Abstract
Recognizing the complexity of coagulation tests and currently used anticoagulants, we developed this illustrated review on bleeding assessment tools and common coagulation screening tests. Quantitative bleeding assessment tools (BATs) are available to standardize the bleeding history and improve the pretest probability prior to coagulation testing. We describe use of BATs and the principles, indications, and limitations of the prothrombin time (PT)/International Normalized Ratio, activated partial thromboplastin time (APTT), and 50:50 mix. Use of these tests to identify coagulation factor deficiencies, specific and nonspecific inhibitors, coagulopathy of liver disease, disseminated intravascular coagulation, and commonly used anticoagulant medications are reviewed. Current literature suggests that unnecessary coagulation testing is rampant. The PT and APTT have astoundingly low sensitivity (1.0%‐2.1%) for detection of clinically significant bleeding disorders. Thus, current guidelines recommend against the use of screening PT and APTT in preoperative patients undergoing noncardiac/vascular surgery.
Keywords: bleeding disorders, clinical laboratory techniques, hemorrhage, International Normalized Ratio, thrombosis
Essentials.
Quantitative bleeding assessment tools standardize the bleeding history and improve the pretest probability of bleeding disorders prior to coagulation testing.
Unnecessary coagulation testing is rampant.
Thorough understanding of common hemostatic tests is essential for appropriate selection and interpretation of tests.









RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
CE and MS developed the concepts and images, wrote the manuscript, and approved the final content.
Elbaz C, Sholzberg M. An illustrated review of bleeding assessment tools and common coagulation tests. Res Pract Thromb Haemost. 2020;4:761–773. 10.1002/rth2.12339
Handling Editor: Pantep Angchaisuksiri
Contributor Information
Carolyne Elbaz, Email: carolyne.elbaz@mail.mcgill.ca, @ElbCarolyne.
Michelle Sholzberg, @sholzberg.
REFERENCES
- 1. Bakhtiari K, Meijers JCM, De Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416–21. [DOI] [PubMed] [Google Scholar]
- 2. Bowman M, Mundell G, Grabell J, Hopman WM, Rapson D, Lillicrap D, et al. Generation and validation of the Condensed MCMDM‐1VWD Bleeding Questionnaire for von Willebrand disease. J Thromb Haemost. 2008;6(12):2062–6. [DOI] [PubMed] [Google Scholar]
- 3. Bowman M, Riddel J, Rand ML, Tosetto A, Silva M, James PD. Evaluation of the diagnostic utility for von Willebrand disease of a pediatric bleeding questionnaire. J Thromb Haemost. 2009;7(8):1418–21. [DOI] [PubMed] [Google Scholar]
- 4. Capoor M, Stonemetz J, Baird J, Ahmed F, Awan A, Birkenmaier C, et al. Prothrombin time and activated partial thromboplastin time testing: a comparative effectiveness study in a million‐patient sample. PLoS ONE. 2015;10(8):e0133317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuker A, Husseinzadeh H. Laboratory measurement of the anticoagulant activity of edoxaban : a systematic review. J Thromb Thrombolysis. 2015;39(3):288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deforest M, Grabell J, Albert S, Young J, Tuttle A, Hopman WM, et al. Generation and optimization of the self‐administered bleeding assessment tool and its validation as a screening test for von Willebrand disease. Haemophilia. 2015;21(5):e384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elbatarny M, Mollah S, Grabell J, Bae S, Deforest M, Tuttle A, et al. Normal range of bleeding scores for the ISTH‐BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378(21):2010–21. [DOI] [PubMed] [Google Scholar]
- 9. Hayward CM, Moffat K, Liu Y. Laboratory investigations for bleeding disorders. Semin Thromb Hemost. 2012;38(7):742–52. [DOI] [PubMed] [Google Scholar]
- 10. Kitchens CS. Prolonged activated partial thromboplastin time of unknown etiology: a prospective study of 100 consecutive cases referred for consultation. Am J Hematol. 1988;27:38–45. [DOI] [PubMed] [Google Scholar]
- 11. Poller L. International Normalized Ratios (INR): the first 20 years. J Thromb Haemost. 2004;2(6):849–60. [DOI] [PubMed] [Google Scholar]
- 12. Quick AJ. The clotting time ‐ an enigma resolved. Am J Clin Pathol. 1974;62(5):670–2. [DOI] [PubMed] [Google Scholar]
- 13. Rodeghiero F, Castaman G, Tosetto A, Batlle J, Baudo F, Cappelletti A, et al. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: An international, multicenter study. J Thromb Haemost. 2005;3(12):2619–26. [DOI] [PubMed] [Google Scholar]
- 14. Rodeghiero F, Tosetto A, Abshire T, Arnold DM, Coller B, James P, et al. ISTH/SSC bleeding assessment tool: A standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063–5. [DOI] [PubMed] [Google Scholar]
- 15. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory Assessment of the Anticoagulant Activity of Direct Oral Anticoagulants: A Systematic Review. Chest. 2017;151(1):127–38. 10.1016/j.chest.2016.08.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
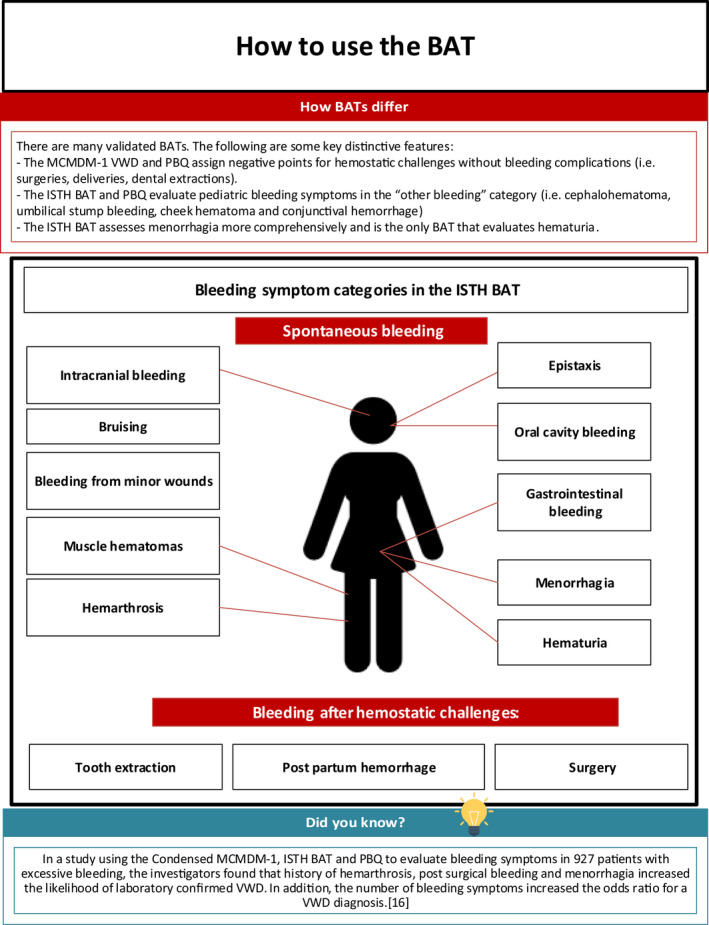
- 16. Spradbrow J, Letourneau S, Grabell J, Liang Y, Riddel J, Hopman W, et al. Bleeding assessment tools to predict von Willebrand disease: Utility of individual bleeding symptoms. Res Pract Thromb Haemost. 2020;4(1):92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM‐1 VWD). J Thromb Haemost. 2006;4(4):766–73. [DOI] [PubMed] [Google Scholar]
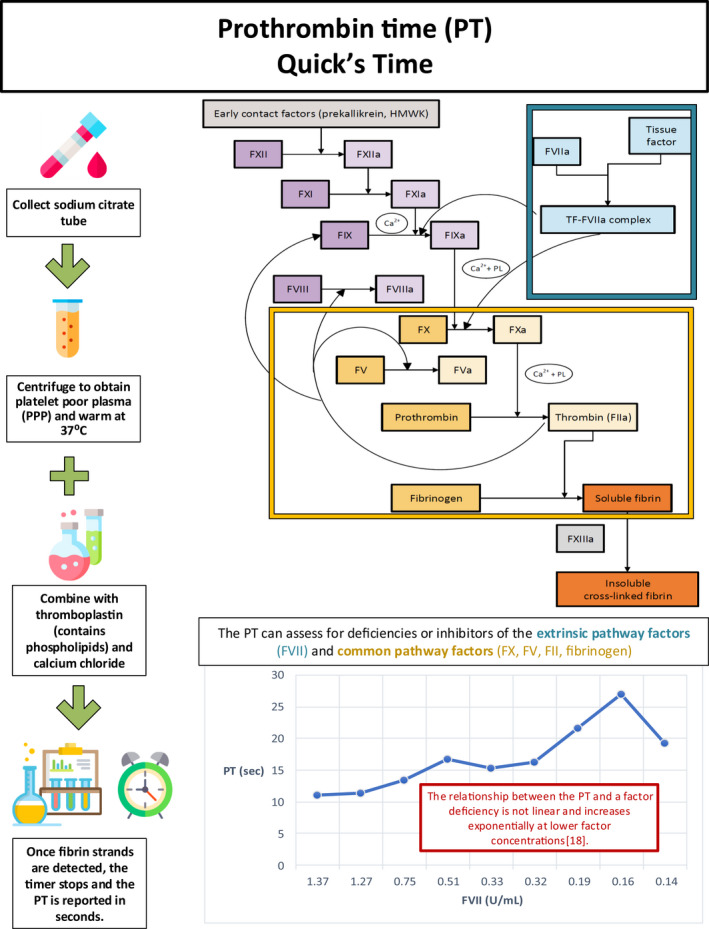
- 18. Local data obtained from St. Michael’s Hospital Laboratory Information System.


