Abstract
Objective(s):
I-123-ioflupane single photon emission computed tomography (FP-CIT-SPECT) has been used to assess dopamine transporter (DAT) loss in Parkinson's disease. The specific binding ratio (SBR), a quantitative parameter of DAT density in the striatum, may be affected by differences in age, sex, and SPECT system. The purpose of this study was to evaluate the utility of FP-CIT-SPECT using the Japanese normal database (NDB) in the diagnosis of Parkinson's disease.
Methods:
To standardize the quantitative outcome measures of DAT density obtained with different SPECT systems, striatal phantoms filled with striatal to background materials at ratios between 8:1 and 1:1 were measured using a gamma camera (ECAM) in our institute. Consecutive fifty patients (23 men and 27 women; age range, 40-86 years) with suspected PD undergoing FP-CIT SPECT brain imaging during the period from April to October 2016 were enrolled in this retrospective study. Their final diagnoses were PD in 28 patients and PD in 22 patients. SBRs of the patients were calculated using either new (Japanese database with different age and sex; NEWver) or old (non-Japanese database not specifying age and sex; OLDver) version software (AZE Virtual Place Hayabusa [DaTView], AZE, Ltd. Tokyo, Japan). The McNemar test was used to compare the diagnostic accuracy between old and new versions.
Results:
Based on the phantom study, the calibrated SBR could be calculated by Y=1.25×Measured SBR+0.78. The sensitivities for OLDver and NEWver were 100% and 93%, respectively (p=0.5), and the specificities were 55% and 100% (p=0.002). The diagnostic accuracy of NEWver (96%) was better than that of OLDver (80%, p<0.001).
Conclusion:
FP-CIT-SPECT using the Japanese NDB improved the diagnostic accuracy of PD by improving specificity.
Key Words: Japanese normal database, Parkinson's disease DAT scan, Specific Binding Ratio
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized pathologically by loss in the substantia nigra and ventral tegmental area. When patients become symptomatic and fulfil the clinical criteria for PD, DAT may be lost in the substantia nigra and ventral tegmental area. Longitudinal studies of DAT scans showed a mean annual decline in striatal radiotracer uptake ranging by 5 to 13% in PD patients, as compared to 0.6–2.5% in healthy controls (1-3).
I-123-ioflupane single photon emission computed tomography (FP-CIT SPECT) is a sensitive method to detect presynaptic dopamine transporter (DAT), which is a biomarker of neurodegenerative parkinsonism. FP-CIT SPECT imaging has been widely used to assess the loss of DAT in PD. Recently, the official International Parkinson and Movement Disorder Society (MDS) presented diagnostic criteria for PD, in which normal FP-CIT-SPECT findings are required for absolute exclusion criteria of PD (4). The functional image diagnosis of FP-CIT SPECT is useful, since the differentiation of early Parkinson's disease and other Parkinson's disease syndromes is difficult (1). The sensitivity and specificity for clinical diagnosis of FP-CIT SPECT have been reported to be useful, and the clinical diagnostic accuracy may be mathematically identical to the diagnostic accuracy by FP-CIT SPECT as shown in the Japanese Parkinson's disease clinical guidelines 2018 of the Japanese Society of Neurology (5-8).
The interpretation of FP-CIT SPECT is based on visual evaluation with the aid of quantitative analysis, but in Japan, assessments of SPECT may also be performed using software such as DaTView (software for calculating specific binding ratio [SBR] of patients) by the Tossici-Bolt method (9). The SBR is calculated by comparing the activity in the striatum and the activity in the background or a reference area due to the non-specific binding. However, the SBR may be affected by differences in age, sex, and SPECT system. Therefore, Matsuda has established a multicenter Japanese large-scale database of FP-CIT SPECT scans from different SPECT scanners in healthy controls across a wide age range and with balanced sex representation (10). However, the diagnostic accuracy of this Japanese normal database (NDB) by SBR has not been evaluated.
The purpose of this study was to evaluate the utility of FP-CIT SPECT using the Japanese NDB in the diagnosis of PD.
Methods
Phantom study
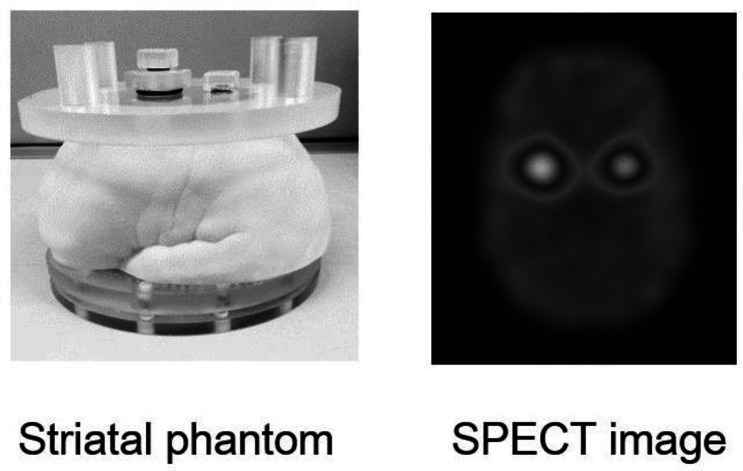
The striatal phantom was initially used to standardize the quantitative outcome measures of DAT density obtained with different SPECT systems. I-123 SPECT images were taken of an anthropomorphic striatal phantom (NMP Business Support Co., Ltd., Hyogo, Japan) consisting of two compartments (striatal compartments (striatum) and brain shell cavity) filled at striatum-to-background concentration ratios of 8, 6, 3, and 1 to 1 with the radiopharmaceutical I-123 FP-CIT (Nihon Medi- Physics Co., Ltd., Tokyo, Japan).
Case study
Fifty patients (23 men and 27 women; age range, 40-86 years) with suspected PD who underwent I-123 FP-CIT SPECT brain imaging during the period from April to October 2016 were selected and enrolled in this retrospective study. Their final diagnoses (28 PD and 22 without PD) were confirmed by broad-certified neurologists as shown in Table 1. The protocol of this study was approved by the Ethics Committee of our institution (No. HS2018-110). Patients were administered 167 MBq of I-123 FP-CIT (I-123 Ioflupane, DaTSCAN, Nihon Medi-Physics Co., Ltd., Tokyo, Japan), and the imaging was acquired three hours after injection. Patients did not undergo any special preparation before the scan.
Table 1.
Patient characteristics
| Characteristics | Patients (n = 50) |
|---|---|
| Number of examinations | 50 |
| Median age, range | 66.7 ± 15.1 (40-86) |
| Sex | |
| Men | 23 |
| Women | 27 |
| Final dia gnosis | |
| PD | 28 |
| AD | 9 |
| ET | 7 |
| Normal | 5 |
| Unclear | 1 |
PD: Parkinson’s disease, AD: Alzheimer's disease, ET: Essential Tremor
SPECT acquisition
An E-CAM (Canon Medical Systems, Otawara, Tochigi, Japan) dual-detector gamma camera system equipped with low-energy high-resolution collimators (LMEGP) was used in both phantom and patient studies. The SPECT data were acquired using dynamic tomography with two repeats, four cycles/repeat, 210 s/cycle on a 128×128 matrix. The reconstructed pixel size was 3.3×3.3 mm, zoom 1.45, and the energy for I-123 was set at 159 keV (±20%). Emission data were reconstructed using a GMS-7700R workstation (Canon Medical Systems, Otawara, Tochigi, Japan). Reconstruction was based on implementation of the ordered subsets - expectation maximization (OS-EM) algorithm (11). A Butterworth filter (photopeak image: order 8, cutoff frequency=0.50 cycles/cm) was used as a pre-filter. The SPECT data were not corrected for attenuation correction and / or scatter correction, since calibrated SBR were almost constant under no correction conditions (10).
Quantitative analysis
All SPECT data were imported into software (AZE Virtual Place Hayabusa [DaTView], AZE, Ltd. Tokyo, Japan) for automatic calculation of SBR. ROI were drawn based on the two-box method developed by Tossici-Bolt et al. (9). The SBR was calculated as follows:
We used a lower SBR value between the right and left striatum in each participant for data analysis.
To analyze the diagnostic accuracy between old and new methods, SBRs of the patients were calculated using either new (Japanese database specifying age and sex; NEWver) or old (non-Japanese database not specifying age and sex; OLDver) version software. In OLDver software, a cut-off value of 4.5 for lower SBR was set for the diagnosis of PD (Tossici-Bolt et. al. (9)). In NEWver software, SBRs were calibrated from SPECT data using a striatal phantom and the SBR was taken as normal from a linear regression line with 95% upper and lower prediction interval (PI) lines reported by Matsuda H et al. (10). A calibrated SBR less than -2SD of SBR of Japanese healthy controls was diagnosed as PD.
Statistical analysis:
The diagnostic accuracies of OLDver and NEWver software were compared using the McNemar test in patients with suspected PD. All statistical analyses were performed with IBM SPSS Statistics (version 23, IBM Corp., Armonk, NY). Probability values <0.05 were considered significant.
Results
Phantom study
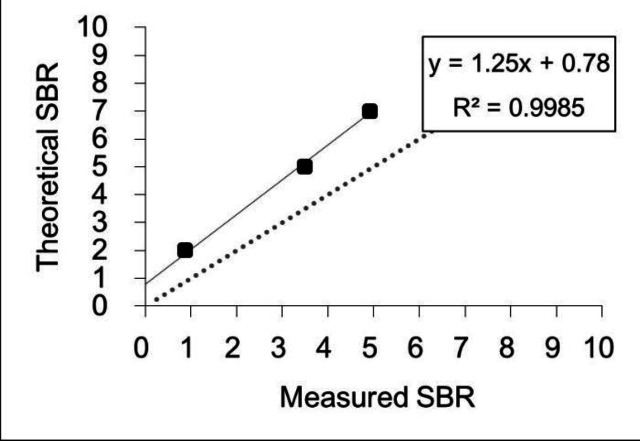
Based on the phantom study, the theoretical SBR obtained by measurements using a well counter and the measured SBR measured by software analysis of SPECT images from a striatal phantom showed a linear relationship for SPECT scanners. Figure 1 and Table 2 show the phantom and SPECT images Linear regression equations for the calibration are listed in Figure 2, also showing the results of the phantom study for SPECT images with NEWver. The calibrated SBR was calculated using the formula:
Figure 1.
Striatal phantom and FP-CIT SPECT image
Table 2.
SBR results by phantom study
| Striatal / BG | Theoretical SBR | Measured SBR |
|---|---|---|
| 8:1 | 7.0 | 4.9 |
| 6:1 | 5.0 | 3.5 |
| 3:1 | 2.0 | 0.9 |
| 1:1 | 0.0 | -0.6 |
Figure 2.
Calibration of SBR using the formula obtained in the phantom study
Y= 1.25×Measured SBR+0.78
This regression formula was obtained by comparing the calibrated SBR to the theoretical SBR. The calibrated SBR values using linear regression equations were used in the clinical study with PD and N-PD.
Clinical study
Figure 3 shows the results of 28 Parkinson’s disease patients and 22 patients without PD (N-PD) evaluated by OLDver. The sensitivity of OLDver was adequate. When these 22 patients with N-PD were evaluated by OLDver, 9 patients were misdiagnosed as PD.
Figure 3.
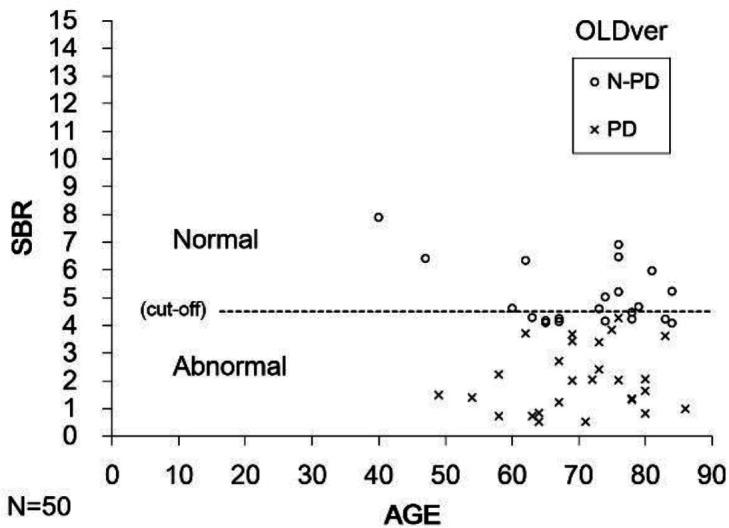
Scatter plot of lower SBR for the left and right striatum as a function of age in 28 patients with PD and 22 N-PD patients in OLDver. The sensitivity of OLDver software was adequate. Nine patients were misdiagnosed as PD. The cut-off value was 4.5
Figure 4 shows the results of SBR evaluated by NEWver. Two patients with PD were misdiagnosed by NEWver, which made the sensitivity of NEWver lower than that of OLDver. In contrast, all 22 patients with N-PD were diagnosed as normal.
Figure 4.
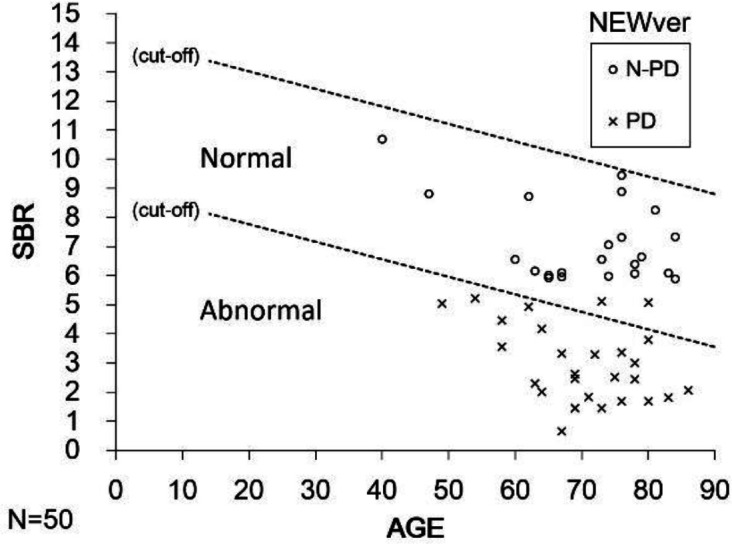
Scatter plot of lower SBR for the left and right striatum as a function of age in 28 PD patients and 22 N-PD patients by NEWver. Two patients with PD were misdiagnosed by NEWver software. The specificity of NEWver software was adequate. Data relevant to cut-off are fitted from the Japanese NDB
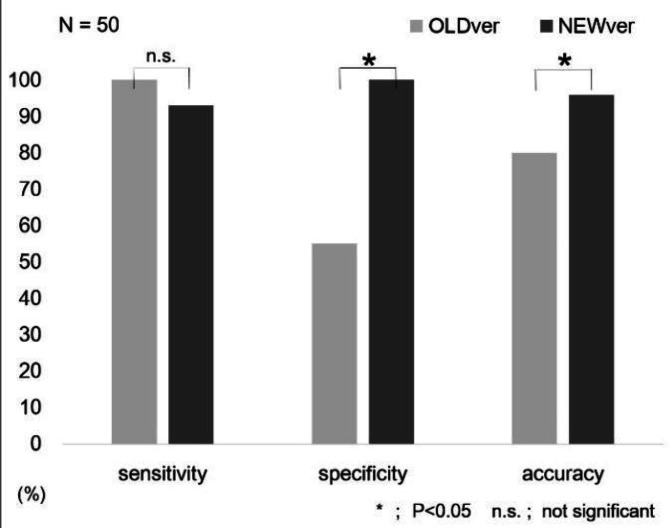
Figure 5 shows the diagnostic utility of OLDver and NEWver. The sensitivities for OLDver and NEWver were 100% and 93%, respectively (p=0.5), and the specificities were 55% and 100% (p=0.002). The diagnostic accuracy of NEWver (96%) was better than that of OLDver (80%, p<0.001).
Figure 5.
Comparison of diagnostic ability for PD of OLDver and NEWver software. Although the sensitivities for OLDver and NEWver showed no significant difference (p=0.5), the specificity and the accuracy of NEWver were significantly better than those of OLDver. * p <0.05, n.s. not significant
Discussion
FP-CIT SPECT has become a common tool in daily clinical routines for PD diagnosis. Image interpretation of SPECT studies has been performed using a combination of evaluation of SBR values for the whole striatum, calculated by computer software, and qualitative assessment of SPECT images. In addition, it has been known that SBR may be affected by differences in age, sex, and SPECT system (10). We compared SBR values by NEWver software, in which SBR was corrected for these influences for PD, with OLDver. The specificity and accuracy were significantly improved in NEWver, while the sensitivity of NEWver was comparable with that of OLDver.
For the harmonization of SBRs among different SPECT cameras and various image reconstruction methods, the usefulness of SBR measurement by phantom calibration was reported by Tossici-Bolt et al. (12, 13). In our study using a similar method, we were able to perform SBR calibration in a phantom study, and calculated a regression equation for the purpose of correcting the underestimation of SBR.
Considering the age-related decline in SBR, we suspected that it is essential to analyze SBRs using the Japanese normal database for better diagnostic accuracy. The age-related decline of SBR was not reflected in the analysis by OLDver, which may have led to many false-positive results of PD, and similar results were noted in this study. To solve these limitations of OLDver, Matsuda H et al. established a new Japanese normal database, and reported that SBR decreases by 6.3% per ten years (10). Many false-positive findings resulted in a lower specificity of OLDver, while reduced false-positive findings with NEWver yielded a higher specificity than OLDver in our study.
Although accurate diagnosis of PD was achieved using NEWver with the Japanese normal database, we should pay attention to patients whose scans show no evidence of dopaminergic deficit (SWEDD) (14), and to those with idiopathic normal pressure hydrocephalus (iNPH) with the ventriculomegaly known to lead to underestimation of SBR (15). In these cases, it is still important to make a diagnosis of PD considering both neurological findings and qualitative evaluation of SPECT images, with careful observation of the striatal shape changes (16). In addition, myocardial innervation imaging using I-123 metaiodobenzylguanidine (MIBG) scintigraphy (17-20) and brain perfusion SPECT (21-24) may be important for the differential diagnosis between PD and other parkinsonism.
There were several limitations in our study. First, we did not have true control groups of patients, such as AD or normal healthy volunteers in our study in which all the enrolled patients were clinically suspected to be PD. For more precise evaluation, recruitment of normal healthy volunteers would be required. Second, separate analysis by gender was not be performed. Since it has been reported that SBR might be affected by serum estrogen and striatum size, separate data analysis by gender should be considered in future studies (24-25).
Conclusion
We concluded that FP-CIT SPECT using software with the Japanese NDB can improve the diagnostic accuracy for Parkinson’s disease by improving specificity.
Acknowledgment
The authors thank Hiroo Kasahara, M.D. and Yoshio Ikeda, M.D, Ph.D. for their valuable help in patient recruiting and management during this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Winogrodzka, A, Booij, J, Wolters, E. C. Disease-related and drug-induced changes in dopamine transporter expression might undermine the reliability of imaging studies of disease progression in Parkinson’s disease. Parkinsonism and Related Disorders. 2005;11(8):475–484. doi: 10.1016/j.parkreldis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, et al. [123I] beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology . 2001;57: 2089–2094. doi: 10.1212/wnl.57.11.2089. [DOI] [PubMed] [Google Scholar]
- 3.Winogrodzka A, Bergmans P, Booij J, van Royen EA, Janssen AG, Wolters EC. [123I] FP-CIT SPECT is a useful method to monitor the rate of dopaminergic degeneration in early-stage Parkinson’s disease. J Neural Transm. 2001;108:1011–1019. doi: 10.1007/s007020170019. [DOI] [PubMed] [Google Scholar]
- 4.R.B. Postuma, D, Berg, M, Stern, W, Poewe, C.W. MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 5.Benamer HTS, Patterson J, Grosset DG, Booij J, Bruin K De, Royen E Van, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: The [123I]-FP-CIT study group. Mov Disord. 2000;15:503–510. [PubMed] [Google Scholar]
- 6.O’Brien JT, Oertel WH, McKeith IG, Grosset DG, Walker Z, Tatsch K, et al. Is ioflupane123I injection diagnostically effective in patients with movement disorders and dementia? Pooled analysis of four clinical trials. BMJ Open. 2014;4:e005122. doi: 10.1136/bmjopen-2014-005122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigo F, Matinella A, Erro R, Tinazzi M. [123I] FP-CIT SPECT (DaTSCAN) may be a useful tool to differentiate between Parkinson’s disease and vascular or drug-induced parkinsonisms: A meta-analysis. Eur J Neurol. 2014;21:1369–1376. doi: 10.1111/ene.12444. [DOI] [PubMed] [Google Scholar]
- 8.Varrone A, Dickson JC, Tossici-bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I] FP-CIT SPECT ( ENC-DAT ): age-related effects , gender differences and evaluation of different methods of analysis. 2013:213–227. doi: 10.1007/s00259-012-2276-8. [DOI] [PubMed] [Google Scholar]
- 9.Tossici-Bolt L, Hoffmann SMA, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I] FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33:1491–1499. doi: 10.1007/s00259-006-0155-x. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda H, Murata M, Mukai Y, Sako K, Ono H, Toyama H, et al. Japanese multicenter database of healthy controls for [123I]FP-CIT SPECT. Eur J Nucl Med Mol Imaging. 2018;45:1405–1416. doi: 10.1007/s00259-018-3976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson HM, Larkin RS. Accelerated Image Reconstruction Using Ordered Subsets of Projection Data. IEEE Trans Med Imaging. 1994;13:601–609. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 12.Tossici-Bolt L, Dickson JC, Sera T, Nijs R De, Bagnara MC, Jonsson C, et al. Calibration of gamma camera systems for a multicentre European 123I-FP-CIT SPECT normal database. Eur J Nucl Med Mol Imaging. 2011;38:1529–1540. doi: 10.1007/s00259-011-1801-5. [DOI] [PubMed] [Google Scholar]
- 13.Tossici-Bolt L, Dickson JC, Sera T, Booij J, Asenbaun-Nan S, Bagnara MC, et al. [ 123 I ] FP-CIT ENC-DAT normal database: the impact of the reconstruction and quantification methods. EJNMMI Phys. 2017;4(1) doi: 10.1186/s40658-017-0175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall VL, Reininger CB, Marquardt M, Patterson J, Hadley DM, Oertel WH, et al. Parkinson's disease is overdiagnosed clinically at baseline in diagnostically uncertain cases:A 3-year European multicenter study with repeat (123I) FP-CIT SPECT. Mov Disord . 2009;24:500–508. doi: 10.1002/mds.22108. [DOI] [PubMed] [Google Scholar]
- 15.Asahi T, Kashiwazaki D, Yoneyama T, Noguchi K, Kuroda S. Importance of 123I-ioflupane SPECT and Myocardial MIBG Scintigraphy to Determine the Candidate of Deep Brain Stimulation for Parkinson’s Disease. Neurologia Medico-Chirurgica . 2016;56(3):125–131. doi: 10.2176/nmc.oa.2015-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami H, Kimura A, Yasumoto T, Miki A, Yamamoto K, Ito N, et al. Usefulness Differs Between the Visual Assessment and Specific Binding Ratio of 123I-Ioflupane SPECT in Assessing Clinical Symptoms of Drug-Naïve Parkinson’s Disease Patients. Front Aging Neurosci. 2018;10:1–8. doi: 10.3389/fnagi.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orimo S, Suzuki M, Inaba A, Mizusawa H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: A systematic review and meta-analysis. Park Relat Disord. 2012;18:494–500. doi: 10.1016/j.parkreldis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Treglia G, Cason E, Stefanelli A, Cocciolillo F, Giuda D Di, Fagioli G, et al. MIBG scintigraphy in differential diagnosis of Parkinsonism: A meta-analysis. Clin Auton Res. 2012;22:43–55. doi: 10.1007/s10286-011-0135-5. [DOI] [PubMed] [Google Scholar]
- 19.Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 2005;109:583–588. doi: 10.1007/s00401-005-0995-7. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Ikemura M, Oka T, Uchihara T, Wakabayashi K, Kakita A, et al. Quantitative correlation between cardiac MIBG uptake and remaining axons in the cardiac sympathetic nerve in Lewy body disease. J Neurol Neurosurg Psychiatry. 2015;86:939–944. doi: 10.1136/jnnp-2015-310686. [DOI] [PubMed] [Google Scholar]
- 21.Bosman T, Laere K Van, Santens P. Anatomically standardised99mTc-ECD brain perfusion SPET allows accurate differentiation between healthy volunteers, multiple system atrophy and idiopathic Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2003;30:16–24. doi: 10.1007/s00259-002-1009-9. [DOI] [PubMed] [Google Scholar]
- 22.Mito Y, Yoshida K, Yabe I, Makino K, Hirotani M, Tashiro K, et al. Brain 3D-SSP SPECT analysis in dementia with Lewy bodies, Parkinson’s disease with and without dementia, and Alzheimer’s disease. Clin Neurol Neurosurg. 2005;107:396–403. doi: 10.1016/j.clineuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Matsui H, Nishinaka K, Oda M, Hara N, Komatsu K, Kubori T, et al. Heterogeneous factors in dementia with Parkinson’s disease: IMP-SPECT study. Park Relat Disord. 2007;13:174–181. doi: 10.1016/j.parkreldis.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Hattori T, Orimo S, Aoki S, Ito K, Abe O, Amano A, et al. Cognitive status correlates with white matter alteration in Parkinson’s disease. Hum Brain Mapp. 2012;33:727–739. doi: 10.1002/hbm.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvälahti E, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002;52(7):759–63. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- 26.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64(4):803–12. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]