could establish the diagnosis in 71 (84.5%) patients with EPE (16), and Zuberi et al. reported the overall specific diagnostic yield of CPB is 78.7% (18). However, after the availability of thoracoscopy, the use of CPB for the diagnosis of cytology negative malignant pleural effusion has constantly been decreasing, especially in the developed world. To use MT in the diagnosis of EPE has been widely accepted, as it allows the operator to visualize any pleural abnormalities directly. The 2010 British Thoracic Society (BTS) guidelines recommended MT as the optimal choice for patients with EPE where a diagnostic pleural aspiration is inconclusive, malignancy is suspected, and Abrams needle biopsy has diagnostic value only in areas with a high incidence of tuberculosis (4). However, the advantages of MT must be weighed against the costs of performing such procedure in terms of training, equipment, and skilled operators as well as the need for chest tube drainage after the procedure, which further increases the hospital stay as well as the healthcare cost. There is a large gap between what is recommended and what is available, not only in China but also in most of the developing world (19).
The reported sensitivity of CPB for the diagnosis of malignant pleural effusion is 48–56%, which is lower than that of cytological examination, thoracoscopy remains the gold standard for the diagnosis of MPE (4). Our study also showed that the ability of MT to diagnose malignant diseases was significantly higher than that of CBP. CPB for mesothelioma is typically low diagnostic accuracy in clinical practice. Attanoos et al. (42) evaluated the diagnostic accuracy of closed and open pleural biopsies in diagnosing malignant pleural mesothelioma, they found that definitive diagnostic accuracy of CPB for mesothelioma was low (16%), while open pleural biopsy produced the highest diagnostic accuracy (100% sensitivity, 95% specificity); McLaughlin et al. indicated that a role for CPB in patients with suspected malignant pleural mesothelioma as it correctly identified 44% of unselected cases (43). Our subgroup analysis showed that the pooled diagnostic accuracy of CPB was lower than MT for mesothelioma (26% vs. 42%).
Nevertheless, some studies have suggested that image guided CPB can achieve a diagnostic yield like thoracoscopy. Koegelenberg et al. evaluated the value of ultrasound-guided CPB in patients with undiagnosed EPE and they found that ultrasound-guided biopsy increased the combined yield for all diagnoses from 48.0% to 90.0% (P<0.001), for malignancy from 31.0% to 89.7% (P<0.001) and for tuberculosis from 77.8% to 88.9% (P=0.688) (44). Botana-Rial et al. analyzed patients who underwent CPB with thoracic ultrasound guidance, performed by an experienced pulmonologist, and they concluded that the diagnostic yields of CPB using thoracic ultrasound for tuberculous pleural effusion and MPE were 89.5% and 77.4%, respectively (14). Ultrasound provides detailed information on the extent of the pleural effusion, enables the clinician to select an optimal biopsy site, allows biopsies to be performed in the lower thoracic parietal pleura, and detects other specific pleural abnormalities, which improve the diagnostic yield of biopsy and minimizes potential complications to the most extent. Otherwise, Management of Malignant Pleural Effusions (An Official ATS/STS/STR Clinical Practice Guideline) was published in July 2018, the new guideline recommended that ultrasound imaging be used to guide pleural interventions in patients with known or suspected MPE, the panel agreed that ultrasound guidance has no significant harms associated with its usage, and has an important benefit of reducing pneumothorax rates (45). Ultrasound-assisted CPB has a high diagnostic yield and is safe and can be considered as a first-line diagnostic tool in undiagnosed pleural effusion.
Our meta-analysis has several limitations. Firstly, differences in study design, in enrolled sample size, in patient’s baseline disease status limit the interpretation of the pooled data. Whether the characteristics of enrolled study subjects are fully representative of patients receiving CPB or MT in clinical practice may limit the accuracy and safety of this quantitative analysis. Secondly, some factors influencing the performance of CPB and MT, including the experience of the operator, the number of biopsies obtained, and the type of thoracoscopy (rigid or semi-rigid), further exploration is needed. Finally, studies were limited to those published in English, which may result in potential language bias.
Conclusions
In summary, our systematic review and meta-analysis confirm the values of CPB and MT in the diagnosis of EPE. CPB could be considered a first diagnostic approach or could be the procedure of choice in case of abnormalities at the CT scan or chest ultrasound. MT is paramount to exclude malignancy (especially mesothelioma) or infections, allowing direct visualization of the pleural cavity and therapeutic pleurodesis at the same time.
Figure S1.
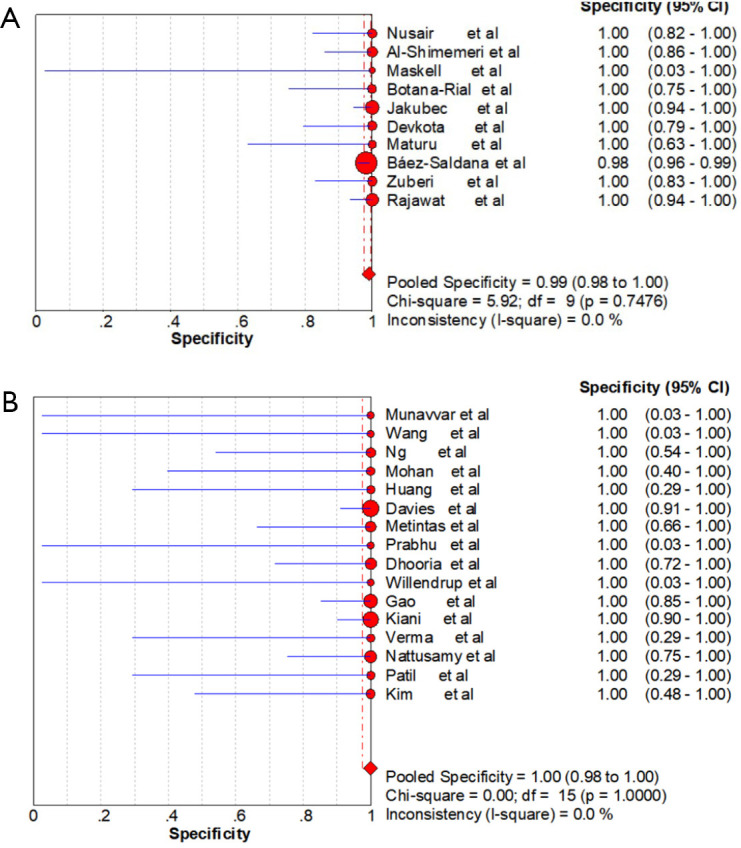
Forest plot of specificity analysis. (A) Forest plot of the pooled specificity analysis of CPB in undiagnosed exudative pleural effusions. (B) Forest plot of the pooled specificity analysis of MT in undiagnosed exudative pleural effusions. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Figure S2.
Forest plot of positive and negative likelihood ratios. (A,B) Forest plot of the pooled positive and negative likelihood ratios of CPB in undiagnosed exudative pleural effusions. (C,D) Forest plot of the pooled positive and negative likelihood ratios of MT in undiagnosed exudative pleural effusions. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Figure S3.
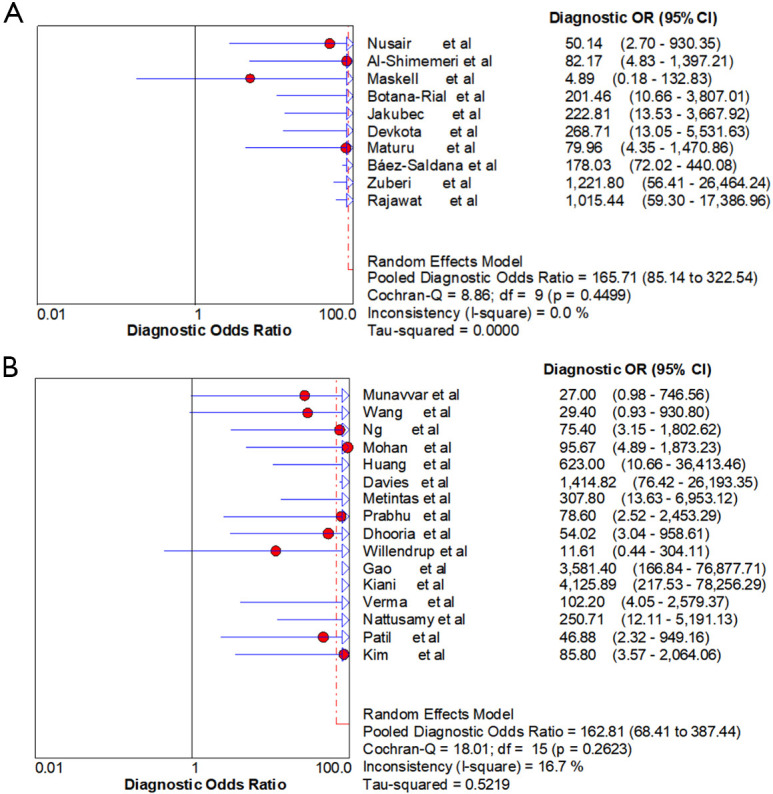
Forest plot of diagnostic odds ratio. (A) Forest plot of the pooled diagnostic odds ratio of CPB in undiagnosed exudative pleural effusions. (B) Forest plot of the pooled diagnostic odds ratio of MT in undiagnosed exudative pleural effusions. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Figure S4.
Summary receiver operating characteristics plot. (A) Summary receiver operating characteristic (SROC) curve of CPB in undiagnosed exudative pleural effusions. (B) SROC curve of MT in undiagnosed exudative pleural effusions. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Figure S5.
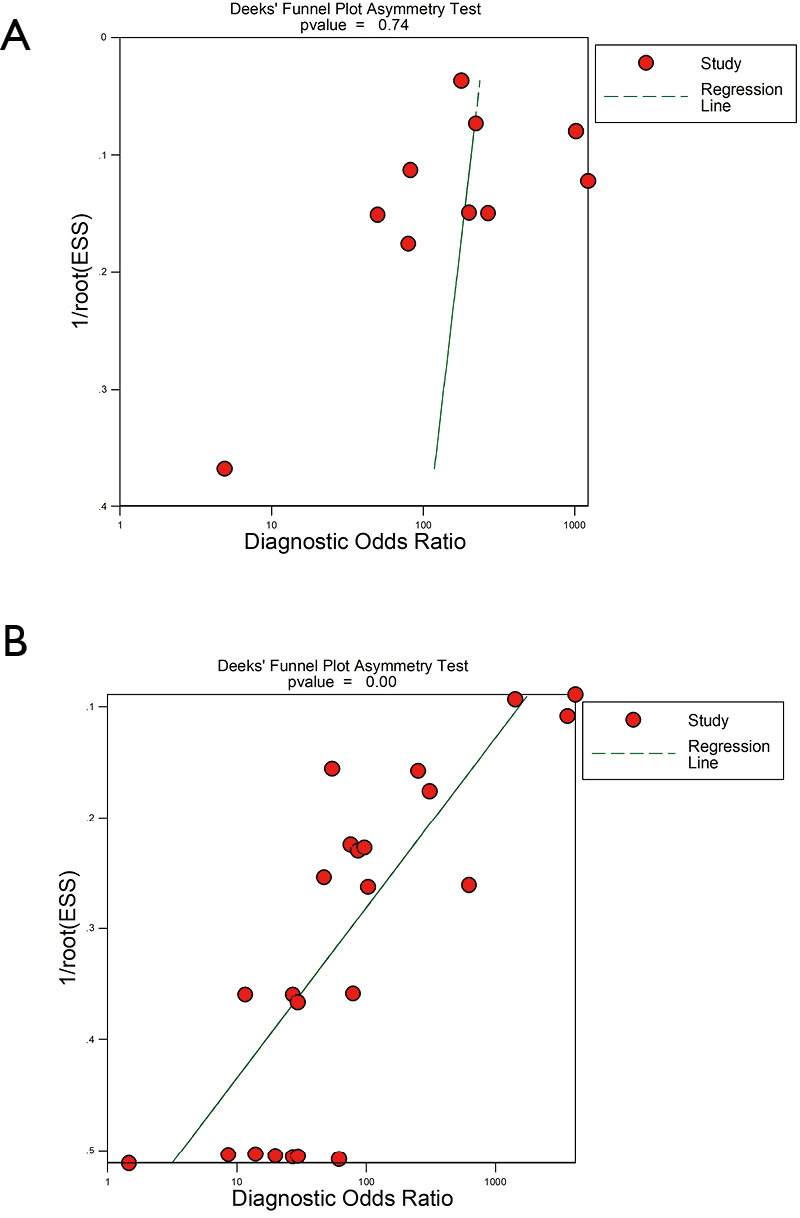
Funnel graph for the assessment of potential publication bias. (A) Funnel graph for the assessment of potential publication bias of CPB. (B) Funnel graph for the assessment of potential publication bias of MT. CPB, closed pleural biopsy; MT, medical thoracoscopy.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81401903, 81572937 and 81572273); the Natural Science Foundation of Jiangsu Province (grant number BK20180139 and BK20161386); Jiangsu Provincial Medical Youth Talent (grant number QNRC2016125), and the Nanjing Medical Science and Technology Development Project (No. ZKX17044), the Jiangsu Provincial Key Research and Development Program (No. BE2016721); Key Project of Natural Science in Universities of Anhui Province (KJ2018A0269), the Talent Introduction Procedure of Wannan Medical College (No. YR201107).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.28). TL serves as the unpaid Associate Editor-in-Chief of Translational Lung Cancer Research. HL serves as the unpaid editorial board member of Translational Lung Cancer Research. YS serves as the unpaid Editor-in-Chief of Translational Lung Cancer Research from Mar 2012 to Mar 2022. The other authors have no conflicts of interest to declare. .
References
- 1.Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest 2010;137:1362-8. 10.1378/chest.09-0884 [DOI] [PubMed] [Google Scholar]
- 2.Enz N, Fragoso F, Gamrekeli A, et al. Carcinoembryonic antigen-positive pleural effusion in early stage non-small cell lung cancer without pleural infiltration. J Thorac Dis 2018;10:E340-3. 10.21037/jtd.2018.04.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hira HS, Ranjan R. Role of percutaneous closed needle pleural biopsy among patients of undiagnosed exudative pleural effusion. Lung India 2011;28:101-4. 10.4103/0970-2113.80319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. 10.1136/thx.2010.136978 [DOI] [PubMed] [Google Scholar]
- 5.Defrancis N, Klosk E, Albano E. Needle biopsy of the parietal pleura. N Engl J Med 1955;252:948-51. 10.1056/NEJM195506022522206 [DOI] [PubMed] [Google Scholar]
- 6.Abrams LD. A pleural-biopsy punch. Lancet 1958;1:30-1. 10.1016/S0140-6736(58)92521-2 [DOI] [PubMed] [Google Scholar]
- 7.Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet 2003;361:1326-30. 10.1016/S0140-6736(03)13079-6 [DOI] [PubMed] [Google Scholar]
- 8.Lee P, Hsu A, Lo C, et al. Prospective evaluation of flex-rigid pleuroscopy for indeterminate pleural effusion: accuracy, safety and outcome. Respirology 2007;12:881-6. 10.1111/j.1440-1843.2007.01144.x [DOI] [PubMed] [Google Scholar]
- 9.Dixon G, de Fonseka D, Maskell N. Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis 2015;7:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhan P, Zhu QQ, Miu YY, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2017;6:23. 10.21037/tlcr.2017.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devkota KC, Chokhani R, Gautam S. Diagnostic yield of pleural biopsy in exudative pleural effusion. Nepal Med Coll J 2014;16:13-6. [PubMed] [Google Scholar]
- 13.Nusair S, Breuer R, Amir G, et al. Closed pleural needle biopsy: predicting diagnostic yield by examining pleural fluid parameters. Respir Med 2002;96:890-4. 10.1053/rmed.2002.1379 [DOI] [PubMed] [Google Scholar]
- 14.Botana-Rial M, Leirofernández V, Represasrepresas C, et al. Thoracic ultrasound-assisted selection for pleural biopsy with Abrams needle. Respir Care 2013;58:1949-54. 10.4187/respcare.02378 [DOI] [PubMed] [Google Scholar]
- 15.Jakubec P, Kolek V, Václavík A, et al. Closed pleural biopsy in the diagnostics of malignant pleural involvement. Vnitr Lek 2014;60:423-30. [PubMed] [Google Scholar]
- 16.Maturu VN, Dhooria S, Bal A, et al. Role of medical thoracoscopy and closed-blind pleural biopsy in undiagnosed exudative pleural effusions: a single-center experience of 348 patients. J Bronchology Interv Pulmonol 2015;22:121. 10.1097/LBR.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 17.Báez-Saldaña R, Rumbo-Nava U, Escobar-Rojas A, et al. Accuracy of closed pleural biopsy in the diagnosis of malignant pleural effusion. J Bras Pneumol 2017;43:424-30. 10.1590/s1806-37562016000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuberi FF, Zuberi BF, Ali SK, et al. Yield of closed pleural biopsy and cytology in exudative pleural effusion. Pak J Med Sci 2016;32:356-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajawat GS, Batra S, Takhar RP, et al. Diagnostic yield and safety of closed needle pleural biopsy in exudative pleural effusion. Avicenna J Med 2017;7:121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munavvar M, Khan MA, Edwards J, et al. The autoclavable semirigid thoracoscope: the way forward in pleural disease? Eur Respir J 2007;29:571-4. 10.1183/09031936.00101706 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Tong ZH, Li HJ, et al. Semi-rigid thoracoscopy for undiagnosed exudative pleural effusions: a comparative study. Chin Med J (Engl) 2008;121:1384-9. 10.1097/00029330-200808010-00010 [DOI] [PubMed] [Google Scholar]
- 22.Ng TH, How SH, Kuan YC, et al. Medical thoracoscopy: Pahang experience. Med J Malaysia 2008;63:298-301. [PubMed] [Google Scholar]
- 23.Sasada S, Kawahara K, Kusunoki Y, et al. A new electrocautery pleural biopsy technique using an insulated-tip diathermic knife during semirigid pleuroscopy. Surg Endosc 2009;23:1901-7. 10.1007/s00464-008-0263-8 [DOI] [PubMed] [Google Scholar]
- 24.Xie Q, Chen Q, Li Y, et al. Application of Flexi-rigid Thoracoscopy Under Local Anesthesia to Diagnose Malignant Pleural Effusion. Zhongguo Fei Ai Za Zhi 2009;12:422. [DOI] [PubMed] [Google Scholar]
- 25.Ishida A, Ishikawa F, Nakamura M, et al. Narrow band imaging applied to pleuroscopy for the assessment of vascular patterns of the pleura. Respiration 2009;78:432-9. 10.1159/000247335 [DOI] [PubMed] [Google Scholar]
- 26.Kannan SK, Lin WJ, Teck TS, et al. Pleuroscopy: early experience in an East malaysian state with high tuberculosis prevalence. J Bronchology Interv Pulmonol 2009;16:250. 10.1097/LBR.0b013e3181ba730a [DOI] [PubMed] [Google Scholar]
- 27.Mohan A, Naik S, Naseer R, et al. Performance characteristics of semirigid thoracoscopy in pleural effusions of undetermined etiology. J Bronchology Interv Pulmonol 2010;17:289. 10.1097/LBR.0b013e3181f9ebca [DOI] [PubMed] [Google Scholar]
- 28.Huang GH, Cheng YX, Su J, et al. Application of flexirigid thoracoscopy in the diagnosis of pleural disease with unknown etiology. Nan Fang Yi Ke Da Xue Xue Bao 2011;31:669-73. [PubMed] [Google Scholar]
- 29.Davies HE, Nicholson JE, Rahman NM, et al. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur J Cardiothorac Surg 2010;38:472-7. 10.1016/j.ejcts.2010.01.057 [DOI] [PubMed] [Google Scholar]
- 30.Khan MA, Ambalavanan S, Thomson D, et al. A comparison of the diagnostic yield of rigid and semirigid thoracoscopes. J Bronchology Interv Pulmonol 2012;19:98-101. 10.1097/LBR.0b013e31824ee45b [DOI] [PubMed] [Google Scholar]
- 31.Prabhu VG, Narasimhan R. The role of pleuroscopy in undiagnosed exudative pleural effusion. Lung India 2012;29:128-30. 10.4103/0970-2113.95304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozman A, Camlek L, Marc-Malovrh M, et al. Rigid versus semi-rigid thoracoscopy for the diagnosis of pleural disease: a randomized pilot study. Respirology 2013;18:704-10. 10.1111/resp.12066 [DOI] [PubMed] [Google Scholar]
- 33.Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014;59:756-64. 10.4187/respcare.02738 [DOI] [PubMed] [Google Scholar]
- 34.Willendrup F, Bodtger U, Colella S, et al. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions in Denmark. J Bronchology Interv Pulmonol 2014;21:215. 10.1097/LBR.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 35.Gao BA, Zhou G, Guan L, et al. Effectiveness and safety of diagnostic flexi-rigid thoracoscopy in differentiating exudative pleural effusion of unknown etiology: a retrospective study of 215 patients. J Thorac Dis 2014;6:438-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiani A, Abedini A, Karimi M, et al. Diagnostic Yield of Medical Thoracoscopy in Undiagnosed Pleural Effusion. Tanaffos 2015;14:227-31. [PMC free article] [PubMed] [Google Scholar]
- 37.Verma A, Taha A, Venkateswaran S, et al. Effectiveness of medical thoracoscopy and thoracoscopic talc poudrage in patients with exudative pleural effusion. Singapore Med J 2015;56:268-73. 10.11622/smedj.2015075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nattusamy L, Madan K, Mohan A, et al. Utility of semi-rigid thoracoscopy in undiagnosed exudative pleural effusion. Lung India 2015;32:119-26. 10.4103/0970-2113.152618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patil CB, Dixit R, Gupta R, et al. Thoracoscopic evaluation of 129 cases having undiagnosed exudative pleural effusions. Lung India 2016;33:502-6. 10.4103/0970-2113.188969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SJ, Choi SM, Lee J, et al. Medical Thoracoscopy in Pleural Disease: Experience from a One-Center Study. Tuberc Respir Dis (Seoul) 2017;80:194-200. 10.4046/trd.2017.80.2.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowditch H. On paracentesis thoracis. Boston Surg J 1857;56:348-54. [Google Scholar]
- 42.Attanoos RL, Gibbs AR. The comparative accuracy of different pleural biopsy techniques in the diagnosis of malignant mesothelioma. Histopathology 2008;53:340-4. 10.1111/j.1365-2559.2008.03099.x [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin KM, Kerr KM, Currie GP. Closed pleural biopsy to diagnose mesothelioma: dead or alive? Lung Cancer 2009;65:388-9. 10.1016/j.lungcan.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 44.Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, et al. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015;70:995-7. 10.1136/thoraxjnl-2014-206567 [DOI] [PubMed] [Google Scholar]
- 45.Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. 10.1164/rccm.201807-1415ST [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as