Abstract
The clinical utility of systematic prostate biopsy in addition to multi-parametric magnetic resonance imagining (mp-MRI) targeted biopsy pathways remains unclear. Despite radiological advancements in mp-MRI and utilisation of international standardised reporting systems (i.e., PI-RADS, LIKERT), undetected clinically significant prostate cancer (csPCa) on imaging persists. This has prevented the widespread adoption of an exclusively targeted biopsy approach. The current evidence on csPCa cancer detection rates in mp-MRI targeted alone and combined with a non-targeted systematic sampling is presented. Arguments for and against routine limited systematic sampling as an adjunct to an mp-MRI targeted biopsy are discussed. Our review will report the clinical utility of a combined sampling strategy on csPCa detection rate. The available evidence suggests that we are yet to reach a stage where non-targeted systematic prostate biopsy can be routinely omitted in mp-MRI targeted prostate biopsy pathways. Research should focus on improving the accuracy of mp-MRI, prostate biopsy techniques, and in identifying those men that will most benefit from a combined prostate biopsy. Such strategies may help future urologists reduce the burden of non-targeted cores in modern mp-MRI prostate biopsy pathways.
Keywords: Prostate cancer, prostate neoplasm, biopsy, magnetic resonance imagining (MRI), grading
Introduction
Conventional transrectal ultrasound guided (TRUS) biopsy for prostate cancer, without a prior multi-parametric magnetic resonance imagining (mp-MRI), has been associated with the underdetection of clinically significant prostate cancer (csPCa) (1,2). The PROMIS trial demonstrated the limited accuracy of standard TRUS biopsy, and validated the benefits of pre-biopsy mp-MRI (1). This has resulted in a recent shift in diagnostic pathway design to incorporate pre-biopsy mp-MRI followed by a targeted biopsy approach as standard of care (3,4).
The case for mp-MRI targeted prostate biopsy was further strengthened by the results of the PRECISION randomised controlled study, in which targeted biopsy alone performed superiorly in the detection of csPCa compared to conventional systematic biopsy (2). In that trial, however, non-targeted systematic biopsies were not performed in the cohort of men with MRI-visible lesions. Despite improvements in mp-MRI performance and standardised radiological reporting, a significant fall in radiologically invisible csPCa, subsequently confirmed on systematic biopsy, has not occurred (5-8).
Research has now focused on the true clinical utility of an additional non-targeted systematic biopsies when performed alongside an mp-MRI targeted prostate biopsy during the same biopsy session (Figure 1). In this article we summarise the current evidence base for and against a “combined biopsy” approach (Table 1), calculating the marginal gains in cancer detection rate in each strategy.
Figure 1.
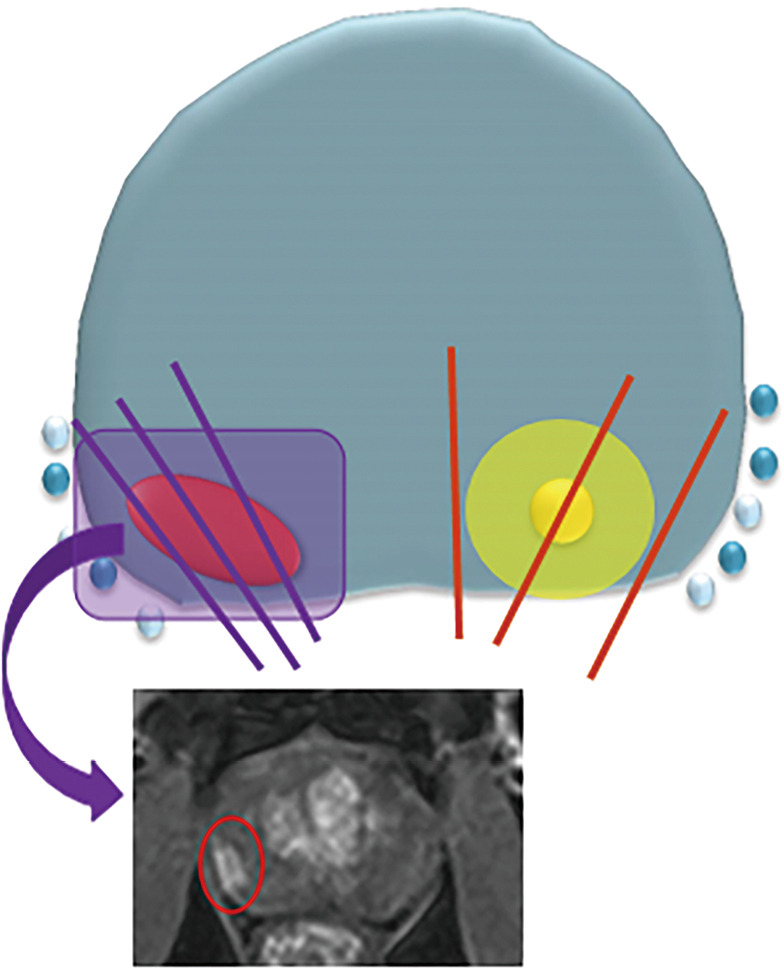
Illustration of targeted and systematic biopsy cores in relation to prior multi-parametric prostate MRI. Graphic representation of targeted cores (purple line) of prostate tumour (red oval) demonstrated on pre-biopsy MRI (red circle). Second prostate tumour (yellow circle) invisible to imaging detected on non-targeted systematic cores (red line).
Table 1. Value of systematic sampling in an mp-MRI targeted prostate biopsy strategy.
| For |
| Added yield of clinically significant cancer detection rate in non-targeted systematic biopsy |
| mp-MRI invisible prostate cancer |
| Final histopathology grade disparity |
| Against |
| Higher yield of clinically insignificant cancer |
| mp-MRI targeted biopsy alone provides a higher yield of significant and low yield of insignificant cancer |
| Increased biopsy related toxicity |
| Cost and reporting impact of additional non-targeted cores |
| Utility in men with a negative prior prostate biopsy |
Evidence against a combined mp-MRI targeted and non-targeted biopsy approach
csPCa detection rate in mp-MRI targeted prostate biopsy alone
The strongest evidence against a combined biopsy strategy is born from the results of mp-MRI targeted prostate biopsy results in the detection of csPCa. The current literature reports the detection rate of csPCa and clinically insignificant prostate cancer (ciPCa) in mp-MRI targeted biopsy as 25–62% and 5.6–23%, respectively (Table 2) (5,7-14).
Table 2. Absolute cancer detection rates of clinically significant and clinically insignificant prostate cancer in mp-MRI targeted prostate biopsies.
| Author | Year | N | Study design | Pre-biopsy MRI | Prostate biopsy approach | Form of targeted biopsy | csPCa in mp-MRI targeted biopsy, % [n] | ciPCa in mp-MRI targeted biopsy, % [n] | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Elkhoury et al. (PAIREDCAP) (9) | 2019 | 300 (248*) | Paired-control trial | mp-MRI 3T | TRPB | C or F | 62% [154] | NR | All biopsy naïve |
| Miah et al. (10) | 2019 | 640 | Prospective cohort study | mp-MRI 3 T | TPB | F | 48.4% [310] | 15.1% [91] | Prior prostate biopsy not excluded. Likert score used for biopsy decision. Targeted only in some men |
| van der Leest et al. (4M) (8) | 2019 | 626 | Prospective cohort study | mp-MRI 3T | TRPB | IB | 25% [159] | 14% [88] | All biopsy naïve |
| Mannaerts et al. (11) | 2019 | 225 | Prospective cohort study | mpMRI | TRPB | F | 44% [113] | 8% [21] | Likert score used for biopsy decision |
| Rouvière et al. (MRI-FIRST) (5) | 2019 | 335 (251*) | Randomised controlled trial | mp-MRI 3 T or 1.5T | TRPB | C | 32.3% [81] | 5.6% | Systematic biopsy in all men regardless of mp-MRI results |
| Kasivisvanathan et al. (PRECISION) (12) | 2018 | 252 | Randomised (non-inferiority) trial | mp-MRI 3 T or 1.5T | TRPB | C or F | 39% [95] | 23% [9] | mp-MRI targeted compared to systematic biopsy |
| Porpiglia et al. (7) | 2017 | 107 | Randomised controlled trial | mpMRI 1.5T | TRPB or TPB | F | 41.1% [44] | 18.5% [10] | TRPB 67.0%, TP 32.1% |
| Siddiqui et al. (13) | 2015 | 1,003 | Prospective cohort study | mpMRI | TRPB | F | 31.3% [314] | – | Excluded if no visible mp-MRI lesion |
| Filson et al. (14) | 2016 | 1042 (825*) | Prospective cohort study | mpMRI | TRPB | F | 27.7% [229] | 15.8% [131] | Prior prostate biopsy not excluded |
*, number of men who proceeded to prostate biopsy of any form. CDR, Cancer detection rate; csPCa, clinically significant prostate cancer (Defined as Gleason ≥3+4 or Gleason Grade Group 2); ciPCa, clinically insignificant prostate cancer; TRPB, transrectal prostate biopsy; TPB, tranperineal prostate biopsy; C, cognitive; F, fusion; IB, in-bore; NR, not reported.
Kasivisvanathan and colleagues’ PECISION trial demonstrated mp-MRI targeted prostate biopsy improved the cancer detection rate of Gleason ≥3+4 disease (39%), whilst reducing the detection of insignificant (Gleason 3+3) cancer (23%) (2). Further, the detection of insignificant disease was significantly higher in the systematic biopsy comparator arm (22% vs. 9%; P<0.001) (12). The MRI-FIRST randomized trial by Rouviere and colleagues confirmed similar favourable detection rates of csPCa (32.3%) and a lower rate of insignificant prostate cancer detection (5.6% vs. 19.5%; P<0.0001) in targeted cores (5). Finally, Porpiglia et al.’s randomised study, utilizing transperineal prostate biopsy, confirmed targeted biopsy had a csPCa detection rate of over 41% (7). In these studies, the higher rate of clinically insignificant disease in systematic biopsy compared to targeted biopsy is considered sufficient evidence to preclude the performance of simultaneous systematic sampling.
Beyond the randomised controlled trial setting, Miah and colleagues’ prospective cohort study of transperineal image-fusion biopsy reported similarly high rates of csPCa (48.4%) and a low rate of insignificant disease (15.1%) detection (10). Of clear methodological distinction from aforementioned studies, the non-targeted biopsy performed in this study did not anatomically overlap in areas with targeted core sampling (10). Thus, preventing the duplication of reported csPCa in systematic cores performed in known regions of interest already sampled by prior targeted cores. Furthermore, van der Leest et al.’s prospective study, in which only biopsy-naïve men underwent an in-bore mp-MRI targeted transrectal biopsy, reported a 25% csPCa and a 14% ciPCa detection rate (8). Figures from both of these studies are highly promising for the replication of such favourable targeted csPCa detection rates in routine clinical practice.
Anatomically, mp-MRI targeted biopsy is superior to systematic biopsy at detecting anterior and apical tumours (15). These are frequently missed on systematic TRUS prostate biopsy (16). It is worth noting that the current literature does not place preference on the form of targeted biopsy (i.e., targeted software fusion versus targeted cognitive fusion) when assessed in terms of csPCa detection rate (5,8,10,12,17). This is supported by the findings of high-level evidence from the FUTURE randomised controlled trial, which demonstrated no significant difference in csPCa detection when comparing targeted software fusion and targeted cognitive fusion biopsies in men with a previous negative systematic non-targeted prostate biopsy (17).
Men with a prior negative biopsy
The detection of csPCa in men with a prior negative prostate biopsy can be significantly increased with MRI-targeted prostate biopsy (16,18). Sonn and colleague’s demonstrated a 20% (21/105) csPCa detection rate using mp-MRI targeted biopsy in men with a prior negative biopsy but continued suspicion secondary to elevated PSA (18).
Furthermore, Patel et al. evaluated the utility of systematic biopsy alongside fusion targeting and found that a prior negative prostate biopsy was significantly associated with the absence of clinically significant cancer in the non-targeted systematic cores (OR 0.46; 95% CI, 0.21–0.99; P=0.046) (19). Whilst no consensus exists on the role of a combined biopsy strategy in men with a prior negative biopsy status, there is growing evidence non-targeted cores in this setting offer little clinical utility (19).
Morbidity and cost of additional non-targeted systematic cores
Evidence from patient reported outcome measures in template-mapping trials, supports the notion that additional cores performed in a prostate biopsy pathway can be detrimental to post-biopsy urinary flow, genitourinary and sexual function (20). In contrast, the reported complication rate of local anaesthetic or sedation for targeted transperineal prostate sampling is low, with a reported post-biopsy urinary retention and sepsis rate of less than 1% (21).
However, a systematic review by Loeb et al. reported greater biopsy related pain with increasing number of cores performed (22). In addition, there is evidence that suggests that patient-reported outcome measures (PROMS), in particular urinary flow and sexual function, are persistently poorer in men who undergo prostate biopsies with a high median number of cores (20,23). Reducing the overall number of cores obtained by limiting non-targeted systematic cores may offer improvements in post-biopsy genitourinary functional outcomes without sacrificing oncological outcomes.
Compared to systematic TRUS biopsy, targeted biopsy using image fusion has been proven to be cost-effective (24). This is despite concerns over higher initial pathway set-up costs and implementation (24). Venderink and colleagues reported the incremental cost-effectiveness ratio of MRI-TRUS image fusion over systematic TRUS biopsy to be $1,470 per quality-adjusted life year gained; thus, deeming image fusion cost-effective (24).
Performing additional non-targeted cores in a targeted biopsy pathway does directly increase the histopathology cost per case by an estimated £112.79/$146.81 (25). In addition, the downstream effects are an increase the technical reporting load on pathologists and a wider clinical reviewing burden on multi-disciplinary meetings.
Evidence for a combined mp-MRI targeted and non-targeted approach
Added value of a csPCa detection in combined biopsy
The additional diagnostic yield of csPCa detection by performing a non-targeted systematic biopsy in addition to an mp-MRI targeted biopsy is reported to be 1.3% to 11% (Table 3) (5,8-11,14,26). A combined biopsy strategy may refer to mp-MRI targeted cores in addition to either sectoral templating or 12-core systematic TRUS. The Ginsburg Study Group on Enhanced Prostate Diagnostics have supported a sectoral templating approach since 2013 (27). On the premise that preferential targeting of the peripheral zones leads to the avoidance of the inherent oversampling of template-mapping sampling and the under-sampling of systemic TRUS in isolation (27).
Table 3. Absolute cancer detection rates of clinically significant cancer in mp-MRI targeted and systematic prostate biopsies.
| Author | Year | n | Study design | Pre-biopsy MRI | Prostate biopsy approach | Form of targeted biopsy | csPCa in targeted biopsy, % [n] | csPCa in systematic biopsy, % [n] | csPCa in combined, % [n] | Value add of combined biopsy, % [n] | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Elkhoury et al. (PAIREDCAP) (9) | 2019 | 300 [248*] | Paired-control trial | mp-MRI 3 T |
TRPB | C or F | 62% [154] | 60% [151] | 70% [178] |
+11% [27] | All biopsy naïve |
| Miah et al. (10) | 2019 | 640 [358*] | Prospective cohort study—targeted biopsy followed by limited systematic | mp-MRI 3 T | TPB | F | 48.4% [310/640] | 17.9% [64/358] | 49.7% [319/640] | + 1.3% [9/640] |
Prior prostate biopsy not excluded. Likert score used. Targeted only in some men |
| van der Leest et al. (4M) (8) | 2019 | 626 | Prospective cohort study—targeted biopsy followed standard 12-core TRPB | mp-MRI 3T | TRPB | C | 25% [159] | 23% [146] | 48% [301] | +7% [21/317] | All biopsy naïve |
| Mannaerts et al. (11) | 2019 | 225 | Prospective cohort study—standard 12-core TRPB followed by targeted biopsy | mp-MRI | TRPB | F | 44% [113] | 43% [110] | 52.4% [118] | + 8% [9] | Likert score used. |
| Rouvière et al. (MRI-FIRST) (5) | 2019 | 335 [251] | Randomised controlled trial (paired diagnostic)—standard 12-core TRPB followed by targeted biopsy | mp-MRI 1.5T or 3T | TRPB | C | 32.3% [81] | 29.9% [75] | 37.5% [94] | + 5.2% [4.8] | Systematic biopsy in all men regardless of mp-MRI results |
| Filson et al. (14) | 2016 | 1042 [825T] | Prospective cohort study—standard 12-core TRPB followed by targeted biopsy | mp-MRI | TRPB | F | 24% [199] | 27.8% [229] | 35% [289] | + 7.27% [60] | Prior prostate biopsy not excluded |
Marginal gains (added value) of combined biopsy. *, number of additional non-targeted systematic biopsy performed. T, number of patients with region of interest on mpMRI who underwent both biopsy. CDR, Cancer detection rate; csPCa, clinically insignificant prostate cancer (defined as Gleason ≥3+4 or Gleason Grade Group 2); TRPB, transrectal prostate biopsy; TPB, transperineal prostate biopsy; C, cognitive; F, fusion.
However, MRI-FIRST, the multicenter, paired diagnostic study by Rouviere and colleagues has provided the first high-level data utilizing a combined TRUS-biopsy strategy (MRI-targeted followed by 12-core systematic) (5). In their study, 251 men underwent combined prostate biopsy; the csPCa detection rate was 29·9% (95% CI, 24.3–36.0) for systematic biopsy and 32.3% (95% CI, 26.5–38.4) for targeted biopsy. csPCa would have been missed in 5.2% of cases if systematic biopsy had not been performed, and in 7.6% if targeted biopsy was not performed. csPCa detection rates were improved when both biopsy methods were combined. However, targeted biopsy detected significantly more grade group ≥3 tumours and significantly fewer grade group 1 tumours.
Elkhoury and colleagues’ recently-published PAIREDCAP trial was paired-cohort study of 248 biopsy-naïve men who underwent a 12-core systematic biopsy followed by two MRI targeted biopsies (targeted cognitive fusion and targeted software fusion) during the same session (9). csPCa was detected in 47% of targeted cognitive fusion biopsies, 54% of targeted software fusion biopsies, and 60% of biopsies obtained via systematic sampling. However, a combined approach resulted in maximal detection, with a 70% (178/248) csPCa detection rate, an additional diagnostic yield of 11% (9).
Using a similar combined targeted and 12-core TRUS approach, Filson and colleagues’ study of 825 men reported csPCa in 24% and 27.8% of the non-targeted and targeted prostate biopsies, respectively (14). The combination of systematic and targeted biopsies detected more csPCa (n=289) than targeting (n=229) or systematic biopsy alone (n=199). 60 patients were found to have csPCa on systematic biopsy that would have been missed by targeted biopsy alone. Additionally, one in eight men without a suspicious lesion on mpMRI were diagnosed with csPCa via systematic biopsy.
Van der Leest et al.’s study utilized an in-bore MRI-guided transrectal biopsy followed by a 12-core TRUS (8). The authors reported a 7.0% (21/317) additional csPCa detection rate when using a combined biopsy approach. Other studies utilizing purely transperineal prostate biopsy have reported the additional diagnostic yield of csPCa at 1.3% (10).
The additional yield of csPCa detected in a combined strategy varies widely across the literature. However, the above studies suggest a significant proportion of csPCa is missed when only a mp-MRI targeted biopsy is performed. The level of acceptable of “missed csPCa” in these pathways is a wider debate yet to gain international consensus.
mp-MRI invisible disease and inter-observer reproducibility
A major limitation of exclusive mp-MRI targeted biopsy is the notion of patients harboring mp-MRI-invisible disease. In men with no identifiable region of interest on mp-MRI, targeted biopsy is not performed and “invisible” disease would otherwise go undetected (28). The PROMIS trial demonstrated that mp-MRI had a sensitivity of 93% and negative predictive value of 89% for predicting csPCa (defined as Gleason ≥4+4 or MCCL ≥6 mm) (1). A recent meta-analysis on the topic reported the median mp-MRI negative predictor value was 82.4% (IQR, 69–92.4%) (28). Unsurprisingly, negative predictor value significantly decreased when baseline cancer prevalence increased (28). mp-MRI invisible disease remains a significant clinical concern amongst urologists in routine clinical practice (29).
Filson’s group identified that 12% of biopsy naïve men with no ROI who underwent a systematic biopsy had clinically significant disease (14). Le and colleagues’ study of 112 whole-mounted prostatectomy specimens detected csPCa in 28% of cases, cancer that was invisible to expert reported mp-MRI (30). Furthermore, Chung et al. retrospectively reviewed 213 radical prostatectomy specimens matched to prior mp-MRI reports (31). The group defined “invisible” prostate cancer was as those graded PIRADS 1 or 2, or those with no MRI-visible region of interest. The group reassuringly found 76.1% of mp-MRI invisible cancer was clinically insignificant disease. However, 6.6% (n=20) of those with negative MRI were found to have ≥ Gleason 8 disease (30).
In van der Leest and colleagues’ study an additional 21 men were diagnosed with csPCa using a combined biopsy strategy (8). However, in 20 of these 21 additional cases the ROI was present on pre-biopsy mp-MRI. Thus, only a single patient (<1%) had mp-MRI invisible cancer detected on a non-targeted systematic biopsy (8).
Persistence of MRI invisible disease has limitations for mp-MRI as a triage biopsy tool in biopsy naïve men. The answer to this may lie in combining MRI-derived parameters (e.g., MRI-prostate volume, PIRADS 1 or 2) in addition to clinical variables (e.g., age, ethnicity) (32). Such modelling produces a binary (yes/no) outcome to the risk of csPCa (32). At present it should be noted, the addition of such modelling into mp-MRI triage has only supported a significant reduction in false-positive rates (32).
Finally, with regard to agreement on mp-MRI ROI score (PIRADS or LIKERT), this has suffered from low inter-observer reproducibility (33). One study found even experienced radiologists, using PIRADS version 2, had poor agreement in the peripheral zone for features relating to diffusion weighted imaging (k=0.53–0.61) (33). With low inter-observer reproducibility, an MRI interpreted as having an ROI by one radiologist may be graded as “negative” by another, failing to trigger a targeted biopsy. In this example case, detection would only be possible via systematic biopsy.
Final histopathology grade disparity
There is some concern that exclusive targeted sampling may underestimate whole gland disease status and thus impact the downstream treatment modality choice by patients (31,34,35). Muthigi and colleagues reviewed the whole mount pathology of 1,003 serial patients in whom patients were upgraded in non-targeted systematic cores alone to csPCa in 13.5% (n=135). Only 1.1% (n=11) resulted in the identification of high-risk disease (Gleason ≥8) (35). When detailing failure of targeted biopsy to detect this disease the authors’ concluded that MRI invisible disease, operating clinician technique failure and intra-lesion Gleason heterogeneity were all significant (35).
Furthermore, when reviewing whole-gland prostatectomy histopathology Siddiqui and colleagues reported that the sensitivity of targeted prostate biopsy in detecting Gleason ≥7 rose from 77% (95% CI, 67–84%) to 85% (95% CI, 76–91%) when a combined strategy was utilized (13).
Finally, the ability to accurately characterize cancer morphology, in particular Gleason 4 subtypes on targeted biopsy alone has been questioned (36). The presence of Gleason pattern 4 cribriform tumours is associated with increased cancer specific mortality and is an adverse independent predictor of metastasis-free survival (36,37). Truong and colleague’s, re-reviewed 694 positive cores for pattern 4 subtypes. The group concluded that a combined biopsy over a targeted only strategy increased absolute cribriform pattern detection by 8.5% (37.1% vs. 28.6%) (34). Awareness of the presence of such morphology has notable downstream effects on the treatment choices and associated risks presented to patients.
Conclusions
We are yet to reach a stage where non-targeted systematic prostate biopsy can be routinely omitted in mp-MRI targeted prostate biopsy pathways. Research should focus on improving the accuracy of mp-MRI, prostate biopsy techniques and in identifying those men likely to most benefit from adding non-targeted systematic biopsies. Such strategies may help future urologists reduce the burden of non-targeted cores in modern mp-MRI prostate biopsy pathways.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: MJC is funded by the Wellcome Trust; LM is a co-founder of Avenda Health; HUA receives funding from Sonacare Medical, Sophiris Inc. and Trod Medical for trials and personal fees for trial/research consultancy from Sophiris Inc. Receives funding for travel, lectures and proctoring fees Sonacare Inc. and BTG Medical (previously Galil). HUA receives funding from the Wellcome Trust. HUA is supported by infrastructure support provided by the NIHR Imperial Biomedical Research Centre.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin J. Connor, Saiful Miah, Taimur T. Shah, Hashim U. Ahmed) for the series “Prostate Imaging and Focal Therapy” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2019.07.16). The series “Prostate Imaging and Focal Therapy” was commissioned by the editorial office without any funding or sponsorship. MJC, SM, TS and HUA served as the unpaid Guest Editors for the series. MJC reports grants from Wellcome Trust, grants from University College London Hospital Charity, DEE reports grants from Imperial Health Charity, grants from The Urology Foundation (TUF), HUM reports grants and personal fees from SonaCare Medical Inc, grants and personal fees from Trod Medical, grants and personal fees from Sophiris Bio Inc, grants and personal fees from Boston Scientific, grants from NIHR UK, grants from Wellcome Trust, grants from Prostate Cancer UK, grants from Imperial Biomedical Research Centre, grants from The Urology Foundation, grants from BMA Foundation, grants from Imperial Healthcare Charity, grants from University College London Hospital Charity, grants from MRC (UK), LM reports other from Avenda Health, The other authors have no other conflicts of interest to declare.
References
- 1.Ahmed HU, Bosaily AE, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 2.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767-77. 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NHS England. Implementing a timed prostate cancer diagnostic pathway. NHS England, 2018. [Google Scholar]
- 4.Mottet N, Bellmunt J, Briers E, et al. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Available online: https://uroweb.org/guideline/prostate-cancer/ [Accessed 14 April 2019].
- 5.Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019;20:100-9. 10.1016/S1470-2045(18)30569-2 [DOI] [PubMed] [Google Scholar]
- 6.Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance‐ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016;122:884-92. 10.1002/cncr.29874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porpiglia F, Manfredi M, Mele F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer. Eur Urol 2017;72:282-8. 10.1016/j.eururo.2016.08.041 [DOI] [PubMed] [Google Scholar]
- 8.van der Leest M, Cornel E, Israel B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol 2019;75:570-8. 10.1016/j.eururo.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 9.Elkhoury FF, Felker ER, Kwan L, et al. Comparison of Targeted vs Systematic Prostate Biopsy in Men Who Are Biopsy Naive: The Prospective Assessment of Image Registration in the Diagnosis of Prostate Cancer (PAIREDCAP) Study. JAMA Surg 2019;154:811-8. 10.1001/jamasurg.2019.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miah S, Hosking-Jervis F, Connor MJ, et al. A multi-centre analysis of detection of clinically significant prostate cancer following transperineal image-fusion targeted and non-targeted systematic prostate biopsy in men at risk. Eur Urol Oncol 2019. Doi: . 10.1016/j.euo.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 11.Mannaerts CK, Kajtazovic A, Lodeizen OA, et al. The added value of systematic biopsy in men with suspicion of prostate cancer undergoing multiparametric MRI-targeted biopsy. Urol Oncol 2019;37:298.e1-9. 10.1016/j.urolonc.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 12.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767-77. 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390-7. 10.1001/jama.2014.17942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance‐ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016;122:884-92. 10.1002/cncr.29874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelsayed GA, Danial T, Kaswick JA, et al. Tumors of the anterior prostate: implications for diagnosis and treatment. Urology 2015;85:1224-8. 10.1016/j.urology.2014.12.035 [DOI] [PubMed] [Google Scholar]
- 16.Rosenkrantz AB, Verma S, Choyke P, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol 2016;196:1613-8. 10.1016/j.juro.2016.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wegelin O, Exterkate L, van der Leest M, et al. The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. Eur Urol 2019;75:582-90. 10.1016/j.eururo.2018.11.040 [DOI] [PubMed] [Google Scholar]
- 18.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance–ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol 2014;65:809-15. 10.1016/j.eururo.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel N, Cricco-Lizza E, Kasabwala K, et al. The Role of Systematic and Targeted Biopsies in Light of Overlap on Magnetic Resonance Imaging Ultrasound Fusion Biopsy. Eur Urol Oncol 2018;1:263-7. 10.1016/j.euo.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 20.Miah S, Eldred-Evans D, Simmons LA, et al. Patient reported outcome measures for transperineal template prostate mapping biopsies in the PICTURE study. J Urol 2018;200:1235-40. 10.1016/j.juro.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 21.Bass EJ, Donaldson IA, Freeman A, et al. Magnetic resonance imaging targeted transperineal prostate biopsy: a local anaesthetic approach. Prostate Cancer Prostatic Dis 2017;20:311. 10.1038/pcan.2017.13 [DOI] [PubMed] [Google Scholar]
- 22.Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol 2013;64:876-92. 10.1016/j.eururo.2013.05.049 [DOI] [PubMed] [Google Scholar]
- 23.Wadhwa K, Carmona-Echeveria L, Kuru T, et al. Transperineal prostate biopsies for diagnosis of prostate cancer are well tolerated: a prospective study using patient-reported outcome measures. Asian J Androl 2017;19:62-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venderink W, Govers TM, de Rooij M, et al. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion. AJR Am J Roentgenol 2017;208:1058-63. 10.2214/AJR.16.17322 [DOI] [PubMed] [Google Scholar]
- 25.Prostate Cancer: Diagnosis and Treatment. London: NICE, 2014. [Google Scholar]
- 26.Miah S, Servian P, Patel A, et al. A prospective analysis of robotic targeted MRI-US fusion prostate biopsy using the centroid targeting approach. J Robot Surg 2020;14:69-74. 10.1007/s11701-019-00929-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuru TH, Wadhwa K, Chang RTM, et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int 2013;112:568-77. 10.1111/bju.12132 [DOI] [PubMed] [Google Scholar]
- 28.Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol 2017;72:250-66. 10.1016/j.eururo.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 29.Marks LS. Some prostate cancers are invisible to magnetic resonance imaging! BJU Int 2016;118:492-3. 10.1111/bju.13440 [DOI] [PubMed] [Google Scholar]
- 30.Le JD, Tan N, Shkolyar E, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol 2015;67:569-76. 10.1016/j.eururo.2014.08.079 [DOI] [PubMed] [Google Scholar]
- 31.Chung DY, Koh DH, Goh HJ, et al. Clinical significance and predictors of oncologic outcome after radical prostatectomy for invisible prostate cancer on multiparametric MRI. BMC Cancer 2018;18:1057. 10.1186/s12885-018-4955-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehralivand S, Shih JH, Rais-Bahrami S, et al. A magnetic resonance imaging–based prediction model for prostate biopsy risk stratification. JAMA Oncol 2018;4:678-85. 10.1001/jamaoncol.2017.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology 2016;280:793-804. 10.1148/radiol.2016152542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truong M, Feng C, Hollenberg G, et al. A comprehensive analysis of cribriform morphology on magnetic resonance imaging/ultrasound fusion biopsy correlated with radical prostatectomy specimens. J Urol 2018;199:106-13. 10.1016/j.juro.2017.07.037 [DOI] [PubMed] [Google Scholar]
- 35.Muthigi A, George AK, Sidana A, et al. Missing the mark: prostate cancer upgrading by systematic biopsy over magnetic resonance imaging/transrectal ultrasound fusion biopsy. J Urol 2017;197:327-34. 10.1016/j.juro.2016.08.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kweldam CF, Wildhagen MF, Steyerberg EW, et al. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol 2015;28:457-64. 10.1038/modpathol.2014.116 [DOI] [PubMed] [Google Scholar]
- 37.Siadat F, Sykes J, Zlotta AR, et al. Not all Gleason pattern 4 prostate cancers are created equal: a study of latent prostatic carcinomas in a cystoprostatectomy and autopsy series. Prostate 2015;75:1277-84. 10.1002/pros.23009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


