Abstract
Purpose:
Mutations in the CLN2 gene lead to a neurodegenerative and blinding lysosomal storage disorder, late infantile neuronal ceroid lipofucionsis, also known as CLN2 disease. The purpose of the current study was to characterize the evolution of CLN2-associated retinal manifestations using the Weill Cornell Batten Scale (WCBS) and the age-association of the retinal degeneration utilizing central subfield thickness (CST) measurements, and then correlate these findings with fundus photography and optical coherence tomography (OCT) to determine a critical period for retinal intervention.
Design:
Retrospective single-center cohort.
Subjects:
Eighty-four eyes of 42 treatment-naïve CLN2 disease patients.
Methods:
Clinical records, fundus photos, and OCT imaging for CLN2 patients collected during examinations under anesthetia were reviewed. Imaging was categorized per WCBS criteria by three masked graders.
Main Outcome Measures:
CLN2-associated retinopathy assessed using WCBS scores, fundus photos and OCT imaging, correlated with patient age,
Results:
Eighty-four eyes from 42 patients had baseline fundus photos, with baseline OCT in 31 eyes of 16 patients. Fundus photos were obtained serially for 26 eyes of 13 patients, and serial OCT in 10 eyes of 5 patients. At baseline, bilateral WCBS scores were highly correlated for OCT and fundus photos, r=0.96 and 0.82, respectively. CST was negatively correlated with left and right eye WCBS OCT scores (r=−0.92 and −0.83; p=<0.001) and fundus photo scores (r=−0.80 and −0.83; p=<0.001). OCT thickness was symmetrical between each eye. Baseline OCT data with age fit using a sigmoid function demonstrated a period of accelerated loss between 48–72 months of age.
Conclusions:
Retinal degeneration associated with CLN2 disease manifests as a progressive, symmetrical decline, which appears to accelerate during a critical period at 48–72 months of age, suggesting intervention with retina-specific CLN2-gene therapy should occur ideally prior to, or as early as possible within this critical period. WCBS is a valuable tool and is highly correlated with the extent of retinal degeneration observed in OCT or fundus photos; using the fellow eye as a control, this grading scale can be used to monitor the effect of CLN2-gene therapy in future trials.
Keywords: Neuronal Ceroid Lipofuscinosis, CLN2, Batten Disease, Optical Coherence Tomography, Retinal Degeneration
INTRODUCTION
Neuronal ceroid lipofuscinosis (NCL) is a heterogeneous collection of lysosomal storage diseases. Collectively, these represent the most common progressive neurodegenerative diseases in childhood with rates ranging across different countries, from 0.56 per 100,000 live births in Italy to 9 per 100,000 in Newfoundland, Canada.1,2,3,4 All but one of the NCL syndromes are autosomal recessively-inherited and result from mutations in one of at least fourteen different genes.5 Previously, NCL disorders were simply classified as congenital or by the age at which the child began to show symptoms, such as infantile, late infantile (LINCL), and juvenile onset. As understanding of NCLs expanded, it was found that while all NCLs demonstrate some clinical and neuropathological similarities, each form represented a different genetic entity with distinct pathophysiological characteristics. Currently, NCL disorders are classified by the gene and mutations involved, as well as the age at clinical manifestation.6 Each gene, CLN (ceroid lipofuscinosis, neuronal), is given a distinct number, designating its subtype. One of the most common is CLN2, in which mutations lead to Jansky-Bielschowksy disease, also specifically referred to as CLN2 disease.7 CLN2 disease is also the most representative of the NCL diseases that occurs in the late infantile period.
CLN2 encodes for the soluble protein tripeptidyl peptidase 1 (TPP1), which as a lysosomal hydrolase, removes 3 amino acids from the N-terminus of small polypeptides.8,9 As such, mutations in CLN2 lead to accumulation of curvilinear profiles in lysosomal residual bodies, which is a defining feature of CLN2 disease.10 This accumulation of lysosomal storage material, due to TPP1 dysfunction, occurs systemically, however the central nervous system (CNS) and retinal tissue appear to be particularly sensitive to it, ultimately leading to degenerative changes in CNS neurons and the retina. Without treatment, initial symptoms, such as delayed acquisition or deterioration of speech, motor decline, seizures and ataxia, tend to occur between 2 and 4 years of age. Often, after age 3, a rapid progressive deterioration of motor function, language, vision, and swallowing occurs, leading to premature death by early adolescence.11,12 Vision loss in children with CLN2 disease tends to develop later in the disease course, well after the motor and developmental deficits have become manifest.13 Although vision loss is apparent later in disease development, pathological changes have been observed by optical coherence tomography (OCT) imaging in children as young as two years of age. Classically, the retinopathy associated with CLN2 disease manifests as a “bull’s eye” maculopathy and atrophy of the retinal pigment epithelium, autofluorescence changes, peripheral retinal pigmentary changes, and marked reduction in electroretinogram.14,15,16,17,18 There are, of course, varying phenotypes and some late-presentation NCL associated with CLN2 mutations in which vision loss may not occur or the onset may not occur until adolescence; however, these cases are rare and outside the scope of the present study which only encountered patients with CLN2 disease occurring in the late infantile period.19,20 Prior reports have established and validated a scoring system (Weill Cornell Batten Severity Score, WCBS) to assess progression of the retinal degeneration in late infantile CLN2 disease patients with confirmed CLN2 mutations. 21 In this study the retinal findings were also correlated with a previously validated measure of neurological function in CLN2 patients, but were not correlated with specific genotypes.
An attractive and potentially effective pharmacological treatment for NCL disorders would be to specifically target the underlying metabolic pathway defect for each precise form of NCL mutation. CLN2 disease is the only form of NCL that currently has an approved pharmacologic treatment in the United States and European Union, namely treatment is enzyme replacement therapy cerliponase alfa, a recombinant human proenzyme of TPP1, which is administered every second week by intracerebroventricular infusions via a reservoir surgically implanted under the scalp. Once activated, it becomes the proteolytic form of TPP1 and in animal models its activity has been shown to reduce the accumulation of lysosomal storage material. In children with CLN2 disease treated with this therapy there is an attenuation of the progressive degeneration of motor and speech function.22 However, ceroliponase alfa infusions do not appear to treat the ophthalmologic manifestations of CLN2 disease. 23,24
Despite slowing the progression of motor and language function decline, evidence is limited that intracerebroventricular treatment with cerliponase alfa has a beneficial effect on many of the clinical consequences of CLN2 disease, including pain, myoclonus, movement disorders, seizures, impaired cognition, ability to perform basic activities of daily living, and visual impairment.25 The required biweekly infusions, and the management of any related complications may present a barrier to treatment access.23
The identification of the causative genes for inherited retinal dystrophies has increased the treatment potential of these disorders using gene therapy. Gene therapy represents the therapeutic delivery of nucleic acid polymers into patient cells with the aim of treating an underlying disease. In 2017, the United States Food and Drug Administration approved the commercial use of gene therapy for RPE65-related retinal dystrophies with voretigene neparvovec. As a classically autosomal recessive disorder, with loss-of-function mutations inhibiting the lysosomal hydrolase TPP1, CLN2 disease stands as an ideal target of gene therapy with insertion of a functional protein coding sequence. A clinical trial of 10 children with CLN2 disease, treated with gene therapy using intracranial injections of an adeno-associated serotype 2 vector that mediates expression of the CLN2 gene (AAV2-CLN2) suggested a slowing of neurological progression of CLN2 over the 18 month study period.26 As progress is made in the central stabilization of CLN2 disease, a greater understanding of the retinal degeneration and vision loss that continues in these children, and efficacious therapies, is needed.27
In the present study we sought to characterize the evolution of CLN2-associated retinopathy using the Weill Cornell Batten Scale (WCBS)21 and the age-association of retinal degeneration in individual eyes utilizing central subfield thickness (CST) measurements, and then correlate these findings with fundus photography and optical coherence tomography (OCT) in order to determine a critical period of ocular retina-directed intervention. We also sought to determine if a specific imaging modality was best suited for grading and monitoring vision-related CLN2 disease, to establish the natural age-related progression of the degeneration, and to assess the symmetry of the degeneration between eyes in the same patient.
METHODS
Forty-two treatment naïve patients with CLN2 disease, genetically confirmed with mutations in CLN2, underwent comprehensive ophthalmic examinations under anesthesia as part of their participation in a natural history trial at Weill Cornell Medical College and New York/Presbyterian Hospital (NCT01035424).
The study protocol was approved by the Institutional Review Boards of Weill Cornell Medical College and New York/Presbyterian Hospital. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. Informed consent was obtained for all patients prior to enrollment.
Examinations and Patient Information:
Patients were referred to our center after undergoing genetic testing to confirm that patients were bi-allelic with at least one of the following mutations: C3670T (c.622 C>T, nonsense Arg208 to stop), G3556C (c.509–1G>C, intron 5, splice), G4655A (c.1094G>A, Cys365Tyr), or G1950C (c. 229 G>C, Gly77Arg). Since testing was performed in multiple laboratories, depending on the source of the referral, labs were not necessarily CLIA-certified. All patients referred to our center with the aforementioned confirmatory genetic testing were included in the study (ie, no patients were excluded if they underwent examinations). Two pairs of patients were siblings.
The ocular examinations included in the current review were conducted as part of the natural history study to support an NIH-sponsored clinical trial, prior to any study-related treatment. This study was performed prior to the approval of enzyme replacement therapy (ERT), and therefore all patients were treatment-naïve with regards to ERT.
Examinations were performed while the child was under general anesthesia, which is required given the difficulties of conducting clinical exams in the context of severe motor and cognitive abnormalities. The following assessments were performed: dilated fundus exam, fundus photography (FP) (RetCam, Clarity Medical Systems Inc, Pleasanton, CA, USA), and spectral-domain optical coherence tomography (OCT) (Heidelberg Engineering, Heidelberg, Germany). Gathered clinical information included age at time of diagnosis, age at time of examination, gender, and laterality of the eye being examined. All 84 eyes of the 42 patients had baseline fundus examination with fundus photos taken, with baseline OCT images acquired in 31 eyes of 16 patients. OCT images were acquired with the Heidelberg Spectralis fast macular protocol consisting of 25 B scans with 512 A scans. Serial examinations including fundus photos were performed on 26 eyes of 13 patients. During these serial examinations, 10 eyes of 5 patients also had OCT images acquired.
Image Selection and Evaluations:
For each eye on each examination date, central subfield thickness (CST), was calculated by Heidelberg software and retinal nerve fiber layer (RNFL) thickness was measured at a point 3mm nasal to the foveal center on an OCT B scan which transected both the fovea and optic nerve. Representative and optimal OCT and fundus photos for each eye at each examination were selected by one masked study member (KDK), who was not involved in the prior study of these patients, based on image signal quality, illumination, and centration. A single image of each modality was selected for each eye at each visit, as opposed to the prior investigation in which WCBS scores were given for a patient based on the collection of all images at a single visit. Each image (fundus photo and OCT scan) was independently interpreted by three retina-trained, masked reviewers (SK, AO, KK). Images were assigned a score from 1 to 5 based on retinal disease severity using the previously established WCBS (full delineation of WCBS score criteria are available in Appendix 1). In the event of a score discrepancy between reviewers, a final designation was determined by one reviewer (SK). The ultimate severity score assigned to each image was then used for statistical analysis and trending progression of the retinal degeneration when serial exams were performed.
Statistical Analysis:
The agreement of scores for each imaging modality, as well as OCT CST and RNFL thickness, was compared using Pearson’s correlation coefficient (r). Inter-observer agreement for WCBS scores for both OCT and fundus photos were assessed with intraclass correlation coefficients. A sigmoidal curve was estimated using a nonlinear regression model to analyze the change in CST over time. Statistical analyses were performed with the use of SAS® software, version 9.3 or higher.
RESULTS
Eighty-four eyes from 42 treatment naïve patients underwent at least one examination. Patients had a mean age of 47 months (±10.4 months) at the time of diagnosis, and 60 months (±13.4 months) at the time of first examination (Table 1). Twenty-four (57%) patients were female.
Table 1:
Baseline Demographics, Imaging, and Follow-up of Patient Cohort
| Description | N = 42 | |
|---|---|---|
| Gender - Female | 24 | 57% |
| Mean Age at Diagnosis (months) | 47 +/− 10.4 | Range 18–67 |
| Mean Age at First Exam (months) | 60 +/− 13.4 | Range 30–103 |
| Mean Serial Exam Follow-up (months) | 15 +/− 8.2 | Range 4–23 |
| Subjects with 1 or more Fundus Photo | 42 | 100% |
| Subjects with 1 or more OCT | 16 | 38% |
| Subjects with serial Fundus Photos | 13 | 31% |
| Subjects with serial OCT | 7 | 17% |
Thirteen patients underwent serial examinations with follow-up time intervals ranging from 4 to 23 months (mean 15.3 months). Fundus photos were obtained for all patients in both eyes at all examinations, including the 13 patients having serial examinations (ranging from 2 to 5 exams). OCT images were acquired in 31 eyes of 16 patients, with five patients having serial examinations (ranging from 2 to 3 exams) that included OCT in addition to fundus photos.
A wide distribution of baseline phenotypes were observed, as assessed by WCBS OCT scores and WCBS fundus photo scores (Table 2). All modalities for monitoring progression of the degeneration showed significant concordance with one another, and also showed symmetry between right and left eyes at initial visit (Table 3). There was a strong correlation between right and left eye WCBS scores for both fundus photographs (r=0.82; [n=42]) and OCT (r=0.96; [n=15]). CST was negatively correlated with WCBS OCT scores (right eye r=−0.83; [n=16] and left eye r=−0.92 [n=15]). CST was also negatively correlated with WCBS photo scores (right eye r= −0.83 [n=16] and left eye r=0.80 [n=15]). CST was highly symmetric between the two eyes (r=0.99). All correlations were significant at p<.001. High correlations were also seen across all visits, and all coefficients were statistically significant at p<.001 (Supplement Table).
Table 2:
Distribution of WCBS Scores for Fundus Photos and OCT at time of Initial Examination
| Photo Right | Photo Left | OCT Right | OCT Left | |
|---|---|---|---|---|
| Number of patients | 42 | 42 | 16 | 15 |
| WCBS 1 | 6 | 8 | 5 | 5 |
| WCBS 2 | 15 | 15 | 6 | 5 |
| WCBS 3 | 10 | 7 | 1 | 1 |
| WCBS 4 | 8 | 8 | 3 | 3 |
| WCBS 5 | 3 | 4 | 1 | 1 |
| Mean Score (StDev) | 2.69 (1.16) | 2.64 (1.27) | 2.31 (1.30) | 2.33 (1.35) |
Table 3:
Correlation Coefficients of WCBS severity scores and central subfield thickness for Right and Left Eyes Among All Imaging Modalities at Initial Examination*
| Pearson’s r N | Photo Right | Photo Left | OCT Right | OCT Left | CST Right | CST Left |
|---|---|---|---|---|---|---|
| Photo Right | 1.00 42 | 0.8242 | 0.8916 | 0.9015 | −0.8316 | −0.85 15 |
| Photo Left | 0.8242 | 1.0042 | 0.8816 | 0.8815 | −0.7816 | −0.8015 |
| OCT Right | 0.8916 | 0.8816 | 1.0016 | 0.9615 | −0.8316 | −0.8515 |
| OCT Left | 0.9015 | 0.8815 | 0.9615 | 1.0015 | −0.9315 | −0.9215 |
| CST Right | −0.8316 | −0.7816 | −0.8316 | −0.9315 | 1.0016 | 0.9915 |
| CST Left | −0.8515 | −0.8015 | −0.8515 | −0.9215 | 0.9915 | 1.0015 |
all p-values <.001
CST=Central Subfield Thickness; OCT=Optical Coherence Tomography; Photo=Fundus Photography
There were moderate to strong correlations between the patient’s age at first examination and both severity scores on fundus photographs and OCT (photo right eye r=0.63; p<.001; n=42 and photo left eye r= 0.54; p<.001; n=42; p<0.001; OCT right eye r =0.76; p<.001; n=16 and OCT left eye r = 0.81, p<.001, n=15). While all correlations were statistically significant, OCT may be more sensitive for accurately discerning early disease severity as a disease biomarker. For all examinations across ages, there were also moderate to strong correlations with right and left WCBS fundus photo scores and right and left WCBS OCT scores (photo right eye r=0.65; n=71 and photo left eye r=0.64; n=71; OCT right eye r=82; n=24 and OCT left eye r=0.83; n=23. All p-values <.0001) (Table 4).
Table 4:
Correlation coefficients of Age with WCBS fundus, WCBS OCT, and CST
| Pearson’s r N | Photo Right | Photo Left | OCT Right | OCT Left | CST Right | CST Left |
|---|---|---|---|---|---|---|
| First Visit | ||||||
| Age | 0.63 | 0.54* | 0.76* | 0.81* | −0.77* | - |
| 42 | 42 | 16 | 15 | 16 | 0.73** | |
| 15 | ||||||
| Any Visit | ||||||
| Age | 0.65 | 0.64 | 0.82 | 0.83 23 | −0.78 | −0.76 |
| 71 | 71 | 24 | 24 | 23 |
All p-values <.0001, except
<.001 and
<.01
There was moderate to substantial inter-observer agreement for WCBS scores for both OCT and fundus photos. Interclass correlation coefficients were 0.8398 and 0.8528 for fundus photo WCBS scores and OCT WCBS scores, respectively.
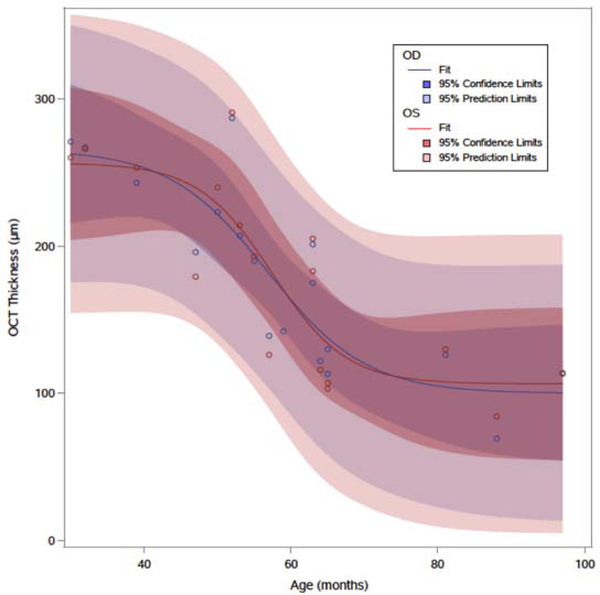
CST was negatively associated with advancing age in both right and left eyes. A sigmoidal curve was estimated using a nonlinear regression model to analyze the change in CST over time (Figure 1), looking only at the initial visit OCT for each eye. A four-parameter logistic function was selected to fit the curve and the resultant equations are provided below for each eye.
Figure 1:
Estimated non-linear regression model correlating right (OD, blue) and left (OS, red) eye OCT central subfield thickness with advancing age. The estimated curve demonstrates a period of accelerated thinning of the central subfield thickness and symmetry in the overall profile and confidence intervals between the two eyes. Age (months), OCT thickness (central subfield thickness, μm).
Left eye:
Right eye:
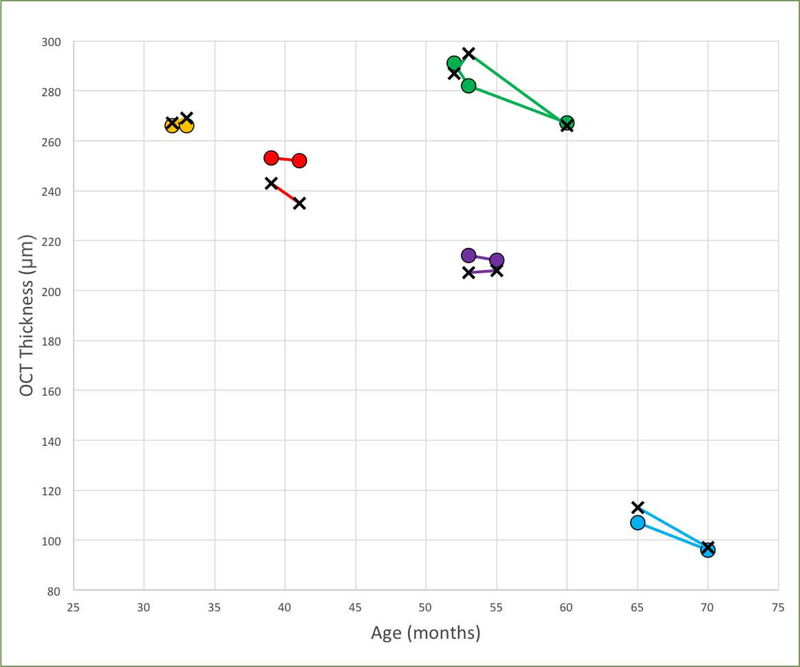
The fitted curve showed significant symmetry between the two eyes in change over time. The estimated sigmoidal curve demonstrated a period of increased loss coming between approximately 48 and 72 months of age, which then slowed again with increasing age (Table 5). There was consistently a loss in CST over time in patients with serial OCT examinations (Figure 2), which also demonstrated symmetry between eyes.
Table 5:
Estimated Decrease in Central Subfield Thickness per 12 Month Interval (μm) using Non-linear Regression Model
| Age (months) | Right Eye | Left Eye |
|---|---|---|
| 36 – 48 | 27.9 | 17.8 |
| 48 – 60 | 67.1 | 73.6 |
| 60 – 72 | 48.0 | 48.7 |
| 72 – 84 | 12.8 | 7.2 |
| 84 – 96 | 2.3 | 0.7 |
Figure 2:
OCT central subfield thickness over time in patients who had serial examinations. Right eyes are plotted with X-marks and left eyes plotted with circles, each color representing a different patient. There is consistent decline over time in patients with serial imaging, and marked symmetry between right and left eyes for each individual patient. Age (months), OCT thickness (central subfield thickness, μm).
Measurements of RNFL thicknesses did not demonstrate any significant correlation with increasing age of the patient, with Pearson’s correlation coefficients of −0.303 (p=0.159) and −0.193 (p=0.389) for right and left eyes, respectively.
DISCUSSION
The current analysis of 84 eyes and serial examinations including fundus photography and OCT represents one of the larger cohorts of CLN2 disease patients assessed, while also providing some early longitudinal framework for the progression of CLN2 disease-associated retinal degeneration. In the present study we delineated the characteristic imaging features and natural history of the retinal degeneration associated with CLN2 disease in patients that were ERT-naïve and had not yet received CNS gene therapy treatment. Multiple modalities were assessed to discern the optimal choice for tracking the degeneration. The findings describe a progressive outer retinal degeneration that is bilateral, symmetric in multiple modes of imaging, and a critical period of accelerated decline between 48 and 72 months of age (Figure 1 and Table 5). Overall, OCT appeared to demonstrate a slightly stronger correlation between both eyes, and greater sensitivity to subtle changes at earlier severity stages compared with fundus photographs alone. The degeneration was well characterized by WCBS scores for both OCT and fundus photos, as well as CST.
The described study included patients ranging from 18 to over 100 months of age, providing a wide spectrum of disease severity and for observing the age-association of the degeneration. All of the patients enrolled were part of the screening study for a phase I CNS gene therapy trial, and therefore there could be a bias in the severity of the disease as effected by differential family response to experimental treatment options. In contrast, we found that our cohort demonstrated a wide distribution in the degree of retinal degeneration as assessed with WCBS scores at the time of initial examination, with the caveat that patients were different ages at the time of this initial exam.
We did not identify any trends in RNFL thickness with advancing age, suggesting that the disease is manifested as a primary retinal degeneration and not a secondary retrograde effect from the CNS degeneration. While there is certainly RNFL thinning in late disease stages, correlated with the presence of optic nerve pallor and diffuse vessel attenuation in WCBS stage 5, this represents only a subset of the present cohort whereas earlier stages show outer retinal loss in absence of concurrent RNFL loss. This aligns with findings in animal models and in those being treated with CNS enzyme therapy: that retinal degeneration occurs independently from the CNS degeneration.22,28 As such, establishing the natural history of the retinal degeneration, and the time window for possible intervention, remains of primary importance for the long-term outcomes of these patients.
The data here demonstrates that retinal degeneration in CLN2 disease appears to progress symmetrically between the eyes.29 This is contrary to some other inherited retinal disorders such as retinitis pigmentosa, in which 20% of patients have been recently found to have asymmetric progression of their degeneration (though subsequent studies tempered that finding with different statistical modeling of their data).30,31 Similarly, Stargardt’s disease progression as evaluated by autofluorescence found discordant right-left progression rates in 17.6% of patients, with particularly high asymmetry in specific variants (late-onset).32 The high degree of symmetry of bilateral progression of patients at different stages of progression in our cohort would allow efficacy of a future therapy to be readily demonstrated with requirement for fewer patients to be enrolled in the study, which could prove critical for orphan diseases such as CLN2 disease. This follows a similar approach to that taken with gene therapy with AAV2-hRPE65v2 for Leber’s Congenital Amaurosis, which has begun advocating bilateral therapy33 after the establishment of an efficacious intervention through observation of unilateral administration for observation of safety and efficacy compared to the fellow eye.34
Our study documented high degrees of inter-eye correlation at each time-point between right and left eyes. While both fundus photo and OCT severity scores between eyes were statistically significant (Tables 3 and 4 comparing inter-eye scores), the sensitivity of OCT imaging may provide a greater ability to assess subtle variations in progression and may be the preferred choice for single-modality examination. OCT has also been recommended in the CLN2 disease management guidelines as it appears to be the most sensitive tool to detect the outer retinal changes and to establish the extent of retinal degeneration.35 While the present study did not measure the width or area of ellipsoid zone loss, particularly with en face imaging, this would potentially be a very sensitive future metric for monitoring disease progression in future studies. OCT CST showed remarkable symmetry between the two eyes at initial visit (r=0.99), again reinforcing the symmetry of disease progression. Prior work suggests that invasive testing (fluorescein and indocyanine green angiography) may not be necessary for monitoring the degeneration in routine cases,21 and as such further validation of the utility of noninvasive testing may help guide the protocols for future interventional studies.
When viewing WCBS scores and CST as a function of patient age, there was manifestation of a consistent phenotype at a specific time-point—ie, unlike many other systemic disease markers, patients of particular ages in this study appeared to show similar stages of the retinal degeneration. This suggests that most of the patients enrolled in the study had similar, or classic, mutations of the CLN2 gene. Most importantly for future studies, data fit using a sigmoid function estimated the greatest loss of CST between approximately 48–72 months of age, identifying a critical period for the retinal degeneration. This suggests that intervention early or ideally prior to, this critical period may mitigate visual decline. Since these patients, by definition, had already been diagnosed with CLN2 disease based on their CNS manifestations contingent to their enrollment to the study cohort, we know that retinal degeneration follows CNS manifestations and that early diagnosis of the CNS disease could provide ample time for retinal therapy prior to onset of significant retinal degeneration.
The conclusions of the present study have a number of limitations inherent to the retrospective nature of this study. While the patients represent a wide range of patient ages, only 13 patients had serial examinations, so the description of the natural history of the disease assumes similar rate of disease progression with advancing age and this is not necessarily an accurate reflection of other disease progression markers. Notably, the work conducted by Nickel et al included more patients with multiple visits and their results of non-visual disease progression were very much aligned with the current study.29 Ideally, a study would assess patients regularly; however, this would require a substantial number of examinations under anesthesia that carry a number of systemic risks and may not be practical or appropriate. All patients being evaluated in this study were part of a natural history study prior to a CNS gene therapy study, not for primary observation of the retinal degeneration. As such, the availability of specific testing devices for the ophthalmic examinations was not identical for each patient. Most notably, not all patients had OCT images acquired at each visit, which reduces the power of the of the study group and the degree of statistical significance for the claims regarding OCT and especially the longitudinal degeneration as evaluated by OCT. Our study is further limited by relying solely on anatomic features of the disease, rather than functional characteristics. This was necessitated by the inability for these young affected individuals to participate in a productive manner as outpatients for clinical examinations. Other studies have noted the need for alternative scales to determine functional visual acuity, due to the progression of the concurrent CNS degeneration.22 An ideal study would also include assessment of functional outcomes, such as electroretinograms, to assess the correlation of anatomic and functional degeneration.
In summary, the present study sought to characterize the evolution of CLN2-associated retinal degeneration using the Weill Cornell Batten Scale (WCBS) and the age-association of the retinal degeneration utilizing central subfield thickness (CST) measurements, and then correlate these findings with fundus photography and optical coherence tomography (OCT) in order to determine a critical period for ocular retina-directed intervention. This study represents one of the largest cohorts of CLN2 disease, treatment naïve patients whose retinal degeneration has been monitored longitudinally. While the study is not without its limitations, implicit to the nature of the cohort and the retrospective nature of the review, it does offer valuable insight into the retinal manifestations of CLN2 disease-associated retinal degeneration and also into the modalities for monitoring disease progression. Our findings suggest that the retinal degeneration associated with CLN2 disease manifests as a progressive, symmetrical decline, appearing to accelerate during a critical period (48–72 months of age) suggesting intervention with retina-specific CLN2-gene therapy occur ideally prior to, or as early as possible within this critical period. WCBS was a valuable tool and highly correlated with the extent of retinal degeneration observed in OCT or fundus photos. WCBS scores and OCT measurements can provide objective biomarkers for the efficacy of retina directed CLN2-gene therapy, where the contralateral eye provides an ideal control.
Supplementary Material
Highlights.
Precis –
CLN2-associated retinal degeneration is symmetrically age-associated and appears to accelerate between 48–72 months of age, suggesting retinal intervention with CLN2-gene therapy occur ideally prior to or early within this critical period.
Acknowledgments
Financial Support:
This study was supported, in part, by NIH 1R01NS061848 and NIH U54NS065768, and the foundations: Nathan’s Battle; Cures Within Reach; Noah’s Hope; and Hope4Bridget. This was also supported in part by an unrestricted department grant from Research to Prevent Blindness.
Abbreviations/acronyms:
- LINCL
Late infantile neuronal ceroid lipofucinosis
- NCL
Neuronal ceroid lipofucinosis
- CST
Central subfield thickness
- OCT
Optical coherence tomography
- WCBS
Weill Cornell Batten scale
- TPP1
Tripeptidyl peptidase 1
- CNS
Central nervous system
- RNFL
Retinal nerve fiber layer
Footnotes
Conflicts of Interest:
Kyle Kovacs- Consultant for Regenxbio
Szilard Kiss- Adverum – Consultant/Advisor, Equity; Regenxbio - Consultant/Advisor, Equity; Genentech/Roche – Consultant/Advisor; Fortress Bio – Consultant/Advisor, Equity; Optos – Consultant/Advisor, Research grant support;
Novartis – Consultant/Advisor; Intellectual Property related to gene and cellular therapy – assigned to Weill Cornell/Cornell University
Samir Patel, Keunpyo Kim, Sherri Van Everen - Employees of Regenxbio
Therese Conner: Employee of Xcenda, previously employee of Regenxbio
Ronald Crystal: Regenxbio – Equity
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cardona F, Rosati E. Neuronal ceroid-lipofuscinoses in Italy: an epidemiological study. Am J Med Genet. 1995;57:142–3. [DOI] [PubMed] [Google Scholar]
- 2.Santavuori P, Haltia M, Rapola J. Infantile type of so-called neuronal ceroid-lipofuscinosis. Dev Med Child Neurol. 1974;16:644–53. [DOI] [PubMed] [Google Scholar]
- 3.Moore SJ, Buckley DJ, MacMillan A, Marshall HD, Steele L, Ray PN, Nawaz Z, Baskin B, Frecker M, Carr SM, Ives E, Parfrey PS. The clinical and genetic epidemiology of neuronal ceroid lipofuscinosis in Newfoundland. Clin Genet. 2008;74:213–22. [DOI] [PubMed] [Google Scholar]
- 4.Williams RE, Adams HR, Blohm M, et al. Management Strategies for CLN2 Disease. Pediatr Neurol 2017. April;69:102–112. [DOI] [PubMed] [Google Scholar]
- 5.Kohlschütter A, Schulz A, Bartsch U, Storch S. Current and Emerging Treatment Strategies for Neuronal Ceroid Lipofuscinoses. CNS Drugs. 2019. April;33(4):315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology. 2012. July 10; 79(2):183–91. [DOI] [PubMed] [Google Scholar]
- 7.Mole SE, Williams RE, Goebel HH. The Neuronal Ceroid Lipofuscinoses (Batten Disease). 2 ed. Oxford, UK: Oxford University Press; 2011. [Google Scholar]
- 8.Mole SE, Cotman SL. Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochim Biophys Acta. 2015;1852(10 Pt B):2237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawlings ND, Barrett AJ. Tripeptidyl-peptidase I is apparently the CLN2 protein absent in classical late-infantile neuronal ceroid lipofuscinosis. Biochim Biophys Acta. 1999, 1429: 496–500. [DOI] [PubMed] [Google Scholar]
- 10.Vines DJ, Warburton MJ. Classical late infantile neuronal ceroid lipofuscinosis fibroblasts are deficient in lysosomal tripeptidyl peptidase I. FEBS Lett. 1999, 443: 131–135. [DOI] [PubMed] [Google Scholar]
- 11.Williams RE, Gottlob I, Lake BD, Goebel HH, Winchester BG et al. Classic late infantile NCL In: Goebel HH, editor. The Neuronal Ceroid Lipofuscinosis (Batten Disease). 1999, IOS Press; pp. 37–54. [Google Scholar]
- 12.Steinfeld R, Heim P, von GH, Meyer K, Ullrich K et al. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am J Med Genet. 2002, 112: 347–354. [DOI] [PubMed] [Google Scholar]
- 13.Worgall S, Kekatpure MV, Heier L, Ballon D, Dyke JP et al. Neurological deterioration in late infantile neuronal ceroid lipofuscinosis. Neurology. 2007, 69: 521–535. [DOI] [PubMed] [Google Scholar]
- 14.Weleber RG, Gupta N, Trzupek KM, Wepner MS, Kurz DE et al. Electroretinographic and clinicopathologic correlations of retinal dysfunction in infantile neuronal ceroid lipofuscinosis (infantile Batten disease). Mol Genet Metab. 2004, 83: 128–137. [DOI] [PubMed] [Google Scholar]
- 15.Collins J, Holder GE, Herbert H, Adams GG. Batten disease: features to facilitate early diagnosis. Br J Ophthalmol. 2006, 90: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly JP, Weiss AH, Rowell G, Seigel GM. Autofluorescence and infrared retinal imaging in patients and obligate carriers with neuronal ceroid lipofuscinosis. Ophthal Genet. 2009, 30: 190198. [DOI] [PubMed] [Google Scholar]
- 17.Weleber RG. The dystrophic retina in multisystem disorders: the electroretinogram in neuronal ceroid lipofuscinoses. Eye (Lond). 1998, 12 (Pt 3b): 580–590. [DOI] [PubMed] [Google Scholar]
- 18.Hainsworth DP, Liu GT, Hamm CW, Katz ML. Funduscopic and angiographic appearance in the neuronal ceroid lipofuscinoses. Retina. 2009, 29: 657–668. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Almomani R, Breedveld GJ, Santen GW, Aten E, Lefeber DJ, Hoff JI, Brusse E, Verheijen FW, Verdijk RM, Kriek M, Oostra B, Breuning MH, Losekoot M, den Dunnen JT, van de Warrenburg BP, Maat-Kievit AJ. Autosomal recessive spinocerebellar ataxia 7 (SCAR7) is caused by variants in TPP1, the gene involved in classic late-infantile neuronal ceroid lipofuscinosis 2 disease (CLN2 disease). Hum Mutat. 2013. May;34(5):706–13. [DOI] [PubMed] [Google Scholar]
- 20.Kohan R, Carabelos MN, Xin W, Sims K, Guelbert N, Cismondi IA, Pons P, Alonso GI, Troncoso M, Witting S, Pearce DA, Dodelson de Kremer R, Oller-Ramírez AM, Noher de Halac I. Neuronal ceroid lipofuscinosis type CLN2: a new rationale for the construction of phenotypic subgroups based on a survey of 25 cases in South America. Gene. 2013. March 1;516(1):114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orlin A, Sondhi D, Witmer MT, Wessel MM, Mezey JG et al. Spectrum of ocular manifestations in CLN2-associated batten (Jansky-Bielschowsky) disease correlate with advancing age and deteriorating neurological function. PLoS One. 2013. August 28;8(8):e73128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz A, Ajayi T, Specchio N, de Los Reyes E, Gissen P, Ballon D, Dyke JP, Cahan H, Slasor P, Jacoby D, Kohlschütter A, CLN2 Study Group. Study of Intraventricular Cerliponase Alfa for CLN2 Disease. N Engl J Med. 2018. May 17; 378(20):1898–1907. [DOI] [PubMed] [Google Scholar]
- 23.Katz ML, Coates JR, Sibigtroth CM, Taylor JD, Carpentier M, Young WM, Wininger FA, Kennedy D, Vuillemenot BR, O’Neill CA. Enzyme replacement therapy attenuates disease progression in a canine model of late-infantile neuronal ceroid lipofuscinosis (CLN2 disease). J Neurosci Res. 2014. November; 92(11):1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical Review Report: Cerliponase Alfa (Brineura): (Biomarin Pharmaceutical (Canada) Inc.): Indication: For the treatment of neuronal ceroid lipofuscinosis type 2 (CLN2) disease, also known as tripeptidyl peptidase 1 (TPP1) deficiency [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019. June Results. [PubMed] [Google Scholar]
- 25.CADTH Common Drug Review. CADTH Canadian Drug Expert Committee Recommendation: Cerliponase Alfa. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019. May. [PubMed] [Google Scholar]
- 26.Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008. May;19(5):463–74. [DOI] [PubMed] [Google Scholar]
- 27.Donsante A, Boulis NM. Progress in gene and cell therapies for the neuronal ceroid lipofuscinoses. Expert Opin Biol Ther. 2018. July;18(7):755–764. [DOI] [PubMed] [Google Scholar]
- 28.Whiting RE, Narfström K, Yao G, Pearce JW, Coates JR, Castaner LJ, Jensen CA, Dougherty BN, Vuillemenot BR, Kennedy D, O’Neill CA, Katz ML. Enzyme replacement therapy delays pupillary light reflex deficits in a canine model of late infantile neuronal ceroid lipofuscinosis. Exp Eye Res. 2014. August;125:164–72. [DOI] [PubMed] [Google Scholar]
- 29.Cabral T, Sengillo JD, Duong JK, Justus S, Boudreault K, Schuerch K, Belfort R Jr, Mahajan VB, Sparrow JR, Tsang SH. Retrospective Analysis of Structural Disease Progression in Retinitis Pigmentosa Utilizing Multimodal Imaging. Sci Rep. 2017. September 4;7(1):10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sujirakul T. et al. Multimodal Imaging of Central Retinal Disease Progression in a 2-Year Mean Follow-up of Retinitis Pigmentosa. Am J Ophthalmol. 2015, 160:786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickel M, Simonati A, Jacoby D, Lezius S, Kilian D, Van de Graaf B, Pagovich OE, Kosofsky B, Yohay K, Downs M, Slasor P, Ajayi T, Crystal RG, Kohlschütter A, Sondhi D, Schulz A. Disease characteristics and progression in patients with late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease: an observational cohort study. Lancet Child Adolesc Health. 2018. August;2(8):582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambertus S, Bax NM, Groenewoud JM, Cremers FP, van der Wilt GJ, Klevering BJ, Theelen T, Hoyng CB. Asymmetric Inter-Eye Progression in Stargardt Disease. Invest Ophthalmol Vis Sci. 2016. December 1;57(15):6824–6830. [DOI] [PubMed] [Google Scholar]
- 33.Bennett J, Wellman J, Marshall KA, McCague S, Ashtari M, DiStefano-Pappas J, Elci OU, Chung DC, Sun J, Wright JF, Cross DR, Aravand P, Cyckowski LL, Bennicelli JL, Mingozzi F, Auricchio A, Pierce EA, Ruggiero J, Leroy BP, Simonelli F, High KA, Maguire AM. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016. August 13;388(10045):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009. November 7;374(9701):1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams RE, Adams HR, Blohm M, Cohen-Pfeffer JL, de Los Reyes E, Denecke J, Drago K, Fairhurst C, Frazier M, Guelbert N, Kiss S, Kofler A, Lawson JA, Lehwald L, Leung MA, Mikhaylova S, Mink JW, Nickel M, Shediac R, Sims K, Specchio N, Topcu M, von Löbbecke I, West A, Zernikow B, Schulz A. Management Strategies for CLN2 Disease. Pediatr Neurol. 2017. April;69:102–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.