Abstract
Haemostasis stops bleeding at the site of vascular injury and maintains the integrity of blood vessels through clot formation. This regulated physiological process consists of complex interactions between endothelial cells, platelets, von Willebrand factor and coagulation factors. Haemostasis is initiated by a damaged vessel wall, followed with a rapid adhesion, activation and aggregation of platelets to the exposed subendothelial extracellular matrix. At the same time, coagulation factors aggregate on the procoagulant surface of activated platelets to consolidate the platelet plug by forming a mesh of cross-linked fibrin. Platelets and coagulation mutually influence each other and there are strong indications that, thanks to the interplay between platelets and coagulation, haemostasis is far more effective than the two processes separately. Clinically this is relevant because impaired interaction between platelets and coagulation may result in bleeding complications, while excessive platelet-coagulation interaction induces a high thrombotic risk. In this review, platelets, coagulation factors and the complex interaction between them will be discussed in detail.
Keywords: Platelet, Coagulation, Haemostasis, Platelet-based coagulation, Clinical disorders
1. The role of platelets in haemostasis
Resting circulating platelets have a discoid shape and do not interact with the intact vessel wall. They are present in high numbers (150-400 billion per liter blood) and continuously assess their environment using a wide array of cell surface receptors and adhesion molecules. Of these, the most abundant are the adhesion and signaling integrin molecules, such as αIIbβ3, αVβ3 and α5β1, α6β1 and α2β1. Furthermore, leucine-rich glycoproteins such as the GPIb-IX-V complex, G protein-coupled receptors such as PAR-1, PAR-4, P2X1, P2Y1 and P2Y12 and prostaglandin receptors such as Thromboxane receptor (TP) and prostacyclin receptor (IP), and immunoreceptors such as GPVI and CLEC-2 play important roles in platelet activation and aggregation. Platelets also contain two unique organelles, α-granules and dense bodies, that contribute to these processes [1]. The α-granules contain membrane bound receptors (αIIbβ3, GPVI, GPIb-IX-V complex, P-selectin and others), coagulation factors (including von Willebrand factor (VWF), factor (F)V, FVIII, protein S, antithrombin (AT), plasminogen/plasmin and protein inhibitors such as C1-inhibitor, protease nexin 1 and 2 (PN1 and PN2)), cytokines and chemokines, growth factors, microbicidal proteins and immune mediators which will be released upon activation [2]. Dense granules, with CD63, LAMP-2, GPIb and αIIbβ3 on their membrane [3], contain high concentrations of adenine nucleotides (ADP/ATP ratio > 1.7), serotonin, histamine, Ca2+, Mg2+, K+, pyrophosphate and polyphosphate (PolyP) [4].
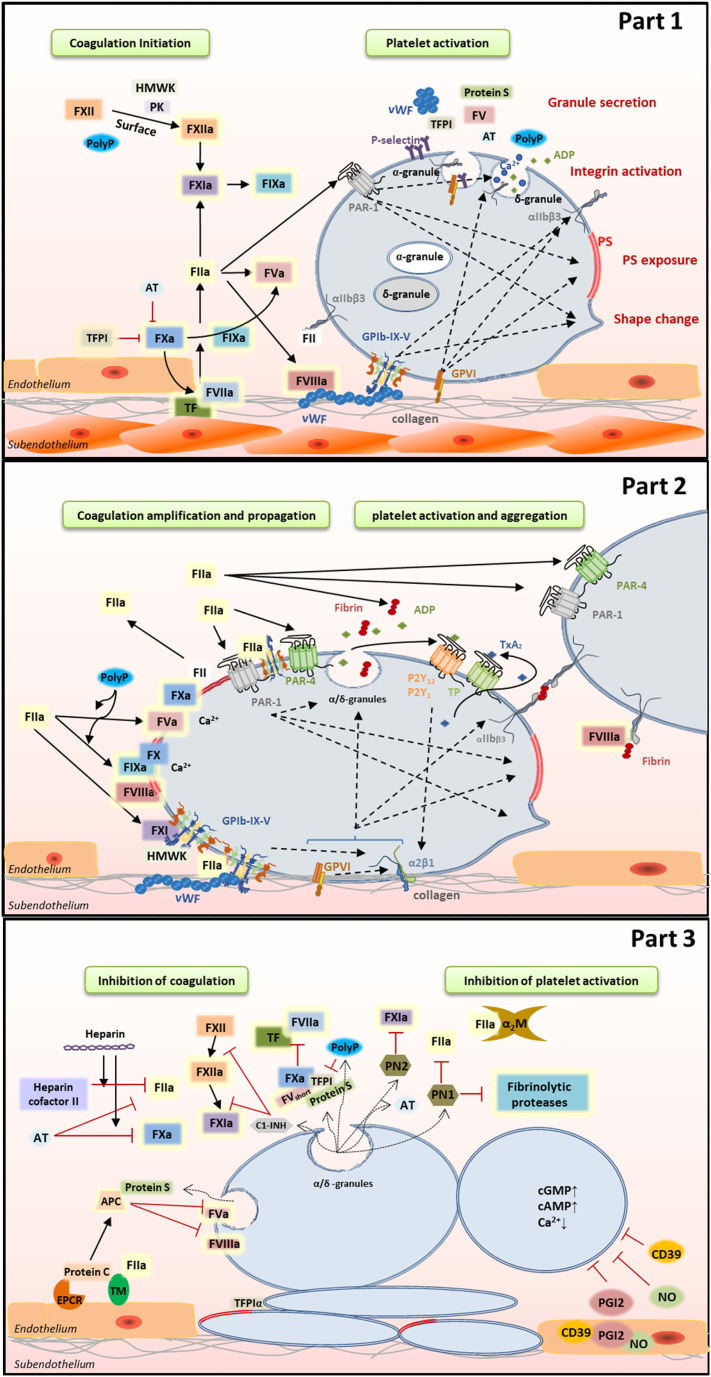
Platelet adhesion to the exposed subendothelial matrix is a multistep process depending on the local shear rate of blood [5]. At venous flow (low shear rates, 100-1000 s-1), platelets can interact with collagen (via GPVI and α2β1), fibronectin (via integrin αIIbβ3, αVβ3 and α5β1) and laminin (via α6β1) present in the extracellular matrix [[6], [7], [8]]. At arterial flow (high shear rates, 1000-4000 s-1), the initial reversible adhesion absolutely depends on the interaction between platelet GPIbα and VWF [9]. Exposed collagen captures VWF from circulating blood, and subsequently, VWF unfolds to expose the A1 domain which is a binding site for the platelet GPIb-IX-V complex [10]. Although VWF-GPIb interactions can resist high shear, the binding is transient and, as a result, fast-flowing platelets will slow down and roll over the vessel wall, allowing interaction of other platelet receptors with matrix proteins leading to stable platelet adhesion (Fig. 1 part 1) [11].
Fig. 1.
Interplay between platelets and coagulation. Part 1: Coagulation initiation and platelet activation. Part 2: Platelet-based amplification and propagation of coagulation and platelet activation and aggregation. Part 3: Inhibition of coagulation and platelet activation.
Initial platelet activation by VWF-GPIbα interaction is enhanced by binding of collagen to platelet GPVI receptors [12]. This interaction induces strong signaling via FcRγ, immunoreceptor tyrosine-based activation motif (ITAM), Src kinases (Fyn and Lyn) and Syk tyrosine kinase resulting in activated phospholipase Cγ (PLCγ) [[13], [14], [15]]. Subsequently, PLCγ hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) in inositol trisphosphate (IP3) and 1,2-diacylglycerol (DAG). IP3 binds to the dense tubular system (DTS) and allows the efflux of Ca2+ from the DTS to the cytoplasm. Membrane bound DAG, together with Ca2+, activates the protein kinase C (PKC) resulting in integrin activation, platelet spreading and granules secretion [11]. Although GPVI plays a crucial role in collagen-dependent thrombus formation [14,16], it shows a low affinity for collagen. The adhesion of platelets on collagen can be considerably enhanced by interactions with another collagen receptor, integrin α2β1. Weak signals through GPIb, GPVI or via interactions of positive feedback mediators ADP and thromboxane A2 (TxA2) with P2Y1/P2Y12 and TP, respectively, change α2β1 from a low-affinity to a high affinity receptor for collagen [[17], [18], [19], [20]]. As a result, α2β1 activation supports firm adhesion of activated platelets to collagen and triggers a weak outside-in signal transduction [21,22]. Under high shear conditions, this α2β1 is essential for the compact thrombus formation and its resistance to shear [23,24]. Together, α2β1 and GPVI synergistically stimulate Ca2+ signaling, phosphatidylserine (PS) exposure, granule secretion and aggregation [14,20]. However, deficiencies of one of these receptors results only in a mild bleeding disorder, suggesting that these receptors can replace each other (Fig. 1 part 2).
On resting platelets, the most abundant platelet membrane receptor αIIbβ3 is in a so-called closed conformation with a low affinity for its ligands VWF, fibronectin and fibrinogen. Upon platelet activation, inside-out signaling drives a conformational change in αIIbβ3 resulting in a high affinity, ‘open’ conformation [[25], [26], [27]]. Subsequently, because of the multiple binding sites for αIIbβ3, fibrinogen and VWF can form bridges between platelets. Binding to αIIbβ3 results in outside-in signaling and, through the involvement of Src family kinases and Syk, in irreversible platelet aggregation and clot retraction [[28], [29], [30], [31], [32]]. Furthermore, granule secretion following platelet activation increases the number of αIIbβ3 on the platelet membrane which further increases the platelet-platelet interactions [1,33] (Fig. 1 part 2).
Furthermore, to prevent unwanted activation of platelets under normal circumstances and to limit the hemostatic response at a site of vascular injury it is essential to have inhibitory mechanisms. Nitric oxide (NO), released from endothelial cells inhibits platelet aggregation through activation of soluble guanylyl cyclase (sGC) and a consequent upregulation of cGMP and activation of protein kinase G (PKG), resulting in phosphorylation of downstream proteins, reduction of Ca2+ levels, inhibition of integrins activation and of granule secretion [34,35]. In addition, prostacyclin (PGI2), synthesized in endothelial cells from arachidonic acid by COX-1 or COX-2 and prostacyclin synthase, binds to IP and stimulates membrane bound adenylyl cyclase which results in the increase of cAMP and activation of protein kinase A (PKA) [[36], [37], [38]]. Activated PKA phosphorylates many signal regulatory proteins, leading to the inhibition of cytoskeleton reorganization and of cytosolic Ca2+ elevation [39,40]. Also, endothelial CD39 (a ectonucleoside triphosphate diphosphohydrolase) prevents the further activation and aggregation of platelets by hydrolyzing ADP, released from activated platelets, to AMP [41]. Mice deficient in CD39 have an increased thrombotic risk [42] (Fig. 1 part 3).
2. The role of coagulation in haemostasis
After a rupture in a blood vessel, blood is exposed to tissue factor (TF)-expressing cells present in the subendothelial tissue (such as smooth muscle cells) or the extracellular matrix (such as fibroblasts). Circulating activated FVIIa binds to TF to form the FVIIa-TF complex (extrinsic coagulation, initiation phase)) that activates FIX and FX. FXa can activate more FVII to FVIIa, accelerating the start of coagulation. In the absence of cofactor FVa, FXa alone can produce trace amounts of thrombin from prothrombin that can : 1) activate FV and FVIII; 2) activate FXI which cleaves FIX to FIXa; 3) activate platelets via PAR-1 and PAR-4 (amplification phase) [43,44]. Furthermore, it was recently reported that besides thrombin, FXa-mediated FV activation is of vital importance in facilitating rapid thrombin generation in the initiation phase of coagulation (Fig. 1 part 1) [45]. After FIX and FVIII activation, the formed tenase complex (FIXa-FVIIIa) converts FX to FXa. Subsequently, the formation of the prothrombinase complex (FXa-FVa) leads to a burst of thrombin which can cleave fibrinogen to fibrin, which stabilizes the thrombus (propagation phase). Ca2+ is essential for assembly of the tenase and prothrombinase complexes to anionic phospholipids (PS) exposed on activated platelets and recently, it was reported that these complexes concentrate in cap regions of balloon-like procoagulant platelets [46] (Fig. 1 part 2).
In the intrinsic pathway, FXII is autoactivated following binding to artificial or biologic surfaces. High-molecular-weight kininogen (HMWK) and prekallikrein facilitate the FXIIa activation which in turn activates FXI. Of note, binding of FXI to polyP appears to be integral to the mechanism for enhancement of FXI activation [47,48]. Also, FVa was described as a cofactor for thrombin induced FXI activation by Maas et al. [49], however, this could not be confirmed by Matafonov et al. [50]. It was assumed that the intrinsic pathway is much less important to haemostasis under normal physiological conditions than the extrinsic pathway. However, there is renewed interest in FXII as (1) collagen, nucleic acids and PolyP were introduced as triggers for FXII autoactivation [[51], [52], [53]], (2) FXII deficiency is not associated with bleeding but reduces thrombosis risk [54,55], and (3) pharmacologic targeting FXII or FXIIa provides protection from thrombosis without increased incidence of bleeding [56,57] (Fig. 1 part 1).
Furthermore, it is also essential to localize clot formation at the site of injury and prevent the formation of clots in healthy vessels. Antithrombin (AT), an important SERPIN present in human plasma, forms stable complexes with predominantly thrombin and FXa, but also to some extent with FIXa, FXIa, FXIIa and Kallikrein, and subsequently the inactivated enzyme-serpin complex will be cleared [43]. Heparin can accelerate this inactivation of coagulation factors by AT [58]. Similar to AT, Heparin cofactor II inhibits thrombin (but no other serine proteases) in the presence of polyanionic molecules such as heparins and dermatan sulfate [59]. In addition, alpha-2-Macroglobulin (α2M) entraps thrombin and prevents activation of other coagulation factors. Another important anticoagulant system is the Protein C axis. Protein C circulating in blood is activated by thrombin bound to thrombomodulin (TM) on the endothelial surface. Activated protein C (APC), associated with its cofactor protein S that is bound to the surface of activated platelets, inactivates FVa and FVIIIa. In large blood vessels, the endothelial protein C receptor (EPCR) supports protein C activation by localizing protein C to the endothelial cell surface and presenting it to the nearby thrombin-TM complex. Furthermore, tissue factor pathway inhibitors (TFPI), a kunitz-type inhibitor that is present in platelets, on the endothelial cell surface and in plasma, binds to the active site of FXa and inhibits its activity. Subsequently the TFPI-FXa complex interacts with the TF-FVIIa complex thereby inhibiting its activity. Protein S, which exists both in plasma and platelet α-granules [60], is a cofactor of TFPI by promoting the interaction between full-length TFPI and FXa [61]. Moreover, a short form of FV prolongs the half-life of TFPI in the circulation and is important as a chaperone for the most active forms of TFPI [62,63] (Fig. 1 part 3).
3. The complexity in platelet-based coagulation
Although platelet plug formation and coagulation are also called primary and secondary haemostasis, respectively, these two processes are initiated simultaneously when blood vessel injury occurs, which means that these two processes are communicating with each other mutually during the whole coagulation process. In the beginning, coagulation factors share (or compete for) membrane receptors (or binding sites) on resting platelets. Once platelets are activated, platelets will provide more binding sites with higher affinity to the activated coagulation factors than to the not-activated coagulation factors [[64], [65], [66], [67], [68]].
Heterogeneity in thrombus composition is promoted by extrinsic (environmental factors such as blood flow dynamics, vascular environment and local availability of platelet agonists), and by intrinsic (platelet size, volume and age, platelet levels of membrane receptors, and levels of cytoplasmic, granular and cytoskeletal proteins and platelet) platelet-specific factors [69]. Procoagulant platelets, exposed to collagen and strongly expressing PS on their surface, serve to sustain the procoagulant response by concentrating coagulation factors and protecting them from inactivation/inhibition [70]. In the initial phase of coagulation, it is now believed that trace amounts of FIXa and FXIa formed play very important roles by diffusing from one surface to another. The initial FIXa formed by TF/FVIIa complex can diffuse to the platelet surface, because FIXa is not rapidly inhibited by AT or other plasma protease inhibitors [71]. The only relevant binding site known for FIX and FIXa on the platelet membrane is PS, and they share 300 low-affinity binding sites on thrombin-activated platelets which can be replaced partly by prothrombin and FX [66]. However, in the presence of FVIII and FX, FIXa binds to ~250 additional high affinity binding sites and the affinity increases 5-fold [66]. Coated platelets, formed after combined stimulation with collagen and thrombin, also express PS on their surface and they retain α-granule derived factors like FV, fibrinogen and thrombospondin on their surface [69,72]. In the alpha granules, FV is stored in complex with the protein multimerin and this platelet derived FV accounts for approximately 20% of the total body pool of FV. In contrast to plasma FV, platelet FV, secreted upon platelet stimulation, is partially activated, exhibiting substantial cofactor activity that is increased two-to-three-fold following activation by thrombin or FXa. Moreover, platelet derived FVa is thought to be (GPI)-anchored and is two to threefold more resistant to APC-catalyzed inactivation [[73], [74], [75]]. Interestingly, platelets also contain FIX, both in alpha granules and diffusely in the platelet cytoplasm and membrane-bounded vesicles, which can be released upon activation [76]. Although the physiological importance of this small amount of FIX is unknown, it may be significant since only a few percent of normal FIX levels are required to support haemostasis. Furthermore, whether or not platelets contain or express FXI is relatively uncertain [[77], [78], [79], [80]], however, recently Zucker et al. reported the presence of FXI in platelet granules and FXI pre-mRNA that is spliced upon platelet activation [81]. Coated platelets may also retain larger amounts of fibrin and FVII, FIX and FX [69,72]. However, recent data suggest that FVIII also binds to less-activated platelets (stimulated with thrombin alone, PS exposure below threshold), and this binding is mediated by fibrin bound to αIIbβ3 [[82], [83], [84]]. Aggregated platelets with active αIIbβ3 on their surface, are proposed to be responsible for contracting and retracting the clot by interacting with fibrin [85,86].
The macrocirculation differs from the microcirculation in vessel wall structure and local hemodynamics [87,88]. However, in both cases there was heterogeneity in the gradient of platelet activation (with a shell of activated, but still P-selectin negative, platelets overlying a core of P-selectin positive platelets), close packaging of the platelets, and the asymmetric distribution of fibrin towards the extravascular side of the plug. Thrombi formed in femoral artery were considerably larger than those required to achieve hemostasis in the arterioles, but a smaller proportion became P-selectin positive. Furthermore, in the microcirculation where flow rates are slower and the vessel wall thinner, hemostatic thrombi tend to project into the vessel lumen. As the vessel wall grows thicker, not only is TF further away from the lumen, but any thrombin formed has a greater distance to diffuse in the tortuous path produced by the narrowing gaps between adjoining platelets. Platelets in the core of the thrombus contact with each other more closely and also release more active content from their granules. This is important because when a thrombus tears apart due to high shear forces, the released polyP of the platelets comes into contact with the circulating blood. Platelet-derived polyP accelerates FV activation, abrogates TFPI activity, enhances fibrin clot structure, and promotes FXI back-activation by thrombin [89] (Fig. 1). It was also proposed that FXII activation is caused by polyP from platelets [90], however, because medium-chain platelet-derived polyP is thousands of times less potent than very long-chain polyP in triggering the contact pathway the physiologically relevance is questioned [89,91]. Interestingly, the membrane-associated polyP largely exceeds the polymer size of platelet-derived polyP, and may play a role in FXII activation [92].
In the later stage of coagulation, anticoagulants proteins released from platelets contribute to restrain excessive coagulation. TFPIα can be produced by megakaryocytes and the platelet TFPIα pool exclusively consist of full-length TFPIα that might be localized in multivesicular bodies or exosomes of platelets instead of α-granules or lysosomes [93]. Platelet TFPIα is slowly released upon activation and can be secreted as a soluble protein [94] or it can bind to the membrane of coated platelets [95]. At the site of vascular injury, local TFPIα concentrations might increase through the release of TFPIα from accumulating platelets within the thrombus and it was speculated that platelet TFPIα is important to prevent systemic coagulation and thrombosis and restrict thrombus formation to the site of the growing platelet plug [96]. Furthermore, platelet protein S can directly bind to stimulated platelets and decease thrombin and FXa generation in an APC /TFPI-independent way [97] (Fig. 1 part 3).
Platelets also harbor a major inhibitor of the contact activation system, C1-inhibitor (C1-INH) as well as fibrinolytic proteases in their α-granules [[98], [99], [100], [101]]. Although platelet-derived C1-INH accounts for only 0.08% of the total circulation pool, this does not preclude an important inhibitory role, as local concentrations within thrombi may be high and plasma-derived C1-INH may not have the capacity to penetrate to the thrombus core [102]. However, at arterial thrombi filtration velocities, the released C1-INH may be rapidly washed out from platelet aggregates which results in the increase of FXIIa prothrombotic activity. This might explain the more important role of FXIIa in arterial thrombosis than in haemostasis [103] (Fig. 1 part 3). Finally, α-granules of platelets also contain SERPINs protease nexin I and II (PN1 and PN2), both released during platelet activation [104,105]. PN1 negatively regulates coagulation and fibrinolysis by inactivating thrombin and fibrinolytic proteases [105,106]. PN2 inhibits FXIa via its kunitz protease inhibitor domain [107], and heparin can accelerate this inhibition [108]. However, FXIa bound to its receptors on activated platelets is completely protected from inactivation by PN2 [67,109] (Fig. 1 part 3).
4. Interaction between coagulation factors and platelet receptors
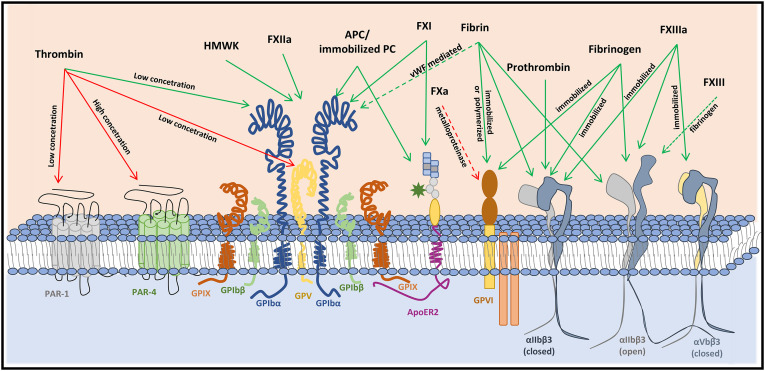
Coagulation factors interact with platelets by binding to platelet receptors directly or indirectly or by cleaving of the platelet receptors (Fig. 2 ). Thrombin (α-thrombin) is one of the most potent physiological agonists of platelets and activates platelets by either proteolytic (cleavage of PAR-1 and PAR-4 [110,111] or GPV [112]) or non-proteolytic (signaling via binding to GPIbα [113]) mechanisms. PAR-1 (~ 2500 copies per platelet) is the high-affinity thrombin receptor responding to nanomolar concentrations of thrombin which results in a transient calcium signal. In contrast, PAR-4 has a low-affinity for thrombin, however, activation of PAR-4 results in a more sustained Ca2+ response. Sub-nanomolar levels of thrombin can also bind a high-affinity binding site (residues 269-287 region) of GPIbα [[114], [115], [116], [117]]. The number of GPIb-complexes with high-affinity binding sites for thrombin is about 1000 [118], which is less than 5% of the total GPIb copies (~25,000) on the platelet membrane [119]. The role of GPIb-IX-V in thrombin-induced platelet activation remains poorly understood. Whereas some proposed that GPIb-IX-V serves as a dock that facilitates thrombin cleavage of PAR receptors [117], others suggested that binding induces platelet activation independent of PARs [113]. Recent research, however, indicated that the mutual cooperativity between thrombin-induced GPIb-IX-V signaling and PAR signaling is required for optimal platelet response to low concentrations of thrombin [120]. Furthermore, prothrombin binds to non-activated αIIbβ3 on resting platelets [121], and this binding accelerates activation of prothrombin by FXa or FXa-FVa, which might be pivotal for the initial platelet-based thrombin generation.
Fig. 2.
Interaction of (anti)coagulation factors with key platelet membrane receptors. Coagulation factors interact with platelets by cleaving (red lines) the receptor directly (solid line) or indirectly (dashed line), or by binding (green line) to their respective receptors.
Once the first layer of platelets forms at the vascular injury site, the recruitment of more platelets to the growing thrombus relies on the formation of fibrinogen bridges. Initially, the integrin αIIbβ3 resides in a low-affinity state, however, inside-out signaling drives a conformational change resulting in binding of fibrinogen, fibrin and other proteins. Bridging of fibrinogen between αIIbβ3 on adjacent platelets results in platelet aggregation. Individual activated αIIbβ3 molecules also attach to fibrin fibers such that aggregated platelets are major components of haemostatic clots. αIIbβ3 binds to different sites on fibrin and fibrinogen [122,123], and the mechanical stability is different for the αIIbβ3-ligand complexes (fibrin polymer > fibrin monomer > fibrinogen) [124]. Furthermore, platelet adhesion and spreading on FXIIIa occur through fibrinogen independent binding of αIIbβ3 and αVβ3 [125]. More recently it was also demonstrated that the zymogen FXIII interacts with platelets, however, strong platelet stimulation, fibrinogen and αIIbβ3 play essential roles in this interaction [126].
Besides αIIbβ3, GPVI has been identified as a receptor for fibrinogen and fibrin and this interaction induces signaling that supports thrombus formation and stabilization [127,128]. However, contrasting observations have been reported on whether fibrin binds to monomeric or dimeric GPVI or to neither form [[129], [130], [131]]. In addition, polymerized fibrin interacts, not directly but with VWF as a linker, with GPIb [[132], [133], [134]]. In this way, fibrin fibers formed on the thrombus surface serve as a scaffold for binding of coagulation factors and stimulate thrombus formation [[135], [136], [137]].
HMWK can bind GPIb-IX-V on unstimulated platelets in a Zn2+-dependent manner [138,139], however, precise binding sites remain to be defined since both anti-GPIb and anti-GPIX antibodies block HMWK binding [140]. HMWK and FXIIa compete with thrombin for binding to GPIb-IX-V and in this way they can inhibit thrombin-induced platelet aggregation [140,141]. PAR-4 is also involved in FXIIa inhibition of thrombin activation of platelets but only at high concentrations [141]. Another coagulation factor interacting with GPIbα in Zn2+-dependent manner is the homodimer FXI that circulates in plasma in a complex with HMWK [142]. More in detail, the apple-3 (A3) domain of FXI interacts with the leucine-rich repeats of GPIbα [143,144], leaving the other FXI monomer free for activation by thrombin [[145], [146], [147]]. Although HMWK is required for optimal FXI binding to GPIbα on activated platelets in suspension, FXI binding to platelets under flow is not enhanced by HMWK [146,148]. Furthermore, FXI binding to platelets is also mediated in part by an interaction with apolipoprotein E receptor 2 (ApoER2, or LRP8) [146,147], and since ApoER2 colocalizes with GPIbα it appears that one FXI homodimer binds simultaneously to both receptors to mediate shear-dependent interactions [146].
Immobilized protein C or APC also mediate platelet binding and activation signaling through ApoER2 and GPIbα under shear conditions [149]. The ability of platelets to bind APC might imply a dual role in haemostasis: stimulating platelet activation and limiting thrombus growth by localizing the anticoagulant role of the protein C system.
Another example of cross-talk between platelet receptors and coagulation is the induction of platelet GPVI shedding by FXa in a metalloproteinase-dependent mechanism in the absence of GPVI ligands and this results in down-regulation of GPVI under procoagulant conditions [150].
5. Bleeding and the interplay between platelets and coagulation
Balanced platelet function and coagulation are crucial for stable blood circulation. If one of the factors involved is not functioning, this will lead to impaired haemostasis, which can clinically express as bleeding complications. Abnormalities in GPIb (Bernard-Soulier syndrome (BSS) caused by mutations within GPIBA, GPIBB and GP9) and αIIbβ3 (Glanzmann thrombasthenia (GT) caused by mutations in ITGA2B and ITGB3) expression on the platelet surface are associated with moderate to severe bleeding symptoms [151]. The impaired prothrombin consumption in BSS patient can be corrected by the addition of human FVIII or FVIII-VWF, which might indicate the essential role of FVIII/VWF-GPIb interaction in the activation of coagulation [152]. In GT, abnormal αIIbβ3 expression results in defective clot retraction due to decreased fibrinogen endocytosis and subsequently decreased binding of fibrinogen to platelets. Both patients with BSS and GT receive good clinical efficacy with recombinant FVIIa (rFVIIa) treatment [151]. The exact working mechanism of rFVIIa is still under debate and the enhancement of thrombin generation was explained by (1) a TF-dependent mechanism where rFVIIa competes with circulation FVII zymogen for TF binding and (2) a TF-independent mechanism where rFVIIa binds to anionic phospholipids, GPIbα or EPCR expressed on activated platelets to localize rFVIIa to the surface of activated platelets [[153], [154], [155], [156], [157], [158]]. rFVIIa can also be used to treat patients with hemophilia. Furthermore, there is a long list of Familial thrombocytopathies (reviewed by Nurden et al. [159,160]), including genetic variation affecting platelet adhesion (platelet-type von Willebrand disease (GP1BA) and GPVI deficiency (GP6)), the secondary platelet activation response (P2Y12 ADP receptor deficiency (P2YR12) and thromboxane A2 receptor deficiency (TBXA2R)), signaling pathways (thromboxane A synthase (TBXAS1) and cytosolic phospholipase A2 (PLA2G4A)) and the procoagulant activity of platelets (Scott Syndrome (ANO6)). Other inherited defects of platelet function are caused by genetic variants affecting granule secretion, such as Hermanski-Pudlack (HPS1, AP3B1, HPS3-6, DTNBP1, BLOC1S3, BLOC1S6), Chediak-Higashi syndrome (LYST), Familial hemophagocytic lymphohistiocytosis types 3-5, Grey platelet syndrome (UNC13D, STX11, STXBP2), Arthrogryposis-renal dysfunction-cholestasis syndrome (VPS33B, VIPAS39) and Quebec platelet syndrome (PLAU). A genetic defect in the NBEAL2 gene is responsible for lack of alpha-granule synthesis, which is known as Gray-platelet syndrome. In addition to inherited thrombocytopathies, there are also several familial thrombocytopenias (FT), including defects in transcription factor defects (GATA1 and FOG1) [161] or variants in genes giving reduced expression of proteins (MYL9, PKC and ALOX12) [162]. Furthermore, variation in the MYH9 and in the FLNA gene leads to cytoskeleton defects and hence macro-thrombocytopenia [163,164]. Other forms of familial thrombocytopenia are X-linked Wiskott-Aldrich syndrome [165], in which the actin polymerization is affected or a genetic variant in ANKRD26, affecting the mitochondrial metabolism [166]. The most obvious platelet defect affecting coagulation is the Scott syndrome. This is a rare inherited bleeding disorder of Ca2+-induced membrane phospholipid scrambling resulting in impaired prothrombin and FX activation due to decreased binding sites for FVa, FVIIIa, FIXa and FXa [[167], [168], [169]]. The disease is caused by mutations in ANO6 (anoctamin 6, also known as TMEM16F) that encodes transmembrane protein 16F (TMEM16F), a phospholipid scramblase that is vital for Ca2+-dependent PS exposure on cell surfaces, and this could explain part of the molecular mechanism in Scott patients [170]. However, recently, a TMEM16F-independent pathway which results in collagen/thrombin-induced PS exposure was discovered [168,171,172]. Patients with Scott syndrome are treated with platelet transfusions. Another platelet defect that affects coagulation is the autosomal dominant Quebec platelet disorder (QPD). QPD is caused by a tandem duplication of a 78 kb genomic segment that includes the PLAU gene, leading to overexpression of urokinase-type plasminogen activator (uPA) [173]. Bleeding is the result of hyperfibrinolysis in the vicinity of platelets caused by increased activation of plasminogen (which is also present within the α-granules) to plasmin. Interestingly, the formed plasmin can also degrade intraplatelet stores of FV, multimerin 1, thrombospondin 1, VWF, fibrinogen, fibronectin, osteonectin and P-selectin [173,174].
Well known coagulation disorders are hemophilia A (FVIII deficiency), hemophilia B (FIX deficiency) and von Willebrand disease. The significant role of platelets in coagulation was shown when measuring thrombin generation (TG) in plasma of VWD3 patients (absent VWF in plasma, platelet and endothelial cells) [175]. Although TG was markedly impaired in the plasma of VWD3 patients, it was close to normal in the presence of platelets [176]. The importance of platelets in coagulation is also clear in congenital FV deficiency (Owren parahemophilia), a rare bleeding disorder (the incidence is 1:1.000.000) inherited in an autosomal recessive trait [177]. In contrast to hemophilia A/B which is usually associated with mild to severe bleeding, people with severe FV deficiency only experience mild to moderate bleeding [178]. This might partly be explained by the existence of functional platelet FV that supports enough thrombin generation to rescue patients with undetectable plasma FV from fatal hemorrhage [179]. Moreover, patients with a FV deficiency have low circulating levels of TFPIα because FV short prolongs the lifetime of TFPI in the circulation.
Despite of these new insights, there is still a paradox that the gold standard platelet function tests and plasma coagulation tests fail to give sufficient insight, and many bleeding complications remain undiagnosed.
6. Thrombosis and the interplay between platelets and coagulation
Arterial thrombosis, manifesting as myocardial infarction or ischaemic stroke, arises from an atherosclerotic plaque disruption (high shear) that triggers platelet aggregation and activation of coagulation and is characterized by platelet rich thrombi that obstruct blood flow. The causal relationship between platelet hyper reactivity and arterial thrombosis has been established in large clinical trials, and four main classes of drugs are currently used clinically, either alone or in combination: P2Y12 antagonists, COX-1-inhibitors (aspirin), PAR-1 antagonists and αIIbβ3 inhibitors [180,181]. However, this strategy also results in an increased risk of hemorrhagic complications. New antiplatelet drug such as inhibitors of phosphatidylinositol 3-kinase-β, protein disulfide-isomerase, activated αIIbβ3, αIIbβ3 outside-in signaling, protease-activated receptors and GPVI-mediated adhesion pathways may pave the way to safer therapies causing minimal perturbation of haemostasis [181,182]. Also recently, targeting components of the intrinsic coagulation pathway (FXI, FXII and PKK) are considered as possible strategies to reduce arterial thrombosis without increasing the bleeding risk [183].
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary lung embolism (PE), arises where shear is low and venous thrombi contain fewer platelets and more fibrin than arterial thrombi. In general, the concentration and function of hemostatic proteins are considered as the main determinants of venous thrombotic risk. Multiple inherited thrombophilic defects, including the factor V Leiden (FVL) and prothrombin G20210A (PT20210A) mutations, along with deficiencies of AT, PC, and PS were discovered that can increase the thrombotic risk. Conclusive evidence for causal involvement of hyper coagulation in venous thrombosis has been delivered by large clinical trials which showed that inhibition of the coagulation pathways with heparins, vitamin K antagonists or with direct anti-coagulants (DOACs) will prevent many venous thrombotic incidents [184]. Despite this conclusive evidence, there is still a missing link that needs to be solved: none of the gold standard coagulation tests gives sufficient insight in the haemostasis to predict a high thrombosis risk in healthy subjects or in clinical populations. Indeed, in the most current view, stasis of blood and the accompanying low oxygen tension (in particular downstream of a venous valve), activation of the endothelium, activation of innate immunity (involving monocytes and neutrophils and platelets), activation of platelets, concentration and nature of microparticles also play a role in the formation of a venous thrombotic complication [44].
Thus, although platelets are widely accepted to play a crucial role in arterial thrombosis, there is increasing evidence that platelets also have a role in the formation of venous thrombi [185,186]. In contrast to arterial thrombosis, where platelets form large aggregates [187], in DVT, platelets are mainly recruited as single cells and adhere either directly to the activated endothelium or to adherent leukocytes forming small heterotypic aggregates [185]. Platelet recruitment to the venous thrombus depends on the interaction between GPIbα and endothelial surface exposed VWF and deficiency in either GPIbα or VWF prevents experimental DVT [185,188]. Additionally, recruitment also depends on binding of platelet CLEC-2 to podoplanin, a mucin-type transmembrane protein expressed in the middle and external layers of the venous wall [189,190]. Interestingly, it has been proposed that hypoxia-induced activation of the endothelial cells renders endothelial cell-cell junctions looser, allowing for platelet penetration into subendothelial spaces where the interaction between CLEC-2 and podoplanin may take place [189]. Furthermore, platelets recruited to the venous wall may release high-mobility group box 1 (HMGB1) that can induce NETosis (formation of neutrophil extracellular traps), resulting in a scaffold for adhering platelets and red blood cells and promoting thrombin generation and fibrin deposition [[190], [191], [192]]. Not surprisingly, recent studies found that aspirin reduces DVT in mice and VTE in patients undergoing orthopedic surgery [[193], [194], [195]].
7. Impact of platelet-coagulation interplay in clinical disorders
There are several clinical conditions with a high prevalence of thrombosis, including cancer [196,197], systemic inflammation (including sepsis) [198], antiphospholipid syndrome (APS) [199], immune thrombocytopenia (ITP) [200], trauma [201], stent implantation [202], blood transfusion [203], liver disease [204], thrombotic microangiopathies (TTP, HUS, HELLP) [205], heparin induced thrombocytopenia (HIT) [206], malaria [207], SARS-CoV-2 (COVID-19) induced infection [208] and many other diseases. All these disorders have a-typical thrombosis, which are not clearly definable with the available coagulation tests or with the available platelet function tests. The interplay between platelets and coagulation could be an important factor because many of these disorders combine thrombocytopenia with prolonged coagulation times but they still have an increased thrombotic risk.
Many functional and structural properties of platelets in combination with the evolution of these cells indicate that platelets belong to the family of innate immune cells [209,210]. Platelets contain many cytokines and chemokines which they secrete after activation. Moreover, they express a number of toll-like receptors (TLR2, TLR3, TLR4, TLR7 and TLR9), and GPIb and GPIX are also members of this TLR superfamily. Through these receptors, platelets can recognize different pathogens such as bacteria, viruses, parasites and fungi. Thrombocytopenia is therefore common in infections and the cause of these drop in numbers is partly explained by increased platelet consumption. Although, the real cause of the high thrombosis risk in many inflammatory diseases with thrombocytopenia remain to be established, it can be speculated that the platelets of these patients are chronically exposed to (auto)inflammatory triggers, leading to a chronic pre-activation state of the platelets. This chronic activation state of platelets may lead to a reduced lifespan because the platelets may lose their granule contents and become more pro-coagulant than platelets of healthy subjects. High numbers of pro-coagulant platelets predispose to a high risk of thrombosis and to thrombocytopenia disorders, because pro-coagulant platelets are more rapidly removed from the body mainly by macrophages in the spleen. Moreover, the endothelial cells of the vessel wall will also respond on the released cytokines and will lose some of their antithrombotic functions such as a drop in thrombomodulin expression. A major complexity of this hypothesis is that there are no reliable tests in the clinical diagnostic settings to measure the interplay between platelets and coagulation since validated coagulation tests (e.g. PT, APTT and thrombin generation) are done in plasma in the absence of platelets, while platelet function testing is done in anti-coagulated blood. Global tests such as TEG and ROTEM have too many shortcomings to be a serious candidate for the measurement of the interplay between platelets and coagulation [211]. Recently, a novel whole blood thrombin generation test (WB-TG) was developed that theoretically should give a precise insight in the interplay between platelets and coagulation, but the test needs to be clinically validated before real studies on the interplay between platelets and coagulation can be initiated [212].
Patients with malignancies have a high incidence of venous thrombosis with a 7-fold and 28-fold elevated risk of venous thromboembolism (VTE) with the highest incidence in pancreatic cancers and lung cancers [196,197]. As a result, venous thrombo-embolic complications are the second most important cause of death in cancer. Despite many speculations, the definitive answer to the cause of the high incidence of venous thrombosis in cancer has not been established. Tumor cells possess the capacity to interact with the haemostatic system in multiple ways, including the production of haemostatic proteins (e.g. TF, thrombin), activation of platelets and the direct adhesion of tumor cells to normal cells, including platelets, endothelial cells and monocytes [213,214]. The interplay between plasma coagulants and blood cells are crucial in this process for the formation of thrombi and future investigation studying the interaction between platelets and coagulation may give further insight in the pathophysiology of thrombosis in cancer.
Similar to cancers, VTE is a common complication in infectious and inflammatory disorders, including sepsis [198]. Systemic inflammation is a potent prothrombotic stimulus because it upregulates procoagulant factors, downregulates natural anticoagulants and inhibits fibrinolytic activity [215]. In addition to modulating plasma coagulation mechanisms, inflammatory mediators increase platelet reactivity. The far majority of studies on the procoagulant state and on platelet hyper-responsiveness have been performed in separate test models and did not measure the effects of inflammation on the interplay between platelets and coagulation and this may result in an underestimation of the thrombotic risk.
Both in cancer and in inflammatory disorders the venous thrombosis risk is high, despite a high prevalence of thrombocytopenia. Initially, this seems paradoxical because there are less platelets available to support the coagulation. However, there is a common link between thrombocytopenia and thrombosis pathophysiology in patients with cancer and in inflammatory disorders. Both processes depend on the activated surface of platelets. During cancer progression or inflammatory triggers, platelets become activated, release part of their granule contents and express P-selectin and eventually negatively charged phosphatidyl serine on their surface. These platelets are procoagulant, however, they are also rapidly removed from the circulation by monocytes in the spleen. The exposure to procoagulant surfaces and the rapid removal of platelets explains the relation between thrombosis and thrombocytopenia related disorders. This theory explains the high incidence of cancer and inflammatory related thrombosis due to pathophysiological modifications of the blood cells and not by modifications of coagulation factors in the blood plasma.
Several global haemostasis tests have been proposed to study the interplay between platelets and coagulation, including bleeding time (obsolete), Global Thrombosis Test (GTT), Thromboelastography (TEG), platelet mapping system and Rotational Thromboelastometry (ROTEM) [216] and clot waveform analysis (CWA) [217]. Although these global tests provide more information than assays measuring platelet function and coagulation separately, a major limitation of these tests is that they lack the precision for a reliable haemostasis test, as they are poorly associated with coagulation deficiencies and platelet function defects. The capacity to detect coagulation defects or platelet function defects is a minimal requirement for a haemostasis test. This may also explain the disappointing relationship between TEG/ROTEM with thrombotic disorders. Although it is still in its infancy, thrombin generation in platelet rich plasma or in whole blood seems to be a more promising approach to study the interplay between coagulation and platelet function, although the real validation of this test in relation to thrombotic disorders remains to be performed [218].
8. Conclusion
The complex interplay between platelets and the coagulation system has been underestimated for many decades. Although the awareness that many steps in platelet thrombus formation are closely connected to the different stages of thrombin formation and physiological coagulation is completely dependent on the expression of procoagulant surfaces, the expression or activation of specific receptors on platelets and the delivery of FV, there are still no accessible tools to study the interplay between platelets and coagulation in clinical research. Many disease- and therapy-related thrombotic events are induced by damaged or affected blood cells rather than by changes in coagulation factors. This may explain the lack of associations between plasma coagulation tests and thrombotic incidents. There are no dedicated tools to study blood cell-mediated thrombotic risk in clinical studies, despite viscoelastic tests, such as TEG and ROTEM. The disadvantage of viscoelastic tests is that they are too a-specific to be a serious alternative for plasma coagulation tests or platelet function tests. Our group recently developed a whole blood thrombin generation test that shows good correlations with plasma thrombin generation tests, with platelet numbers and with the use of different platelet inhibitors [212]. Although the WB-TG seems to be the first serious method to study involvement of blood cells in thrombosis, the technique is in its infancy. Many clinical validation studies are required before serious conclusions can be drawn regarding the additional value for WB-TG as tool to measure the interplay between blood cells and coagulation.
9. Future considerations
We recommend studying thrombotic risk with whole blood coagulation and whole blood platelet function tests, if possible, in the absence of anticoagulants. Whole blood thrombin generation may be a step forward for research to study the interaction between blood cells and the coagulation cascade in cancer and inflammation induced thrombosis, although it will only give partial insights in the thrombosis pathophysiology.
Practice Points
-
-
Thanks to the interplay between platelets and coagulation, haemostasis is far more effective than the two processes separately.
-
-
Current diagnostic tests are incapable of measuring the interactions between platelets and coagulation and this may lead to underdiagnosis or overdiagnosis of defects.
-
-
The importance of the interplay between platelets and coagulation may be underestimated in clinical conditions with high prevalence of thrombosis, including cancer, systemic inflammation, and others.
Research Agenda
-
-
Development of diagnostic tests that can measure platelets, coagulation, and the interplay.
-
-
Studying the interplay between platelets and coagulation can be used to screen for patients with bleeding or for the prediction of the thrombotic risk or the recurrence of thrombosis.
Declaration of Competing Interest
Yaqiu Sang reports a grant from the China Scholarship Council.
Acknowledgment
Yaqiu Sang was supported by the China Scholarship Council (CSC) via the State Scholarship Fund (File No. 201606790009).
References
- 1.Harrison P., Cramer E.M. Platelet alpha-granules. Blood Rev. 1993;7:52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- 2.Thomas S.G. In: Platelets. Michelson A.D., editor. 2019. 3 - The structure of resting and activated platelets; pp. 47–77. [Google Scholar]
- 3.Nishibori M., Cham B., McNicol A., Shalev A., Jain N., Gerrard J.M. The protein CD63 is in platelet dense granules, is deficient in a patient with Hermansky-Pudlak syndrome, and appears identical to granulophysin. J. Clin. Invest. 1993;91:1775–1782. doi: 10.1172/JCI116388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNicol A., Israels S.J. Platelet dense granules: structure, function and implications for haemostasis. Thromb. Res. 1999;95:1–18. doi: 10.1016/s0049-3848(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 5.Reininger A.J. Function of von Willebrand factor in haemostasis and thrombosis. Haemophilia. 2008;14:11–26. doi: 10.1111/j.1365-2516.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 6.Hindriks G., Ijsseldijk M.J.W., Sonnenberg A., Sixma J.J., Degroot P.G. Platelet-adhesion to laminin - role of Ca2+ and Mg2+ ions, share rate, and platelet membrane-glycoproteins. Blood. 1992;79:928–935. [PubMed] [Google Scholar]
- 7.Ruggeri Z.M., Mendolicchio G.L. Adhesion mechanisms in platelet function. Circ. Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 8.Houdijk W.P., PGd Groot, Nievelstein P.F., Sakariassen K.S., Sixma J.J. Subendothelial proteins and platelet adhesion. von Willebrand factor and fibronectin, not thrombospondin, are involved in platelet adhesion to extracellular matrix of human vascular endothelial cells. Arteriosclerosis. 1986;6:24–33. doi: 10.1161/01.atv.6.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Ulrichts H., Udvardy M.S., Lenting P.J., Pareyn I., Vandeputte N., Vanhoorelbeke K., et al. Shielding of the A1 domain by the D ’ D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J. Biol. Chem. 2006;281:4699–4707. doi: 10.1074/jbc.M513314200. [DOI] [PubMed] [Google Scholar]
- 10.Farndale R.W., Silijander P.R.M., Onley D.J., Sundaresan P., Knight C.G., Barnes M.J. In: Proteases and the Regulation of Biological Processes. Saklatvala J., Nagase H., Salvesen G., editors. Portland Press Ltd; London: 2003. Collagen-platelet interactions: recognition and signalling; pp. 81–94. [Google Scholar]
- 11.Broos K., Feys H.B., De Meyer S.F., Vanhoorelbeke K., Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–167. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Arthur J.F., Gardiner E.E., Matzaris M., Taylor S.G., Wijeyewickrema L., Ozaki Y., et al. Glycoprotein VI is associated with GPIb-IX-V on the membrane of resting and activated platelets. Thromb. Haemost. 2005;93:716–723. doi: 10.1160/TH04-09-0584. [DOI] [PubMed] [Google Scholar]
- 13.Quek L.S., Pasquet J.M., Hers I., Cornall R., Knight G., Barnes M., et al. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–4253. [PubMed] [Google Scholar]
- 14.Nieswandt B., Watson S.P. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 15.Li Z., Delaney M.K., O’Brien K.A., Du X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moroi M., Jung S.M. Platelet glycoprotein VI: its structure and function. Thromb. Res. 2004;114:221–233. doi: 10.1016/j.thromres.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Jung S.M., Moroi M. Signal-transducing mechanisms involved in activation of the platelet collagen receptor integrin alpha(2)beta(1) J. Biol. Chem. 2000;275:8016–8026. doi: 10.1074/jbc.275.11.8016. [DOI] [PubMed] [Google Scholar]
- 18.Lecut C., Schoolmeester A., Kuijpers M.J.E., Broers J.L.V., van Zandvoort M., Vanhoorelbeke K., et al. Principal role of glycoprotein VI in alpha 2 beta 1 and alpha IIb beta 3 activation during collagen-induced thrombus formation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1727–1733. doi: 10.1161/01.ATV.0000137974.85068.93. [DOI] [PubMed] [Google Scholar]
- 19.Cruz M.A., Chen J.M., Whitelock J.L., Morales L.D., Lopez J.A. The platelet glycoprotein Ib-von Willebrand factor interaction activates the collagen receptor alpha 2 beta 1 to bind collagen: activation-dependent conformational change of the alpha 2-I domain. Blood. 2005;105:1986–1991. doi: 10.1182/blood-2004-04-1365. [DOI] [PubMed] [Google Scholar]
- 20.Kuijpers M.J.E., Schulte V., Bergmeier W., Lindhout T., Brakebusch C., Offermanns S., et al. Complementary roles of platelet glycoprotein VI and integrin alpha 2 beta 1 in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003;17:685–687. doi: 10.1096/fj.02-0381fje. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson B.T., Jarvis G.E., Watson S.P. Activation of GPVI by collagen is regulated by alpha(2)beta(1) and secondary mediators. J. Thromb. Haemost. 2003;1:1278–1287. doi: 10.1046/j.1538-7836.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- 22.Farndale R.W., Sixma J.J., Barnes M.J., De Groot P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004;2:561–573. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 23.He L., Pappan L.K., Grenache D.G., Li Z.Z., Tollefsen D.M., Santoro S.A., et al. The contributions of the alpha(2)beta(1) integrin to vascular thrombosis in vivo. Blood. 2003;102:3652–3657. doi: 10.1182/blood-2003-04-1323. [DOI] [PubMed] [Google Scholar]
- 24.Siljander P.R.M., Munnix I.C.A., Smethurst P.A., Deckmyn H., Lindhout T., Ouwehand W.H., et al. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood. 2004;103:1333–1341. doi: 10.1182/blood-2003-03-0889. [DOI] [PubMed] [Google Scholar]
- 25.Nesbitt W.S., Kulkarni S., Giuliano S., Goncalves I., Dopheide S.M., Yap C.L., et al. Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J. Biol. Chem. 2002;277:2965–2972. doi: 10.1074/jbc.M110070200. [DOI] [PubMed] [Google Scholar]
- 26.Banno A., Ginsberg M.H. Integrin activation. Biochem. Soc. Trans. 2008;36:229–234. doi: 10.1042/BST0360229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shattil S.J., Kim C., Ginsberg M.H. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson S.P., Nesbitt W.S., Kulkarni S. Signaling events underlying thrombus formation. J. Thromb. Haemost. 2003;1:1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 29.Blockmans D., Deckmyn H., Vermylen J. Platelet actviation. Blood Rev. 1995;9:143–156. doi: 10.1016/0268-960x(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 30.Brass L.F., Zhu L., Stalker T.J. Minding the gaps to promote thrombus growth and stability. J. Clin. Invest. 2005;115:3385–3392. doi: 10.1172/JCI26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S.L., Brugge J.S., et al. Coordinate interactions of Csk, Src, and Syk kinases with alpha IIb beta 3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payrastre B., Missy K., Trumel C., Bodin S., Plantavid M., Chap H. The integrin alpha IIb/beta 3 in human platelet signal transduction. Biochem. Pharmacol. 2000;60:1069–1074. doi: 10.1016/s0006-2952(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 33.Rendu F., Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12:261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 34.Michelson A.D., Benoit S.E., Furman M.I., Breckwoldt W.L., Rohrer M.J., Barnard M.R., et al. Effects of nitric oxide/EDRF on platelet surface glycoproteins. Am. J. Physiol.-Heart Circul. Physiol. 1996;270 doi: 10.1152/ajpheart.1996.270.5.H1640. (H1640-H8) [DOI] [PubMed] [Google Scholar]
- 35.Beaulieu L.M., Freedman J.E. In: Platelets 3rd edition. Michelson A.D., editor. Academic Press; 2013. Inhibition of Platelet Function by the endothelium; pp. 313–342. [Google Scholar]
- 36.McAdam B.F., Catella-Lawson F., Mardini I.A., Kapoor S., Lawson J.A., FitzGerald G.A. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: The human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. U. S. A. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y., Wang M., Yu Y., Lawson J., Funk C.D., FitzGerald G.A. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J. Clin. Invest. 2006;116:1391–1399. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz U.R., Walter U., Eigenthaler M. Taming platelets with cyclic nucleotides. Biochem. Pharmacol. 2001;62:1153–1161. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 39.Harbeck B., Huttelmaier S., Schluter K., Jockusch B.M., Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J. Biol. Chem. 2000;275:30817–30825. doi: 10.1074/jbc.M005066200. [DOI] [PubMed] [Google Scholar]
- 40.Cavallini L., Coassin M., Borean A., Alexandre A. Prostacyclin and sodium nitroprusside inhibit the activity of the platelet inositol 1,4,5-trisphosphate receptor and promote its phosphorylation. J. Biol. Chem. 1996;271:5545–5551. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- 41.Marcus A.J., Safier L.B., Hajjar K.A., Ullman H.L., Islam N., Broekman M.J., et al. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. Thromboregulation by endothelial cells. J. Clin. Invest. 1991;88:1690–1696. doi: 10.1172/JCI115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinsky D.J., Broekman M.J., Peschon J.J., Stocking K.L., Fujita T., Ramasamy R., et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J. Clin. Invest. 2002;109:1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffbrand A.V., Higgs D.R., Keeling D.M., Mehta A.B. 7th ed. Wiley Blackwell; 2016. Postgraduate Haematology. [Google Scholar]
- 44.Versteeg H.H., Heemskerk J.W., Levi M., Reitsma P.H. New fundamentals in hemostasis. Physiol. Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 45.Schuijt Tim J., Bakhtiari K., Daffre S., DePonte K., Wielders Simone J.H., Marquart J.A., et al. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system. Circulation. 2013;128:254–266. doi: 10.1161/CIRCULATIONAHA.113.003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Podoplelova N.A., Sveshnikova A.N., Kotova Y.N., Eckly A., Receveur N., Nechipurenko D.Y., et al. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 2016;128:1745–1755. doi: 10.1182/blood-2016-02-696898. [DOI] [PubMed] [Google Scholar]
- 47.Choi S.H., Smith S.A., Morrissey J.H. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng Y., Verhamme I.M., Smith S.B., Sun M.-F., Matafonov A., Cheng Q., et al. The dimeric structure of factor XI and zymogen activation. Blood. 2013;121:3962–3969. doi: 10.1182/blood-2012-12-473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maas C., Meijers J.C.M., Marquart J.A., Bakhtiari K., Weeterings C., de Groot P.G., et al. Activated factor V is a cofactor for the activation of factor XI by thrombin in plasma. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9083–9087. doi: 10.1073/pnas.1004741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matafonov A., Sarilla S. Sun M-f, Sheehan JP, Serebrov V, Verhamme IM, et al. Activation of factor XI by products of prothrombin activation. Blood. 2011;118:437–445. doi: 10.1182/blood-2010-10-312983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith S.A., Mutch N.J., Baskar D., Rohloff P., Docampo R., Morrissey J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith S.A., Choi S.H., Davis-Harrison R., Huyck J., Boettcher J., Rienstra C.M., et al. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Meijden P.E.J., Munnix I.C.A., Auger J.M., Govers-Riemslag J.W.P., Cosemans J.M.E.M., Kuijpers M.J.E., et al. Dual role of collagen in factor XII–dependent thrombus formation. Blood. 2009;114:881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 54.Kleinschnitz C., Stoll G., Bendszus M., Schuh K., Pauer H.-U., Burfeind P., et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J. Exp. Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratnoff O.D., Colopy J.E. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J. Clin. Invest. 1955;34:602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson M., Rayzman V., Nolte M.W., Nickel K.F., Björkqvist J., Jämsä A., et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3006804. 222ra17-ra17. [DOI] [PubMed] [Google Scholar]
- 57.Revenko A.S., Gao D., Crosby J.R., Bhattacharjee G., Zhao C., May C., et al. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–5311. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin L., Abrahams J.P., Skinner R., Petitou M., Pike R.N., Carrell R.W. The anticoagulant activation of antithrombin by heparin. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tollefsen D.M., Pestka C.A., Monafo W.J. Activation of heparin cofactor II by dermatan sulfate. J. Biol. Chem. 1983;258:6713–6716. [PubMed] [Google Scholar]
- 60.Schwarz H., Heeb M., Wencel-Drake J., Griffin J. Identification and quantitation of protein S in human platelets. Blood. 1985;66:1452–1455. [PubMed] [Google Scholar]
- 61.Hackeng T.M., Seré K.M., Tans G., Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broze G.J., Jr., Girard T.J. Factor V, tissue factor pathway inhibitor, and east Texas bleeding disorder. J. Clin. Invest. 2013;123:3710–3712. doi: 10.1172/JCI71220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahlbäck B., Guo L.J., Livaja-Koshiar R., Tran S. Factor V-short and protein S as synergistic tissue factor pathway inhibitor (TFPIα) cofactors. Res Pract Thromb Haemost. 2017;2:114–124. doi: 10.1002/rth2.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tracy P.B., Peterson J.M., Nesheim M.E., McDuffie F.C., Mann K.G. Interaction of coagulation factor V and factor Va with platelets. J. Biol. Chem. 1979;254:10354–10361. [PubMed] [Google Scholar]
- 65.Ahmad S.S., Scandura J.M., Walsh P.N. Structural and functional characterization of platelet receptor-mediated factor VIII binding. J. Biol. Chem. 2000;275:13071–13081. doi: 10.1074/jbc.275.17.13071. [DOI] [PubMed] [Google Scholar]
- 66.Ahmad S.S., Rawala-Sheikh R., Walsh P.N. Comparative interactions of factor IX and factor IXa with human platelets. J. Biol. Chem. 1989;264:3244–3251. [PubMed] [Google Scholar]
- 67.Sinha D., Seaman F.S., Koshy A., Knight L.C., Walsh P.N. Blood coagulation factor XIa binds specifically to a site on activated human platelets distinct from that for factor XI. J. Clin. Invest. 1984;73:1550–1556. doi: 10.1172/JCI111361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh P.N. Platelet coagulation-protein interactions. Semin. Thromb. Hemost. 2004;30:461–471. doi: 10.1055/s-2004-833481. [DOI] [PubMed] [Google Scholar]
- 69.van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat. Rev. Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 70.Beth A., Bouchard J.R.S., Paula B. In: Platelets. Michelson A.D., editor. 2013. Tracy, 21-Interactions between platelets and the coagulation system. [Google Scholar]
- 71.Monroe D.M. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002;22:1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 72.Mattheij N.J.A., Swieringa F., Mastenbroek T.G., Berny-Lang M.A., May F., Baaten C.C.F.M.J., et al. Coated platelets function in platelet-dependent fibrin formation via integrin αIIbβ3 and transglutaminase factor XIII. Haematologica. 2016;101:427–436. doi: 10.3324/haematol.2015.131441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood J.P., Fager A.M., Silveira J.R., Tracy P.B. Platelet-derived factor Va expressed on the surface of the activated platelet is GPI-anchored. Blood. 2008;112:219–220. [Google Scholar]
- 74.Camire R.M., Kalafatis M., Simioni P., Girolami A., Tracy P.B. Platelet-derived factor Va/Va Leiden cofactor activities are sustained on the surface of activated platelets despite the presence of activated protein C. Blood. 1998;91:2818–2829. [PubMed] [Google Scholar]
- 75.Gould W.R., Silveira J.R., Tracy P.B. Unique in vivo modifications of coagulation factor V produce a physically and functionally distinct platelet-derived cofactor: characterization of purified platelet-derived factor V/Va. J. Biol. Chem. 2004;279:2383–2393. doi: 10.1074/jbc.M308600200. [DOI] [PubMed] [Google Scholar]
- 76.Romp K.G., Monroe D.M., Hoffman M. Platelets contain releasable coagulation-factor-IX antigen. Blood Coagul. Fibrinolysis. 1993;4:905–910. [PubMed] [Google Scholar]
- 77.Gailani D., Zivelin A., Sinha D., Walsh P.N. Do platelets synthesize factor XI? J. Thromb. Haemost. 2004;2:1709–1712. doi: 10.1111/j.1538-7836.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- 78.Hsu T.-C., Shore S.K., Seshsmma T., Bagasra O., Walsh P.N. Molecular cloning of platelet factor XI, an alternative splicing product of the plasma factor XI gene. J. Biol. Chem. 1998;273:13787–13793. doi: 10.1074/jbc.273.22.13787. [DOI] [PubMed] [Google Scholar]
- 79.Podmore A., Smith M., Savidge G., Alhaq A. Real-Time quantitative PCR analysis of factor XI mRNA variants in human platelets. J. Thromb. Haemost. 2004;2:1713–1719. doi: 10.1111/j.1538-7836.2004.00924.x. [DOI] [PubMed] [Google Scholar]
- 80.Martincic D., Kravtsov V., Gailani D. Factor XI messenger RNA in human platelets. Blood. 1999;94:3397–3404. [PubMed] [Google Scholar]
- 81.Zucker M., Hauschner H., Seligsohn U., Rosenberg N. Platelet factor XI: intracellular localization and mRNA splicing following platelet activation. Blood Cells Mol. Dis. 2018;69:30–37. doi: 10.1016/j.bcmd.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Phillips J.E., Lord S.T., Gilbert G.E. Fibrin stimulates platelets to increase factor VIIIa binding site expression. J. Thromb. Haemost. 2004;2:1806–1815. doi: 10.1111/j.1538-7836.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- 83.Pratt K.P. fVIII binds platelets + fibrin: no PS! Blood. 2015;126:1158–1159. doi: 10.1182/blood-2015-07-657924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gilbert G.E., Novakovic V.A., Shi J., Rasmussen J., Pipe S.W. Platelet binding sites for factor VIII in relation to fibrin and phosphatidylserine. Blood. 2015;126:1237–1244. doi: 10.1182/blood-2015-01-620245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoenwaelder S.M., Ono A., Nesbitt W.S., Lim J., Jarman K., Jackson S.P. Phosphoinositide 3-kinase p110 beta regulates integrin alpha IIb beta 3 avidity and the cellular transmission of contractile forces. J. Biol. Chem. 2010;285:2886–2896. doi: 10.1074/jbc.M109.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heemskerk J.W., Mattheij N.J., Cosemans J.M. Platelet-based coagulation: different populations, different functions. J. Thromb. Haemost. 2013;11:2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- 87.Michelson AD, Cattaneo M, Frelinger A, and Newman P. Platelets. 2019: Elsevier Science.
- 88.Welsh J.D., Poventud-Fuentes I., Sampietro S., Diamond S.L., Stalker T.J., Brass L.F. Hierarchical organization of the hemostatic response to penetrating injuries in the mouse macrovasculature. J. Thromb. Haemost. 2017;15:526–537. doi: 10.1111/jth.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morrissey J.H., Choi S.H., Smith S.A. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012;119:5972–5979. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Müller F., Mutch N.J., Schenk W.A., Smith S.A., Esterl L., Spronk H.M., et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Faxälv L., Boknäs N., Ström J.O., Tengvall P., Theodorsson E., Ramström S., et al. Putting polyphosphates to the test: evidence against platelet-induced activation of factor XII. Blood. 2013;122:3818–3824. doi: 10.1182/blood-2013-05-499384. [DOI] [PubMed] [Google Scholar]
- 92.Verhoef J.J.F., Barendrecht A.D., Nickel K.F., Dijkxhoorn K., Kenne E., Labberton L., et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood. 2017;129:1707–1717. doi: 10.1182/blood-2016-08-734988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wood J.P., Ellery P.E.R., Maroney S.A., Mast A.E. Biology of tissue factor pathway inhibitor. Blood. 2014;123:2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Novotny W., Girard T., Miletich J., Broze G.J. Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- 95.Maroney S.A., Haberichter S.L., Friese P., Collins M.L., Ferrel J.P., Dale G.L., et al. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winckers K., Thomassen S., Ten Cate H., Hackeng T.M. Platelet full length TFPI-α in healthy volunteers is not affected by sex or hormonal use. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168273. e0168273-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stavenuiter F., Davis N.F., Duan E., Gale A.J., Heeb M.J. Platelet protein S directly inhibits procoagulant activity on platelets and microparticles. Thromb. Haemost. 2013;109:229–237. doi: 10.1160/TH12-08-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caliezi C., Wuillemin W.A., Zeerleder S., Redondo M., Eisele B., Hack C.E. C1-esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol. Rev. 2000;52:91–112. [PubMed] [Google Scholar]
- 99.Davis A.E. The pathophysiology of hereditary angioedema. Clin. Immunol. 2005;114:3–9. doi: 10.1016/j.clim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Schmaier A.H., Smith P.M., Colman R.W. Platelet C1- inhibitor. A secreted alpha-granule protein. J. Clin. Invest. 1985;75:242–250. doi: 10.1172/JCI111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pixley R.A., Schapira M., Colman R.W. The regulation of human factor XIIa by plasma proteinase inhibitors. J. Biol. Chem. 1985;260:1723–1729. [PubMed] [Google Scholar]
- 102.Mutch N.J. In: Platelets. Michelson A.D., editor. Academic Press; 2019. 23 - Regulation of fibrinolysis by platelets; pp. 417–431. [Google Scholar]
- 103.Zakharova N.V., Artemenko E.O., Podoplelova N.A., Sveshnikova A.N., Demina I.A., Ataullakhanov F.I., et al. Platelet surface-associated activation and secretion-mediated inhibition of coagulation factor XII. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116665. e0116665-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Nostrand W., Schmaier A., Farrow J., Cunningham D. Protease nexin-II (amyloid beta-protein precursor): a platelet alpha-granule protein. Science. 1990;248:745–748. doi: 10.1126/science.2110384. [DOI] [PubMed] [Google Scholar]
- 105.Boulaftali Y., Adam F., Venisse L., Ollivier V., Richard B., Taieb S., et al. Anticoagulant and antithrombotic properties of platelet protease nexin-1. Blood. 2010;115:97–106. doi: 10.1182/blood-2009-04-217240. [DOI] [PubMed] [Google Scholar]
- 106.Boulaftali Y., Ho-Tin-Noe B., Pena A., Loyau S., Venisse L., François D., et al. Platelet protease nexin-1, a serpin that strongly influences fibrinolysis and thrombolysis. Circulation. 2011;123:1326–1334. doi: 10.1161/CIRCULATIONAHA.110.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu W., Li H., Navaneetham D., Reichenbach Z.W., Tuma R.F., Walsh P.N. The kunitz protease inhibitor domain of protease nexin-2 inhibits factor XIa and murine carotid artery and middle cerebral artery thrombosis. Blood. 2012;120:671–677. doi: 10.1182/blood-2012-03-419523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y., Scandura J.M., Van Nostrand W.E., Walsh P.N. The mechanism by which heparin promotes the inhibition of coagulation factor XIa by protease nexin-2. J. Biol. Chem. 1997;272:26139–26144. doi: 10.1074/jbc.272.42.26139. [DOI] [PubMed] [Google Scholar]
- 109.Scandura J.M., Zhang Y., Van Nostrand W.E., Walsh P.N. Progress curve analysis of the kinetics with which blood coagulation factor XIa is inhibited by protease nexin-2. Biochemistry. 1997;36:412–420. doi: 10.1021/bi9612576. [DOI] [PubMed] [Google Scholar]
- 110.Kahn M.L., Nakanishi-Matsui M., Shapiro M.J., Ishihara H., Coughlin S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kahn M.L., Zheng Y.W., Huang W., Bigornia V., Zeng D.W., Moff S., et al. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 112.Ramakrishnan V., DeGuzman F., Bao M., Hall S.W., Leung L.L., Phillips D.R. A thrombin receptor function for platelet glycoprotein Ib–IX unmasked by cleavage of glycoprotein V. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1823–1828. doi: 10.1073/pnas.98.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frédéric A., Marie-Claude G., Martine J.-P. Glycoprotein Ib-mediated platelet activation. Eur. J. Biochem. 2003;270:2959–2970. doi: 10.1046/j.1432-1033.2003.03670.x. [DOI] [PubMed] [Google Scholar]
- 114.López J.A., Andrews R.K., Afshar-Kharghan V., Berndt M.C. Bernard-Soulier Syndrome. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- 115.Gralnick H.R., Williams S., McKeown L.P., Hansmann K., Fenton J.W., Krutzsch H. High-affinity a-thrombin binding to platelet glycoprotein Iba: Identification of two binding domains. Proc. Natl. Acad. Sci. U. S. A. 1994;91:6334–6338. doi: 10.1073/pnas.91.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dörmann D., Clemetson K.J., Kehrel B.E. The GPIb thrombin-binding site is essential for thrombin-induced platelet procoagulant activity. Blood. 2000;96:2469–2478. [PubMed] [Google Scholar]
- 117.De Candia E., Hall S.W., Rutella S., Landolfi R., Andrews R.K., De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J. Biol. Chem. 2001;276:4692–4698. doi: 10.1074/jbc.M008160200. [DOI] [PubMed] [Google Scholar]
- 118.Workman E.F., White G.C., Lundblad R.L. Structure-function relationships in the interaction of alpha-thrombin with blood platelets. J. Biol. Chem. 1977;252:7118–7123. [PubMed] [Google Scholar]
- 119.BM C., Cheryl G., Arnold K., Heddy Z., Dominique F., CP A. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur. J. Biochem. 1985;151:637–649. doi: 10.1111/j.1432-1033.1985.tb09152.x. [DOI] [PubMed] [Google Scholar]
- 120.Estevez B., Kim K., Delaney M.K., Stojanovic-Terpo A., Shen B., Ruan C., et al. Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood. 2016;127:626–636. doi: 10.1182/blood-2015-04-638387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Byzova T.V., Plow E.F. Networking in the hemostatic system: integrin αIIbβ3 binds prothrombin and influences its activation. J. Biol. Chem. 1997;272:27183–27188. doi: 10.1074/jbc.272.43.27183. [DOI] [PubMed] [Google Scholar]
- 122.Podolnikova N.P., Gorkun O.V., Loreth R.M., Yee V.C., Lord S.T., Ugarova T.P. A cluster of basic amino acid residues in the γ370−381 sequence of fibrinogen comprises a binding site for platelet integrin αIIbβ3 (Glycoprotein IIb/IIIa) Biochemistry. 2005;44:16920–16930. doi: 10.1021/bi051581d. [DOI] [PubMed] [Google Scholar]
- 123.Podolnikova N.P., Yakovlev S., Yakubenko V.P., Wang X., Gorkun O.V., Ugarova T.P. The interaction of integrin αIIbβ3 with fibrin occurs through multiple binding sites in the αIIb β-propeller domain. J. Biol. Chem. 2014;289:2371–2383. doi: 10.1074/jbc.M113.518126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Höök P., Litvinov R.I., Kim O.V., Xu S., Xu Z., Bennett J.S., et al. Strong binding of platelet integrin αIIbβ3 to fibrin clots: potential target to destabilize thrombi. Sci. Rep. 2017;7:13001. doi: 10.1038/s41598-017-12615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Magwenzi S.G., Ajjan R.A., Standeven K.F., Parapia L.A., Naseem K.M. Factor XIII supports platelet activation and enhances thrombus formation by matrix proteins under flow conditions. J. Thromb. Haemost. 2011;9:820–833. doi: 10.1111/j.1538-7836.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 126.Kotova Y.N., Podoplelova N.A., Obydennyy S.I., Kostanova E.A., Ryabykh A.A., Demyanova A.S., et al. Binding of coagulation factor XIII zymogen to activated platelet subpopulations: roles of integrin αIIbβ3 and fibrinogen. Thromb. Haemost. 2019;119:906–915. doi: 10.1055/s-0039-1683912. [DOI] [PubMed] [Google Scholar]
- 127.Inoue O., Suzuki-Inoue K., McCarty O.J.T., Moroi M., Ruggeri Z.M., Kunicki T.J., et al. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107:1405–1412. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mammadova-Bach E., Ollivier V., Loyau S., Schaff M., Dumont B., Favier R., et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683–691. doi: 10.1182/blood-2015-02-629717. [DOI] [PubMed] [Google Scholar]
- 129.Induruwa I., Moroi M., Bonna A., Malcor J.-D., Howes J.-M., Warburton E.A., et al. Platelet collagen receptor Glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. J. Thromb. Haemost. 2018;16:389–404. doi: 10.1111/jth.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slater A., Perrella G., Onselaer M.-B., Martin E.M., Gauer J.S., Xu R.-G., et al. Does fibrin(ogen) bind to monomeric or dimeric GPVI, or not at all? Platelets. 2019;30:281–289. doi: 10.1080/09537104.2018.1508649. [DOI] [PubMed] [Google Scholar]
- 131.Rayes J., Watson S.P., Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J. Clin. Invest. 2019;129:12–23. doi: 10.1172/JCI122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar R., Béguin S., Hemker H.C. The effect of fibrin clots and clot-bound thrombin on the development of platelet procoagulant activity. Thromb. Haemost. 1995;73:962–968. [PubMed] [Google Scholar]
- 133.Béguin S., Kumar R., Keularts I., Seligsohn U., Coller B.S., Hemker H.C. Fibrin-dependent platelet procoagulant activity requires GPIb receptors and von Willebrand factor. Blood. 1999;93:564–570. [PubMed] [Google Scholar]
- 134.Loscalzo J., Inbal A., Handin R.I. von Willebrand protein facilitates platelet incorporation in polymerizing fibrin. J. Clin. Invest. 1986;78:1112–1119. doi: 10.1172/JCI112668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iino M., Takeya H., Takemitsu T., Nakagaki T., Gabazza E.C., Suzuki K. Characterization of the binding of factor Xa to fibrinogen/fibrin derivatives and localization of the factor Xa binding site on fibrinogen. Eur. J. Biochem. 1995;232:90–97. doi: 10.1111/j.1432-1033.1995.tb20785.x. [DOI] [PubMed] [Google Scholar]
- 136.Konings J., Govers-Riemslag J.W.P., Philippou H., Mutch N.J., Borissoff J.I., Allan P., et al. Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118:3942–3951. doi: 10.1182/blood-2011-03-339572. [DOI] [PubMed] [Google Scholar]
- 137.van Geffen J.P., Swieringa F., Heemskerk J.W.M. Platelets and coagulation in thrombus formation: aberrations in the Scott syndrome. Thromb. Res. 2016;141 doi: 10.1016/S0049-3848(16)30355-3. (S12-S6) [DOI] [PubMed] [Google Scholar]
- 138.Gustafson E.J., Schutsky D., Knight L.C., Schmaier A.H. High molecular weight kininogen binds to unstimulated platelets. J. Clin. Invest. 1986;78:310–318. doi: 10.1172/JCI112567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meloni F.J., Schmaier A.H. Low molecular weight kininogen binds to platelets to modulate thrombin-induced platelet activation. J. Biol. Chem. 1991;266:6786–6794. [PubMed] [Google Scholar]
- 140.Bradford H.N., Dela Cadena R.A., Kunapuli S.P., Dong J.-F., López J.A., Colman R.W. Human kininogens regulate thrombin binding to platelets through the Glycoprotein Ib-IX-V complex. Blood. 1997;90:1508–1515. [PubMed] [Google Scholar]
- 141.Bradford H.N., Pixley R.A., Colman R.W. Human factor XII binding to the Glycoprotein Ib-IX-V complex inhibits thrombin-induced platelet aggregation. J. Biol. Chem. 2000;275:22756–22763. doi: 10.1074/jbc.M002591200. [DOI] [PubMed] [Google Scholar]
- 142.Thompson R.E., Mandle R., Jr., Kaplan A.P. Association of factor XI and high molecular weight kininogen in human plasma. J. Clin. Invest. 1977;60:1376–1380. doi: 10.1172/JCI108898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baglia F.A., Gailani D., López J.A., Walsh P.N. Identification of a binding site for Glycoprotein Ibα in the Apple 3 domain of factor XI. J. Biol. Chem. 2004;279:45470–45476. doi: 10.1074/jbc.M406727200. [DOI] [PubMed] [Google Scholar]
- 144.Baglia F.A., Shrimpton C.N., Emsley J., Kitagawa K., Ruggeri Z.M., López J.A., et al. Factor XI interacts with the leucine-rich repeats of Glycoprotein Ibα on the activated platelet. J. Biol. Chem. 2004;279:49323–49329. doi: 10.1074/jbc.M407889200. [DOI] [PubMed] [Google Scholar]
- 145.Ho D.H., Badellino K., Baglia F.A., Sun M.-F., Zhao M.-M., Gailani D., et al. The Role of High Molecular Weight Kininogen and Prothrombin as Cofactors in the Binding of Factor XI A3 Domain to the Platelet Surface. J. Biol. Chem. 2000;275:25139–25145. doi: 10.1074/jbc.M001890200. [DOI] [PubMed] [Google Scholar]
- 146.White-Adams T.C., Berny M.A., Tucker E.I., Gertz J.M., Gailani D., Urbanus R.T., et al. Identification of coagulation factor XI as a ligand for platelet apolipoprotein E receptor 2 (ApoER2) Arterioscler. Thromb. Vasc. Biol. 2009;29:1602–1607. doi: 10.1161/ATVBAHA.109.187393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lisman T. Factor XI binding to platelets. Arterioscler. Thromb. Vasc. Biol. 2009;29:1409-10. [DOI] [PubMed]
- 148.Emsley J., McEwan P.A., Gailani D. Structure and function of factor XI. Blood. 2010;115:2569–2577. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.White T.C., Berny M.A., Tucher E.I., Urbanus R.T., De Groot P.G., Fernandez J.A., et al. Protein C supports platelet binding and activation under flow: role of glycoprotein Ib and apolipoprotein E receptor 2. J. Thromb. Haemost. 2008;6:995–1002. doi: 10.1111/j.1538-7836.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- 150.Al-Tamimi M., Grigoriadis G., Tran H., Paul E., Servadei P., Berndt M.C., et al. Coagulation-induced shedding of platelet glycoprotein VI mediated by factor Xa. Blood. 2011;117:3912–3920. doi: 10.1182/blood-2010-08-301523. [DOI] [PubMed] [Google Scholar]
- 151.Cattaneo M, Congenital Disorders of Platelet Function. In: A.D. Michelson (Editor). Platelets. 2013. pp. 1019-47.
- 152.Bellucci S., Girma J.P., Lozano M., Meyer D., Caen J.P. Impaired prothrombin consumption in Bernard-Soulier syndrome is corrected in vitro by human factor VIII. Thromb. Haemost. 1997;77:383–386. [PubMed] [Google Scholar]
- 153.Weeterings C., de Groot P.G., Adelmeijer J., Lisman T. The glycoprotein Ib-IX-V complex contributes to tissue factor–independent thrombin generation by recombinant factor VIIa on the activated platelet surface. Blood. 2008;112:3227–3233. doi: 10.1182/blood-2008-02-139113. [DOI] [PubMed] [Google Scholar]
- 154.Lisman T., Adelmeuer J., Cauwenberghs S., Van Pampus E.C.M., Heemskerk J.W.M., De Groot P.G. Recombinant factor VIIa enhances platelet adhesion and activation under flow conditions at normal and reduced platelet count. J. Thromb. Haemost. 2005;3:742–751. doi: 10.1111/j.1538-7836.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 155.van’t Veer C., Golden N.J., Mann K.G. Inhibition of thrombin generation by the zymogen factor VII: implications for the treatment of hemophilia A by factor VIIa. Blood. 2000;95:1330–1335. [PubMed] [Google Scholar]
- 156.Butenas S., Brummel K.E., Branda R.F., Paradis S.G., Mann K.G. Mechanism of factor VIIa–dependent coagulation in hemophilia blood: Presented in part at the 42nd Annual Meeting of the American Society of Hematology, December 1-5, 2000, San Francisco. CA. Blood. 2002;99:923–930. doi: 10.1182/blood.v99.3.923. [DOI] [PubMed] [Google Scholar]
- 157.Monroe D.M., Hoffman M., Oliver J.A., Roberts H.R. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br. J. Haematol. 1997;99:542–547. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 158.Fager AM and Hoffman M. Endothelial protein C receptor is expressed on procoagulant platelets and contributes to factor VIIa binding and activity. Blood. 2014;124:4224-.
- 159.Nurden A.T., Freson K., Seligsohn U. Inherited platelet disorders. Haemophilia. 2012;18(Suppl. 4):154–160. doi: 10.1111/j.1365-2516.2012.02856.x. [DOI] [PubMed] [Google Scholar]
- 160.Nurden A.T., Nurden P. Congenital platelet disorders and understanding of platelet function. Br. J. Haematol. 2014;165:165–178. doi: 10.1111/bjh.12662. [DOI] [PubMed] [Google Scholar]
- 161.Millikan P.D., Balamohan S.M., Raskind W.H., Kacena M.A. Inherited thrombocytopenia due to GATA-1 mutations. Semin. Thromb. Hemost. 2011;37:682–689. doi: 10.1055/s-0031-1291378. [DOI] [PubMed] [Google Scholar]
- 162.Kaur G., Jalagadugula G., Mao G., Rao A.K. RUNX1/core binding factor A2 regulates platelet 12-lipoxygenase gene (ALOX12): studies in human RUNX1 haplodeficiency. Blood. 2010;115:3128–3135. doi: 10.1182/blood-2009-04-214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Balduini C.L., Pecci A., Savoia A. Recent advances in the understanding and management of MYH9-related inherited thrombocytopenias. Br. J. Haematol. 2011;154:161–174. doi: 10.1111/j.1365-2141.2011.08716.x. [DOI] [PubMed] [Google Scholar]
- 164.Nurden P., Debili N., Coupry I., Bryckaert M., Youlyouz-Marfak I., Solé G., et al. Thrombocytopenia resulting from mutations in filamin A can be expressed as an isolated syndrome. Blood. 2011;118:5928–5937. doi: 10.1182/blood-2011-07-365601. [DOI] [PubMed] [Google Scholar]
- 165.Nurden A., Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. J. Thromb. Haemost. 2011;9(Suppl. 1):76–91. doi: 10.1111/j.1538-7836.2011.04274.x. [DOI] [PubMed] [Google Scholar]
- 166.Noris P., Perrotta S., Seri M., Pecci A., Gnan C., Loffredo G., et al. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: analysis of 78 patients from 21 families. Blood. 2011;117:6673–6680. doi: 10.1182/blood-2011-02-336537. [DOI] [PubMed] [Google Scholar]
- 167.Zwaal R.F.A., Comfurius P., Bevers E.M. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 1636;2004:119–128. doi: 10.1016/j.bbalip.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 168.Munnix I.C.A., Harmsma M., Giddings J.C., Collins P.W., Feijge M.A.H., Comfurius P., et al. Store-mediated calcium entry in the regulation of phosphatidylserine exposure in blood cells from Scott patients. Thromb. Haemost. 2003;89:687–695. [PubMed] [Google Scholar]
- 169.Ahmad S.S., Rawala-Sheikh R., Ashby B., Walsh P.N. Platelet receptor-mediated factor X activation by factor IXa. High-affinity factor IXa receptors induced by factor VIII are deficient on platelets in Scott syndrome. J. Clin. Invest. 1989;84:824–828. doi: 10.1172/JCI114242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Suzuki J., Umeda M., Sims P.J., Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 171.van Kruchten R., Mattheij N.J.A., Saunders C., Feijge M.A.H., Swieringa F., Wolfs J.L.N., et al. Both TMEM16F-dependent and TMEM16F-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood. 2013;121:1850–1857. doi: 10.1182/blood-2012-09-454314. [DOI] [PubMed] [Google Scholar]
- 172.Rosing J., Bevers E., Comfurius P., Hemker H., van Dieijen G., Weiss H., et al. Impaired factor X and prothrombin activation associated with decreased phospholipid exposure in platelets from a patient with a bleeding disorder. Blood. 1985;65:1557–1561. [PubMed] [Google Scholar]
- 173.Blavignac J., Bunimov N., Rivard G.E., Hayward C.P. Quebec platelet disorder: update on pathogenesis, diagnosis, and treatment. Semin. Thromb. Hemost. 2011;37:713–720. doi: 10.1055/s-0031-1291382. [DOI] [PubMed] [Google Scholar]
- 174.Mumford A.D., Frelinger A.L., 3rd, Gachet C., Gresele P., Noris P., Harrison P., et al. A review of platelet secretion assays for the diagnosis of inherited platelet secretion disorders. Thromb. Haemost. 2015;114:14–25. doi: 10.1160/TH14-11-0999. [DOI] [PubMed] [Google Scholar]
- 175.Sadler J.E., Budde U., Eikenboom J.C.J., Favaloro E.J., Hill F.G.H., Holmberg L., et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J. Thromb. Haemost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 176.Szanto T., Nummi V., Jouppila A., Brinkman H.J.M., Lassila R. Platelets compensate for poor thrombin generation in type 3 von Willebrand disease. Platelets. 2020;31:103–111. doi: 10.1080/09537104.2019.1581922. [DOI] [PubMed] [Google Scholar]
- 177.Owren P. Parahaemophilia: haemorrhagic diathesis due to absence of a previously unknown clotting factor. Lancet. 1947;249:446–448. [PubMed] [Google Scholar]
- 178.Lak, Sharifian, Peyvandi, and Mannucci. Symptoms of inherited factor V deficiency in 35 Iranian patients. Br. J. Haematol. 1998;103:1067-9. [DOI] [PubMed]
- 179.Duckers C., Simioni P., Spiezia L., Radu C., Dabrilli P., Gavasso S., et al. Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mild bleeding symptoms. Blood. 2010;115:879–886. doi: 10.1182/blood-2009-08-237719. [DOI] [PubMed] [Google Scholar]
- 180.King S.B., Smith S.C., Hirshfeld J.W., Jacobs A.K., Morrison D.A., Williams D.O., et al. 2007 Focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 181.McFadyen J.D., Schaff M., Peter K. Current and future antiplatelet therapies: emphasis on preserving haemostasis. Nat. Rev. Cardiol. 2018;15:181–191. doi: 10.1038/nrcardio.2017.206. [DOI] [PubMed] [Google Scholar]
- 182.Mackman N., Bergmeier W., Stouffer G.A., Weitz J.I. Therapeutic strategies for thrombosis: new targets and approaches. Nat. Rev. Drug Discov. 2020;19:333–352. doi: 10.1038/s41573-020-0061-0. [DOI] [PubMed] [Google Scholar]
- 183.Grover S.P., Mackman N. Intrinsic Pathway of Coagulation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019;39:331–338. doi: 10.1161/ATVBAHA.118.312130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pabinger I., Riedl J. Direct oral anticoagulants: now also for prevention and treatment of cancer-associated venous thromboembolism? Hematol. Am Soc Hematol Educ Program. 2017;2017:136–143. doi: 10.1182/asheducation-2017.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.von Brühl M.L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Montoro-García S., Schindewolf M., Stanford S., Larsen O.H., Thiele T. The Role of Platelets in Venous Thromboembolism. Semin. Thromb. Hemost. 2016;42:242–251. doi: 10.1055/s-0035-1570079. [DOI] [PubMed] [Google Scholar]
- 187.Massberg S., Grahl L., von Bruehl M.L., Manukyan D., Pfeiler S., Goosmann C., et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 188.Brill A., Fuchs T.A., Chauhan A.K., Yang J.J., De Meyer S.F., Köllnberger M., et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Payne H., Ponomaryov T., Watson S.P., Brill A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. 2017;129:2013–2020. doi: 10.1182/blood-2016-09-742999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Budnik I., Brill A. Immune Factors in Deep Vein Thrombosis Initiation. Trends Immunol. 2018;39:610–623. doi: 10.1016/j.it.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dyer M.R., Chen Q., Haldeman S., Yazdani H., Hoffman R., Loughran P., et al. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci. Rep. 2018;8:2068. doi: 10.1038/s41598-018-20479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stark K., Philippi V., Stockhausen S., Busse J., Antonelli A., Miller M., et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood. 2016;128:2435–2449. doi: 10.1182/blood-2016-04-710632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tarantino E., Amadio P., Squellerio I., Porro B., Sandrini L., Turnu L., et al. Role of thromboxane-dependent platelet activation in venous thrombosis: Aspirin effects in mouse model. Pharmacol. Res. 2016;107:415–425. doi: 10.1016/j.phrs.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 194.Jameson S.S., Baker P.N., Deehan D.J., Port A., Reed M.R. Evidence-base for aspirin as venous thromboembolic prophylaxis following joint replacement. Bone Joint Res. 2014;3:146–149. doi: 10.1302/2046-3758.35.2000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mistry D.A., Chandratreya A., Lee P.Y.F. A Systematic Review on the Use of Aspirin in the Prevention of Deep Vein Thrombosis in Major Elective Lower Limb Orthopedic Surgery: An Update from the Past 3 Years. Surg J (N Y). 2017;3 doi: 10.1055/s-0037-1615817. e191-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Noble S., Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br. J. Cancer. 2010;102(Suppl. 1) doi: 10.1038/sj.bjc.6605599. S2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mukai M., Oka T. Mechanism and management of cancer-associated thrombosis. J. Cardiol. 2018;72:89–93. doi: 10.1016/j.jjcc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 198.Gando S., Levi M., Toh C.-H. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037. doi: 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- 199.Schreiber K., Sciascia S., de Groot P.G., Devreese K., Jacobsen S., Ruiz-Irastorza G., et al. Antiphospholipid syndrome. Nat Rev Dis Primers. 2018;4:17103. doi: 10.1038/nrdp.2017.103. [DOI] [PubMed] [Google Scholar]
- 200.Nørgaard M. Thrombosis in patients with primary chronic immune thrombocytopenia. Thromb. Res. 2012;130(Suppl. 1) doi: 10.1016/j.thromres.2012.08.282. (S74-S5) [DOI] [PubMed] [Google Scholar]
- 201.Olson E.J., Bandle J., Calvo R.Y., Shackford S.R., Dunne C.E., Van Gent J.-M., et al. Heparin versus enoxaparin for prevention of venous thromboembolism after trauma: A randomized noninferiority trial. J. Trauma Acute Care Surg. 2015;79:961–969. doi: 10.1097/TA.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 202.Gori T., Polimeni A., Indolfi C., Räber L., Adriaenssens T., Münzel T. Predictors of stent thrombosis and their implications for clinical practice. Nat. Rev. Cardiol. 2019;16:243–256. doi: 10.1038/s41569-018-0118-5. [DOI] [PubMed] [Google Scholar]
- 203.Ghazi L., Schwann T.A., Engoren M.C., Habib R.H. Role of blood transfusion product type and amount in deep vein thrombosis after cardiac surgery. Thromb. Res. 2015;136:1204–1210. doi: 10.1016/j.thromres.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 204.Lisman T., Porte R.J. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost. 2017;1:150–161. doi: 10.1002/rth2.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bayer G., von Tokarski F., Thoreau B., Bauvois A., Barbet C., Cloarec S., et al. Etiology and outcomes of thrombotic microangiopathies. Clin. J. Am. Soc. Nephrol. 2019;14:557–566. doi: 10.2215/CJN.11470918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arepally G.M. Heparin-induced thrombocytopenia. Blood. 2017;129:2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moxon C.A., Wassmer S.C., Milner D.A., Jr., Chisala N.V., Taylor T.E., Seydel K.B., et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood. 2013;122:842–851. doi: 10.1182/blood-2013-03-490219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cognasse F., Laradi S., Berthelot P., Bourlet T., Marotte H., Mismetti P., et al. Platelet Inflammatory Response to Stress. Front. Immunol. 2019;10:1478. doi: 10.3389/fimmu.2019.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hamzeh-Cognasse H., Damien P., Chabert A., Pozzetto B., Cognasse F., Garraud O. Platelets and infections - complex interactions with bacteria. Front. Immunol. 2015;6:82. doi: 10.3389/fimmu.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Harrison P., Mackie I., Mumford A., Briggs C., Liesner R., Winter M., et al. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br. J. Haematol. 2011;155:30–44. doi: 10.1111/j.1365-2141.2011.08793.x. [DOI] [PubMed] [Google Scholar]
- 212.Wan J., Konings J., Yan Q., Kelchtermans H., Kremers R., de Laat B., et al. A novel assay for studying the involvement of blood cells in whole blood thrombin generation. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Levi M. Disseminated Intravascular Coagulation in Cancer: An Update. Semin. Thromb. Hemost. 2019;45:342–347. doi: 10.1055/s-0039-1687890. [DOI] [PubMed] [Google Scholar]
- 214.Mitrugno A., Tassi Yunga S., Sylman J.L., Zilberman-Rudenko J., Shirai T., Hebert J.F., et al. The role of coagulation and platelets in colon cancer-associated thrombosis. Am. J. Phys. Cell Phys. 2019;316:C264–c73. doi: 10.1152/ajpcell.00367.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Esmon C.T. Inflammation and thrombosis. J. Thromb. Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 216.Paniccia R., Priora R., Liotta A.A., Abbate R. Platelet function tests: a comparative review. Vasc. Health Risk Manag. 2015;11:133–148. doi: 10.2147/VHRM.S44469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lancé M.D. A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thromb. J. 2015;13:1. doi: 10.1186/1477-9560-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ninivaggi M., Apitz-Castro R., Dargaud Y., de Laat B., Hemker H.C., Lindhout T. Whole-blood thrombin generation monitored with a calibrated automated thrombogram-based assay. Clin. Chem. 2012;58:1252–1259. doi: 10.1373/clinchem.2012.184077. [DOI] [PubMed] [Google Scholar]