Abstract
The toxicokinetics (TK) of hydrolyzed fumonisin B1 (HFB1) were evaluated in 16 broiler chickens after being fed either a control or a fumonisins-contaminated diet (10.8 mg fumonisin B1, 3.3 mg B2 and 1.5 mg B3/kg feed) for two weeks, followed by a single oral (PO) or intravenous (IV) dose of 1.25 mg/kg bodyweight (BW) of HFB1. Fumonisin B1 (FB1), its partially hydrolyzed metabolites pHFB1a and pHFB1b, and fully hydrolyzed metabolite HFB1, were determined in chicken plasma using a validated ultra-performance liquid chromatography–tandem mass spectrometry method. None of the broiler chicken showed clinical symptoms of fumonisins (FBs) or HFB1 toxicity during the trial, nor was an aberration in body weight observed between the animals fed the FBs-contaminated diet and those fed the control diet. HFB1 was shown to follow a two-compartmental pharmacokinetic model with first order elimination in broiler chickens after IV administration. Toxicokinetic parameters of HFB1 demonstrated a total body clearance of 16.39 L/kg·h and an intercompartmental flow of 8.34 L/kg·h. Low levels of FB1 and traces of pHFB1b were found in plasma of chickens fed the FBs-contaminated diet. Due to plasma concentrations being under the limit of quantification (LOQ) after oral administration of HFB1, no toxicokinetic modelling could be performed in broiler chickens after oral administration of HFB1. Moreover, no phase II metabolites, nor N-acyl-metabolites of HFB1 could be detected in this study.
Keywords: mycotoxins, toxicokinetics, fumonisins metabolites, feeding trial, broiler chicken
1. Introduction
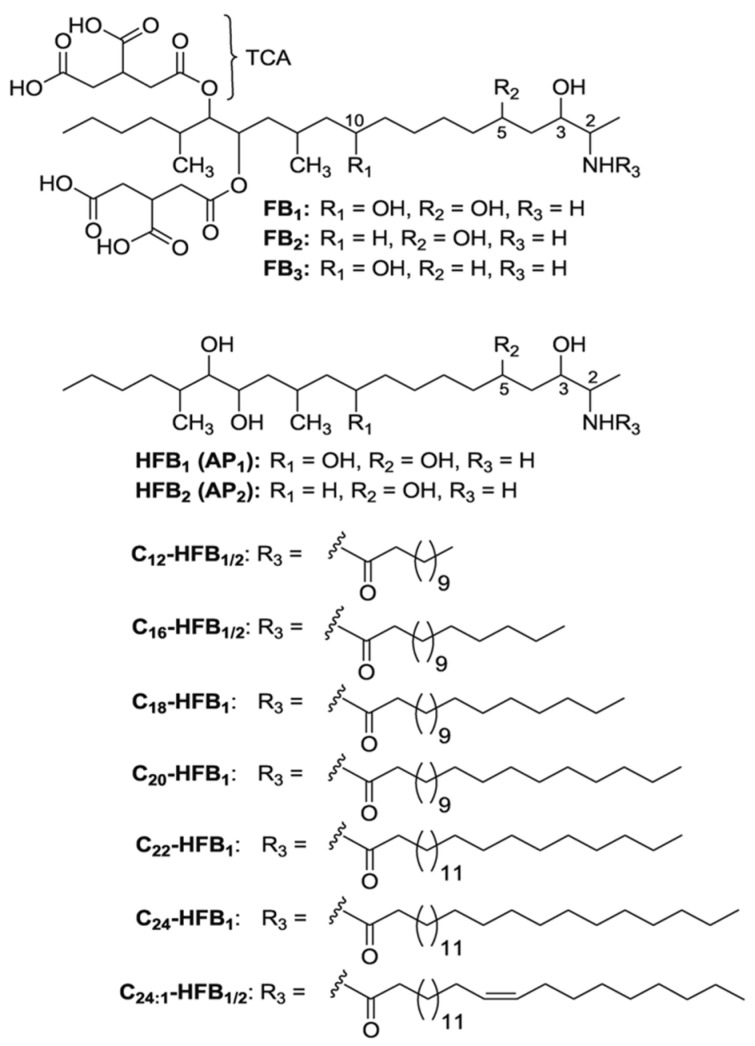
Fumonisins (FBs) are secondary fungal metabolites of Fusarium verticillioides, F. proliferatum or other Fusarium species, that resemble each other structurally [1,2]. Fumonisin B1 (FB1), B2 (FB2) and B3 (FB3) represent the most important of the more than 15 FBs analogues that have been described [3]. FBs can be divided into four main categories, namely A, B, C and P [4]. Since FB1 is the most prevalent and toxic analogue, toxicological assessment of FBs has mostly been done for FB1. FB1 is a diester of propane-1,2,3-tricarboxylic acid (TCA), which has a long aminopolyol side-chain, namely 2-amino-12,16-dimethyl-3,5,10,14,15-pentahydroxyeicosane [3]. FB2 and FB3 differ structurally from FB1 in the number and position of hydroxyl groups, since a free hydroxyl group is lacking on either the C-10 or the C-5 position, as shown in Figure 1 [2]. The primary amino group is known for the biological activity of FBs, as acetylation of FB1 to fumonisin A1 hampers the cytotoxicity as well as the inhibition of ceramide synthase [3].
Figure 1.
Structures of the fumonisins FB1, FB2 and FB3 with tricarballylic acid side-chains (TCA), and of the hydrolyzed fumonisins HFB1 and HFB2 (or aminopentols, AP1 and AP2, respectively) and the corresponding N-acyl-derivatives [5]. Copyright © 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
FBs reveal distinct structural similarities to sphingolipids, due to their long-chain base backbones. Hence, FBs have the ability to completely inhibit sphinganine N-acyl transferase (ceramide synthase), resulting in a disruption of the ceramide and sphingolipid metabolism [6,7,8]. Blockage of the ceramide synthase enzyme not only leads to the inhibition of sphingolipids synthesis, but also induces an accumulation of free sphinganine (Sa) and sphingosine (So) in tissues, serum and urine [2,4]. This associated increase of the Sa:So ratio, observed in tissues and body fluids after exposure to FBs, is a suitable biomarker of effect in animals and humans [2,9,10]. In most animal species, the liver, as well as the kidneys and the intestinal tract, are the main target organs of FBs toxicity [2,11,12]. Generally, poultry are rather resistant to FBs in comparison to mammals, especially pigs and horses [13,14,15,16,17,18]. Only during the first three days of life (≥125 mg/kg feed) was an increase in mortality after FB1 exposure observed in broiler chickens [13], as well as in growing ducks aged 12–14 weeks (20 mg/kg feed) [16]. Feeding a FB1-contaminated diet (100–400 mg/kg feed) to broilers resulted in black, sticky diarrhea in the first 2 weeks of life [15]. Besides, broiler chickens fed an FBs-contaminated diet in doses from 100 to 400 mg FB1/kg feed for 2 to 3 weeks showed a dose-dependent decrease in feed intake and bodyweight gain [13,15]. No negative effects on performance were observed under experimental conditions, at concentration levels which were below or approached the European maximum guidance level in feed (20 mg FB1 + FB2/kg feed) [19]. However, it was recently demonstrated that even low to moderate dietary levels of FBs negatively affect enterocyte viability and proliferation, and the production of pro-inflammatory cytokines, and alter the intestinal barrier function, and therefore increase the susceptibility of avian species to important enteric infectious diseases, such as coccidiosis and necrotic enteritis [20,21,22,23]. Furthermore, the mRNA expression of genes encoding for the intestinal cytochrome P450 (CYP450), the drug-metabolizing enzyme CYP1A4, and the drug transporter mechanisms, such as multiple drug resistance protein 1 (MDR1 or P-glycoprotein, P-gp), in broiler chickens were upregulated following exposure to 25 mg FB1 + FB2/kg feed for 15 days, which can affect the pharmacokinetic (PK) or toxicokinetic (TK) properties of other xenobiotics substrates [24].
Therefore, taking into account that mycotoxin contamination of feed is a continuous feed safety issue, resulting in economic losses during animal production [25], methods for the detoxification of feed have been developed. For FBs, these imply feed processing techniques, such as alkaline cooking (nixtamalization) used during the production of masa and tortillas for human consumption, and the application of mycotoxin binders/modifiers in animal feed. Mycotoxin binders, such as clays, are highly effective against aflatoxins, however, their detoxification capacity wit regards to FBs is rather limited. In contrast, following the application of FB esterase FumD (EC 3.1.1.87), enzymatic degradation results in conversion of FBs to the less toxic partially hydrolyzed FB1 (pHFB1), or fully hydrolyzed HFB1, by cleavage of the tricarballylic acid esters at the C-14 and/or C-15 position. FB esterase FumD is an enzyme of bacterial FB catabolism [26,27], which has recently been commercialized as FUMzyme® (BIOMIN GmbH, Getzersdorf, Austria) and authorized by the EU for usage in poultry and pigs. Enzymatic hydrolysis has been shown to be an effective detoxification method in broilers, since it prevents the FB-associated disruption of the sphingolipid metabolism, characterized by a reduced serum and hepatic Sa:So ratio and a counteracting FB-induced intestinal up-regulation of cytokine gene expression [interleukin-8 (IL-8) and IL-10] [28]. Besides, in accordance with different mammalian species [29,30,31], it has been demonstrated that the intestinal microbiota of broiler chickens and turkeys also has a limited capacity to hydrolyze FBs [28,32].
The only known metabolic pathway of FB1 is the hydrolyzation (hydrolysis) of its side-chains in vertebrates [11]. HFB1 is not only a 10-fold less potent inhibitor of ceramide synthase than FB1, but it is also a substrate for ceramide synthase in rat liver microsomes [5,26,31,33,34]. Acetylation of HFB1 at the primary amino group, with fatty acids of variable chain lengths, results in the formation of N-acyl-HFB1 (NAHFB1) metabolites, which are also known to be ceramide analogues. The in vivo formation of NAHFB1 has been demonstrated in rats [5]. Furthermore, phase II conjugation processes of FB1, e.g., sulphatation as proposed by Hopmans et al. (1997) [35], or glucuronidation, have not yet been identified. However, no data are available on the in vivo TK of HFB1, nor on the formation of NAHFB1 in chickens [36]. Furthermore, FB1 is not absorbed in vitro by human colon adenocarcinoma Caco-2 cells, also suggesting a low oral bioavailability. The oral bioavailability of FB1 in chickens is only 0.7% [36]. In contrast, for HFB1 a high transcellular passage has been observed in vitro, particularly from the basolateral to the apical side, suggesting that HFB1 is effluxed by P-gp [33,37]. Remarkably, this P-gp transport mechanism was upregulated following exposure to the toxic FBs parent molecules, which could affect the TK properties of the HFB1 metabolite, as it is a P-gp substrate. Since exposure to HFB1 is linked with the hydrolysis of FB1 by intestinal enzymes or by a feed additive enzyme, the aim of this study was to investigate not only the TK parameters of HFB1 alone, but also the impact of prior exposure to FBs on these parameters.
2. Results and Discussion
During the animal experiment, no signs of illness due to FBs or HFB1 toxicity were observed in any of the broiler chickens. All birds were alert, showed a normal feed intake and normal droppings, and no regurgitation was observed. The average bodyweight (BW) was similar (p = 0.820) in both groups (control and FBs diet), 974 ± 109 and 1036 ± 110 g at day 21, respectively. This matched expectations, since the contaminated feed contained 10.8 mg FB1, 3.3 mg FB2 and 1.5 mg FB3/kg feed, and therefore approached, but was below, the EU guidance level of 20 mg FB1 + FB2/kg [19]. Additionally, HFB1 is generally known to be less toxic than its parent toxin FB1 [26,27], and poultry species are known to be able to tolerate high doses of FB1, especially regarding performance response, with no effect on growth of broiler chickens up to 75–100 mg FB1/kg feed [15].
To the authors’ knowledge, for the first time, the TK properties of HFB1 were determined in broiler chickens, both after feeding a control and FBs-contaminated diet. In pigs, biotransformation of FB1 into pHFB1, and further to HFB1, by their digestive microbiota and liver has been observed after being fed an FBs-contaminated diet containing 45 mg FB1, 8.6 mg FB2 and 4.6 mg FB3/kg for 10 days, together with a persistence of low levels of pHFB1 in most organs for several days [30]. However, only low concentrations of pHFB1a and pHFB1b have been detected in the plasma of broiler chickens, fed an FBs-contaminated diet with 10 mg FBs/kg for 14 days [28]. The formation of pHFB1a, pHFb1b and HFB1 after FB1 exposure has been described in turkeys [32], monkeys [29] and rats [31] as well. Accordingly, in this study, only traces (>limit of quantification; LOQ) of pHFB1b (0.94–1.34 ng/mL and 0.93–2.43 ng/mL, respectively) were detected in a few samples of broiler chickens fed the FBs-contaminated diet, and subsequently administered an IV or PO bolus of HFB1, respectively. Although, the presence of pHFB1a was detected in some samples of broilers fed the FBs-contaminated diet (>limit of detection; LOD), it could not be quantified (<LOQ). No pHFB1a or pHFB1b was detected in chickens fed the control diet. Low levels of FB1 were observed in chickens fed the FBs-contaminated diet, with average background levels at all timepoints of 4.89 ± 5.91 ng/mL and 2.67 ± 3.70 ng/mL, in broiler chickens administered HFB1 IV and PO, respectively. No FB1 was detected in the plasma of animals fed the control diet. Consequently, it can be concluded that the observed pHFB1 in the plasma of animals fed the FBs-contaminated diet resulted from hydrolysis of the FB1 present in the diet. However, the intestinal microbiota of broiler chickens and turkeys seems to have a rather limited capacity to hydrolyze FBs [28,32]. HFB1 initiated lesser inflammatory responses than FB1 in an in vitro co-culture model of porcine intestinal epithelial and immune cells [38]. In vivo toxicity of the intermediate, pHFB1, has only been investigated in rats so far, resulting in no observed in vivo toxicity [31]. On the other hand, hepatic and renal lesions, in terms of single-cell necrosis, mitosis, apparent collapse of the centrilobular parenchyma of the liver and single-cell necrosis of the outer medulla, and cytoplasmic vacuolation of the kidney, have been reported in rats fed nixtamalized feed containing HFB1 [39], while no hepatotoxicity or pathological changes of the liver, brain, heart, kidneys, thymus or mesenteric lymph nodes examined in mice fed purified HFB1 have been detected [40]. This different outcome could probably be explained by the presence of residual, partially hydrolyzed or masked FB1, following the process of nixtamalization [5]. However, no significant influence of HFB1 on the intestinal lesion score of the villi morphology in piglets exposed to HFB1 has been observed [41].
No N-acyl or phase II metabolites of HFB1 were detected in any of the samples in this study. N-acyl-metabolites of HFB1 were highly cytotoxic in human colonic HT29 cells, and were also characterized as a potent inhibitor of ceramide synthase [34]. HFB1 acylation has been evaluated in rats, and although N-acyl-metabolites were detected, no toxicity was observed in vivo [5]. While N-acylation of FB1 and HFB1 occurred in human cell lines and in rats [33,34], the occurrence of these metabolites in avian species has not yet been observed, matching the absence of these metabolites in this study.
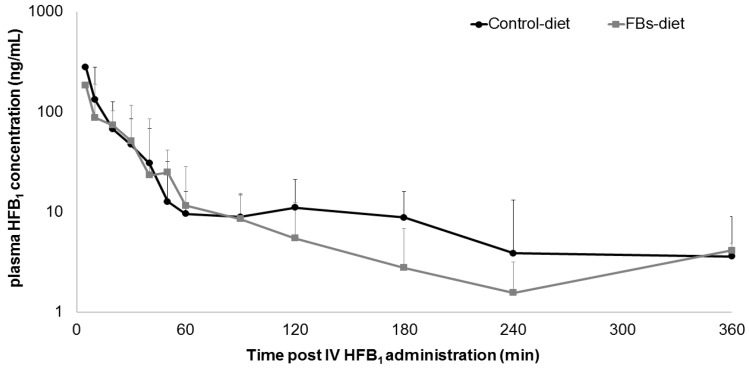
Comparative HFB1 plasma concentration–time profiles, following IV HFB1 administration to broiler chickens fed either a control diet or a FBs-contaminated diet, are shown in Figure 2. Visual inspection of the goodness-of-fit plots of the individual, model-predicted concentrations (IPRED) versus the observed HFB1 plasma concentrations (Cobs) revealed an appropriate structural model for most individuals (Figure S1A). The QQ-plots of the conditionally weighted residuals of Cobs demonstrated the normal distribution of the weighted residuals (Figure S1B). The addition of the covariate experimental diet (control versus FBs-contaminated) did not significantly improve the -2 log likelihood-ratio (2LL) of any of the fixed effect parameters’ volume of distribution of the central compartment (Vc), volume of distribution of the peripheral compartment (Vp), total body clearance (Cl), or intercompartmental flow (Q), and was therefore not retained in the final model.
Figure 2.
Comparative plasma concentration−time profile of HFB1 after intravenous (IV) administration of 1.25 mg HFB1/kg bodyweight to broiler chickens fed either a control diet (n = 8) or a fumonisins (FBs)-contaminated diet (10.8 mg FB1, 3.3 mg FB2 and 1.5 mg FB3/kg feed, n = 8) for two weeks. Values are presented as mean + standard deviation (SD).
The toxicokinetic parameters of HFB1 following IV administration are shown in Table 1, and are described by a two-compartment model. The mean plasma concentration of HFB1 in broiler chickens fed an FBs-contaminated diet for 2 weeks following IV administration (plasma concentration at time 0; C0) was 339.59 ng/mL. The total exposure of HFB1 in broiler chickens after IV administration, from timepoint 0 to infinity (area under the plasma-concentration time curve from time 0 to infinity; AUC0-inf), was 76.26 ng·h/mL. Regarding volume of distribution (Vd), values of 3.68 L/kg and 5.04 L/kg have been determined for the central and peripheral compartments, respectively, thus resulting in a Vd of 8.7 L/kg at steady state (Vss). Accordingly, pigs given a single HFB1 IV bolus showed a Vd of 11.0 L/kg, in a one-compartmental model [42]. Furthermore, a short distribution half-life (T1/2α) of 0.09 h, followed by a longer elimination half-life (T1/2β) of 0.69 h, was revealed. In barrows given a single IV bolus, of 0.056 mg/kg BW, of HFB1, a T1/2α of 0.05 h and a T1/2β of 1.01 ± 0.80 h was observed [42]. The mean residence time (MRT) of HFB1 after IV administration to broiler chickens was 0.53 h, and the elimination rate constant (ke) was 4.4 h−1. In comparison to pigs, with a total body clearance of 6.7 L·kg/h in barrows after IV HFB1 administration, broilers seem to have a higher total body clearance (16.39 L/kg·h). The latter could concur with the oral bioavailability of parental FB1 being higher in swine than in poultry, with values of 3.1% [42] and 0.7% [36], respectively, and with poultry being rather resistant to FBs toxicity when compared to pigs or other mammals [13,14,15,16,17,18].
Table 1.
Population toxicokinetic results of intravenous (IV) administration of HFB1 (1.25 mg/kg BW) to broiler chickens [n = 16, 8 animals fed a control diet and 8 animals fed a fumonisins (FBs)-contaminated diet prior HFB1 administration].
| Θ | Tvθ | CV (%) | ω |
|---|---|---|---|
| Vc (L/kg) | 3.68 | 23.47 | 0.095 |
| Vp (L/kg) | 5.04 | 41.47 | 0.009 |
| VSS (L/kg) | 8.72 | 28.75 | / |
| Cl (L/kg·h) | 16.39 | 12.67 | 0.126 |
| Q (L/kg·h) | 8.34 | 41.37 | 0.391 |
| AUC0-inf (ng·h/mL) | 76.26 | 12.67 | / |
| C0 (ng/mL) | 339.59 | 23.47 | / |
| Ke (1/h) | 4.45 | 26.27 | / |
| MRT (h) | 0.53 | 31.56 | / |
| T1/2α (h) | 0.09 | 33.26 | / |
| T1/2β (h) | 0.69 | 40.41 | / |
Θ: fixed effect parameter; Tvθ: population typical value of the fixed effect parameter; CV: coefficient of variation; ω: variance of the interindividual variability (only for fixed parameters). Addition of the covariate experimental diet (control versus FBs-contaminated) did not significantly improve the –2 log likelihood (–2LL) of any of the fixed effect parameters, and was therefore not retained in the final model. Vc: volume of distribution of the central compartment; Vp: volume of distribution of the peripheral compartment; Vss: volume of distribution at steady state; Cl: total body clearance; Q: intercompartmental flow, AUC0-inf: area under the plasma concentration–time curve from time 0 to infinity; C0: plasma concentration at time 0 following IV administration; Ke: elimination rate constant; MRT: mean residence time; T1/2α: distribution half-life, and T1/2β: elimination half-life.
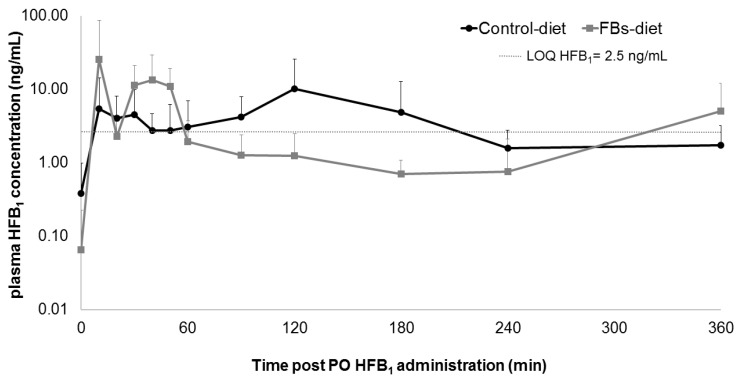
Plasma concentrations of HFB1 after oral administration were only above the LOQ in a limited number of samples, and are shown in Figure 3. Consequently, no TK modelling could be performed on these data. Still, the rather fast appearance of HFB1 in the systemic circulation after oral administration indicates that the ingested mycotoxin is mainly absorbed in the proximal part of the small intestines.
Figure 3.
Comparative plasma concentration−time profiles of HFB1 after oral (PO) administration of 1.25 mg HFB1/kg bodyweight to broiler chickens fed either a control diet (n = 8) or a fumonisins (FBs)-contaminated diet (10.8 mg FB1, 3.3 mg FB2 and 1.5 mg FB3/kg feed, n = 8) for two weeks. Values are presented as means + standard deviatie (SD).
The Tmax of HFB1 (10 min) after oral HFB1 administration in the FBs-fed group was reached remarkably fast, when compared to studies of oral FB1 intake in laying hens, turkeys and ducks, where respective Tmax values of 60 min, 180 min and 60–120 min were observed [43].
Previously, a disturbance of intestinal homeostasis of chickens fed an FBs-contaminated diet (18.6 mg FB1 + FB2/kg feed) was demonstrated [21]. In another study, broiler chickens were either fed an FBs-contaminated (8.70 mg FB1/kg, 4.15 mg FB2/ kg and 1.44 mg FB3/kg feed) or a control diet, resulting in FB1 influencing the expression of intestinal P-gp [24]. With HFB1 being a substrate of this receptor [24], another aim of this research was to examine whether prolonged FB1 exposure would influence the uptake of its metabolite HFB1 in the intestines of broiler chickens. Regarding the difference in plasma concentrations between the FBs and the control diet fed groups, after oral administration of HFB1, in this study, one could presume that a prolonged FBs exposure actually does influence the intestinal uptake of HFB1. But as mentioned before, the quantity of plasma concentration measurements above the LOQ, after oral HFB1 administration, was insufficient to allow the drawing of conclusions from those results. The latter is also held responsible for the fact that no calculation of oral bioavailability of HFB1 could be made. Nevertheless, a high transcellular passage, particularly from the basolateral to the apical side, was revealed for HFB1 in human colon adenocarcinoma Caco-2 cells, suggesting that HFB1 is effluxed by P-gp [33,37]. Since an increased expression of multi-drug resistance protein 1 (MDR1), encoding for P-gp, could be observed in the intestine of broiler chickens fed an FBs-contaminated diet [24], oral bioavailability of HFB1 could vary, leaving one with the need for more research on the oral bioavailability of HFB1 in avian species. In broiler chickens, the oral bioavailability of FB1, being mainly negatively charged in the duodenum and jejunum due to the pH which therefore limits oral absorption by passive non-ionic transcellular diffusion [43], is known to be rather low [44]. Similarly, in this study only very low plasma levels of HFB1 were detected in broiler chickens following oral administration, suggesting a very low oral bioavailability. That said, in rats, the bioavailability of HFB1 was thought to be greater than that of FB1, since about 2.5-fold greater amounts of 14C-HFB1, compared to 14C-FB1, were excreted in urine after oral administration of 0.69 µmol 14C-FB1 and 14C-hydrolyzed FB1/kg BW [45], increasing the likelihood that HFB1 can be absorbed by the gut, and in the meantime allowing the subsequent metabolism of N-acyl-HFB1 and the enhancing of its possible toxicological effects.
3. Conclusions
While HFB1 is shown to be less toxic than its parental toxin FB1, its toxicokinetic pathways in avian species are still widely unexplored. In this study, toxicokinetic parameters following administration of a single IV bolus of HFB1 to broiler chickens have been determined (Vc, Vp, Vss, Cl, Q, AUC0-inf, C0, ke, MRT, T1/2α and T1/2β). The plasma concentrations of HFB1 after oral administration were only above the LOQ in a limited number of samples. Consequently, no TK modelling could be performed on these data. Traces of pHFB1b (0.94–1.34 ng/mL and 0.93–2.43 ng/mL, respectively) have been detected in a few samples of broiler chickens fed the FBs-contaminated diet, and subsequently administered an IV bolus or PO bolus of HFB1, respectively. Using UPLC-HRMS, no phase II metabolites or N-acyl metabolites of HFB1 could be detected in any of the samples.
4. Materials and Methods
4.1. HFB1 for Animal Trial
HFB1 for oral (PO) and intravenous (IV) administration was prepared as previously described [31] with some minor changes. HFB1 for PO administration was obtained by enzymatic treatment of F. verticillioides culture material with the FB carboxylesterase FumD (80 U enzyme/g culture material) for 30 min, and subsequently purified and lyophilized [31]. Per 250 g of culture material, 2550 µmol HFB1 was obtained.
HFB1 for IV administration was prepared by alkaline hydrolysis of FB1 analytical standard (Biopure, Tulln, Austria). Therefore, 5 mL of FB1 solution (7.4 mg/mL water) was hydrolyzed with 4 mL of 2.5M NaOH by shaking for 7.5 h. After completing the conversion, the formed HFB1 was recovered by liquid–liquid extraction (eight times with acetonitrile (ACN)). The achieved HFB1 solution was purified over an SPE C18 column to remove salts and tricarballylic acids, and subsequently diluted in a 0.9% NaCl solution up to a concentration of 4.18 mg HFB1/mL, with traces of pHFB1a (0.0045 mg/mL). HFB1 for PO and IV administration was stored at 2–8 °C.
4.2. Feed Preparation and Experimental Diets
A commercial broiler starter diet was fed to all chickens (Vanden Avenne, Ooigem, Belgium) during the first week of the experiment. Later, this diet is referred to as control diet. From day 8 until day 24 chickens were fed either the control diet without mycotoxins [commercial broiler grower diet (Vanden Avenne)], or a grower diet experimentally contaminated with FBs. None of the diets contained a mycotoxin detoxifier. Screening of the control starter and grower feeds for mycotoxin contamination was executed by a liquid chromatography tandem mass spectrometer (LC-MS/MS) method, as described by Monbaliu et al. [46]. The levels of all tested mycotoxins were below the decision limit (CCα) in the control starter and grower diets. More specifically, the CCα values of FB1, FB2 and FB3 were 58, 45 and 42 µg/kg feed, respectively.
To produce the grower diet experimentally contaminated with FBs, FBs culture material was mixed with 500 g of control feed. Lyophilized FBs culture material of F. verticillioides (M-3125) [47] (7.37 g FB1/g and 2.93 g FB2/g) was obtained from Biopure, Romer Labs Diagnostic GmbH (Tulln, Austria) and stored at 2–8 °C. This premix was then blended with 5 kg of control feed to ensure a homogeneous distribution of the mycotoxins. The premix was blended for 20 min into the total quantity of feed necessary for the trial. To test the homogeneity of FBs in feed, samples were taken at three different points in the batch and analyzed for FBs as described for the control diets. The FBs-contaminated diet contained 10.8 mg FB1, 3.3 mg FB2 and 1.5 mg FB3/kg feed.
4.3. Animal Experiment
A total of 16 one-day-old broiler chickens (Ross 308) were obtained from a commercial hatchery (Vervaeke-Belavi, Tielt, Belgium), and were fed a starter diet without mycotoxins during the first week. Subsequently, animals were randomly divided into two experimental groups of eight animals (4 male/4 female). While one group was being fed a control grower diet from day 8 onwards, the other group was given a FBs-contaminated grower diet. An 18 h/6 h light/darkness program was applied. Feed and drinking water were provided ad libitum.
A toxicokinetic (TK) study of HFB1 was performed following a two-way cross-over design. Each broiler chicken was administered a bolus of HFB1 (1.25 mg/kg bodyweight) either orally (PO) or intravenously (IV) (vena cutanea ulnaris superficialis or wing vein). At day 21, four chickens of each group were administered HFB1 IV, and the four other animals received HFB1 PO. After a wash out and recovery period of two days, the protocol was repeated at day 24 in a cross-over design. Animals were feed-deprived overnight (8 h) prior to, and until 3 h after, HFB1 administration. After each bolus dosing, blood was collected into heparinized tubes by direct venipuncture of the leg vein (vena metatarsalis plantaris superficialis) before (0 h) and at different time points after HFB1 administration: 5 min, 10 min, 20 min, 30 min, 40 min, 50 min, 1 h, 1.5 h, 2 h, 3 h, 4 h, 6 h and 9 h post-administration (p.a.). Blood samples were centrifuged (2851× g, 10 min, 4 °C) and plasma was stored at ≤−15 °C until analysis.
The animal experiment was approved by the Ethical Committee of the Faculty of Veterinary Medicine and the Faculty of Bioscience Engineering of Ghent University (EC 2015/10, approval date: 9 March 2015).
4.4. Plasma Fumonisins Analysis: FB1, FB2, FB3, HFB1, pHFB1a, pHFB1b, Phase II Metabolites and N-Acyl Metabolites
FB1, and its partially hydrolyzed metabolites pHFB1a + b and hydrolyzed metabolite HFB1, in plasma, were determined using a validated sensitive and specific UPLC-MS/MS method as described by De Baere et al. (2018) [48].
4.4.1. Preparation of Standard Solutions
Stock solutions of FB1, FB2 and FB3 (1 mg/mL) were made in water/ACN (50/50, v/v) and kept at 2–8 °C. Working solutions of 10 µg/mL, 1 µg/mL and 0.1 µg/mL were made by adequate dilution of the stock solution in water/ACN (50/50, v/v). The standard mixture solution contained FB1, HFB1, pHFB1a and pHFB1b and was adequately diluted in water/ACN (50/50, v/v) in order to prepare working solutions necessary for the preparation of calibrator and quality control (QC) samples. A working solution of 1 µg/mL of 13C34-FB1 was made in water/ACN (50/50, v/v) to obtain the internal standard (IS). All working solutions were kept at 2–8 °C.
4.4.2. Plasma Sample Pre-Treatment
To 100 µL of plasma 12.5 µL of the IS working solution (1 µg/mL) was added. Then, after vortex mixing the sample was transfered onto an OstroTM 96-well plate. Next, 300 µL of 1% formic acid (FA) in ACN was added, after which the sample was aspirated 3 times in order to stimulate protein precipitation. By the application of a vacuum (67.7 kPa) for 10 min, the sample was run through the 96-well plate. An aliquot of 2.5-µL was injected onto the LC-MS/MS instrument.
4.4.3. UPLC-MS/MS Method for Quantification
Briefly, the LC system was composed of an Acquity UPLC H-Class Quaternary Solvent Manager and Flow-Through-Needle Sample Manager with temperature-controlled tray and column oven from Waters (Zellik, Belgium). Chromatographic separation was accomplished on an Acquity UPLC HSS T3 column (100 mm × 2.1 mm i.d., dp: 1.8 µm) (Waters, Zellik, Belgium) combined with an Acquity HSS T3 1.8 μm Vanguard pre-column (Waters, Zellik, Belgium). The mobile phase A was composed of 0.3% FA and 10 mM NH4FA in water, and the mobile phase B was ACN. A gradient elution of 0–0.5 min (90% A, 10% B), 5.5 min (linear gradient to 90% B), 5.5–7.5 min (10% A, 90% B), 7.7 min (linear gradient to 90% A) and 7.7–10.0 min (90% A, 10% B) was performed. The flow-rate was set at 0.4 mL/min. The temperatures of the column oven and autosampler tray were adjusted to 40 °C and 8 °C, respectively. The UPLC column effluent was coupled to a Xevo TQ-S® MS/MS system, supplied with a positive electrospray ionization (ESI) probe (all from Waters). A divert valve was utilized and the UPLC effluent was routed to the mass spectrometer from 2.5 to 4.5 min. Optimization of instrument parameters was obtained by directly infusing working solutions of 1 µg/mL of FB1, FB2, FB3, the IS and a diluted standard mixture solution, which contained FB1, HFB1, pHFB1a and pHFB1b at concentrations of 0.5, 0.86, 1.43 and 2.5 µg/mL, respectively, combined with the mobile phase (50% A, 50% B, flow-rate: 200 µL/min). The flow-rate was set at 10 µL/min. MS/MS acquisition was operated in the multiple reaction monitoring (MRM) mode [48].
4.4.4. UPLC-HR-MS Analysis for Identification
To determine potential HFB1 phase II and N-acyl metabolites in plasma samples of broiler chickens, an Acquity I-Class UPLC interfaced to a Synapt G2-Si HDMS instrument (Waters, Zellik, Belgium) was used [48].
4.5. Toxicokinetic Modelling
Plasma concentration–time data were analyzed with a nonlinear mixed-effects modelling approach using quasi-random parametric expectation maximization (QRPEM) as an estimation method in Phoenix NLME® (Certara, Cary, NC, USA). The structural toxicokinetic model for the IV data was a two-compartmental model with first order elimination as shown in Equation (1).
| (dA1/dt) × 1/Vc = −Cl × C − Q × (C − C2) (dA2/dt) × 1/Vp = Q × (C − C2) |
(1) |
where dA1/dt is the rate of the decrease of the amount of mycotoxin in the plasma or central compartment, dA2/dt is the rate of decrease of the amount of mycotoxin in the peripheral compartment, Vc is the volume of distribution of the central compartment, Vp is the volume of distribution of the peripheral compartment, Cl is total body clearance, Q is the intercompartmental flow and C and C2 are the concentrations of the toxin in the central and peripheral compartments, respectively.
Interindividual variability was expressed using an exponential error model according to Equation (2):
| Pi = θP × eηPi | (2) |
where Pi is the parameter in the ith bird, θP is the typical value of the parameter in the population, and ηPi is a random variable in the ith bird, with a mean of zero and a variance of ω². Interindividual variability is reported as ω. Residual variability (ε), with a mean of zero and a variance of σ², was best described with a multiplicative error model in Equation (3).
| Cobs = Cpred × (1 + ε) | (3) |
where Cobs is the observed concentration for the individual and Cpred is the model-predicted concentration plus the error value (ε).
Structural and error model selection was guided by visual inspection of goodness-of-fit plots (e.g., observed vs predicted plasma concentrations, weighted residuals versus predicted concentrations, and weighted residuals versus time), –2LL, Akaike information criterion (AIC) and Bayesian information criterion (BIC) as well as precision of the parameter estimates. The models were chosen based on the smaller values of –2LL, AIC and BIC, the better precision of estimates, and the superior goodness-of-fit plots.
The evaluated covariate was the prior feeding with FBs-contaminated feed versus feeding with uncontaminated control feed (categorical variable). A stepwise forward–backward process was used to evaluate whether inclusion of the covariates significantly improved the model fit using a –2LL test. A decrease in –2LL with a p-value < 0.01 was considered significant for addition, and p < 0.001 for exclusion, of the covariate.
The following fixed effect parameters were determined: Vc, Vp, Cl and Q. The computed secondary parameters were: C0, AUC0-inf, Ke, MRT, T1/2α and T1/2β.
Acknowledgments
The technical assistance of J. Muyle was gratefully appreciated. M. Eeckhout and M. Van Hecke from the Department of Applied Biosciences, Faculty of Bioscience Engineering, Ghent University are acknowledged for the preparation of the experimental diets. S. De Saeger and C. Detavernier from the Centre of Excellence in Mycotoxicology & Public Health, Department of Bioanalysis, Faculty of Pharmaceutical Sciences, Ghent University, are acknowledged for the mycotoxin analyses of the feed samples. The authors gratefully appreciated the excellent assistance of many PhD students from the Department of Pharmacology, Toxicology and Biochemistry. G. Bichl of BIOMIN Holding GmbH is also greatly acknowledges for her help contributing this research. G. Antonissen was supported by a postdoctoral fellowship from Research Foundation—Flanders (12V6418N).The Laboratory of Pharmacology and Toxicology is part of the Ghent University expertise centre MSmall. The authors acknowledge the Hercules Synpat G2-Si HDMS instrument infrastructure funding AUGE/13/13.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/12/6/413/s1, Figure S1: Visual evaluation of the population model of HFB1 after intravenous (IV) dosing: scatter plot of the population dependent variable (DV), namely observed plasma concentration (Cobs), versus the individually predicted plasma concentration values (IPRED) (A) and QQ plot of the conditionally weighted residuals of Cobs (B).
Author Contributions
G.A., S.D.B. and S.C. conceived and designed the experiments; G.A. performed the animal experiment; S.D.B. performed the analytical experiments; B.N. and D.S. contributed to the synthesis of the FB1, pHFB1a, pHFB1b and HFB1 standard mixture; G.A., S.D.B. and M.D. analyzed the data; G.A., S.D.B. and D.d.H. drafted the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research was funded supported by BIOMIN Holding GmbH, Austria.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Key Contribution
For the first time toxicokinetic parameters of hydrolyzed fumonisin B1 (HFB1) were calculated in broiler chicken, after a feeding trial of a control or a fumonisin B1 contaminated diet and followed by a single oral or intravenous bolus of HFB1.
References
- 1.Gelderblom W.C., Jaskiewicz K., Marasas W.F., Thiel P.G., Horak R.M., Vleggaar R., Kriek N.P. Fumonisins-novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988;54:1806–1811. doi: 10.1128/AEM.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voss K., Smith G., Haschek W. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed. Sci. Technol. 2007;137:299–325. doi: 10.1016/j.anifeedsci.2007.06.007. [DOI] [Google Scholar]
- 3.Humpf H.-U., Voss K.A. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 2004;48:255–269. doi: 10.1002/mnfr.200400033. [DOI] [PubMed] [Google Scholar]
- 4.Stockmann-Juvala H., Savolainen K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008;27:799–809. doi: 10.1177/0960327108099525. [DOI] [PubMed] [Google Scholar]
- 5.Seiferlein M., Humpf H.-U., Voss K.A., Sullards M.C., Allegood J.C., Wang E., Merrill A.H. Hydrolyzed fumonisins HFB1 and HFB2are acylatedin vitroandin vivoby ceramide synthase to form cytotoxicN-acyl-metabolites. Mol. Nutr. Food Res. 2007;51:1120–1130. doi: 10.1002/mnfr.200700118. [DOI] [PubMed] [Google Scholar]
- 6.Merrill A.H., Sullards M.C., Wang E., Voss K.A., Riley R.T. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ. Health Perspect. 2001;109:283–289. doi: 10.1289/ehp.01109s2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley R.T., Enongene E., Voss K.A., Norred W.P., Meredith F.I., Sharma R.P., Spitsbergen J., Williams D.E., Carlson D.B., Merrill A.H. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 2001;109:301–308. doi: 10.1289/ehp.01109s2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang E., Norred W.P., Bacon C.W., Riley R.T., Merrill A.H. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Boil. Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 9.Benlasher E., Geng X., Nguyen N.T.X., Tardieu D., Bailly J.-D., Auvergne A., Guerre P. Comparative Effects of Fumonisins on Sphingolipid Metabolism and Toxicity in Ducks and Turkeys. Avian Dis. 2012;56:120–127. doi: 10.1637/9853-071911-Reg.1. [DOI] [PubMed] [Google Scholar]
- 10.Qiu M., Liu X. Determination of sphinganine, sphingosine and Sa/So ratio in urine of humans exposed to dietary fumonisin B1. Food Addit. Contam. 2001;18:263–269. doi: 10.1080/02652030117470. [DOI] [PubMed] [Google Scholar]
- 11.Bouhet S., Oswald I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007;51:925–931. doi: 10.1002/mnfr.200600266. [DOI] [PubMed] [Google Scholar]
- 12.Grenier B., Applegate T. Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javed T., Bennett G.A., Richard J.L., Dombrink-Kurtzman M.A., Côté L.M., Buck W.B. Mortality in broiler chicks on feed amended withFusarium proliferatum culture material or with purified fumonisin B1 and moniliformin. Mycopathologia. 1993;123:171–184. doi: 10.1007/BF01111269. [DOI] [PubMed] [Google Scholar]
- 14.Kubena L., Harvey R., Buckley S., Bailey R., Rottinghaus G. Effects of long-term feeding of diets containing moniliformin, supplied by Fusarium fujikuroi culture material, and fumonisin, supplied by Fusarium moniliforme culture material, to laying hens. Poult. Sci. 1999;78:1499–1505. doi: 10.1093/ps/78.11.1499. [DOI] [PubMed] [Google Scholar]
- 15.le Doux D.R., Brown T.P., Weibking T.S., Rottinghaus G. Fumonisin Toxicity in Broiler Chicks. J. Veter Diagn. Investig. 1992;4:330–333. doi: 10.1177/104063879200400317. [DOI] [PubMed] [Google Scholar]
- 16.Tardieu D., Bailly J.D., Benard G., Tran T.S., Guerre P. Toxicity of maize containing known levels of fumonisin B1 during force-feeding of ducks. Poult. Sci. 2004;83:1287–1293. doi: 10.1093/ps/83.8.1287. [DOI] [PubMed] [Google Scholar]
- 17.Tran S.T., Auvergne A., Benard G., Bailly J.D., Tardieu D., Babile R., Guerre P. Chronic effects of fumonisin B1 on ducks. Poult. Sci. 2005;84:22–28. doi: 10.1093/ps/84.1.22. [DOI] [PubMed] [Google Scholar]
- 18.Weibking T.S., le Doux D.R., Bermudez A.J., Turk J.R., Rottinghaus G.E., Wang E., Merrill A.H. Effects of Feeding Fusarium moniliforme Culture Material, Containing Known Levels of Fumonisin B1, on the Young Broiler Chick. Poult. Sci. 1993;72:456–466. doi: 10.3382/ps.0720456. [DOI] [PubMed] [Google Scholar]
- 19.Commission of the European Communities Comission recommendation 2006/576/ec of 17 August 20076 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products. Off. J. Eur. Union. 2006;229:7–9. [Google Scholar]
- 20.Antonissen G., Martel A., Pasmans F., Ducatelle R., Verbrugghe E., Vandenbroucke V., Li S., Haesebrouck F., van Immerseel F., Croubels S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins. 2014;6:430–452. doi: 10.3390/toxins6020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonissen G., Croubels S., Pasmans F., Ducatelle R., Eeckhaut V., Devreese M., Verlinden M., Haesebrouck F., Eeckhout M., de Saeger S., et al. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Veter Res. 2015;46:98. doi: 10.1186/s13567-015-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonissen G., van Immerseel F., Pasmans F., Ducatelle R., Janssens G.P.J., de Baere S., Mountzouris K., Su S., Wong E.A., de Meulenaer B., et al. Mycotoxins Deoxynivalenol and Fumonisins Alter the Extrinsic Component of Intestinal Barrier in Broiler Chickens. J. Agric. Food Chem. 2015;63:10846–10855. doi: 10.1021/acs.jafc.5b04119. [DOI] [PubMed] [Google Scholar]
- 23.Grenier B., Schwartz-Zimmermann H.E., Caha S., Moll W.-D., Schatzmayr G., Applegate T. Dose-Dependent Effects on Sphingoid Bases and Cytokines in Chickens Fed Diets Prepared with Fusarium Verticillioides Culture Material Containing Fumonisins. Toxins. 2015;7:1253–1272. doi: 10.3390/toxins7041253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonissen G., Devreese M., de Baere S., Martel A., van Immerseel F., Croubels S. Impact of Fusarium mycotoxins on hepatic and intestinal mRNA expression of cytochrome P450 enzymes and drug transporters, and on the pharmacokinetics of oral enrofloxacin in broiler chickens. Food Chem. Toxicol. 2017;101:75–83. doi: 10.1016/j.fct.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Bryden W. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed. Sci. Technol. 2012;173:134–158. doi: 10.1016/j.anifeedsci.2011.12.014. [DOI] [Google Scholar]
- 26.Heinl S., Hartinger D., Thamhesl M., Vekiru E., Krska R., Schatzmayr G., Moll W.-D., Grabherr R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010;145:120–129. doi: 10.1016/j.jbiotec.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Hartinger D., Moll W.-D. Fumonisin elimination and prospects for detoxification by enzymatic transformation. World Mycotoxin J. 2011;4:271–283. doi: 10.3920/WMJ2011.1285. [DOI] [Google Scholar]
- 28.Grenier B., Schwartz-Zimmermann H., Gruber-Dorninger C., Dohnal I., Aleschko M., Schatzmayr G., Moll W.-D., Applegate T.J. Enzymatic hydrolysis of fumonisins in the gastrointestinal tract of broiler chickens. Poult. Sci. 2017;96:4342–4351. doi: 10.3382/ps/pex280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shephard G.S., Thiel P., Sydenham E., Vleggaar R., Alberts J. Determination of the mycotoxin fumonisin B1 and identification of its partially hydrolysed metabolites in the faeces of non-human primates. Food Chem. Toxicol. 1994;32:23–29. doi: 10.1016/0278-6915(84)90032-2. [DOI] [PubMed] [Google Scholar]
- 30.Fodor J., Balogh K., Weber M., Mézes M., Kametler L., Pósa R., Mamet R., Bauer J., Horn P., Kovács F., et al. Absorption, distribution and elimination of fumonisin B1 metabolites in weaned piglets. Food Addit. Contam. Part A. 2007;25:88–96. doi: 10.1080/02652030701546180. [DOI] [PubMed] [Google Scholar]
- 31.Hahn I., Nagl V., Schwartz-Zimmermann H., Varga E., Schwarz C., Slavik V., Reisinger N., Malachová A., Cirlini M., Generotti S., et al. Effects of orally administered fumonisin B1 (FB1), partially hydrolysed FB1, hydrolysed FB1 and N-(1-deoxy-D-fructos-1-yl) FB1 on the sphingolipid metabolism in rats. Food Chem. Toxicol. 2015;76:11–18. doi: 10.1016/j.fct.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Masching S., Naehrer K., Schwartz-Zimmermann H.E., Sărăndan M., Schaumberger S., Dohnal I., Nagl V., Schatzmayr D. Gastrointestinal Degradation of Fumonisin B1 by Carboxylesterase FumD Prevents Fumonisin Induced Alteration of Sphingolipid Metabolism in Turkey and Swine. Toxins. 2016;8:84. doi: 10.3390/toxins8030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrer H., Humpf H.-U., Voss K. In vivo formation of N-acyl-fumonisin B1. Mycotoxin Res. 2014;31:33–40. doi: 10.1007/s12550-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humpf H.-U., Schmelz E.M., Meredith F.I., Vesper H., Vales T.R., Wang E., Menaldino D.S., Liotta D.C., Merrill A.H. Acylation of Naturally Occurring and Synthetic 1-Deoxysphinganines by Ceramide Synthase. J. Boil. Chem. 1998;273:19060–19064. doi: 10.1074/jbc.273.30.19060. [DOI] [PubMed] [Google Scholar]
- 35.Hopmans E.C., Hauck C.C., Hendrich S., Murphy P.A. Excretion of Fumonisin B1, Hydrolyzed Fumonisin B1, and the Fumonisin B1−Fructose Adduct in Rats. J. Agric. Food Chem. 1997;45:2618–2625. doi: 10.1021/jf960886j. [DOI] [Google Scholar]
- 36.Vudathala D.K., Prelusky D.B., Ayroud M., Trenholm H.L., Miller J.D. Pharmacokinetic fate and pathological effects of14C-fumonisin B1 in laying hens. Nat. Toxins. 1994;2:81–88. doi: 10.1002/nt.2620020206. [DOI] [PubMed] [Google Scholar]
- 37.Harrer H., Laviad E.L., Humpf H.-U., Futerman A.H. Identification ofN-acyl-fumonisin B1 as new cytotoxic metabolites of fumonisin mycotoxins. Mol. Nutr. Food Res. 2012;57:516–522. doi: 10.1002/mnfr.201200465. [DOI] [PubMed] [Google Scholar]
- 38.Gu M.J., Han S.E., Hwang K., Mayer E., Reisinger N., Schatzmayr D., Park B.-C., Han S.H., Yun C.-H. Hydrolyzed fumonisin B1 induces less inflammatory responses than fumonisin B1 in the co-culture model of porcine intestinal epithelial and immune cells. Toxicol. Lett. 2019;305:110–116. doi: 10.1016/j.toxlet.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Voss K.A., Bacon C.W., Meredith F.I., Norred W.P. Comparative Subchronic Toxicity Studies of Nixtamalized and Water-extracted Fusarium. Food Chem. Toxicol. 1996;34:623–632. doi: 10.1016/0278-6915(96)00024-5. [DOI] [PubMed] [Google Scholar]
- 40.Howard P.C.C., Howarda P., Coucha L.H., Pattonb R.E., Eppleyc R.M., Doergea D.R., Churchwella M.I., Marques M.M., Okerbergb C.V. Comparison of the Toxicity of Several Fumonisin Derivatives in a 28-Day Feeding Study with Female B6C3F1 Mice. Toxicol. Appl. Pharmacol. 2002;185:153–165. doi: 10.1006/taap.2002.9529. [DOI] [PubMed] [Google Scholar]
- 41.Grenier B., Bracarense A.-P.F., Schwartz-Zimmermann H., Trumel C., Cossalter A.-M., Schatzmayr G., Kolf-Clauw M., Moll W.-D., Oswald I.P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012;83:1465–1473. doi: 10.1016/j.bcp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Schertz H., Kluess J., Frahm J., Schatzmayr D., Dohnal I., Bichl G., Schwartz-Zimmermann H., Breves G., Dänicke S. Oral and Intravenous Fumonisin Exposure in Pigs—A Single-Dose Treatment Experiment Evaluating Toxicokinetics and Detoxification. Toxins. 2018;10:150. doi: 10.3390/toxins10040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonissen G., Devreese M., van Immerseel F., de Baere S., Hessenberger S., Martel A., Croubels S. Chronic Exposure to Deoxynivalenol Has No Influence on the Oral Bioavailability of Fumonisin B1 in Broiler Chickens. Toxins. 2015;7:560–571. doi: 10.3390/toxins7020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerre P. Fusariotoxins in Avian Species: Toxicokinetics, Metabolism and Persistence in Tissues. Toxins. 2015;7:2289–2305. doi: 10.3390/toxins7062289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dantzer W.R., Hopper J., Mullin K., Hendrich S., Murphy P.A. Excretion of14C-Fumonisin B1,14C-Hydrolyzed Fumonisin B1, and14C-Fumonisin B1-Fructose in Rats. J. Agric. Food Chem. 1999;47:4291–4296. doi: 10.1021/jf981340v. [DOI] [PubMed] [Google Scholar]
- 46.Monbaliu S., van Poucke C., Detavernier C., Dumoulin F., van de Velde M., Schoeters E., van Dyck S., Averkieva O., van Peteghem C., de Saeger S. Occurrence of Mycotoxins in Feed as Analyzed by a Multi-Mycotoxin LC-MS/MS Method. J. Agric. Food Chem. 2010;58:66–71. doi: 10.1021/jf903859z. [DOI] [PubMed] [Google Scholar]
- 47.Leslie J.F., Doe F.J., Plattner R.D., Shackelford D.D., Jonz J. Fumonisin B1 production and vegetative compatibility of strains from Gibberella fujikuroi mating population ?A? (Fusarium moniliforme) Mycopathologia. 1992;117:37–45. doi: 10.1007/BF00497277. [DOI] [PubMed] [Google Scholar]
- 48.de Baere S., Croubels S., Novak B., Bichl G., Antonissen G. Development and Validation of a UPLC-MS/MS and UPLC-HR-MS Method for the Determination of Fumonisin B1 and Its Hydrolysed Metabolites and Fumonisin B2 in Broiler Chicken Plasma. Toxins. 2018;10:62. doi: 10.3390/toxins10020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.