Abstract
Background
Complement may contribute to platelet destruction in immune thrombocytopenia (ITP), but serum complement levels of ITP patients are not well defined. This study characterized C3, C4, and CH50 levels from 108 ITP patients in comparison with 120 healthy subjects.
Methods
Results of complement testing performed using commercially available turbidimetric immunoassays were retrospectively analyzed. Mean complement levels in patients with ITP were compared with levels from a sample of 120 healthy subjects, and subgroups of ITP patients were compared. Regression analyses evaluated for relations between low complement levels and disease severity and response to ITP treatments.
Results
One hundred eight patients with ITP were included. Mean C3, C4, and CH50 were significantly lower in patients with ITP compared with healthy subjects, largely driven by the 32% of patients with ITP with substantial reductions in one or more assays. Patients requiring treatment had lower mean C4 (18.1 vs 23.1 mg/dL; P = .042) and CH50 (50.4 vs 63.0 mg/dL; P = .004). Mean C3 was higher in splenectomized versus nonsplenectomized patients (120.6 vs 101.0 mg/dL; P = .035). In multivariable analyses, reduced complement did not predict treatment response to corticosteroids, intravenous immunoglobulin, or thrombopoietin receptor agonists but low C4 levels did predict more severe ITP (relative to nonsevere disease, odds ratio for severe/refractory disease: 6.28; 95% confidence interval, 0.75‐52.54; P = .090). Complement levels in patients with ITP were generally consistent over repeat measurements.
Conclusions
Complement levels are reduced in one‐third of patients with ITP and are associated with more severe disease. Additional study is needed to evaluate if hypocomplementemia is predictive of response to emerging complement‐directed therapies.
Keywords: blood platelets, complement C3, complement C4, complement hemolytic activity assay, idiopathic, immune thrombocytopenia, purpura, sutimlimab, thrombocytopenic
Essentials.
Complement may contribute to platelet destruction in immune thrombocytopenia (ITP) but this process is poorly understood.
This study evaluated C3, C4, and CH50 in 108 patients with ITP, compared with 120 healthy subjects.
All complement assays were significantly lower in the ITP group versus the healthy subject group.
Reductions in ≥1 complement assay were found in 32% of patients with ITP.
1. INTRODUCTION
Immune thrombocytopenia (ITP) is a relatively common autoimmune disorder resulting in increased bleeding risk, fatigue, and reduced quality of life. 1 Multiple pathophysiologic mechanisms have been demonstrated to contribute to the disease process, including activation of complement by autoantibodies bound to the platelet surface. 2 , 3 , 4 In recognition of this pathophysiologic mechanism, novel therapeutics targeting the complement pathway in ITP are currently in clinical trials. Preliminary results of a phase I trial of sutimlimab (BIVV009), a monoclonal antibody inhibiting C1s, were recently presented, describing a response rate of 50% in 8 patients refractory to multiple other treatments and a meaningful rise in the platelet count occurring within 8 hours of infusion. 5 Additionally, clinical benefit was seen in refractory patients with ITP treated with TNT003 (another C1s inhibitor) as well as a C1 esterase inhibitor. 6 , 7 Despite ongoing clinical studies of complement inhibitors and evidence of the complement‐fixing nature of glycoprotein‐specific platelet autoantibodies, there are limited data describing serum complement levels in patients with ITP or any relation that may exist between these levels and the severity of disease or response to current ITP therapies. Furthermore, we hypothesize that patients with ITP with complement‐mediated platelet destruction as a major pathophysiologic component, as indicated by reduced serum complement levels, may be more likely to respond to emerging complement‐inhibitory therapies. Because clinical serum complement evaluation has been a routine part of initial patient evaluation at our ITP center for the past 3 years (with repeat measurements performed at provider discretion), we sought to evaluate the serum complement levels of C3, C4, and total hemolytic complement (CH50) in patients with ITP and their relation to clinical features. These 3 assays were selected for evaluation because they are the standard complement assays obtained for clinical purposes and are widely available and used in clinical settings.
2. METHODS
This study was approved by the Institutional Review Board (approval 2018P000964) of the Massachusetts General Hospital. Patients aged ≥ 18 treated by hematology providers at the Massachusetts General Hospital with a diagnosis of ITP and with at least 1 measurement of C3, C4, or CH50 obtained between January 1, 2016, and March 29, 2019, were identified via query of the Partners Healthcare Research Patient Data Registry (RPDR). The RPDR is a large patient data repository containing comprehensive electronic health record data for over 6 million patients in the Partners Healthcare system. 8 Satisfaction of the 2011 American Society of Hematology (ASH) ITP diagnostic criteria were required for inclusion of patients with ITP. 9 Exclusion criteria included remission of ITP, concurrent hemolytic anemia (Evans syndrome), rheumatologic disorder (such as systemic lupus erythematosus), or any other clinical disorder known to be complement mediated or result in a reduction of serum complement levels. Data collected for analysis included dates and results of complement testing (including disease status and platelet count at time of testing), patient demographics, and disease characteristics (date of ITP diagnosis, treatment history, and treatment at time of complement testing). ASH 2011 ITP guidelines of disease severity classification and response to treatment were used for data analysis. 9 ASH 2011 guidelines classify active ITP into 3 severity categories: patients with nonsevere ITP have not developed bleeding symptoms requiring treatment; patients with severe ITP have developed bleeding symptoms requiring treatment; and patients with refractory ITP have undergone splenectomy but subsequently developed bleeding symptoms requiring additional treatment.
Serum C3, C4, and CH50 were measured using a commercially available turbidimetric immunoassay (Optilite System, Binding Site, Birmingham, UK). 10 Results from patients with ITP at our institution were collected and compared with results from 120 healthy subjects obtained from the assay manufacturer. For patients with ITP with >1 measurement of a given complement assay, the first measurement was used for analyses. Wilcoxon rank‐sum tests and t tests were used to compare patients with ITP with healthy subjects and compare ITP patient subgroups, depending on the distribution of the data. ITP patient subgroups analyzed included those receiving treatment versus not receiving treatment, splenectomized versus not splenectomized, and positive for platelet autoantibodies versus negative for platelet autoantibodies (measured using the PakAuto direct glycoprotein‐specific platelet autoantibody assay evaluating for anti‐glycoprotein (GP) IIb/IIIa, anti‐GPIb/IX, and anti‐GPIa/IIa antibodies; Immucor, Brookfield, WI, USA). Additionally, because corticosteroids can potentially lower complement production, 11 , 12 complement levels in patients with ITP receiving corticosteroids were compared with those not receiving corticosteroids.
Multivariable logistic regression was used to model the probability of low complement levels based on disease severity (nonsevere vs severe or refractory) and platelet count, as well as model the probability of response to treatment (corticosteroids, intravenous immunoglobulin [IVIG], or thrombopoietin receptor agonists) based on complement levels. Low complement levels were defined in binary fashion as levels below the lower limit of the reference range of a given assay (C3, 81.1 mg/dL; C4, 12.9 mg/dL; CH50, 41.7 U/mL). For patients with multiple measurements of a given complement assay, consistency of measurement was evaluated over time. Statistical analysis was performed, and graphs for figures were prepared using Stata version 14.2 (StataCorp LLC, College Station, TX, USA), Prism 7 (GraphPad, Inc, La Jolla, CA, USA), and Microsoft Excel 360 (Microsoft Corp., Redmond, WA, USA).
3. RESULTS AND DISCUSSION
Of the 111 ITP patients identified from the RPDR query, 108 patients were included in the analysis. Three were excluded because of concomitant autoimmune hemolytic anemia and/or systemic lupus erythematosus. Of these 108 patients, 98 had one or more C3 assays, 97 had ≥1 C4 assays, and 102 had ≥1 CH50 assays performed; 93 patients had all 3 assays performed. Characteristics of patients with ITP are detailed in Table 1.
Table 1.
Baseline characteristics of cohort of patients with ITP (N = 108)
| Baseline characteristic | Value |
|---|---|
| Age, y, median (range) | 53 (18‐89) |
| Female, % | 54 |
| Platelet count (×109/L) at time of complement testing, median (range) | 66 (2‐595) |
| ITP duration in years at time of complement testing, median (range) | 8.0 (0.0‐59.6) |
| Post‐splenectomy, n (%) | 17 (16) |
| Platelet autoantibody a positive, n (%) | 59 (75) |
| Anti‐GPIIb/IIIa antibodies a , n (%) | 54 (71) |
| Anti‐GPIb/IX antibodies, a n (%) | 47 (63) |
| Anti‐GPIa/IIa antibodies, a % | 31 (41) |
| On ITP treatment at time of complement testing, b n (%) | 56 (52) |
| Corticosteroids, n (%) | 18 (17) |
| Romiplostim, n (%) | 19 (18) |
| Eltrombopag, n (%) | 16 (15) |
| Rituximab, n (%) | 2 (2) |
| Fostamatinib, n (%) | 2 (2) |
| Other, n (%) | 8 (7) |
Abbreviations: GP, glycoprotein; ITP, immune thrombocytopenia.
Direct glycoprotein‐specific platelet autoantibody testing (PakAuto assay; Immucor, Brookfield, WI, USA) available in 76 patients at or before the time of complement testing included.
Fourteen patients were receiving >1 agent at the time of complement testing.
Thirty‐two percent of patients with ITP had levels measured below the lower limit of the reference range for at least 1 complement assay, and 10% of patients with ITP had reduced levels of all 3 assays. Mean serum C3, C4, and CH50 levels for patients with ITP and healthy controls are described in Table 2. Figure 1 demonstrates the difference in distribution between the 108 patients with ITP and the 120 healthy controls for C3 (Figure 1A), C4 (Figure 1B), and CH50 (Figure 1C). The means of the 2 groups were significantly different for all 3 assays (C3 and C4, P < .001 by Wilcoxon rank‐sum test; CH50, P = .005 by 2‐tailed unpaired t test), with patients with ITP having a lower mean level of complement than healthy controls. Subgroup analysis was performed on patients with ITP requiring treatment (N = 56) versus those not requiring treatment (N = 52), as well as those who were splenectomized (N = 17) versus those not (N = 91), demonstrating significantly lower serum C4 and C50 in patients with ITP requiring treatment as opposed to those who did not (Table 3) and significantly higher serum C3 in splenectomized patients (Table 3). There were no significant differences in any of the evaluated complement assays based on platelet autoantibody positivity versus negativity, or patients receiving versus not receiving corticosteroids.
Table 2.
Comparison of complement assay results in patients with ITP (N = 108) versus healthy subjects (N = 120)
| Assay | Patients with ITP | Healthy subjects | P value |
|---|---|---|---|
| Mean serum C3, mg/dL (95% CI) | 104.2 (97.6‐110.8) | 116.8 (113.0‐120.3) | <.001 |
| Mean serum C4, mg/dL (95% CI) | 20.4 (17.7‐23.2) | 24.1 (22.83‐25.33) | <.001 |
| Mean serum CH50, U/mL (95% CI) | 62.9 (59.6‐66.1) | 68.4 (66.2‐71.1) | .005 |
D’Agostino & Pearson normality testing performed on all groups; CH50 results are parametric (and so compared with 2‐tailed unpaired t test), and C3 and C4 results are nonparametric (and so compared with Wilcoxon rank‐sum test). The reference ranges for each assay are as follows: C3, 81.1‐157.0 mg/dL; C4, 12.9‐39.2 mg/dL; CH50, 41.7‐68.7 U/mL.
Abbreviations: CI, confidence interval; ITP, immune thrombocytopenia.
Figure 1.
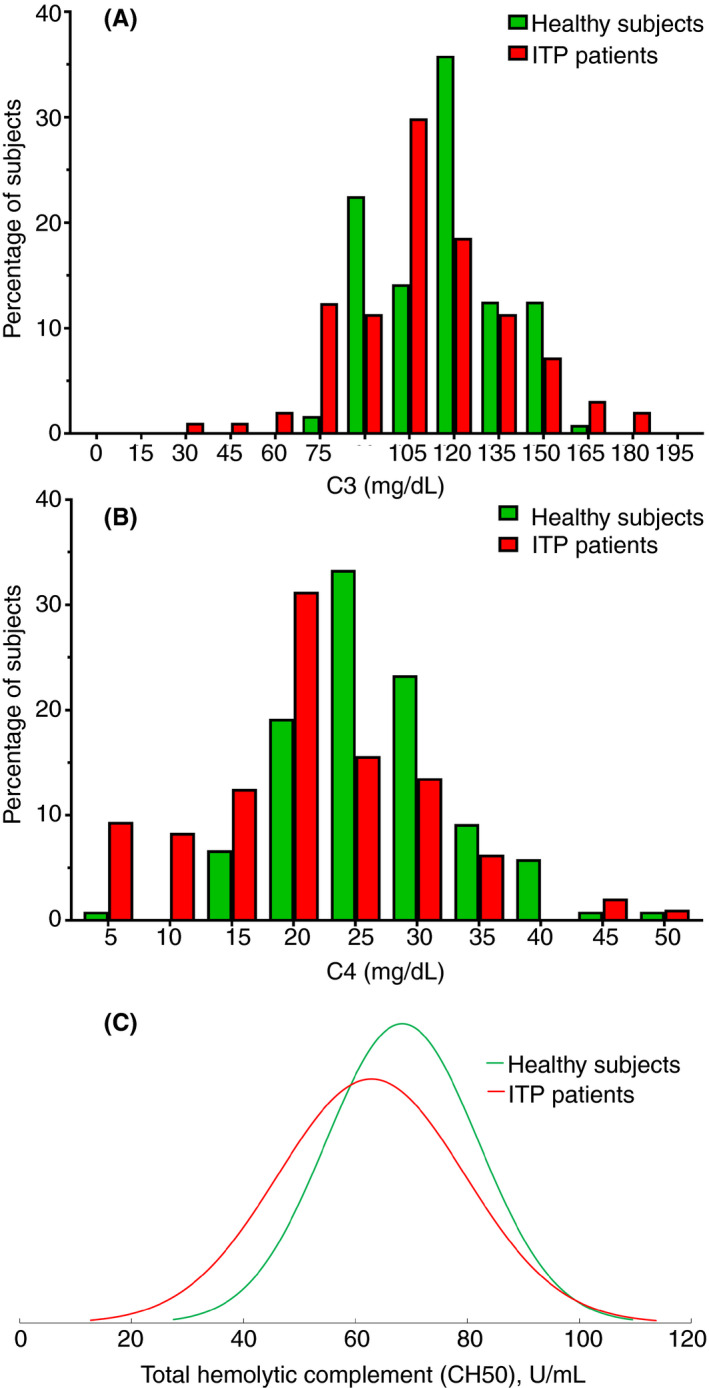
Distributions of C3, C4, and CH50 measurements in patients with ITP (red) versus healthy subjects (green). (A) C3 (nonparametric). (B) C4 (nonparametric). (C) CH50 (parametric). (A) and (B) are interleaved histograms (bin size 15 for A and 5 for B) with results for each group (ITP patients and healthy subjects) paired at each bin to facilitate comparison. Values on the X axis are the center value for a given bin. ITP, immune thrombocytopenia
Table 3.
Subgroup analyses of ITP patients
| Assay | Treated | Not treated | P value | Splenectomized | Not splenectomized | P value |
|---|---|---|---|---|---|---|
| Mean serum C3, mg/dL (95% CI) | 103.8 (93.2‐114.3) | 104.7 (96.9‐112.5) | .90 | 120.6 (92.2‐148.9) | 101.0 (95.2‐106.8) | .035 |
| Mean serum C4, mg/dL (95% CI) | 18.1 (15.0‐21.2) | 23.1 (18.3‐27.8) | .04 | 21.6 (14.0‐29.3) | 20.2 (17.2‐23.2) | .952 |
| Mean serum CH50, U/mL (95% CI) | 50.4 (43.7‐57.2) | 63.0 (59.2‐66.9) | .004 | 55.0 (44.3‐65.6) | 56.6 (52.1‐61.2) | .667 |
Comparison of complement assay results in patients with ITP requiring treatment (N = 56) versus patients with ITP not requiring treatment (N = 52) and splenectomized patients with ITP (N = 17) versus nonsplenectomized patients with ITP (N = 91). Groups compared with Wilcoxon rank‐sum test.
Abbreviations: CI, confidence interval; ITP, immune thrombocytopenia.
Multivariable logistic regression analyses including age, sex, splenectomy status, disease severity, platelet count at time of complement assay, and results of complement testing demonstrated a relation between low C4 levels and presence of severe or refractory disease (relative to nonsevere disease, odds ratio [OR] for severe/refractory disease 6.28; 95% confidence interval [CI], 0.75‐52.54; P = .09) and low C3 levels and platelet count (OR for low C3 per 10 × 109/L reduction in platelet count, 1.04; 95% CI, 0.99‐1.08, P = .06). Univariable logistic regression had similar findings, suggesting little impact of covariates on the observed relationships (relation of low C4 levels and presence of severe or refractory disease; OR 5.76; 95% CI, 0.72‐46.11; P = .09; OR for low C3 per 10 × 109/L reduction in platelet count, 1.04; 95% CI 1.00‐1.08; P = .06). Both of these relations nearly missed statistical significance at an alpha of .05. Both univariable and multivariable logistic regression analyses including age, sex, splenectomy status, platelet count, treatment response, and results of complement testing did not demonstrate a relation between complement levels and response to corticosteroids, IVIG, or thrombopoietin receptor agonists.
Fifty‐two patients with ITP had multiple (median, 3; range, 2‐10) C3 and C4 measurements; consistency over time (all values normal or all values low) was noted in 81% of patients for both C3 and C4. Forty‐nine patients with ITP had multiple (median, 3; range, 2‐10) CH50 measurements; consistency over time was noted in 74% of patients.
Using commercially available clinical complement assays in a large cohort of patients with ITP, we found that approximately one‐third of patients with ITP have low levels in ≥1 assays and 1 in 10 patients have low levels in all 3 assays. While the mean levels of all 3 serum complement assays were statistically significantly lower in patients with ITP than controls, the absolute difference in the groups was not great, reflective of the fact that the difference was largely driven by the one‐third of patients with substantial reductions. There were no recurring or distinguishing clinical features of those patients with reductions in all 3 assays. We additionally observed relationships (although they were not statistically significant at an alpha of .05) between low C4 and more severe disease, low C3 and reduced platelet counts, and higher C3 in those patients who are post‐splenectomy, all consistent with prior studies finding that complement may play an important role in platelet destruction in a significant subset of patients with ITP. Furthermore, patients requiring treatment had significantly lower C4 and CH50 relative to those who did not. There were no significant differences in complement levels in patients with detectable platelet autoantibodies versus those without detectable autoantibodies, which is counterintuitive given that fixation of complement by platelet autoantibodies bound to the platelet surface 2 , 3 , 4 is believed to be the mechanism of complement‐mediated platelet destruction in this disease. Repeat serum complement measurements were performed primarily at the discretion of the treating hematologist and were relatively consistent over time in those with repeat measurements; this suggests that informative levels may be able to be drawn at random times rather than only at specific milestones (such as initial diagnosis, prior to initiation of therapy, etc). An exception to this may be possible changes that occur with splenectomy; further evaluation of complement levels before and after splenectomy in patients with ITP is needed to answer this question.
Serum complement levels did not predict response to existing ITP therapies that do not target the complement pathway, but we hypothesize that the subset of patients with ITP with reduced complement levels may be more likely to respond to complement‐inhibitory therapies such as sutimlimab. In the preliminary published results of a phase I study of sutimlimab for ITP (for which hypocomplementemia was not an inclusion criterion), 4 of 8 patients achieved the primary end point of a platelet count > 30×109/L and a > 2‐fold increase from baseline at 2 consecutive visits 7 days apart by day 14 after sutimlimab initiation. 5 Mean C4 levels rose considerably in the cohort receiving this agent. While additional data describing the safety and efficacy of sutimlimab is needed, low serum complement levels as measured by clinical complement assays may serve as a predictive biomarker for response to this agent. Routine measurement of complement levels is currently not indicated for diagnosis of ITP or its management, given the lack of any relation of hypocomplementemia to response to available therapies, but could potentially be useful if complement‐directed treatments become available.
The primary limitation of this study is its retrospective nature, which meant that complement assays were not drawn at uniform points in each patient’s disease course or relative to the timing of treatment administration. We did not measure the levels of all or even most complement proteins, as we focused our evaluation on widely available commercial complement assays. These assays represent the most clinically relevant complement testing, and this methodology aligned with our goal of evaluating the potential utility of complement assays as a predictive biomarker. Additional studies are needed to validate our findings and to assess reduced serum complement as a predictive biomarker for complement‐inhibitory treatment in ITP.
RELATIONSHIP DISCLOSURES
DK: research: Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Immunovant, Kezar, Principia, Protalex, Rigel, Takeda (Bioverativ); consulting: Actelion (Syntimmune), Agios, Alnylam, Amgen, Argenx, Bristol Myers Squibb (BMS), Caremark, CRICO, Daiichi Sankyo, Dova, Genzyme, Immunovant, Incyte, Kyowa‐Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Platelet Disorder Support Association, Principia, Protalex, Protalix, Rigel, Sanofi, Genzyme, Shionogi, Shire, Takeda (Bioverativ), UCB, Up‐To‐Date, Zafgen. HA‐S: consultancy: Agios, Dova; research funding: Agios, Dova, Amgen. AC declares nothing to report.
AUTHOR CONTRIBUTIONS
AC contributed to data collection, data analysis, writing the first draft of the manuscript, and final approval; DK contributed to study design, critical revision of the manuscript, and final approval; HA‐S contributed to study design, data collection, data analysis, creation of the tables and figures, critical revision of the manuscript, and final approval.
ACKNOWLEDGMENTS
H. Al‐Samkari is the recipient of the National Hemophilia Foundation‐Shire Clinical Fellowship Award, the Harvard KL2/Catalyst Medical Research Investigator Training Award, and the American Society of Hematology Scholar Award.
Cheloff AZ, Kuter DJ, Al‐Samkari H. Serum complement levels in immune thrombocytopenia: Characterization and relation to clinical features. Res Pract Thromb Haemost. 2020;4:807–812. 10.1002/rth2.12388
Handling Editor: Neil Zakai
Contributor Information
Abraham Z. Cheloff, @abrahamcheloff.
Hanny Al‐Samkari, Email: hal-samkari@mgh.harvard.edu, @hannyalsamkari.
REFERENCES
- 1. Efficace F, Mandelli F, Fazi P, Santoro C, Gaidano G, Cottone F, et al. Health‐related quality of life and burden of fatigue in patients with primary immune thrombocytopenia by phase of disease. Am J Hematol. 2016;91:995–1001. [DOI] [PubMed] [Google Scholar]
- 2. Najaoui A, Bakchoul T, Stoy J, Bein G, Rummel MJ, Santoso S, et al. Autoantibody‐mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur J Haematol. 2012;88:167–74. [DOI] [PubMed] [Google Scholar]
- 3. Peerschke EI, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurata Y, Curd JG, Tamerius JD, McMillan R. Platelet‐associated complement in chronic ITP. Br J Haematol. 1985;60:723–33. [DOI] [PubMed] [Google Scholar]
- 5. Broome CM, Roeth A, Kuter DJ, Scully M, Smith R, Wang J, et al. Inhibition of the classical pathway of complement with sutimlimab in chronic immune thrombocytopenic purpura patients without adequate response to two or more prior therapies. Blood. 2019;134:898. [Google Scholar]
- 6. Peerschke EI, Panicker S, Bussel J. Classical complement pathway activation in immune thrombocytopenia purpura: inhibition by a novel C1s inhibitor. Br J Haematol. 2016;173:942–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roesch E, Broome CM. Complement blockade with C1 esterase inhibitor in refractory immune thrombocytopenia. Am J Hematol Oncol. 2016;12:20–5. [Google Scholar]
- 8. Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a research patient data repository. AMIA Annu Symp Proc. 2006;1044. [PMC free article] [PubMed] [Google Scholar]
- 9. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther M, et al. The American Society of Hematology evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;2011(117):4190–207. [DOI] [PubMed] [Google Scholar]
- 10. Nespola B, Comitogianni H, Jahn I, Goetz J. Evaluation of the Optilite((R)) analyser for determination of total complement activity and C3 and C4 fractions. Ann Biol Clin (Paris). 2019;77:447–52. [DOI] [PubMed] [Google Scholar]
- 11. Engelman RM, Rousou JA, Flack JE 3rd, Deaton DW, Kalfin R, Das DK. Influence of steroids on complement and cytokine generation after cardiopulmonary bypass. Ann Thorac Surg. 1995;60:801–4. [DOI] [PubMed] [Google Scholar]
- 12. Lappin DF, Whaley K. Modulation of complement gene expression by glucocorticoids. Biochem J. 1991;280(Pt 1):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


