Abstract
The objectives of this study were to evaluate temporal changes in lubricin, hyaluronan (HA), and HA molecular weight (MW) distributions in three distinct models of equine joint injury affecting the carpal (wrist), tarsal (ankle), and femoropatellar (knee) joints. To establish ranges for lubricin, HA, and HA MW distributions across multiple joints, we first evaluated clinically healthy, high-motion equine joints. Synovial fluid was collected from high-motion joints in horses without clinical signs of joint disease (n=11 horses, 102 joints) and from research horses undergoing carpal osteochondral fragmentation (n=8), talar cartilage impact injury (n=7), and femoral trochlear ridge full-thickness cartilage injury (n=22) prior to and following arthroscopically-induced joint injury. Lubricin and HA concentrations were measured via enzyme-linked immunosorbent assays, and gel electrophoresis was performed to evaluate HA MW distributions. Synovial fluid parameters were analyzed via linear regression models, revealing that lubricin and HA concentrations were conserved across healthy, high-motion joints. Lubricin concentrations increased post-injury in all osteoarthritis models (carpal fragmentation p=0.001; talar impact p<0.001; femoral trochlear ridge cartilage defect p=0.03). Sustained loss of HA was noted post-arthroscopy following carpal osteochondral fragmentation (p<0.0001) and talar impact injury (p<0.001). Lubricin may be elevated to compensate for loss of HA and to protect cartilage post-injury. Further investigation into the mechanisms regulating lubricin and HA following joint injury and their effects on joint homeostasis is warranted, including whether lubricin has value as a biomarker for post-traumatic osteoarthritis.
Keywords: osteoarthritis, proteoglycan 4, hyaluronic acid, horse, PTOA
Introduction
Acute joint injury, including soft tissue destabilization and articular surface injury, increases the risk of developing osteoarthritis by 10 to 20-fold, respectively1. In early post-traumatic osteoarthritis (PTOA), varying degrees of articular cartilage damage can be apparent as a result of the initial trauma, with progression of cartilage degradation over time secondary to altered biomechanics, impaired joint lubrication, and up-regulation of inflammatory mediators2,3. The principal lubricating molecules in synovial fluid, lubricin and hyaluronan, are essential for mitigation of the effects of inflammatory cytokines, maintaining low-friction joint lubrication, and protecting articular cartilage from degeneration4. Lubricin functions as the primary boundary lubricant, while hyaluronic acid confers viscoelastic properties to synovial fluid5.
The autosomal recessive disease camptodactyly-arthropathy-coxa vara-pericarditis (CACP) in humans is characterized by a genetic mutation within the PRG4 gene leading to a deficiency in lubricin6. Studies utilizing lubricin-deficient synovial fluid from CACP patients and lubricin knock-out mice have shown inferior boundary lubricating abilities and a propensity to develop osteoarthritic changes at an early age, respectively7,8. Additionally, a subset of people with naturally-occurring osteoarthritis (OA) of the knee have been identified as having lubricin-deficient synovial fluid and similarly have decreased cartilage lubricating ability9. Further, losses of HA, particularly high molecular weight (MW) HA, have been associated with increased friction coefficients in mixed or viscoelastic lubrication regimes10,11, and elevated friction due to loss of synovial fluid lubricants is thought to contribute to increased cartilage wear and joint degeneration12.
Loss of synovial fluid lubricin has been detected in human knee OA associated with anterior cruciate ligament injury13, knee destabilization models in the rat14,15, and in a subset of people with chronic knee OA9. However, recent work has identified increased lubricin concentrations secondary to osteochondral fragmentation in the equine carpus16,17, naturally-occurring acute and chronic joint injury in the equine carpal and metacarpophalangeal joints10, and following repair of experimentally-induced cartilage defects in the femoropatellar joint of the horse18. Lubricin synovial fluid concentrations are also elevated in humans following intra-articular tibial fracture19 and in advanced knee OA20. To date, it remains unclear whether these variations in lubricin activity are unique to specific joints or are predominantly a function of the underlying injury mechanism.
A recent study evaluating a variety of equine joints identified variations in gene expression, cell density, and response to joint trauma in articular cartilage, suggesting that inherent differences between joints may be significant factors in the development and progression of OA21. In humans, it is well documented that the rate of occurrence and type of OA varies significantly between joints, with a higher incidence of OA in the knee, hand, and hip compared to the ankle, wrist, elbow, and shoulder22. Biomechanical differences exist between the various joint types, and complex joints, such as the human knee or equine stifle, may be significantly influenced by peri- and intra-articular soft tissue structures. These biomechanical variations significantly affect the loading and shear forces exerted on the articular structures and likely play a role in the development of OA and specific pathology commonly noted in each joint type23.
Given this knowledge, it would be of value to investigate both healthy and OA synovial fluid obtained from biomechanically distinct human joints; however, human studies are often restricted to data from end-stage OA joints, thus minimizing the amount of data about disease progression and intervention that can be obtained24. Obtaining healthy tissues and fluids from age-matched controls presents an additional difficulty in human studies. Thus, a multitude of translational animal OA models have been developed in both small and large animal species. Given the similarities in joint biomechanics and cellular and biochemical composition, equine models are suggested to most closely represent the disease process in humans24,25. Horses are also similar to humans in that the prevalence and joint specificity of OA varies based on athletic use. As OA significantly affects quality of life and is a source of wastage in the equine industry26, utilization of equine models offers a dual benefit for both species.
The overarching goal of this study was to determine if elevations in lubricin are specific to intra-articular fracture or fragmentation-type joint injuries. In addition, we investigated whether lubricin, HA, and HA MW distributions varied between equine joints and type of joint trauma. Synovial fluid was evaluated in three distinct equine joint injury models: carpal osteochondral fragmentation, talar cartilage impact injury, and full-thickness femoral lateral trochlear ridge cartilage defects. In order to draw meaningful conclusions from data obtained from different joints and injury models, we also aimed to establish normal ranges for and identify associations between lubricin, HA, and HA MW distributions in healthy, high-motion equine joints (tarsocrural, antebrachiocarpal, middle carpal, metacarpo/metatarso-phalangeal and femorotibial).
Methods
Samples
Synovial fluid samples were obtained from four different equine cohorts: (Cohort 1) horses donated for research purposes unrelated to clinical signs of joint disease (n=11), and research animals with joint-injuries induced in the (Cohort 2) carpus (n=8), (Cohort 3) tarsus (n=7), or (Cohort 4) stifle (n=22). The Cornell University Institutional Animal Care and Use Committee approved all experimental protocols, briefly described below, prior to the onset of each original study.
Multi-joint synovial fluid harvest (Cohort 1)
Synovial fluid was obtained via needle arthrocentesis immediately post-euthanasia from 11 horses, ranging in age from 1–15 years old (mean 8.7 years) that were euthanized for reasons unrelated to this study. The cohort consisted of castrated males (n=8) and intact females (n=3), and was predominantly composed of Thoroughbreds (n=9 Thoroughbreds; n=1 Standardbred; n=1 Quarter Horse). The horses were sound at the walk with no clinical evidence of osteoarthritis. High-motion joints, including the tarsocrural (n=22), antebrachiocarpal (n=17), middle carpal (n=18), metacarpophalangeal (n=18), metatarsophalangeal (n=18), and femorotibial (n=9) joints were sampled via direct aspiration of synovial fluid immediately after euthanasia, resulting in a total of 102 joint samples. Centesis of all joints of interest was attempted in all horses. Maximal synovial fluid aspiration was performed in each joint and, when possible, joints were sampled bilaterally. After collection, synovial fluid was centrifuged at 4,000 × g for 60 minutes to remove cellular debris. The supernatant was retained, and the synovial fluid was frozen in aliquots of 1.5 mL at −80°C until sufficient samples were obtained for evaluation. Synovial fluid total protein (TP) was measured using a refractometer.
Osteochondral fragmentation (Cohort 2)
Synovial fluid samples from the middle carpal joints of eight young adult (2–6 years old) Thoroughbred horses (n=3 castrated males; n=5 intact females) were obtained. Horses were sound, based on AAEP subjective lameness assessment by an equine veterinarian, and were confirmed to have no radiographic evidence of carpal OA prior to study enrollment in the osteochondral fragment-treadmill exercise model of PTOA16. Briefly, each horse’s forelimbs were randomly assigned to either sham operation (arthroscopic evaluation only) or osteochondral fragmentation in which an 8mm osteochondral fragment was arthroscopically created on the distal dorsal margin of the radial carpal bone. Horses were exercised on a treadmill (30 minutes/day; 5 days/week) starting 2 weeks after osteochondral fragmentation until cessation of the study. Serial synovial fluid samples were obtained throughout the study (days 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, and 70). Post-mortem examination of the injured joints was performed for OA induction assessment.
Cartilage impact injury (Cohort 3)
A cohort of 7 young adult horses (2–5 years old; n=5 castrated males; n=2 intact females) of various breeds (n=4 Thoroughbreds, n=1 Quarter Horse, n=1 Standardbred, and n=1 Mixed breed) underwent cartilage impact injury to the medial trochlear ridge of the talus by use of a spring-loaded impacting device27. Horses were sound, based on AAEP subjective assessment by an equine veterinarian, and had no radiographic evidence of tarsal OA prior to inclusion in the study. Synovial fluid was sampled from the impacted tarsocrural joint on days 0, 4, 7, 14, 43, 71, and 168 post-arthroscopy. Contralateral limb sham-operation was not performed in this group of horses. Synovial fluid total protein was reported as < 2.5 g/dl or as a discrete number if the value was greater than 2.5 g/dl. Post-mortem examination of the injured joints was performed for OA induction assessment.
Full thickness cartilage defect (Cohort 4)
Synovial fluid samples were obtained from a cohort of 22 young adult (2–6 years old; n=5 castrated males; n=17 intact females) research horses of various breeds (n=14 Quarter Horses; n=3 Paint Horses; n=2 Appaloosas; n=1 Tennessee Walking Horse; n=1 Saddlebred; and n=1 Paso Fino) enrolled in a cartilage repair study28. Horses were sound, based on AAEP subjective assessment by an equine veterinarian, and had no radiographic evidence of stifle OA prior to inclusion in the study. Briefly, two 15mm diameter full-thickness femoral lateral trochlear ridge cartilage defects were created in one randomly assigned femoropatellar joint in each horse. Synovial fluid samples were obtained on day 0, 84 days after creation of the cartilage defect via a second-look arthroscopic surgery, and 397 days post-injury at necropsy. Synovial fluid total protein was reported as < 2.5 g/dl or as a discrete number if the value was greater than 2.5 g/dl. Contralateral limb sham-operation was not performed in this group of horses. Post-mortem examination of the injured joints was performed for OA induction assessment.
Biochemistry
Lubricin characterization
Lubricin was quantified as previously described using a sandwich ELISA with peanut agglutinin (PNA) as the capture reagent (L0881; Sigma-Aldrich, St. Louis, MO) and monoclonal antibody 9G3 (MABT401; EMD Millipore, Darmstadt, Germany) as the detection antibody, with purified equine synovial fluid lubricin as the standard16. Equine synovial fluid samples from all horses at all time points were diluted 1:1,000 in phosphate buffered saline (PBS), and samples were measured in duplicate on high binding 96-well plates (Costar #3590; Corning Inc., Corning, NY). Absorbance was measured at 450nm with wavelength correction set at 540nm. To evaluate ELISA specificity for high molecular weight lubricin rather than degradation products, select samples were assessed for size relative to full-length, purified equine synovial fluid lubricin. Following electrophoresis on 3–8% NuPAGE tris-acetate gels (Life Technologies, Carlsbad, CA) and transfer to nitrocellulose, Western and lectin blotting with mAb 9G3, polyclonal antibody PA3–118 (Thermo Fisher Scientific, Waltham, MA), and biotinylated PNA (BA-0074; Vector Laboratories, Burlingame, CA) was performed (Supplemental Figure 1).
Hyaluronan characterization
A commercially available sandwich ELISA (Hyaluronan DuoSet ELISA, R&D Systems, Minneapolis, USA) was used to quantify HA concentration in synovial fluid. This assay utilizes recombinant human aggrecan as the HA capture reagent and biotinylated recombinant human aggrecan to detect bound HA using streptavidin conjugated to horseradish peroxidase. Synovial fluid samples from all horses at all time points were diluted 1:80,000 in 5% Tween 20 in PBS and measured in duplicate. Absorbance was measured at 450nm with wavelength correction set at 540nm. HA MW distribution was determined by gel electrophoresis as previously described29. Briefly, synovial fluid samples were diluted (1:20 in PBS) and treated overnight with proteinase K (Roche Applied Science, Mannheim, Germany) prior to electrophoresis (50V for 8 hours) on a 1% agarose gel. HiLadder (0.5–1.5 MDa) and Mega-HA Ladder (1.5–6.1 MDa) (Amsbio LLC, Cambridge, MA) were used for MW reference. Gels were stained with 0.005% Stains-All (Sigma-Aldrich, St. Louis, MO) in 50% ethanol, then de-stained with a 10% ethanol solution prior to image acquisition (Bio-Rad VersaDoc Imaging System, Hercules, CA). Where necessary, gel images were combined using the Stitching plugin30 prior to quantification of band intensity with ImageJ software31.
Statistical analysis
Multi-joint samples
Student’s t-tests were used to confirm the absence of significant differences between left and right limbs. Samples were then grouped by joint for the remaining analyses. Shapiro-Wilk goodness-of-fit tests were performed to assess the data for normality. As HA and lubricin were not normally distributed, log transformations were performed to satisfy regression assumptions. Linear, mixed effect models were used to evaluate differences in lubricin, HA, and HA MW distribution between joints utilizing joint, age, sex, and total protein as fixed effects. Random effects included the horse and each individual joint nested within the respective horse to account for the lack of independence between these factors. Post hoc Tukey’s HSD comparisons were performed to further evaluate significant differences between joints.
Experimental OA samples
Shapiro-Wilk goodness-of-fit tests were performed to confirm normal distribution of the data. To evaluate differences in lubricin, HA, and HA MW distribution between joints and to assess the influence of joint and post-injury sampling time on synovial fluid parameters while controlling for the hierarchical nature of the data, linear, mixed effect models were used. Post hoc Dunnett’s tests were performed to further evaluate significant differences between sampling time-points, with the baseline (time 0) sample used as the control comparison. All statistical analyses were performed using JMP Pro 14 (SAS Institute Inc., Cary, NC), and significance was set at p<0.05.
Results
Multi-joint comparison
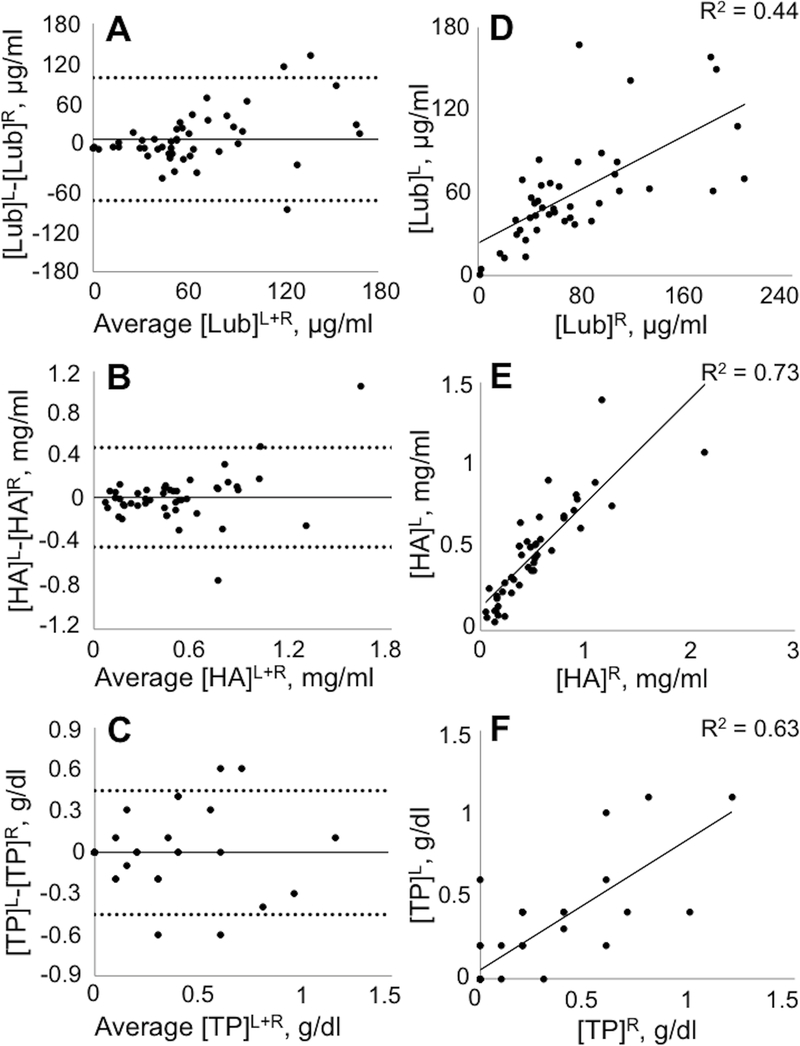
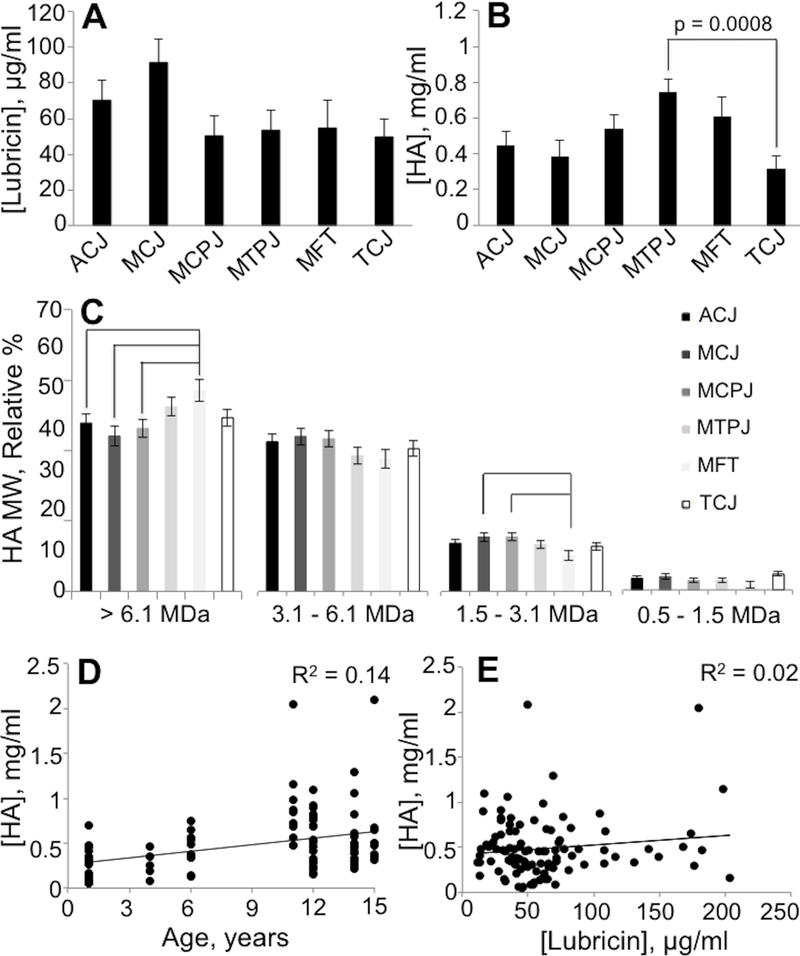
A total of 102 joints from 11 horses (Table S-1) were analyzed for between-joint comparisons (Table 1). Lubricin, HA, HA MW proportions, and total protein were similar between left and right limbs for each respective joint (Figure 1). Lubricin concentrations did not vary significantly across the sampled joints (p=0.13; Table 2). However, HA concentrations did vary between joints (p=0.002), with the highest HA concentration in the metatarsophalangeal joint and the lowest HA concentration in the tarsocrural joint (Figure 2). The relative proportion of high molecular weight HA MW > 61 MDa was increased in the medial femorotibial joint compared to the antebrachiocarpal joint (p=0.3), middle carpal joint (p=0.001), and the metacarpophalangeal joint (p=0.01). For the lowest HA MW category 1.5 – 3.1 MDa, the relative proportion was lower in the medial femorotibial joint compared to the middle carpal joint (p=0.01) and the metacarpophalangeal joint (p=0.02). Relative proportions of HA MW did not vary by joint for the MW categories 3.1 – 6.1 MDa or 0.1 – 1.5 MDa. Both total protein (p=0.01) and age (p<0.0001) were significant predictors for HA concentration but not for lubricin concentration (Table 2).
Table 1.
Synovial fluid lubricin concentrations, hyaluronic acid concentrations, and relative percentages of hyaluronic acid molecular weight categories in sampled high-motion equine joints. Data are presented as least square means ± standard error of the mean.
| Joint | [Lubricin], μg/ml | [HA], mg/ml | >6.1 MDa | 3.1 – 6.1 MDa | 1.5 – 1.3 MDa | 0.5 – 1.5 MDa | [TP], g/dl |
|---|---|---|---|---|---|---|---|
| ACJ (n=17) | 72.2 ± 9.7 | 0.46 ± 0.06 | 48.3 ± 2.1 | 37.1 ± 1.4 | 11.8 ± 0.9 | 2.82 ± 0.6 | 0.24 ± 0.06 |
| MCJ (n=18) | 99.4 ± 13.5 | 0.48 ± 0.06 | 44.4 ± 2.1 | 38.7 ± 1.5 | 13.8 ± 0.7 | 3.14 ± 0.6 | 0.53 ± 0.05 |
| MCPJ (n=18) | 49.4 ± 8.8 | 0.50 ± 0.06 | 47.7 ± 1.9 | 36.8 ± 1.6 | 13.0 ± 0.7 | 2.53 ± 0.3 | 0.02 ± 0.05 |
| MTPJ (n=18) | 52.2 ± 9.9 | 0.72 ± 0.11 | 54.0 ± 1.5 | 32.3 ± 1.2 | 11.2 ± 0.5 | 2.6 ± 0.3 | 0.04 ± 0.05 |
| MFT (n=9) | 55.1 ± 8.1 | 0.59 ± 0.20 | 54.1 ± 1.8 | 34.7 ± 1.5 | 10.0 ± 0.7 | 1.13 ± 0.2 | 0.37 ± 0.08 |
| TCJ (n=22) | 48.2 ± 4.5 | 0.28 ± 0.03 | 50.4 ± 2.3 | 34.6 ± 2.1 | 10.9 ± 0.9 | 4.13 ± 0.8 | 0.11 ± 0.05 |
Figure 1.
Graphical representation of similarity of synovial fluid parameters between left versus right healthy, high-motion equine joints. Bland-Altman plots of individual differences between left and right joints for lubricin (Lub) (A), hyaluronan (HA) (B), and total protein (TP) (C) concentrations plotted against their mean. The solid line represents the mean difference, and the dashed lines represent ±2 standard deviations of the individual difference. Data for left (y axis) and right (x axis) joints plotted for lubricin (D), hyaluronan (E), and total protein (F) concentrations. n = 54–59 joints.
Table 2.
Linear, least square regression models for log-transformed synovial fluid parameters (lubricin and hyaluronic acid concentrations) in high-motion equine joints. Intercept omitted for clarity.
| Log [Lubricin] (n=102) | Log [HA] (n=102) | |||||
|---|---|---|---|---|---|---|
| Predictors | Estimate | Sth. Error | P-value | Estimate | Std. Error | P-value |
| Joint | 0.13 | < 0.01 | ||||
| ACJ | 0.20 | 0.16 | −0.10 | 0.15 | ||
| MCJ | 0.45 | 0.19 | −0.21 | 0.17 | ||
| MCPJ | −0.25 | 0.17 | 0.11 | 0.15 | ||
| MTPJ | −0.22 | 0.16 | 0.47 | 0.15 | ||
| MFT | −0.03 | 0.21 | 0.18 | 0.19 | ||
| Age (years) | 0.01 | 0.01 | 0.61 | 0.06 | 0.01 | < 0.01 |
| Sex (female) | 0.01 | 0.08 | 0.86 | −0.01 | 0.07 | 0.90 |
| TP (g/dl) | 0.18 | 0.28 | 0.52 | 0.62 | 0.24 | 0.01 |
| R2 (Adj. R2) | 0.75 (0.73) | 0.87 (0.87) | ||||
Figure 2.
Graphical representation of similarity of lubricin concentration (A), hyaluronan concentration (B), and relative proportions of hyaluronan molecular weight (C) in synovial fluid from various healthy, high-motion equine joints. Least square means ± standard error of the mean are plotted. Lines indicate a significant difference between joints. Scatterplots of hyaluronan versus age (D) and lubricin (E).
Experimental OA Samples
Lubricin characterization
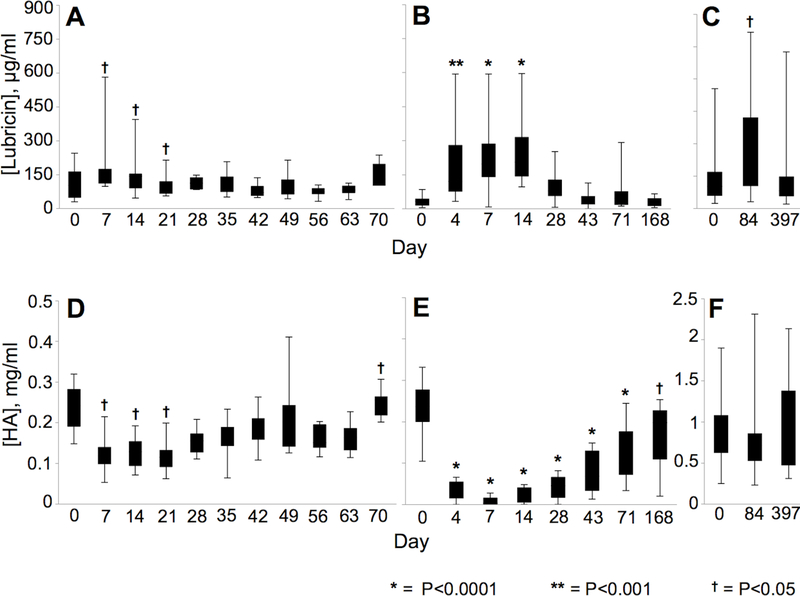
The intra-assay CV was 9.6%. Lubricin concentrations increased post-injury in synovial fluid samples from all joint injury models (Table 3). In the osteochondral fragment model, middle carpal joint injury, middle carpal joint synovial fluid lubricin concentrations were increased from pre-injury levels on day 7 (p=0.005), day 14 (p=0.04), and day 21 (p=0.03) (Figure 3). In the cartilage impact model, tarsocrural joint synovial fluid lubricin concentrations varied by day (p<0.0001) and were elevated from pre-injury levels at day 4 (p=0.0006), 7 (p<0.0001), and 14 (p<0.0001) post-injury. Horses with full-thickness femoral cartilage defects had elevated lubricin concentrations at the second time point (day 84, p=0.04) as compared to the pre-injury sample obtained prior to creation of the full-thickness cartilage defect (day 0). Day 84 samples also showed a trend for elevated lubricin compared to samples obtained at day 397 (p=0.06). Synovial fluid lubricin immunoblots revealed increased signal intensity for both mucin-domain reagents (mAb 9G3 and PNA) following joint injury, paralleling the sandwich ELISA results (Figure S-1A,D; B,E). In addition to the high MW lubricin band, the C-terminal antibody immunoblot revealed a strong signal at ~ 220 kDa which was also increased post-injury (Figure S-1C,F). These results suggest that there is a lower, ~ 220 kDa band, which may represent a non- or under-glycosylated variant of lubricin or a non-O-glycosylated lubricin cleavage product.
Table 3.
Linear, least square regression models for synovial fluid parameters (lubricin and hyaluronic acid concentrations) in each post-traumatic osteoarthritis model: A. osteochondral fragmentation in the equine carpus; B. cartilage impact injury in the equine tarsus; C. full-thickness cartilage defect in the equine femoropatellar joint. Variations in samples size can be accounted for via exhaustion of banked synovial fluid samples at various time-points. Intercept omitted for clarity.
|
A. Osteochondral
fragmentation |
||||||
| Lubricin (n=160) | HA (n=168) | |||||
| Predictors | Estimate | Std. Error | P-value | Estimate | Std. Error | P-value |
| Day | 0.001 | <0.0001 | ||||
| 0 | −69.18 | 28.34 | 0.031 | 0.015 | ||
| 7 | 75.66 | 26.09 | −0.080 | 0.015 | ||
| 14 | 50.04 | 27.40 | −0.064 | 0.015 | ||
| 21 | 44.87 | 24.29 | −0.054 | 0.014 | ||
| 28 | 30.89 | 24.31 | −0.027 | 0.014 | ||
| 35 | −18.66 | 23.51 | −0.004 | 0.014 | ||
| 42 | 0.24 | 24.29 | 0.031 | 0.014 | ||
| 49 | −23.39 | 23.51 | 0.052 | 0.014 | ||
| 56 | −59.73 | 24.31 | 0.010 | 0.014 | ||
| 63 | −46.37 | 23.51 | 0.006 | 0.014 | ||
| R2 (Adj. R2) | 0.52 (0.45) | 0.68 (0.63) | ||||
|
B. Cartilage
impact |
||||||
| Lubricin (n=106) | HA (n=108) | |||||
| Predictors | Estimate | Std. Error | P-value | Estimate | Std. Error | P-value |
| Day | <0.0001 | <0.0001 | ||||
| 0 | −81.48 | 25.07 | 0.15 | 0.01 | ||
| 4 | 75.69 | 25.58 | −0.06 | 0.01 | ||
| 7 | 104.84 | 26.56 | −0.08 | 0.01 | ||
| 14 | 112.59 | 29.97 | −0.08 | 0.01 | ||
| 28 | −27.25 | 29.12 | −0.05 | 0.01 | ||
| 43 | −72.43 | 27.28 | −0.01 | 0.01 | ||
| 71 | −37.74 | 31.73 | 0.04 | 0.01 | ||
| TP (g/dl) | 9.60 | 9.29 | 0.3 | 0.00 | 0.00 | 0.08 |
| R2 (Adj. R2) | 0.58 (0.51) | 0.90 (0.88) | ||||
|
C. Cartilage
defect |
||||||
| Lubricin (n=64) | HA (n=65) | |||||
| Predictors | Estimate | Std. Error | P-value | Estimate | Std. Error | P-value |
| Day | 0.03 | 0.6 | ||||
| 0 | −41.44 | 26.49 | 0.00 | 0.07 | ||
| 84 | 74.90 | 26.90 | −0.06 | 0.07 | ||
| R2 (Adj. R2) | 0.49 (0.48) | 0.45 (0.43) | ||||
Figure 3.
Effect of time post-surgery on lubricin and hyaluronan concentrations in each post-traumatic osteoarthritis model. Observed means ± standard error of the means are plotted. Symbols (* = P<0.0001; ** = P<0.001; † = P<0.05) indicate significant differences from pre-surgery (day 0). Lubricin concentrations increased following carpal osteochondral fragmentation (A), talar cartilage impact (B), and trochlear ridge full-thickness cartilage defect creation (C). Hyaluronan depletion is shown following carpal osteochondral fragmentation (D), and talar cartilage impact (E); however, loss of hyaluronan was not detected following creation of full-thickness cartilage defects in the trochlear ridge (F).
Hyaluronan characterization
The intra-assay CV was 7.1%. HA concentrations decreased post-injury in both the carpal fragmentation (p<0.0001) and talar impact (p<0.0001) models (Table 3). HA concentrations in middle carpal joint synovial fluid were significantly decreased following intra-articular fracture on days 7 (p=0.002), 14 (p=0.02), and 21 (p=0.04) post-injury (Figure 3). By the final sampling time point (day 70), HA concentrations had rebounded and were higher than pre-injury values (p=0.02). HA concentrations remained significantly decreased from day 4 to 168 days post-injury in the talar impact group. Altered HA concentrations were not detected in full-thickness cartilage defect synovial fluid samples on days 84 (p=0.89) and 397 (p=0.88) post-injury.
Alterations in relative proportions of HA MW were not detected at any day across HA MW categories in the talar cartilage impact model (> 6.1 MDa, p=0.19; 3.1 – 6.1 MDa, p=0.67; 1.5 – 3.1 MDa, p=0.96; 0.5 – 1.5 MDa, p=0.7) or the femoral full-thickness cartilage defect model (> 6.1 MDa, p=0.34; 3.1 – 6.1 MDa, p=0.25; 1.5 – 3.1 MDa, p=0.66; 0.5 – 1.5 MDa, p=0.19). HA MW data was not available for samples from the carpal osteochondral fragment model due to limited remaining synovial fluid volumes.
Discussion
Synovial fluid lubricin increased in all equine injury models, confirming that elevations in lubricin are not specific to intra-articular fracture. Rather, increased synovial fluid lubricin concentration appears to be a conserved response to various types of injury, including osteochondral fragmentation and two distinct models of cartilage injury across three different high-motion joints. Conversely, hyaluronan was decreased post-arthroscopy in both osteochondral fragmentation and cartilage impact injury models. The disparate responses between lubricin and HA are supported by the lack of correlation between the two lubricating molecules in this study and suggest that the mechanisms that regulate lubricin and HA synovial fluid homeostasis are distinct from one another. While the precise relationship between HA and lubricin has not been fully elucidated, it is well accepted that HA and lubricin primarily function under different loading circumstances in the joint where HA is essential for lubrication under high motion and low-load circumstances, and lubricin primarily enables low-friction lubrication while joints are under high load. Additionally, a synergistic relationship between lubricin and HA has been suggested4, and it is possible that lubricin may be elevated secondarily to HA losses to restore synovial fluid lubricating ability in joint injury.
The mechanisms responsible for increased synovial fluid lubricin following joint injury are not fully understood. A prior study revealed increased PRG4 synovial membrane gene expression in horses with naturally occurring carpal osteoarthritis, suggesting that lubricin upregulation may be a protective response to joint injury16. Another possibility is that the increased lubricin detected in synovial fluid may be lubricin that is degraded, sheared off the articular cartilage surface, or otherwise non-functional; however, synovial fluid lubricating function was not impaired in horses with elevated lubricin following experimental carpal fragmentation17. Data suggests that synovial fluid lubricin concentrations may correlate with measures of joint instability, such as anterior-posterior laxity in humans with knee injury32, and in vitro studies show that both compression and increased dynamic shear stress can increase lubricin production33–35. Therefore, increased lubricin could be secondary to altered mechanical loading or a result of inflammation induced by joint trauma.
Based on our evaluation of healthy equine joints, lubricin concentrations were conserved amongst the sampled high-motion joints. In healthy joints, neither age, joint, nor synovial fluid total protein were predictive of lubricin concentrations. Elevated lubricin expression has been previously associated with fibrillated cartilage and cartilaginous deposits in subchondral bone adjacent to regions of exposed articular bone in human OA36, suggesting the possible involvement of lubricin in the reparative process of damaged joints10,16. Further investigation of lubricin in healthy joints and those with subclinical joint disease is needed to determine the utility of lubricin as a sensitive biomarker for early cartilage injury or subclinical joint disease. The absence of variation as a function of age or joint might represent an advantage of synovial fluid lubricin as a potential biomarker for joint disease as compared to HA.
Marked losses of HA were noted in both the osteochondral fragmentation and cartilage impact models in the immediate postoperative period. This dramatic and acute response may be related to rapid degradation of HA secondary to acute joint injury18. However, arthroscopic lavage alone is not benign, and mechanical washout of HA may instead be the major factor underpinning this finding in the early postoperative period37–39. While HA concentrations after osteochondral fragmentation increased back to pre-injury levels within the study timeframe (<70 days), HA losses were still evident 168 days post-injury in the cartilage impact model. In our multi-joint comparison, synovial fluid from healthy tarsocrural joints had the lowest concentrations of HA amongst all sampled high-motion joints, demonstrating an inherent variation between joints, which could explain the disparity in HA rebound timeframes. This variation in response could also be a specific finding secondary to the focal cartilage impact injury inducing a more sustained loss of HA.
In contrast to the osteochondral fragmentation and cartilage impact injury models, HA depletion was not detected following the creation of full-thickness cartilage defects in the femoropatellar joint. However, the first sampling time point (day 84) was nearly 3 months after the initial joint injury. As prior studies have shown normalization of HA concentrations 3 months after experimental cartilage injury and repair in the equine stifle18 and no disparity between HA concentration in healthy joints and those with naturally-occurring OA greater than 3 weeks duration10, the timing of synovial fluid collection in this particular model limits our ability to draw significant conclusions about HA activity following creation of cartilage defects. Additional studies with earlier and more frequent sampling would be useful to further investigate this.
Variations in relative proportions of HA MW were noted for the MW categories of >6.1 MDa and 1.5 – 3.1 MDa, but only in relation to medial femorotibial joints. As the medial femorotibial joint has a smaller sample size as compared to other joints, it is unclear whether this relationship would hold true with increased sample sizes. Additionally, given the weak correlations between relative proportions of HA MW and HA concentration, these differences could also be related to the relative insensitivity of gel electrophoresis to detect lower MW HA. In humans with OA or rheumatoid arthritis, HA molecules can be degraded or cleaved early in production such that there are increased relative concentrations of low MW HA40. The biologic activity of low MW HA is under question; however, there is a large body of evidence to suggest that hyaluronan fragments induce inflammatory cytokines, further perpetuating the cycle of joint inflammation post-injury41,42. More sensitive methods for detection of HA size are being evaluated43 and may provide additional insight into HA MW distribution following various types of joint injury.
In this study, banked synovial fluid samples obtained from three distinct joint injury models were evaluated to determine whether lubricin was increased, decreased or unchanged from baseline values prior to induction of injury. Each joint injury model involved repeated arthrocentesis through the respective study time periods to monitor serial alterations in lubricin and hyaluronan. While frequent arthrocentesis alone can induce an inflammatory response, sampling without administration of any concurrent medications at the relative frequency of ≥ 1 week performed here has not been shown to induce clinically significant synovial fluid alterations44 and is consistent with recommendations of spacing serial arthrocenteses ≥ 1 week apart to avoid this as a confounding factor45. A limitation of the study is that all injury models were not performed concurrently, and banked synovial fluid samples were stored at −80°C for different durations of time. However, all synovial fluid samples were similarly obtained via direct aspiration, processed to remove cellular debris, and stored at −80°C until processing. In addition, horses in each joint injury model were confirmed to be free of clinical joint disease based on initial subjective lameness exam and radiographs of the joint of interest. Evidence of induced joint disease was confirmed on post-mortem examination of the injured joint and the contralateral joint16, 27, 28. Joint samples collected from horses for comparison of high-motion joints were evaluated, and the average lubricin concentrations (64.9 ± 4.18 ug/ml) and the average HA concentrations (0.47 ± 0.03 mg/ml) were within published ranges for healthy equine joints16,46.
An additional limitation of this study is that the lubricin sandwich ELISA is not able to discern whether the lubricin detected is intact, as both mAb 9G3 and PNA react against epitopes within the mucin-rich domain of lubricin47,48. While this is a limitation of several prior studies, it would be ideal to be able to detect both the N- and C-termini of lubricin using a sandwich ELISA. Unfortunately, we are unaware of any N-terminal antibodies that cross-react with equine lubricin. In order to address this potential limitation, we performed immunoblotting using mAb 9G3, PNA and a C-terminal antibody, PA3–118. Given that the C-terminal antibody immunoblot revealed an ~ 220 kDa band that did not correspond to any reactivity with the mucin-domain reagents, it is possible that this lower MW band could represent a non- or under-glycosylated variant of lubricin or a non-O-glycosylated lubricin cleavage product. However, we can conclude, based on the absence of any mAb 9G3 or PNA signal at this ~ 220 kDa band, that this is not a cleavage product that was being detected with our lubricin sandwich ELISA.
The response to joint trauma is influenced by a combination of factors, including the type of injury, the anatomy of the joint, and the biomechanical forces acting upon that joint. The three joints evaluated in this study are all considered high-motion joints, but do vary anatomically and biomechanically. The equine femoropatellar joint is structurally similar to humans in that the patella lies in the intertrochlear groove and glides between the lateral and medial trochlear ridges during flexion and extension of the knee. The equine tarsocrural joint has been described as having primarily helical motion or screw action during flexion and extension, with the distal aspect of the tibia rotating around the talus49. While the femoropatellar and tarsocrural joints share biomechanical similarities, the femoropatellar joint is a non-weight bearing joint and the tarsocrural joint is a weight-bearing joint. The middle carpal joint is a weight-bearing joint and functions in a hinge fashion with only approximately 45 degrees of articulation50. The variations in the underlying biomechanics of the joints evaluated here may have had some impact on the response to joint trauma; however, the increased lubricin concentrations observed in response to joint injury were conserved across all joints.
This study evaluated serial concentrations of lubricin, HA, and relative HA MW distributions across three distinct equine joint injury models. Despite conflicting literature about lubricin in the PTOA joint, here we demonstrate that synovial fluid lubricin is increased from baseline pre-injury values in three distinct models of equine PTOA, including intra-articular fracture, cartilage impact injury, and full-thickness cartilage defects. Increases in synovial fluid lubricin in response to PTOA spanned three separate high-motion joints, including the carpus (wrist), tarsus (ankle) and femoropatellar joint (knee). Whereas lubricin concentrations were increased in all three models, synovial fluid HA was decreased in both intra-articular fracture and cartilage impact injury. Lubricin may be elevated post-injury to protect joints from inflammation and to maintain joint lubrication by compensating for loss of HA17. Further investigation into the mechanisms regulating synovial fluid lubricin concentrations following joint injury is warranted, including whether lubricin has potential value as a biomarker for PTOA.
Supplementary Material
Figure S-1 (Supplemental Figure 1). Synovial fluid western and lectin blots. Serial, reduced, synovial fluid samples (0.2 μl) from a representative horse undergoing carpal osteochondral fragment injury (A-C) and a representative horse undergoing tarsal impact injury (D-F) were loaded onto 3-8% NuPAGE tris-acetate gels. Gels were transferred to nitrocellulose and blotted with anti-lubricin mAb 9G3 for the mucin domain (A,D), peanut agglutinin lectin for O-glycans (B,E), and anti-lubricin pAb PA3-118 for the C-terminus (C,F). Although the band intensities differ over time, consistent with increases in lubricin post-injury, the molecular weight profile of lubricin does not change. Western blots and lectin blots do not show evidence of lubricin degradation post-injury.
Table S-1 (Supplemental Table 1). Breakdown of the source of synovial fluid samples collected from the 11 horses in Cohort 1 and utilized for the multi-joint analysis. MCPJ (metacarpophalangeal joint), MTPJ (metatarsophalangeal joint), MCJ (middle carpal joint), ACJ (antebrachiocarpal joint), TCJ (tarsocrural joint), FPJ (femoropatellar joint).
Acknowledgements
The authors would like to acknowledge the Cleveland Clinic Program for Excellence in Glycoscience and the NHLBI Award number PO1HL107147 for providing detailed protocols related to HA gel electrophoresis and Stains-All staining. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K08AR068469 (HR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Reesink has a provisional patent on the production and use of recombinant glycoproteins related to lubricin and similar mucins.
References
- 1.Anderson DD, Chubinskaya S, Guilak F, et al. 2011. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 29:802–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman BD, Mangiapani DS, Zeitler E, et al. 2014. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther 16:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldring MB, Goldring SR. 2010. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann NY Acad Sci 1192:230–37. [DOI] [PubMed] [Google Scholar]
- 4.Jay GD, Torres JR, Warman ML, et al. 2007. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA 104:6194–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jay GD, Elsaid KA, Zack J, et al. 2004. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol 31:557–64. [PubMed] [Google Scholar]
- 6.Marcelino J, Carpten JD, Suwairi WM, et al. 1999. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23:319–22. [DOI] [PubMed] [Google Scholar]
- 7.Jay GD, Torres JR, Rhee DK, et al. 2007. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum 56:3662–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee DK, Marcelino J, Al-Mayouf S, et al. 2005. Consequences of disease-causing mutations on lubricin protein synthesis, secretion, and post-translational processing. J Biol Chem 280:31325–32. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig T, McAllister JR, Lun V, et al. 2012. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4. Arthritis Rheum 64:3963–71. [DOI] [PubMed] [Google Scholar]
- 10.Antonacci JM, Schmidt TA, Serventi LA, et al. 2012. Effects of equine joint injury on boundary lubrication of articular cartilage by synovial fluid: role of hyaluronan. Arthritis Rheum 64:2917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwiecinski JJ, Dorosz SG, Ludwig TE, et al. 2011. The effect of molecular weight on hyaluronan’s cartilage boundary lubricating ability – alone and in combination with proteoglycan 4. Osteoarthritis Cartilage 19:1356–62. [DOI] [PubMed] [Google Scholar]
- 12.Atarod M, Ludwig TE, Frank CB, et al. 2015. Cartilage boundary lubrication of ovine synovial fluid following anterior cruciate ligament transection: a longitudinal study. Osteoarthritis Cartilage 23:640–47. [DOI] [PubMed] [Google Scholar]
- 13.Elsaid KA, Fleming BC, Oksendahl HL, et al. 2008. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum 58:1707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teeple E, Elsaid KA, Fleming BC, et al. 2008. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res 26:231–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsaid KA, Machan JT, Waller K, et al. 2009. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting TNF-a on chondroprotection in an animal model. Arthritis Rheum 60;2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reesink HL, Watts AE, Mohammed HO, et al. 2017. Lubricin/proteoglycan 4 increases in both experimental and naturally occurring equine osteoarthritis. Osteoarthritis Cartilage 25:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feeney E, Peal BT, Inglis JE, Su J, et al. 2019. Temporal changes in synovial fluid composition and elastoviscous lubrication in the equine carpal fracture model. J Orthop Res 37: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grissom MJ, Temple-Wong MM, Adams MS, et al. 2014. Synovial fluid lubricant properties are transiently deficient after arthroscopic articular cartilage defect repair with platelet-enriched fibrin alone and with mesenchymal stem cells. Orthop J Sports Med 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballard BL, Antonacci JM, Temple-Wong MM, et al. 2012. Effect of tibial plateau fracture on lubrication function and composition of synovial fluid. J Bone Joint Surg 94:e64(1–9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu CP, Reddi AH, Komvopoulos K, et al. 2010. Friction coefficient and superficial zone protein are increased in patients with advanced osteoarthritis. Arthritis Rheum 62:2680–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novakofski KD, Berg LC, Bronzini I, et al. 2015. Joint-dependent response to impact and implications for post-traumatic osteoarthritis. Osteoarthritis Cartilage 23:1130–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushnaghan J, Dieppe P. 1991. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis 50:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawcak CE. 2016. Biomechanics in joints In: McIlwraith CW, Frisbie DD, Kawcak CE, van Weeren PR, editors. Joint disease in the horse. St. Louis: Elsevier; p 25–32. [Google Scholar]
- 24.McCoy AM. 2015. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol 52:803–18. [DOI] [PubMed] [Google Scholar]
- 25.McIlwraith CW, Frisbie DD, Kawcak CE. 2012. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res 1:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennel JC, Egenvall A, Bonnett BN, et al. 2005. Specific causes of morbidity among Swedish horses insured for veterinary care between 1997 and 2000. Vet Rec 157:470–7. [DOI] [PubMed] [Google Scholar]
- 27.Delco ML, Bonnevie ED, Alexander PG, et al. 2017. An in vivo large animal model to study impact-induced cartilage injury and the development of early posttraumatic ankle osteoarthritis. Osteoarthritis Cartilage 25:s309–310. [Google Scholar]
- 28.Nixon AJ, Sparks HD, Begum L, et al. 2017. Matrix-induced autologous chondrocyte implantation (MACI) using a cell-seeded collagen membrane improves cartilage healing in the equine model. J Bone Joint Surg 99:1987–98. [DOI] [PubMed] [Google Scholar]
- 29.Lee HG, Cowman MK. 1994. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem 219:278–87. [DOI] [PubMed] [Google Scholar]
- 30.Preibisch S, Saalfeld S, Tomancak P. 2009. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25:1463–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa H, Matsumoto K, Terabayashi N, et al. 2017. Association of lubricin concentration in synovial fluid and clinical status of osteoarthritic knee. Mod Rheumatol 27(3):489–492. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa H, Kozhemyakina E, Hung HH, et al. 2014. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev 28(2):127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones ARC, Chen S, Chai DH, et al. 2009. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum 60(1):133–142. [DOI] [PubMed] [Google Scholar]
- 35.Nugent GE, Aneloski NM, Schmidt TA, et al. 2006. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum 54(6):1888–1896. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D, Johnson LJ, Hsu H, Spector M. 2007. Cartilaginous deposits in subchondral bone in regions of exposed bone in osteoarthritis of the human knee: histomorphometric study of PRG4 distribution in osteoarthritic cartilage. J Orthop Res 25:873–83. [DOI] [PubMed] [Google Scholar]
- 37.Hempfling H 2007. Intra-articular hyaluronic acid after knee arthroscopy: a two-year study. Knee Surg Sports Traumatol Arthrosc 15:537–46. [DOI] [PubMed] [Google Scholar]
- 38.de Rezende MU, de Campos GC. Viscosupplementation. 2012. Rev Bras Ortop 47:160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teeple E, Karamchedu NP, Larson KM, et al. 2016. Arthroscopic irrigation of the bovine stifle joint increased cartilage surface friction and decreases superficial zone lubricin. J Biomech 49:3106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowman MK, Lee HG, Schwertfeger KL, et al. 2015. The content and size of hyaluronan in biological fluids and tissues. Front Immunol 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheibner KA, Lutz MA, Boodoo S, et al. 2006. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177:1272–81. [DOI] [PubMed] [Google Scholar]
- 42.Iacob S, Knudson CB. 2006. Hyaluronan fragments activate nitric oxide synthase and the production of nitric oxide by articular chondrocytes. Int J Biochem Cell Biol 38:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivas F, Zahid OK, Reesink HL, et al. 2018. Label-free analysis of physiological hyaluronan size distribution with a solid-state nanopore sensor. Nat Commun 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rinnovati R, Bonelli F, Tognetti R, et al. 2017. Effect of repeated arthrocentesis on cytology of synovial fluid. J Equine Vet Sci 57:112–115. [Google Scholar]
- 45.van den Boom R, van de Lest CHA, Bull S, et al. 2005. Influence of repeated arthrocentesis and exercise on synovial fluid concentrations of nitric oxide, prostaglandin E2 and glycosaminoglycans in healthy equine joints. Equine Vet J 37(3): 250–256. [DOI] [PubMed] [Google Scholar]
- 46.Tulamo RM, Heiskanen T, Salonen M. 1994. Concentration and molecular weight distribution of hyaluronate in synovial fluid from clinically normal horses and horses with diseased joints. Am J Vet Res 55:710–15. [PubMed] [Google Scholar]
- 47.Ai M, Cui Y, Sy M, et al. 2015. Anti-lubricin monoclonal antibodies created using lubricin-knockout mice immunodetect lubricin in several species and in patients with healthy and diseased joints. PLoS One 10(2): e0116237, 10.1371/journal.pone.0116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svala E, Jin C, Rüetschi U, et al. 2017. Characterisation of lubricin in synovial fluid from horses with osteoarthritis. Equine Vet J 49:116–123. [DOI] [PubMed] [Google Scholar]
- 49.Lanovaz JL, Khumsap S, Clayton HM, et al. 2002. Three-dimensional kinematics of the tarsal joint at the trot. Equine Vet J 34:308–313. [DOI] [PubMed] [Google Scholar]
- 50.Lee H, Kirkland WG, Whitmore RN, et al. 2014. Comparison of equine articular cartilage thickness in various joints. Connect Tissue Res 55(5–6):339–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S-1 (Supplemental Figure 1). Synovial fluid western and lectin blots. Serial, reduced, synovial fluid samples (0.2 μl) from a representative horse undergoing carpal osteochondral fragment injury (A-C) and a representative horse undergoing tarsal impact injury (D-F) were loaded onto 3-8% NuPAGE tris-acetate gels. Gels were transferred to nitrocellulose and blotted with anti-lubricin mAb 9G3 for the mucin domain (A,D), peanut agglutinin lectin for O-glycans (B,E), and anti-lubricin pAb PA3-118 for the C-terminus (C,F). Although the band intensities differ over time, consistent with increases in lubricin post-injury, the molecular weight profile of lubricin does not change. Western blots and lectin blots do not show evidence of lubricin degradation post-injury.
Table S-1 (Supplemental Table 1). Breakdown of the source of synovial fluid samples collected from the 11 horses in Cohort 1 and utilized for the multi-joint analysis. MCPJ (metacarpophalangeal joint), MTPJ (metatarsophalangeal joint), MCJ (middle carpal joint), ACJ (antebrachiocarpal joint), TCJ (tarsocrural joint), FPJ (femoropatellar joint).