Abstract
Background
Intracranial hemorrhage (ICH) is a common and often devastating outcome in patients with brain tumors. Despite this, there is little evidence to guide anticoagulation management following an initial ICH event.
Objectives
To analyze the risk of recurrent hemorrhagic and thrombotic outcomes after an initial ICH event in patients with brain tumors and prior venous thromboembolism (VTE).
Patients and Methods
A retrospective cohort study was performed. Radiographic images obtained after initial ICH were reviewed for the primary outcomes of recurrent ICH and VTE.
Results and Conclusions
A total of 79 patients with brain tumors who developed ICH on anticoagulation for VTE were analyzed. Fifty‐four patients (68.4%) restarted anticoagulation following ICH. The cumulative incidence of recurrent ICH at 1 year was 6.1% (95% confidence interval [CI], 1.5‐15.3) following reinitiation of anticoagulation. Following a major ICH (defined as an ICH >10 mL in size, causing symptoms, or requiring intervention), the rate of recurrent ICH upon reexposure to anticoagulation was 14.5% (95% CI, 2.1‐38.35), whereas the rate of recurrent ICH following smaller ICH was 2.6% (95% CI, 0.2%‐12.0%). Mortality following a recurrent ICH on anticoagulation was 67% at 30 days. The cumulative incidence of recurrent VTE was significantly lower in the restart cohort compared to patients who did not restart anticoagulation (8.1% vs 35.3%; P = .003). We conclude that resumption of anticoagulation is lowest among patients with metastatic brain tumors with small initial ICH. Following an initial major ICH, resumption of anticoagulation was associated with a high rate of recurrent ICH.
Keywords: anticoagulation, brain tumors, intracranial hemorrhage, venous thromboembolism
Essentials.
Intracranial hemorrhage (ICH) is common in patients with brain tumors on anticoagulation for venous thrombosis.
Severity of initial ICH appears to predict risk of recurrent ICH with resumption of anticoagulation.
Recurrent thrombosis is more common in patients who do not restart anticoagulation.
1. INTRODUCTION
Intracranial hemorrhage (ICH) is a common and potentially life‐threatening complication of primary and metastatic brain tumors, occurring spontaneously at rates between 10% and 20%. 1 , 2 , 3 , 4 Anticoagulation with low‐molecular‐weight heparin (LMWH) does not increase the risk of ICH in patients with brain metastases but is associated with a >3‐fold increased risk of ICH in patients with primary brain tumors. 5 , 6 Emerging data suggest that direct oral anticoagulants (DOACs) do not increase the risk of ICH in patients with primary or secondary brain tumors. 7
Despite evidence supporting the safety of anticoagulation with brain tumors, there is little evidence to guide anticoagulant management following an ICH event. In a recent systematic review of studies of adult patients who developed anticoagulation‐associated ICH, the reinstitution of anticoagulation was associated with a lower risk of venous thromboembolism (VTE) and similar rates of recurrent ICH. 8 However, this analysis included a total of 3 patients with brain tumors out of a total population of 1899. In light of the lack of data to guide anticoagulation management in patients with brain tumors who develop ICH, we analyzed the risk of recurrent hemorrhagic and thrombotic outcomes after an initial ICH event in the setting of brain tumors.
2. METHODS
2.1. Study design
The protocol was approved by the institutional review board at the Dana‐Farber Harvard Cancer Center. Data were extracted from the electronic medical record at Beth Israel Deaconess Medical Center from 2011 to 2019, as previously reported. 1 , 7 , 9 The database was updated using search criteria for the International Classification of Diseases, Ninth Revision and Tenth Revision codes for “malignant neoplasm of brain” and “malignant neoplasm of brain, metastatic.” The electronic medical record was manually reviewed for each case to ensure eligibility. Inclusion criteria for all cases included diagnosis of a solid tumor with histologically confirmed brain metastases or a World Health Organization grade III or IV glioma, including glioblastoma multiforme, anaplastic astrocytoma, or anaplastic oligodendroglioma; receiving anticoagulation following an objectively confirmed VTE by radiographic studies; diagnosis of ICH as confirmed by review by a collaborating neuro‐oncologist; and clinical care for brain tumor received at Beth Israel Deaconess Medical Center for at least 2 months.
The initial ICH events were reviewed by the neuro‐oncologist for hemorrhage classification and to calculate bleed volume using the one‐half ABC technique. 10 Intracranial hemorrhages were classified as trace, measurable, or major. 1 , 2 Trace hemorrhages were either too small to be measured or measured <1 mL in volume. Measurable ICHs were classified as those that measured ≥1 mL but <10 mL in volume. Major ICH was defined as those that measured ≥10 mL in volume; required surgical intervention; or were associated with clinical symptoms, focal neurologic deficits, or changes in cognitive function. Baseline demographics were recorded at time of brain tumor diagnosis and patients were recorded as having hypertension if hypertension was recorded in their past medical history and they required pharmacologic treatment. Patients were defined as having chronic kidney disease if they had an estimated glomerular filtration rate <60 mL/min/1.73 m2.
2.2. Study end points
All available radiology reports, including magnetic resonance imaging of the brain; computed tomography scan of the head; computed tomography scans of the chest, abdomen, and pelvis; and upper‐ and lower‐extremity ultrasounds from the date of the initial ICH, were reviewed to identify instances of hemorrhage and thrombosis. If a radiology report indicated hemorrhage or blood products, information surrounding the event, such as indication for imaging or subsequent management, was collected from the electronic medical record. Any patient with a bleed that occurred within 4 weeks after neurosurgery was excluded. The primary radiographic image of each identified instance of hemorrhage was reviewed by the neuro‐oncologist to confirm recurrent ICH. The investigators reviewing and adjudicating cases of recurrent ICH and VTE were blinded to anticoagulation resumption status.
2.3. Statistical analysis
The primary end points of the study were recurrent ICH and VTE within 12 months from the initial ICH (time 0 representing the date of the initial ICH). Variables were compared between the restart and nonrestart anticoagulation cohorts using Fisher’s exact test for categorical variables and the Wilcoxon rank‐sum test for nonnormally distributed continuous data. The Kaplan‐Meier method was used to estimate survival, and the log‐rank test was used to compare survival between groups. The cumulative incidence of recurrent ICH and VTE were determined, with death considered a competing risk. 11 Gray’s test was used to compare the cumulative incidence of recurrent ICH and VTE between the groups. Two‐sided P values <0.05 were considered statistically significant. Patients who were still alive were censored at the date of last contact. Patients who were discharged to hospice were considered deceased at the date of last contact.
3. RESULTS
A total of 79 patients with a primary or metastatic brain tumor receiving anticoagulationfor VTEand later diagnosed with ICH were analyzed. Fifty‐four patients (68.4%) restarted anticoagulation after ICH. Twenty‐five patients did not restart anticoagulation. Baseline demographics including tumor diagnosis, age, and comorbidities that portend an increased risk of ICH such as hypertension, chronic kidney disease, and concomitant aspirin use were similar between the 2 cohorts (Table 1). A similar percentage of ICH were intratumoral in the restart group (42 of 52; 78%) and the nonrestart group (18 of 25; 72%). In the restart group, the remaining ICH included intraparenchymal hemorrhage (n = 7), subarachnoid hemorrhage (n = 2), and subdural hematoma (n = 3); in the nonrestart group, the ICH included intraparenchymal hemorrhage (n = 2) and subarachnoid hemorrhage (n = 3). Median time from VTE to initial ICH was 72 days in the restart cohort compared to 81 days in patients who did not resume anticoagulation (Wilcoxon P = .6). At the time of initial ICH, median platelet count was lower in patients who subsequently did not restart anticoagulation (190 × 109/L vs 241 × 109/L; Wilcoxon P = .03). The median prothrombin time (PT) was 13.0 seconds in both cohorts. Of the 31 major ICH events, 23 patients met criteria for major ICH based on volume and symptoms, with the remaining 8 patients on the basis of clinical symptoms alone. In patients who restarted anticoagulation, 12 of 54 (22%) initial ICH events occurred in the setting of tumor progression; in the nonrestart group, 7 of 25 (28%) initial ICH events occurred in the setting of tumor progression.
Table 1.
Baseline demographics based on resumption of anticoagulation status
| Characteristic | Resumption Status | |
|---|---|---|
| Yes (n = 54) | No (n = 25) | |
| Males, n (%) | 35 (65) | 16 (64) |
| Mean age at time of ICH (range) | 61 (28‐84) | 62 (31‐84) |
| Brain tumor type, n (%) | ||
| Breast cancer | 2 (4) | 2 (8) |
| Glioma | 12 (22) | 9 (36) |
| Melanoma | 8 (15) | 2 (8) |
| Non–small cell lung cancer | 20 (37) | 6 (24) |
| Other | 8 (15) | 4 (16) |
| Renal cell carcinoma | 4 (7) | 2 (8) |
| DVT characteristics, n (%) | ||
| Proximal | 15 (28) | 6 (24) |
| Distal | 3 (6) | 1 (4) |
| PE characteristics, n (%) | ||
| Lobar or more central | 17 (31) | 7 (28) |
| Segmental | 12 (22) | 6 (24) |
| Subsegmental | 1 (4) | 2 (8) |
| Symptomatic VTE, n (%) | ||
| Yes | 45 (83) | 16 (64) |
| Initial ICH event classification, n (%) | ||
| Major | 16 (30) | 15 (60) |
| Measurable | 11 (20) | 4 (16) |
| Trace | 27 (50) | 6 (24) |
| Labs closest to time of ICH (medians) | ||
| Platelet count (× 109/L) | 241 | 190 |
| Prothrombin time (s) | 13.0 | 13.0 |
| Anticoagulant, n (%) | ||
| LMWH | 50 (93) | … |
| DOAC | 4 (7) | … |
| Anticoagulant dose, n (%) | ||
| Therapeutic | 52 (96) | … |
| Prophylactic | 2 (4) | … |
| Comorbidities, n (%) | ||
| Hypertension | 19 (35) | 8 (32) |
| Chronic kidney disease | 5 (9) | 1 (4) |
| Aspirin | 4 (7) | 1 (4) |
| Prior antiangiogenic therapy, n (%) | 4 (7) | 6 (24) |
| Prior radiation therapy, n (%) | 37 (69) | 18 (72) |
Abbreviations: DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; ICH, intracranial hemorrhage; LMWH, low‐molecular‐weight heparin; PE, pulmonary embolus; VTE, venous thromboembolism.
3.1. Recurrent ICH following reinitiation of anticoagulation
Of the 54 patients who restarted systemic anticoagulation, 50 (93%) received LMWH, and the remainder received a DOAC. The vast majority of patients were restarted on therapeutic‐dose anticoagulation, and only 2 patients (4%) received a prophylactic dose of LMWH. Median time to resumption of anticoagulation was 3 days.
The cumulative incidence of recurrent ICH at 1 year was 6.1% (95% confidence interval [CI], 1.5‐15.3) in the restart cohort compared to 4.2% (95% CI, 0.3‐18.3) in patients who did not restart anticoagulation (Figure 1). Median time from anticoagulation restart to recurrent ICH was 36 days (range, 20‐119 days). All recurrent ICH events met criteria for classification as a major hemorrhage on the basis of clinical symptoms (with a volume range of 8‐9 mL, excluding a single case of intraventricular hemorrhage). None of the patients was thrombocytopenic at time of recurrent ICH (range, 195 × 109/L to 233 × 109/L). Recurrent ICH was associated with a 30‐day mortality of 67%.
Figure 1.
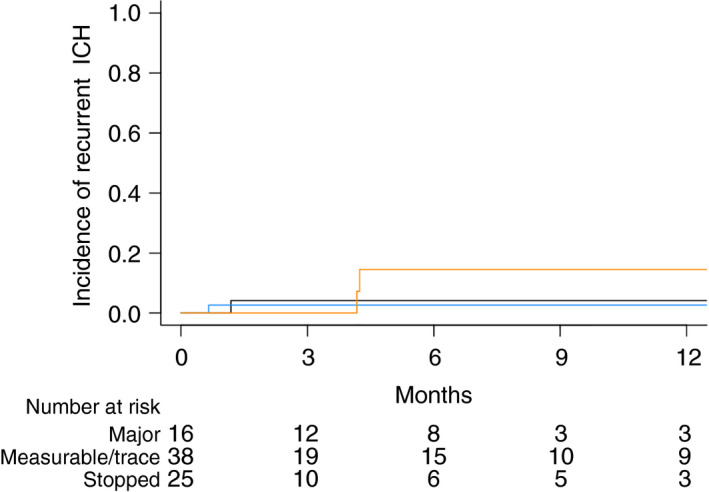
Cumulative incidence of recurrent ICH in patients with brain tumors according to anticoagulation resumption status. Restart cohort after a major ICH shown in orange, restart after a trace/measurable ICH shown in blue, and no anticoagulation resumption shown in black. ICH, intracranial hemorrhage
The risk of recurrent major ICH following reinitiation of anticoagulation appeared dependent on classification of ICH at time of diagnosis (ie, trace, measureable, or major). A total of 16 of 31 patients with major ICH restarted anticoagulation, and among these patients 2 developed subsequent ICH (Figure 1; cumulative incidence, 14.5%; 95% CI, 2.1‐38.3). Among the 38 patients with measurable or trace ICH (7 gliomas and 31 brain metastases) who restarted anticoagulation, 1 patient with glioma subsequently developed ICH (cumulative incidence, 2.6%; 95% CI, 0.2‐12.0). In a multivariate analysis based on tumor type and initial ICH size (measurable and trace initial events), there was no statistical difference between primary brain tumors and brain metastases (P = .11).
There was a single recurrent ICH event among the patients who did not restart anticoagulation with a cumulative incidence of recurrent ICH of 6.7% (95% CI, 0.3‐27.5). This was a patient with melanoma with multiple brain metastases who had a recurrent event 17 days after an initial major ICH. In the restart cohort, all 3 patients who developed recurrent ICH were in the setting of glioma; 2 patients had major ICH, and 1 had a measurable initial ICH. Two patients restarted therapeutic anticoagulation, and 1 restarted prophylactic LMWH. Median time from initial ICH to restart was 36 days. All recurrent ICH events occurred in the absence of clear tumor progression.
3.2. Recurrent VTE following ICH
The cumulative incidence of recurrent VTE at 1 year was significantly lower in the restart cohort compared to the cohort of patients who did not restart anticoagulation (8.1 vs 35.3; P = .003; Figure 2). There were 4 VTE events in the restart cohort, 3 deep vein thromboses (DVT), and 2 pulmonary emboli (PE). One patient had concurrent DVT and PE. The cancer diagnoses of those who developed recurrent VTE on anticoagulation were glioma, sarcoma, non–small lung cancer, and germ cell tumor. Two of the DVTs were associated with inferior vena cava (IVC) filters among the 5 total who underwent IVC filter placement in the restart cohort.
Figure 2.
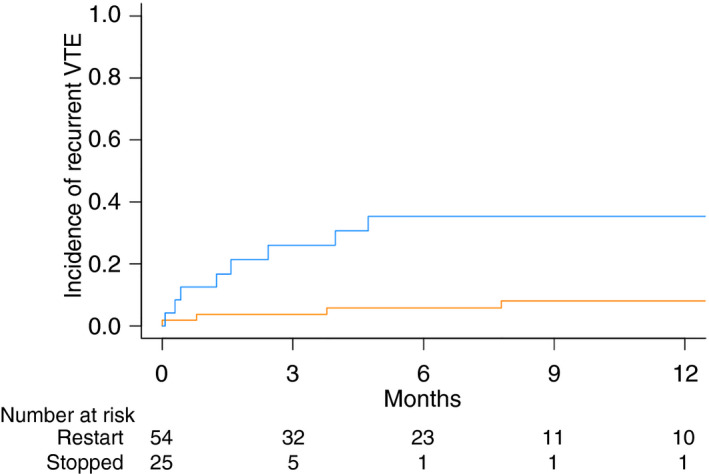
Cumulative incidence of recurrent venous thromboembolism in patients with brain tumors with ICH according to anticoagulation resumption status. Restart cohort in orange, no anticoagulation cohort in blue. ICH, intracranial hemorrhage
There were 9 VTE events in patients who did not restart anticoagulation, 7 DVTs and 2 PEs. The cancer diagnoses of those who developed recurrent VTE without anticoagulation included non–small cell lung cancer (3), renal cell carcinoma (2), glioma, melanoma, breast cancer, and sarcoma. Seven patients (28%) in the cohort who did not resume anticoagulation underwent IVC filter placement and the majority of patients with an IVC filter later developed DVT irrespective of anticoagulation status (5/7; 71%). The 2 PEs were both submassive events requiring intensive care unit admission. No recurrent VTE events were fatal. Median time from initial ICH to VTE was 69.5 days in the restart cohort compared to 48 days in patients who did not restart anticoagulation.
3.3. Survival outcomes
Post–initial ICH survival was statistically similar between the cohorts (Figure 3). Median post–initial ICH survival was 185 days in the restart cohort compared to 79 days in patients who did not restart anticoagulation (log‐rank P = .15).
Figure 3.
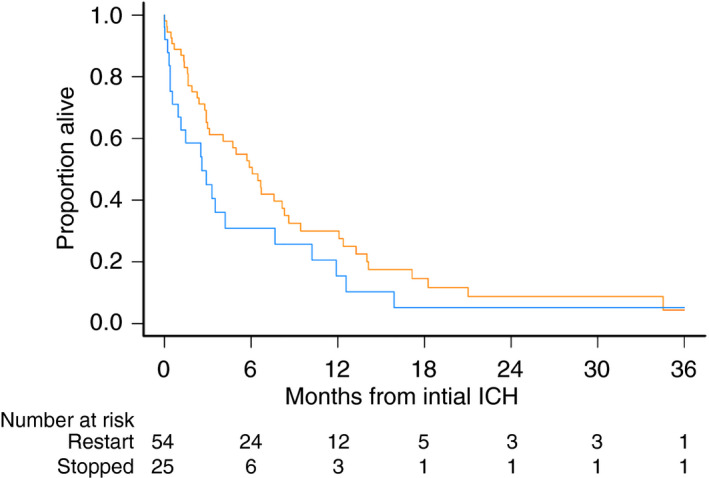
Survival after initial ICH in patients with brain tumors according to resumption of anticoagulation status. Restart cohort in orange, no anticoagulation cohort in blue. ICH, intracranial hemorrhage
4. DISCUSSION
Anticoagulation management after an initial ICH event inVTE patients with primary and metastatic brain tumors is challenging. Concerns about recurrent ICH must be weighed against the significant risk of VTE in this population. To better inform clinical decision making, it is important to establish rate estimates of recurrent ICH following restarting anticoagulation and rate of recurrent VTE with or without anticoagulation.
In the current study, we observed that recurrent VTE events are less frequent and less severe in patients who restart anticoagulation following ICH. Approximately 35% of patients who did not resume anticoagulation subsequently developed recurrent VTE events, which is similar to the 27% recurrence rate of VTE previously observed in patients with glioblastoma. 12 Regardless of anticoagulation administration, 75% of patients in our cohort who underwent IVC filter placement subsequently developed a DVT. The value of IVC filters in the absence of anticoagulation continues to be debated. 13 Population‐based studies failed to demonstrate a reduction in mortality 14 or risk of subsequent PE. 15 However, among the 790 patients with an apparent contraindication for anticoagulation, there was an observed trend toward prevention of PE at 90 days (hazard ratio [HR], 0.17; 95% CI, 0.03‐10.05; P = .056) without an impact on 30‐day survival (HR, 0.99). 15 In the Computerized Registry of Patients With Venous Thromboembolism (RIETE), the use of IVC filters following a major hemorrhage was associated with a lower 30‐day mortality (HR, 0.41; 95% CI, 0.24‐70) as well as fatal hemorrhage (HR, 0.16; 95% CI, 0.05‐0.52). 16 However, a fatal PE also occurred in 4 of 43 (9%) of patients diagnosed with an intracranial hemorrhage. 16
There was only 1 recurrent ICH following reinitiation of anticoagulation following smaller ICH (trace or measurable). This observation is in keeping with larger, noncancer cohorts who develop ICH and require long‐term anticoagulation, where the resumption of anticoagulation has been associated with a reduced risk of thrombotic events without an increased risk of recurrent ICH. 17 , 18 Considering the limited number of patients with glioma with small ICH who restarted anticoagulation, the clearest signal of safety was in those with brain metastases and trace or measureable ICH. However, based on the observed a rate of recurrent major ICH in excess of 10% among those who restarted anticoagulation following a major ICH, the safety of resuming anticoagulation in this population is questionable, especially in patients with glioma.
The appropriate management of anticoagulation following an initial major ICH is unclear. There are emerging data regarding the relative safety of DOACs compared to other anticoagulants in terms of risk of ICH in both patients without cancer 19 , 20 and those with brain tumors. 7 However, there were too few patients in the current study who restarted DOACs to draw any conclusions whether their use provides additional safety over LWMH. At least in the setting of acute VTE in patients with cancer with severe thrombocytopenia, reduced‐intensity LMWH has shown a favorable risk‐benefit profile. 9
Strengths of this study include a blinded review of radiology images to minimize classification bias as well as strict criteria for ICH that include an objective definition of major hemorrhage based on hemorrhage volume. Selection bias is an implicit limitation of this type of study; anticoagulation was resumed more frequently in patients with smaller ICH or when the treating physician otherwise deemed it reasonable to resume. Even with such a selection bias, there was an unacceptably high rate of recurrent major ICH following an initial major ICH following the resumption of therapeutic anticoagulation. Similarly, there is also concern for lead‐time bias in that median survival tended to be longer in the restart cohort than those who did not resume anticoagulation, suggesting this may have been a less advanced patient population. The cumulative incidence of recurrent ICH accounted for death as a competing risk for recurrent ICH. Nonetheless, the high rate of recurrent ICH with the resumption of anticoagulation is likely an underestimation of the true recurrent event rate considering patients needed to have survived the initial event and be considered robust enough to re‐initiate therapeutic anticoagulation. While we feel that the rate of recurrent ICH following an initial major ICH with the resumption of anticoagulation is alarming, we also acknowledge limitations due to a small sample size and number of events. However, to appropriately power a study based on the observed ICH rates, analyses would require approximately 300 patients with brain tumors who developed an ICH on anticoagulation and then restarted anticoagulation—a number that exceeds the cumulative number of published cases.
In those patients with brain tumors who are diagnosed with an ICH while receiving anticoagulation, the decision to restart anticoagulation remains a difficult one. Withholding anticoagulation is associated with a high rate of recurrent VTE. In cases of small ICH (especially brain metastases), anticoagulation can be considered. However, following a major ICH, the risk of recurrent ICH is sufficiently high that we suggest caution when considering reinitiation of therapeutic LMWH anticoagulation.
RELATIONSHIP DISCLOSURE
JIZ has received prior research funding from Quercegen Pharma and Incyte and has served on scientific advisory boards for Portola Pharmaceuticals, BMS, Janssen, Bayer, Pfizer, and Seattle Genetics. All other authors declare nothing to report.
AUTHOR CONTRIBUTIONS
The manuscript was principally authored by BJC, JIZ, and MP. The final version was reviewed by all authors. The study was designed by BJC and JIZ. Data collection performed by BJC, EJU, CM, and GW, with analysis performed BJC, MP, DSN, and JIZ.
ACKNOWLEDGMENTS
JIZ is supported NHLBI Consortium Linking Oncology and Thrombosis (U01HL143365).
Carney BJ, Uhlmann EJ, Puligandla M, et al. Anticoagulation after intracranial hemorrhage in brain tumors: Risk of recurrent hemorrhage and venous thromboembolism. Res Pract Thromb Haemost. 2020;4:860–865. 10.1002/rth2.12377
Handling Editor: Dr Neil Zakai.
Contributor Information
Charlene Mantia, @CharleneMantia.
Jeffrey I. Zwicker, Email: jzwicker@bidmc.harvard.edu.
REFERENCES
- 1. Donato J, Campigotto F, Uhlmann EJ, Coletti E, Neuberg D, Zwicker J. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood. 2015;126:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017;129:3379–85. [DOI] [PubMed] [Google Scholar]
- 3. Weinstock MJ, Uhlmann EJ, Zwicker JI. Intracranial hemorrhage in cancer patients treated with anticoagulation. Thromb Res. 2016;140(suppl 1):S60–S65. [DOI] [PubMed] [Google Scholar]
- 4. Khoury MN, Missios S, Edwin N, Sakruti S, Barnett G, Stevens G, et al. Intracranial hemorrhage in setting of glioblastoma with venous thromboembolism. Neuro Oncol Prac. 2015;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunter BD, Minichiello T, Bent S. Anticoagulation for the treatment of venous thromboembolism in patients with brain metastases: a meta‐analysis and systematic review. J Thromb Thrombolysis. 2017;44:392–8. [DOI] [PubMed] [Google Scholar]
- 6. Zwicker JI, Karp Leaf R, Carrier M. A meta‐analysis of intracranial hemorrhage in patients with brain tumors receiving therapeutic anticoagulation. J Thromb Haemost. 2016;14:1736–40. [DOI] [PubMed] [Google Scholar]
- 7. Carney BJ, Uhlmann EJ, Puligandla M, Mantia C, Weber GM, Neuberg DS, et al. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost. 2019;17:72–6. [DOI] [PubMed] [Google Scholar]
- 8. Murthy SB, Gupta A, Merkler AE, Navi BB, Mandava P, Iadecola C, et al. Restarting anticoagulant therapy after intracranial hemorrhage: a systematic review and meta‐analysis. Stroke. 2017;48:1594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mantha S, Miao Y, Wills J, Parameswaran R, Soff GA. Enoxaparin dose reduction for thrombocytopenia in patients with cancer: a quality assessment study. J Thromb Thrombolysis. 2017;43:514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy‐to‐use predictor of 30‐day mortality. Stroke. 1993;24:987–93. [DOI] [PubMed] [Google Scholar]
- 11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 12. Edwin NC, Khoury MN, Sohal D, McCrae KR, Ahluwalia MS, Khorana AA. Recurrent venous thromboembolism in glioblastoma. Thromb Res. 2016;137:184–8. [DOI] [PubMed] [Google Scholar]
- 13. Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320:1583–94. [DOI] [PubMed] [Google Scholar]
- 14. Turner TE, Saeed MJ, Novak E, Brown DL. Association of inferior vena cava filter placement for venous thromboembolic disease and a contraindication to anticoagulation with 30‐day mortality. JAMA Network Open. 2018;1:e180452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brunson A, Ho G, White R, Wun T. Inferior vena cava filters in patients with cancer and venous thromboembolism (VTE) does not improve clinical outcomes: a population‐based study. Thromb Res. 2017;153:57–64. [DOI] [PubMed] [Google Scholar]
- 16. Mellado M, Trujillo‐Santos J, Bikdeli B, Jimenez D, Nunez MJ, Ellis M, et al. Vena cava filters in patients presenting with major bleeding during anticoagulation for venous thromboembolism. Intern Emerg Med. 2019;14(7):1101–12. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen PB, Larsen TB, Skjoth F, Gorst‐Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation. 2015;132:517–25. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen PB, Larsen TB, Skjoth F, Lip GY. Outcomes associated with resuming warfarin treatment after hemorrhagic stroke or traumatic intracranial hemorrhage in patients with atrial fibrillation. JAMA Intern Med. 2017;177:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolfe Z, Khan SU, Nasir F, Raghu Subramanian C, Lash B. A systematic review and Bayesian network meta‐analysis of risk of intracranial hemorrhage with direct oral anticoagulants. J Thromb Haemost. 2018;16:1296–306. [DOI] [PubMed] [Google Scholar]
- 20. van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–75. [DOI] [PubMed] [Google Scholar]


