Abstract
Coronavirus disease 2019 (COVID-19) is a viral pandemic precipitated by the severe acute respiratory syndrome coronavirus 2. Since previous reports suggested that viral entry into cells may involve angiotensin converting enzyme 2, there has been growing concern that angiotensin converting enzyme inhibitor (ACEI) and angiotensin II receptor blocker (ARB) use may exacerbate the disease severity. In this retrospective, single-center US study of adult patients diagnosed with COVID-19, we evaluated the association of ACEI/ARB use with hospital admission. Secondary outcomes included: ICU admission, mechanical ventilation, length of hospital stay, use of inotropes, and all-cause mortality. Propensity score matching was performed to account for potential confounders. Among 590 unmatched patients diagnosed with COVID-19, 78 patients were receiving ACEI/ARB (median age 63 years and 59.7% male) and 512 patients were non-users (median age 42 years and 47.1% male). In the propensity matched population, multivariate logistic regression analysis adjusting for age, gender and comorbidities demonstrated that ACEI/ARB use was not associated with hospital admission (OR 1.2, 95%CI 0.5 to 2.7, p = 0.652). CAD and CKD/end stage renal disease [ESRD] remained independently associated with admission to hospital. All-cause mortality, ICU stay, need for ventilation, and inotrope use was not significantly different between the 2 study groups. In conclusion, among patients who were diagnosed with COVID-19, ACEI/ARB use was not associated with increased risk of hospital admission.
Coronavirus disease 2019 (COVID-19) is a viral pandemic precipitated by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerging in late December 2019 from Wuhan, Hubei Province, China.1 Since then, the number of cases has exponentially increased around the globe, with over 9 million confirmed cases as of June 27, 2020.2 Early in the pandemic, it was postulated that the use of renin-angiotensin-aldosterone-system (RAS) antagonists may independently affect health outcomes in patients with COVID-19. This hypothesis originates from the intricate interplay between the SARS-CoV-2 and RAS system; membrane bound angiotensin-converting enzyme 2 (ACE2) has been suggested to play an important role for SARS-CoV-2 entry into human cells. However, direct evidence for infection of cardiac tissue by SARS-CoV-2 and expression levels of ACE2 in different cardiac cell types remain unknown. Furthermore, patients with comorbidities including hypertension, diabetes mellitus, and chronic kidney disease (CKD) have higher circulating ACE2 expression, leading to a potentially additive effect with ACEI/ARB use.3 Yet some experts contend that ACEIs/ARBs may be beneficial in these patients4 —the main substrate of ACE2 is angiotensin II, converting it to angiotensin 1-7 which causes vasodilation and hypotension. There remains a lack of clinical data regarding association of ACEI/ARB use and outcomes in humans infected with SARS-CoV-2.
Given its prevalent usage in the United States coupled with the growing concern that ACEI/ARB use may exacerbate viral disease, there is an urgent need to address the inquiry about continuing ACEI/ARB in patients diagnosed with COVID-19. In the current report, we present a retrospective cohort study evaluating the association of ACEI/ARB use with hospital admission in patients with COVID-19 in the United States.
Methods
This is a retrospective cohort study of all consecutive adult patients (≥18 years) who presented to the outpatient, emergency room, or inpatient setting and were diagnosed with COVID-19 between March 1st, 2020 and April 15th, 2020 at the University of California, Los Angeles (UCLA) Health System. The UCLA Health Care System includes 2 academic medical centers (UCLA Ronald Reagan and UCLA Santa Monica) and multiple ambulatory outpatient sites dispersed throughout Los Angeles County. COVID-19 was diagnosed based on a positive reverse transcription-polymerase chain reaction of a nasopharyngeal swab or a bronchoalveolar lavage. This study was exempt from patient informed consent under oversight by the institutional review board of the University of California, Los Angeles.
Data including patient demographics, clinical presentation, laboratory values, past medical history, and outpatient medications were collected from the electronic medical record. Patient demographic information included age, gender, race, and body mass index. The patient's clinical presentation included symptoms (subjective fevers, chills, night sweats, cough, sore throat, congestion, dyspnea, chest pain, myalgia, malaise, headaches, abdominal pain, diarrhea, nausea/vomiting, loss of smell/taste, decrease appetite, dizziness, syncope, altered mental status), approximate start of symptoms onset and COVID-19 testing date were collected. Comorbidities of interest included history of cardiovascular disease and associated risk-factors (hypertension, diabetes, obesity, prior/present smoking, coronary artery disease [CAD], myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, cardiomyopathy, congestive heart failure [CHF], valvular disease, valvular intervention, atrial fibrillation and other arrhythmias including cardiac arrest, heart block, and cardiac device implantation, cerebrovascular accident [CVA], peripheral artery disease), hypothyroidism, reactive airway disease, chronic lung disease, sleep apnea, CKD and ESRD, organ transplant, deep venous or pulmonary thromboembolism, liver disease, malignancy, gastrointestinal bleeding, inflammatory bowel disease, hematologic disorders, rheumatologic disorders, prior surgeries, and depression, which were abstracted from the electronic medical record. Use of outpatient medications, specifically cardiovascular medications which included ACEIs, ARBs, beta blockers, calcium channel blockers, diuretics, statins, anti-platelet therapy, diabetic and immunosuppressive medications were collected from electronic medical records. For those patients that were hospitalized serial cardiovascular (troponin and brain natriuretic peptide) and inflammatory markers (D-Dimer, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], and Interleukin-6, procalcitonin) were abstracted from electronic medical records.
A patient's first interaction with a healthcare system to discuss COVID-19 symptoms and testing via phone call, telemedicine visit, outpatient clinic visit, or emergency room visit was defined as the index healthcare COVID-19 contact. Patients who were on an ACEI/ARB at the time of their index healthcare COVID-19 contact were classified as ACEI/ARB group. Those patients who were not on an ACEI/ARB at the time of their index healthcare COVID-19 contact were defined as the non-ACEI/ARB group. Patients in whom ACEI/ARB was discontinued after hospitalization they were included in the ACEI/ARB group for the primary outcome analysis. CVA was defined as a prior stroke or transient ischemic attack. Chronic lung disease was defined as a diagnosis of asthma, chronic obstructive lung disease, bronchitis or emphysema. CKD was defined as estimated glomerular filtration rate of <60 and ESRD disease was defined as estimated glomerular filtration rate of <15 or treatment with dialysis.
The primary outcome was inpatient admission. Emergency room visit with subsequent discharge was not considered inpatient admission. Secondary outcomes included: need for intensive care unit (ICU) admission, need for endotracheal intubation and mechanical ventilation, length of hospital stay, all-cause mortality and cardiac and inflammatory biomarkers.
Given significant differences in age and other comorbidities between the 2 study groups propensity score-matched cohorts were created to adjust for expected confounders associated with increased likelihood of ACEI/ARB use. Propensity scores derived from logistic regression were used to match ACEI/ARB and non-ACEI/ARB using a matching caliper size of 0.2. Variables used for matching included: age, hypertension, dyslipidemia, diabetes/pre-diabetes, CAD, CHF, CVA, chronic lung disease, and CKD/ESRD. Sensitivity analysis was performed to test the robustness of model by evaluating different combination of confounding variables in the propensity regression model. Of note, propensity matching without hypertension as a covariate resulted in significant imbalance for other cardiovascular comorbidities, therefore it was included in the model as a covariate. Matching was considered robust and successful if no significant difference was observed between the 2 study groups for the covariate using non-parametric testing. Any imbalanced and clinically important variables were further adjusted in the logistic regression model.
Continuous variables were expressed as median and interquartile range (IQR), and categorical variables were expressed as number and percentage (%). Statistical differences between the 2 groups were analyzed using the Mann-Whitney U test for continuous variables, while categorical variables were compared using Fisher's exact test for both unmatched and matched cohorts. The risk for the primary outcome and corresponding odds ratio (OR) and 95% confidence intervals (CI) were calculated using binary logistic regression models comparing the ACEI/ARB group versus the non-ACEI/ARB group in both unmatched and matched cohorts after adjusting for significant comorbidities. Covariates in the model included gender in addition to variables used in propensity matching, age was categorized into 10-year increments in regression model. Area under curve (AUC) was calculated using receiver operating curves (ROC) to evaluate predictive value of age and number of comorbidities. A 2-side p value of less than 0.05 was considered statistically significant. Data were analyzed using SPSS Statistics (Version 25.0, IBM, Armonk) and R (Version 3.3.3, The R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall, this study included 590 COVID-19 positive patients in the unmatched cohort (median age 46 years, [IQR 33 to 60] years, 48.8% male) with 78 (13.2%) patients in the ACEI/ARB group and 512 (86.8%) patients in the non-ACEI/ARB group. The propensity score-matched cohort included 149 patients (median age 64 years, [IQR 53 to 77] years, 55.7% male) of which 78 (52.3%) patients were included in the ACEI/ARB group and 71 (47.7%) in the non-ACEI/ARB group. The characteristics of the 2 study groups at the time of index healthcare COVID-19 contact are listed in Table 1 . After propensity score matching, no significant difference in baseline demographics and comorbidities were present between the 2 study groups (Table 1). Symptoms associated with being COVID-19 positive were overall similar between the 2 study groups. However, before propensity matching headaches were observed significantly more in those in the non-ACEI/ARB group as compared to those in the ACEI/ARB group (p = 0.01), but this association lost statistical significance after propensity matching (p = 0.15, Table 2 ).
Table 1.
Baseline characteristics of patients in ACEI/ARB and non-ACEI/ARB groups before and after propensity score matching
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| ACEI/ARB | Non-ACEI/ARB | p value | ACEI/ARB | Non-ACEI/ARB | p value | |
| n = 78 | n = 512 | n = 78 | n = 71 | |||
| Variables | ||||||
| Age (years), median (IQR) | 63 (55-75) | 42 (32-55) | <0.001 | 65 (55-75) | 64 (51-77) | 0.658 |
| Age >60 years | 51 (66.2%) | 99 (19.3%) | <0.001 | 77 (98.7%) | 68 (95.8%) | 0.348 |
| Men | 46 (59.7%) | 242 (47.1%) | 0.051 | 47 (60.3%) | 36 (50.7%) | 0.253 |
| Number of comorbidities, median (IQR) | 3 (3-4) | 1 (0-2) | <0.001 | 3 (3-4) | 3 (2-4) | 0.041 |
| Coronary Artery Disease | 15 (19.5%) | 16 (3.2%) | <0.001 | 16 (20.5%) | 10 (14.1%) | 0.388 |
| Myocardial infarction | 7 (9.1%) | 5 (1.1%) | <0.001 | 8 (10.3%) | 3 (4.3%) | 0.217 |
| Percutaneous Coronary Intervention/Coronary Artery Bypass Grafting | 13 (17.9%) | 11 (2.2%) | <0.001 | 13 (14.8%) | 7 (9.9%) | 0.472 |
| Peripheral Arterial Disease | 5 (8.3%) | 6 (1.4%) | 0.005 | 6 (7.7%) | 4 (5.6%) | 0.748 |
| Stroke/Transient Ischemic Attack | 9 (15%) | 10 (2.2%) | <0.001 | 9 (11.7%) | 7 (9.9%) | 0.795 |
| Cardiomyopathy | 8 (10.5%) | 9 (1.8%) | <0.001 | 9 (11.7%) | 4 (5.7%) | 0.203 |
| Heart failure | 7 (15.6%) | 14 (4.3%) | 0.008 | 10 (12.8%) | 8 (11.3%) | 0.807 |
| Heart failure with reduced ejection fraction | 3 (9.1%) | 5 (2.2%) | 0.064 | 4 (11.8%) | 3 (7.3%) | 0.695 |
| Left ventricle ejection fraction, median (IQR) | 63 (58-68) | 63 (58-65) | 0.704 | 63 (54-67) | 65 (58-66) | 0.878 |
| Atrial Fibrillation | 8 (12.7%) | 15 (3.2%) | <0.001 | 8 (12.5%) | 9 (12.9%) | 0.979 |
| Other atrial/ventricular arrhythmias | 3 (5.1%) | 12 (2.6%) | 0.215 | 3 (4.8%) | 6 (8.6%) | 0.498 |
| Permanent pacemaker or Implantable Cardioverter Defibrillator | 5 (8.3%) | 2 (0.5%) | <0.001 | 5 (8.2%) | 2 (3.1%) | 0.262 |
| Hypertension | 73 (96.1%) | 77 (15.7%) | <0.001 | 74 (96.1%) | 63 (88.7%) | 0.119 |
| Dyslipidemia | 51 (67.1%) | 145 (29.7%) | <0.001 | 66 (84.6%) | 56 (78.9%) | 0.472 |
| Diabetes/Pre-diabetes | 41 (53.9%) | 114 (23.2%) | <0.001 | 41 (52.6%) | 40 (56.3%) | 0.742 |
| Smoking, current or past | 11 (15.9%) | 64 (13.4%) | 0.363 | 13 (14.8%) | 18 (25.4%) | 0.112 |
| Obesity | 26 (36.6%) | 88 (18.3%) | 0.001 | 30 (34.1%) | 19 (26.8%) | 0.388 |
| Hypothyroidism | 16 (21.1%) | 33 (6.8%) | <0.001 | 17 (22.1%) | 11 (15.7%) | 0.402 |
| Chronic lung disease | 15 (19.7%) | 50 (10.3%) | 0.011 | 15 (19.5%) | 13 (18.3%) | 0.732 |
| Obstructive Sleep Apnea | 11 (17.5%) | 19 (4.1%) | <0.001 | 11 (17.2%) | 6 (8.6%) | 0.134 |
| Chronic Kidney Disease/End Stage Renal Disease | 14 (18.4%) | 25 (5.1%) | <0.001 | 15 (19.5%) | 14 (19.7%) | 0.991 |
| Organ Transplantation | 5 (6.8%) | 9 (1.9%) | 0.028 | 5 (6.7%) | 4 (5.8%) | 0.994 |
| Deep Vein or Pulmonary Thromboembolism | 4 (6.2%) | 15 (3.3%) | 0.11 | 4 (6.2%) | 6 (8.8%) | 0.997 |
| Cancer | 15 (19.7%) | 22 (4.5%) | <0.001 | 15 (19.5%) | 6 (8.5%) | 0.063 |
| Active Cancer | 2 (2.8%) | 5 (1.1%) | 0.215 | 2 (2.9%) | 1 (1.6%) | 0.972 |
| Immunologic diseases | 2 (3.3%) | 16 (3.5%) | 0.978 | 2 (3.2%) | 4 (6.1%) | 0.681 |
| Hematologic diseases | 7 (11.3%) | 14 (3.1%) | 0.007 | 7 (11.1%) | 6 (8.8%) | 0.773 |
| Rheumatologic diseases | 7 (11.5%) | 17 (3.7%) | 0.015 | 7 (11.3%) | 4 (5.8%) | 0.348 |
IQR = interquartile range.
Table 2.
Symptoms reported in ACEI/ARB and non-ACEI/ARB groups before and after propensity score matching
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| ACEI/ARB | Non-ACEI/ARB | p value | ACEI/ARB | Non-ACEI/ARB | p value | |
| n = 78 | n = 512 | n = 78 | n = 71 | |||
| Symptoms | ||||||
| Fever | 39 (62.9%) | 310 (67.1%) | 0.567 | 39 (61.9%) | 44 (67.7%) | 0.493 |
| Cough | 47 (77.1%) | 364 (79.1%) | 0.708 | 47 (75.8%) | 48 (76.2%) | 0.961 |
| Sore throat | 10 (16.1%) | 124 (27.4%) | 0.064 | 10 (16.1%) | 12 (21.1%) | 0.645 |
| Congestion/Rhinorrhea | 16 (28.1%) | 160 (35.8%) | 0.302 | 16 (28.1%) | 17 (29.9%) | 0.841 |
| Dyspnea | 28 (44.4%) | 153 (33.4%) | 0.091 | 28 (43.8%) | 16 (25.4%) | 0.041 |
| Chest Pain | 6 (10.1%) | 74 (16.2%) | 0.257 | 6 (9.8%) | 10 (16.1%) | 0.422 |
| Dizziness | 5 (9.1%) | 24 (5.6%) | 0.357 | 5 (8.9%) | 2 (3.3%) | 0.261 |
| Syncope | 2 (3.6%) | 6 (1.4%) | 0.225 | 3 (5.4%) | 4 (6.7%) | 0.982 |
| Myalgias | 28 (45.2%) | 219 (47.9%) | 0.787 | 28 (44.4%) | 28 (45.2%) | 0.936 |
| Fatigue | 21 (36.8%) | 164 (36.3%) | 0.934 | 21 (36.8%) | 19 (31.1%) | 0.563 |
| Headache | 7 (12.3%) | 126 (28.1%) | 0.011 | 7 (12.3%) | 14 (23.3%) | 0.151 |
| Gastrointestinal symptoms | 26 (42.6%) | 179 (39.3%) | 0.613 | 26 (42.6%) | 20 (32.3%) | 0.267 |
| Altered mental status | 4 (8.2%) | 11 (2.9%) | 0.081 | 5 (10.1%) | 10 (18.9%) | 0.267 |
In the propensity matched cohort, use of cardiovascular and diabetes medications was not different between the 2 groups except significantly higher use of diuretics, statins and immunosuppressive medications in the ACEI/ARB group as compared to the non-ACEI/ARB group (p <0.05) (Table 3 ). In the unmatched cohort, there was no significant difference in cardiac biomarkers or inflammatory biomarkers between the ACEI/ARB and non-ACEI/ARB groups (Table 4 ). After propensity matching, those in the ACEI/ARB group were found to have significantly lower median peak inflammatory markers of ESR, procalcitonin, and fibrinogen as compared to the non-ACEI/ARB group (Table 4).
Table 3.
Outpatient medication use amongst ACEI/ARB and non-ACEI/ARB groups before and after propensity score matching
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| ACEI/ARB | Non-ACEI/ARB | p value | ACEI/ARB | Non-ACEI/ARB | p value | |
| n = 78 | n = 512 | n = 78 | n = 71 | |||
| Medications | ||||||
| Beta Blockers | 24 (31.2%) | 30 (6.2%) | <0.001 | 24 (31.2%) | 17 (23.9%) | 0.362 |
| Calcium Channel Blockers | 20 (25.6%) | 33 (6.8%) | <0.001 | 20 (25.6%) | 24 (33.8%) | 0.287 |
| Diuretics | 29 (37.2%) | 18 (3.7%) | <0.001 | 29 (37.2%) | 12 (16.9%) | 0.006 |
| Spironolactone | 1 (1.6%) | 8 (1.7%) | 0.999 | 1 (1.6%) | 1 (1.4%) | 0.987 |
| Vasodilators | 3 (3.8%) | 7 (1.4%) | 0.148 | 3 (3.8%) | 4 (5.6%) | 0.709 |
| Immunosuppressive Treatment | 15 (19.3%) | 26 (5.3%) | <0.001 | 17 (19.3%) | 4 (5.6%) | 0.017 |
| Statin Therapy | 48 (62.3%) | 53 (11.1%) | <0.001 | 48 (62.3%) | 28 (40.3%) | 0.008 |
| Aspirin | 27 (42.9%) | 31 (6.6%) | <0.001 | 30 (34.5%) | 16 (22.5%) | 0.115 |
| P2Y12 receptor blockers | 5 (8.2%) | 5 (1.1%) | 0.003 | 5 (8.2%) | 5 (7.4%) | 0.991 |
| Direct Oral Anticoagulants | 6 (10.2%) | 12 (2.8%) | 0.014 | 6 (10.2%) | 6 (9.8%) | 0.945 |
| Diabetes medications | 23 (30.3%) | 27 (5.6%) | <0.001 | 27 (31.4%) | 14 (20.2%) | 0.143 |
Table 4.
In-hospital labs upon admission amongst ACEI/ARB and non-ACEI/ARB groups before and after propensity score matching
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| ACEI/ARB | Non-ACEI/ARB | p value | ACEI/ARB | Non-ACEI/ARB | p value | |
| n = 35 | n = 87 | n = 35 | n = 28 | |||
| In-Hospital Labs, median (IQR) | ||||||
| Peak Troponin | 0.04 (0.04-0.04) | 0.04 (0.04-0.04) | 0.562 | 0.04 (0.04-0.06) | 0.04 (0.04-0.09) | 0.184 |
| Brain Natriuretic Peptide | 76.3 (42.5-262) | 69.1 (29.7-246) | 0.449 | 76.2 (42.5-357) | 213 (74.3-387) | 0.123 |
| Procalcitonin | 0.11 (0.10-0.35) | 0.14 (0.10-0.76) | 0.235 | 0.11 (0.10-0.42) | 0.41 (0.10-3.19) | 0.02 |
| D-Dimer | 1492 (882-3201) | 1459 (710-3319) | 0.954 | 1515 (912-3537) | 2285 (889-4242) | 0.372 |
| Erythrocyte Sedimentation Rate | 35.5 (22.8-62.3) | 67.1 (40.2-103) | 0.098 | 35.5 (22.8-62.3) | 81 (55.3-105) | 0.041 |
| C-Reactive Protein | 7.81 (3.18-14.9) | 9.9 (2.9-17.7) | 0.302 | 7.8 (3.18-14.93) | 11.1 (4.7-18.6) | 0.235 |
| Interleukin-6 | 13.1 (5.5-24.4) | 19.3 (7.1-43.2) | 0.219 | 13.2 (5.54-24.1) | 28.5 (13.8-81.3) | 0.061 |
| Fibrinogen | 476 (415-476) | 680 (645-795) | 0.311 | 497 (415-497) | 673 (645-707) | 0.046 |
A total of 122 (20.6%) patients were hospitalized during the study period (Table 5 ). In the unmatched cohort, a higher proportion of patients who were receiving ACEI/ARB medications in the outpatient setting were admitted as compared to those not taking either of these classes of medications (44.9% vs 17.4%; p <0.001). In the adjusted matched cohort, there was no significant difference in the admission rate for those in the ACEI/ARB group versus the non-ACEI/ARB group (44.9% vs 39.4%; p = 0.512) (Table 5). Similarly, in the propensity matched cohort, there was no statistically significant difference in the incidence of ICU admission, endotracheal intubation, hospital length of stay, inotrope use, and all-cause mortality for the ACEI/ARB versus non-ACEI/ARB group, respectively (Table 5).
Table 5.
Outcomes amongst ACEI/ARB and non-ACEI/ARB groups before and after propensity score matching
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| ACEI/ARB | Non-ACEI/ARB | p value | ACEI/ARB | Non-ACEI/ARB | p value | |
| n = 78 | n = 512 | n = 78 | n = 71 | |||
| Primary Outcome | ||||||
| Admission to hospital | 35 (44.9%) | 87 (17.3%) | <0.001 | 35 (44.9%) | 28 (39.4%) | 0.512 |
| Secondary Outcomes | ||||||
| Intensive Care Unit Admission | 13 (16.7%) | 32 (6.2%) | 0.028 | 13 (16.7%) | 13 (18.3%) | 0.831 |
| Mechanical Ventilation | 7 (9.1%) | 22 (4.3%) | 0.057 | 6 (7.7%) | 10 (14.1%) | 0.291 |
| Length of Hospital Stay, median (IQR) | 8 (4-14) | 6 (3-12) | 0.178 | 7.2 (4-14) | 6.5 (3-14) | 0.351 |
| Inotrope use | 2 (2.6%) | 14 (2.7%) | 0.844 | 2 (2.8%) | 6 (8.5%) | 0.152 |
| All-cause mortality | 1 (1.3%) | 5 (1.2%) | 0.707 | 1 (1.5%) | 3 (4.8%) | 0.357 |
IQR = interquartile range.
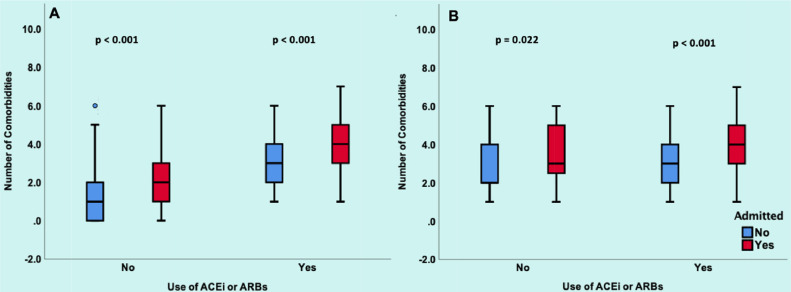
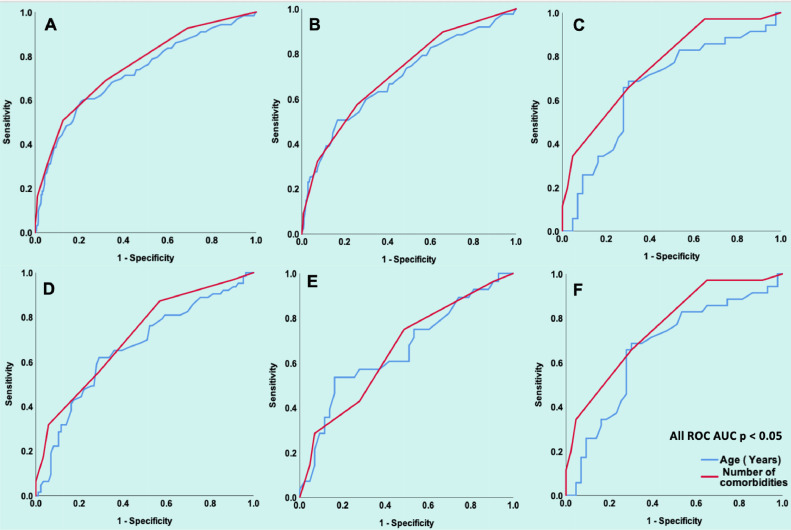
The number of comorbidities was significantly higher in patients who were admitted versus those who were not admitted both in unmatched (median 3, IQR [1 to 4] vs median 1, IQR [0 to 2]; p <0.001) and matched (median 4, IQR [3 to 5] vs median 3, IQR [2 to 4]; p <0.001) study cohorts and this remained significant on stratification by use of ACEI/ARB (Figure 1 ). ROCs showed that AUC for age and number of comorbidities was predictive of admission to hospital in both unmatched and matched study cohorts in all-comers and on stratification by ACEI/ARB use (all p <0.05) (Figure 2 ).
Figure 1.
Association of ACEI/ARB use with number of comorbidities.
Panel A shows the unmatched population. For patients not on ACEI/ARB, the number of comorbidities was higher in admitted patients (median 2, IQR 1 to 3) compared to those not admitted (median 1, IQR 0 to 2, p <0.001). For patients on ACEI/ARB, the number of comorbidities was higher in admitted patients (median 4, IQR 3 to 5) compared to those
who were not admitted (median 3, IQR 2 to 4, p <0.001). Panel B shows the matched population. For patients not on ACEI/ARB, number of comorbidities was higher in admitted patients (median 3, IQR 2.25 to 5) compared to those not admitted (median 2 IQR 2 to 4, p <0.022). For patients on ACEI/ARB, number of comorbidities was higher in admitted patients (median 4, IQR 3 to 5) compared to those not admitted (median 3, IQR 2 to 4, p <0.001). In both the matched and unmatched cohort, ACEI/ARB use was associated with increased number of comorbidities compared to nonuse in both hospitalized and nonhospitalized patients.
IQR = interquartile range.
Figure 2.
Age and Number of Comorbidities in Relation to Hospital Admission. Panel A shows ROC for age (AUC 0.72, 95%CI: 0.67 to 0.78, p <0.001) and number of comorbidities (AUC 0.75, 95%CI: 0.70 to 0.80, p <0.001) in all unmatched patients (n = 590). Panel B shows ROC for age (AUC 0.69, 95%CI: 0.63 to 0.76, p <0.001) and number of comorbidities (AUC 0.72, 95%CI: 0.66 to 0.78, p <0.001) in all unmatched patients not on an ACEI/ARB (n = 590). Panel C shows the ROC for age (AUC 0.66, 95%CI: 0.54 to 0.79, p = 0.013) and number of comorbidities (AUC 0.76, 95%CI: 0.66 to 0.87, p = 0.13) in all unmatched patients on a ACEI/ARB (n = 78).
Panel D shows the ROC for age (AUC 0.66, 95%CI: 0.57 to 0.75, p = 0.001) and number of comorbidities (AUC 0.71, 95%CI: 0.63 to 0.80, p < 0.001) in all matched patients (n = 149). Panel E shows the ROC for age (AUC 0.66, 95%CI: 0.53 to 0.79, p = 0.024) and number of comorbidities (AUC 0.66, 95%CI: 0.53 to 0.79, p = 0.027 in all matched patients not on an ACEI/ARB (n = 71). Panel F shows the ROC for age (AUC 0.66, 95%CI: 0.54 to 0.79, p = 0.013) and number of comorbidities (AUC 0.76, 95%CI: 0.66 to 0.87, p <0.001) in all matched patients on an ACEI/ARB (n = 78). AUC = area under the curve; CI = confidence interval; ROC = receiver operating curve.
In the multivariate logistic regression analysis of the unmatched cohort, use of ACEI/ARB in COVID-19 positive patients was not associated with hospital admission (OR 1.2; 95%CI 0.5 to 2.6, p = 0.677) when compared to those not on an ACEI/ARB (Table 6 ). Significant factors associated with admission to hospital in this multivariate unmatched model, included: age in 10-year increments (OR 1.3, 95%CI 1.1 to 1.5, p = 0.001), male gender (OR 2.1, 95%CI 1.3 to 3.4, p = 0.003), history of CAD (OR 3.2, 95%CI 1.2 to 9.1, p = 0.024), chronic lung disease (OR 2.2, 95%CI 1.2 to 4.3, p = 0.017), and CKD/ESRD (OR 6.2, 95%CI 2.5 to 15.5, p <0.001) (Table 6).
Table 6.
Multivariate logistic regression model for various predictors of hospital admission in matched and unmatched cohorts
| Unmatched (n = 559) |
Matched (n = 145) |
|||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | OR | 95%CI | p value | |
| Predictors | ||||||
| ACEI/ARB use | 1.2 | 0.5-2.6 | 0.677 | 1.2 | 0.5-2.7 | 0.652 |
| Age in 10-year increments | 1.3 | 1.1-1.5 | 0.001 | 1.2 | 0.9-1.5 | 0.203 |
| Male gender | 2.1 | 1.3-3.4 | 0.003 | 1.4 | 0.6-3.2 | 0.376 |
| Hypertension | 1.4 | 0.7-2.9 | 0.322 | 0.6 | 0.1-2.8 | 0.529 |
| Dyslipidemia | 0.6 | 0.3-1.1 | 0.113 | 0.9 | 0.3-2.5 | 0.783 |
| Diabetes/Pre-diabetes | 1.5 | 0.8-2.5 | 0.184 | 1.2 | 0.5-3.1 | 0.676 |
| Coronary artery disease | 3.2 | 1.2-9.1 | 0.024 | 4.1 | 1.2-13.2 | 0.022 |
| Congestive heart failure | 1.5 | 0.5-4.4 | 0.505 | 1.4 | 0.4-5.3 | 0.617 |
| Stroke/Transient Ischemic Attack | 1.9 | 0.6-6.4 | 0.302 | 1.9 | 0.5-7.2 | 0.339 |
| Chronic lung disease | 2.2 | 1.2-4.3 | 0.017 | 1.9 | 0.7-5.2 | 0.191 |
| Chronic Kidney Disease/End Stage Renal Disease | 6.2 | 2.5-15.5 | <0.001 | 5.6 | 1.8-17.2 | 0.002 |
CI = confidence interval, OR = odds ratio.
In the multivariate logistic regression analysis of the propensity matched cohort, use of ACEI/ARB in COVID-19 positive patients was also not associated with hospital admission (OR 1.2; 95%CI 0.5 to 2.7, p = 0.652) when compared to those not on an ACEI/ARB (Table 6). Significant factors independently associated with admission to hospital in this matched model included: CAD (OR 4.1, 95%CI 1.2 to 13.2, p = 0.022) and CKD/ESRD (OR 5.6, 95%CI 1.8 to 17.2, p = 0.002) (Table 6).
Discussion
In this single-center, retrospective, US based cohort study of 590 consecutive patients with confirmed diagnosis of COVID-19, baseline use of ACEI/ARB was not associated with increased risk of hospital admission. Two recently published studies from China evaluated the association of ACEI/ARB with all-cause in-hospital mortality in patients with COVID-19.5 , 6 Our study evaluated the impact of outpatient ACEI/ARB use on outcomes in patients who tested positive for SARS-CoV-2. Our study adds to the growing COVID-19 literature, and further strengthens the recommendations put forth by professional societies, including American College of Cardiology, American Heart Association, and Heart Failure Society of America to continue ACEI/ARBs among patients with co-existing hypertension and COVID-197.
Hypertension and use of ACEI/ARB is more common in patients who are older and have other major CV comorbidities including CAD, MI, CHF, CVA and CKD, therefore, these key confounders must be accounted for in the studies evaluating role of ACEI/ARB on outcomes.6 , 8 In our study, we found that ACEI/ARB users had more comorbidities than non-ACEI/ARB users. This relationship of increased number of comorbidities with ACEI/ARB use was also seen when stratified by primary outcome of in-hospital admission (Figure 1). After our study was propensity matched for known covariates of poor outcomes in COVID-19,5 , 6 we found no differences in the rate of hospital admissions in ACEI/ARB users versus nonusers. There were also no differences between groups in secondary outcomes, focusing on clinical markers of inpatient prognosis, such as ICU admission, length of hospital stay, mechanical ventilation, inotrope use and all-cause mortality. However; we did find that CAD and CKD/ESRD remained significant independent determinants of admission to hospital in the multivariate analysis.
Interestingly, after propensity matching, median peak inflammatory markers, including ESR, CRP, Interleukin-6, and D-dimer laboratory values were higher in the non-ACEI/ARB group. This finding is consistent with recently published data by Zhang et al.5 These results may partially be explained by the intricate interaction of ACEI/ARB with the RAS system. ACEI/ARB upregulates membrane-bound ACE2, which in turn converts angiotensin II to angiotensin-(1 to 7), attenuating the vasoconstrictive and inflammatory effects of angiotensin II.9 Studies investigating ACEI/ARB use have shown an association with reduction of various inflammatory markers including fibrinogen, D-dimer and CRP.10 Therefore, ACEI/ARB use during COVID-19 infection may potentially lead to lower levels although in our study they did not translate into improved clinical outcomes.
Our study adds to the growing body of evidence that in patients with COVID-19 there is no adverse association of ACEI/ARB use with clinical outcomes.5 , 6 Zhang et al showed a decreased mortality in patients on ACEI/ARB, while the study by Li et al showed no significant difference in mortality in patients with and without ACE or ARB use. The theoretical concerns of ACEI/ARB facilitating SARS-CoV-2 entry into human cells by way of increased ACE2 expression, have not been confirmed in any clinical studies. While future prospective and randomized-controlled trials are needed, this study demonstrates that continuation of ACEI/ARB therapy appears to be safe and warranted. This could be due to downregulation of ACE2 in cardiac tissue as suggested by Nicin et al or due to genetic variance and polymorphism of ACE2 in humans.11 Additionally, discontinuing ACEI/ARB therapy has potential downstream effects such as clinical deterioration in patients with CHF, myocardial infarction and rebound hypertension.
This study has several limitations. First, this cohort was obtained from a single University Health System. While the study does encompass a heterogeneous population, further geographically and racially diverse studies to provide greater generalizability. Second, our sample size was modest, but the findings of our study are consistent with those from other studies evaluating inpatients with COVID-19 and include data verified by physician review. The study sample is not large enough to evaluate differential effects between ACEI/ARBs, varying indications for these medications, such as heart failure with reduced ejection fraction or hypertension or the role of race. Finally, the study was a retrospective analysis and thus prone to inherit biases such as unmeasured confounding between the 2 study groups. Although, we performed propensity matching to account for these disparities, further large-scale prospective and randomized controlled are needed to better understand the efficacy of ACEI/ARB use in patients with COVID-19.
Among patients who were diagnosed with COVID-19, ACEI/ARB use was not associated with increased risk of hospital admission. Cardiovascular comorbidities are major determinants of admission to hospital in patients with COVID-19. While future prospective and randomized-controlled trials are needed, this study adds to a growing body of evidence that continuation of ACEI/ARB therapy, appears to be safe and further supports current societal recommendations by American College of Cardiology/American Heart Association/and Heart Failure Society of America to continue ACEI/ARB therapy in a heterogenous COVID-19 patient population.
Authors’ Contribution
David Bae: Conceptualization, Methodology, Investigation, Data Curation, Writing – Original draft preparation, Visualization. David Tehrani: Conceptualization, Methodology, Investigation, Data Curation, Writing – Original draft preparation, Visualization. Soniya Rabadia: Conceptualization, Methodology, Investigation, Data Curation, Writing – Review & Editing, Visualization. Marlene Frost: Writing – Review & Editing. Rushi Parikh: Writing – Review & Editing. Marcella Calfon-Press: Writing – Review & Editing. Olcay Aksoy: Writing – Review & Editing. Soban Umar: Writing – Review & Editing. Reza Ardehali: Writing – Review & Editing. Amir Rabbani: Writing – Review & Editing. Pooya Bokhoor: Writing – Review & Editing. Ali Nsair: Writing – Review & Editing. Jesse Currier: Writing – Review & Editing. Jonathan Tobis: Writing – Review & Editing. Gregg Fonarow: Writing – Review & Editing. Ravi Dave: Writing – Review & Editing. Asim Rafique: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing – Review & Editing, Visualization, Supervision.
Acknowledgments
None.
Disclosures
Dr. Fonarow discloses consulting for Abbott, Amgen, AstraZeneca, Bayer, Janssen, Merck, and Novartis. The remaining authors report that they have no relationships with industry relevant to the contents of this manuscript to disclose.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Funding: None.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2020.07.007.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao G, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;S1473-3099:30120–30121. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anguiano L, Riera M, Pascual J, Valdivielso J, Barrios C, Betriu A, Mojal S, Fernandez E, Soler M. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant. 2015;30:1176–1185. doi: 10.1093/ndt/gfv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang P, Zhu L, Cai J, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, Shen ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized With COVID-19. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317134. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiology. 2020 doi: 10.1001/jamacardio.2020.1624. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. Available at:https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Accessed April 18, 2020. [DOI] [PMC free article] [PubMed]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotension-(1-7) in essential hypertension. J Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Ceconi C, Fox KM, Remme WJ, Simoons ML, Deckers JW, Bertrand M, Parrinello G, Kluft C, Blann A, Cokkinos D, Ferrari R. ACE inhibition with perindopril and biomarkers of atherosclerosis and thrombosis: results from the PERTINENT study. Atherosclerosis. 2009;204:273–275. doi: 10.1016/j.atherosclerosis.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41:1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.