Abstract
Background:
Comorbidity between posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) was commonly overlooked by studies examining resting-state functional connectivity (rsFC) patterns in PTSD. The current study used a data-driven approach to identify rsFC biomarkers to (a) differentiate PTSD (with or without MDD) from trauma-exposed healthy controls (TEHCs), (b) compare PTSD alone with comorbid PTSD+MDD, and (c) explore the clinical utility of the identified biomarkers by testing their associations with clinical symptoms and treatment response.
Method:
Resting-state magnetic resonance images were obtained from 51 individuals with PTSD alone, 52 with PTSD+MDD, and 76 TEHCs. Fifty-five of the 103 PTSDs were enrolled in prolonged exposure treatment. A support vector machine (SVM) model was used to identify rsFC biomarkers differentiating PTSD (with or without MDD) from TEHCs, and PTSD alone from PTSD+MDD. The associations between the identified features and symptomatology were tested with Pearson correlations.
Results:
The SVM models achieved 70.6% accuracy in discriminating between PTSD and TEHCs, and 76.7% accuracy in discriminating between PTSD alone and PTSD+MDD for out-of-sample prediction. Within-network connectivity in the executive control network, prefrontal network, and salience network discriminated PTSD from TEHCs. The basal ganglia network played an important role in differentiating PTSD alone from PTSD+MDD. PTSD scores were inversely correlated to within-executive control network connectivity (p<.001), and executive control network connectivity correlated positively with treatment response (p<.001).
Conclusion:
Results suggest that unique brain-based abnormalities differentiate PTSD from TEHC and PTSD from PTSD+MDD, and demonstrate clinical utility in predicting levels of symptomatology and treatment response.
Keywords: Posttraumatic stress disorder, major depressive disorder, treatment outcome, fMRI classification, resting state functional MRI, machine learning, support vector machine
Posttraumatic stress disorder (PTSD) is a debilitating condition commonly observed in individuals following traumatic exposure, with estimated lifetime prevalence of 6.8% (1). PTSD is highly heterogeneous (2) and frequently comorbid with major depression disorder (MDD) (3), complicating our ability to identify its brain mechanisms and identify novel therapeutic targets. Accumulating resting-state functional connectivity (rsFC) studies implicate altered within-network connectivity in the salience network (SN), default mode network (DMN), executive control network (ECN), as well as between these networks (4,5). Within the SN, which typically includes the anterior cingulate cortex (ACC) and anterior insula, studies have found enhanced connectivity between amygdala and insula nodes in individuals with PTSD relative to trauma-exposed (TEHC) and non-trauma exposed healthy controls (HC) (5–7). It was hypothesized that such enhanced connectivity attests to hypervigilance (6,7), whereas decreased connectivity between DMN nodes (e.g., medial prefrontal cortex [PPC], precuneus, the ventromedial prefrontal cortex [vmPFC], and hippocampus) in individuals with PTSD (5,8,9) reflects depersonalization/derealization symptoms (10). It has been further suggested that these altered rsFC patterns may represent neurobiological correlates of increased salience processing and hypervigilance, at the cost of awareness of internal thoughts and autobiographical memory in PTSD (4). Individuals with PTSD also showed decreased connectivity within the ECN (or frontal parietal network, which includes portions of the lateral prefrontal cortex and posterior parietal cortex), potentially representing diminished emotion regulation abilities (i.e., inability to downregulate negative emotions) (11,12).
Although the preponderance of data supports a view of PTSD as being associated with altered within- and between-network connectivity in SN, DMN, and ECN, divergent findings have also been reported (4). Within the SN, connectivity between the amygdala and dorsal anterior cingulate (dACC) has been shown in various studies to be higher (13), lower (6), or unaltered (7) in individuals with PTSD compared to controls (TEHC and HC). Both higher (5,14) and lower (8) between-network connectivity of DMN nodes, such as the PCC/precuneus, and the SN have been demonstrated in PTSD compared to controls. Some studies have reported reduced connectivity between amygdala and the inferior frontal gyrus (IFG), vmPFC, and middle frontal cortex (6,13,14), whereas others found no differences in connectivity between the amygdala and vmPFC pathway (7,15).
A potential reason for these divergent findings might be the high comorbidity rates between PTSD and MDD, which have been largely overlooked by existing connectivity data analyses. PTSD and MDD co-occur in as many as 52% of cases (16,17), and this comorbidity is associated with significantly greater subjective distress and impairment than either condition alone (e.g., 7–10), demonstrating a more chronic course of impairment (18). These clinical differences suggest that corresponding underlying neurobiological differences may be present as well. Meta-analyses on connectivity abnormalities in MDD (19–22) suggest that MDD is characterized by hypoconnectivity within the ECN and between frontoparietal systems and parietal regions of the dorsal attention network (DAN). MDD was also associated with hyperconnectivity within the DMN, and with hyperconnectivity between ECN control systems and regions of the DMN. It is an open question whether individuals with PTSD+MDD show more connectivity abnormalities that are similar to those documented among individuals with MDD than do individuals with PTSD without MDD comorbidity.
To date, only a few studies (23,24) have assessed whether individuals with PTSD+MDD exhibit connectivity differences relative to individuals with PTSD alone. Kennis et al. (23) found in PTSD+MDD vs. PTSD alone increased connectivity between subgenual and perigenual ACC, as well as decreased connectivity of the subgenual ACC with the thalamus. Yet this study focused on the insula and ACC as seed regions, and did not address potential alterations in pathways involving the nucleus accumbents (NAcc). Zhu et al. (24) found that PTSD+MDD, compared to PTSD alone, was associated with multifaceted functional connectivity alterations, including decreased connectivity across multiple amygdala and striatal-subcortical pathways. These findings suggest that individuals with comorbid PTSD+MDD may show dysfunctions that characterize both individuals with PTSD and those with MDD, but it was not possible to draw definitive conclusions because of the small sample size.
Little is known about the clinical utility of the altered within- and between-networks connectivity identified so far in the literature on PTSD. The few available findings suggest that rsFC of the PCC with the perigenual anterior cingulate and the right amygdala is associated with current PTSD symptoms, and that correlation with the right amygdala predicts future PTSD symptoms, but no treatment effect has been studied (25). Another study showed that neural circuitry changes may be associated with treatment response but did not investigate the ability of baseline biomarkers to predict treatment response (26). Closing this gap in the literature by investigating the clinical utility of identified biomarkers is of critical importance in the progress towards personalized PTSD treatments (27).
To address these gaps in knowledge, the present study has the following four aims: (a) identify network connectivity differences distinguishing individuals with PTSD (with and without comorbid MDD) from TEHC; (b) identify network connectivity differences distinguishing individuals with PTSD without MDD from those with PTSD+MDD; (c) examine the clinical utility of the features identified through aims (a) and (b) by examining their associations with MDD and PTSD symptomatology; and (d) test the utility of the identified network connectivity features in predicting subsequent treatment outcome in a sub-sample receiving prolonged exposure (PE) therapy. These four aims are critical for developing a better understanding of the unique neuropathology of PTSD patients and to identify novel therapeutic targets.
To identify the network connectivity features (aims (a) and (b)), the present study used a support vector machine (SVM) model, which is a multivariate pattern recognition machine learning (ML) technique especially well-suited for discriminating high-dimensional rsFC fMRI data. ML approaches have two main advantages over standard univariate analytical methods that are typically used in neuroimaging. First, the traditional approaches are based on average estimates of differences at the group level. By contrast, ML approaches make possible inferences at the level of the individual rather than the group. In an effort to increase the translational applicability of the results to clinical practice where decisions are made about individual patients, not groups, there has been a recent shift toward the use of multivariate ML techniques (28–32). Findings based on ML approaches are expected to have higher translational applicability to everyday decision making in clinical practice. Second, ML approaches are more sensitive to differences that are subtle and spatially distributed by taking inter-regional correlations into account. Such spatially distributed patterns in the brain might be undetectable using group comparisons. Thus, ML approaches provide an optimal framework for investigating psychiatric disorders that affect a distributed network of regions (Nicholson et al., 2019; Orrù et al., 2012; Fu & Costafreda, 2013; Wolfers et al., 2015).
Previous studies comparing traditional and ML approaches with group classification based on resting-state data suggest that ML approaches are more sensitive to the subtle and spatially diffuse alterations typically observed in psychiatric disorders, and therefore may be better suited to the development of a real-world clinical diagnostic tools, than are standard mass-univariate techniques (28). Previous studies suggest SVM ability to discriminate between trauma-exposed individuals and between trauma-exposed and non-traumatized healthy controls with high levels of accuracy (67.57% −91%) ( 40,42). Studies further suggest its ability to predict long-term response to antidepressant medication (21).
Method
Participants
We combined data from three studies conducted at the New York State Psychiatric Institute (NYSPI). The studies were approved by the NYSPI Institutional Review Board, and all participants provided written informed consent after receiving an explanation of the procedures. rsFC fMRI was conducted in a total of 179 individuals: 51 with PTSD alone, 52 with PTSD+MDD, and 76 TEHCs. Detailed inclusion and exclusion criteria for each study appear in Table S1, in the online supplements. Briefly, all participants met the DSM-IV-TR criterion A1 and A2 (35) or DSM-5 (36) PTSD criterion A for adult traumatic events. Clinical evaluators administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (37) and the Clinician-Administered PTSD Scale (CAPS) (38) to establish psychiatric diagnoses and assess PTSD severity. All participants in the PTSD+MDD group, but not in the PTSD alone or TEHC, also met SCID DSM-IV or DSM-5 criteria for a major depressive episode (35). Exclusion criteria for participants in the TEHC group consisted of current or past Axis I disorders, including substance use disorders and the use of any psychotropic medications. Exclusion criteria for all groups included any condition that would rule out MRI administration.
A subsample of 55 patients with PTSD (33 PTSD-alone, 22 PTSD-MDD) underwent PE treatment conducted by one of two trained therapists adhering to a 10-week standard PE protocol (39). The detailed PE treatment protocol was described in Helpman et al. (40) and Zhu et al. (24).
Seed-based functional connectivity analyses
Neuroimaging data acquisition, preprocessing of imaging data, and seed-based functional connectivity analyses appear in the online supplements. rsFC analyses were carried out using a seed-based approach implemented in the CONN-fMRI Functional Connectivity toolbox v13(41). ROI-to-ROI connectivity analysis was performed using 43 ROIs previously identified as important in PTSD and MDD (see online supplements). The mean BOLD time series was computed across all voxels within each ROI. Bivariate regression analyses were used to determine the linear association of the BOLD time series between each pair of regions for each individual. The resultant correlation coefficients were transformed into z-scores using Fisher’s transformation to satisfy normality assumptions.
Statistical Analyses
Clinical Variables
We used SPSS software (SPSS Inc. Chicago, IL, USA) for statistical analyses. T-tests were used to test the differences in clinical symptoms and age between groups. Chi-square test was used to analyze differences in gender and race.
Machine Learning Analysis: SVM
Linear kernel SVM has emerged as one of the most popular supervised machine learning (ML) methods, with learning algorithms aimed at classification used in neuroimaging (42) and psychiatry studies (43). SVM uses a well-defined dataset to create a decision function or “hyperplane” that can best distinguish between categories, which can then be used to predict to which predefined group a new observation belongs. SVM can effectively handle high-dimensional data and is less prone to overfitting of the data (44). SVM classifies data points by maximizing the margin between classes in a high-dimensional space (45). It constructs an optimal classifier through a “training phase,” in which key brain features are identified to distinguish between two groups (such as patients vs. controls), which is then applied to categorize new, unseen data in the “testing phase.” Comparison studies between multivariate pattern recognition methods showed that SVM reduces the effect of noisy features that are highly correlated with each other in the presence of a large number of features (45). SVM can be combined with different methods for dimensionality reduction and feature selection to improve diagnostic accuracy (45). SVM was applied using the Statistics and Machine Learning Toolbox in Matlab. The main steps of the SVM method included: (a) preprocessing of features (regressing out age, gender, and dataset and normalizing each feature to [−1 1]), (b) feature extraction and selection within each cross validation (using an embedded feature selection method, which combines filter- and wrapper-based approaches, to select the most discriminative features), (c) training the SVM classifier model by 10-fold cross-validation, using the training data, and (d) evaluating the performance of the SVM model, using the 10% holdout evaluation data (46). For more information regarding each of the steps, see online supplements.
Correlation Analysis
We used SPSS software to calculate the correlations between identified features and clinical symptoms. Because some of the studies used CAPS-IV and others used CAPS-5, we used the index developed by Powers et al. (47) to convert the two versions of the CAPS into a common one for analysis. To correct for multiple correlations, we used an alpha of 0.0028 (0.05/18) for the 18 network connectivities identified based on the SVM implementation for the first comparison (PTSD with and without MDD vs. TEHC), and 0.0025 (0.05/20) for the 20 network connectivities for the second comparison (PTSD-alone vs. PTSD-MDD). We regressed out age, gender, and sites/scanners as covariates during the feature preprocessing, so no covariate was used during the correlation analysis. Combining data from different scanners with different scanning parameters and field strengths is common in the neuroimaging literature (48), and can yield reliable data (49), as long as the data are regressed out for scanner type. To examine associations between the identified features and treatment outcome in the sub-sample receiving PE, we also tested the correlations between pre-treatment rsFC features and reduction in PTSD symptoms from pre- to post-treatment in both CAPS and HAM-D.
Results
Demographics and clinical characteristics of the participants
The PTSD and the TEHC groups were not significantly different in gender (58 males for the PTSD group; 36 males for the control group; χ2(1)=1.4, p=.23) and age (42.22 years for the PTSD group; 40.80 years for the control group; t(177) = −0.63, p=.53). Patients with PTSD showed a significantly higher total CAPS score than TEHC (t=−15.92, p<.0001). We repeated the analyses to test potential differences between the PTSD with and without MDD comorbidity. No significant differences were found for age (t(101)= −1.17, p=.24), CAPS (t(101)= −0.79, p=.93), or gender (χ2(1) = 0.013, p=.91) between the two groups. As expected, the PTSD+MDD had higher HAM-D scores than the PTSD without MDD (M =18.67 and 12.82, respectively; t(101)= −4.8, p<.0001). Detailed demographic and clinical data are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the three groups
| TEHC | PTSD-alone | PTSD+MDD | |
|---|---|---|---|
| N | 76 | 51 | 52 |
| Gender, N (%) | |||
| Male | 36 (47.36%) | 29 (56.86%) | 29 (55.76%) |
| Female | 40 (52.64%) | 22 (43.14%) | 23 (44.24%) |
| Race, N (%) | |||
| Caucasian | 21 (27.63%) | 12 (23.52%) | 17 (32.69%) |
| African-American | 23 (30.26%) | 27 (52.94%) | 22 (42.30%) |
| Hispanic | 26 (34.21%) | 0 (0%) | 5 (9.61%) |
| Others | 6 (7.89%) | 12 (23.52%) | 8 (15.38%) |
| Age, mean years (SD) | 40.8 (15.8) | 40.6 (13.8) | 43.9 (14.7) |
| HAM-D, mean (SD) | 2.97 (3.5) | 12.8 (6.2) | 18.7 (6.1) |
| Total CAPS, mean (SD) | 6.3 (6.8) | 56.8 (23.5) | 57.2 (28.9) |
Discrimination between individuals with PTSD-all and TEHC
The classification of PTSD vs. TEHC revealed 18 final features (for the full list, see Figure 1) as the final selected subset based on the SVM implementation (AUC = 0.87, L = 0.16, validation testing set: accuracy = 70.6%). The most discriminative features differentiating PTSD from TEHC included within network connectivity in executive control network (ECN) including ECN.LPFCr-ECN.PPCr, ECN.LPFCl-ECN.PPCl, and within salience network (SN) (SN.ACC-SN.AInsulal, CMA-SN.AInsular; see Table 2 for network abbreviations). Compared with PTSD, TEHC showed stronger connectivity in the within ECN and SN networks. All abbreviations appear in Table 1.
Figure 1.
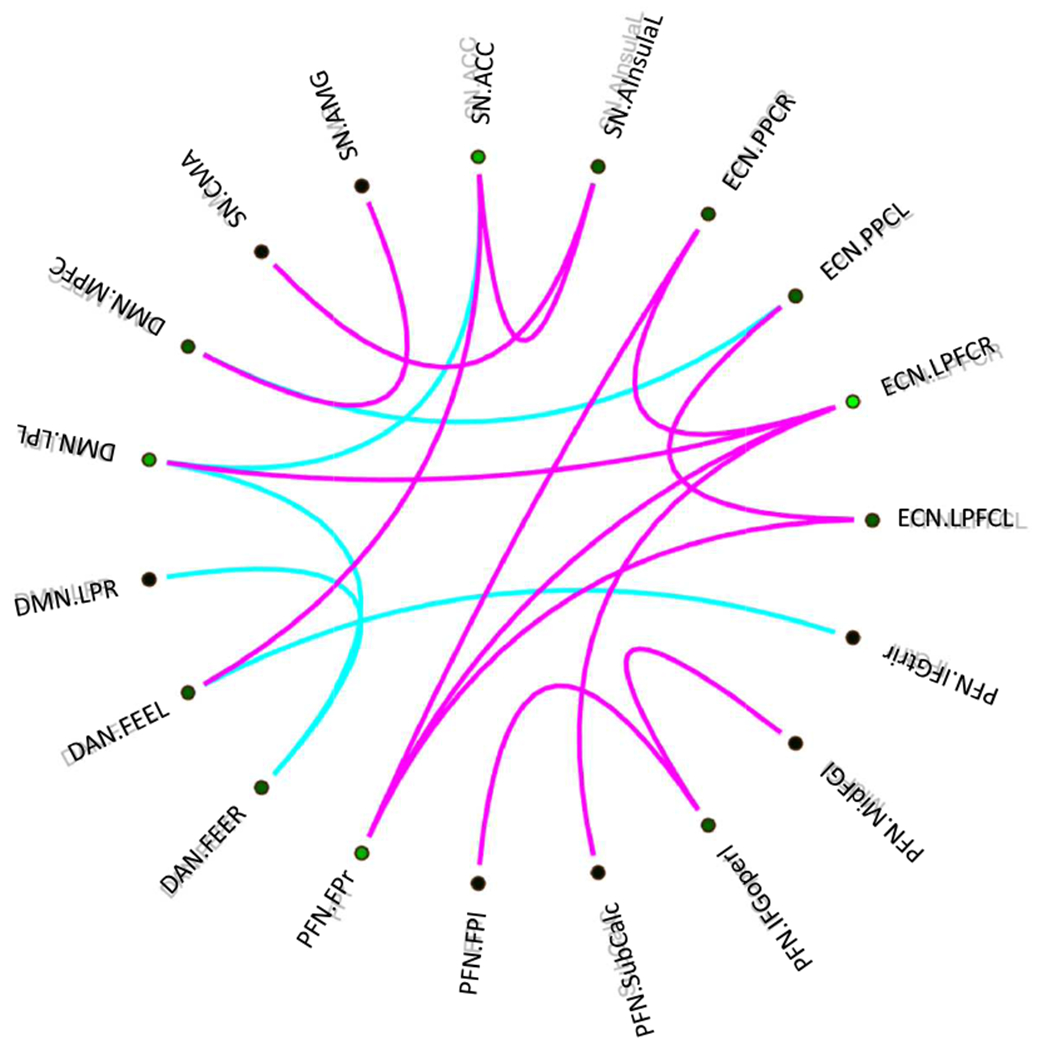
The most discriminative networks from differentiating PTSD-all from TEHC. Purple: TEHC>PTSD, blue: TEHC<PTSD. The figure represents the connectogram of the most discriminative multivariate features (spatial functional connectivity). The abbreviations are listed in Table 1.
Table 2:
Abbreviation of network names
| Network | Seed names | Abbreviation | Coordinates (x,y,z) |
|---|---|---|---|
| ECN | Networks.Executive Control.Lateral prefrontal cortex Left | ECN.LPFCl | −43,33,28 |
| Networks. Executive Control.Lateral prefrontal cortex Right | ECN.LPFCr | 41,38,30 | |
| Networks. Executive Control.Posterior parietal cortex Left | ECN.PPCl | −46,−58,49 | |
| Networks. Executive Control.Posterior parietal cortex Right | ECN.PPCr | 52,−52,45 | |
| SN | Networks.Salience.Anterior Cingulate Cortex | SN.ACC | 0,22,35 |
| Networks.Salience.AInsula Left | SN.Insulal | −44,13,1 | |
| Networks.Salience.AInsula Right | SN.Insular | 47,14,0 | |
| Networks.Salience.Rostral Prefrontal Left | SN.RPFCl | −32,45,27 | |
| Networks.Salience.Rostral Prefrontal Right | SN.PRFCr | 32,46,27 | |
| Networks.Salience.Supramarginal gyrus Left | SN.SMGl | −60,−39,31 | |
| Networks.Salience.Supramarginal gyrus Right | SN.SMGr | 62,−35,32 | |
| Atlas.Amygdala | AMG | ±23,−4,−18 | |
| Atlas.Basolateral amygdala | BLA | ±27,−7,−10 | |
| Atlas.Central medial amygdala | CMA | ±23,−6,−20 | |
| DMN | Networks.DefaultMode.MPFC | DMN.mPFC | 1,55,−3 |
| Networks.DefaultMode.lateral parietal Left | DMN.LPl | −39,−77,33 | |
| Networks.DefaultMode.lateral parietal Right | DMN.LRr | 47,−67,29 | |
| Networks.DefaultMode.Posterior Cingulate Cortex | DMN.PCC | 1,−61,38 | |
| Atlas.Anterior hippocampus | HIPA | ±30,−15,−18 | |
| Atlas.Posterior hippocampus | HIPP | ±29,−38,2 | |
| Atlas.Precuneus Cortex | Precuneus | 0,−65,41 | |
| BGN | Atlas.Nucleus Accumbens | NAcc | 10,12,−7 |
| Atlas.Thalamus | THA | ±10,−17,9 | |
| DAN | Networks.DorsalAttention.Frontal eye fields Left | DAN.FEFl | −27,−9,64 |
| Networks.DorsalAttention.Frontal eye fields Right | DAN.FEFr | 30,−6,64 | |
| Networks.DorsalAttention.Intraparietal sulcus Left | DAN.IPSl | −39,−43,52 | |
| Networks.DorsalAttention.Intraparietal sulcus Right | DAN.IPSr | 39,−42,54 | |
| PFN | Atlas.Superior Frontal Gyrus Right | SFGr | 16,18,61 |
| Atlas.Superior Frontal Gyrus Left | SFGl | −16,18,61 | |
| Atlas.Middle Frontal Gyrus Right | MidFGr | 43,18,45 | |
| Atlas.Middle Frontal Gyrus Left | MidFGl | −43,18,45 | |
| Atlas.Inferior Frontal Gyrus, pars triangularis Right | IFGtrir | 46,27,27 | |
| Atlas.Inferior Frontal Gyrus, pars triangularis Left | IFGtril | −46,27,27 | |
| Atlas.Inferior Frontal Gyrus, pars opercularis Right | IFGoperr | 54,16,19 | |
| Atlas.Inferior Frontal Gyrus, pars opercularis Left | IFGoperl | −54,16,19 | |
| Atlas.Frontal Pole Right | FPr | 31,59,13 | |
| Atlas.Frontal Pole Left | FPl | −31,59,13 | |
| Atlas.Subcallosal Cortex | SubCalC | 0,21,−13 | |
| Atlas.Frontal Orbital Cortex Right | OFCr | 32,24,−15 | |
| Atlas.Frontal Orbital Cortex Left | OFCl | −32,24,−15 | |
| SMN | Networks.SensoriMotor.Lateral Left | SMN.Ll | −55,−12,29 |
| Networks.SensoriMotor.Lateral Right | SMN.Lr | 56,−10,29 | |
| Networks.SensoriMotor.Superior | SMN.S | 0,−31,67 |
Discriminative features also emerged between network connectivity between SN-DAN, SN-DMN, DMN-DAN, and DMN-ECN. Compared with patients with PTSD, TEHC showed lower connectivity in the DMN-DAN, SN-DMN, but higher connectivity in SN-DAN, SN-DMN, and DMN-ECN. For a full list of areas see Table S2.
Discrimination of PTSD+MDD individuals from PTSD alone
The classification of PTSD alone vs. PTSD+MDD revealed 20 final features (for the full list, see Figure 2) as the final selected subset based on the SVM implementation (training set AUC = 0.85, L = 0.15, validation testing set: accuracy = 76.7%). The most discriminative features differentiating PTSD alone from PTSD+MDD included within-network connectivity in the basal ganglia network (BGN; Nacc-THA), within DAN (DAN.FEFl-DAN.IPSr), and within SN (CMA-SN.RPFCl). PTSD-alone showed higher within connectivity than PTSD-MDD in BGN, but lower connectivity in within ECN, SN, and DAN.
Figure 2.
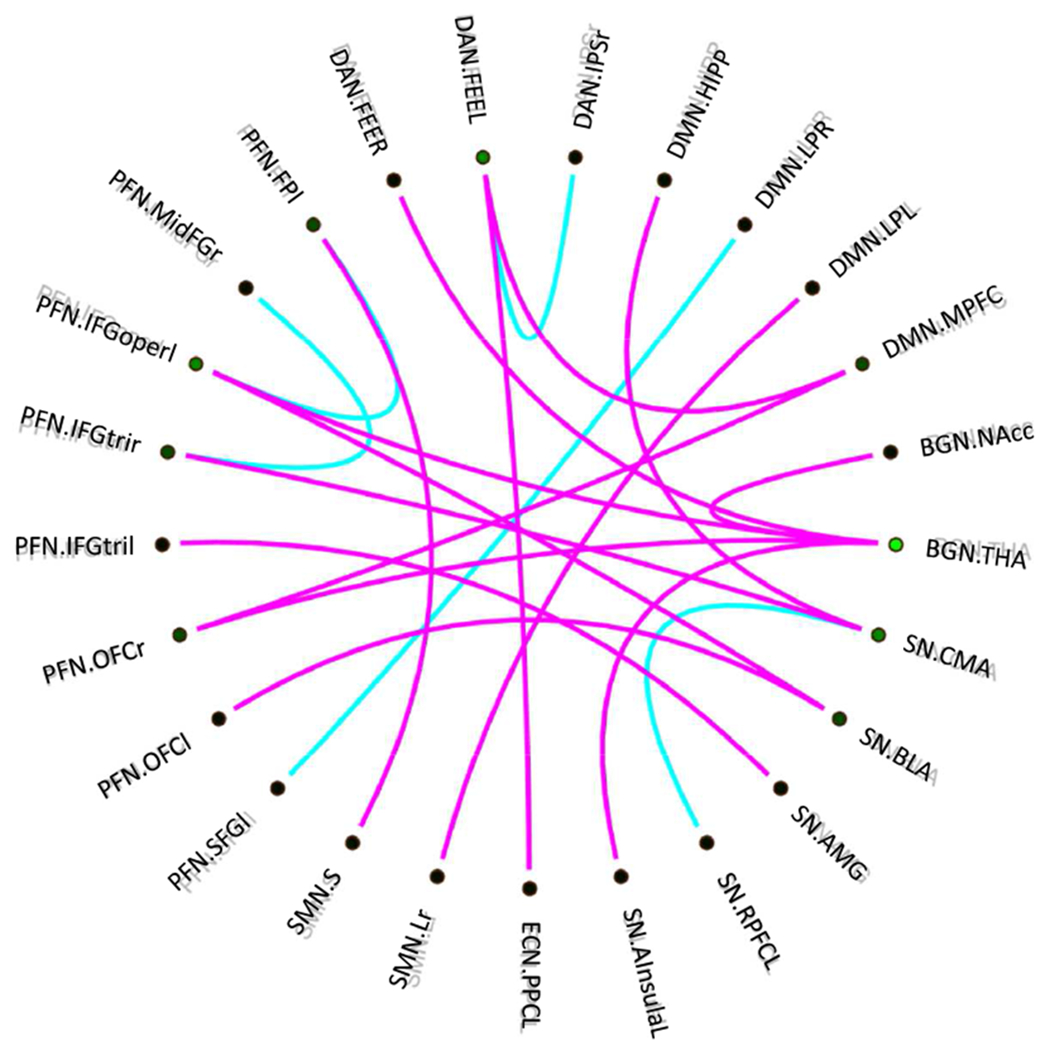
The most discriminative networks from differentiating PTSD-alone from PTSD-MDD. Purple: PTSD-alone>PTSD-MDD, blue: PTSD-alone<PTSD-MDD. The figure represents the connectogram of the most discriminative multivariate features (spatial functional connectivity). The abbreviations are listed in Table 1.
Also, discriminative features emerged between BGN and other related networks including BGN-DAN, and BGN-SN as well as other between-networks connectivity including SN-DMN, DAN-ECN, and SMN-DMN. PTSD-alone showed higher connectivity in BGN-other networks, SN-DMN, DAN-ECN, and SMN-other networks. For a full list of areas see Table S3.
Associations between the identified biomarkers at baseline and PTSD and MDD symptomatology at baseline
We examined the correlations between rsFC features identified above and CAPS and HAMD symptom severity at baseline.
Associations between the identified biomarkers from PTSD-all and TEHC classification and symptomatology.
CAPS: Significant negative correlation was found between baseline CAPS scores and within ECN connectivity with p<0.001 (ECN.LPFCR-ECN.PPCR: r=−0.302, FPr-ECN.PPC: r=−0.248, ECN.LPFCL-ECN.PPCL: r=−0.237). HAMD: Significant negative correlation was found between baseline HAM-D scores and within ECN connectivity with p<0.001 (FPr-ECN.LPFCR: r=−0.239, p=0.002).
Associations between the identified biomarkers from PTSD+MDD and PTSD-alone classification and symptomatology.
CAPS: No significant correlation was found in any networks. HAM-D: No significant correlation was found in any networks.
The utility of baseline biomarkers in predicting PE treatment outcome, calculated as changes in symptoms from pre- to post-treatment
We examined the correlations between the baseline rsFC features identified above and the changes in CAPS and HAM-D symptom severity from baseline to post-treatment.
Features identified from PTSD-all and TEHC classification.
CAPS: Higher within ECN connectivity (ECN.LPFCr-ECN.PPCr: r=0.455, p<0.001, FPr-ECN.LPFCr: r=0.415, p=0.002) correlated with greater PTSD CAPS symptom reduction. HAM-D: No significant correlation was found.
Features identified from PTSD-alone and PTSD-MDD classification.
CAPS: No significant association was found. HAMD: No significant association was found.
Trend level findings. Associations with symptomatology at baseline.
PTSD-all vs. TEHC: A trending negative correlation was found between baseline CAPS scores and within-SN connectivity (SN.ACC-SN.Insulal: r=−0.205, p=0.007). A trending positive correlation was found between baseline CAPS scores and within-DMN-DAN connectivity (DMN.LPR-DAN.FEFr:r=0.207, p=0.007). PTSD+MDD vs. PTSD-alone: A trending negative correlation was found between baseline CAPS scores and Nacc-THA (r=−0.203, p=0.041). A trending negative correlation was found also between baseline HRSD scores and THA-DAN.FEFr connectivity (r=−0.182, p=0.066). Associations with treatment response. A trending positive correlation was found between greater PTSD CAPS symptom reduction and within-ECN (FPr-ECN.PPCr: r=0.365, p=0.006) and within-SN connectivity (SN.ACC-SN. Ainsulal:r=0.284, p=0.035). For correlations with specific clusters of the CAPS see Tables S4–7.
Discussion
The present study identified functional connectivity biomarkers differentiating individuals with PTSD, PTSD+MDD, and TEHC, and demonstrated their clinical utility. SVM models were able to discriminate with a high level of accuracy between individuals with PTSD and TEHC, and between individuals with PTSD alone and those with comorbid PTSD+MDD. Specifically, we achieved 70.6% accuracy in discriminating between individuals with PTSD and TEHC, and 76.7% accuracy in discriminating between individuals with PTSD and those with PTSD+MDD for out-of-sample prediction. Within- and between-networks connectivity features differentiating PTSD from TEHC (Figure 1) and PTSD alone from PTSD+MDD (Figure 2) were consistent with at least some of the previous reports characterizing connectivity abnormalities in PTSD and attest to the importance of MDD-related abnormalities in differentiating between PTSD alone and PTSD+MDD. The identified altered connectivity features characterizing individuals with PTSD (with or without MDD comorbidity) compared with TEHC demonstrated clinical utility, as evident by the associations between these features and symptomatology and ability to predict treatment response.
The findings attest to the ability to differentiate between PTSD and TEHC with a relatively high level of accuracy. Consistent with at least some of the literature, among the most discriminative features were altered within-network connectivity in the SN and the ECN, as well as altered SN-DMN between-networks connectivity (4,5). The findings are consistent with some previous reports suggesting enhanced connectivity between amygdala and insula nodes within the SN (6,7) but not with other studies (4,50). In addition to the networks described in the literature on PTSD, the current findings also demonstrate the role of the triple network alteration, consisting of the ECN, the SN, and the DMN. It has been suggested that the SN integrates sensory, emotional, and cognitive information, acts as an interface between the DMN and the ECN to integrate and balance internal mental processes with external stimulus-driven cognitive and affective processes (51,52), and may be useful in differentiating individuals with PTSD from controls (53). Individuals with PTSD showed higher DMN-DAN network connectivity, which may reflect the abnormal cognitive function associated with PTSD. Currently, the diagnosis of PTSD relies on subjective reporting of symptoms. The altered network connectivities identified here may eventually be used to develop objective biomarkers for PTSD to help clinicians improve the accuracy of PTSD diagnosis.
The findings further demonstrate the ability to differentiate between PTSD+MDD from PTSD alone, with 76.7% accuracy. Among the most discriminative rsFC abnormalities in PTSD+MDD vs. PTSD alone were those related to reward dysfunctions, which are typical of patients with MDD (54). Individuals with PTSD+MDD vs. PTSD alone showed rsFC abnormalities within the BGN, which has been found to underlie reward behavior in prior reports (55). BGN comprises the striatum (subdivided into the caudate nucleus and putamen), globus pallidus, and thalamus (56). Altered BGN connectivity in individuals with PTSD+MDD, as opposed to those with PTSD alone, may underlie impaired motivation and a high prevalence of addictions and substance use in this subpopulation (57). The findings also attest to the importance of identifying not only within-network but also between-networks impairments, indicating both altered BGN within-network connectivity and altered connectivity between BGN and other related networks (BGN-DAN and BGN-SN) in PTSD+MDD vs. PTSD alone.
Unique brain-based biomarkers differentiating PTSD alone from PTSD+MDD may help explain divergent findings in PTSD connectivity studies enrolling heterogeneous populations, mixing individuals with PTSD alone and those with PTSD+MDD. Including different proportions of individuals with PTSD alone and PTSD+MDD may influence which networks show the most altered connectivity. Moreover, features differentiating PTSD+MDD from PTSD alone may be useful in identifying novel therapeutic targets, which are much needed in this comorbid subgroup that is frequently non-responsive to treatment and shows poor prognosis (18). Currently, targets of intervention for PTSD include fear processing pathways but do not address MDD-related deficits. Potentially distinct patterns of brain regions may be involved in fear and reward processing in individuals with PTSD alone and those with comorbid PTSD+MDD. PTSD may be associated with decreased connectivity of pathways that are key to fear processing and fear expression, such as the BLA-orbitalfrontal cortex (OFC) and CMA-thalamus, respectively (58). PTSD comorbidity with MDD may be associated with decreased connectivity of pathways, which are key to the reward system, for example, decreased connectivity across multiple amygdala and striatal-subcortical pathways: BLA-OFC; NAcc-thalamus; and NAcc-hippocampus (59). Thus, it has been suggested that comorbid PTSD+MDD is associated with multifaceted functional connectivity alterations in both fear and reward systems (24). The present findings support this suggestion. The fact that available treatments do not focus on the specific patterns of alteration that characterize individuals with PTSD+MDD may explain the poor prognosis of currently available treatments for this subpopulation, compared to that of patients with PTSD alone (24). Given the importance of the BGN and reward-related abnormalities in PTSD+MDD vs. PTSD alone, new therapeutic solutions for individuals with PTSD+MDD are needed, which target the altered BGN, such as those focusing on dopaminergic targets.
Several post hoc explanations may be suggested why classification accuracy for PTSD vs. PTSD+MDD was higher than for PTSD vs. non-PTSD. One explanation is that the NAcc may play a critical role in differentiating those with and without MDD comorbidity, resulting in higher heterogeneity within the PTSD diagnosis (that is, between PTSD+MDD vs. PTSD alone) than between individuals who were exposed to trauma and developed PTSD and those who did not develop PTSD (24). This and other post hoc explanations should be considered with caution, however, because the difference between the clarification accuracy of PTSD vs. TEHC and PTSD alone vs. PTSD+MDD was only of 6.1% (70.6% and 76.7%, respectively).
Findings demonstrate the clinical utility of the identified biomarkers discriminating PTSD-all from TEHC. Specifically, significant associations were found between alteration in within ECN-connectivity and PTSD and MDD symptoms, such that higher connectivity was associated with more severe symptoms. The identified biomarkers were also capable of predicting treatment response: lower within-ECN connectivity were associated with greater PTSD symptom reduction. This finding is consistent with a previous report demonstrating decreased connectivity within the ECN in patients with PTSD, potentially representing diminished emotion regulation abilities (i.e., inability to downregulate negative emotions) (4). Interestingly, the identified biomarkers discriminating PTSD alone from PTSD+MDD were not significantly associated with symptomatology and treatment response. One potential post-hoc explanation is that the received treatment focused on PTSD, and that treatment focusing on MDD may have yielded different results.
Several limitations should be noted. First, the present study combined data from three separate trials to increase sample size, with some differences between the trials in their inclusion and exclusion criteria, as well as differences between scanners in spatial or temporal signal-to-noise ratio. Additionally, we relied exclusively on differences between DSM disorders, despite the potential interest in within-disorder variance, including categorization options that transcend the boundaries of clinical diagnosis. Future studies should implement unsupervised machine learning approaches that can complement the current findings by determining the extent to which the biomarkers identified here for PTSD+MDD and PTSD are indeed those that create distinct subpopulations of patients. This could determine whether the identified data-driven biotypes of homogeneous patterns of dysfunctional connectivity match those found in the present study. Future studies with larger samples should also explore the association between the different clusters of PTSD symptoms and the identified resting-state features. Finally, it should be noted that because a trauma-unexposed subgroup was not included, the effect of trauma exposure could not be tested.
These caveats notwithstanding, the current findings suggest that unique sets of brain-based biomarkers differentiate between PTSD (with and without comorbid MDD) and TEHC, as well as between PTSD alone and PTSD+MDD. Certain connectivity alterations in the PTSD+MDD comorbid population vs. PTSD alone may explain inconsistencies between previous studies that enrolled diverse participant populations. The present findings suggest that brain function abnormalities observed in PTSD+MDD vs. PTSD alone during fMRI resting state were those related to cortical-limbic dysregulation, which are the basis of MDD etiology and describe altered connections. The findings also stress the importance of the triple network in PTSD. The findings further demonstrate the clinical utility of the identified connectivity alterations, especially within ECN, by demonstrating its associations with PTSD and MDD symptoms, and its ability to predict subsequent treatment response. Taken together, the findings support the potential of resting-state fMRI to inform accurate future clinical assessment of psychopathology in individuals at high risk of developing PTSD following exposure to trauma, by the development of objective biomarkers indicative of the diagnostic heterogeneity of psychopathology and of treatment prognosis. Such objective biomarkers may facilitate the early identification of heterogeneous subtypes of illness. Neuroimaging techniques hold the promise to aid in the clinical assessment of individual psychiatric patients, particularly in cases in which a clear differential diagnosis is difficult to establish because of comorbidity. If these findings are replicated in future research, they can make an important contribution to accurate diagnosis and help identify precise targets for maximally efficient treatment of PTSD+MDD and PTSD alone.
Supplementary Material
Acknowledgments
Disclosures and acknowledgements: Work on this paper was supported by NIMH grant R01 MH111596 (Dr. Rutherford), R01MH105355 (Dr. Neria), R01MH072833 (Dr. Neria), and 5T32MH020004-20 (Dr. Pickover). All authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sigal Zilcha-Mano, Department of Psychology, University of Haifa, Mount Carmel, Haifa 31905, Israel.
Xi Zhu, Department of Psychiatry, Columbia University, New York, New York; New York State Psychiatric Institute, Columbia University Medical Center, New York, New York.
Benjamin Suarez-Jimenez, Department of Psychiatry, Columbia University, New York, New York; New York State Psychiatric Institute, Columbia University Medical Center, New York, New York.
Pickover Alison, Department of Psychiatry, Columbia University, New York, New York; New York State Psychiatric Institute, Columbia University Medical Center, New York, New York
Shachaf Tal, Department of Psychology, University of Haifa
Such Sara, New York State Psychiatric Institute, Columbia University Medical Center, New York, New York
Caroline Marohasy, New York State Psychiatric Institute, Columbia University Medical Center, New York, New York
Marika Chrisanthopoulos, Columbia University College of Physicians and Surgeons; New York State Psychiatric Institute
Chloe Salzman, Columbia University College of Physicians and Surgeons; New York State Psychiatric Institute
Amit Lazarov, Department of Psychiatry, Columbia University Medical Center, New York, NY, USA; School of Psychological Sciences, Tel-Aviv University, Tel-Aviv, Israel
Yuval Neria, Department of Psychiatry, Columbia University, New York, New York; New York State Psychiatric Institute, Columbia University Medical Center, New York, New York.
Bret R. Rutherford, Columbia University College of Physicians and Surgeons; New York State Psychiatric Institute.
Reference
- 1.Kessler RC, Chiu WT, Demler O, Walters EE (2005): Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galatzer-Levy IR, Bryant RA (2013): 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci 8: 651–662. [DOI] [PubMed] [Google Scholar]
- 3.Stein DJ, McLaughlin KA, Koenen KC, Atwoli L, Friedman MJ, Hill ED, et al. (2014): DSM-5 and ICD-11 definitions of posttraumatic stress disorder: Investigating “narrow” and “broad” approaches. Depress Anxiety 31: 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2016): Aberrant resting-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depress Anxiety 33: 592–605. [DOI] [PubMed] [Google Scholar]
- 5.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I (2012): Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med 74: 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I (2012): Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinak CA, Angstadt M, Welsh RC, Kennedy A, Lyubkin M, Martis B, Phan KL (2011): Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front psychiatry 2: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. (2009): Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J psychiatry Neurosci JPN 34: 187. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen AC, Etkin A (2013): Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 38: 1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, Lanius RA (2015): Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr Scand 132: 29–38. [DOI] [PubMed] [Google Scholar]
- 11.New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, et al. (2009): A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry 66: 656–664. [DOI] [PubMed] [Google Scholar]
- 12.Xiong K, Zhang Y, Qiu M, Zhang J, Sang L, Wang L, et al. (2013): Negative emotion regulation in patients with posttraumatic stress disorder. PLoS One 8: e81957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown VM, LaBar KS, Haswell CC, Gold AL, Workgroup M-AM, Beall SK, et al. (2014): Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, et al. (2014): Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol Med 44: 1927–1936. [DOI] [PubMed] [Google Scholar]
- 15.Kennis M, Rademaker AR, van Rooij SJH, Kahn RS, Geuze E (2015): Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum Brain Mapp 36: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA (2013): The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. J Trauma Stress 26: 299–309. [DOI] [PubMed] [Google Scholar]
- 17.Campbell DG, Felker BL, Liu C-F, Yano EM, Kirchner JE, Chan D, et al. (2007): Prevalence of depression–PTSD comorbidity: Implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med 22: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green BL, Krupnick JL, Chung J, Siddique J, Krause ED, Revicki D, et al. (2006): Impact of PTSD comorbidity on one-year outcomes in a depression trial. J Clin Psychol 62: 815–835. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA psychiatry 72: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, Liu J, Liu T, Ma L, Wang W, Shi S, et al. (2019): Altered Resting-State Functional Activity in Medication-Naive Patients with First-Episode Major Depression Disorder versus Healthy Control: A Quantitative Meta-Analysis. Front Behav Neurosci 13: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Wang Y, Wu X, Huang H, Jia Y, Zhong S, et al. (2018): Shared and specific functional connectivity alterations in unmedicated bipolar and major depressive disorders based on the triple-network model. Brain Imaging Behav 1–14. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Yu L, Wu F, Wu H, Wang J (2019): Altered whole brain functional connectivity pattern homogeneity in medication-free major depressive disorder. J Affect Disord 253: 18–25. [DOI] [PubMed] [Google Scholar]
- 23.Kennis M, Rademaker AR, van Rooij SJH, Kahn RS, Geuze E (2013): Altered functional connectivity in posttraumatic stress disorder with versus without comorbid major depressive disorder: A resting state fMRI study. F1000Research 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, et al. (2017): Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress Anxiety 34: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. (2010): Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand 121: 33–40. [DOI] [PubMed] [Google Scholar]
- 26.Titcombe-Parekh RF, Chen J, Rahman N, Kouri N, Qian M, Li M, et al. (2018): Neural circuitry changes associated with increasing self-efficacy in Posttraumatic Stress Disorder. JPsychiatr Res 104: 58–64. [DOI] [PubMed] [Google Scholar]
- 27.King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, et al. (2016): Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress Anxiety 33: 289–299. [DOI] [PubMed] [Google Scholar]
- 28.Gong Q, Li L, Du M, Pettersson-Yeo W, Crossley N, Yang X, et al. (2014): Quantitative prediction of individual psychopathology in trauma survivors using resting-state FMRI. Neuropsychopharmacology 39: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson AA, Densmore M, McKinnon MC, Neufeld RWJ, Frewen PA, Theberge J, et al. (2019): Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: a multimodal neuroimaging approach. Psychol Med 49: 2049–2059. [DOI] [PubMed] [Google Scholar]
- 30.Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A (2012): Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev 36: 1140–1152. [DOI] [PubMed] [Google Scholar]
- 31.Fu CHY, Costafreda SG (2013): Neuroimaging-based biomarkers in psychiatry: clinical opportunities of a paradigm shift. Can J Psychiatry 58: 499–508. [DOI] [PubMed] [Google Scholar]
- 32.Wolfers T, Buitelaar JK, Beckmann CF, Franke B, Marquand AF (2015): From estimating activation locality to predicting disorder: a review of pattern recognition for neuroimaging-based psychiatric diagnostics. Neurosci Biobehav Rev 57: 328–349. [DOI] [PubMed] [Google Scholar]
- 33.Gong Q, Li L, Tognin S, Wu Q, Pettersson-Yeo W, Lui S, et al. (2014): Using structural neuroanatomy to identify trauma survivors with and without post-traumatic stress disorder at the individual level. Psychol Med 44: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Wu Q, Zhu H, He L, Huang H, Zhang J, Zhang W (2016): Multimodal MRI-based classification of trauma survivors with and without post-traumatic stress disorder. Front Neurosci 10: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Association AP, Association AP (2000): Diagnostic and statistical manual of mental disorders (revised 4th ed.). Washington, DC: Author. [Google Scholar]
- 36.Association AP (2013): Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, Williams JBW (1996): Structured clinical interview for DSM-IV axis I disorders research version (SCID-I). New York, NY Biometrics Res New York State Psychiatr Inst. [Google Scholar]
- 38.Weathers FW, Keane TM, Davidson JRT (2001): Clinician-Administered PTSD Scale: A review of the first ten years of research. Depress Anxiety 13: 132–156. [DOI] [PubMed] [Google Scholar]
- 39.Foa E, Hembree E, Rothbaum BO (2007): Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. Oxford University Press. [Google Scholar]
- 40.Helpman L, Papini S, Chhetry BT, Shvil E, Rubin M, Sullivan GM, et al. (2016): PTSD remission after prolonged exposure treatment is associated with anterior cingulate cortex thinning and volume reduction. Depress Anxiety 33: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitfield-Gabrieli S, Nieto-Castanon A (2012): Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2: 125–141. [DOI] [PubMed] [Google Scholar]
- 42.Craddock RC, Holtzheimer III PE, Hu XP, Mayberg HS (2009): Disease state prediction from resting state functional connectivity. Magn Reson Med An Off J Int Soc Magn Reson Med 62: 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L (2005): Support Vector Machines: Theory and Applications, vol. 177 Springer Science & Business Media. [Google Scholar]
- 44.Mourao-Miranda J, Reinders A, Rocha-Rego V, Lappin J, Rondina J, Morgan C, et al. (2012): Individualized prediction of illness course at the first psychotic episode: a support vector machine MRI study. Psychol Med 42: 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira F, Mitchell T, Botvinick M (2009): Machine learning classifiers and fMRI: a tutorial overview. Neuroimage 45: S199–S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyrba M, Grothe M, Kirste T, Teipel SJ (2015): Multimodal analysis of functional and structural disconnection in A lzheimer’s disease using multiple kernel SVM. Hum Brain Mapp 36: 2118–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powers A, Fani N, Murphy L, Briscione M, Bradley B, Tone EB, et al. (2019): Attention bias toward threatening faces in women with PTSD: eye tracking correlates by symptom cluster. Eur J Psychotraumatol 10: 1568133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adhikari BM, Jahanshad N, Shukla D, Turner J, Grotegerd D, Dannlowski U, et al. (2019): A resting state fMRI analysis pipeline for pooling inference across diverse cohorts: an ENIGMA rs-fMRI protocol. Brain ImagingBehav 13: 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Sullivan EV (2012): Combining atlas-based parcellation of regional brain data acquired across scanners at 1.5 T and 3.0 T field strengths. Neuroimage 60: 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Block SR, King AP, Sripada RK, Weissman DH, Welsh R, Liberzon I (2017): Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cogn Affect Behav Neurosci 17: 422–436. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Li L, Li B, Feng N, Li L, Zhang X, et al. (2017): Decreased triple network connectivity in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. Sci Rep 7: 12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15: 483–506. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Li L, Li B, Zhang X, Lu H (2017): Decreased triple network connectivity in patients with post-traumatic stress disorder. Medical Imaging 2017: Biomedical Applications in Molecular, Structural, and Functional Imaging, vol. 10137 10137: 101370Q. [Google Scholar]
- 54.Admon R, Pizzagalli DA (2015): Dysfunctional reward processing in depression. Curr Opin Psychol 4: 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt A, Denier N, Magon S, Radue E- W, Huber CG, Riecher-Rossler A, et al. (2015): Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Transl Psychiatry 5: e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kandel ER, Schwartz JH, Jessell TM, Jessell D of B and MBT, Siegelbaum S, Hudspeth AJ (2000): Principles of Neural Science, vol. 4 McGraw-hill; New York. [Google Scholar]
- 57.Anderson RE, Hruska B, Boros AP, Richardson CJ, Delahanty DL (2018): Patterns of co-occurring addictions, posttraumatic stress disorder, and major depressive disorder in detoxification treatment seekers: Implications for improving detoxification treatment outcomes. J Subst Abuse Treat 86: 45–51. [DOI] [PubMed] [Google Scholar]
- 58.Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004): Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43: 897–905. [DOI] [PubMed] [Google Scholar]
- 59.Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK (2009): Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry 66: 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


