Abstract
There is currently debate about human coronavirus (HCoV) seasonality and pathogenicity, as epidemiological data are scarce. Here, we provide epidemiological and clinical features of HCoV patients with acute respiratory infection (ARI) examined in primary care general practice. We also describe HCoV seasonality over six influenza surveillance seasons (week 40 to 15 of each season) from the period 2014/2015 to 2019/2020 in Corsica (France). A sample of patients of all ages presenting for consultation for influenza-like illness (ILI) or ARI was included by physicians of the French Sentinelles Network during this period. Nasopharyngeal samples were tested for the presence of 21 respiratory pathogens by real-time RT-PCR. Among the 1389 ILI/ARI patients, 105 were positive for at least one HCoV (7.5%). On an annual basis, HCoVs circulated from week 48 (November) to weeks 14–15 (May) and peaked in week 6 (February). Overall, among the HCoV-positive patients detected in this study, HCoV-OC43 was the most commonly detected virus, followed by HCoV-NL63, HCoV-HKU1, and HCoV-229E. The HCoV detection rates varied significantly with age (p = 0.00005), with the age group 0–14 years accounting for 28.6% (n = 30) of HCoV-positive patients. Fever and malaise were less frequent in HCoV patients than in influenza patients, while sore throat, dyspnoea, rhinorrhoea, and conjunctivitis were more associated with HCoV positivity. In conclusion, this study demonstrates that HCoV subtypes appear in ARI/ILI patients seen in general practice, with characteristic outbreak patterns primarily in winter. This study also identified symptoms associated with HCoVs in patients with ARI/ILI. Further studies with representative samples should be conducted to provide additional insights into the epidemiology and clinical features of HCoVs.
Keywords: human coronavirus, acute respiratory infection, influenza, general practice, respiratory pathogens, influenza season
1. Introduction
Coronaviruses (CoVs) are an enveloped, single positive-strand RNA species of viruses belonging to the Coronaviridae family, which infect birds and mammals. In animals, CoVs cause respiratory, enteric, cardio-vascular, and neurological disorders [1]. In humans, these viruses result in respiratory and gastro-intestinal symptoms, ranging from cold symptoms to severe diseases [2,3].
CoVs recognized to infect humans belong to the genera Alphacoronavirus and Betacoronavirus [4]. Seven CoV species are known to cause human infection, of which four HCoVs (namely HCoV 229E, NL63, OC43, and HKU1) are known as non-severe acute respiratory syndrome (SARS)-like CoVs. HCoV-229E and HCoV-NL63 belong to the genus Alphacoronavirus. The genus Betacoronavirus includes HCoV-HKU1, HCoV-OC43, SARS-CoV-1 [5], the Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) [6], and the SARS-CoV-2, which is currently associated with a global outbreak [7].
The four non-SARS/MERS species circulate widely in humans and infect individuals of all ages [8,9]. HCoV-229E and HCoV-OC43 were identified in 1967 and were primarily associated with mild upper respiratory tract infections [10,11]. HCoV-NL63 was identified in 2004 from a 7-month-old child suffering from bronchiolitis and conjunctivitis [12] and HCoV-HKU1 was discovered in 2005 in Hong Kong and isolated from patients with pneumonia [13]. In general, the four common circulating HCoVs mostly infect humans during the winter season (December–April) [14], whereas circulation of HCoV-HKU1 has been observed during the spring–summer period [9].
The World Health Organization has highlighted the need to improve epidemiological surveillance and knowledge of the health burden imposed by non-influenza respiratory viruses [15]. HCoVs are generally associated with mild upper respiratory tract infections [16], but severe infections with HCoV-229E, HCoV-NL63, and HCoV-OC43 have been reported [17,18,19].
In the context of the spread of SARS-CoV-2 in the community, a better understanding of the seasonality and clinical features of patients with confirmed HCoVs could be useful for mathematical modelling and clinical diagnosis. There is currently a debate about HCoV seasonality and pathogenicity, as epidemiological data are scarce and mostly from hospitalized populations. Here, we document the epidemiological and clinical features of HCoV patients with acute respiratory infection (ARI) observed in general practice. We also describe HCoV seasonality over six influenza surveillance seasons (2014/2015 to 2019/2020) in Corsica, France.
2. Materials and Methods
2.1. Clinical Samples
Nasopharyngeal samples were collected: i) as part of the community influenza surveillance conducted in collaboration with the French Sentinelles Network from patients seen in general practice, consulting for influenza-like illness (ILI) or ARI (for patients aged >65 years old) during six influenza seasons (week 40 to 15 of each season) from 2014 to 2020 in Corsica, France; and ii) from ARI patients enrolled throughout mainland France by general practitioners (GPs) of the French Sentinelles Network, during an epidemiological study of the risk factors for seasonal influenza (IRIIS study; 2014–2016 influenza seasons (week 40 to 15)) [20]. Notably, to ensure that the selection of ILI/ARI patients remained random, each GP was required to include, each week, the first two patients unrelated to one another, consulting within <48 h since symptom onset and consenting to provide a nasopharyngeal specimen. Each patient could be included only once a year (Table 1 and Figure 1). The surveillance uses a specific definition of ILI for patient recruitment: sudden onset of fever >39 °C with myalgia and respiratory signs, diagnosed by the physician. The case definition of ARI was “any person with a sudden onset of symptoms and at least one of the following four systemic symptoms: fever or feverishness, malaise, headache, myalgia, and at least one of the following three respiratory symptoms: cough, sore throat, or shortness of breath.”
Table 1.
Samples from 2014/2015 to 2019/2020 included in the study.
| Origin of Samples | Period (Week 40 to 15) | N | HCoVs | 229E | OC43 | NL63 | HKU1 | Not Typed |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Influenza Surveillance | 2014/2015 to 2019/2020 | 811 | 57 (7.0) | 12 (21.0) | 20 (35.1) | 11 (19.3) | 11 (19.3) | 3 (5.3) |
| IRIIS | 2015/2016 to 2016/2017 | 578 | 48 (8.3) | 6 (12.0) | 8 (16.0) | 10 (20.0) | 9 (18.0) | 17 (34.0) |
| Total | 2014/2015 to 2019/2020 | 1389 | 105 * (7.5) | 18 (17.1) | 28 (26.6) | 21 (20.0) | 20 (20.0) | 20 (18.1) |
* Among the HCoV-positive patients, two were co-infected by two HCoVs (number of HCoVs detected = 107).
Figure 1.
Diagram of the data flow summarizing the patient subsets used for each analysis.
2.2. Viral Diagnostic Testing
Viral genome was extracted from 200 μL of patient samples using the QIAamp MinElute Virus Spin Kit (Qiagen, France). Routine molecular testing by real-time RT-PCR (rtRT-PCR) using an FTD Respiratory pathogens 21 Kit (Fast Track Diagnostics, Luxembourg) was employed from 2017/2018 onwards to detect: influenza viruses (subtypes A and B: flu A and flu B), human rhinovirus (HRV), human coronaviruses NL63 (HCoV-NL63), 229E (HCoV-229E), OC43 (HCoV-OC43), and HKU1 (HCoV-HKU1), respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human adenovirus (HAdV), human parainfluenza virus (HPIV), and human bocavirus (HBoV). Samples collected in 2014/2015 and 2016/2017 were analysed by ARGENE® Respiratory Range (Marcy l’Etoile, France). This rRT-PCR did not provide details for HCoVs and HAdV, HBoV, and HPIV were not analysed. Thus, samples still available were retrospectively analysed for CoV-229E, CoV-OC43, and CoV-NL63 by FTD.
All nasopharyngeal samples collected by the French Sentinelles Network during the 2019/2020 season were analysed for SARS-CoV-2. RdRp-IP1 and RdRp quantitative rtRT-PCR was used for detection of SARS-CoV-2. The RdRp rtRT-PCR corresponds to the Charité protocol [21]. When a sample was positive for RdRp-IP1, quantification of the number of RNA copies was performed according to a scale ranging from 103 to 107 copies per μL [22]. All analyses were conducted by the Laboratory of Virology, University of Corsica.
2.3. Seasonality of HCoVs in ILI/ARI Patients in General Practice
The HCoV seasonality in Corsica from 2014/2015 to 2019/2020 was studied using samples collected by GPs of the Corsican Sentinelles Network, as constant and homogeneous monitoring of this population of ILI/ARI patients has been conducted throughout six influenza seasons (Table 1 and Figure 1).
To study the weekly number of HCoVs detected among ILI/ARI patients seen in general practice during the six influenza seasons, we gathered all samples collected by GPs for influenza surveillance and for the IRIIS study (Table 1 and Figure 1).
2.4. Ethical Considerations
The samples were obtained as part of influenza surveillance. The protocol was conducted in agreement with the Helsinki Declaration. We obtained authorization from the French Data Protection Agency (CNIL#471393). The IRIIS study was approved by the Ethics Committee (CPP SUD MEDITERRANEE V, 01/09/2014; ref. number 14.078).
2.5. Statistical Analysis
Differences according to viral infection and epidemiological data (sex, age, clinical symptoms, and risk factors) were analysed and tested using Fisher’s exact test or Chi-square test. Results were considered statistically significant when the p value was lower than 0.05. All statistical analyses were performed using R software version 3.6.1 (R Foundation, Vienna, Austria).
3. Results
3.1. HCoV Prevalence over Six Influenza Seasons among Patients with ILI/ARI Seen in General Practice in Corsica
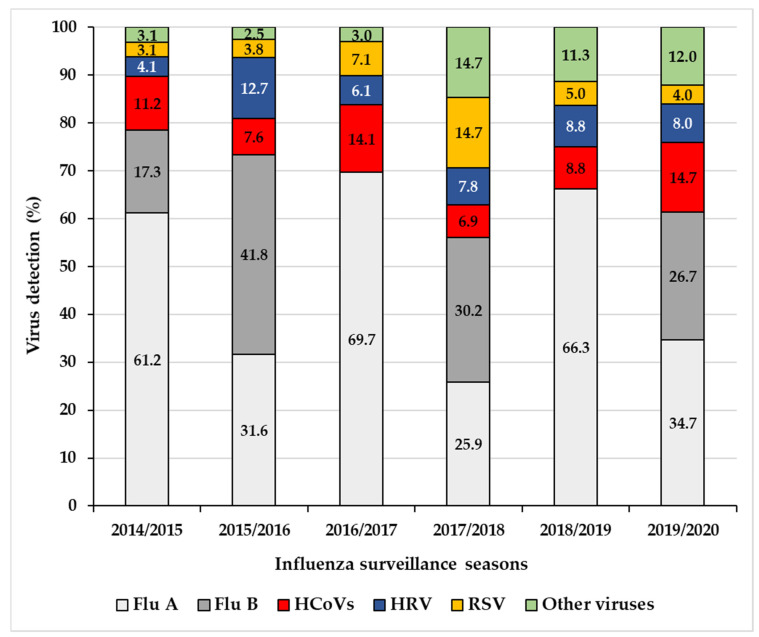
During the six-year study, among the 811 ILI/ARI patients enrolled by the Corsican Sentinelles Network, 63.3% (n = 513) were positive for at least one of the respiratory viruses analysed (Table 2). HCoVs were the third most frequently detected viruses (7.0%; n = 57) after influenza viruses subtypes A (32.4%; n = 263) and B (12.9%; n = 105) (Table 2). The number of HCoV infections ranged from 4.5% in 2014/2015 (6 of 133) to 10.0% in 2016/2017 (14 of 140) (Table 2). The contribution of HCoVs to the total number of viral detections (n = 547) (Figure 2) in the 811 ILI/ARI patients during each influenza season (week 40 to week 15) ranged from 6.9% (8 of 116) in 2017/2018 to 14.7% (11 of 75) in 2019/2020.
Table 2.
Chronological distribution of the different viruses detected during the influenza surveillance seasons.
| Overall | Flu A | Flu B | HCoVs | HRV | RSV | Other Viruses | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | n | % | n | % | n | % | n | % | n | % | |
| 2014/2015 | 151 | 60 | 39.7 | 17 | 11.3 | 11 | 7.3 | 4 | 2.6 | 3 | 2.0 | 3 | 2.0 |
| 2015/2016 | 133 | 25 | 18.8 | 33 | 24.8 | 6 | 4.5 | 10 | 7.5 | 3 | 2.3 | 2 | 1.5 |
| 2016/2017 | 140 | 69 | 49.3 | 0 | 0.0 | 14 | 10.0 | 6 | 4.3 | 7 | 5.0 | 3 | 2.1 |
| 2017/2018 | 150 | 30 | 20.0 | 35 | 23.3 | 8 | 5.3 | 9 | 6.0 | 17 | 11.3 | 17 | 11.3 |
| 2018/2019 | 118 | 53 | 44.9 | 0 | 0.0 | 7 | 5.9 | 7 | 5.9 | 4 | 3.4 | 9 | 7.6 |
| 2019/2020 | 119 | 26 | 21.8 | 20 | 16.8 | 11 | 9.2 | 6 | 5.0 | 3 | 2.5 | 9 | 7.6 |
| TOTAL | 811 | 263 | 32.4 | 105 | 12.9 | 57 | 7.0 | 42 | 5.2 | 37 | 4.6 | 43 | 5.3 |
Flu A: Influenza virus A; Flu B: Influenza virus B; HCoVs: Human coronaviruses; HRV: Human rhinovirus; RSV: Respiratory syncytial virus.
Figure 2.
Percentages of viral respiratory infections during each influenza season (week 40 to15) from 2014/2015 to 2019/2020, based on 811 ILI/ARI patients enrolled by the general practitioners of the Corsican Sentinelles Network.
3.2. Seasonality of HCoVs
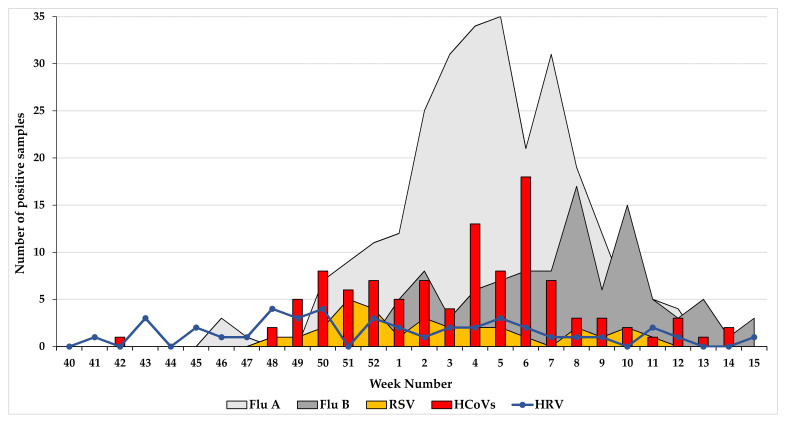
The seasonal distribution per week of HCoV, influenza viruses (A and B), RSV, and HRV was studied in 1389 ILI/ARI patients (Table 1 and Figure 3). Among the 1389 patients, 59.6% (n = 828) were positive for at least one respiratory virus, of which 39.6% (n = 328) were positive for influenza A virus, 25.6% (n = 212) for influenza B virus, and 12.6% (n = 105) for HCoVs. HCoVs were detected during the entire winter season (weeks 48–14), with the highest number of HCoVs detected in week 6 (Figure 3). Notably, week 6 had a decrease in influenza A virus detection (Figure 3). Since the number of HCoVs detected by species per season was low, the seasonality of HCoVs was established from all viruses detected per week over the six influenza seasons. The HCoV peak trend detection among week 4 to 7 was clearly observed during three influenza seasons (2014/2015; 2015/2016; 2019/2020).
Figure 3.
Analysis of the weekly distribution of Human Coronaviruses (HCoVs), influenza viruses (A and B), RSV, and HRV. Flu A: Influenza virus A; Flu B: Influenza virus B; HCoVs: Human coronaviruses; HRV: Human rhinovirus; RSV: Respiratory syncytial virus.
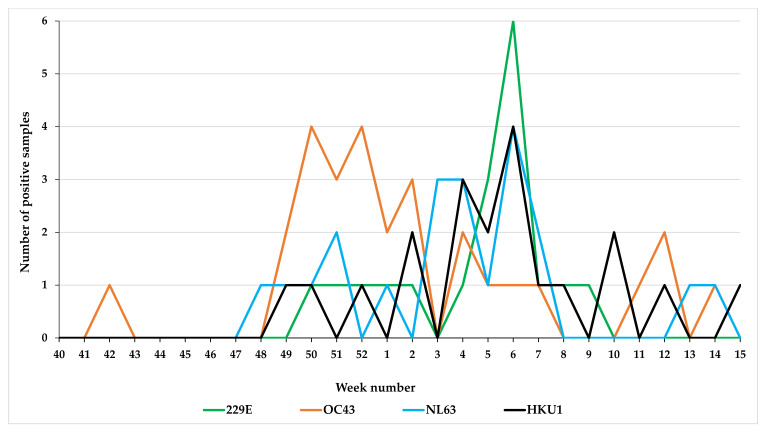
Among the 105 HCoV cases, 82.8% (n = 87) were typed. Of these 32.2% (n = 28) of cases were infected with HCoV-OC43, 24.1% (n = 21) with HCoV-NL63, 22.9% (n = 20) with HCoV-HKU1, and 20.6% (n = 18) with HCoV-229E (Table 1). The weekly number of HCoV types detected among the 1389 ILI/ARI patients is illustrated in Figure 3. A degree of synchrony was observed during all winter seasons and in the timing of the peak (week 6) for HCoV-HKU1, NL63, and 229E, whereas HCoV-OC43 infections occurred earlier with a peak in weeks 50–52 (Figure 4).
Figure 4.
Weekly prevalence of seasonal human coronaviruses (HCoVs) detected among ILI/ARI patients.
3.3. Co-Infection in HCoV-Positive Patients
Among the 1389 ILI/ARI patients, 105 (7.5%) were positive for at least one HCoV. Among them, 82 (78.1%) were infected with one HCoV, while 23 (21.9%) were infected with at least two different respiratory viruses (including two co-infections with three viruses) (Table 3). Of these, the most frequently observed co-pathogens were influenza viruses (flu A: n = 9/23; 39.1%, and flu B: n = 8/23; 34.8%) (Table 3). Two co-infection combinations were detected: HCoV-229E/NL63 and HCoV-HKU1/OC43.
Table 3.
Co-pathogen detection in human coronavirus-positive patients.
| Co-Pathogen | HCoVs | 229E | OC43 | NL63 | HKU1 | HCoV Without Species Determination |
|---|---|---|---|---|---|---|
| Flu A | 9 | 5 | 1 | / | 2 | 1 |
| Flu B | 8 | / | 1 | 2 | 3 | 2 |
| RSV | 3 | / | / | 1 | 2 | / |
| HRV | 1 | 1 | / | / | / | / |
| HMPV | 1 | / | / | / | 1 | / |
| HAdV | 1 | / | / | 1 | / | / |
| HCoV-229E | / | 1 | / | / | ||
| HCoV-OC43 | / | / | 1 | / | ||
| HCoV-NL63 | 1 | / | / | / | ||
| HCoV-HKU1 | / | 1 | / | / | ||
| HCoV without species determination | / | / | / | / | ||
| Total | 23 * | 7 | 3 | 5 | 9 | 3 |
* Two co-infections with three viruses (A(H1N1)pdm09/HKU/HMPV and BYAM/NL63/HAdV) and two co-infections with two HCoVs (HCoV-229E/NL63 and HCoV-HKU1/OC43). Flu A: Influenza virus A; Flu B: Influenza virus B; HCoVs: Human coronaviruses; HRV: Human rhinovirus; RSV: Respiratory syncytial virus; HMPV: Human metapneumovirus; HAdV: Human adenovirus.
3.4. Age Distribution of HCoV-Positive Patients
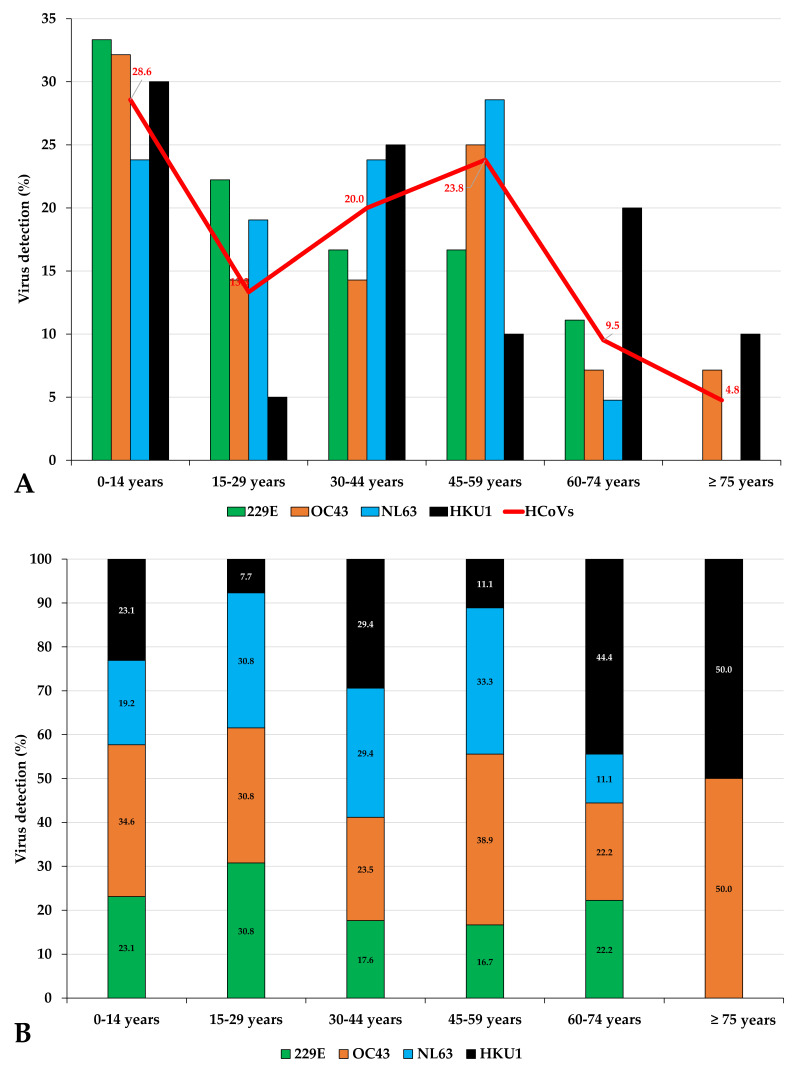
The demographic data and clinical characteristics of the 105 confirmed HCoV-positive patients are summarized in Table 4. The age of patients infected with HCoV varied from 1 month to 90 years with mean and median ages of 34 years. The male to female ratio of HCoV-infected patients was 1.06 (54/51). HCoV infections were observed in all age groups (Table 4 and Figure 5). However, the detection rates in these different age groups varied significantly (p = 0.00005) with the age group of 0–14 years accounting for 28.6% (n = 30) of HCoV-positive patients. In the remaining groups, detection rates were observed of 13.3% (n = 14) in the 15–29 age group, 20% (n = 21) in the 30–44 age group, 23.8% (n = 25) in the 45–59 age group, 9.5% (n = 10) in the 60–74 age group, and 4.8% (n = 5) in the group aged ≥75 years old.
Table 4.
Age, sex and clinical characteristics of ARI/ILI patients and their positivity for human coronaviruses and influenza viruses.
| Collected Variable | All Patients ARI./ILI | Patients positive | Patients HCoVs Positive | Negative for All Viruses Tested | ||||||||||||||||||||||
| Influenza Virus A | Influenza Virus B | All HCoVs Positive Patients* | p-Value | Single HCoV | Co-Pathogen | p-Value | p-Value | |||||||||||||||||||
| n | % | n | % | n | % | n | % | FluA/HCoV | FluB/HCoV | n | % | n | % | singleHCoV/ Copathogen | n | % | HCoV/ Negative | |||||||||
| Number of HCoVs positive patients | 1389 | 100.0 | 328 | 23.6 | 212 | 15.3 | 105 | 7.6 | 82 | 78.1 | 23 | 21.9 | 561 | 40.4 | ||||||||||||
| Age | Median Age (years) | 34 | 36 | 28.5 | 34 | / | / | 39.5 | 11.0 | / | 36.0 | / | ||||||||||||||
| Mean Age (Min-Max) (years) | 34.96 (0.08–97.0) | 34.53 (0.08–89) | 31.02 (0.67–91) | 34.61 (0.08–90) | 37.43 (0.50–90.0) | 24.5 (0.08–80) | 36.73 (0.25–90) | |||||||||||||||||||
| Age IQR (years) | 13–53 | 13–51 | 10–48 | 11–52 | 16.0–53.5 | 3.5–44.0 | 17.0–54.0 | |||||||||||||||||||
| n | % | n | % | n | % | n | % | p-Value | n | % | p-Value | n | % | p-Value | n | % | ||||||||||
| 0–14 years | 378 | 27.2 | 88 | 26.8 | 73 | 34.4 | 30 | 28.6 | 0.00005 | 0.908 | 0.535 | 18 | 22.0 | 0.00137 | 12 | 52.2 | 0.00005 | 0.008 | 124 | 22.1 | 0.406 | |||||
| 15–29 years | 210 | 15.1 | 42 | 12.8 | 37 | 17.5 | 14 | 13.3 | 13 | 15.9 | 1 | 4.3 | 0.295 | 93 | 16.6 | |||||||||||
| 30–44 years | 303 | 21.8 | 84 | 25.6 | 42 | 19.8 | 21 | 20.0 | 17 | 20.7 | 4 | 17.4 | 1 | 135 | 24.1 | |||||||||||
| 45–59 years | 268 | 19.3 | 69 | 21.0 | 34 | 16.0 | 25 | 23.8 | 22 | 26.8 | 3 | 13.0 | 0.267 | 104 | 18.5 | |||||||||||
| 60–74 years | 149 | 10.7 | 32 | 9.8 | 16 | 7.5 | 10 | 9.5 | 8 | 9.8 | 2 | 8.7 | 1 | 73 | 13.0 | |||||||||||
| ≥ 75 | 81 | 5.8 | 13 | 4.0 | 10 | 4.7 | 5 | 4.8 | 4 | 4.9 | 1 | 4.3 | 1 | 32 | 5.7 | |||||||||||
| Sex | Female | 758 | 54.6 | 184 | 56.1 | 110 | 51.9 | 51 | 48.6 | 0.782 | 0.216 | 0.633 | 44 | 53.7 | 0.435 | 7 | 30.4 | 0.0174 | 0.06 | 311 | 55.4 | 0.202 | ||||
| Male | 631 | 45.4 | 144 | 43.9 | 102 | 48.1 | 54 | 51.4 | 38 | 46.3 | 16 | 69.6 | 250 | 44.6 | ||||||||||||
| n | % | NA | n | % | NA | n | % | NA | n | % | NA | n | % | NA | n | % | NA | n | % | NA | ||||||
| Symptoms | Fever | 1298 | 95.2 | 25 | 321 | 98.5 | 2 | 205 | 97.2 | 1 | 94 | 91.3 | 2 | 0.00131 | 0.0439 | 72 | 88.9 | 1 | 22 | 100.0 | 1 | 0.198 | 513 | 93.4 | 12 | 0.400 |
| Cough | 1087 | 87.8 | 151 | 247 | 92.2 | 60 | 173 | 88.7 | 17 | 84 | 89.4 | 11 | 0.397 | 1 | 66 | 90.4 | 9 | 18 | 85.7 | 2 | 0.688 | 418 | 82.9 | 57 | 0.128 | |
| Sore throat | 659 | 53.2 | 151 | 125 | 46.6 | 60 | 101 | 51.8 | 17 | 64 | 68.1 | 11 | 0.000465 | 0.011 | 55 | 75.3 | 9 | 9 | 42.9 | 2 | 0.006 | 267 | 53.0 | 57 | 0.0068 | |
| Dyspnea | 241 | 19.5 | 151 | 49 | 14.9 | 0 | 31 | 14.6 | 0 | 32 | 30.5 | 0 | 0.000817 | 0.00152 | 29 | 35.4 | 0 | 3 | 13.0 | 0 | 0.04 | 91 | 16.2 | 0 | 0.000963 | |
| Rhinorrhea | 932 | 75.3 | 151 | 206 | 76.9 | 60 | 150 | 76.9 | 17 | 83 | 88.3 | 11 | 0.017 | 0.0257 | 64 | 87.7 | 9 | 19 | 90.5 | 2 | 1 | 355 | 70.4 | 57 | 0.000201 | |
| Myalgia | 666 | 69.0 | 424 | 123 | 70.7 | 154 | 120 | 74.1 | 50 | 52 | 70.3 | 31 | 1 | 0.533 | 41 | 71.9 | 25 | 11 | 64.7 | 6 | 0.561 | 288 | 71.5 | 158 | 0.889 | |
| Headache | 863 | 69.7 | 151 | 194 | 72.4 | 60 | 147 | 75.4 | 17 | 73 | 77.7 | 11 | 0.343 | 0.769 | 59 | 80.8 | 9 | 14 | 66.7 | 2 | 0.233 | 346 | 68.7 | 57 | 0.0866 | |
| Malaise | 85 | 12.0 | 681 | 26 | 12.7 | 124 | 13 | 14.3 | 121 | 3 | 3.2 | 11 | 0.0104 | 0.00844 | 2 | 2.7 | 9 | 1 | 4.8 | 2 | 0.536 | 36 | 14.9 | 319 | 0.002 | |
| Conjunctivitis | 345 | 24.8 | 0 | 67 | 20.4 | 0 | 59 | 27.8 | 0 | 32 | 30.5 | 0 | 0.0446 | 0.692 | 24 | 29.3 | 0 | 8 | 34.8 | 0 | 0.616 | 141 | 25.1 | 0 | 0.275 | |
| Vomiting | 151 | 10.9 | 0 | 32 | 9.8 | 0 | 20 | 9.4 | 0 | 15 | 14.3 | 0 | 0.208 | 0.252 | 12 | 14.6 | 0 | 3 | 13.0 | 0 | 1 | 66 | 11.8 | 0 | 0.514 | |
| Diarrhea | 154 | 11.1 | 0 | 23 | 7.0 | 0 | 23 | 10.8 | 0 | 10 | 9.5 | 0 | 0.401 | 0.845 | 9 | 11.0 | 0 | 1 | 4.3 | 0 | 0.442 | 68 | 12.1 | 0 | 0.512 | |
| Other symptoms | 184 | 21.0 | 512 | 44 | 16.2 | 56 | 21 | 18.3 | 97 | 18 | 26.9 | 38 | 0.0521 | 0.192 | 15 | 30.6 | 33 | 3 | 16.7 | 5 | 0.757 | 82 | 25.0 | 233 | 0.759 | |
| Anormal lung auscultation | 88 | 26.3 | 1055 | 24 | 25.3 | 233 | 8 | 17.0 | 165 | 8 | 38.1 | 84 | 0.282 | 0.0709 | 6 | 46.2 | 69 | 2 | 25.0 | 15 | 0.4 | 34 | 27.2 | 436 | 0.309 | |
| Other variables | Risk factors | 321 | 23.5 | 22 | 59 | 18.3 | 6 | 41 | 19.4 | 1 | 16 | 15.2 | 0 | 0.555 | 0.4 | 13 | 15.9 | 0 | 3 | 13.0 | 0 | 1 | 145 | 26.4 | 11 | 0.0182 |
| Oseltamivir | 241 | 18.0 | 49 | 88 | 27.7 | 10 | 29 | 14.0 | 5 | 14 | 13.6 | 2 | 0.00348 | 1 | 12 | 15.0 | 2 | 2 | 8.7 | 0 | 0.73 | 88 | 16.4 | 26 | 0.558 | |
| Antibiotics | 173 | 13.3 | 86 | 43 | 14.2 | 25 | 18 | 8.8 | 7 | 10 | 9.9 | 4 | 0.31 | 0.833 | 7 | 8.9 | 3 | 3 | 13.6 | 1 | 0.45 | 73 | 14.1 | 43 | 0.338 | |
| Hospitalization | 39 | 2.9 | 32 | 12 | 3.8 | 10 | 7 | 3.4 | 5 | 2 | 1.9 | 0 | 0.532 | 0.7 | 2 | 2.4 | 0 | 0 | 0.0 | 0 | 1 | 15 | 2.7 | 10 | 1 | |
* Overall HCoV infections also include strains without species determination (N = 20).
Figure 5.
Age distribution of patients with human coronavirus infection (A) and the distribution of coronavirus subtype infections in the different age groups (B).
HCoV-OC43 and HCoV-HKU1 were detected in patients of all ages (Table 5 and Figure 5). The oldest patients (≥75 years old) were infected by HCoV-OC43 and HCoV-HKU1 only (Figure 5A), and HCoV-HKU1 was the most prevalent among patients aged ≥60 years old (Figure 5B), but a significant difference in the age-group distribution of HCoV species was not observed (Table 5).
Table 5.
Description of HCoV-positive patients according to the viral strains detected.
| Collected Variable | Distribution of HCoV Strain | |||||||||||||
| 229E | OC43 | NL63 | HKU1 | p-Value Variation between Species | ||||||||||
| n | % | n | % | n | % | n | % | |||||||
| Number of HCoVs positive patients | 18 | 17.1 | 28 | 26.7 | 21 | 20.0 | 20 | 19.0 | ||||||
| Age | Median Age (years) | 27 | 31 | 40 | 34.5 | / | ||||||||
| Mean Age (Min-Max) (years) | 31.1 (4–69) | 33.1 (0.5–90.0) | 33.72 (0.08–68) | 36.99 (0.83–80) | ||||||||||
| Age IQR (years) | 11.25–47.5 | 7–48.25 | 15–52 | 4–60.25 | ||||||||||
| n | % | p-Value | n | % | p-Value | n | % | p-Value | n | % | p-Value | |||
| 0–14 years | 6 | 33.3 | 0.156 | 9 | 32.1 | 0.0721 | 5 | 23.8 | 0.0721 | 6 | 30.0 | 0.224 | 0.927 | |
| 15–29 years | 4 | 22.2 | 4 | 14.3 | 4 | 19.0 | 1 | 5.0 | 0.46 | |||||
| 30–44 years | 3 | 16.7 | 4 | 14.3 | 5 | 23.8 | 5 | 25.0 | 0.75 | |||||
| 45–59 years | 3 | 16.7 | 7 | 25.0 | 6 | 28.6 | 2 | 10.0 | 0.44 | |||||
| 60–74 years | 2 | 11.1 | 2 | 7.1 | 1 | 4.8 | 4 | 20.0 | 0.38 | |||||
| ≥ 75 | 0 | 0.0 | 2 | 7.1 | 0 | 0.0 | 2 | 10.0 | 0.3 | |||||
| Sex | Female | 2 | 11.1 | 0.0000005 | 15 | 53.6 | 0.79 | 13 | 61.9 | 0.217 | 11 | 55.0 | 0.752 | 0.00209 |
| Male | 16 | 88.9 | 13 | 46.4 | 8 | 38.1 | 9 | 45.0 | ||||||
| n | % | NA | n | % | NA | n | % | NA | n | % | NA | |||
| Symptoms | Fever | 17 | 94.4 | 0 | 26 | 92.9 | 0 | 16 | 84.2 | 2 | 19 | 95.0 | 0 | 0.140 |
| Cough | 14 | 100.0 | 4 | 22 | 91.7 | 4 | 17 | 89.5 | 2 | 15 | 78.9 | 1 | 0.261 | |
| Sore throat | 11 | 78.6 | 4 | 14 | 58.3 | 4 | 15 | 78.9 | 2 | 11 | 57.9 | 1 | 0.307 | |
| Dyspnea | 3 | 16.7 | 0 | 4 | 14.3 | 0 | 7 | 33.3 | 0 | 8 | 40.0 | 0 | 0.138 | |
| Rhinorrhea | 13 | 92.9 | 4 | 23 | 95.8 | 4 | 16 | 84.2 | 2 | 16 | 84.2 | 1 | 0.509 | |
| Myalgia | 7 | 77.8 | 9 | 10 | 66.7 | 13 | 13 | 76.5 | 4 | 12 | 66.7 | 2 | 0.861 | |
| Headache | 13 | 92.9 | 4 | 20 | 83.3 | 4 | 14 | 73.7 | 2 | 13 | 68.4 | 1 | 0.322 | |
| Malaise | 0 | 0.0 | 4 | 0 | 0.0 | 4 | 0 | 0.0 | 2 | 3 | 15.8 | 1 | 0.0247 | |
| Conjunctivitis | 4 | 22.2 | 0 | 11 | 39.3 | 0 | 6 | 28.6 | 0 | 6 | 30.0 | 0 | 0.656 | |
| Vomiting | 1 | 5.6 | 0 | 5 | 17.9 | 0 | 4 | 19.0 | 0 | 2 | 10.0 | 0 | 0.543 | |
| Diarrhea | 1 | 5.6 | 0 | 1 | 3.6 | 0 | 2 | 9.5 | 0 | 3 | 15.0 | 0 | 0.517 | |
| Other symptoms | 4 | 30.8 | 5 | 3 | 15.0 | 8 | 2 | 15.4 | 8 | 3 | 25.0 | 8 | 0.669 | |
| Anormal lung auscultation | 1 | 33.3 | 15 | 4 | 66.7 | 22 | 1 | 16.7 | 15 | 2 | 33.3 | 14 | 0.343 | |
| Other variables | Risk factors | 0 | 0.0 | 0 | 3 | 10.7 | 0 | 3 | 14.3 | 0 | 5 | 25.0 | 0 | 0.138 |
| Oseltamivir | 3 | 16.7 | 0 | 2 | 7.1 | 0 | 3 | 15.8 | 2 | 4 | 20.0 | 0 | 0.607 | |
| Antibiotics | 2 | 11.1 | 0 | 1 | 3.6 | 0 | 3 | 15.0 | 1 | 1 | 5.9 | 3 | 0.515 | |
| Hospitalization | 1 | 5.6 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0.275 | |
3.5. Clinical Characteristics of ARI/ILI Patients and Comparison of Patients according to Their Viral Infection
The epidemiological and clinical features of patients were compared according to their viral infection: influenza A cases, influenza B cases, and HCoV cases (single or co-infection). Fever and malaise were less frequent in HCoV patients than in influenza patients, while sore throat, dyspnoea, rhinorrhoea, and conjunctivitis were more frequently associated with HCoV positivity (Table 4). Sore throat and dyspnoea were more frequently detected in patients with HCoV single infection (75.3%, 55/82) than in patients with HCoV co-infection (42.9%, 9/23; p = 0.006) (Table 4). Notably, oseltamivir was prescribed more frequently to influenza A patients (Table 4).
Lastly, when comparing epidemiological and clinical features of patients according to the HCoV strain detected, there was a significant effect on the prevalence of sex (p = 0.002), with the HCoV-229E strain detected more frequently in male patients (88.9%) (Table 5). A significant difference in the prevalence of malaise (p = 0.025) among HCoV strain patients was also identified, as this symptom was exclusively related to HCoV-HKU1 patients (15.8%, 3/20) (Table 5).
3.6. Clinical Description of SARS-CoV-2-Positive Patients Seen in General Practice
Among the 119 ARI patient samples collected during the 2019/2020 influenza season and tested retrospectively for SARS-CoV-2 from week 40 to week 15, four (3.3%) were positive for SARS-CoV-2. These four infections were detected during weeks 11 (n = 1), 14 (n = 2), and 15 (n = 1). Fever, cough, and headache were the most commonly declared symptoms, while anosmia was reported for one patient. The mean age of the four patients was 60.75 years and the sex ratio was 1:1 (two women and two men).
4. Discussion
Data from continuous surveillance are very important for identifying the pattern of HCoV epidemiology. This report is the first to describe patterns of circulation of the four common HCoV strains in French ILI/ARI patients seen in general practice over six influenza seasons. HCoV was the third most detected respiratory virus in ARI/ILI patients after the influenza A and B viruses. Similar to their susceptibility to other respiratory viruses, young children aged <14 years old had the highest risk of HCoV infection. Sore throat, dyspnoea, and conjunctivitis were more frequently described among HCoV cases than among influenza cases.
Similar to other countries in the northern hemisphere, we reported a winter seasonal prevalence pattern for the four HCoV strains [23], with the highest number of cases presenting in February [9,24]. In our study, the peak in February was attributable to the 229E, NL63, and HKU1 strains, whereas the OC43 strain had peaks in December. Overall, among the HCoV-positive patients in this study, the OC43 strain was the most commonly detected virus, followed by the NL63, HKU1, and 229E strains, in agreement with previous studies [9,25,26]. HCoV-OC43 is routinely associated with ~5% of acute respiratory infections and was the most commonly detected virus in HCoV patients [27,28,29]. A seroconversion study in hospitalized children reported that HCoV-OC43 and HCoV-NL63 might induce cross-immunity, and therefore reduce the subsequent number of clinically identified HCoV-HKU1 and HCoV-229E infections [30].
We confirmed that, similar to other respiratory pathogens, HCoVs were widespread in all age groups [31]. A significant difference in the age distribution of HCoV patients was noted, with the youngest (0–14 years old) patients displaying a threefold higher level of infection than those aged 60–74 years old and a sevenfold higher infection level than patients aged ≥75 years old. Among HCoV patients aged <60 years old, the most frequently detected viruses were HCoV-OC43 and HCoV-NL63, while among patients aged ≥60 years old, HCoV-HKU1 was the most frequently detected. This trend of HCoVs infection has already been reported in other studies [14,25,26,29]. A recent surveillance study on the occurrence and hospitalization rates in children with respiratory infections over 9 years reported that HCoVs were involved in 9.1% of episodes [25]. To date, in contrast to that observed for HCoVs, few SARS-CoV-2 have been observed in children. Official national statistics in Italy reported that 1% of total cases diagnosed country-wide were below 20 years [32]. It could be possible that pre-existing cross-immunity provides protection and/or reduces the severity of COVID-19, thereby reducing the number of children who are tested/hospitalized [33]. The fewer number of HCoV infections in older than younger patients may result from higher antibody levels or other immune mechanisms of protection [34]. The lower number of HCoV infections in older than younger patients may result from higher antibody levels or other immune mechanisms of protection [34]. It is well known that HCoVs co-circulate endemically with other common respiratory viruses and co-infections are frequently observed. In our study, six other common respiratory viruses were detected simultaneously and 21.9% of the HCoV-positive patients were co-infected by at least one of the other respiratory viruses. The occurrence of co-infection of HCoV, including other HCoVs, RSV, influenza A and B viruses, HAdV, and HMPV has been reported [29,35]. Similar to other studies, we observed that influenza and RSV were the most common respiratory viruses co-infected with HCoV [27,36]. We did not observe co-infection of SARS-CoV-2 and another respiratory virus among the four COVID-19 patients detected. A recent co-infection of SARS-CoV-2 and HCoV-HKU1 has been reported [37].
In this study, we compared the demographic data and clinical characteristics of HCoV patients with those of influenza A and B patients, respectively (Table 4). Sore throat, dyspnoea, rhinorrhoea, and conjunctivitis were more often observed in HCoV cases than in influenza cases. While dyspnoea was reported as not common in seasonal influenza viruses [38], studies of hospitalized patients reported the role of HCoV in causing lower respiratory illness [13,26]. Fever was more often observed in influenza cases, as reported [23]. These observations may be of importance, especially when HCoVs and influenza virus co-circulate. The clinical impact of HCoVs in co-detection is not fully understood, with previous studies observing unchanged morbidity or increased illness severity [39]. When we compared the clinical presentations of patients with a single HCoV infection with those with co-infection, sore throat and dyspnoea were more commonly reported in cases with a single infection.
A growing number of studies are currently describing the clinical features due to SARS-CoV-2 infection [40,41,42,43,44]. Although most of the COVID-19 studies have been conducted on hospitalized patients some comparisons with data of the present study might be useful. Fever and cough were the most common symptoms reported for SARS-CoV-2 and HCoV patients [45]. Compared to symptoms observed in HCoV patients included in the present study, SARS-CoV-2 patients seem to show less headache (6.3%–33.9% vs. 80.8%), rhinorrhea (4% vs. 87.7%) and myalgia (11.1–34.8% vs. 71.9%) [45]. Sore throat, which was one of the most detected symptoms among HCoVs patients, was less common among COVID-19 patients [43]. About half of COVID-19 patients had an underlying disease [45], whereas in our study less than a quarter of the HCoV patients suffered from an underlying disease.
This study has some limitations. First, we used two ILI/ARI patient samples, which can introduce bias, even if the methodology of enrolment was very similar. Second, the number of HCoV patients included did not allow the identification of meaningful associations by subanalyses. Third, we studied HCoV circulation during influenza season and not on annual basis. As previously described, HCoVs co-circulate and showed marked winter seasonality, and are not detected in the summer period [14]. Lastly, we did not identify the species of 20 HCoV cases because relevant samples were not available.
In conclusion, this study demonstrates that HCoV subtypes appear in ARI/ILI patients seen in general practice, with characteristic outbreak patterns primarily in winter. HCoVs were detected at a significantly higher rate in children aged <14 years old than in older patients aged ≥60 years. Some clinical manifestations seem to differentiate HCoV patients from influenza patients. Further studies with representative samples should be conducted to provide additional insights into the epidemiology and clinical features of HCoVs.
Acknowledgments
We thank all general practitioners and paediatricians participating in the French Sentinelles network.
Author Contributions
Conceptualization, A.F. and L.C.; methodology, L.C. and S.M.; validation, T.B., R.C. and A.F.; investigation, S.M.; data curation, A.F.; Laboratory analysis: N.V. and S.M.; Statistical analyses: L.C. and A.F.; writing—original draft preparation, A.F., L.C. and S.M.; writing—review and editing, R.C.; visualization, A.F.; funding acquisition, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by public funds from University of Corsica, Santé publique France and the Collectivité de Corse (grant number 2016-0033; 2019-0034; 2019-0031). The funding was not specific for the study described in this article.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit this article for publication. All researchers’ decisions have been entirely independent from funders.
References
- 1.Cui J., Li F., Shi Z. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Genet. 2018;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabeça T.K., Granato C., Bellei N.C.J. Epidemiological and clinical features of human coronavirus infections among different subsets of patients. Influ. Other Respir. Viruses. 2013;7:1040–1047. doi: 10.1111/irv.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esper F., Ou Z., Huang Y.T. Human coronaviruses are uncommon in patients with gastrointestinal illness. J. Clin. Virol. 2010;48:131–133. doi: 10.1016/j.jcv.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman S., Netland J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Genet. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . SARS (Severe Acute Respiratory Syndrome) WHO; Geneva, Switzerland: 2020. [Google Scholar]
- 6.Zaki A.M., Van Boheemen S., Bestebroer T., Osterhaus A., Fouchier R. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Coronavirus Disease (COVID-19) Outbreak. WHO; Geneva, Switzerland: 2020. [Google Scholar]
- 8.Graham N.M.H. THE EPIDEMIOLOGY OF ACUTE RESPIRATORY INFECTIONS IN CHILDREN AND ADULTS: A GLOBAL PERSPECTIVE. Epidemiol. Rev. 1990;12:149–178. doi: 10.1093/oxfordjournals.epirev.a036050. [DOI] [PubMed] [Google Scholar]
- 9.Friedman N., Alter H., Hindiyeh M., Mendelson E., Shemer-Avni Y., Mandelboim M. Human Coronavirus Infections in Israel: Epidemiology, Clinical Symptoms and Summer Seasonality of HCoV-HKU1. Viruses. 2018;10:515. doi: 10.3390/v10100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamre D., Procknow J.J. A New Virus Isolated from the Human Respiratory Tract? Exp. Boil. Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 11.Almeida J.D., Tyrrell D.A.J. The Morphology of Three Previously Uncharacterized Human Respiratory Viruses that Grow in Organ Culture. J. Gen. Virol. 1967;1:175–178. doi: 10.1099/0022-1317-1-2-175. [DOI] [PubMed] [Google Scholar]
- 12.Van Der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J.M., Wolthers K.C., Dillen P.M.E.W.-V., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo P.C.Y., Lau S.K.P., Chu C.-M., Chan K.-H., Tsoi H.-W., Huang Y., Wong B.H.L., Poon R.W.S., Cai J.J., Luk W.-K., et al. Characterization and Complete Genome Sequence of a Novel Coronavirus, Coronavirus HKU1, from Patients with Pneumonia. J. Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaunt E.R., Hardie A., Claas E.C.J., Simmonds P., Templeton K.E. Epidemiology and Clinical Presentations of the Four Human Coronaviruses 229E, HKU1, NL63, and OC43 Detected over 3 Years Using a Novel Multiplex Real-Time PCR Method. J. Clin. Microbiol. 2010;48:2940–2947. doi: 10.1128/JCM.00636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang J.W., Lam T.T.-Y., Zaraket H., Lipkin W.I., Drews S.J., Hatchette T.F., Heraud J.-M., Koopmans M.P.G., Abraham A., Baraket A., et al. Global epidemiology of non-influenza RNA respiratory viruses: Data gaps and a growing need for surveillance. Lancet Infect. Dis. 2017;17:e320–e326. doi: 10.1016/S1473-3099(17)30238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean A., Quach C., Yung A., Semret M. Severity and Outcome Associated With Human Coronavirus OC43 Infections Among Children. Pediatr. Infect. Dis. J. 2013;32:325–329. doi: 10.1097/INF.0b013e3182812787. [DOI] [PubMed] [Google Scholar]
- 17.Gagneur A., Vallet S., Talbot P.J., Legrand-Quillien M., Picard B., Payan C., Sizun J. Outbreaks of human coronavirus in a pediatric and neonatal intensive care unit. Eur. J. Pediatr. 2008;167:1427–1434. doi: 10.1007/s00431-008-0687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hand J., Rose E.B., Salinas A., Lu X., Sakthivel S.K., Schneider E., Watson J.T. Severe Respiratory Illness Outbreak Associated with Human Coronavirus NL63 in a Long-Term Care Facility. Emerg. Infect. Dis. 2018;24:1964–1966. doi: 10.3201/eid2410.180862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak R., Prost K., Yip L., Williams V., Leis J.A., Mubareka S. Severity of coronavirus respiratory tract infections in adults admitted to acute care in Toronto, Ontario. J. Clin. Virol. 2020;126:104338. doi: 10.1016/j.jcv.2020.104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minodier L., Masse S., Capai L., Blanchon T., Ceccaldi P., Werf S., Hanslik T., Charrel R., Falchi A. Risk factors for seasonal influenza virus detection in stools of patients consulting in general practice for acute respiratory infections in France, 2014-2016. Influ. Other Respir. Viruses. 2019;13:398–406. doi: 10.1111/irv.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO . Real-Time Rt-Pcr-Assays for the Detection of SARS-CoV-2 Institut Pasteur Paris. WHO; Geneva, Switzerland: 2020. [Google Scholar]
- 23.Zimmermann P., Curtis N. Coronavirus Infections in Children Including COVID-19. Pediatr. Infect. Dis. J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jevšnik M., Uršič T., Žigon N., Lusa L., Krivec U., Petrovec M. Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMC Infect. Dis. 2012;12:365. doi: 10.1186/1471-2334-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimdal I., Moe N., Krokstad S., Christensen A., Skanke L.H., Nordbø S.A., Døllner H. Human Coronavirus in Hospitalized Children With Respiratory Tract Infections: A 9-Year Population-Based Study From Norway. J. Infect. Dis. 2019;219:1198–1206. doi: 10.1093/infdis/jiy646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monto A.S., Dejonge P.M., Callear A.P., Bazzi L.A., Capriola S.B., Malosh R.E., Martin E.T., Petrie J.G. Coronavirus Occurrence and Transmission Over 8 Years in the HIVE Cohort of Households in Michigan. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Z.-Q., Chen D.-H., Tan W.-P., Qiu S.-Y., Xu D., Liang H.-X., Chen M.-X., Li X., Lin Z.-S., Liu W.-K., et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: A study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur. J. Clin. Microbiol. Infect. Dis. 2017;37:363–369. doi: 10.1007/s10096-017-3144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varghese L., Zachariah P., Vargas C., LaRussa P., Demmer R.T., Furuya Y.E., Whittier S., Reed C., Stockwell M.S., Saiman L. Epidemiology and Clinical Features of Human Coronaviruses in the Pediatric Population. J. Pediatr. Infect. Dis. Soc. 2017;7:151–158. doi: 10.1093/jpids/pix027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I., Watson J.T. Human coronavirus circulation in the United States 2014–2017. J. Clin. Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dijkman R., Jebbink M.F., Gaunt E.R., Rossen J.W., Templeton K.E., Kuijpers T.W., Van Der Hoek L. The dominance of human coronavirus OC43 and NL63 infections in infants. J. Clin. Virol. 2012;53:135–139. doi: 10.1016/j.jcv.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vabret A., Brouard J., Petitjean J., Eugene-Ruellan G., Freymuth F. Human coronavirus infections: Importance and diagnosis. La Presse Médicale. 1998;27:1813–1817. [PubMed] [Google Scholar]
- 32.Parri N., Magistà A.M., Marchetti F., Cantoni B., Arrighini A., Romanengo M., Felici E., Urbino A., Da Dalt L., Verdoni L., et al. Characteristic of COVID-19 infection in pediatric patients: Early findings from two Italian Pediatric Research Networks. Eur. J. Nucl. Med. Mol. Imaging. 2020:1–9. doi: 10.1007/s00431-020-03683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickbakhsh S., Ho A., Marques D.F.P., McMenamin J., Gunson R.N., Murcia P.R. Epidemiology of Seasonal Coronaviruses: Establishing the Context for the Emergence of Coronavirus Disease 2019. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorse G.J., Donovan M.M., Patel G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J. Med. Virol. 2020;92:512–517. doi: 10.1002/jmv.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X., Lu R., Wang Z., Zhu N., Wang W., Julian D., Chris B., Lu J., Tan W. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PLoS ONE. 2012;7:e32174. doi: 10.1371/journal.pone.0032174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debiaggi M., Canducci F., Ceresola E.R., Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol. J. 2012;9:247. doi: 10.1186/1743-422X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaung J., Chan D., Pada S., Tambyah P.A. Coinfection with COVID-19 and Coronavirus HKU1—The critical need for repeat testing if clinically indicated. J. Med. Virol. 2020 doi: 10.1002/jmv.25890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang C., Yao X., Zhao Y., Wu J., Huang P., Pan C., Liu S., Pan C. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect. 2020 doi: 10.1016/j.micinf.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asner S.A., Michelle E., Tran D., Smieja M., Merglen A., Mertz D. Clinical Disease Severity of Respiratory Viral Co-Infection versus Single Viral Infection: A Systematic Review and Meta-Analysis. PLoS ONE. 2014;9:e99392. doi: 10.1371/journal.pone.0099392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L., Li S.-B., Wang H.-Y., Zhang S., Gao H.-N., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368:606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ge H., Wang X., Yuan X., Xiao G., Wang C., Deng T., Yuan Q., Xiao X. The epidemiology and clinical information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]