Abstract
The neoteric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been jeopardizing the world with the symptoms of seasonal flu. The virus contagion predicted to have been originated from Wuhan, China has by far trapped 4,198,418 cases from 212 countries in the world with two international conveyances with 284,102 deaths as of 11 May 2020 (10:18 GMT). Researchers around the globe have indulged in deciphering viral mode in the body for devising a cure. Affirmations from autopsies and preliminary findings on SARS-CoV-2 hypothesized on viral pathogenesis within the host, for instance, source of inflammation in lungs and pneumonia. This hypothesis assigns the platelets as agents of infection after viral entry. Presently, curbing infection to stall the spread of SARS-CoV-2 is the prima facie intervention employed, worldwide. However, public health authorities must monitor the state of affairs scrupulously, as the deeper our understanding of this novel virus and its associated outbreak, the better we can deal with it. Knowing this idea might be far-fetched, yet this postulate would serve as the groundwork for the present situation.
Keywords: COVID-19, Platelets, Pathogenesis, Lung inflammation, Thrombocytopenia
Introduction
Present pandemic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the third zoonotic infectious disease crossing species barrier in the past 18 years [1]. It is a positive-sense RNA virus with ~140 nm diameter envelope, spikes around like a crown under a microscope. Bats are predicted as a powerhouse of SARS-CoV-2, and repetitive spillovers urge zoonotic diseases in mere future [2]. As of 11 May 2020, 3.4% of 4,198,418 cases have died worldwide in comparison to the seasonal-flu that effectuated the death of <1% [3]. Infection escalates via droplets, touching contaminants followed by touching eyes, nose and mouth with no link to age and gender. The virus may be transmitted via the faecal-oral mode as it is seen in faeces and aerosolized transmission by contaminated waterworks [3] within incubation of two days-two weeks (5-d-median). The virus invades mucosal layer angiotensin-converting enzyme 2 (ACE2) cells in lungs with basic case reproduction rate (BCR) of 2 to 6.47 [4]. Amongst the registered cases 10–15% show severe pneumonia, multiple organ failure (MOF) and acute respiratory distress (ARDS) as a precursor of intravascular coagulopathy [5]. The disease's severity highlights the need for biomarkers in the affected individuals. Some studies listed platelet counting as economic, ubiquitous and ephemeral in discriminating the coronavirus disease (COVID) into severe/moderate as thrombocytopenia is linked to COVID-19 [6].
Platelets possess Fc receptors for immunocomplexes, and surface receptors for pathogens [7]. Involvement of these receptors on platelets by immune complexes initiates intracellular signalling events that result in to platelet stimulation and aggregation. Interestingly, these events manifest in vivo, significantly in response to pathological immune complexes, and association of these receptors on platelets has been coupled to disease pathology. These receptors benefit the host by boosting immunity and protecting pathogens from leukocytes, antiviral-agent traps. The adhesiveness of platelets weakens endothelial-microbe association and enables the infection to migrate [8]. Severe acute respiratory syndrome (SARS) infection, and assisted ventilation disrupts lung-endothelium, aiding platelet activation. Lungs produce ~50% of total platelets [9], allowing virion to directly infect and produce inflammation resulting in hyaline and thick alveolar wall formation [8]. Hence, observing the interaction of SARS-CoV-2 with platelets before-and-after entry is crucial. We have postulated virion propagation and causes of pneumonia in COVID-19.
Infection’s prime site
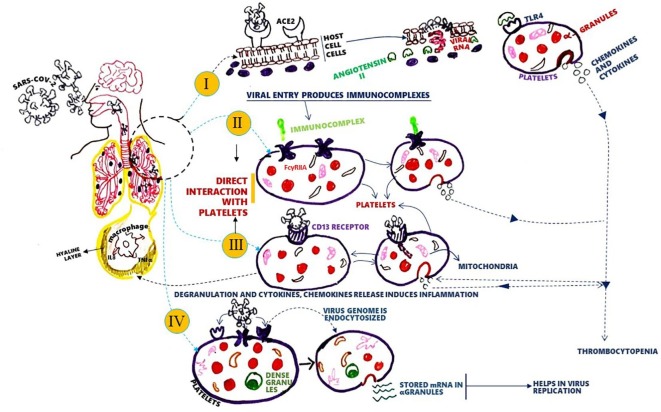
Conventional studies revealed enhancement in platelets with a concomitant decrease in megakaryocytes (MKs) within the blood, leaving the lungs. Platelets and MKs are fully packed with growth factors and cytokines responsible for inflammation (Fig. 1 ). Mouse studies showed that >10 million platelets were produced every hour from lungs that posed as the primary site for platelet biogenesis [9] with an accumulation of the required components of immune responses for instances spliceosomes, immune receptors and RNA’s from MKs [10]. The presence of pattern recognition and cytokine receptors on MKs may influence thrombopoiesis, thereby contributing to the plausible symptoms of infection, for instance, thrombocytopenia [11]. Therefore, we hypothesize that due to their omnipresence and opulence platelets enact as the primary source of cytokine storm during infection.
Fig. 1.
SAR-CoV-2 and platelet receptor interaction to induce thrombocytopenia and pneumonia in COVID-19. The virus can degranulate platelets to produce chemokines and cytokines responsible for inflammation in the lungs, as lungs are the site of platelet biogenesis. Degranulation reduces platelet count and causes thrombocytopenia. Degranulation can happen via: I. Interaction of SARS-CoV-2 with ACE-2 receptor increases angiotensin II, which binds to TLR4 and degranulate platelets. II & III. The virus may directly interact with surface receptors like FcγRIIA or CD 13, resulting in degranulation. IV. Platelet might act as virus production house by translating endocytosed viral genomes using its stored mRNA's.
SARS-CoV-2-platelet communion
These tiny-cells exhibited the presence of ample surface receptors to bind or permit entry of viruses, especially the toll-like receptor family (TLRs). Studies showed an interaction between angiotensin II and TLR 4 caused pro-inflammation by kinases and transcription factors [12]. Evidence showed platelet TLR 4 receptor contributed to functional impairment and platelets stacking in the lung and liver. Possibly, SARS-CoV-2 entry from ACE-2 can release intracellular angiotensin II [13], triggering platelet degranulation. This, in turn, led to inflammation and loss of platelets via deposition in peripheral microvascular beds, thereby heralding symptom-thrombocytopenia and intravascular coagulopathy in COVID-19. Platelets also express human aminopeptidase-N(CD13) metalloprotease receptor of HCoV-229E. It is predicted that SARS-CoV-2 might be similar to HCoV-229E owing to 82% homology [14]. Plausibly the virion interacted with CD13 of platelets, degranulate and released inflammatory cytokines.
Platelets extravascular activity
Like Influenza-virus, it is possible the platelets could link to immunocomplexes and get activated by local agonists resulting in the inflammatory response. Vaccination showed increased inflammatory CD14, high CD16+and thrombo-inflammation in activated platelets by FcγRIIA-immune-complexes’ association. Platelets also stock mRNAs, enabling their feasibility to translate SARS-CoV-2 RNA and shield it from guardian cells [15].
Conclusion
Considering the onset of COVID-19, many researchers traced its pathogenesis. Although many unanswered questions strangle around disease mechanisms, the virus is presumed to attack endothelial cells of lung capillaries, causing a rise in plasma of the alveolar cavity. To retort SARS-CoV-2, cells like erratic platelets might release assorted pro-inflammatory cytokines, enabling recruitment of neutrophils and monocytes to clear viral residues ensuring inflammation. Platelets reveal the dual role of either suppressing or supporting certain viruses. Thrombocytopenia, ubiquitously present during viral infections, raises a major question “whether thrombocytopenia is a mechanism to protect the host or the virus? After multiple cross-checks on platelet's role in viral infections due to COVID-19, we hypothesized that platelets might worsen SARS-Cov-2 infections and cause pneumonia or fatality. Employing drugs like monomeric IV. 3 mAb and antagonist IAXO-102 revealed inactivation of platelets and elucidated the mechanism of lung inflammation in COVID-19. However, enhanced knowledge on platelets and its immunoregulatory role during SARS-CoV-2 may go a long way in providing insights on the pandemic and aid in designing therapeutic approaches. Still, umpteen experimental trials are required to assess direct interaction of virus-platelets or the message sent by damaged endothelial cells that led to inflammation.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank ICGEB authorities especially to the Director ICGEB, New Delhi for providing the necessary infrastructure and ICGEB core funds for this research work i.e., to analyze and screen the variants for COVID19 disease. We also thank Director-General, ICGEB Trieste, Italy and Department of Biotechnology (DBT) for supporting the research activities at ICGEB. We also thank Dr. K. Sasikala, Rtd. Professor and Emeritus, School of Life Science, Bharathiar University, Coimbatore – 641046, India, and Dr. Suresh Easwaran, ENT Surgeon, Government Head Quarters Hospital, Kanchipuram, Tamil Nadu, India, for their valuable suggestions and discussions to write the manuscript.
Author contributions
HKB, ME and TK have done the literature search and drafted the paper. AM, BB, and MS helped with the literature search and revised the manuscript. SC, MS put forward the hypothesis and revised the manuscript. VA, PM, HKB, ME, AM and BB provided ideas about the mechanism of platelet receptor interaction and revised the paper. All authors approved the final version of the hypothesis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110098.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References:
- 1.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382(8):760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease 2019 (COVID-19) situation report – 92. April 22, 2020. https://www.who.int/docs/default-source/coronaviruse/20200312-sitrep-92-covid-19.pdf?sfvrsn=e2bfc9c0_2 [accessed 22.04.2020].
- 4.Cheng Z.J., Shan J. 2019 novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattiuzzi C., Lippi G. Which lessons shall we learn from the 2019 novel coronavirus outbreak? Ann Transl Med. 2020;8(3):48. doi: 10.21037/atm.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippi G., Plebani M., Michael H.B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assinger A. Platelets and infection – an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefrançais E., Ortiz-Muñoz G., Caudrillier A. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zufferey A., Speck E.R., Machlus K.R. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 2017;1:1773–1785. doi: 10.1182/bloodadvances.2017007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu L.M., Lin E., Mick E. Interleukin 1 receptor 1 and interleukin 1β regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler Thromb Vasc Biol. 2014;34:552–564. doi: 10.1161/ATVBAHA.113.302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaipan C., Soilleux E.J., Simpson P. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biancardi V.C., Bomfim G.F., Reis W.L., Al-Gassimi S., Nunes K.P. The interplay between Angiotensin II, TLR4 and hypertension. Pharmacol Res. 2017;120:88–96. doi: 10.1016/j.phrs.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020:1–4. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniak S., Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123:2605–2613. doi: 10.1182/blood-2013-09-526277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.