Abstract
Hepatitis B virus (HBV) chronic infection is a critical risk factor for hepatocellular carcinoma. The innate immune response to HBV infection is a matter of debate. In particular, viral escape mechanisms are poorly understood. Our study reveals that HBV RNAs are not immunostimulatory in immunocompetent myeloid cells. In contrast, HBV DNA from viral particles and DNA replication intermediates are immunostimulatory and sensed by cyclic GMP-AMP Synthase (cGAS) and Stimulator of Interferon Genes (STING). We show that primary human hepatocytes express DNA sensors to reduced levels compared to myeloid cells. Nevertheless, hepatocytes can respond to HBV relaxed-circular DNA (rcDNA), when transfected in sufficient amounts, but not to HBV infection. Finally, our data suggest that HBV infection does not actively inhibit the DNA-sensing pathway. In conclusion, in infected hepatocytes, HBV passively evades recognition by cellular sensors of nucleic acids by (i) producing non-immunostimulatory RNAs, (ii) avoiding sensing of its DNAs by cGAS/STING without active inhibition of the pathway.
Keywords: hepatitis B virus (HBV), innate immunity, STING, cGAS, innate immune sensing, interferon response
1. Introduction
Innate immunity is the first line of defense against pathogens. Detection of pathogen-associated molecular patterns (PAMPs) by cellular pathogen recognition receptors (PRRs) triggers signaling pathways leading to interferon (IFN) production, which induces antiviral interferon-stimulated genes (ISGs) and pro-inflammatory cytokines.
In viral infections, viral RNA or DNA are common PAMPs that can be detected by cytosolic PRRs. Members of the RIG-I-like Receptor (RLR) family such as Retinoic Acid Inducible Gene I (RIG-I) and Melanoma Differentiation-Associated protein 5 (MDA5) sense foreign RNAs and activate Mitochondrial Antiviral Signaling Protein (MAVS), while foreign viral DNA is recognized by sensors like cyclic GMP-AMP Synthase (cGAS). Activated cGAS produces 2’3’-cyclic GMP-AMP (cGAMP), which activates Stimulator of Interferon Genes (STING). STING or MAVS activation can both lead to the activation of Interferon Regulatory Factor 3 (IRF3), which promotes IFN gene transcription [1]. To evade antiviral innate responses, viruses have evolved escape strategies involving the inhibition of innate signaling pathways by viral proteins, or the shielding of the viral genome from innate sensors (reviewed in [1,2]).
To date, more than 250 million people are chronically infected with hepatitis B virus (HBV), which is a high risk factor for liver cirrhosis and hepatocellular carcinoma. Its interplay with the signaling pathways leading to IFN production is still a matter of debate. Some publications have described a type I and III IFN or pro-inflammatory response to HBV infection in cultured hepatocytes [3,4,5,6,7]. HBV may also induce pro-inflammatory cytokines in immune cells such as Kupffer cells, the liver macrophages, even if they are not productively infected [8,9,10]. The induction of an innate response to HBV in infected hepatocytes has however been questioned by other reports [9,11,12,13,14], in line with initial studies in chimpanzees [15] and patients [16,17]. Therefore, HBV has been proposed to be a stealth virus in infected hepatocytes, but the mechanisms behind the lack of innate immune responses are not fully understood.
HBV particles contain a partially double-stranded DNA, the relaxed-circular DNA (rcDNA), which is repaired into a covalently closed circular DNA (cccDNA) in the nuclei of infected cells. The cccDNA is then transcribed into mRNAs and pregenomic RNA (pgRNA) that are exported to the cytoplasm. The pgRNA is encapsidated into newly synthesized capsids, where it is reverse transcribed into rcDNA. Both HBV RNAs and DNAs are therefore potential PAMPs. HBV pgRNA has been proposed to be sensed by the RIG-I- [4] or MDA5- pathways [3]. Surprisingly, MDA5 was also reported to bind HBV DNA [3]. Recently, sensing of HBV DNA by the cGAS/STING pathway was suggested [11,18] but the expression and functionality of this pathway in hepatocytes is unclear [9,11,14,18,19]. Therefore, the immunostimulatory potential of HBV nucleic acids and the PRR involved require further investigation.
HBV could inhibit the innate immune response to escape its antiviral effects, as suggested by several publications [6,11,20,21,22,23,24,25,26,27,28,29]. In particular, the regulatory Hepatitis B virus X protein (HBx) has been described to inhibit the MAVS pathway [21,27], while the viral polymerase was reported to inhibit STING [30] and the phosphorylation of IRF3 [24,25]. Verrier et al. [11] suggested the down-regulation of cGAS, STING and TANK-binding kinase 1 (TBK1) by HBV [11]. On the other hand, several publications claimed an absence of inhibition of the innate response by HBV [9,12,31,32]. Most of them tested several RNA-sensing pathways but the DNA-sensing pathway has been less extensively investigated.
In this study, we first assessed the immunostimulatory potential of naked HBV DNAs and RNAs in monocyte-derived dendritic cells (MDDCs) as a model for highly immunocompetent cells. For the first time, we could demonstrate that HBV RNAs are not immunostimulatory. On the contrary, naked HBV DNA can elicit a strong innate immune response, mediated by the cGAS/STING pathway. In hepatocytes, this pathway is expressed at a low level but retains its ability to sense naked HBV DNA, when present in sufficient amounts. However, this pathway is not activated upon productive HBV infection although our data suggest that the virus does not actively suppress it.
2. Materials and Methods
2.1. Cells and Ethics Statements
HepG2-hNTCP cells and HepaRG-hNTCP cells are derived from HepG2 cells and from HepaRG cells respectively and have been stably transduced with a lentiviral vector expressing HBV receptor, the sodium-taurocholate cotransporting polypeptide (NTCP) [33]. HepG2.2.15 cells are derived from HepG2 cells and have been stably transfected with HBV genome [34]. HepG2 H1.3deltaX cells are derived from HepG2 cells and contain the stable integration of a 1.3-fold HBV genome carrying premature stop codon mutations in both 5’ and 3’ parts of the HBx open reading frame [35]. HepAD38 cells are derived from HepG2 cells and contain a stable integration of an HBV genome under the control of a tetracycline repressor promoter [36]. In the absence of tetracycline these cells express all HBV RNAs and produce HBV particles. HepG2-hNTCP, HepAD38 and HepG2 H1.3deltaX were maintained in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal calf serum (FCS) and 2 mM L-Glutamine (complete DMEM). HepaRG-hNTCP were grown in William’s E medium supplemented with 10% FCS, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 5 × 10−5 M hydrocortisone hemisuccinate and 5 μg/mL insulin.
THP-1 cells are a human monocytic leukaemia cell line and can be differentiated into macrophage-like cells using phorbol 12-myristate 13-acetate (PMA) [37]. THP-1 cells deficient for cGAS (CGAS), STING (TMEM173) and MAVS (MAVS) were previously described [38]. THP-1 cells were cultivated in RPMI supplemented with 10% fetal calf serum (FCS) and 2 mM L-Glutamine. For differentiation, 40 ng/mL of PMA were added for 48 h.
Cell lines were authenticated as described in the Table 1 and regularly tested to exclude any mycoplasma contamination using a PCR-based method with the PCR Mycoplasma Test Kit II from AppliChem.
Table 1.
Cell lines, suppliers and authentication methods.
| Name | Citation | Supplier | Authentication Test Method |
|---|---|---|---|
| HepG2 | Figure 3 | Gift from Prof S.Urban, Heidelberg, Germany | Cell line authentication performed by Eurofins Genomics, using Applied BiosystemsTM AmpFLSTRTM IdentifilerTM Plus PCR Amplification Kit with 16 markers |
| HepG2-hNTCP | Figures 3–6 | Gift from Prof S.Urban, Heidelberg, Germany [33] | Cell line authentication performed by Eurofins Genomics, using Applied BiosystemsTM AmpFLSTRTM IdentifilerTM Plus PCR Amplification Kit with 16 markers |
| HepaRG-hNTCP | Figure 3 | Gift from Prof S.Urban, Heidelberg, Germany | Multiplexion cell contamination assay (Heidelberg, Germany) as described [39] |
| HepAD38 | Figure 1 (for HBV RNAs and replication intermediate extraction) | Gift from Prof E Hild, Langen, Germany | Release of HBV particles (qPCR + infectivity test) |
| THP1 | Figures 2 and 3 | Gift from Prof V. Hornung, Munich, Germany | Cell line authentication by Eurofins Genomics on 24.03.2017 |
| THP1 ΔSTING | Figure 2 | Gift from Prof V. Hornung, Munich, Germany | Western blot |
| THP1 ΔcGAS | Figure 2 | Gift from Prof V. Hornung, Munich, Germany | Western blot |
| THP1 ΔMAVS | Figure 2 | Gift from Prof V. Hornung, Munich, Germany | Western blot |
Primary human hepatocytes (PHH) were isolated from liver specimens resected from patients undergoing partial hepatectomy because of hepatocellular carcinoma or severe cirrhosis. The patients were tested negative for HBV, Hepatitis C virus (HCV) and Human immunodeficiency virus (HIV). Approval from the local and national ethics committees (Ethic committee from the Goethe University of Frankfurt, agreement number 343/13, and the Hannover medical school, agreement number 252-2008) and informed consent from patients were obtained. PHH were isolated with a two-step perfusion method and cultured as described previously [40]. Alternatively, cryopreserved plateable PHH were purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA). These cryopreserved PHH were isolated from dead patients who were tested negative for HBV, HCV, HIV-1 and 2, Human T- cell leukemia virus (HTLV) 1 and 2 and Cytomegalovirus (CMV). PHH were maintained in Williams’ Medium E supplemented with Serum-free Hepatocyte Maintenance Supplement Pack (Thermo Fisher Scientific CM4000) according to the provider’s instructions.
Cryopreserved human Kupffer cells were purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA) and handled according to the provider’s instructions.
Monocyte-derived dendritic cells (MDDCs) or monocyte-derived macrophages (MDMs) were generated from human peripheral blood mononuclear cells (PBMC) using respectively 25 U/mL GM-CSF (Leukine®Sargramostim, Genzyme, Cambridge, MA, USA) and 800 U/mL IL-4 (PeproTech, Rocky Hill, NJ, USA) or 50 U/mL GM-SCF. The PBMCs were isolated from human buffy coats of anonymous blood donors purchased from the German Red Cross Blood Donor Service Baden-Württemberg Hessen, Germany.
2.2. HepG2-hNTCP Overexpressing cGAS and STING
HepG2-hNTCP were transduced with pLX304-based lentiviral vectors encoding V5-tagged cGAS and selected with 20 µg/mL of blasticidin. For each experiment, the selected cells were freshly transduced with pLX304-based lentiviral vectors encoding V5-tagged STING and were used 6 days post-transduction for experiments.
2.3. Hepatitis B Virus Production and Infection
Preparation of Hepatitis B Virus wild type (HBVwt) inoculum from HepAD38 cells or HBV deficient for HBx (HBV X-) from HepG2 H1.3deltaX was performed as previously described [41]. Viral particles were titered by qPCR quantification of viral RC-DNA with the primers HBV_RC_F and HBV_RC_R (Table 2). For infection, HepG2-hNTCP and PHH were seeded on collagen-coated plates. HBV infections were performed in presence of 4% Polyethylene glycol 8000 and 2% DMSO unless otherwise indicated. When indicated, HBV entry inhibitor Myrcludex B [42] (1 µM) was added. Twenty-four hours after viral inoculation, the medium was changed and complete DMEM supplemented with 2% DMSO was added.
Table 2.
Oligonucleotides.
| Name | Sequence (5′–3′) | Supplier | Use |
|---|---|---|---|
| HBV_RC_F | CACTCTATGGAAGGCGGGTA | Eurofins Genomics | qPCR for quantification of HBV stocks |
| HBV_RC_R | TGCTCCAGCTCCTACCTTGT | Eurofins Genomics | qPCR for quantification of HBV stocks |
| HBV RNA 3 F | GCTTTCACTTTCTCGCCAAC | Eurofins Genomics | RT-qPCR |
| HBV RNA 3 R | GAGTTCCGCAGTATGGATCG | Eurofins Genomics | RT-qPCR |
| ISG54_F | GGTGGCAGAAGAGGAAGATT | Eurofins Genomics | RT-qPCR |
| ISG54_R | TAGGCCAGTAGGTTGCACAT | Eurofins Genomics | RT-qPCR |
| IFNλ-1_F | CGCCTTGGAAGAGTCACTCA | Eurofins Genomics | RT-qPCR |
| IFNλ-1_R | GAAGCCTCAGGTCCCAATTC | Eurofins Genomics | RT-qPCR |
| cGAS_F | CAAGAAGGCCTGCGCATTCA | Eurofins Genomics | RT-qPCR |
| cGAS_R | GAGAAGGATAGCCGCCATGT | Eurofins Genomics | RT-qPCR |
| STING_F | GATATCTGCGGCTGATCCTG | Eurofins Genomics | RT-qPCR |
| STING_R | GCTGTAAACCCGATCCTTGA | Eurofins Genomics | RT-qPCR |
| IFI16_F | CGCTTGAAGACCTGGCTGAA | Eurofins Genomics | RT-qPCR |
| IFI16_R | TGACAGTGCTGCTTGTGGAG | Eurofins Genomics | RT-qPCR |
| PQBP1_F | TCTGGAGCCTGAACCAGAGGAA | Eurofins Genomics | RT-qPCR |
| PQBP1_R | TCCAACCTGGTGGCCTCGTAGT | Eurofins Genomics | RT-qPCR |
| RIG-I_F | CCTACCTACATCCTGAGCTACAT | Eurofins Genomics | RT-qPCR |
| RIG-I_R | TCTAGGGCATCCAAAAAGCCA | Eurofins Genomics | RT-qPCR |
| MAVS_F | GGTGCTCACCAAGGTGTCTG | Eurofins Genomics | RT-qPCR |
| MAVS_R | AGGAGGTGCTGGCACTGATG | Eurofins Genomics | RT-qPCR |
| MDA5_F | AGAGTGGCTGTTTACATTGCC | Eurofins Genomics | RT-qPCR |
| MDA5_R | GCTGTTCAACTAGCAGTACCTT | Eurofins Genomics | RT-qPCR |
| TLR3_F | ACCTCCAGCACAATGAGCTA | Eurofins Genomics | RT-qPCR |
| TLR3_R | TCCAGCTGAACCTGAGTTCC | Eurofins Genomics | RT-qPCR |
| RPL13A_F | CCT GGA GGA GAA GAG GAA AGA GA | Eurofins Genomics | RT-qPCR |
| RPL13A_R | TTG AGG ACC TCT GTG TAT TTG TCA A | Eurofins Genomics | RT-qPCR |
| TBP_F | GGAGCTGTGATGTGAAGT | Eurofins Genomics | RT-qPCR |
| TBP_R | TACGTCITCTTCCTGAATCC | Eurofins Genomics | RT-qPCR |
| HBV_quant_F | TGTCAACACTAATATGGGCCTAA | Eurofins Genomics | quantification of HBV RNA and DNA |
| HBV_quant_R | AGGGGCATTTGGTGGTCTAT | Eurofins Genomics | quantification of HBV RNA and DNA |
| HBV_st | TGTCAACACTAATATGGGCCTAAAGTTCAGGCAACTCTTGTGGTTTCACATTTCTTGTCTCACTTTTGGAAGAGAAACAGTTATAGAGTATTTGGTGTCTTTCGGAGTGTGGATTCGCACTCCTCCAGCTTATAGACCACCAAATGCCCCT | Integrated DNA Technologies, BvBA | standard for quantification of HBV RNA and DNA |
| MVAgfp_F | AGCACGACTTCTTCAAGTCC | Eurofins Genomics | quantification of MVAgfp DNA |
| MVAgfp_R | GTTGTAGTTGTACTCCAGCTTG | Eurofins Genomics | quantification of MVAgfp DNA |
| MVAgfp_st | AGCACGACTTCTTCAAGTCCGCCATGCCCGAAGGCTACGTCCAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTACAAGACCCGCGCCGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGGGCATCGACTTCAAGGAGGACGGCAACATCCTGGGGCACAAGCTGGAGTACAACTACAAC | Integrated DNA Technologies, BvBA | standard for quantification of MVAgfp DNA |
| SeV_F | TTCATTATCATCCCGTGAGA | Eurofins Genomics | quantification of SeV RNA |
| SeV_R | CCAGTGATCCATCATCAATC | Eurofins Genomics | quantification of SeV RNA |
| SeV_st | TTCATTATCATCCCGTGAGATCAGGAACCTGAGGGTTATCACAAAAACTTTATTAGACAGGTTTGAGGATATTATACATAGTATAACGTATAGATTCCTCACCAAAGAGATAAAGATTTTGATGAAGATTTTAGGGGCAGTCAAGATGTTCGGGGCCAGGCAAAATGAATACACGACCGTGATTGATGATGGATCACTGG | Integrated DNA Technologies, BvBA | standard for quantification of SeV RNA |
(Eurofins Genomics, Ebersberg, Germany; Integrated DNA Technologies, BvBA, Coralville, IA, USA).
2.4. Other Viruses and Viral Vectors
Sendai virus, strain Cantell, was provided by G. Kochs, Medical Center-University of Freiburg, Germany.
The DNA virus MVA-gfp based on Modified vaccinia Ankara was kindly provided by Gerd Sutter, GSF-Institute for Molecular Virology, Munich, Germany [43,44].
2.5. Quantitative Reverse Transcription PCR (RT-qPCR)
For RT-qPCR analysis, total RNAs were extracted from cell pellets using NucleoSpin® RNA Plus Kit (Macherey Nagel, Düren, Germany). RT-qPCRs were performed on a LightCycler 480 (Roche, Basel, Switzerland) using QuantiTect SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany) and specific primers (Table 2). For analysis of HBV RNAs, primer sets that specifically amplify the 3.5 kb, 2.4 kb and 2.1 kb HBV RNAs (HBV RNA 3 F, HBV RNA 3 R, Table 2) were used. Unless otherwise stated, Ribosomal Protein L13 A (RPL13A) was used as a reference gene to normalize the samples. For each sample, the relative amount of RNA was calculated using the formula 2-ΔCt with ΔCt = (Ct gene of interest − Ct RPL13A). When indicated, relative change to the control condition was calculated using the formula 2- ΔΔ Ct with ΔΔCt = ΔCt sample − ΔCt control.
For Figure 3, the expression levels of the genes of interest and of 2 reference genes, RPL13A and TBP (TATA-box binding protein), were calculated using a standard curve generated with serial dilutions of THP1 RNA. For each sample, the values of the genes of interest were normalized to the geometric mean of the 2 reference genes. The limit of detection for each sample and each gene of interest was calculated as the RNA amount measured in the last detectable standard dilution of THP1 for the gene of interest divided by the geometric mean of the expression levels of the 2 reference genes for each sample.
2.6. Reagents
2′3′ cGAMP was purchased from Invivogen (San Diego, CA, USA) and used at a final concentration of 2 µg/mL in cell supernatant. HT-DNA (herring testis DNA) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.7. Preparation and Quantification of Viral Nucleic Acids for Transfection Experiments
For viral nucleic acid transfection experiments, total nucleic acids from HBV, Sendai Virus (SeV) and Modified vaccinia Ankara (MVA)-gfp viral stocks were extracted using the High Pure Viral Nucleic Acid Kit (Roche, Basel, Switzerland). When indicated, samples were digested with 1 mg/mL of RNAseA for 1 h at 37 °C followed by 15 min inactivation at 72 °C and an addition of RNAseIN (Promega, Madison, WI, USA), or with DNAse using the Turbo DNA-free kit (Ambion, Thermo Fisher Scientific, Waltham, MA, USA) accordingly to the manufacturer’s instructions.
Total RNAs from HepAD38 or HepG2 cells were extracted using the NucleoSpin® RNA Plus Kit (Macherey Nagel, Düren, Germany) and any DNA contaminants were digested with the Turbo DNA-free kit (Ambion, Thermo Fisher Scientific, Waltham, Massachusetts, USA).
HBV replication intermediates were extracted from the cytoplasmic fraction of HepAD38 cells as described in [45] with minor modifications. Cells were incubated for 45 min on ice in hypotonic buffer (10 mM Hepes pH 8, 1.5 mM MgCl2, 10 mM KCl). One percent of NP-40 was then added and the samples were vortexed for 30 s. Nuclei were pelleted by centrifugation at 6000 g at 4 °C for 5 min. The supernatants (cytoplasmic fractions) were treated with 8U/ml of DNAseI in presence of 3.3 mM of MgOAc for 90 min at 37 °C in order to keep only encapsidated DNA. After proteinase K digestion, the DNA was extracted with phenol-chloroform and treated with RNAseA as described earlier.
HBV DNA from particles, replication intermediates and MVA-gfp DNA copy numbers were determined by qPCR, HBV RNA and SeV RNA copy numbers were quantified using one-step reverse transcription qPCR (RT-qPCR) using specific primers and serial dilutions of standard DNA oligonucleotides (Table 2). For RNA samples, the results are expressed as cDNA-equivalent since the copy number of the viral RNAs is assumed to be proportional to the respective specific cDNA quantification. RT-qPCRs and qPCRs were performed using QuantiTect SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany) respectively with and without RT on a LightCycler 480 (Roche, Basel, Switzerland).
2.8. Transfections
Viral nucleic acids or HT-DNA (Sigma-Aldrich, St. Louis, MO, USA) were transfected into MDDCs or PMA-differentiated THP1 using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) or in PHH and HepG2-hNTCP-derived cell lines using X-tremeGENE HP reagent (Merck, Darmstadt, Germany) according to the manufacturer’s instructions.
For MDDC transfection experiments (Figure 1), Table 3 indicates the copy number/cell of viral DNAs (DNA copy/cell, determined by qPCR) or of viral RNAs (expressed as cDNA-equivalent copy/cell, determined by RT-qPCR). The indicated copy numbers/cell refers to the undiluted nucleic acids. SeV RNAs, MVA-gfp DNA and HBV DNA from viral particles were quantified from undigested nucleic acid preparations. HBV DNA replication intermediates were quantified from RNAse-digested samples. HBV RNA from viral particles or from total HepAD38 RNAs were quantified from DNase-digested samples to avoid any DNA contamination, and a control without RT was performed.
Table 3.
Quantification of viral nucleic acids used for MDDC transfection experiments.
| SeV n.ac. from Particles | MVA n.ac. from Particles | HBV n.ac. from Particles | HepaD38 RNA | HBV R.I. | |
|---|---|---|---|---|---|
| Viral DNA (copies/cell) | 5.0E+03 | 5.0E+03 | 5.0E+03 | ||
| Viral RNA (cDNA-equivalent copies/cell) | 2.8E+01 | 2.3E+01 | 1.3E+04 |
(n.ac: nucleic acids; R.I.: replication intermediates).
2.9. Western Blots
Cell samples were lysed using a TritonX-100 based Lysis Buffer (100 mM NaCl, 10 mM EDTA, 20 mM TRIS pH7.5, 1% TritonX-100, 1% Sodiumdesoxycholate). Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Specific proteins were detected using the antibodies listed in the Table 4. Secondary antibodies conjugated to the horseradish peroxidase (HRP; GE Healthcare, Chicago, IL, USA) were used for chemiluminescence detection.
Table 4.
Antibodies used for Western blots.
| Name | Supplier | Cat No. | Clone No. |
|---|---|---|---|
| Anti phospho S386-IRF3 (rabbit) | Abcam | ab76493 | EPR2346 |
| Anti IRF3 (rabbit) | Epitomics | 2241-1 | EP2419Y |
| Anti beta-actin (mouse) | Sigma-Aldrich | A5441 | AC-15 |
| Anti beta-tubulin (mouse) | Sigma-Aldrich | 075K4875 | TUB 2.1 |
| Anti GAPDH (rabbit) | Cell Signaling Technology | 2118 | 14C10 |
| Anti MAVS (rabbit) | Abcam | Ab25084 | |
| Anti STING (rabbit) | Cell Signaling Technology | 13647 | D2P2F |
| Anti cGAS (rabbit) | Cell Signaling Technology | 15102 | D1D3G |
| Anti-mouse HRP | Cell Signaling Technology | 7076S | |
| Anti-rabbit HRP | Cell Signaling Technology | 7074S |
(Abcam, Cambridge, United Kingdom; Epitomics, Burlingame, CA, USA; Sigma-Aldrich, St. Louis, MO, USA; Cell Signaling Technology, Danvers, MA, USA).
2.10. IRF3 Nuclear Translocation Assays
HepG2-hNTCP overexpressing cGAS and STING were infected or not with HBV (MOI 10000) in the presence of 2% DMSO. Four days post infection (dpi), the DMSO was removed. Six dpi, the cells were seeded on collagen-coated coverslips in 48-well plates and one day later transfected or not with 2 µg/well of HT-DNA. Sixteen hours post-transfection the samples were stained for IRF3 and HBV core (HBc) and imaged with a Zeiss LSM 510-META Confocal laser scanning microscope (Oberkochen) as described below.
2.11. Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 15 min, washed 3 times with PBS, permeabilized with 0.25% Triton-X100 for 7 min at room temperature and blocked for 1 h in PBS-0.1% Tween (PBST) containing 5% of bovine serum albumin (BSA). The coverslips were then incubated for 2 h at room temperature with the primary antibodies, washed 3 times in PBST, and incubated for 1 hour with the secondary antibodies (Table 5). The coverslips were washed 3 times in PBST and the DNA was stained with Hoechst reagent.
Table 5.
Antibodies used for immunofluorescence.
| Name | Citation | Supplier | Cat No. | Clone No. |
|---|---|---|---|---|
| Anti IRF3 (rabbit) | IRF3 translocation assay, Figure 6 | Cell Signaling Technology | 11904S | D6I4C |
| anti HBc (mouse) | IRF3 translocation assay, Figure 6 | gift from Prof. S. Urban, Heidelberg | M312 | |
| anti-rabbit Alexa Fluor 488 | IRF3 translocation assay, Figure 6 | Invitrogen | A11008 | |
| anti-mouse Alexa Fluor 555 | IRF3 translocation assay, Figure 6 | Thermo Fischer Scientific | A21422 | |
| Anti-HBc (rabbit) | Figure S5 | Dako, Agilent | B0586 | |
| Goat anti-rabbit IgG(H+L), Alexa Fluor 546 | Figure S5 | Thermo Fischer Scientific | A-11010 |
(Cell Signaling Technology, Danvers, Massachusetts, USA; Invitrogen, Carlsbad, CA, USA; Thermo Fischer Scientific, Waltham, MA, USA; Dako, Agilent, Santa Clara, CA, USA).
2.12. Microscope Image Acquisition
For the IRF3 translocation assay (Figure 6) the samples were mounted with Mowiol medium. They were imaged using a Zeiss LSM 510-META Axiovert 200M Confocal laser scanning microscope, with a 40× objective (EC "Plan-Neofluar" 40×/1,30 Oil DIC) and an Axiocam camera with the acquisition software ZEN 2009, Version: 5.5.0.451.
Imaging of the MVA-gfp-infected HepG2-hNTCP (Figure S2) was performed in the culture plates with cell medium using a Nikon Eclipse TS100 inverted microscope with a 10× objective (Achromat, 10×/0,25 Ph1 DL) and a Nikon DS-Vi1 camera, with the acquisition software NIS-Elements F 4.30.00 (Build 1020) 64 bit.
For HBcAg staining in PHH (Figure S5), stained cells were imaged in PBS without mounting medium using an LEICA DM IRB microscope with a 20× objective (N PLAN L 20*/0.40 CORR PH 1) and am HAMAMATSU camera with the acquisition software HoKawo 2.12.
All microscope acquisitions were performed at room temperature. Contrast/brightness of the images were adjusted with Fiji ImageJ (version 1.52p; open source software).
3. Results
3.1. HBV DNAs but not RNAs are Immunostimulatory
We first investigated whether different species of HBV nucleic acids (DNAs or RNAs from viral particles or from HBV-producing cells) are immunostimulatory. To this aim, we used monocyte-derived dendritic cells (MDDCs) as a model for highly sensitive immune cells that most likely express all nucleic acid sensors. The MDDCs were transfected with ten-fold serial dilutions of quantified viral nucleic acids and ISG54 mRNA induction was measured by RT-qPCR. ISG54 mRNA was chosen as a marker for activation of nucleic acid-sensing pathways, as it is a direct target gene of IRF3-dependent transcription [46,47]. The copy number of the viral nucleic acids (DNA or RNA) used for MDDC transfection was determined as described in the material and methods section and is indicated in Table 3.
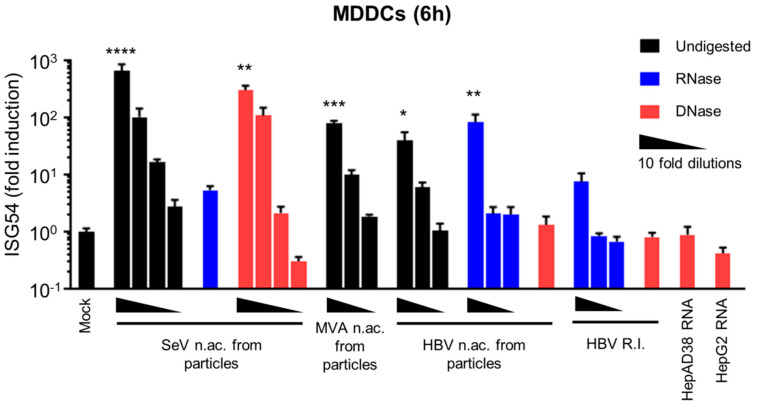
To validate the system, we first used total nucleic acids isolated from viral preparations of the RNA virus SeV or of a Gfp-expressing vector based on the DNA virus Modified vaccinia Ankara (MVA-gfp). SeV RNA activates RIG-I signaling and is used as a model to stimulate cytoplasmic sensing of RNA [48]. MVA-gfp DNA activates cGAS/STING signaling and is employed as a model to stimulate cytoplasmic sensing of DNA [49]. We observed a high ISG54 induction upon transfection of MDDCs with SeV nucleic acids (Figure 1) demonstrating the functionality of the RIG-I pathway. This response is abrogated upon RNAse digestion of SeV nucleic acids, while it is unaffected by DNAse digestion, proving that this response is specific for RNA. Nucleic acids from MVA-gfp particles also induce a dose-dependent ISG54 response, validating the functionality of the cGAS/STING pathway.
Figure 1.
HBV DNAs but not RNAs are immunostimulatory. Total nucleic acids (n.ac.) were extracted from SeV, MVA-gfp or HBV particles. HBV DNA replication intermediates (HBV R.I.) were extracted from HepAD38 cytoplasm. Total RNAs were extracted from HepAD38 or HepG2 cells. When indicated, the nucleic acids were digested with RNAse (blue) or DNAse (red; black bars: undigested). Human MDDCs were transfected with 10-fold dilutions of the nucleic acids (quantified in Table 3). ISG54 mRNA level was quantified by RT-qPCR 6 h post-transfection. Average and SEM of three technical replicates of at least two donors are shown. Levels of significance over mock transfection: **** p < 0.0001, *** p < 0.001, ** p < 0.01 and * p < 0.05 (Kruskal–Wallis test with Dunn’s multiple comparisons test).
Interestingly, nucleic acids extracted from HBV viral preparations induced an ISG54 response, which was unaffected by RNAse digestion but is abrogated by DNAse digestion (Figure 1), demonstrating that HBV DNA contained in the viral particles was immunostimulatory. HBV viral preparations also contained small amounts of HBV RNAs (Table 3), confirming the reports by Cheng et al. [9] However, DNAse-digested nucleic acids from HBV particles do not induce an ISG54 response (Figure 1), suggesting that these particle-associated HBV RNAs do not account for the immunostimulatory activity of HBV nucleic acids from particles.
In HBV-infected cells, various DNA replication intermediates are produced in the cytoplasm upon reverse-transcription of the pgRNA [50] and may also act as PAMPs. To test their immunostimulatory potential, we extracted HBV DNA replication intermediates from the cytoplasmic fraction of HepAD38 cells after removal of any cellular or viral RNA contamination using RNAse (as described in Materials and Methods and in [45]). HepAD38 contain an integrated copy of the HBV genome and produce all viral RNAs and DNA replication intermediates, as well as functional HBV particles [36]. When HBV DNA replication intermediates were transfected into MDDCs, the kinetic of ISG54 induction varied depending on the donors. The analysis at 6 h (Figure 1) shows a slight but not statistically significant ISG54 induction. However, when a second time point was included in the analysis (6 h + 24 h, Figure S1), a significant ISG54 induction was observed with the highest concentration (5000 copies/cell) of HBV replication intermediates. Intriguingly, the response was apparently weaker than when using the same copy number (5000 copies/cell) of HBV rcDNA (Figure 1). However, we cannot exclude that different DNA structures are transfected with different efficiencies, leading to apparent differences in their immunostimulatory potential. We therefore conclude that HBV replication intermediates are immunostimulatory but we cannot affirm that the difference with HBV DNA from viral particles is biologically relevant.
Furthermore, we tested the immunostimulatory potential of HBV RNAs from HBV-producing cells, using total RNAs from HepAD38 cells. However, no ISG54 induction was detected upon transfection of 1.3 × 104 cDNA-equivalent copies/cells of HepAD38 RNAs into MDDCs at 6 h or 24 h (Figure 1, Figure S1 and Table 3). Considering that only 28 cDNA-equivalent copies/cells of SeV RNAs induce a robust ISG54 response at 6 h in MDDCs (Figure 1, undiluted sample, Table 3), which is 4.6 × 102 times less than the amount of HBV RNAs used, we conclude that that HBV RNAs (mRNAs or pgRNA) are not immunostimulatory.
In summary, naked HBV DNA from particles and DNA replication intermediates from HBV-producing cells have the potential to elicit an innate response whereas HBV RNAs from HBV-producing cells are not immunostimulatory.
3.2. Virion-Associated HBV DNA is Sensed by the cGAS/STING Pathway
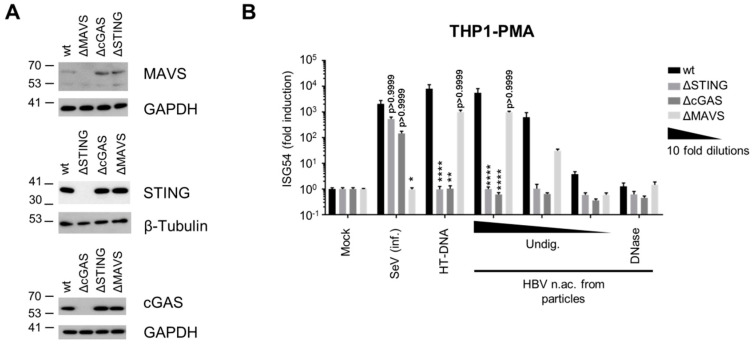
To identify which PRRs and pathways sense and respond to HBV DNA, we used a panel of THP-1 knock-out (KO) cell lines deficient for key nodes of the sensing pathways, cGAS, STING or MAVS (THP-1 wt, ΔSTING, ΔcGAS and ΔMAVS respectively, Figure 2A). As expected, KO of STING or cGAS did not significantly affect the ISG54 response to SeV infection, while MAVS KO abrogated it (Figure 2B). On the contrary, STING or cGAS KOs abrogated the innate response to transfection with the cGAS agonist herring testes DNA (HT-DNA) while the transfected DNA was well sensed by MAVS KO, proving the validity of the chosen assay system. Similar to MDDCs, transfection of HBV nucleic acids from viral particles in THP1 wt strongly induced a dose-dependent ISG54 response, which was abrogated by DNAse digestion. Interestingly, STING and cGAS KOs totally abrogated the response to HBV nucleic acids, while MAVS KO had no significant effect. These results indicate that HBV DNA is sensed through the cGAS/STING pathway, while the RLR pathway is not involved. In addition, this further confirms that virion-associated HBV RNAs do not account for the innate response.
Figure 2.
HBV DNA is sensed by the cGAS/STING pathway. (A) Genome editing of THP1 CRISPR/Cas9 KO for STING, cGAS or MAVS was controlled by Western Blot. (B) PMA-differentiated WT or KO THP1 cells were infected with SeV, transfected with HT-DNA (5 pg/cell) or with serial dilutions of undigested (Undig.) or DNAse-digested HBV nucleic acids (n.ac.) from particles. ISG54 mRNA fold induction to the mock was determined by RT-qPCR 24 h post-transfection. Average and SEM of three independent experiments in triplicates are shown. **** p < 0.0001, ** p < 0.01 and * p < 0.05 (Kruskal–Wallis test with Dunn’s test; for each stimulus, the knock-out cells are compared to the WT cells).
3.3. Hepatocytes Express Low Levels of the DNA Sensors Compared to Immune Cells
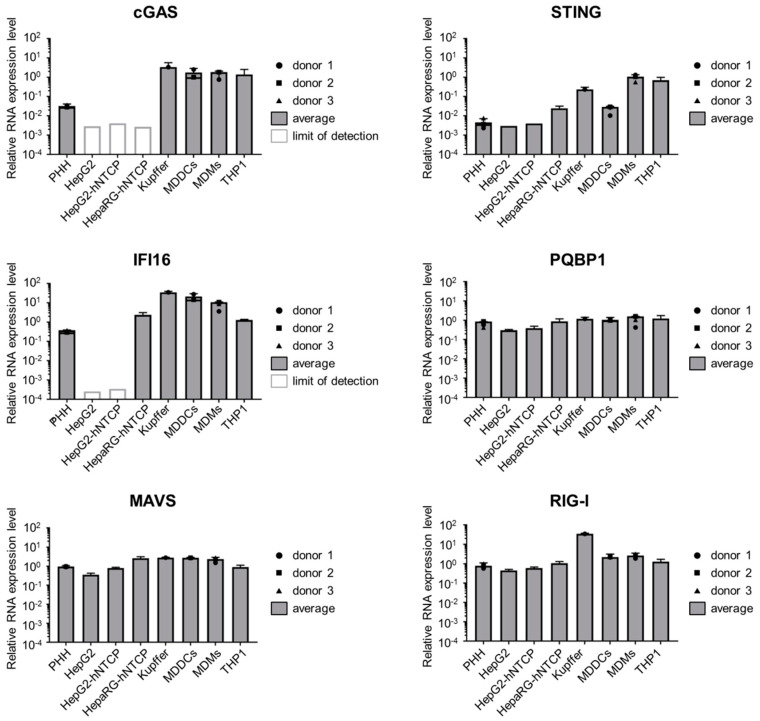
Having proven that HBV DNA is able to stimulate the cGAS/STING pathway, we analyzed the expression of this pathway in hepatocytes. To this aim, we first measured by RT-qPCR the RNA level of cGAS, STING as well as other components of PRR pathways in hepatic cell lines (HepG2 and HepaRG, overexpressing or not the viral receptor NTCP) and in PHH and compared them to primary immune cells (MDDCs, monocyte-derived macrophages (MDM), Kupffer cells) and THP1 cells (Figure 3). cGAS RNAs are expressed to a similar level in all the tested immune cells, however its expression is strongly reduced in hepatic cells although still detectable in PHH. STING shows a variable expression pattern in immune cells (high in THP1, MDMs and Kupffer cells, lower in MDDCs as seen earlier in [51]). In hepatic cells, STING expression is generally lower than in MDDCs, although it is always above the detection limit. Gamma-interferon-inducible protein 16 (IFI16), proposed as an immune sensor of retroviral DNA intermediates and a cGAS cooperative factor for activating STING [52], is expressed in PHH and HepaRG-hNTCP, although it is slightly reduced compared to immune cells, but is undetectable in the HepG2-derived cell lines. PQBP1, a cofactor of the cGAS/STING pathway involved in sensing HIV-1 reverse-transcribed DNA [53], is expressed to similar levels in all tested cell types. Finally, RIG-I and MAVS are expressed to similar levels in immune and hepatic cells. In conclusion, some components of the DNA-sensing pathways, and in particular the cGAS/STING pathway are considerably less expressed in hepatocytes than in immune cells, while components of the RNA-sensing pathways are expressed to comparable levels.
Figure 3.
Hepatocytes express a low level of components of the DNA-sensing pathway. cGAS, STING, IFI16, PQBP1, MAVS and RIG-I mRNAs were quantified by RT-qPCR in the indicated cell types and normalized to the geometric mean of RPL13A and TBP mRNAs. For primary cells, the average and SEM of technical triplicates from 3 (PHH and MDMs), 2 (MDDCs) or 1 (Kupffer cells) donors are shown.
3.4. Hepatocytes are Competent for Sensing HBV DNA
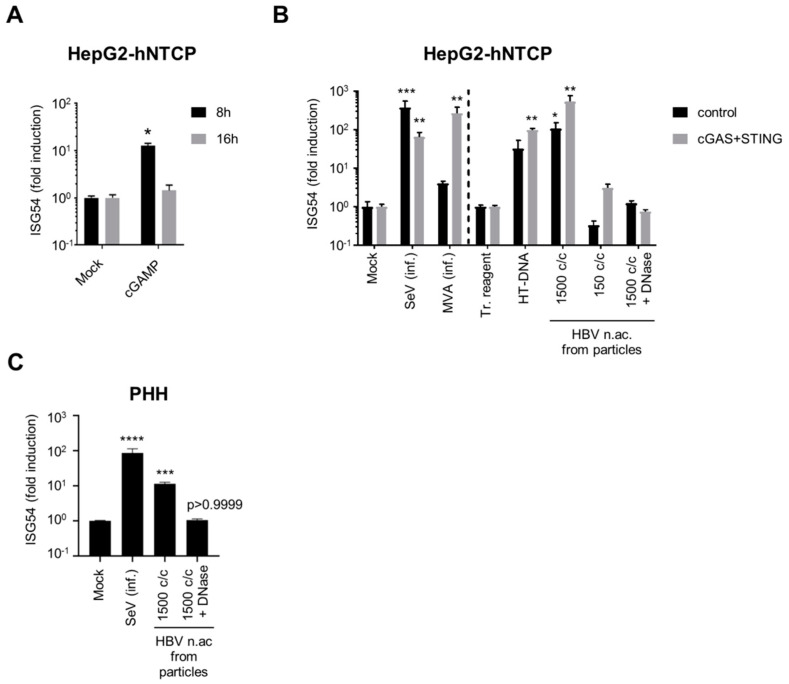
We next tested whether the low levels of cGAS/STING correlate with an altered functionality of the pathway in hepatocytes. To this aim, we first stimulated HepG2-hNTCP with the STING agonist cGAMP (Figure 4A). Interestingly, ISG54 was transiently but significantly induced in HepG2-hNTCP eight hours after cGAMP stimulation, indicating the functionality of STING and of the downstream pathway. We next tested the complete cGAS/STING pathway using infection with MVA-gfp, or transfection with HT-DNA and with HBV nucleic acids from viral particles. To determine if the levels of cGAS and STING are limiting for DNA sensing, we overexpressed cGAS and STING in HepG2-hNTCP using lentiviral vectors and compared them with cells transduced with a control vector (Figure 4B). The infection efficiency of MVA-gfp was controlled by the GFP expression (Figure S2). Since the transfection reagent alone induced a weak ISG54 response compared to an untransfected control, we compared the transfected samples to a mock-transfected control (transfection reagent only), while the samples infected with SeV or MVA, which have not been treated with a transfection reagent, were compared to an uninfected/untransfected control (mock). Interestingly, the control-transduced HepG2-hNTCP cells were able to significantly respond to transfection with HBV nucleic acids from viral particles (Figure 4B). The response to HBV nucleic acids transfection was dose-dependent (1500 copies/cell induce ISG54 by 2 log, while 150 copies/cells are insufficient), suggesting that the amount of foreign DNA in hepatocytes needs to reach a threshold to activate the DNA-sensing pathway. This induction was abrogated by DNAse digestion, demonstrating the specificity of the response for DNA. Transduction with cGAS and STING vectors increased the RNA level of cGAS and STING by 1 to 2 log compared to the control vector (Figure S3) and enhanced the response to all DNA stimuli but not to SeV infection. This shows that HepG2-hNTCP are able to sense HBV DNA from particles, to some extent, but cGAS and STING expressions are limiting.
Figure 4.
Hepatocytes respond to foreign DNA. HepG2-hNTCP (A), HepG2-hNTCP-control or -cGAS/STING (B) or PHH (C) were stimulated with cGAMP (A), infected (inf.) with SeV or MVA-gfp, or transfected with HT-DNA or HBV nucleic acids (c/c: copies/cells) (B,C). ISG54 mRNA fold inductions to mock (A,B left part, C) or to transfection reagent-treated cells (tr. reagent) (B, right part) was determined by RT-qPCR. Average and SEM of 4 (A), 2 (B) independent experiments in triplicates or of 4 donors in duplicates (C). Levels of significance compared to respective controls (A,B left part, C: mock; B, right part: transfection reagent-treated cells): **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05 (2-way ANOVA with Sidak’s multiple comparisons (A); Kruskal–Wallis with Dunn’s test (B,C)).
We next assessed the functionality of the DNA-sensing pathway in PHH (Figure 4C). Interestingly, transfection of PHH with undigested, but not with DNAse-digested HBV DNA (1500 copies/cell), induced ISG54 in all four donors, indicating that PHH are able to sense HBV DNA and that the response is specific for DNA.
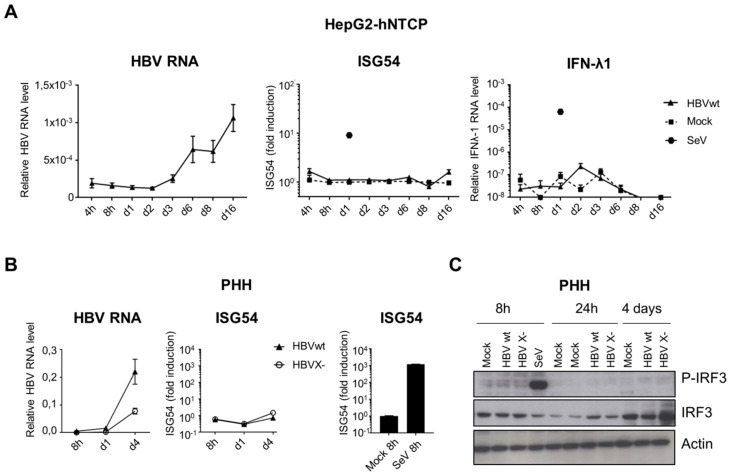
3.5. HBV DNA is not Sensed During Productive Infection of Hepatocytes
In parallel, we analyzed the activation of the nucleic acid-sensing pathways upon productive infection of hepatocytes with HBV (Figure 5). In addition to ISG54 induction, we also investigated IRF3 activation by phosphorylation, as an upstream event common to both RNA and DNA cytosolic-sensing pathways. IFN-λ1 mRNA expression, previously proposed to be induced by HBV [4,5], was also analyzed. Although HepG2-hNTCP and PHH were efficiently infected, as demonstrated by the accumulation of HBV RNAs over time, we observed no induction of ISG54 or IFN-λ1 (5A and 5B). No IRF3 phosphorylation was detected upon HBVwt infection of PHH (Figure 5C). In addition, depletion of the viral HBx protein (HBV X- mutant), previously proposed to inhibit IRF3-dependant innate-sensing pathways [21,27], did not restore the activation of the pathway in infected PHH (Figure 5B,C). However, the HBV X- mutant replicates less efficiently than HBVwt. Therefore we cannot rule out that higher MOIs of HBV X- may induce an innate response. In contrast, infection with SeV induces a robust innate response in these cells.
Figure 5.
HBVwt or HBV X- infection do not activate nucleic acid-sensing pathways in hepatocytes. HepG2-hNTCP (A) or PHH (B,C) were infected with HBVwt (MOI 100), HBV X- (MOI 100) or SeV (MOI 0,13). HBV, ISG54 or IFN-λ1 RNAs were quantified by RT-qPCR (A,B). Relative expression levels to the reference gene RPL13A were calculated. For ISG54, fold induction to the respective mock-control is shown for each time point. Average and standard error of the mean (SEM) of three independent (HepG2-hNTCP) or 1 (PHH) experiment in technical triplicates are shown. (C) Phospho- or total IRF3 protein levels were analyzed by Western blot in HBV-infected PHH (HBVwt or HBV X-, MOI 100).
In conclusion, hepatocytes are able to sense and respond to naked HBV DNA but we confirmed that this viral DNA is not sensed upon productive HBV infection. Furthermore, the absence of IRF3 phosphorylation in HBV-infected PHH suggests either an absence of viral DNA sensing by PRRs or, alternatively, an inhibition of the signaling pathways upstream of IRF3 phosphorylation. Alternatively, it is possible that the amount of HBV DNA present in infected hepatocytes is not sufficient to be sensed, since the efficiency of the cGAS/STING pathway is poor in these cells.
3.6. HBV Does not Inhibit the Innate Immune Response to Foreign DNA in Hepatocytes
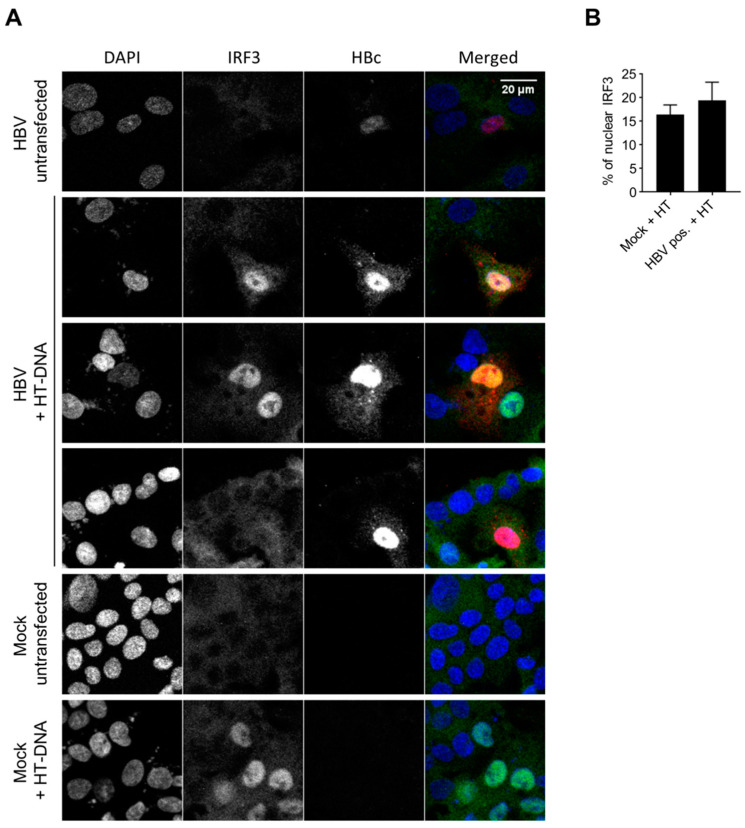
The hypothesis of an inhibition of the DNA-sensing pathway by the virus is still a matter of debate. Previous studies showed an inhibition of STING and IRF3 phosphorylation by HBV polymerase [24,30] and the down-regulation of cGAS and STING upon HBV infection [11]. On the contrary, another report concluded on the absence of an inhibition of the DNA-sensing pathway upon HBV expression in HepAD38 cells [12]. We therefore addressed this controversial question in HBV-infected HepG2-hNTCP. In order to focus only on infected cells, we immunostained HBc as an infection marker, and analyzed IRF3 nuclear translocation after HT-DNA transfection. IRF3 is translocated to the nucleus upon stimulation of the cGAS/STING pathway. To get a higher percentage of IRF3 nuclear translocation, we used HepG2-hNTCP overexpressing cGAS and STING (Figure S4). No nuclear staining was observed for IRF3 in the unstimulated cells, independent of HBV infection (Figure 6; row “HBV untransfected”), consistent with the lack of an innate response to HBV infection. In the mock samples, HT-DNA transfection induced IRF3 nuclear translocation in 16.4% of the cells (Figure 6B, graph). We observed a similar rate of IRF3 translocation in HBc-positive, HBV-infected cells transfected with HT-DNA (19.4%). The ratio +/- standard deviation of IRF3 nuclear translocation in (HT-DNA-transfected HBV-infected cells) / (HT-DNA-transfected mock-infected cells) is 1.18 +/- 0.55. This indicates that HBV infection does not inhibit the innate immune response to foreign DNA in HepG2-hNTCP overexpressing cGAS and STING. However, because of the low percentage of IRF3 nuclear localization upon HT-DNA transfection in the absence of cGAS/STING overexpression, we could not perform this experiment in PHH. Therefore, we cannot confirm that HBV does not interfere with the DNA-sensing pathway in cells expressing a low level of sensors.
Figure 6.
HBV does not inhibit the innate response to foreign DNA. HepG2-hNTCP-cGAS-STING were infected or not with HBVwt (MOI 10000). Seven days post-infection (dpi), the DNA-sensing pathway was stimulated with HT-DNA. Sixteen hours post-HT-DNA-transfection, IRF3 (green), HBc (red) and DNA (blue) were stained. (A) Representative images. (B) Percentage of nuclear IRF3 counted in HT-DNA transfected cells. For HBV-infected cells, the analysis is restricted to the HBc-positive cells (productive infection). Average and SEM of two independent experiments in duplicates are shown. At least 67 cells per condition and replicate were counted (total: 1153 mock-infected cells; 333 HBc-positive cells).
In addition, we measured the level of cGAS and STING mRNAs in mock-infected versus HBV-infected PHH (MOI 5000). Immunofluorescence staining of HBc indicated that the majority of the cells were infected (Figure S5A). However, productive HBV infection did not affect the levels of cGAS or STING compared to uninfected PHH or PHH infected in the presence of the entry inhibitor Myrcludex B (Figure S5B,C).
4. Discussion
In this study, we investigated the evasion of HBV from nucleic acid sensors during infection of hepatocytes. We first demonstrated that naked HBV RNAs are not immunostimulatory. In contrast, we found that naked HBV DNAs, and particularly the DNA from viral particles, can be sensed by the cGAS/STING pathway. We showed that in hepatocytes, including PHH, this pathway is expressed at a low level, but is able to respond to high amounts of transfected HBV DNA associated with particles, although hepatocytes do not respond to productive HBV infection. We further demonstrated that HBV does not actively inhibit the innate response to foreign DNA in infected hepatocytes overexpressing cGAS and STING.
We showed for the first time that naked HBV RNAs, including mRNAs and the pgRNA, are not immunostimulatory in transfected MDDCs, used as a model for highly immune competent cells. All HBV RNAs are transcribed by the cellular RNA polymerase Pol II, similar to cellular mRNAs. It is known that cellular mRNAs bear specific features that avoid their recognition by the RLR sensors [54,55]. Binding to the RLR sensor, RIG-I is inhibited by a capping of the cellular mRNA, both by the 7-methyl guanosine cap (Cap0) and 2’-O-methylation (Cap1) [56]. Interestingly, HBV RNAs have been described to bear a Cap0 [57,58]. The presence of a Cap1 on HBV RNAs has not been formally studied, but could account additionally for the absence of recognition by RIG-I. In addition, HBV RNAs contain N6-methyladenosine (m6A) RNA methylation [59], which has been shown to impair RIG-I binding [60]. In addition, we demonstrated that HBV RNA species are associated with HBV particles, confirming observation from Cheng et al. [9], but that they do not account for the observed ISG54 induction when MDDCs are transfected with HBV nucleic acids from particles (Figure 1). The origin of these particle-associated RNAs is unclear and may arise from incomplete reverse-transcription of the pgRNA. Alternatively, RNAs from HBV-producing cells may remain associated with the viral particles during the preparation of the viral stocks.
On the contrary, we demonstrated that HBV DNA isolated from viral particles is immunostimulatory in myeloid as well as hepatic cells including PHH. The majority of the HBV DNA represents the most likely rcDNA as it is known that mature nucleocapsids are secreted as double-stranded DNA-containing virions [61]. However, we cannot exclude that also immature virions containing single-stranded DNA have been released as it was observed with certain HBc mutants [62]. CRISPR-Cas9 knockout cells revealed that virion-associated HBV DNA is sensed by the cGAS/STING pathway, while MAVS is dispensable, ruling out the possible sensing of HBV DNA by MDA5 suggested by Lu et al. [3] Our results confirm and extend earlier studies showing the role of the cGAS/STING pathway in HBV DNA sensing [11,18]. In addition, we demonstrated that the other HBV DNA species tested, HBV replication intermediates, are immunostimulatory as well. Although we primarily used myeloid cells as a model to study the immunostimulatory potential of viral nucleic acids in cells expressing most of the PRRs, a direct role for these cells in the innate response to HBV in vivo has been suggested [8,9,10]. The hypothesis that Kupffer cells or dendritic cells may engulf viral particles or viral debris and respond to them deserves further investigation. Our data show that myeloid cells can mount an innate immune response against HBV DNAs when actively introduced into cell cytoplasm by transfection. However, so far, our preliminary experiments have indicated no innate immune response of THP-1, MDDCs or Kupffer cells to inoculation with HBV particles, neither to naked HBV DNA in the absence of transfection reagent and therefore do not allow us to confirm this hypothesis.
In hepatocytes, the activity of the cGAS/STING pathway is controversial. Several publications have indicated low expression and functionality of these factors in hepatocytes [9,14,19], on the contrary, Verrier et al. [11] proposed that both proteins are expressed and able to sense foreign DNA. Here we confirm that STING is expressed in hepatocytes including PHH but show that its expression is reduced compared to immune cells. cGAS RNA was detected in PHH, but was under the detection limit in HepG2-derived cell lines. Nevertheless, we show that PHH and HepG2-hNTCP are able to sense HBV DNA when transfected in sufficient amounts (Figure 4). Using THP-1 KO cell lines as a model to dissect the possible sensing pathway(s) involved (Figure 2), we demonstrated that HT-DNA and HBV DNA sensing was totally abrogated by cGAS or STING KO, demonstrating that the cGAS/STING pathway is required for HBV DNA sensing and that other possible DNA sensors expressed in THP1 are not sufficient to sense these DNA species in the absence of cGAS or STING. However, we do not exclude that other DNA sensors such as IFI16, which was recently suggested to sense HBV cccDNA in the nucleus of hepatocytes [63], might act as cofactors of the cGAS/STING pathway. These data suggest that in hepatocytes, the cGAS/STING pathway is very likely responsible for the observed sensing of virion-associated HBV DNA, even if cGAS and STING RNA expression is low or below the detection limit of our assay. Furthermore, we demonstrated that the efficiency of the innate response to transfected DNA in hepatocytes is not optimal and can be improved by overexpressing cGAS and STING (Figure 4B). In summary, we propose a central role of the cGAS/STING pathway in HBV DNA sensing. We demonstrated that, in hepatocytes, this pathway is expressed at a relatively low level but is sufficient for sensing high amounts of naked HBV DNA from particles.
The possible inhibition of innate immune pathways by HBV as a way to escape the interferon response is still a matter of debate. The viral regulatory protein HBx has been described to interfere with MAVS [21,27] leading to a reduced innate immune response to different stimuli in cells transfected with plasmids encoding HBx or the HBV genome. Here we observed no innate immune response in PHH infected with an HBV X- mutant (Figure 5B,C), suggesting that HBx is not responsible for the lack of innate immune response to HBV in infected hepatocytes. Although we cannot exclude that a higher MOI of HBV X- may have led to an innate immune response in PHH, we further showed that MAVS is not involved in sensing HBV nucleic acids, while cGAS and STING are (Figure 2). This confirms the hypothesis that HBx is not responsible for the absence of innate response to HBV infection in hepatocytes.
Several publications have proposed that HBV does not inhibit innate immune responses. Many of them focused on RNA-sensing pathways [9,31,32], while the DNA-sensing pathway has been less investigated and is still a matter of debate. Liu et al. suggested that HBV replication dampened STING-mediated innate signaling [30]. In addition, Verrier et al. [11] proposed that HBV infection decreases the expression levels of STING, cGAS and TBK1. On the contrary, Guo et al. [12] reported that an induction of HBV replication in HepAD38 cells did not affect the response to foreign DNA. Here, we demonstrated that IRF3 is not phosphorylated in HBV-infected hepatocytes (Figure 5). This indicates that, if the absence of activation of the DNA-sensing pathway following HBV infection is due to an active inhibition by the virus, this viral inhibition would target a step upstream of IRF3 phosphorylation. We then demonstrated that HBV infection does not alter IRF3 nuclear translocation in response to HT-DNA transfection in HepG2-hNTCP overexpressing cGAS and STING (Figure 6). In addition, we observed no significant change in cGAS and STING expression levels in PHH infected with a high MOI of HBV (Figure S5). Altogether, our data point towards an absence of inhibition of the cGAS-STING DNA-sensing pathway by HBV.
In the absence of active inhibition, HBV evasion from the DNA-sensing pathway has to be passive. Since we observed that hepatocytes can sense and respond to naked HBV DNA, we hypothesize that a shielding of viral rcDNA and replication intermediates in the viral capsids may be the reason why HBV DNA is not sensed upon infection. This hypothesis has been suggested in an immortalized mouse hepatocyte cell line model where HBV capsids are destabilized [64]. Nevertheless, it is difficult to accurately quantify and compare the copy numbers of HBV DNA in infected hepatocytes and in our hepatocyte transfection assay. Therefore we cannot exclude that upon infection, the levels of intracellular HBV DNA are not sufficient to activate the cGAS/STING pathway. Both hypotheses may coexist and ensure that the virus is not detected under conditions where small amounts of viral DNA are released in the cytoplasm from defective capsids.
During the preparation of this manuscript, a report was published suggesting that HBV stimulates the TLR-2 pathway leading to NF-κB activation and production of pro-inflammatory cytokines [7]. The authors suggested that a receptor-binding mechanism might be involved in this response. This finding is not contradictory with our results since we demonstrated that upon HBV infection, viral nucleic acids were not detected in hepatocytes, but we cannot exclude that another viral factor might activate a pro-inflammatory response.
5. Conclusions
In conclusion, we showed for the first time that HBV RNAs are not immunostimulatory. Furthermore, we indicated that different forms of HBV DNA can be sensed by the cGAS/STING pathway. This pathway is expressed at a low level but is active in hepatocytes and able to respond to naked HBV DNA. However, upon infection, the virus escapes this response, apparently without actively inhibiting the DNA-sensing pathway. The shielding of the viral DNA in the capsids combined to the low functionality of the cGAS/STING pathway likely allow HBV to escape cGAS/STING sensing.
Acknowledgments
The authors thank Nina Hein-Fuchs and Esther Schnellbächer for experimental support.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/6/592/s1. Figure S1: Two-time-point analysis of the immunostimulatory potential of viral nucleic acids in MDDCs. Figure S2: Infection control for MVA-gfp infection of HepG2-hNTCP. Figure S3: cGAS and STING overexpression in HepG2-hNTCP. Figure S4: Experimental design for Figure 6. Figure S5: HBV does not affect the expression level of cGAS or STING. PHH were infected with HBVwt (MOI 5000).
Author Contributions
Conceptualization, R.K. and L.L.-R.; methodology, L.L.-R., R.K., M.B., M.R.; validation, L.L.-R., M.B.; formal analysis, L.L.-R.; investigation, L.L.-R., M.B., S.M., B.Q.; resources, F.W.R.V., J.L., V.H., S.U.; writing—original draft preparation, L.L.-R.; writing—review and editing, R.K., M.B., L.L.-R.; visualization, L.L.-R.; supervision, R.K. and L.L.-R.; project administration, L.L.-R., R.K.; funding acquisition, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG) SPP1923 Project KO4573/1-1 to R.K and German Center for Infection Research (DZIF) HZI2010Z10 to R.K. V.H. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project number 272983813 – TRR 179.
Conflicts of Interest
S.U. is a coapplicant and coinventor on patents protecting Myrcludex B as an HBV/HDV entry inhibitor. The rest of the authors have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.García-Sastre A. Ten Strategies of Interferon Evasion by Viruses. Cell Host Microbe. 2017;22:176–184. doi: 10.1016/j.chom.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan Y.K., Gack M.U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol. 2016;14:360–373. doi: 10.1038/nrmicro.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H.-L., Liao F. Melanoma Differentiation–Associated Gene 5 Senses Hepatitis B Virus and Activates Innate Immune Signaling To Suppress Virus Replication. J. Immunol. 2013;191:3264–3276. doi: 10.4049/jimmunol.1300512. [DOI] [PubMed] [Google Scholar]
- 4.Sato S., Li K., Kameyama T., Hayashi T., Ishida Y., Murakami S., Watanabe T., Iijima S., Sakurai Y., Watashi K., et al. The RNA Sensor RIG-I Dually Functions as an Innate Sensor and Direct Antiviral Factor for Hepatitis B Virus. Immunity. 2015;42:123–132. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Shlomai A., Schwartz R.E., Ramanan V., Bhatta A., De Jong Y.P., Bhatia S.N., Rice C.M. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc. Natl. Acad. Sci. USA. 2014;111:12193–12198. doi: 10.1073/pnas.1412631111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luangsay S., Gruffaz M., Isorce N., Testoni B., Michelet M., Faure-Dupuy S., Maadadi S., Ait-Goughoulte M., Parent R., Rivoire M., et al. Early inhibition of hepatocyte innate responses by hepatitis B virus. J. Hepatology. 2015;63:1314–1322. doi: 10.1016/j.jhep.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Trippler M., Real C.I., Werner M., Luo X., Schefczyk S., Kemper T., Anastasiou O.E., Ladiges Y., Treckmann J., et al. Hepatitis B virus particles activate toll-like receptor 2 signaling initial upon infection of primary human hepatocytes. Hepatology. 2020 doi: 10.1002/hep.31112. [DOI] [PubMed] [Google Scholar]
- 8.Hösel M., Quasdorff M., Wiegmann K., Webb D., Zedler U., Broxtermann M., Tedjokusumo R., Esser K., Arzberger S., Kirschning C.J., et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 9.Cheng X., Xia Y., Serti E., Block P.D., Chung M., Chayama K., Rehermann B., Liang T.J. Hepatitis B virus evades innate immunity of hepatocytes but activates cytokine production by macrophages. Hepatology. 2017;66:1779–1793. doi: 10.1002/hep.29348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boltjes A., Van Montfoort N., Biesta P.J., Brouw M.L.O.D., Kwekkeboom J., Van Der Laan L.J.W., Janssen H.L.A., Boonstra A., Woltman A.M. Kupffer Cells Interact With Hepatitis B Surface Antigen In Vivo and In Vitro, Leading to Proinflammatory Cytokine Production and Natural Killer Cell Function. J. Infect. Dis. 2014;211:1268–1278. doi: 10.1093/infdis/jiu599. [DOI] [PubMed] [Google Scholar]
- 11.Verrier E.R., Yim S.-A., Heydmann L., El Saghire H., Bach C., Turon-Lagot V., Mailly L., Durand S.C., Lucifora J., Durantel D., et al. Hepatitis B Virus Evasion From Cyclic Guanosine Monophosphate–Adenosine Monophosphate Synthase Sensing in Human Hepatocytes. Hepatology. 2018;68:1695–1709. doi: 10.1002/hep.30054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F., Tang L., Shu S., Sehgal M., Sheraz M., Liu B., Zhao Q., Cheng J., Zhao X., Zhou T., et al. Activation of Stimulator of Interferon Genes in Hepatocytes Suppresses the Replication of Hepatitis B Virus. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00771-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winer B.Y., Gaska J.M., Lipkowitz G., Bram Y., Parekh A., Parsons L., Leach R., Jindal R., Cho C.H., Shrirao A., et al. Analysis of Host Responses to Hepatitis B and Delta Viral Infections in a Micro-scalable Hepatic Co-culture System. Hepatology. 2019;71:14–30. doi: 10.1002/hep.30815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomsen M.K., Nandakumar R., Stadler D., Malo A., Valls R.M., Wang F., Reinert L.S., Dagnaes-Hansen F., Hollensen A.K., Mikkelsen J.G., et al. Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatology. 2016;64:746–759. doi: 10.1002/hep.28685. [DOI] [PubMed] [Google Scholar]
- 15.Wieland S., Thimme R., Purcell R.H., Chisari F.V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl. Acad. Sci. USA. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacey A.R., Norris P.J., Qin L., Haygreen E.A., Taylor E., Heitman J., Lebedeva M., DeCamp A., Li D., Grove D., et al. Induction of a Striking Systemic Cytokine Cascade prior to Peak Viremia in Acute Human Immunodeficiency Virus Type 1 Infection, in Contrast to More Modest and Delayed Responses in Acute Hepatitis B and C Virus Infections. J. Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn C., Peppa D., Khanna P., Nebbia G., Jones M., Brendish N., Lascar R.M., Brown D., Gilson R., Tedder R.J., et al. Temporal Analysis of Early Immune Responses in Patients With Acute Hepatitis B Virus Infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Dansako H., Ueda Y., Okumura N., Satoh S., Sugiyama M., Mizokami M., Ikeda M., Kato N. The cyclic GMP-AMP synthetase-STING signaling pathway is required for both the innate immune response against HBV and the suppression of HBV assembly. FEBS J. 2016;283:144–156. doi: 10.1111/febs.13563. [DOI] [PubMed] [Google Scholar]
- 19.Faure-Dupuy S., Lucifora J., Durantel D. Interplay between the Hepatitis B Virus and Innate Immunity: From an Understanding to the Development of Therapeutic Concepts. Viruses. 2017;9:95. doi: 10.3390/v9050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng J., Imanishi H., Morisaki H., Liu W., Nakamura H., Morisaki T., Hada T. Recombinant HBsAg inhibits LPS-induced COX-2 expression and IL-18 production by interfering with the NFkappaB pathway in a human monocytic cell line, THP-1. J. Hepatol. 2005;43:465–471. doi: 10.1016/j.jhep.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Wei C., Ni C., Song T., Liu Y., Yang X., Zheng Z., Jia Y., Yuan Y., Guan K., Xu Y., et al. The Hepatitis B Virus X Protein Disrupts Innate Immunity by Downregulating Mitochondrial Antiviral Signaling Protein. J. Immunol. 2010;185:1158–1168. doi: 10.4049/jimmunol.0903874. [DOI] [PubMed] [Google Scholar]
- 22.Real C.I., Lu M., Liu J., Huang X., Trippler M., Hossbach M., Deckert J., Jahn-Hofmann K., Ickenstein L.M., John M.J., et al. Hepatitis B virus genome replication triggers toll-like receptor 3-dependent interferon responses in the absence of hepatitis B surface antigen. Sci. Rep. 2016;6:24865. doi: 10.1038/srep24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J., Meng Z., Jiang M., Pei R., Trippler M., Broering R., Bucchi A., Sowa J.-P., Dittmer U., Yang D., et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2008;49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 24.Yu S., Chen J., Wu M., Chen H., Kato N., Yuan Z. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J. Gen. Virol. 2010;91:2080–2090. doi: 10.1099/vir.0.020552-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Ryu W.-S. Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion. PLoS Pathog. 2010;6:e1000986. doi: 10.1371/journal.ppat.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao G., An B., Zhou H., Wang H., Xu Y., Xiang X., Dong Z., An F., Yu D., Wang W., et al. Impairment of the retinoic acid-inducible gene-I-IFN-? signaling pathway in chronic hepatitis B virus infection. Int. J. Mol. Med. 2012;30:1498–1504. doi: 10.3892/ijmm.2012.1131. [DOI] [PubMed] [Google Scholar]
- 27.Kumar M., Jung S.Y., Hodgson A.J., Madden C.R., Qin J., Slagle B.L. Hepatitis B Virus Regulatory HBx Protein Binds to Adaptor Protein IPS-1 and Inhibits the Activation of Beta Interferon. J. Virol. 2011;85:987–995. doi: 10.1128/jvi.01825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan Y., Cao W., Han T., Ren S., Feng J., Chen T., Wang J., Broering R., Lu M., Zhu Y. Inducible Rubicon facilitates viral replication by antagonizing interferon production. Cell. Mol. Immunol. 2017;14:607–620. doi: 10.1038/cmi.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutgehetmann M., Bornscheuer T., Volz T., Allweiss L., Bockmann J.-H., Pollok J.M., Lohse A.W., Petersen J., Dandri M. Hepatitis B Virus Limits Response of Human Hepatocytes to Interferon-α in Chimeric Mice. Gastroenterology. 2011;140:2074–2083. doi: 10.1053/j.gastro.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Li J., Chen J., Li Y., Wang W., Du X., Song W., Zhang W., Lin L., Yuan Z. Hepatitis B Virus Polymerase Disrupts K63-Linked Ubiquitination of STING To Block Innate Cytosolic DNA-Sensing Pathways. J. Virol. 2015;89:2287–2300. doi: 10.1128/jvi.02760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suslov A., Boldanova T., Wang X., Wieland S.F., Heim M.H. Hepatitis B Virus Does Not Interfere With Innate Immune Responses in the Human Liver. Gastroenterology. 2018;154:1778–1790. doi: 10.1053/j.gastro.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 32.Mutz P., Metz P., Lempp F.A., Bender S., Qu B., Schöneweis K., Seitz S., Tu T., Restuccia A., Frankish J., et al. HBV Bypasses the Innate Immune Response and Does Not Protect HCV From Antiviral Activity of Interferon. Gastroenterology. 2018;154:1791–1804.e22. doi: 10.1053/j.gastro.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M., Stindt J., Königer C., Nassal M., Kubitz R., et al. Hepatitis B and D Viruses Exploit Sodium Taurocholate Co-transporting Polypeptide for Species-Specific Entry into Hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Sells M.A., Chen M.L., Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucifora J., Arzberger S., Durantel D., Belloni L., Strubin M., Levrero M., Zoulim F., Hantz O., Protzer U. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J. Hepatol. 2011;55:996–1003. doi: 10.1016/j.jhep.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Ladner S.K., Otto M.J., Barker C.S., Zaifert K., Wang G.H., Guo J.T., Seeger C., King R.W. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: A novel system for screening potential inhibitors of HBV replication. Antimicrob. Agents Chemother. 1997;41:1715–1720. doi: 10.1128/aac.41.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 38.Mankan A.K., Schmidt T., Chauhan D., Goldeck M., Höning K., Gaidt M., Kubarenko A.V., Andreeva L., Hopfner K.-P., Hornung V. Cytosolic RNA:DNA hybrids activate the cGAS –STING axis. EMBO J. 2014;33:2937–2946. doi: 10.15252/embj.201488726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt M., Pawlita M. High-throughput detection and multiplex identification of cell contaminations. Nucleic Acids Res. 2009;37:e119. doi: 10.1093/nar/gkp581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auth M.-K.H., Boost K.A., Leckel K., Beecken W.-D., Engl T., Jonas D., Oppermann E., Hilgard P., Markus B.H., Bechstein W.-O., et al. Controlled and reversible induction of differentiation and activation of adult human hepatocytes by a biphasic culture technique. World J. Gastroenterol. 2005;11:2080–2087. doi: 10.3748/wjg.v11.i14.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivière L., Gerossier L., Ducroux A., Dion S., Deng Q., Michel M.-L., Buendia M.-A., Hantz O., Neuveut C. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J. Hepatology. 2015;63:1093–1102. doi: 10.1016/j.jhep.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Lempp F.A., Urban S. Inhibitors of Hepatitis B Virus Attachment and Entry. Intervirology. 2014;57:151–157. doi: 10.1159/000360948. [DOI] [PubMed] [Google Scholar]
- 43.Staib C., Drexler I., Ohlmann M., Wintersperger S., Erfle V., Sutter G. Transient Host Range Selection for Genetic Engineering of Modified Vaccinia Virus Ankara. BioTechniques. 2000;28:1137–1142. doi: 10.2144/00286st04. [DOI] [PubMed] [Google Scholar]
- 44.Cosma A., Bühler S., Nagaraj R., Staib C., Hammarin A.-L., Wahren B., Goebel F.D., Erfle V., Sutter G. Neutralization Assay Using a Modified Vaccinia Virus Ankara Vector Expressing the Green Fluorescent Protein Is a High-Throughput Method To Monitor the Humoral Immune Response against Vaccinia Virus. Clin. Diagn. Lab. Immunol. 2004;11:406–410. doi: 10.1128/CDLI.11.2.406-410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducroux A., Benhenda S., Rivière L., Semmes O.J., Benkirane M., Neuveut C. The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1. PLoS Pathog. 2014;10:e1004343. doi: 10.1371/journal.ppat.1004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grandvaux N., Servant M.J., Tenoever B., Sen G.C., Balachandran S., Barber G.N., Lin R., Hiscott J. Transcriptional Profiling of Interferon Regulatory Factor 3 Target Genes: Direct Involvement in the Regulation of Interferon-Stimulated Genes. J. Virol. 2002;76:5532–5539. doi: 10.1128/JVI.76.11.5532-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakaya T., Sato M., Hata N., Asagiri M., Suemori H., Noguchi S., Tanaka N., Taniguchi T. Gene Induction Pathways Mediated by Distinct IRFs during Viral Infection. Biochem. Biophys. Res. Commun. 2001;283:1150–1156. doi: 10.1006/bbrc.2001.4913. [DOI] [PubMed] [Google Scholar]
- 48.Strähle L., Marq J.-B., Brini A., Hausmann S., Kolakofsky D., Garcin D. Activation of the Beta Interferon Promoter by Unnatural Sendai Virus Infection Requires RIG-I and Is Inhibited by Viral C Proteins. J. Virol. 2007;81:12227–12237. doi: 10.1128/JVI.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai P., Wang W., Cao H., Avogadri F., Dai L., Drexler I., Joyce J.A., Li X.-D., Chen Z., Merghoub T., et al. Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway. PLoS Pathog. 2014;10:e1003989. doi: 10.1371/journal.ppat.1003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nassal M. Hepatitis B viruses: Reverse transcription a different way. Virus Res. 2008;134:235–249. doi: 10.1016/j.virusres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Paijo J., Döring M., Spanier J., Grabski E., Nooruzzaman M., Schmidt T., Witte G., Messerle M., Hornung V., Kaever V., et al. cGAS Senses Human Cytomegalovirus and Induces Type I Interferon Responses in Human Monocyte-Derived Cells. PLoS Pathog. 2016;12:e1005546. doi: 10.1371/journal.ppat.1005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schott K., Riess M., König R. Role of Innate Genes in HIV Replication. Curr. Top. Microbiol. Immunol. 2018;419:69–111. doi: 10.1007/82_2017_29. [DOI] [PubMed] [Google Scholar]
- 53.Yoh S.M., Schneider M., Seifried J., Soonthornvacharin S., Akleh R.E., Olivieri K.C., de Jesus P.D., Ruan C., de Castro E., Ruiz P.A., et al. PQBP1 Is a Proximal Sensor of the cGAS-Dependent Innate Response to HIV-1. Cell. 2015;161:1293–1305. doi: 10.1016/j.cell.2015.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kell A.M., Gale M. RIG-I in RNA virus recognition. Virology. 2015;479–480:110–121. doi: 10.1016/j.virol.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramanathan A., Robb G.B., Chan S.-H. mRNA capping: Biological functions and applications. Nucleic Acids Res. 2016;44:7511–7526. doi: 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devarkar S.C., Wang C., Miller M.T., Ramanathan A., Jiang F., Khan A.G., Patel S.S., Marcotrigiano J. Structural basis for m7G recognition and 2’-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl. Acad. Sci. USA. 2016;113:596–601. doi: 10.1073/pnas.1515152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beck J., Nassal M. Hepatitis B virus replication. World J. Gastroenterol. 2007;13:48–64. doi: 10.3748/wjg.v13.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lien J.M., Aldrich C.E., Mason W.S. Evidence that a capped oligoribonucleotide is the primer for duck hepatitis B virus plus-strand DNA synthesis. J. Virol. 1986;57:229–236. doi: 10.1128/jvi.57.1.229-236.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imam H., Khan M., Gokhale N.S., McIntyre A.B.R., Kim G.-W., Jang J.Y., Kim S.-J., Mason C.E., Horner S.M., Siddiqui A. N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle. Proc. Natl. Acad. Sci. USA. 2018;115:8829–8834. doi: 10.1073/pnas.1808319115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durbin A.F., Wang C., Marcotrigiano J., Gehrke L. RNAs Containing Modified Nucleotides Fail To Trigger RIG-I Conformational Changes for Innate Immune Signaling. mBio. 2016;7 doi: 10.1128/mBio.00833-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ning X., Nguyen D., Mentzer L., Adams C., Lee H., Ashley R., Hafenstein S., Hu J. Secretion of Genome-Free Hepatitis B Virus—Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus. PLoS Pathog. 2011;7:e1002255. doi: 10.1371/journal.ppat.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan T.T., Sahu G.K., Whitehead W.E., Greenberg R., Shih C. The Mechanism of an Immature Secretion Phenotype of a Highly Frequent Naturally Occurring Missense Mutation at Codon 97 of Human Hepatitis B Virus Core Antigen. J. Virol. 1999;73:5731–5740. doi: 10.1128/JVI.73.7.5731-5740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Zhao X., Wang Z., Shu W., Li L., Li Y., Guo Z., Gao B., Xiong S. Nuclear Sensor Interferon-Inducible Protein 16 Inhibits the Function of Hepatitis B Virus Covalently Closed Circular DNA by Integrating Innate Immune Activation and Epigenetic Suppression. Hepatology. 2020;71:1154–1169. doi: 10.1002/hep.30897. [DOI] [PubMed] [Google Scholar]
- 64.Cui X., Clark D.N., Liu K., Xu X.-D., Guo J.-T., Hu J. Viral DNA-Dependent Induction of Innate Immune Response to Hepatitis B Virus in Immortalized Mouse Hepatocytes. J. Virol. 2016;90:486–496. doi: 10.1128/JVI.01263-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.