Abstract
Topic:
To review the available literature on the prevalence, incidence, natural history, and exudative conversion rates of subclinical (treatment naïve) non-exudative macular neovascularization (MNV) in patients with age-related macular degeneration (AMD).
Clinical Relevance:
Non-exudative MNV is now known to be more prevalent in patients with AMD than initially thought and is bringing new insights into both the natural history and management of this very prevalent disease.
Methods:
We conducted a literature search on PubMed, Scopus and Web of Science, along with a manual search, from January 2014 to June 2019. We included studies that used optical coherence tomography angiography (OCTA) as a primary diagnostic tool to evaluate subclinical (treatment naïve), non-exudative, neovascular AMD.
Results:
Of the 258 screened articles, 12 were included. The prevalence of subclinical non-exudative neovascular AMD in the fellow eyes of patients with unilateral exudative AMD ranged from 6.25% to 27%. Although these lesions were not associated with a significant decrease in visual acuity, the presence of non-exudative MNV seems to be an important predictor of exudative disease. Incidence of exudation in the reviewed studies ranged from 20–80% (follow-up 6 months to 2 years). There is some evidence that non-exudative MNV may slow down the growth of adjacent geographic atrophy (GA). As long as exudation does not occur, it appears that subclinical non-exudative MNV is not responsible for the deterioration of visual function.
Conclusions:
Non-exudative MNV is an asymptomatic condition. While non-exudative MNV appears to be a precursor andfor the formation of exudative neovascular AMD, there is evidence suggesting a protective effect in slowing the progression of GA. Early detection of non-exudative MNV before exudation develops should result in better monitoring of patients who are at high risk of conversion to exudative AMD.While no controlled clinical trial has been performed to provide definitive recommendations, the authors of the studies included in this review agree that non-exudative lesions should not be treated until symptomatic exudation develops. Moreover, the existence of a non-exudative form of neovascular AMD would suggest that the term neovascular AMD should be preceded by either exudative or non-exudative when describing this neovascular stage of AMD.
Keywords: OCTA, non-exudative, subclinical, macular neovascularization, age-reated macular degeneration
Précis
- Non-exudative neovascular lesions are a risk factor for exudative disease, appear to have a protective effect in slowing the progression of atrophy and should not be treated until exudation appears.
INTRODUCTION
Optical coherence tomography (OCT) angiography (OCTA) has improved our ability to non-invasively detect and characterize macular neovascularization (MNV).1,2,11,12,3–10 Prior to OCTA, the onset of MNV in clinical practice corresponded to the onset of exudation or hemorrhage as detected by funduscopic exam, fluorescein angiography (FA), indocyanine green angiography (ICGA), or structural OCT scanning. The characteristic findings when using these diagnostic strategies include the presence of macular exudates and hemorrhage on fundoscopic evaluation, leakage on FA,13 hot spot or plaques on ICGA,14,15 and the presence of intra-retinal, sub-retinal fluid, or fluid beneath the retinal pigment epithelium (RPE) on structural OCT imaging.16 Currently, in most clinical practices, the presence of macular fluid seen on structural OCT imaging serves as the basis for treatment decisions using vascular endothelial growth factor (VEGF) inhibitors16,17 in patients with exudative MNV. However, MNV can be present even in the absence of detectable macular fluid by OCT imaging or leakage by FA.1,2,11,12,17,3–10
The first reports of non-exudative MNV came from postmortem eyes with AMD in the 1970’s. Green et al18 and Sarks et al19 described abnormal choroidal vessels passing through breaks in Bruch’s membrane without evidence of overlying hemorrhage or exudation. They hypothesized that these neovascular lesions pre-dated exudation. Histopathological studies of autopsy eyes with AMD without clinical signs of exudation have confirmed the presence of these subclinical fibrovascular lesions beneath the RPE.20,21 In the 1990’s, Chang et al22 reported the clinicopathologic correlations from a case of non-exudative MNV. In this case, a patient developed MNV that was observed with ICGA, but the MNV was not detected on FA. ICGA was able to detect a subclinical MNV by the presence of a hyperfluorescent plaque with late staining. This MNV persisted without signs of exudation for four years. Schneider et al23 and Hanutsaha et al24 used ICGA to detect the presence of subclinical, non-exudative MNV and found that eyes with these lesions, seen on ICGA as focal spots or ICGA plaques, were associated with an increased risk of exudation during the follow-up period.
Despite these findings, the use of ICGA to identify non-exudative MNV in clinical practice was not routinely performed due to the invasive nature of this imaging modality, its risk of an allergic reaction, the time and expense, and the fact that the risks and costs outweighed the potential benefits of early detection since no therapy existed for these non-exudative lesions. Because of these limitations, ICGA monitoring of eyes with non-exudative AMD never became a routine screening technique. However, OCTA is a faster, less expensive, non-invasive, contrast-free macular angiographic technique that can be used to monitor patients with non-exudative AMD and to detect non-exudative MNV at follow-up visits.1,2,11,12,3–10
Currently, with the increased availability of OCTA, studies have begun to explore the exact incidence, prevalence, and natural history of non-exudative MNV in asymptomatic eyes with AMD.1,2,11,12,3–10 The aim of this paper is to review the available literature and describe the available data on the prevalence, incidence, natural history, and exudative conversion rates of treatment naïve non-exudative subclinical MNV in patients with AMD.
METHODS
We conducted a systematic review in accordance with the guidelines presented by the Cochrane Collaboration25. Ethics Committee of Centro Hospitalar São João/Faculty of Medicine of the University of Porto ruled that approval was not required for this study”.
Terminology
For the purpose of this paper the following terminology will be used:
Non-Exudative AMD: No evidence of macular fluid on OCT imaging or leakage by FA
Exudative Neovascular AMD: Evidence of MNV on OCTA and macular fluid by structural OCT, evidence of leakage on FA, with or without evidence of a plaque or hot spot on ICGA
Non-Exudative, Neovascular AMD: Evidence of MNV by OCTA or the presence of a plaque or hot spot on ICGA, but no leakage on FA and no macular fluid by structural OCT imaging. May refer to a lesion that is treatment naïve or a lesion that had exudation, but is now fluid-free after anti-VEGF therapy
Subclinical (Treatment Naïve), Non-Exudative, Neovascular AMD: Asymptomatic MNV that has never received treatment and has never shown evidence of exudation
Search Strategy
We conducted a systematic review and comprehensive search across several electronic databases from 1st January 2014 to 30th June2019 – Pubmed, SCOPUS and Web of Science – using the following query: ((non-exudative) OR (nonexudative) OR (subclinical) OR (asymptomatic) OR (quiescent) OR (remission)) AND ((choroidal neovascularization) OR (CNV) OR (neovascular) OR (neovascularization) OR (macular neovascularization) or (MNV)) AND ((age-related macular degeneration) OR (AMD)). Furthermore, we conducted a hand-search of references of the included studies and reviews.
Eligibility Criteria
In this review, we focused on studies describing the natural history of subclinical, treatment naïve, non-exudative neovascular AMD. Identified publications were screened manually based on the title and abstract in accordance with Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines26.
We included studies that fulfilled all the inclusion criteria listed below:
Studies analyzing subclinical (treatment naïve), non-exudative, neovascular AMD.
Studies using OCTA as primary diagnostic tool.
Prospective and retrospective studies presenting a cohort, case–control or cross-sectional study design. Case reports and synthesis studies (systematic reviews and meta-analysis) were excluded. Bibliographic references from relevant reviews and meta-analyses were reviewed for additional relevant studies. Non-human studies were also excluded.
We placed no restrictions or limits during the search process (language, time or country of origin).
Data Extraction
The primary quantitative outcome was the prevalence of treatment naïve non-exudative neovascular AMD. In addition, we recorded, when available, the percentage of conversion to exudative disease, the follow up time, and the predictive factors and biomarkers for this conversion. Other insights in the natural history and imaging techniques of non-exudative neovascular AMD were also extracted.
Synthesis of Evidence
Narrative synthesis of evidence was undertaken for all included studies. Data regarding subclinical, non-exudative neovascular AMD were manually extracted and categorized as follows: prevalence, funduscopic and OCT characterization/classification, SS-OCTA versus SD-OCTA for detecting MNV, segmentation for the visualization of MNV, incidence of conversion into exudative MNV, predictors of exudation, relation with GA, and treatment insights. Meta-analytic methods could not be employed due to heterogeneity of study design and outcomes.
RESULTS
Figure 1 represents the search strategy and its results. A total of 12 studies were identified after a careful evaluation (Table 1). The main findings of these studies are described below.
Figure 1. Flow chart of the search strategy.
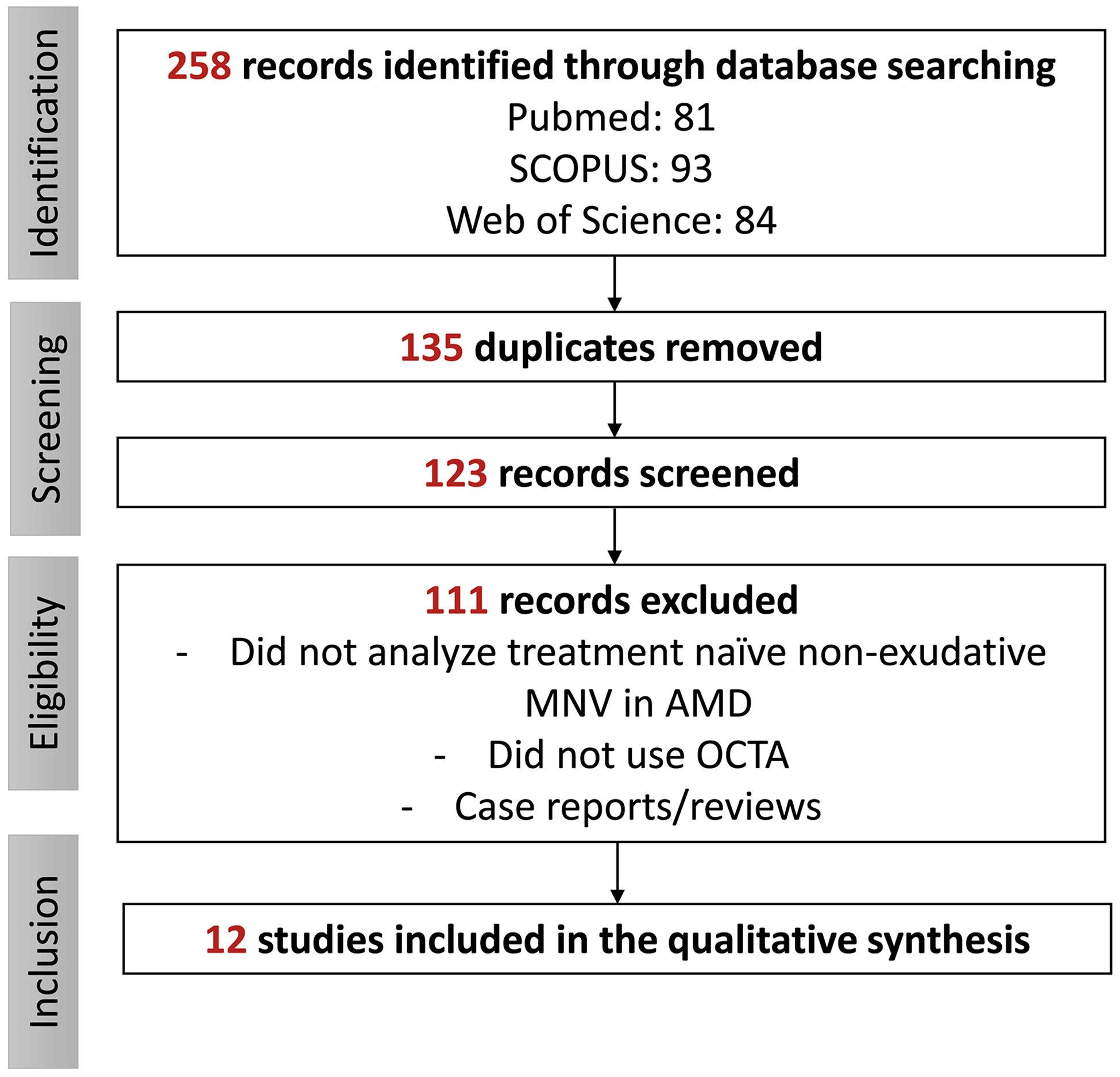
AMD: age-related macular degeneration. MNV: macular neovascularization. OCTA: optical coherence tomography angiography.
Table 1.
Summary of the studies that documented the prevalence of subclinical nonexudative macular neovascularization and/or incidence of exudation.
| Author (year) | Type of study | N. eyes/Pts | Study summary | Methods used to detect MNV | Prevalence of nonexudative MNV | Incidence of exudation; follow up | Predictors/associations with exudation in subclinical NV |
|---|---|---|---|---|---|---|---|
| Palejwala et al (2015)12 | Observational, prospective | 32/32 | Patients with unilateral exudative AMD in fellow eye. | SD-OCTA, SD-OCT | 6.25% (2/32) | NA | NA |
| Choi et al (2015)8 | Observational, prospective | 12/7 | 63 eyes from 32 normal subjects and 12 eyes from 7 patients with non-exudative AMD with geographic atrophy. | SS-OCTA, SS-OCT, FA, ICGA | 16.7% (2/12) | NA | NA |
| Carnevali et al (2016)4 | Observational, prospective | 22/20 | Diagnostic tool validity assessment. | SD-OCTA, SD-OCT, FA, ICGA | NA | NA | NA |
| Roisman et al (2016)2 | Observational, prospective | 11/11 | Asymptomatic, iAMD in one eye and exudative AMD in the fellow eye. | SS-OCTA, SD-OCT, FA, ICGA | 27% (3/11) | NA | NA |
| Capuano et al (2017)10 | Observational, retrospective | 644/399 | Unilateral or bilateral geographic atrophy secondary to AMD. | SD-OCT, SD-OCTA, FA, ICGA | 11% (73/644) | 26% (5/19) by 45.7 ± 14.7 months (overall rate based on the total follow-up interval) | - In the 2 cases that developed exudation, capillaries and inner and margin loops typical of active neovascularization developed within the neovascular lesion. |
| Yanagi et al (2017)*3 | Observational, prospective | 76/76 | Patients with unilateral exudative AMD or PCV in the fellow eye. | SS-OCTA, SD-OCT, FA, ICGA | 18% (14/76) | NA | - Pachychoroid pigment epitheliopathy was the only risk factor associated with the presence of non-exudative MNV. |
| De Oliveira Dias et al (2017)**11 | Observational, prospective | 160/160 | Patients with iAMD or GA secondary to AMD in one eye and exudative AMD in the fellow eye. | SS-OCTA, SD OCT | 14.4% (23/160) | 24% by one year using Kaplan-Meier plot (cumulative, range 1–31 months) | - For eyes with subclinical MNV at the time of first OCTA imaging, the incidence of exudation was 21.1%, and for eyes without subclinical MNV, the incidence was 3.6%. - After the detection of subclinical MNV, the risk of exudation was 15.2 times greater than in eyes without subclinical MNV. |
| Treister et al (2018)***9 | Observational, retrospective | 34/34 | Patients with unilateral exudative AMD in the fellow eye. | SD-OCTA | 15% (5/34) | NA | |
| Yanagi et al (2018)*7 | Observational, prospective | 95/95 | Patients with unilateral exudative AMD or PCV in fellow eye. | SS-OCTA, SD-OCT, FA, ICGA | 19% (18/95) | 22.2% (4/18) by 6 months (overall rate based on the total follow-up interval) | - The probability of developing exudation was significantly higher in eyes with baseline non-exudative MNV (22%) compared with eyes without any MNV (2%; p<0.05). |
| Bailey et al (2019)6 | Observational, prospective | 63/63 | Patients with unilateral exudative AMD in fellow eye. | SD OCTA, SD OCT | 8% (5/63) | 80% (8/10) by 2 years (overall rate based on the total follow-up interval) | - A non-exudative MNV was associated with an 18.1-fold increase of subsequently developing exudation. |
| Heiferman et al (2019)***5 | Observational, prospective | 34/34 | Patients with unilateral exudative AMD in fellow eye. | SD OCTA, SD OCT | 15% (5/34) | 20% (1/5) by 15.2±3.27 months (overall rate based on the total follow-up interval) | - GA grew at a rate of 0.82±1.20mm2/year in four eyes without subclinical MNV and 0.02mm2/year in one eye with subclinical MNV. |
| Yang et al (2019)**1 | Observational, prospective | 227/227 | Patients with unilateral exudative AMD in fellow eye. | SS-OCTA | 13.2% (30/227) | 34.5% by 2 years using Kaplan Meier plot | - The relative risk of exudation after detection of subclinical MNV was 13.6 times greater than in the absence of subclinical MNV (P<0.001). - There was no significant risk of exudation based on lesion size alone (p=0.91). |
Extensions of the previous studies (non-independent samples). Pts: patients. AMD: age-related macular degeneration. iAMD: intermediate age-related macular degeneration. OCT: optical coherence tomography angiography. OCTA: optical coherence tomography angiography. SS: swept source. SD: spectral domain. FA: fluorescein angiography. ICGA: Indocyanine green angiography. MNV: macular neovascularization. GA: geographic atrophy. PCV: polypoidal choroidal vasculopathy. NA: non-available.
Prevalence of Subclinical (Treatment naïve), Non-Exudative, Neovascular AMD
The prevalence of treatment naïve non-exudative neovascular AMD has been documented predominantly in the fellow eyes of patients with unilateral exudative neovascular AMD. We identified 9 studies that documented the prevalence of treatment naïve non-exudative MNV in these patients (seven were prospective and two were retrospective). Three studies (Treister et al9, Yanagi et al3, De Oliveira Dias et al11) had follow-up secondary publications with non-independent samples (the extensions of the mentioned studies were reported by Heiferman et al5, Yanagi7 et al and Yang et al1, respectively). The estimated prevalence of subclincal non-exudative neovascular AMD in the fellow eyes of patients with unilateral exudative AMD ranged from 6.25% to 27%.
We found three studies describing the prevalence of these lesions in eyes with geographic atrophy (GA). De Oliveira Dias et al11 and Yang et al1 characterized the presence of treatment naïve non-exudative MNV in eyes with GA and found the prevalence, incidence, and risk of exudation was no different than in eyes with intermediate AMD. Capuano et al10 analyzed 644 eyes from 399 consecutive patients with unilateral or bilateral GA secondary to AMD, and identified 73 eyes from 71 patients (11%) with treatment naïve non-exudative MNV. The values of prevalence per study are detailed in Table 1.
Functional Characterization of Treatment naïve, Non-exudative, Neovascular AMD
Although most of the studies reviewed did not specify the best-corrected visual acuity (BCVA) of the eyes with non-exudative MNV, they are mostly described as “asymptomatic” or “subclinical”1,5,9,11, and we may thus infer that there is not a significant decrease in the BCVA with the exception of those with central involving concomitant GA. Roisman et al2 described three cases of non-exudative neovascular AMD without GA: the BCVA was 20/20 in one case and 20/30 in the remaining two. Palejwala et al12 also detailed two cases of non-exudative MNV in AMD, and their BCVA was 20/25 and 20/32. Bailey et al6 prospectively followed 10 patients with non-exudative MNV and concluded that the detection of non-exudative MNV was not associated with any reduction in visual acuity, with the exception of a single case in which visual acuity loss was attributable to central GA.
Funduscopic and OCT Characterization/Classification of Non-Exudative Neovascular AMD
The typical funduscopic findings in patients with non-exudative MNV are macular drusen and pigmentary abnormalities2 that appear in the structural OCT as RPE elevations and intra-retinal hyper-reflective foci with no evidence of intraretinal or subretinal fluid.3,7 The presence of a double-layer sign on structural OCT scans has been shown to be a useful strategy for identifying non-exudative MNV.27 The double-layer sign is comprised of 2 highly reflective layers that correspond to a separation between the retinal pigment epithelium (RPE) and another highly reflective layer beneath the RPE, which is presumed to be Bruch’s membrane. This double-layer sign represents a low-lying, irregular pigment epithelial detachment. The specificity and sensitivity for identifying non-exudative MNV can be as high as 88%.27 However, on OCTA imaging, the neovascular complexes can be directly visualized thus achieving superior sensivity and specificity values for identifying these lesions. These findings show that non-exudative MNV is typically a type 1 neovascular lesion without evidence of intra-retinal or sub-retinal fluid9,11. However, Treister et al9, Bayley et al6 de Oliveira Dias et al11, and Yang et al1 also described cases of non-exudative MNV lesions appearing as type 3 neovascular lesions.
SS-OCTA and SD-OCTA for the Detection of Subclinical, Non-Exudative Neovascular AMD
OCTA has become an invaluable imaging technique because it directly visualizes MNV without the use of intravenous dyes. The two types of OCTA instruments used to detect non-exudative MNV are spectral domain OCTA (SD-OCTA) and swept source OCTA (SS-OCTA). Both use Fourier domain detection techniques, but the SD-OCT instruments use a broadband near-infrared super luminescent diode as a light source with a center wavelength of approximately 840 nm and a spectrometer as the detector while SS-OCT devices use a tunable swept source laser, currently with a center wavelength of approximately 1,060 nm, and a single photodiode detector.28
The sensitivity of OCTA to detect MNV using FA and ICGA as the “goldstandard” ranged from from 50–100%29 Previous reports have compared the performance of both SD-OCTA and SS-OCTA for detecting MNV and concluded that SS-OCTA yielded larger MNV areas for the MNV and had the ability of deliver angiograms with better image quality compared with SD-OCTA.30,31 These authors support that SS-OCTA may provide a more accurate representation of MNV when compared with SD-OCTA.30,31
Segmentation for Visualization of MNV in Neovascular AMD Using SS-OCTA
The strategy for visualizing the non-exudative MNV requires the use of a decorrelation algorithm and segmentation slabs that follow certain boundary layers. Different OCTA instruments use different OCTA algorithms and different strategies for defining segmentation slabs.
. Parravano et al32 compared three different automatically segmented slabs to assess type 1 MNV using OCTA). The outer retina to choriocapillaris (ORCC) slab was defined as the slab that extended from the outer retina to 8 μm beneath Bruch’s membrane,1,11,33 the choriocapillaris slab extended from the RPE to 20 μm below the Bruch’s membrane, and the RPE to RPEfit slab extended from the RPE to Bruch’s membrane. This study showed that the RPE-RPEfit had the highest interreader agreement when grading type1 MNV compared with the gradings from the other slabs.
For the studies using different OCTA instruments, slabs similar to the ORCC slab were used with segmentation strategies that include the outer retina to the choriocapillaries. Treister et al9 created two en face slabs, 90 μm below the inner plexiform layer (IPL) to 50 μm below the RPE and 72 μm below the IPL to 34 μm below the RPE.9 Yanagi et al7 visualized the MNV using automated layer segmentation from the outer retina, 70.2 μm below the junction between IPL and the inner nuclear layer, to Bruch’s membrane and the choriocapillaris slab, Bruch’s membrane to 10.4 μm below Bruch membrane.7 Both of these studies showed that these slabs could detect subclinical non-exudative MNV. In summarya slab containing the outer retina to the choriocapillaris may be the best slab for the detection of both types 1 and 2 MNV.34 However, device specifications should be taken into consideration when choosing the slab for detecting non-exudative MNV. Challenging cases may require manual review of the segmentation.
Incidence of Conversion into Exudative Neovascular AMD
We identified 6 studies that documented the incidence of exudation in eyes with treatment naïve, non-exudative, neovascular AMD with variable follow-up times (detailed in Table 1). De Oliveira Dias et al11 found a cumulative incidence of exudation of 24% by one year in patients with non-exudative MNV. This study was extended,1 and after two years of follow-up, the cumulative incidence of exudation was 34.5%. Yanagi et al7 described an incidence of exudation of 22.2% by 6 months. Capuano et al10 reported an incidence of exudation of 26.3% in a cohort of eyes with non-exudative MNV and GA with a mean of 20 months of follow-up. Hiferman et al5 reported an indicence of exudation of 20% after a mean follow-up of 15 months. Interestingly, Bailey et al6 found an incidence of exudation of 80% at two years.
The differences in the incidence of non-exudative MNV among these studies may be explained by the different populations, the number of participants, the severity and duration of AMD in the patient cohort, and the different follow up times.
Subclinical, Non-Exudative Neovascular AMD as Predictor for Exudation
Several studies have shown that treatment naïve non-exudative MNV is a harbinger of exudative disease. De Oliveira Dias et al,11 in a study that included 134 eyes, found that the risk of exudation at one year increased dramatically by a factor of 15.2 (95% confidence interval, 4.2–55.4) compared with eyes without treatment naïve non-exudative MNV (p<0.001, time-dependent Cox proportional hazards regression). This study was extended,1 and at two years of follow up, the relative risk of exudation after detection of subclinical MNV was 13.6 times greater (95% confidence interval, 4.9–37.7) than in the absence of subclinical MNV (p<0.001). Yanagi et al7 analyzed 95 eyes from 95 patients with exudative AMD or polypoidal choroidal vasculopathy and also found that the probability of developing exudation at 6 months was significantly higher in eyes with baseline non-exudative MNV (22% conversion rate) compared with eyes without any MNV (2.6% conversion rate; odds ratio 10.3; 95% confidence interval 1.34–124.0; p =0.01, Fisher exact test). In addition, the authors verified that the period between diagnosis and fellow eye involvement was shorter in eyes with non-exudative MNV than those without (p=0.01, log-rank test). Bailey et al,6 in their study, also concluded that eyes with OCTA-detected non-exudative MNV developed exudation at a faster rate (p< 0.0001, log-rank test) than study eyes without non-exudative MNV (Cox proportional hazard ratio was 18.1).
Predictors of Conversion from Non-Exudative to Exudative Neovascular AMD
Some studies have tried to find characteristics of subclinical non-exudative MNV that could serve as predictors of near-future exudation. The reviewed studies suggest some predictors that are detailed bellow.
Yanaggi et al7 followed patients with non-exudative MNV and found that in the 3 eyes that developed exudation in which OCTA images were available within 6 months before the onset of exudation, there was an increase in the size of the vascular network. In another case, OCTA imaging was available only at the time of exudation; the MNV had also increased. In contrast, there was no change in the size and shape of nonexudative neovascularization in the fellow eyes that did not develop exudative changes. Bailey et al6 described vessel area growth in 5 out of 6 non-exudative MNV lesions that later developed exudation. Capuano et al10 also described similar results in 2 cases that showed an enlarged total area of neovascularization, as well as morphological changes such as the sprouting of new vascular loops and tiny capillaries before exudation. Heiferman et al5 found an increase in the surface area on non-exudative MNV that converted to exudative AMD. However, Yang et al1 found no size or growth rate dependent correlation between eyes that developed exudation and eyes that remained non-exudative. Treister et al9 quantified choriocapillaris nonperfusion in SD-OCTA scans and found no significant difference in areas of non-perfusion eyes with exudative MNV compared with their fellow eyes with subclinical, non-exudative MNV (p=0.115). In their follow-up study,5 the authors confirmed this previous result and did not find a correlation between the baseline area of the choriocapillaris nonperfusion percentages and the development of exudation in eyes with subclinical, nonexudative MNV. However, this study has several limitations. As previously mentioned, SD-OCTA uses a shorter wavelength that doesn’t penetrate the RPE as well as SS-OCTA with an increased sensitivity roll-off into the choroid, which results in a decrease in the likelihood of detecting the weaker signals from beneath the RPE.28 In addition, the authors used a strategy to image the choriocapillaris using a global thresholding algorithm that had not been validated. Future studies will need to study the relationship between choriocapillaris flow deficits and the presence of subclinical non-exudative MNV to determine whether there’s a relationship between the extent of surrounding flow deficits and exudation.
Non-Exudative Neovascularization slows the Growth of Geographic Atrophy
As previously mentioned, non-exudative MNV may exist not only in eyes with intermediate AMD, but also in eyes with GA. Capuano et al10 followed 19 eyes with GA and non-exudative MNV for 27–65 months. In his study, the mean growth of atrophy from baseline to last examination was 5.08 ± 3.51 mm2, giving a lesion growth rate of 1.38 ± 0.93 mm2/year. Moreover, in 92% of cases, the non-exudative MNV was located in the adjacent area in which there was no progression of atrophy, and in 85% of eyes, there was a “sharp” demarcation in the structural SD-OCT images between the areas of atrophic and non-atrophic retina as seen by the enhanced choroidal signal due to RPE loss in the GA. Interestingly, 5 of the 19 patients (26.3%) developed exudative disease. Heiferman et al5 described in their study that GA grew at a rate of 0.82±1.20mm2/year in four eyes without non-exudative MNV and 0.02mm2/year in one eye with non-exudative MNV. Choi et al8 described two cases of patients with nonexudative AMD and GA where the MNV lesion existed beneath the surviving RPE, and they hypothesized that non-exudative MNV may be associated with improved survival of the RPE. Larger longitudinal studies are needed to confirm whether the growth of GA is slowed by the presence of adjacent subclinical non-exudative MNV.
Should Subclinical, Non-Exudative, Neovascular AMD be treated?
Questions have been raised as to whether non-exudative MNV should be treated with anti-VEGF therapy. While no controlled clinical trial has been performed to provide definitive recommendations, the reviewed studies all recommend that non-exudative MNV AMD should be closely monitored, but the clinician should abstain from treatment with anti-VEGF therapy, even if the lesion is growing until there is evidence of exudative disease. De Oliveira Dias et al11 suggest close monitoring in order to detect early signs of exudation that may affect visual function. Previously reviewed studies have documented that nonexudative MNV may persist for two years or longer without developing exudation and affecting vision. Moreover, it has been suggested that these type 1 neovascular lesions may be an attempt by the eye to recapitulate the choriocapillaris and provide beneficial nutritional support for the overlying RPE and photoreceptors.1,11
DISCUSSION
In this review we present the most recent information about the natural history of subclinical non-exudative MNV in AMD using OCTA.
The prevalence of non-exudative MNV in the fellow eyes of patients with exudative MNV secondary to AMD in the analyzed studies ranged between 6.25% and 27%. Non-exudative MNV is a prevalent condition that requires prompt diagnosis and monitoring with OCTA because these lesions have been consistently associated with a higher risk of developing exudative disease regardless of whether these eyes have intermediate or late non-exudative AMD. From the presented data, eyes with both GA and treatment naïve non-exudative MNV have the same risk of developing exudation as eyes with drusen and treatment naïve non-exudative MNV. However, the presence of these non-exudative neovascular lesions in eyes with GA may have a protective effect against progression of GA into the area with MNV. Following this rational, it could be possible that stable, non-exudative MNV may not only slow the progression of GA, but they could possibly prevent GA altogether.
Globally, in the reviewed studies, about 25% of non-exudative MNV lesions became exudative (range 6–20 months). Close monitoring of these eyes may allow an early detection of exudation and prompt anti-VEGF therapy. Post-hoc analysis of pivotal “wet-AMD” clinical trials have elucidated the incidence of exudation in fellow-eyes. Although the rate of exudation that we identified demonstrated some variation, it was not far from the rates previously found in larger studies. In CATT35, the overall incidence of MNV and exudation in the fellow eye after 1 year was 7.9% in patients treated with ranibizumab and 7.2% in patients treated with bevacizumab; after 2 years these rates were 20.6% and 16.6%, respectively. In the MARINA trial36, the exudation rates at 1 year for the 0.3-mg and 0.5-mg ranibizumab groups were 20% and 21%, respectively, and 15.9% and 23.8% at 12 and 24 months in the ANCHOR trial. In a post hoc analysis of the VIEW 1 and VIEW 2 studies,37 the exudation rates at 96 weeks were 27% for the 0.5-mg ranibizumab group (every 4 weeks), 24% for the 2mg-aflibercept group (every 4 weeks), 22% for the 0.5-mg aflibercept group (every 4 weeks) and 23% for the 2-mg aflibercept group (every 8 weeks after 3 doses at 4-week intervals). Fellow eye exudative conversion in large trials exhibit similar rates as those found in the reviewed studies. A possible explanation to this finding is that a proportion of these fellow eyes in clinical trials had non-exudative MNV lesions that are more prone to develop exudation. To clarify this findings, future controlled trials should include OCTA monitoring of fellow eyes so that non-exudative lesions can be identified, and their natural history may be further evauated.
The presence of non-exudative MNV seems to be an important predictive factor for exudative disease. The presence of a double-layer sign on structural OCT imaging is a good indicator for the presence of these lesions, however it is not as sensitive and specific as SS-OCTA; its presence should prompt the search of subclinical non-exudative MNV using OCTA.27 Probably, these lesions precede exudation for most type 1 MNV, and this exudation will result in visual impairment that requires anti-VEGF therapy. High priority should be placed on identifying biomarkers that may predict the exudative conversion of these neovascular lesions. Some authors have shown that enlargement of non-exudative lesions may predict exudation while others have not. Perhaps the rate of growth could be used in future studies to identify the lesions at a higher risk of exudation. While there are no biomarkers at this time that have been found to accurately predict the near onset of an exudative change, there is a treatment that appears to promote exudation. In a recently completed Phase 2 study of a complement C3 inhibitor known as APL-2, there appeared to be a dose dependent increase in exudation in eyes with GA treated with the drug.38 This exudation may have arose from pre-existing subclinical MNV, which would suggest a immunological trigger for the conversion of non-exudative MNV to exudative MNV.
As mentioned previously, non-exudative MNV may also occur in eyes with GA. These eyes are also more likely to develop exudative disease compared with eyes with GA that don’t have non-exudative MNV. Thus, it is important to closely monitor these lesions. Interestingly, the majority of the non-exudative neovascular lesions found in the reviewed series were located adjacent to atrophic areas. One possibility is that non-exudative MNV arises adjacent to the GA because of choriocapillaris impairment while another possibility is that non-exudative MNV arose first and the GA developed in the area that wasn’t protected by the neovascular lesion. While histopathological and OCTA studies have shown choriocapillaris changes in AMD and around GA, there is an ongoing debate as to whether the choriocapillaris or the RPE are the primary site of injury. McLeod et al39 demonstrated a relationship between RPE atrophy and choriocapillaris degeneration in eyes with GA concluding that a loss of RPE precedes the loss of the choriocapillaris while Biesmeier et al40 concluded that it is probably the loss of choriocapillaris that precedes RPE degeneration. Previous research using OCTA had shown the presence of abnormalities in choriocapillaris perfusion around the edge of GA41–44. In addition, Thulliez et al45 and Nassisi et al46 demonstrated marked choriocapillaris deficits around GA, but there was a better correlation between the enlargement rate of GA and choriocapillaris flow deficits further away from the edge of GA. These findings support the hypothesis that choriocapillaris loss at a distance may precede RPE changes. Thus, non-exudative MNV may actually be considered a protective factor as it may prevent further development and progression of atrophy and thus preserve foveal function by supplying oxygen to the hypoxic outer retina that results from decreased choriocapillaris perfusion. Another possibility is that the non-exudative MNV evolved before GA even developed and the MNV protects against both the development and enlargement of the GA into the region occupied by the MNV.
In summary, non-exudative MNV in AMD is a subclinical, asymptomatic condition that usually occurs in eyes with good visual acuity.All the authors of the studies included in this review agree that these non-exudative lesions should not be treated until symptomatic exudation develops; nonetheless, it is important to state that no randomized clinical trial has been performed to support or confirm these recommendations. These lesions may persist for many years without affecting vision and could provide nutritional support to the overlying RPE and photoreceptors. There is some evidence that MNV may be protective by slowing down the growth of GA growth or preventing the appearance of GA. As long as exudation does not occur, it appears that non-exudative MNV is not responsible for the deterioration of visual function.
Future directions
Future efforts should focus on understanding the anatomic changes and biological signals responsible for the formation, growth, and exudative conversion of these subclinical, neovascular lesions. The recent insights from OCTA studies have shown that large MNV complexes do not disappear with repeated anti-VEGF therapy, which suggests that these neovascular lesions are already mature and do not respond to repeated injections. Thus, we would not expect these subclinical MNV lesions to regress with anti-VEGF therapy, and why would we want them to regress since non-exudative MNV appears to slow down GA growth? The goal of any future treatments will not be to eliminate non-exudative MNV, but to promote their maturation and prevent exudation. Some papers have reported that frequent anti-VEGF therapy may lead to the increased formation of macular atrophy.47,48 Thus, the complete suppression of anti-VEGF after controlling the exudation may be more harmful in the long-term than promoting the maturation and stabilization of MNV. Treating the exudative disease by initially promoting the regression of the leaky immature vessels could probably be complemented by a second step by promoting the stabilization and maturation of the MNV lesion. There are several factors that promote this process.49 Transforming growth factor (TGF) β participates in mural cell proliferation and migration as well as deposition of extracellular matrix. Platelet derived growth factor (PDGF) participates in the recruitment, migration, and proliferation of both pericytes and smooth muscle cells, as does the bioactive lipid sphingosine-1-phosphate. Angiopoietin-1 helps stabilize vessels, promotes pericyte adhesion, and affects vascular tight junctions. For instance, supplemental angiopoietin 1 has been shown to promote blood vessel remodeling and formation, and these blood vessels are nonleaky, noninflammatory, functional, and stable.50 All these molecular pathways may be potential targets for maintaining beneficial, viable non-exudative MNV.
Limitations
Our study is limited by the quality of the reviewed studies. Most studies consist of case series with one prospective series following 227 at-risk eyes for 2 years or longer,1 but there are no controlled trials. Additional prospective studies are needed to understand the triggers responsiple for the formation, growth, and exudation of these subclinical lesions.
By performing this review, we were aware to the fact that a lot of terms have been used to classify non-exudative neovascular AMD (subclinical, quiescent, asymptomatic, remission), and our intention was to provide terminology that could be used in future reports. The existence of a non-exudative form of neovascular AMD would suggest that the term neovascular AMD should be preceded by either exudative or non-exudative when describing this neovascular stage of AMD.
CONCLUSION
In this review we presented the results from the recent studies with OCTA that address the natural history of subclinical, non-exudative, neovascular AMD. While non-exudative MNV appears to be a precursor and a very important risk factor for the formation of exudative neovascular AMD, there is evidence to suggest a protective effect that slows the progression of GA. Early detection of non-exudative MNV before exudation develops should result in better monitoring of patients with AMD who are at high risk of conversion to exudative AMD. This monitoring should involve both home monitoring, as well as more frequent clinic visits. Timely anti-VEGF treatment prior to vision loss should result in better visual outcomes. Future studies will help us understand the factors that promote the exudative conversion of these MNV so that we can better understand disease progression and design preventive strategies to prevent exudation.
Footnotes
Conflicts of Interest: No conflicting relationship exists for any author.
REFERENCES
- 1.Yang J, Zhang Q, Motulsky EH, et al. Two-Year Risk of Exudation in Eyes with Non-Exudative AMD and Subclinical Neovascularization Detected with Swept Source OCT Angiography. Am J Ophthalmol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roisman L, Zhang Q, Wang RK, et al. Optical Coherence Tomography Angiography of Indocyanine Green Angiographic Plaques in Asymptomatic Intermediate Age- Related Macular Degeneration HHS Public Access. Ophthalmology. 2016;123:1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanagi Y, Mohla A, Lee W-K, et al. Prevalence and Risk Factors for Nonexudative Neovascularization in Fellow Eyes of Patients With Unilateral Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Investig Opthalmology Vis Sci. 2017;58:3488. [DOI] [PubMed] [Google Scholar]
- 4.Carnevali A, Cicinelli MV, Capuano V, et al. Optical Coherence Tomography Angiography: A Useful Tool for Diagnosis of Treatment-Naïve Quiescent Choroidal Neovascularization. Am J Ophthalmol. 2016;169:189–198. [DOI] [PubMed] [Google Scholar]
- 5.Heiferman MJ, Fawzi AA. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS One. 2019;14:e0217805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey ST, Thaware O, Wang J, et al. Detection of Nonexudative Choroidal Neovascularization and Progression to Exudative Choroidal Neovascularization Using OCT Angiography. Ophthalmol Retin. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagi Y, Mohla A, Lee SY, et al. Incidence of Fellow Eye Involvement in Patients With Unilateral Exudative Age-Related Macular Degeneration. JAMA Ophthalmol. 2018;136:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi W, Moult EM, Waheed NK, et al. Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology. 2015;122:2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treister AD, Nesper PL, Fayed AE, et al. Prevalence of Subclinical CNV and Choriocapillaris Nonperfusion in Fellow Eyes of Unilateral Exudative AMD on OCT Angiography. Transl Vis Sci Technol. 2018;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capuano V, Miere A, Querques L, et al. Treatment-Naïve Quiescent Choroidal Neovascularization in Geographic Atrophy Secondary to Nonexudative Age-Related Macular Degeneration. Am J Ophthalmol. 2017;182:45–55. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural History of Subclinical Neovascularization in Nonexudative Age-Related Macular Degeneration Using Swept-Source OCT Angiography. Ophthalmology. 2017;125:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palejwala NV, Jia Y, Gao SS, et al. Detection of non-exudative choroidal neovascularization in age- related macular degeneration with optical coherence tomography angiography. 2015;35:2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63:Suppl:1–139. [PubMed] [Google Scholar]
- 14.Yannuzzi L, Slakter J, Sorenson J, et al. Digital Indocyanine Green Videoangiography and Choroidal Neovascularization. 1992;12:191–223. [PubMed] [Google Scholar]
- 15.Guyer DR, Yannuzzi LA, Slakter JS, et al. Classification of choroidal neovascularization by digital indocyanine green videoangiography. Ophthalmology. 1996;103:2054–60. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld PJ. Optical Coherence Tomography and the Development of Antiangiogenic Therapies in Neovascular Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2016;57:OCT14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Querques G, Srour M, Massamba N, et al. Functional characterization and multimodal imaging of treatment-naive “quiescent” choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54:6886–6892. [DOI] [PubMed] [Google Scholar]
- 18.Green WR, Key SN. Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkes S New vessel formation beneath the retinal pigment epithelium in senile eyes. Br J Ophthalmol. 1973;57:951–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spraul CW, Grossniklaus HE. Characteristics of Drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol (Chicago, Ill 1960). 1997;115:267–73. [DOI] [PubMed] [Google Scholar]
- 21.Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92:615–27. [PubMed] [Google Scholar]
- 22.Chang T, Freund KB, Cruz Z de la, et al. Clinicopathologic correlation of choroidal neovascularization demonstrated by indocyanine green angiography in a patient wih retention of good vision for almost four years. Retina. 1994;14:114–124. [DOI] [PubMed] [Google Scholar]
- 23.Schneider U, Gelisken F, Inhoffen W, Kreissig I. Indocyanine green angiographic findings in fellow eyes of patients with unilateral occult neovascular age-related macular degeneration. Int Ophthalmol. 1997;21:79–85. [DOI] [PubMed] [Google Scholar]
- 24.Hanutsaha P, Guyer DR, Yannuzzi LA, et al. Indocyanine-green videoangiography of drusen as a possible predictive indicator of exudative maculopathy. Ophthalmology. 1998;105:1632–1636. [DOI] [PubMed] [Google Scholar]
- 25.TC, Collaboration. Cochrane Handbook for systematic reviews of interventions version 5.1.0 2011.
- 26.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Motulsky EH, Goldhardt R, et al. Predictive Value of the OCT Double-Layer Sign for Identifying Subclinical Neovascularization in Age-Related Macular Degeneration. Ophthalmol Retin. 2018;3:211–219. [DOI] [PubMed] [Google Scholar]
- 28.Potsaid B, Gorczynska I, Srinivasan VJ, et al. Ultrahigh speed Spectral / Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second. Opt Express. 2008;16:15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrott-Reynolds R, Cann R, Cronbach N, et al. The diagnostic accuracy of OCT angiography in naive and treated neovascular age-related macular degeneration: a review. Eye. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novais EA, Mehreen Adhi, Moult EM, et al. Choroidal neovascularization analyzed on ultra-high speed swept source optical coherence tomography angiography compared to spectral domain optical coherence tomography angiography. Am J Ophthalmol. 2016;164:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller AR, Roisman L, Zhang Q, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Investig Ophthalmol Vis Sci. 2017;58:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parravano M, Borrelli E, Sacconi R, et al. A Comparison Among Different Automatically Segmented Slabs to Assess Neovascular AMD using Swept Source OCT Angiography. Transl Vis Sci Technol. 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Zhang A, Lee CS, et al. Projection Artifact Removal Improves Visualization and Quantitation of Macular Neovascularization Imaged by Optical Coherence Tomography Angiography. Ophthalmol Retin. 2016;1:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motulsky EH, Zheng F, Shi Y, et al. Anatomic localization of type 1 and type 2 macular neovascularization using swept-source OCT angiography. Ophthalmic Surg Lasers Imaging Retin. 2018;49:878–886. [DOI] [PubMed] [Google Scholar]
- 35.Maguire MG, Daniel E, Shah AR, et al. Incidence of Choroidal Neovascularization in the Fellow Eye in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2013;120:2035–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbazetto IA, Saroj N, Shapiro H, et al. Incidence of New Choroidal Neovascularization in Fellow Eyes of Patients Treated in the MARINA and ANCHOR Trials. Am J Ophthalmol. 2010;149:939–946.e1. [DOI] [PubMed] [Google Scholar]
- 37.Parikh R, Avery RL, Saroj N, et al. Incidence of New Choroidal Neovascularization in Fellow Eyes of Patients Treated for Age-Related Macular Degeneration. JAMA Ophthalmol. 2019;137:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao DS, Grossi FV., El Mehdi D, et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2019. [DOI] [PubMed] [Google Scholar]
- 39.McLeod DS, Grebe R, Bhutto I, et al. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biesemeier A, Taubitz T, Julien S, et al. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35:2562–2573. [DOI] [PubMed] [Google Scholar]
- 41.Nassisi M, Shi Y, Fan W, et al. Choriocapillaris impairment around the atrophic lesions in patients with geographic atrophy: A swept-source optical coherence tomography angiography study. Br J Ophthalmol. 2019;103:911–917. [DOI] [PubMed] [Google Scholar]
- 42.Choi W, Moult EM, Waheed NK, et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology. 2015;122:2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kvanta A, De Salles CC, Amrén U, Bartuma H. Optical coherence tomography angiography of the foveal microvasculature in geographic atrophy. Retina. 2017;37:936–942. [DOI] [PubMed] [Google Scholar]
- 44.Sacconi R, Corbelli E, Carnevali A, et al. Optical Coherence Tomography Angiography in Geographic Atrophy. Retina. 2018;38:2350–2355. [DOI] [PubMed] [Google Scholar]
- 45.Thulliez M, Zhang Q, Shi Y, et al. Correlations between Choriocapillaris Flow Deficits around Geographic Atrophy and Enlargement Rates Based on Swept-Source OCT Imaging. Ophthalmol Retin. 2019;3:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nassisi M, Baghdasaryan E, Borrelli E, et al. Choriocapillaris flow impairment surrounding geographic atrophy correlates with disease progression Bhattacharya S, ed. PLoS One. 2019;14:e0212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gemenetzi M, Lotery AJ, Patel PJ. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye. 2017;31:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enslow R, Bhuvanagiri S, Vegunta S, et al. Association of Anti-VEGF Injections with Progression of Geographic Atrophy. Ophthalmol Eye Dis. 2016;8:OED.S38863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabral T, Mello LGM, Lima LH, et al. Retinal and choroidal angiogenesis: A review of new targets. Int J Retin Vitr. 2017;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, Park DY, Park DY, et al. Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage. Investig Ophthalmol Vis Sci. 2014;55:2191–2199. [DOI] [PubMed] [Google Scholar]


