Abstract
Malaria continues to be one of the top infectious agents contributing to morbidity and mortality in sub-Saharan Africa. Annually, Botswana accounts only for a small proportion of cases (<<1%). Despite significantly reduced incidence rate, the country still experiences sporadic outbreaks that hamper the goal of malaria elimination. This review evaluated previous and current biological factors that impact malaria in Botswana, specifically focussing on the vectors, the parasite and the host. This was accomplished via a literature review evaluating these variables in Botswana. Current literature suggests that Anopheles arabiensis is the main malaria vector in the country. Several other potential vectors have been found widely distributed throughout Botswana in high numbers, yet remain largely unstudied with regards to their contribution to the country's malaria burden. We also report the most up to date list of all Anopheles species that have been found in Botswana. Plasmodium falciparum is responsible for the vast majority of symptomatic malaria in the country and some drug resistance markers have been documented for this species. Plasmodium vivax has been reported in asymptomatic subjects, even though a large proportion of the Botswana population appears to be Duffy antigen negative. Very little is known about the true distribution of P. vivax and no point of care testing infrastructure for this species exists in Botswana, making it difficult to tailor treatment to address possible recrudescence or relapse. Due to a genetically diverse population with a substantial Khoisan contribution into the Bantu genetic background, several phenotypes that potentially impact prevalence and severity of malaria exist within the country. These include sickle cell trait, Glucose-6-Phosphate Dehydrogenase deficiency, and Duffy negativity. This review highlights the information that currently exists on malaria in Botswana. It also postulates that a comprehensive understanding of these aforementioned biological factors may help to explain malaria persistence in Botswana.
Keywords: Malaria, Botswana, Vectors, Anopheles, Plasmodium, Human genetics
1. Introduction
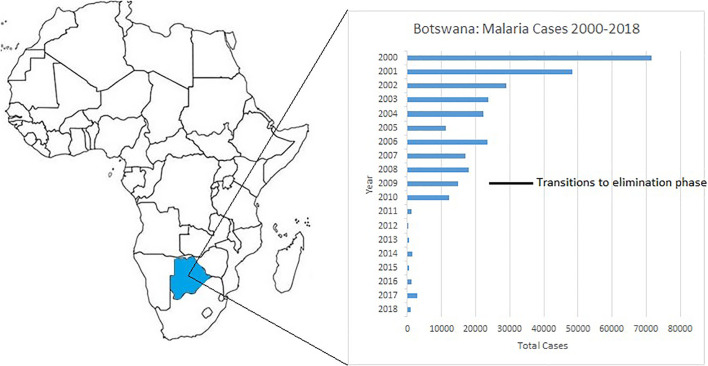
Despite over a century of data driven education, research, and elimination efforts (N'ajera-Morrondo, 1991), malaria claimed 405,000 lives in 2018 when 228 million cases of infection were recorded worldwide (World Health Organization, 2019). Botswana accounted for a very small fraction of these mortalities and infections, 9 and 879, respectively, in the same year (World Health Organization, 2019). These numbers are extraordinary in comparison to previous estimations from the early 2000s, where annual malaria cases in Botswana frequently exceeded 70,000 (World Health Organization, 2015a) in a population of 2.3 million. A change in focus from a program solely concentrated on vector control, to one that had improved access to diagnostic tools, initiation of contact tracing, free distribution of insecticidal nets in specific endemic regions in conjunction with indoor residual spraying (IRS), and access to free antimalarial chemotherapy circa 2010 by Botswana's Ministry of Health (Moakofhi et al., 2018; World Health Organization, 2015a), greatly reduced the country's malaria burden (Fig. 1 ). This allowed the country to adopt a national malaria elimination strategy in 2009 and attain the targets therein over several years (Republic of Botswana, 2018). Despite these major improvements, the World Health Organization (WHO) has raised concerns about the epidemic propensity of malaria in Botswana and in other southern African countries. During the 2016/2017 malaria season, Botswana experienced an 80% increase in annual malaria cases, which mirrored intensified transmission in other southern African countries (World Health Organization, 2018).
Fig. 1.
Map of Africa showing Botswana's geographical position and malaria cases over an 18 year period. Years 2000–2013 (World Health Organization, 2015a), 2014–2018 (World Health Organization, 2019).
1.1. Country profile
Botswana is a landlocked country that is surrounded by Namibia, Zambia, Zimbabwe, and South Africa. It is comprised of large swaths of desert, such as the Kgalagadi, as well as the world renowned Okavango Delta. Temperatures in the summer can rise above 45 °C and sub-freezing temperatures are occasionally noted in the winter months of June–August. Rainfall ranges between 650 mm in the Northern portions of the country, to 250 mm in the extreme Southern sections of the Kgalagadi (Thomson et al., 2005). Although rainfall usually occurs between November and April, Botswana is vulnerable to intermittent and periodic drought (Thomson et al., 2005) associated with the El Niño Southern Oscillation (ENSO) pattern.
Malaria transmission in Botswana is seasonal and highly unstable. Transmission typically occurs between November and April during the rainy season when extreme flooding creates prime breeding grounds for malarial vectors (Republic of Botswana, 2018). Peak malaria transmission usually occurs in March–April due to increased rainfall and temperatures in the previous months (Thomson et al., 2005). Spatial-statistical models have identified summer rainfall, mean annual temperature and altitude to be the main environmental predictors of malaria risk in Botswana, which explains the annual fluctuations registered in the last years (Craig et al., 2007). These environmental variables make the more South and Southeastern regions prone to fluctuating levels of transmission and sporadic epidemics (Botswana Ministry of Health, 2010; Craig et al., 2007). Whereas, malaria in the Northwestern region is considered endemic with sustained transmission annually (World Health Organization, 2015a). Malaria incidence in the region increases during La Niña Southern Oscillations episodes, and decreases with El Niño, despite some incongruences (Craig et al., 2007; Kgoroebutswe et al., 2020).
Of the five Plasmodium species known to infect humans (Plasmodium falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi), P. falciparum is the primary species responsible for morbidity and mortality in sub-Saharan Africa (World Health Organization, 2019) and Botswana. However, recent surveys have demonstrated a much higher prevalence of P. vivax in Botswana than previously expected, which is congruent with findings in neighboring countries (Baird, 2007; Howes et al., 2015; Motshoge et al., 2016). Like many countries in the Southern Africa Development Community (SADC), Anopheles arabiensis has been demonstrated to be a major vector spreading malaria in Botswana (Botswana Ministry of Health, 2010). However, multiple studies have found several other malaria vectors in Botswana which have received little to no national attention, likely due to the fact that they have not been found to carry Plasmodium within the country's borders (Abdulla-Khan, 1995; Cornel et al., 2018; Kgoroebutswe et al., 2020; Koekemoer et al., 1998; Kyalo et al., 2017; Pachka et al., 2016; Tawe et al., 2017). Though, variances in collection techniques, mosquito species screenings and whether trapped mosquitoes were tested for sporozoites may have impacted evidence of their vectorial capacity in the country.
1.2. Demographics
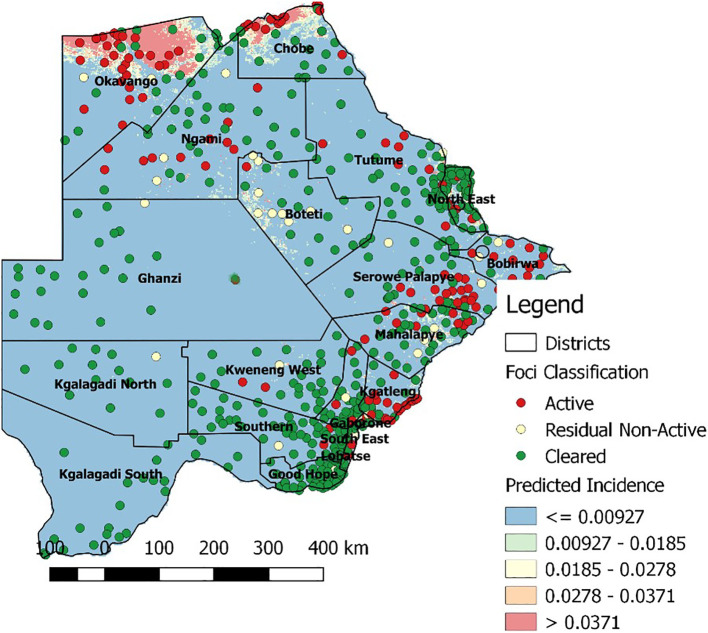
Botswana houses approximately 2.3 million individuals of a unique genetic background (Statistics Botswana, 2018). Nearly 1.5 million of these residents are at risk for malarial infection (World Health Organization, 2019), with the highest incidence found in the northern part of the country (Fig. 2 ). Khoisan and Bantu are the two major ancestral groups of today's Botswana people. Admixture has occurred between the two ethnic groups to various extents (Schuster et al., 2010). As a consequence, there is a wider spectrum of genotypes and phenotypes when considering genetic characteristics such as Duffy antigen, sickle cell trait, glucose-6-phosphate dehydrogenase (G6PD), and detoxifying liver enzymes (Howes et al., 2011; Motshoge et al., 2018; Tawe et al., 2018a, Tawe et al., 2018b). These human genetic factors play a pivotal role in susceptibility to malaria infection and associated morbidity (Haldane, 1949; Sirugo et al., 2014), as well as for metabolism of medications currently on the market (Tawe et al., 2018a, Tawe et al., 2018b).
Fig. 2.
Map showing predicted malaria incidence in Botswana. The map was kindly provided by the Botswana Ministry of Health and Wellness.
To date, relatively little has been published on malaria in Botswana. Here, we summarize those publications and consider current factors affecting malaria elimination in Botswana via a literature review over the past nine decades. We specifically considered studies involving malaria vector species, Plasmodium species, and human genetics and how these work in concert to either support or handicap Botswana's goal of malaria elimination.
2. Methods
2.1. Article selection
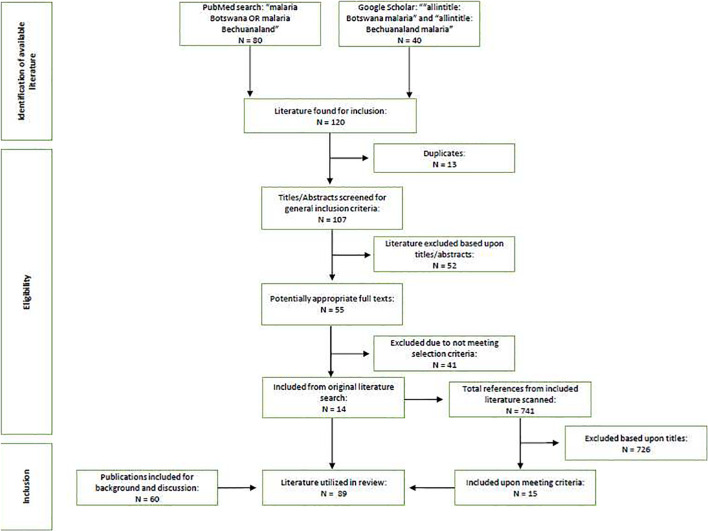
Two search engines, PubMed and Google Scholar, were utilized in the review of the widely available literature. All publications were included that matched the selection criteria published between 1 January 1937 and 5 May 2020. The criteria were defined as: any primary data or information in the context of Botswana involving the vectors of malaria, the parasite, or the host's interaction with malaria. The search query: “malaria Botswana OR malaria Bechuanaland” was used for PubMed. The search query: “allintitle: Botswana malaria” and “allintitle: Bechuanaland malaria” were utilized for Google Scholar. Each publication was examined for primary data or findings that matched at least one of our three selection criteria. If met, then it was included in the review (Fig. 3 ). The titles of the references from these publications were also scanned for keywords matching our selection criteria and included if they met one of them. Authors included selected publications that did not meet selection criteria at their discretion in order to provide background information on a specific topic, or to further elaborate on a topic when no literature on Botswana were available for reference.
Fig. 3.
Flowchart of the literature search/review strategy.
3. Results and discussion
3.1. Malaria vectors
3.1.1. Anopheles gambiae
Vectors within the Anopheles gambiae sensu lato (s.l.) complex are widely recognized as the primary contributors to malaria transmission in sub-Saharan Africa. Two species within this complex, An. gambiae sensu stricto (s.s.) Giles and An. arabiensis Patton, are accepted as the most efficient and widespread vectors in this region (Coetzee et al., 2000). Anopheles gambiae s.s. tends to prefer humid zones, whereas An. arabiensis dominates in arid savannas that lack large bodies of water (Onyabe and Conn, 2001). Historically An. gambiae s.s., an endophilic and endophagic mosquito, inhabited Southern Africa, though it was never officially identified in Botswana (Mastbaum, 1944). But, aggressive indoor residual spraying (IRS) with dichlorodiphenyltrichloroethane (DDT) is thought to have effectively rendered this species extinct in Botswana, whereas An. arabiensis persisted (Hansford, 1972). Anopheles arabiensis accounts for the majority of malaria transmission within the SADC region (World Health Organization, 2015a). To various degrees, An. arabiensis when compared to similar vectors within the An. gambiae complex is described to as partially zoophilic, exophagic and exophilic. Wide variances in feeding and resting patterns have been recorded, mostly depending upon geographical location (Sinka et al., 2010). These behavioral inconsistences may be controlled genetically rather than environmentally. Karyotype inversions in this species appear to alter resting behavior and anthropophily, allowing for rapid propagation of a trait if selective pressure is applied (Coluzzi et al., 1979; Main et al., 2016). Selection for exophilic phenotypes may account for poor outcomes of IRS campaigns against this vector (Coluzzi et al., 1979; Main et al., 2016). In Botswana, the majority of studies on An. arabiensis have been completed in the Northwestern portion of the country. Two studies (Chirebvu et al., 2014; Chirebvu et al., 2016) conducted over a 5 year period in the village of Tubu, which is situated along the banks of the Okavango Delta, identified An. arabiensis as the primary indoor resting vector (Chirebvu et al., 2016). They also found it to be highly prevalent at a larval stage (Chirebvu et al., 2014). Additional studies in this village found samples to be primarily zoophagic, with only 27% of blood fed mosquitos testing positive for human blood (Chirebvu et al., 2014). A study in seven other districts found a similar average human blood fed frequency of 28% for this species (Tawe et al., 2017). Though, these mosquitoes were collected via pyrethrum spray catches (PSC), suggesting that the aforementioned frequency of 27–28% does not reflect the integration of anthropophily for the vector, given its known behavioral heterogeneity and the likelihood of blood fed mosquitos resting outdoors where PSC would fail to collect them. Additional studies examining the outdoor/indoor resting and feeding behavior of An. arabiensis are required to better understand how this vector behaves in Botswana. Studies evaluating post blood meal behavior of all anopheline species via exit window traps or as detailed in Odiere et al. (2007), where clay pots are utilized to probe the behavior of outdoor resting vectors, could fill major gaps in the current literature.
Anopheles arabiensis infected with P. falciparum in Botswana can range between 0 and 3% prevalence in PSC collections, depending on the region, time of year, annual rainfall and human parasite reservoir (Tawe et al., 2017). This delineates An. arabiensis as the only vector with confirmed Plasmodium infection in the country. This relatively limited prevalence (0–3%) of infected vectors by site contributes to high levels of malaria transmission during peak malaria season and could explain the epidemic nature of the disease in certain areas (Mbogo et al., 1995), especially when considering that infected vectors have been shown to congregate in specific areas in other countries (Kulkarni et al., 2006). Anopheles arabiensis has been described in all of Botswana's westernmost districts, placing over half of the country's populationat at risk to malaria due the presence of malaria infected vectors, given the presence of a Plasmodium ridden host (Statistics Botswana, 2018). Furthermore, the Ministry of Health in Botswana has detected in vivo pyrethroid resistance in this species in all malaria endemic districts, and resistance to DDT in one district (Republic of Botswana, 2018). Since IRS coverage and availability is relatively high in high risk areas in Botswana (World Health Organization, 2019), An. arabiensis should be considered the top priority vector with regards to elimination efforts in the region based upon currently available literature.
3.1.2. Anopheles funestus
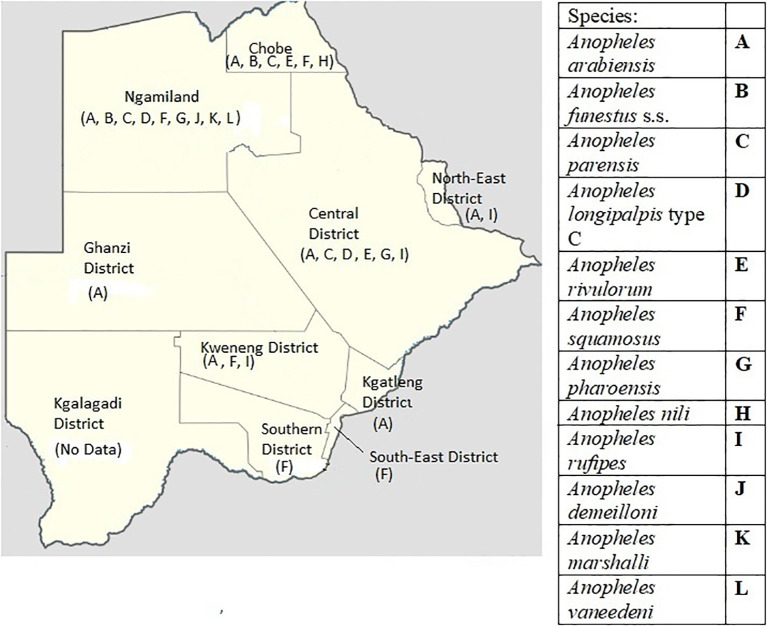
Surveys in Botswana and surrounding countries have revealed the presence of An. funestus s.l., the second most important taxon of malaria vectors in Africa (Abdulla-Khan, 1995; Coetzee and Fontenille, 2004; Kyalo et al., 2017; Kgoroebutswe et al., 2020; Tawe et al., 2017). This group of species is of particular concern due to documented insecticide resistance and for possibly causing the largest malaria epidemic in South Africa in over 50 years (Hargreaves et al., 2000). Four studies from the past three decades: a PhD thesis published in 1995, a study from 1998, a survey completed in 2017, and a study published in 2020, confirmed the group's presence in Botswana (Abdulla-Khan, 1995; Kgoroebutswe et al., 2020; Koekemoer et al., 1998; Tawe et al., 2017). The 1995 thesis identified An. funestus s.s., the major vector within the group, in 3 districts and An. rivulorum in 1 district (Abdulla-Khan, 1995) (Fig. 4 ). The 1998 study found An. vaneedeni in one district (Koekemoer et al., 1998) (Fig. 4). The study in 2017 confirmed the presence of An. parensis in 4 districts, An. longipalpis type C in 2 districts, as well as several non-vector species within the group (Tawe et al., 2017) (Fig. 4). Of the two species described in the 2017 study, only An. parensis was found to have taken a human blood meal. The most recent study in 2020 found An. longipalpus type C in one district, and An. parensis in two (Kgoroebutswe et al., 2020) (Fig. 4). Similar to surrounding countries, An. funestus s.l. was assumed to have been reduced to negligible levels with the widespread use of IRS since the 1950s (Hargreaves et al., 2000). Though, limited data suggests that An. funestus s.l. persisted in Botswana as late as 1984 (Kyalo et al., 2017). We propose two hypotheses to explain its current presence in the country. The first is that An. funestus s.l. persisted post IRS implementation, but at negligible levels and has only recently returned to a significant population density. Alternatively, An. funestus s.l. migrated from proximal regions in surrounding countries. Nonetheless, non-negligible numbers now exist within several districts. This could prove to be of paramount importance if these mosquitos were found to harbor and effectively transmit Plasmodium sporozoites in Botswana, due to evidence of endophilic and limited anthropophilic behavior (Baird, 2007; Tawe et al., 2017). At this time, only one of the species in the group, An. funestus s.s., has been thoroughly implicated as a malarial vector in multiple regions of Africa (Chirebvu et al., 2014; Coetzee and Fontenille, 2004). Outside of An. funestus s.s., An. vaneedeni was found to carry P. falciparum just across the border in the Mpumalanga and KwaZulu-Natal regions of South Africa (Burke et al., 2017). Also, sporadic studies have found An. parensis and An. longipalpus type C to carry P. falciparum in other countries, though very little evidence exists to confirm their status as malaria vectors (Burke et al., 2019; Ogola et al., 2018). Finally, An. rivulorum has been incriminated as a vector in Tanzania (Wilkes et al., 1996). Further studies assessing transmission of Plasmodium by this group of species in Botswana are required in order to determine if it plays a role in disease propagation. If found, Botswana may need to adjust elimination strategies to address this vectors.
Fig. 4.
Published potential/known malaria vectors' distribution in Botswana (Abdulla-Khan, 1995; Chirebvu et al., 2016; Cornel et al., 2018; Kgoroebutswe et al., 2020; Koekemoer et al., 1998; Kyalo et al., 2017; Pachka et al., 2016; Tawe et al., 2017).
3.1.3. Other Anopheles
In addition to An. gambiae and An. funestus species members, several other potential malaria vectors have been identified in Botswana that have been found to harbor malaria parasites in other countries. These include: An. squamosus, An. pharoensis, An. nili, An. rufipes, An. demeilloni, An. marshalli, and An. ziemanni (Abdulla-Khan, 1995; Chirebvu et al., 2016; Cornel et al., 2018; Koekemoer et al., 1998; Pachka et al., 2016). None of these mosquitos have been tested for the presence of Plasmodium infection in Botswana, so their role as vectors is not confirmed in the country. Of particular note is the presence of An. squamosus and An. pharoensis, due to them being found in a 43:1 and 28:1 ratio, respectively, to An. arabiensis when collected via nighttime outdoor carbon dioxide traps in villages on the western side of the Okavango Delta (Cornel et al., 2018; Pachka et al., 2016). To date, neither of these mosquitos has been implicated in the transmission of malaria in Botswana. However, substantial evidence in other African countries points to both of these mosquitoes as potential secondary vectors (Carrara et al., 1990; Stevenson et al., 2016). Their relative abundance and attraction to carbon dioxide in outdoor settings may indicate that these mosquitos are generally exophagic, possibly even more so than An. arabiensis. Additionally, a novel study in the Sahel region of Mali demonstrated that both of these vectors, after taking human blood meals, potentially travel 100–200 km (Huestis et al., 2019) via air currents. This is of particular importance in districts that experience sporadic epidemics, if the mosquitoes in Botswana were found to behave in a similar manner. Furthermore, the study from Mali found that An. arabiensis did not travel via the same mechanism, even though it is a known local vector within that study region (Huestis et al., 2019). Further research analyzing the infection status of An. squamosus and An. pharoensis, as well as their migratory behavior, are needed to truly understand the possible role these mosquitos play in malarial transmission in Botswana. Of the other seven potential vectors found in Botswana, An. ziemanni has been noted in small numbers in two unrelated studies in one district (Cornel et al., 2018; Pachka et al) (Fig. 4). Anopheles nili and An. rufipes were reported in the previously mentioned thesis (Abdulla-Khan, 1995). Anopheles demeilloni and An. marshallii were identified as a minor species via indoor PSC in a study looking for An. arabiensis (Chirebvu et al., 2016). All of these Anopheles species have been found to carry Plasmodium in other countries (Antonio-Nkondjio et al., 2005; Carrara et al., 1990; Daygena et al., 2017; Kyalo et al., 2017; Tabue et al., 2014; Tabue et al., 2017; Wilkes et al., 1996). As these species were found in minute quantities compared to other vectors, there is a low likelihood of them contributing significantly to malaria transmission. It is important to note that very few published entomological studies completed in the last 25 years in Botswana even assessed the presence of additional vectors outside of An. gambiae s.l.. This may have resulted in significantly underrepresenting the true distribution and density of these mosquitoes.
3.1.4. Non-vector anophelines
Additional species of Anopheles are present in Botswana, though their capacity as vectors is not confirmed in Africa. However, disregarding these species in future vector studies should be considered erroneous due to the potential of emerging vectorial behavior or ability. In Box 1 we include the most up to date list of Anopheles species found in Botswana since 1935.
Box 1. List of all 31 anophelines that have been identified in Botswana since 1935 (Abdulla-Khan, 1995; Abdulla-Khan et al., 1998; Chirebvu et al., 2016; Cornel et al., 2018; Kgoroebutswe et al., 2020; Koekemoer et al., 1998; Kyalo et al., 2017; Pachka et al., 2016; Tawe et al., 2017). *Underlined species are vectors in Africa.
| An. arabiensis, An. demeilloni, An. funestus s.s., An. longipalpis, An. marshallii, An. parensis, An. pharoensis, An. rivulorum, An. squamosus, An. vaneedeni, An. ziemanni, An. nili,An. argentolobatus, An. caliginosus, An. coustani, An. distinctus, An. leesoni, An. listeri, An. pretoriensis, An. quadriannulatus, An. rhodesiensis, An. rufipes, An. seretsei, An. tchekedii, An. tenebrosus, An. walravensi, An. wellcomei ugandae, An. theileri, An. implexus, An. kingi, An. maculipalpis. |
Alt-text: Box 1
3.2. Plasmodium species
3.2.1. Malaria parasites found in Botswana
Of the five species of Plasmodia generally accepted to exploit humans for schizogony (Antinori et al., 2012), the three most predominant in Africa have been found in Botswana: P. falciparum, P. vivax and P. malariae (Motshoge et al., 2016). Given the presence of P. vivax, a small reservoir of P. ovale is also likely, and has been found in surrounding countries (Amanfo et al., 2016; Haiyambo et al., 2019; Ukpe, 1998; Wolfe, 1968). Here we discuss two of these species, P. falciparum and P. vivax, due to their prevalence, prognosis, and inclusion in malaria treatment guidelines in Botswana (Republic of Botswana, 2015).
Plasmodium falciparum, best known for being the etiological factor in malaria tropica, contributes to the majority of the malarial morbidity and mortality in Africa (World Health Organization, 2019) and this is reflected also in Botswana. Rapid progression of the disease makes immediate diagnosis and treatment of this species the primary variable for good patient outcomes (Mohandas and An, 2012).
Of the other two Plasmodium species identified, P. vivax is the most prevalent. Motshoge et al. (2016) demonstrated that, in asymptomatic children, it is far more prevalent than even P. falciparum at an average national prevalence of 4.66% and 0.16%, respectively (Motshoge et al., 2016). These data contradict the official data from Ministry of Health and Wellness where malaria in Botswana is reported being almost 100% P. falciparum (World Health Organization, 2019). However, it important to note that data reported by WHO are on symptomatic patients based on RDT positivity, while research outputs (Motshoge et al., 2016) are on the asymptomatic reservoir analyzed through molecular techniques. This is not surprising given the differences in life cycles of the two parasites, which directly impacts the severity of symptoms depending upon the species as well as the likelihood of patients presenting themselves for treatment (Antinori et al., 2012). In fact, subjects infected with P. falciparum are far more likely to develop symptomatic malaria in the low endemic context of Botswana, where malaria immunity is generally absent or very low (Tawe et al., 2018a). Biological determinants were long thought to limit P. vivax's pathogenicity. However, global attention has shifted towards this organism due to reports on fatalities and poor patient outcomes if radical cure is not achieved (Baird, 2007; Howes et al., 2015). High levels of Duffy negativity in Africa, an antigen originally thought to be essential for erythrocyte invasion, initially led to P. vivax being discarded as a pathogen of major concern in the continent. However, international literature now points to P. vivax having the ability to invade red blood cells, no matter the Duffy status (Howes et al., 2015; Popovici et al., 2020). In Botswana, where Duffy negativity rates are not fully known, but estimated between 30 and 80% (Howes et al., 2011), asymptomatic P. vivax prevalence is quite high (Motshoge et al., 2016). Nevertheless, there is no evidence of symptomatic P. vivax infections or confirmed cases of P. vivax relapse. Though, a literature review on imported cases from Botswana to the United States of Ameria in the last 20 years revealed sporadic cases of P. vivax and unknown Plasmodium spp., other than P. falciparum, thus confirming a low but historical circulation of non-falciparum parasites (Causer et al., 2002; Cullen and Arguin, 2014; Filler et al., 2003; Holtz et al., 2001;Williams et al., 1999). The vectors that carry P. vivax in the country have not been identified, but may include An. arabiensis, members of the An. funestus group, and An. pharoensis, based upon data from other African countries (Howes et al., 2015).
Only one publication has described malarial parasites outside of P. falciparum in Botswana (Motshoge et al., 2016). Studies quantifying the current human parasitic reservoir, as well as identifying the primary vector of this species, are needed in order to direct medical interventions.
3.2.2. Diagnostic methods
Identification of the Plasmodium species via microscopy and/or molecular methods is of the utmost importance in order to ensure proper treatment and patient outcomes (World Health Organization, 2015b). Historically, the vast majority of malaria in Botswana was diagnosed based upon symptoms alone. Only the turn of the millennia saw a push for the widespread implementation of microscopy and rapid diagnostic tests (RDTs) in the country (Moakofhi et al., 2018). As of 2013, 89% of diagnoses were made via one of these two diagnostic methods, in comparison to 6% six years earlier. The latter of these two tests is preferred by health workers due to its ease of use and the lack of trained microscopists (Moakofhi et al., 2018). For example, between 2013 and 2014, 94% of patients were diagnosed via RDT and 6% via microscopy (Moakofhi et al., 2018). Though, RDT stock outs have been noted by the Ministry of Health (Botswana Ministry of Health, 2010). Overall, the number of positive RDTs confirmed by microscopy continues to decline due to diagnostic noncompliance, despite recommendations that all positive samples undergo microscopy to confirm Plasmodium species and parasitemia (Moakofhi et al., 2018). The lack of microscopy implementation stands as a barrier for elimination efforts, especially due to recent studies demonstrating a much higher prevalence of alternative Plasmodium species, particularly P. vivax, than originally expected (Motshoge et al., 2016). This is noteworthy because many RDTs utilized within the country lack the capacity to identify species other than P. falciparum and only indicate a mixed infection. This makes it difficult for clinicians to tailor treatment to specific species, which can cause relapse.
3.2.3. Malaria treatment
Until 1997, chloroquine was the first line treatment for uncomplicated P. falciparum malaria in Botswana, but was replaced with sulfadoxine-pyrimethamine due to anecdotal reports of reduced efficacy (Botswana Ministry of Health, 2010). Circa 2006, drug efficacy reports showed high failure rates in patients treated with sulfadoxine-pyrimethamine (Botswana Ministry of Health, 2010), which led to confirmed cases of P. falciparum by RDT or microscopy being treated with artemether-lumefantrine (Botswana Ministry of Health, 2010). Additionally, a single dose of primaquine as a gametocytocide, in uncomplicated cases, was adopted in 2010 in accordance with WHO guidelines (Botswana Ministry of Health, 2010). Until 2015, quinine was the chemotherapy of choice for severe malaria. Parenteral artesunate was then adopted in line with WHO recommendations (Botswana Ministry of Health, 2010; Botswana Ministry of Health, 2015; World Health Organization, 2015b). No medication shortages were noted by the Ministry of Health (Botswana Ministry of Health, 2015).
Unlike P. falciparum, P. vivax requires treatment to clear both the blood infection and for radical cure of liver hypnozoites that may cause relapse. This can only be achieved via a fourteen day course of primaquine (Ashley et al., 2014). This medication can result in haemolytic toxicity in individuals that are G6PD deficient when administered the WHO recommended dose of 0.25–0.5 mg/kg over the two week period (World Health Organization, 2015b). In a setting such as Botswana, where G6PD deficiency rates are relatively low, phenotyping patients prior to treatment with the two week course of primaquine may be required in order to avoid malpractice and to better align with national guidelines (Motshoge et al., 2018; Republic of Botswana, 2015). Currently, testing for G6PD is not conducted, nor are patients treated with full two week courses of primaquine due to a lack of point of care diagnostics for G6PD and RDTs that effectively identify P. vivax (Republic of Botswana, 2015).
3.2.4. Drug resistance surveillance
A recent report on drug resistance markers in P. falciparum demonstrated that the parasite harbors certain variation in several of the genes analyzed. In the study, 95.8% of samples showed N86 polymorphism at Plasmodium falciparum multidrug resistance 1 gene (pfmdr1) (Tawe et al., 2018a, Tawe et al., 2018b) known to confer sensitivity to 4-aminoquinolines (Howes et al., 2015) (chloroquine and amodiaquine) but also associated to a reduced sensitivity to lumefantrine (Wongsrichanalai et al., 2002). This suggests that a similar situation to that seen in Malawi (Kublin et al., 2003) has unfolded in Botswana with regards to chloroquine. More importantly, P. falciparum may be generally tolerant to lumefantrine, which is one of the compounds utilized in first line treatment together with the highly efficacious artemether. The same study found two synonymous mutations - V555V and R513R - in the propeller domain of the P. falciparum Kelch13 gene (pfk13) (Tawe et al., 2018a, Tawe et al., 2018b) which is implicated in the resistance to artemisinin. It should be noted that neither of these genetic variants were associated with a delayed parasite clearance phenotype. However, this indicates that molecular assessment and continuous surveillance of the pfk13 and pfmdr1 genes as drug resistance markers in Botswana is of paramount importance. No documented drug surveillance conducted by local Ministry of Health and Wellness for P. vivax has been reported in Botswana.
3.3. Human genetics
Botswana is home to a population that arises from two distinct ancestries, the Bantu and Khoisan speaking peoples, with remarkably diverse genetic and linguistic backgrounds (Schlebusch et al., 2016). It is generally accepted that these two populations converged approximately 2000 years ago with the arrival of the Bantu speaking peoples from the North, which contributed to the diversification and admixture between the two ancestral populations (Schlebusch et al., 2016). Since malaria is acknowledged to have applied significant selective pressure to humans (Kwiatkowski, 2005), genetic differences in Duffy status, haemoglobin S frequency and G6PD deficiency have been noted based upon geographic location and ancestral background (Jenkins et al., 1968; Jenkins et al., 1987; Motshoge et al., 2018; Prugnolle et al., 2013). This is especially pertinent since the Bantu originated from a highly endemic malaria setting, whereas the Khoisan were likely only exposed to sporadic outbreaks that did not apply chronic selective pressure and therefore lack of malaria-resistance alleles (Maingard, 1937; Schuster et al., 2010). Additionally, the recent imposed sedentarism to the traditionally nomadic hunter-gatherers Khoisan communities of the Kalahari region of Botswana (Kent, 1995; Winters, 2015; Ikeya, 2018) may also have an impact on malaria acquisition risk. Recent reports have also indicated that this genetic diversity plays a significant role in pharmacogenetics pertaining also to antimalarial drugs used in Botswana (Tawe et al., 2018a, Tawe et al., 2018b). In Botswana, the peculiar genetic admixture between these two populations clusters specific genotypes more than other Southern African countries (Retshabile et al., 2018). In particular, this genetic structure has potential implications on susceptibility and resistance to infectious diseases and treatment outcomes (Retshabile et al., 2018; Tawe et al., 2018a, Tawe et al., 2018b; Thami and Chimusa, 2019).
Of final note, the original occupants of the region, the Khoisan, appear to have been occasionally exposed to malaria “in exceptionally rainy seasons” (Maingard, 1937) and had traditional medicines to treat its symptoms. A report from the 1930s described a tribe of Khoisan that had lost many of its children to the disease during a flood year. This same group utilized the roots of Tarchonanthus camphoratus to treat the fevers and headaches associated with malaria (Maingard, 1937).
3.3.1. Sickle cell anemia
In many African countries overall childhood mortality is high; but in children with sickle cell disease it is higher still (50–90%). Though, limited temporal data exists on the continent (Grosse et al., 2011). The frequency and distribution of the haemoglobin S (HbS) allele, and its overlap with that of malaria, acts as the geographical confirmation of the malaria hypothesis for selection of this allele (Haldane, 1949). In Botswana, the frequency of the HbS allele is estimated to be between 0 and 2%, though this is a very rough estimation given a lack of data for the region (Piel et al., 2010). However, these limited data support a distribution of this allele that generally reflects the malaria endemicity in the country, with higher frequencies seen in the North and lower in the South. Lastly, a survey conducted in 1967 was unable to identify any sickled cells in a sample of Khoisan and Bantu individuals in the Kgalagadi (Jenkins et al., 1968). Additional surveys would be needed in order to measure HbS allele frequency in Botswana according to the geographical context and malaria endemicity.
3.3.2. G6PD deficiency
Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency is common in Africa due to high levels of selective pressure from Plasmodium and conferred advantage against clinical malaria in heterozygous females (Sirugo et al., 2014). In Botswana, data exist on the frequency of the African G6PD A- allele. Early publications from the 1960s and 1970s could only find relatively low G6PD deficiency in the Khoisan peoples they studied (1–5% with a higher percentage in the Northwest), and surprisingly no deficiency in the Bantu (Jenkins et al., 1968). This finding is somewhat congruent with a recent study that discovered the G6PD deficiency rate in a pattern that peaks in the Northwest (3.37%) and decreases steadily with latitude until reaching its minimum in the Southeast of the country (Motshoge et al., 2018). Interestingly, these latter results reflect the distribution of malaria endemicity in Botswana. Glucose-6-Phosphate Dehydrogenase testing should be considered if radical cure for P. vivax via primaquine is ever utilized in Botswana, especially with regards to the use of a point of care diagnostic tool for G6PD deficiency, prior to treatment. It is worth noting that single dose primaquine, that is gametocytocidal for P. falciparum, has been shown to be safe for G6PD deficient persons (World Health Organization, 2015b).
3.3.3. Duffy
Very little published data exists on the Duffy status of individuals in Botswana relative to their respective genetic backgrounds. In general, literature shows that there is a lack of the African-specific Duffy negativity among Khoisan (Nurse et al., 1977; Petersen et al., 2013; Schuster et al., 2010), consistent with the evolutionary history of this population. While, the Bantu expanded to East and Southern Africa from a region between southern Nigeria and Cameroon (Nurse and Philippson, 2005) where malaria was (and is still) endemic, which correlates with having Duffy negativity at a very high rate. Thus, Duffy negativity rate in Botswana mostly depends from the admixture history between the two main populations. One survey based on 79 unrelated subjects showed that 75.9% of North-eastern individuals from Botswana were Duffy negative (Fy(a-b-)) (Chasko et al., 1979). Another study by these authors found a similar frequency of the same phenotype in individuals from the Bakgalagadi tribe, which consists of peoples of mostly Bantu origin in the Southwest of the country (Jenkins et al., 1987). A recent global report on Duffy, which summed data from several surrounding countries, predicted a gradual decline in the frequency of the negative phenotype in the Southwest direction, especially as one crosses the border into Namibia (Howes et al., 2011). Given that the Bantu peoples originated from a region with near 100% Duffy negativity in Central-West Africa (Howes et al., 2011) and are found in increasing quantities in the Northeast of Botswana, this matches the ethnographic distribution in Botswana and surrounding countries. This hypothesis also aligns with a model recently proposed in the literature on the origin of P. vivax in West Africa (Prugnolle et al., 2013), where Duffy negativity is at its highest level, then decreasing in frequency from the epicentre south-eastwards. Despite being originally thought to provide protection against P. vivax, Duffy negative individuals appear to still be at risk for P. vivax infection (Howes et al., 2015), since it is now proven that for red cell invasion by P. vivax Duffy is not an absolute requirement (Popovici et al., 2020). However, there is no available data on whether P. vivax infected subjects from Botswana are Duffy-negative or Duffy-positive.
4. Malaria and COVID-19
As we write, the SARS-CoV-2 epidemic is ongoing in Botswana. The COVID-19 pandemic has forced countries to prioritize containment and mitigation measures such as social distancing and lockdowns as part of the primary response in reducing the number of cases. These measures have resulted in readjustment of resources that were initially allocated towards routine public health programs such as immunization and surveillance. Given that majority of malaria cases occur in Sub-Saharan Africa, one of the poorest regions in the world, the further readjusting of already limited resources is likely to put a strain on Governments in the provision of quality healthcare.
Furthermore, the similarity of the most symptoms associated between COVID-19 and malaria (such as fever and fatigue) has the potential to misdiagnose individuals. This places further strain on healthcare resources. It is important to note that all efforts towards malaria elimination should still continue in order to not erode all efforts and milestones that have been achieved.
5. Conclusions
Botswana has made massive strides over the past 50 years with malaria control initiatives and has come remarkably close to elimination with the turn of the millennia. However, an increase of more than 80% of malaria cases between 2015 and 2017 led to the WHO placing Botswana in the top three most concerning E-2020 countries in 2018 (World Health Organization, 2018). This review surveyed the literature describing three major biological factors that impact malaria in Botswana: vector diversity, Plasmodium species, and host genetics. To date, a very limited number of pertinent primary publications assess these three factors in Botswana. Additional studies that describe the behavior of primary vectors and the vectorial capacity of other mosquito species, in concert with research assessing and characterizing the parasite reservoir, are warranted in order to direct elimination efforts and to understand transmission in Botswana. Finally, future studies into the unique and diverse genetic background of Batswana should also be considered for insights into its complex interaction with malaria. Overall, these three major biological factors should be considered in order to craft an effective elimination strategy for the country.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]; by Wellcome Trust [grant # Z/212638] and from the Botswana-University of Pennsylvania Partnership, Penn Center for AIDS Research [grant # P30 AI045008]. We would like to thank the entire joint University of Botswana-University of Pennsylvania molecular laboratory for their continuous support and dialogue. As well as staff from the Botswana-University of Pennsylvania Partnership and University of Botswana for their assistance. Finally, we would like to acknowledge all of the talented students that always brighten our lab.
References
- Abdulla-Khan R. A Survey of the Anopheline Mosquito Fauna of Botswana, with Special Reference to the Malaria Vectors. 1995. http://wiredspace.wits.ac.za/handle/10539/20555
- Abdulla-Khan R., Coetzee M., Hunt R.H. Description of Anopheles (Cellia) seretsei sp. nov. from Kasane, Botswana. J. Am. Mosq. Control Assoc. 1998;14:248–252. [PubMed] [Google Scholar]
- Amanfo S.A., Mduluza T., Midzi N., Cavanagh D.R., Mutapi F. Seroepidemiology of Plasmodium species infections in Zimbabwean population. Malar. J. 2016;15:267. doi: 10.1186/s12936-016-1325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori S., Galimberti L., Milazzo L., Corbellino M. Biology of human malaria plasmodia including Plasmodium Knowlesi. Mediterr. J. Hematol. Infect. Dis. 2012 doi: 10.4084/MJHID.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio-Nkondjio C., Simard F., Awono-Ambene P., Ngassam P., Toto J.-C., Tchuinkam T., et al. Malaria vectors and urbanization in the equatorial forest region of South Cameroon. Trans. R. Soc. Trop. Med. Hyg. 2005;99:347–354. doi: 10.1016/j.trstmh.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Ashley E.A., Recht J., White N.J. Primaquine: the risks and the benefits. Malar. J. 2014;13:418. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J.K. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–539. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Botswana Ministry of Health Malaria Strategic Plan- 2010-2015. 2010. https://endmalaria.org/sites/default/files/botswa2010-2015.pdf
- Burke A., Dandalo L., Munhenga G., Dahan-Moss Y., Mbokazi F., Ngxongo S., et al. A new malaria vector mosquito in South Africa. Sci. Rep. 2017;7:43779. doi: 10.1038/srep43779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A., Dahan-Moss Y., Duncan F., Qwabe B., Coetzee M., Koekemoer L., et al. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar. J. 2019;18:257. doi: 10.1186/s12936-019-2889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara G.C., Petrarca V., Niang M., Coluzzi M. Anopheles pharoensis and transmission of Plasmodium falciparum in the Senegal River delta, West Africa. Med. Vet. Entomol. 1990;4:421–424. doi: 10.1111/j.1365-2915.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Causer L.M., Newman R.D., Barber A.M., Roberts J.M., Stennies G., Peter Bloland, et al. Vol. 51. 2002. Malaria Surveillance --- United States, 2000. MMWR Surveillance Summaries; pp. 9–21. [Google Scholar]
- Chasko W.J., Nurse G.T., Harpending H.C., Jenkins T. Botswana Notes and Records. Vol. 11. 1979. Sero-genetic studies on the “Masarwa” of northeastern Botswana; pp. 15–23. [Google Scholar]
- Chirebvu E., Chimbari M.J., Ngwenya B.N. Assessment of risk factors associated with malaria transmission in Tubu Village, Northern Botswana. Malaria Res. Treat. 2014 doi: 10.1155/2014/403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirebvu E., Chimbari M.J., Ngwenya B.N., Sartorius B. Clinical malaria transmission trends and its association with climatic variables in Tubu Village, Botswana: a retrospective analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0139843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee M., Fontenille D. Advances in the study of Anopheles funestus, a major vector of malaria in Africa. Insect Biochem. Mol. Biol. 2004;34:599–605. doi: 10.1016/j.ibmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Coetzee M., Craig M., le Sueur D. Parasitol Today (Regul Ed) Vol. 16. 2000. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex; pp. 74–77. [DOI] [PubMed] [Google Scholar]
- Coluzzi M., Sabatini A., Petrarca V., Di Deco M.A. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Cornel A.J., Lee Y., Almeida A.P.G., Johnson T., Mouatcho J., Venter M., et al. Mosquito community composition in South Africa and some neighboring countries. Parasit. Vectors. 2018;11:331. doi: 10.1186/s13071-018-2824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M.H., Sharp B.L., Mabaso M.L., Kleinschmidt I. Developing a spatial-statistical model and map of historical malaria prevalence in Botswana using a staged variable selection procedure. Int. J. Health Geogr. 2007;6:44. doi: 10.1186/1476-072X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.A., Arguin P.M. Malaria surveillance – United States, 2012. MMWR Surveill. Summ. 2014;63:1–22. [PubMed] [Google Scholar]
- Daygena T.Y., Massebo F., Lindtjørn B. Variation in species composition and infection rates of Anopheles mosquitoes at different altitudinal transects, and the risk of malaria in the highland of Dirashe Woreda, South Ethiopia. Parasit. Vectors. 2017;10:343. doi: 10.1186/s13071-017-2288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler S., Causer L.M., Newman R.D., Barber A.M., Roberts J.M., MacArthur J., et al. Malaria surveillance --- United States, 2001. MMWR Surveill. Summ. 2003;52:1–14. [PubMed] [Google Scholar]
- Grosse S.D., Odame I., Atrash H.K., Amendah D.D., Piel F.B., Williams T.N. Sickle cell disease in Africa. Am. J. Prev. Med. 2011;41:398–405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiyambo D.H., Uusiku P., Mumbengegwi D., Pernica J.M., Bock R., Malleret B., et al. Molecular detection of P. vivax and P. ovale foci of infection in asymptomatic and symptomatic children in Northern Namibia. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane J.B.S. The rate of mutation of human genes. Hereditas. 1949;35:267–273. [Google Scholar]
- Hansford C.F. Recent trends in the control and treatment of malaria. S. Afr. Med. J. 1972;46:635–637. [PubMed] [Google Scholar]
- Hargreaves K., Koekemoer L.L., Brooke B.D., Hunt R.H., Mthembu J., Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med. Vet. Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- Holtz T.H., Kachur S.P., MacArthur J.R., Robertys J.M., Barber A.M., Steketee R.W., et al. Malaria surveillance --- United States, 1998. MMWR Surveill. Summ. 2001;50:1–18. [PubMed] [Google Scholar]
- Howes R.E., Patil A.P., Piel F.B., Nyangiri O.A., Kabaria C.W., Gething P.W., et al. The global distribution of the Duffy blood group. Nat. Commun. 2011;2:266. doi: 10.1038/ncomms1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes R.E., Reiner R.C., Battle K.E., Longbottom J., Mappin B., Ordanovich D., et al. Plasmodium vivax transmission in Africa. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis D.L., Dao A., Diallo M., Sanogo Z.L., Samake D., Yaro A.S., et al. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature. 2019:1–5. doi: 10.1038/s41586-019-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya K. Settlement patterns and Sedentarization among the san in the central Kalahari (1930-1996) Senri Ethnological Studies. 2018;99:177–196. [Google Scholar]
- Jenkins T., Blecher S.R., Smith A.N., Anderson C.G. Some hereditary red-cell traits in Kalahari bushmen and bantu: hemoglobins, glucose-6-phosphate dehydrogenase deficiency, and blood groups. Am. J. Hum. Genet. 1968;20:299–309. [PMC free article] [PubMed] [Google Scholar]
- Jenkins T., Speirs J., Dunn D.S., Nurse G.T. Serogenetic and haematological studies on the Kgalagadi of Botswana. Ann. Hum. Biol. 1987;14:143–153. doi: 10.1080/03014468700006872. [DOI] [PubMed] [Google Scholar]
- Kent S. Unstable households in a stable Kalahari Community in Botswana. Am. Anthropol. 1995;97:297–312. [Google Scholar]
- Kgoroebutswe T.K., Ramatlho P., Reeder S., Makate N., Paganotti G.M. Distribution of Anopheles mosquito species, their vectorial role and profiling of knock-down resistance mutations in Botswana. Parasitol. Res. 2020 doi: 10.1007/s00436-020-06614-6. [DOI] [PubMed] [Google Scholar]
- Koekemoer L.L., Coetzee M., Hunt R.H. Hpall endonuclease distinguishes between two species in the Anopheles funestus group. Insect Mol. Biol. 1998;7:273–277. doi: 10.1046/j.1365-2583.1998.00072.x. [DOI] [PubMed] [Google Scholar]
- Kublin J.G., Cortese J.F., Njunju E.M., Mukadam R.A., Wirima J.J., Kazembe P.N., et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- Kulkarni M.A., Kweka E., Nyale E., Lyatuu E., Mosha F.W., Chandramohan D., et al. Entomological evaluation of malaria vectors at different altitudes in Hai district, northeastern Tanzania. J. Med. Entomol. 2006;43:580–588. doi: 10.1603/0022-2585(2006)43[580:eeomva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D.P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyalo D., Amratia P., Mundia C.W., Mbogo C.M., Coetzee M., Snow R.W. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. Wellcome Open Res. 2017 doi: 10.12688/wellcomeopenres.12187.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main B.J., Lee Y., Ferguson H.M., Kreppel K.S., Kihonda A., Govella N.J., et al. The genetic basis of host preference and resting behavior in the major African malaria vector, Anopheles arabiensis. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingard J.F. Some notes on health and disease among the bushmen of the southern Kalahari. Bantu Studies. 1937;11:285–295. [Google Scholar]
- Mastbaum O. Unpublished Document; The Botswana National Archives and Records Services: 1944. Clinical and on Entomological Surveys in Botswana: Report by the Chief Medical Officer. [Google Scholar]
- Mbogo C.N., Snow R.W., Khamala C.P., Kabiru E.W., Ouma J.H., Githure J.I., et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am. J. Trop. Med. Hyg. 1995;52:201–206. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- Moakofhi K., Edwards J.K., Motlaleng M., Namboze J., Butt W., Obopile M., et al. Public Health Action. 2018. Advances in malaria elimination in Botswana: a dramatic shift to parasitological diagnosis, 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., An X. Malaria and human red blood cells. Med. Microbiol. Immunol. 2012;201:593–598. doi: 10.1007/s00430-012-0272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motshoge T., Ababio G.K., Aleksenko L., Read J., Peloewetse E., Loeto M., et al. Molecular evidence of high rates of asymptomatic P. vivax infection and very low P. falciparum malaria in Botswana. BMC Infect. Dis. 2016 doi: 10.1186/s12879-016-1857-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motshoge T., Ababio G., Aleksenko L., Souda S., Muthoga C.W., Mutukwa N., et al. Prevalence of G6PD deficiency and associated haematological parameters in children from Botswana. Infect. Genet. Evol. 2018;63:73–78. doi: 10.1016/j.meegid.2018.05.014. [DOI] [PubMed] [Google Scholar]
- N'ajera-Morrondo J.A. Malaria control : history shows it's possible. World health 1991 ; Sep-Oct : 4–5. 1991. https://apps.who.int/iris/handle/10665/48350
- Nurse D., Philippson G. The bantu languages. Bull. Sch. Orient. Afr. Stud. 2005;68:500–502. [Google Scholar]
- Nurse G.T., Botha M.C., Jenkins T. Sero-genetic studies on the san of south West Africa. Hum. Hered. 1977;27:81–98. doi: 10.1159/000152855. [DOI] [PubMed] [Google Scholar]
- Odiere M., Bayoh M.N., Gimnig J., Vulule J., Irungu L., Walker E. Sampling outdoor, resting anopheles gambiae and other mosquitoes (Diptera: Culicidae) in Western Kenya with clay pots. J. Med. Entomol. 2007;44:14–22. doi: 10.1603/0022-2585(2007)44[14:soraga]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogola E.O., Fillinger U., Ondiba I.M., Villinger J., Masiga D.K., Torto B., et al. Insights into malaria transmission among Anopheles funestus mosquitoes. Kenya. Parasit Vectors. 2018;11:577. doi: 10.1186/s13071-018-3171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyabe D.Y., Conn J.E. The distribution of two major malaria vectors, Anopheles gambiae and Anopheles arabiensis, in Nigeria. Mem. Inst. Oswaldo Cruz. 2001;96:1081–1084. doi: 10.1590/s0074-02762001000800009. [DOI] [PubMed] [Google Scholar]
- Pachka H., Annelise T., Alan K., Power T., Patrick K., Véronique C., et al. Rift Valley fever vector diversity and impact of meteorological and environmental factors on Culex pipiens dynamics in the Okavango Delta. Botswana. Parasit Vectors. 2016;9:434. doi: 10.1186/s13071-016-1712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen D.C., Libiger O., Tindall E.A., Hardie R.-A., Hannick L.I., Glashoff R.H., et al. Complex patterns of genomic admixture within southern Africa. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel F.B., Patil A.P., Howes R.E., Nyangiri O.A., Gething P.W., Williams T.N., et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat. Commun. 2010;1:104. doi: 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici J., Roesch C., Rougeron V. The enigmatic mechanisms by which Plasmodium vivax infects Duffy-negative individuals. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prugnolle F., Rougeron V., Becquart P., Berry A., Makanga B., Rahola N., et al. Diversity, host switching and evolution of Plasmodium vivax infecting African great apes. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8123–8128. doi: 10.1073/pnas.1306004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Republic of Botswana . National Malaria Programme; Gaborone: 2015. Revised Guidelines for the Diagnosis and Treatment of Malaria in Botswana. [Google Scholar]
- Republic of Botswana . National Malaria Programme; Gaborone: 2018. National Plan for Insecticide Resistance Prevention and Management in Malaria Vectors 2018-2021. [Google Scholar]
- Retshabile G., Mlotshwa B.C., Williams L., Mwesigwa S., Mboowa G., Huang Z., et al. Whole-exome sequencing reveals uncaptured variation and distinct ancestry in the southern African population of Botswana. Am. J. Hum. Genet. 2018;102:731–743. doi: 10.1016/j.ajhg.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlebusch C.M., Prins F., Lombard M., Jakobsson M., Soodyall H. The disappearing san of southeastern Africa and their genetic affinities. Hum. Genet. 2016;135:1365–1373. doi: 10.1007/s00439-016-1729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S.C., Miller W., Ratan A., Tomsho L.P., Giardine B., Kasson L.R., et al. Complete Khoisan and bantu genomes from southern Africa. Nature. 2010;463:943–947. doi: 10.1038/nature08795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M.E., Bangs M.J., Manguin S., Coetzee M., Mbogo C.M., Hemingway J., et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirugo G., Predazzi I.M., Bartlett J., Tacconelli A., Walther M., Williams S.M. G6PD A- deficiency and severe malaria in the Gambia: heterozygote advantage and possible homozygote disadvantage. Am. J. Trop. Med. Hyg. 2014;90:856–859. doi: 10.4269/ajtmh.13-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Botswana Annual report 2017/18. 2018. http://www.statsbots.org.bw/sites/default/files/documents/Statistics%20Botswana%20Annual%20Report%202018.pdf
- Stevenson J.C., Simubali L., Mbambara S., Musonda M., Mweetwa S., Mudenda T., et al. Detection of Plasmodium falciparum infection in Anopheles squamosus (Diptera: Culicidae) in an area targeted for malaria elimination, Southern Zambia. J. Med. Entomol. 2016;53:1482–1487. doi: 10.1093/jme/tjw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabue R.N., Nem T., Atangana J., Bigoga J.D., Patchoke S., Tchouine F., et al. Anopheles ziemanni a locally important malaria vector in Ndop health district, north west region of Cameroon. Parasit. Vectors. 2014;7:262. doi: 10.1186/1756-3305-7-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabue R.N., Awono-Ambene P., Etang J., Atangana J., Antonio-Nkondijo C., Toto J.C., et al. Role of Anopheles (Cellia) rufipes (Gough, 1910) and other local anophelines in human malaria transmission in the northern savannah of Cameroon: a cross-sectional survey. Parasit. Vectors. 2017;10:22. doi: 10.1186/s13071-016-1933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawe L., Ramatlho P., Waniwa K., Muthoga C.W., Makate N., Ntebela D.S., et al. Preliminary survey on Anopheles species distribution in Botswana shows the presence of Anopheles gambiae and Anopheles funestus complexes. Malar. J. 2017;16:106. doi: 10.1186/s12936-017-1756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawe L., Menegon M., Ramatlho P., Muthoga C.W., Mutukwa N., Vurayai M., et al. Molecular surveillance of Plasmodium falciparum drug resistance markers in clinical samples from Botswana. Am. J. Trop. Med. Hyg. 2018;99:1499–1503. doi: 10.4269/ajtmh.18-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawe L., Motshoge T., Ramatlho P., Mutukwa N., Muthoga C.W., Dongho G.B.D., et al. Human cytochrome P450 2B6 genetic variability in Botswana: a case of haplotype diversity and convergent phenotypes. Sci. Rep. 2018;8:4912. doi: 10.1038/s41598-018-23350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thami P.K., Chimusa E.R. Population structure and implications on the genetic architecture of HIV-1 phenotypes within southern Africa. Front. Genet. 2019 Sep 27;10:905. doi: 10.3389/fgene.2019.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson M.C., Mason S.J., Phindela T., Connor S.J. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am. J. Trop. Med. Hyg. 2005;73:214–221. [PubMed] [Google Scholar]
- Ukpe I.S. Plasmodium ovale in South Africa. Trans. R. Soc. Trop. Med. Hyg. 1998;92:574. doi: 10.1016/s0035-9203(98)90925-7. [DOI] [PubMed] [Google Scholar]
- Wilkes T.J., Matola Y.G., Charlwood J.D. Anopheles rivulorum, a vector of human malaria in Africa. Med. Vet. Entomol. 1996;10:108–110. doi: 10.1111/j.1365-2915.1996.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Williams H.A., Roberts J., Kachur P., Barber A.M., Barat L.M., Bloland P.B., et al. Malaria surveillance – United States, 1995. MMWR Surveill. Summ. 1999;48:1–21. [PubMed] [Google Scholar]
- Winters O.J. 2015. The Botswana Bushmen’s Fight for Water & Land Rights in the Central Kalahari Game Reserve; pp. 285–299. Consilience, no. 13. [Google Scholar]
- Wolfe H.L. Plasmodium ovale in Zambia. Bull. World Health Organ. 1968;39:947–948. [PMC free article] [PubMed] [Google Scholar]
- Wongsrichanalai C., Pickard A.L., Wernsdorfer W.H., Meshnick S.R. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Guidelines for the Treatment of Malaria. 2015. Global malaria programme.https://apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng.pdf?sequence=1 Accessed 12 Nov 2019. [Google Scholar]
- World Health Organization World Malaria Report. 2015. https://apps.who.int/iris/bitstream/handle/10665/200018/9789241565158_eng.pdf;jsessionid=D4F6FF52429056815942B06C6DFED967?sequence=1 Accessed 3 Oct 2019.
- World Health Organization World Malaria Report. 2018. https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1 Accessed 3 Oct 2019.
- World Health Organization World Malaria Report. 2019. http://www.who.int/malaria/publications/world-malaria-report-2019/en/ Accessed 30 Jan 2020.