Abstract
Alzheimer’s disease (AD) is diagnosed using neuropsychological testing, supported by amyloid and tau biomarkers and neuroimaging abnormalities. The cause of neuropsychological changes is not clear since they do not correlate with biomarkers. This study investigated if changes in cellular metabolism in AD correlate with neuropsychological changes. Fibroblasts were taken from 10 AD patients and 10 controls. Metabolic assessment included measuring total cellular ATP, extracellular lactate, mitochondrial membrane potential (MMP), mitochondrial respiration and glycolytic function. All participants were assessed with neuropsychological testing and brain structural MRI. AD patients had significantly lower scores in delayed and immediate recall, semantic memory, phonemic fluency and Mini Mental State Examination (MMSE). AD patients also had significantly smaller left hippocampal, left parietal, right parietal and anterior medial prefrontal cortical grey matter volumes. Fibroblast MMP, mitochondrial spare respiratory capacity (MSRC), glycolytic reserve, and extracellular lactate were found to be lower in AD patients. MSRC/MMP correlated significantly with semantic memory, immediate and delayed episodic recall. Correlations between MSRC and delayed episodic recall remained significant after controlling for age, education and brain reserve. Grey matter volumes did not correlate with MRSC/MMP. AD fibroblast metabolic assessment may represent an emergent disease biomarker of AD.
Keywords: Alzheimer’s disease, mitochondrial spare respiratory capacity, mitochondrial, membrane potential, glycolytic reserve, semantic memory, phonemic fluency, episodic memory, neuropsychology, neuroimaging
1. Background
Alzheimer’s disease (AD) is the most common cause of dementia worldwide and in 2018 was estimated to cost the global economy 1 trillion US dollars [1]. The clinical symptoms of the disease are the progressive loss of different aspects of cognitive function until a patient becomes completely dependent on the care of family members and healthcare workers [2]. Median survival after diagnosis is 7 to 10 years for people in their 60s to 70s, and 3 years for people in their 90s [3].
The disease is characterized pathologically by the presence of extracellular amyloid plaques comprising mainly of the amyloid beta protein; and intracellular neurofibrillary tangles (NFT) made mainly of the cytoskeletal protein tau [4]. To date the cause of AD still remains poorly understood. As the buildup of amyloid appears to be a key step in the development of both familial and sporadic forms of the disease, the amyloid cascade hypothesis has become the leading theory for the cause of the condition [5]. In brief, this hypothesis states that the key step in developing AD is the accumulation of amyloid beta through reduced breakdown and clearance, and/or increased production. Strategies aimed at reducing the amyloid load in the brain, however, have failed to control the disease [6] and have resulted in a large number of clinical trials that have failed to achieve primary outcome measures [7]. Even in pre-clinical carriers of dominantly inherited AD mutations, amyloid removal therapies have not slowed disease progression [8]. Furthermore, brain amyloid load does not correlate with clinical symptoms [9]. This has led researchers to investigate alternative pathophysiological mechanisms [10].
AD is clinically defined by distinctive changes in cognitive status identified by neuropsychological assessment. Brain imaging changes and amyloid and tau protein levels in the cerebrospinal fluid are used to confirm the diagnosis in vivo [11]. The changes seen in a patient’s ability to perform a cognitive task are often difficult to explain from a cellular perspective. Tau deposition does explain elements of the observed neuropsychological abnormalities [12], but does not fully account for all cognitive changes observed in AD patients [13,14].
Cognitive processing, such as that required while performing memory tasks, puts an increased metabolic demand on the brain [15]. This is evidenced by neuroimaging studies of the brain which use tracers of metabolism such as 2-[18F]fluoro-2-Deoxy-D-glucose (FDG) that have shown poor glucose utilization in patients who perform poorly on memory tasks [16,17]. Positron-emission tomography (PET) imaging studies that use oxygen-15 labelled water also show reduced uptake when AD patients perform cognitive tasks [18]. These imaging studies suggest that any deficit in metabolic function, such as deficits in mitochondrial respiration or glycolysis, are likely to affect an individual’s performance on cognitive tasks. It is therefore possible that mitochondrial respiration or glycolytic dysfunction might contribute to cognitive deficits in AD, making these cellular processes suitable pharmacological targets to improve the cognitive symptoms of AD.
Cellular metabolic changes within the brains of patients with AD and in peripheral cell populations are seen very early in the condition, and often precede the development of both amyloid plaques and NFT. Abnormalities have been shown in many metabolic pathways in AD [19]. Mounting evidence suggests that deficits in glycolysis and the function of mitochondria, specifically how they control oxidative phosphorylation, are likely to be key in the development and establishment of AD [20,21,22].
A mitochondrial cascade hypothesis has been suggested for the aetiology of AD, and states that people who inherit mitochondrial genes that predispose them to lower mitochondrial respiration rates may be more likely to develop the condition [23]. In animal models of AD, changes in mitochondrial function are seen prior to amyloid deposition [20,24], and cell models show changes in mitochondrial function and oxidative stress without the presence of amyloid [25], giving further evidence for the key role of mitochondrial dysfunction in AD.
Impairment of glycolysis is also seen early in patients with AD. A 2-[18F]fluoro-2-Deoxy-D-glucose positron-emission tomography (FDG-PET) imaging of the brain shows a reduction in glucose metabolism [26]. In particular, there is a reduction in aerobic glycolysis in brain areas susceptible to amyloid deposition [27], and in those regions where high levels of tau accumulation are seen [28].
We have previously shown that fibroblasts from sporadic AD (sAD) patients have multiple mitochondrial structural and functional abnormalities, and that these can be ameliorated by treatment with ursodeoxycholic acid (UDCA) [29]. Other studies have shown that glycyl-l-histidyl-l-lysine (GHK-Cu), by increasing gene expression, has an effect on improving mitochondrial activity and influencing cognitive decline [30]. In our previous study we showed sAD fibroblasts to have deficits in mitochondrial membrane potential (MMP) and mitochondrial spare respiratory capacity (MSRC). MSRC refers to the difference in oxidative phosphorylation rates between the basal level of mitochondrial respiration and the maximal level a cell can achieve [31]. In essence, MSRC measures the cellular reserve respiratory capacity. In animal models of AD it has been shown that deficits in MSRC cause cognitive deficits that treatment with the antioxidant pyrroloquinoline quinone can improve [32]. It has not been previously shown, however, whether changes in MSRC in human cell lines obtained from sAD patients correlate with their performance on neuropsychological tests.
As deficits in both glycolysis and mitochondrial function have been shown in AD patients and models, in this proof of concept study we explored metabolic function in fibroblasts from sporadic AD patients and its role in the cognitive decline experienced by these patients. To this end, we assessed mitochondrial functional changes in a larger cohort of patients compared to the findings described in our previous study [29]. We have then assessed additional metabolic parameters in the sAD fibroblasts including glycolytic function and cellular ATP levels, which have previously not been described. Finally, we investigated whether these metabolic abnormalities detected in sAD fibroblasts correlated with neuropsychological and neuroimaging features typical of the early stages of this disease.
2. Results
2.1. Patient Demographic Details
Skin biopsies were taken from ten sAD patients (mean age 61.3 years, 6 male) and ten controls (mean age 66.7 years, 5 male). Body mass index (BMI) did not differ significantly between the groups (sAD mean 27.8 kg/m2 SD 5.37 kg/m2 Controls mean 28.2 kg/m2 SD 4.00 kg/m2 t-test p = 0.44). Table 1 shows patient and control demographic data.
Table 1.
Patient Demographic Information and contemporary treatment status.
| Patient Number | Age (Years) |
Sex | MMSE | Length of Education (Years) | AD Treatment (in Disease Cohort, at Time of Biopsy) |
|---|---|---|---|---|---|
| 1 | 63 | Male | 20 | 16 | None |
| 2 | 57 | Male | 18 | 15 | Donepezil |
| 3 | 53 | Male | 14 | 11 | Donepezil |
| 4 * | 60 | Male | 18 | 9 | None |
| 5 * | 59 | Female | 23 | 11 | Galantamine |
| 6 * | 63 | Female | 26 | 10 | Donepezil |
| 7 * | 60 | Male | 18 | 11 | Memantine |
| 8 | 60 | Male | 18 | 11 | None |
| 9 | 79 | Female | 28 | 15 | None |
| 10 | 61 | Female | 25 | 11 | Donepezil |
| Group Mean (Standard Dev) |
61.33 (7.19) |
20.8 (4.4) |
12.0 (2.4) |
||
| Controls | |||||
| 1 | 66 | Male | 29 | 11 | NA |
| 2 * | 54 | Male | 27 | 17 | NA |
| 3 * | 53 | Male | 29 | 16 | NA |
| 4 * | 56 | Male | 24 | 11 | NA |
| 5 | 61 | Female | 30 | NA | NA |
| 6 | 54 | Female | 29 | 17 | NA |
| 7 * | 100 | Female | 24 # | 14 | NA |
| 8 | 75 | Female | 28 | 18 | NA |
| 9 | 73 | Female | 26 | 12 | NA |
| 10 | 75 | Male | 27 | 10 | NA |
| Group Mean (Standard Dev) |
65.77 (14.7) |
27.6 (1.8) |
14 (3.1) |
# For control 7 some items of the Mini Mental State Examination (MMSE) could not be tested due to sensory impairment * indicates fibroblast lines in which Mitochondrial Membrane Potential (MMP) and Mitochondrial Spare Respiratory Capacity (MRSC) values were published in our previous paper [29].
2.2. Neuropsychological Profiles
Neuropsychological profiling of controls and patients used in this study was performed as part of the VPH-DARE research project (http://www.vph-dare.eu/), (see Methods section for additional details). For the present study, a subgroup of neuropsychological tests was selected to be correlated with metabolic findings. The tests which can detect the earliest typical cognitive impairments in mild sAD were selected. These included assessment of semantic memory, immediate and delayed episodic recall. Phonemic fluency was also selected as dysfunction on this cognitive test is seen later in AD but not in its earlier stages [33]. All 10 controls and 10 patients with AD were assessed. The Mini-Mental State Examination (MMSE) [34] was also used, as this test is useful in staging disease severity in sAD [34,35].
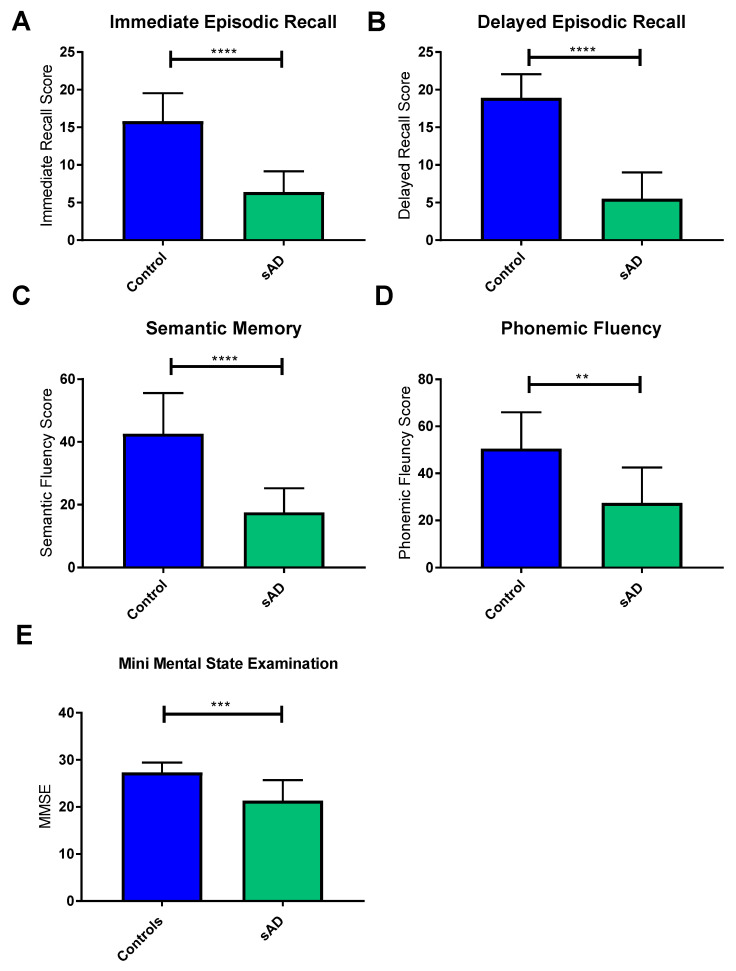
Patients with sAD performed at a lower level than controls on all tests (see Figure 1). The most significant differences were seen in the tests of semantic memory (mean score in controls 42.6 points, mean score in sAD 17.5 points, difference between means 25.1 points, SD 4.774 p < 0.0001); immediate (mean score in controls 15.8 points, mean score in sAD 6.4 points, difference between means 25.1 points, SD 1.468 p < 0.0001); and delayed recall of the Prose Memory test (mean score in controls 18.6 points, mean score in sAD 5.5 points, difference between means 13.4 points, SD 1.488 p < 0.0001). The phonemic fluency test performance was also significantly different between the 2 groups (mean score in controls 50.5 points, mean score in sAD 27.4 points, difference between means 23.1 points, SD 6.837 p = 0.0033), but to a lesser extent than the other neuropsychological tests. As expected, MMSE scores of sAD patients were significantly lower than those of controls (mean score in controls 27.3 points, mean score in sAD 21.3 points, mean difference 6.0 points, SD 1.54 p = 0.0011).
Figure 1.
Mean scores of sporadic AD (sAD) patients and controls on cognitive tests included in the neuropsychological assessment. Graphs show mean with error bars indicating standard deviation. **** p < 0.0001; *** p < 0.001; ** p < 0.01. Controls are indicated by blue bars and sAD patients indicated by green bars. Significant reductions were seen in sAD immediate recall (A), delayed recall (B), semantic memory (C), phonemic fluency (D) and Mini Mental State Examination (E) when compared to controls.
2.3. Neuroimaging Profiles
Volumetric structural MRI scans were acquired on 9 controls and 10 patients with sAD. One control did not complete their MRI scan as an incidental finding (of no diagnostic significance for this study) on initial scans meant the participant could no longer take part in the VPH-DARE@IT original study. For the remaining participants, the left and right parietal lobes, anterior medial pre-frontal cortex and posterior cingulate gyrus, and left hippocampal grey matter volumes were extracted as these brain areas are affected early in AD [36].
Comparisons between the 2 groups showed no significant differences between the grey matter volumes of the preselected areas when performing a t-test. Brain grey matter volumes can be affected by age, education and brain reserve [37,38]. For these reasons a further analysis of the 2 groups was completed controlling for these covariates. A significant difference emerged in the volume of the left hippocampus, left parietal, right parietal and anterior medial prefrontal cortex in patients with sAD after controlling for confounding variables. Table 2 highlights the F-test and p-values for these differences. No significant difference was seen in the posterior cingulate cortex grey matter volume between the 2 groups.
Table 2.
Differences in Control and sAD brain volumes. This table shows the significance of the differences in brain volumes between controls and patients with sAD when controlling for age, years of education and brain reserve.
| Brain Volume | F-Test | p-Value |
|---|---|---|
| Left Hippocampal Volume | 9.420 | 0.001 |
| Left Parietal Volume | 7.882 | 0.002 |
| Right Parietal Volume | 10.051 | <0.0001 |
| Anterior Medial Prefrontal Cortex | 0.056 | 0.017 |
| Posterior Cingulate Cortex | 0.752 | 0.575 |
2.4. Fibroblast Metabolic Assessment
Mitochondrial function was investigated in both sAD patients and controls. We measured elements of both mitochondrial function and glycolysis. Five metabolic parameters were chosen that best describe different factors influencing the ability of the fibroblast to meet its energy demands. Mitochondrial parameters included: total cellular ATP, as this is a global marker of the energetic status of the cell; MMP and MSRC. MMP and MSRC were selected as these parameters are important in maintaining the rate of ATP production as cellular energy demand changes. These are also the two parameters which we have previously identified as being relevant to the mitochondrial phenotype in sAD fibroblasts [29]. Measures of glycolysis included glycolytic reserve and extracellular lactate levels. Glycolytic reserve, like MSRC and MMP for mitochondrial function, is a measure of cellular metabolic flexibility and deficits in this parameter are likely to contribute to an inability to maintain cellular energy production. Extracellular lactate is the final breakdown product of glycolysis and contributes information about how well the cell can utilize glucose as a metabolite.
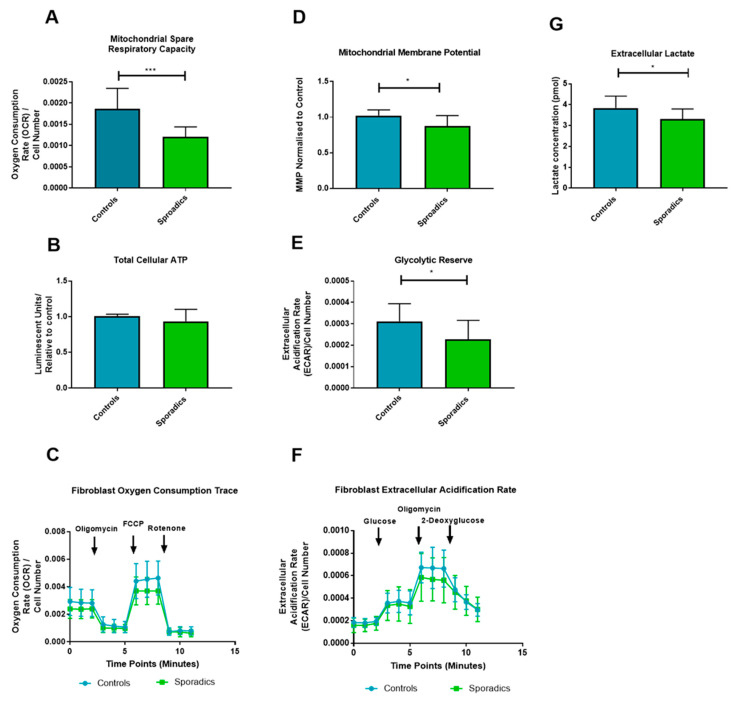
First, we assessed MMP and MRSC as we have done in our previous work [29]. In this cohort, MRSC was significantly reduced (36% reduction p < 0.0001) in sAD when compared to controls (Figure 2A). MMP was also significantly reduced in the sAD fibroblast lines (14% reduction p = 0.011) (See Figure 2B). Figure 2C shows a representative oxygen consumption trace for the mitochondrial stress test experiment.
Figure 2.
Oxidative phosphorylation and Glycolytic fibroblast Assessment: *** = p < 0.001; * = p < 0.05. Significant reductions in mitochondrial spare respiratory capacity (A), Mitochondrial Membrane potential (D), Glycolytic Reserve (E) and Extracellular lactate (G) was detected in sAD when compared to controls. No significant difference was seen in total cellular ATP (B). OCR and ECAR traces for mitochondrial stress test and glycolysis stress test are displayed in panels (C) and (F) respectively. For all panels controls are represented in blue and sAD are represented in green. Graphs represent mean with error bars indicating standard deviation.
Next, we assessed total cellular ATP, which showed no significant difference between sAD patient fibroblasts and controls when comparing mean values (p = 0.165, Figure 2D). The final metabolic assessment of the fibroblast lines involved assessment of glucose metabolism using the glycolysis stress test programme on the Seahorse analyzer and extracellular lactate measurement. A significantly lower glycolytic reserve was found in sAD fibroblasts when compared to controls (see Figure 1E, 25.8% reduction, p = 0.031). No significant differences were seen in maximum glycolytic rate (p = 0.792), or basal glycolytic rate (p = 0.381). Figure 1F shows the extracellular acidification rate (ECAR) trace for the glycolysis stress test, comparing the group of controls against the sAD fibroblast group. Measurement of extracellular lactate levels revealed significantly lower lactate levels released from sAD fibroblasts (14% reduction, p = 0.0227, Figure 2G).
2.5. Neuropsychological/Metabolic Correlations
Next, we sought to correlate the neuropsychological scores of the sAD patients and controls combined with their metabolic parameters measured in fibroblasts. As five neuropsychological measures and five fibroblast metabolic markers had been assessed to identify differences in the control and sAD fibroblast groups, we controlled for the effect of multiple comparisons by adjusting what we deemed to be a significant association to p ≤ 0.01 (0.05/5). Using this new statistical threshold, only MSRC and MMP metabolic tests and immediate, delayed and semantic memory neuropsychological assessments were identified as significantly different between control and sAD groups. Correlations were, therefore, performed only for these data sets.
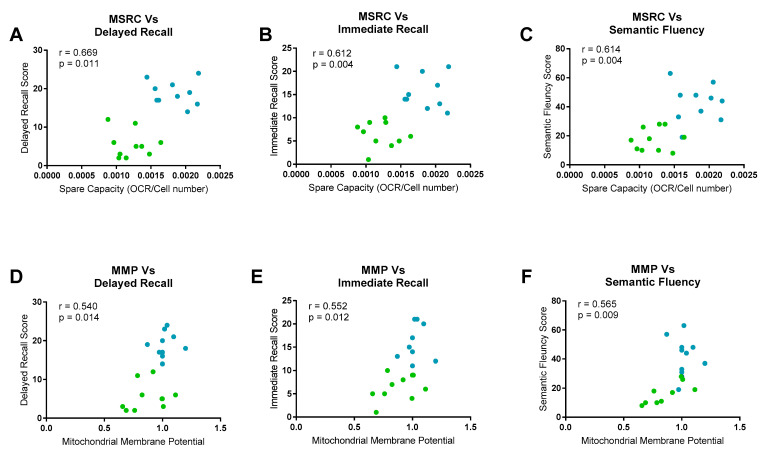
MSRC had a significant positive correlation with immediate episodic recall (r = 0.612, p = 0.004), delayed episodic recall (r = 0.669, p = 0.001) and semantic memory scores (r = 0.614, p = 0.003). MMP correlated only with semantic memory scores (r = 0.565, p = 0.009). Immediate episodic recall (r = 0.552, p = 0.0134) and delayed episodic recall correlated positively with MMP, but at a lower level of significance (r = 0.540, p = 0.015). Figure 3 displays these correlations graphically.
Figure 3.
Neuropsychological Fibroblast Metabolism Correlations. Significant correlations were seen between mitochondrial spare respiratory capacity (MSRC) and delayed recall (A), Immediate recall (B) and semantic fluency (C). Mitochondrial membrane potential (MMP) correlated significantly with semantic fluency (F), and with delayed recall (D) and immediate recall (E) but to a lesser extent. For all panels controls are represented in blue and sAD are represented in green. Correlation coefficients and p-values for each correlation are displayed.
Age, years of education and brain reserve can potentially confound the correlations between neuropsychological measurements and the fibroblast metabolic markers, as all three factors have been shown to affect either performance on neuropsychological tests [39,40] or mitochondrial function [41]. To control for the effect of these three covariates, a further analysis was performed taking these parameters into account. This further analysis showed that the correlation between delayed episodic recall and MSRC was still significant at the more conservative significance threshold. Both immediate episodic recall (p = 0.013) and semantic memory (p = 0.012) correlations with MSRC were no longer significant once covariates were included in the analysis at the new more stringent p-value. All correlations seen between the neuropsychological tests and MMP were no longer significant after controlling for age, education and brain reserve. Table 3 displays the new correlation coefficients and p-values for the neuropsychological data.
Table 3.
Neuropsychological fibroblast metabolism correlations corrected for age, years of education and grey matter reserve.
| Psychological Test | Fibroblast Marker | R Value | p-Value |
|---|---|---|---|
| Immediate Episodic Recall | |||
| MSRC | 0.605 | 0.013 | |
| MMP | 0.196 | 0.466 | |
| Delayed Episodic Recall | |||
| MSRC | 0.695 | 0.003 | |
| MMP | 0.204 | 0.448 | |
| Semantic Memory | |||
| MSRC | 0.610 | 0.012 | |
| MMP | 0.345 | 0.191 |
2.6. Neuroimaging/Metabolic Correlations
Volumetric imaging for areas of the brain known to be affected by AD were correlated with metabolic markers of the disease. Only the grey matter volumes that had shown significant differences between the sAD and control groups (with a p-value equal to or less than 0.01) were included. None of the grey matter volumes showed a significant correlation with changes in cellular metabolism. Table 4 shows the results of the correlations.
Table 4.
Grey Matter Volume Fibroblast metabolic correlations: No significant correlations were seen between MMP nor MSRC and any grey matter volume when controlling for age, length of disease or brain reserve.
| Grey Matter Volume | Fibroblast Marker | R Value | p-Value |
|---|---|---|---|
| Left Hippocampal Volume | |||
| MSRC | 0.371 | 0.157 | |
| MMP | 0.041 | 0.881 | |
| Left Parietal Volume | |||
| MSRC | 0.341 | 0.196 | |
| MMP | −0.047 | 0.862 | |
| Right Parietal Volume | |||
| MSRC | 0.418 | 0.107 | |
| MMP | −0.043 | 0.875 |
3. Discussion
This proof of concept study shows that fibroblast MSRC correlates with established neuropsychological abnormalities that are affected early in AD. This is one of the first studies to show a functional biomarker of AD correlating with a marker of fibroblast mitochondrial function. MSRC is a measure of the ability of mitochondria within cells to increase the production of ATP in response to an increased energy demand. The correlation of MSRC with neuropsychological scores potentially helps to describe the cellular pathology underlying these neuropsychological changes seen in AD. This is the first study in humans to show that peripheral cell MSRC correlates with neuropsychological profiles. Correcting the abnormality in MSRC could be a future therapeutic approach in AD. The fact that this abnormality in mitochondrial function correlates with a clinical biomarker of AD also opens the avenue for monitoring drug response in future clinical trials.
MSRC has been shown to be abnormal in multiple diseases including acute myeloid leukaemia (AML) [42], Parkinson’s disease [43,44] and motor neuron disease (MND) [45]. MSRC deficits may not be disease specific, but the combination of cellular metabolic changes seen here may represent a cellular metabolic profile specific to sAD. This metabolic phenotype could potentially represent a biomarker that can stratify and define a specific subset of sAD patients that might then respond to a personalised therapeutic approach. Previous research has shown that the metabolic profile identified in fibroblasts from sporadic Parkinson’s disease patients differs from that identified here in fibroblasts from sAD [46,47].
Reductions in MSRC and MMP were seen in all sAD fibroblast lines when compared to controls at a group level. These data reinforce and extend the findings reported in our previous paper [29], and show that the finding of reduced MSRC and MMP is reproducible and more robust in sporadic AD fibroblasts when sample size is increased. Extracellular lactate levels and cellular glycolytic reserves, both markers of glycolytic function, were significantly reduced in all sAD fibroblasts. These measures, however, did not meet the statistical significance for correlation with neuropsychological markers of the disease. The deficits in markers of mitochondrial function and glycolysis in the fibroblasts reflect a lack of flexibility of metabolic pathways in sAD. This lack of flexibility is likely to be very important when performing cognitive tasks that depend on the coordination of multiple brain regions. Taken together, these results suggest that sAD fibroblasts may have limited ability to respond to increased cellular energy demand.
MMP did correlate with neuropsychological markers, but correlations did not survive the correction for confounding factors unlike MSRC, for which correlation with delayed episodic recall (a core feature in the clinical diagnostic criteria for sAD) remained significant. Semantic memory and immediate episodic memory did not significantly correlate with MSRC at the more conservative significance threshold after confounder consideration, but p-values for both of these correlations were less than 0.02. Potentially, these correlations would reach significance level with an increased sample size. The difference in MMP between control and sAD groups was much smaller than the difference seen in MSRC. MMP has a key role in maintaining many pathways in the mitochondria outside that of ATP generation, such as apoptosis fates [48]. It is likely that several mechanisms not affected by sAD in the fibroblast cell help to maintain MMP levels, which may explain the reduced variability seen in this parameter in sAD and the lack of a significant correlation with neuropsychological scores.
Baseline ATP levels were not significantly different between the two groups. These results suggest that, in a controlled environment when cells are not stressed, they can maintain ATP levels similar to that of controls. Interestingly though, the functional capacity of mitochondria is impaired, as described above, suggesting a potential inability to respond appropriately to increased ATP demand. The measurements of ATP we performed in this study only gives information about the total cellular ATP level. We did not investigate the rate of ATP breakdown nor how ATP levels change when the cell is under stressed conditions or the ratio of ATP/ADP in the cell. This may explain why no significant correlations were seen with ATP measurements, but were seen with the other markers of mitochondrial respiration.
It is interesting that the correlation between scores on phonemic fluency, a neuropsychological test not affected early in sAD, and cell metabolism markers did not reach significance threshold. It. The medial temporal lobe is important in semantic memory processing [49], an area of cognition that is impaired very early in sAD [50]. This is not the case for phonemic fluency which is supported by processes associated with frontal executive regions [51]. It could be that the correlations we see between neuropsychological tests and cellular metabolic function may reflect which areas of the brain are more susceptible to metabolic failure. It has been previously shown that areas of the brain that are affected early in sAD express lower levels of electron transport chain (ETC) genes when compared to controls [52], suggesting that this might be the case. Data from FDG-PET studies also support the concept of focal brain hypometabolism in sAD, with areas such as the medial temporal lobes, precuneus and lateral parietal lobes all preferentially affected [53].
We did not see a significant correlation between mitochondrial function or glycolysis and brain structural imaging markers of sAD. This may be explained by the fact that multiple factors can affect grey matter volume such as age, levels of education and brain reserve. It has been previously shown that smaller grey matter volumes in the frontal lobes associated with ageing are associated with worse performance on frontal lobe cognitive tests [54]. The neuropsychological assessment performed in this study mainly focused on temporal lobe cognitive functioning and not frontal lobe function. Potentially, the effect of brain ageing on grey matter volumes may mask any effect metabolic function may have.
The limitations of our study include a small sample size, and the fact that we have used fibroblasts to measure metabolic function. It could also be argued that a correlation between metabolic function of central nervous system cells and neuropsychological parameters would be more meaningful. This is not the first study, however, to show that the metabolism of peripheral cells outside the nervous system is affected in sAD. White blood cells [55], platelets [56] and fibroblasts have been shown to have metabolic abnormalities in multiple studies [46,47,57,58]. Identifying that fibroblast mitochondrial abnormalities correlate with neuropsychological markers of AD opens up avenues to use fibroblast metabolic parameters as biomarkers of sAD, and also as a high throughput drug screening model, as well as potential outcome measures of therapeutic efficacy. Replication of the findings of this study in larger cohorts and by other groups is needed, however, to consolidate the evidence that fibroblast metabolic function is a reliable biomarker of sAD.
Our patient cohort were selected at an early stage of sAD to try and reduce group variability. To understand the use of fibroblast metabolic abnormalities as a biomarker, further work investigating cohorts of patients with amnestic mild cognitive impairment (MCI), the prodromal stage of sAD, would be advantageous as well as investigating these parameters in more advanced sAD patients.
Future work could extend the findings of metabolic-neuropsychological correlations by creating neuronal lineage cells via cellular reprogramming methods such as that described by Takahashi et al. [59]. This type of model would allow for direct comparison between human nervous system cells and human nervous system function in a context where reprogrammed cells would maintain their ageing phenotype [60].
4. Methods
4.1. Patient Details
Skin biopsies and fibroblast metabolic assessments were performed as part of the MODEL-AD research study (Yorkshire and Humber Research and Ethics Committee number: 16/YH/0155). Due to the initial success of fibroblast metabolic assessment, the initial cohort of four controls and four patients (previously reported in [29]) with sAD was expanded to ten healthy controls (mean age: 65.77 years, SD 14.70 years) and 10 patients with sAD (mean age: 61.33 years, SD 7.19 years). All patients had been involved in the EU-funded Framework Programme 7 Virtual Physiological Human: Dementia Research Enabled by IT (VPH-DARE@IT) initiative (http://www.vph-dare.eu/). A diagnosis of sAD was made based on clinical criteria [11]. Ethical approval for the neuropsychological and neuroimaging measures collected was gained from Yorkshire and Humber Regional Ethics Committee, Reference number: 12/YH/0474. Informed written consent was obtained from each participant. Investigations were carried out following the rules of the Declaration of Helsinki of 1975 (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/), revised in 2013.
4.2. Neuropsychological Testing
A battery of tests was devised to detect impaired performance in the cognitive domains most susceptible to sAD neurodegeneration. This included tests of short- and long-term memory, attention and executive functioning, language and semantics, and visuoconstructive skills. A detailed description of each task is provided elsewhere [61]. Most tests were exclusively used as part of the clinical assessment of patients and controls. Three tests were also included as part of the experimental protocol. Immediate and delayed recall on the Logical Memory test were used as measures of episodic memory, the cognitive domain centrally defined by diagnostic criteria for a diagnosis of sAD. The performance on the Category Fluency test was used as an index of semantic memory, the cognitive domain affected by the accumulation of neurofibrillary pathology in the transentorhinal cortex [50]. Finally, the Letter Fluency test (phonemic fluency) was used as a methodological control, since performance on this task is often within normal age limits in patients with mild sAD [33,62].
4.3. MRI Acquisition and Processing
An MRI protocol of anatomical scans was acquired on a Philips Ingenia 3 T scanner. Several acquisitions (including T1-weighted, T2-weighted, FLAIR and diffusion-weighted sequences) were used for diagnostic purposes. Three-dimensional T1-weighted images (voxel size: 0.94 mm × 0.94 mm × 1.00 mm; repetition time: 8.2 s; echo delay time: 3.8 s; field of view: 256 mm; matrix size: 256 × 256 × 170) were also used for the calculation of hippocampal volumes. These were processed with the Similarity and Truth Estimation for Propagated Segmentations routine [63]. This procedure, available at niftyweb.cs.ucl.ac.uk, allows automated segmentation of the left and right hippocampus in the brain’s native space using multiple reference templates. Hippocampal volumes were then quantified using Matlab (version R2014a) and the “get totals” script (http://www0.cs.ucl.ac.uk/staff/G.Ridgway/vbm/get_totals.m). Fractional measures were also obtained dividing hippocampal volumes by the volume of the total intracranial space. The left hippocampal volume and left hippocampal ratio were chosen as structural markers of AD as evidence suggests the left hippocampus is affected early in the progression of the disease [64]. Native space maps created to extract hippocampal volume from MRI images are shown in Supplementary Figure S1. Additional regions of interest (listed in Table 2) were then defined as binary image masks. Segmented grey matter maps were registered to the Montreal Neurological Institute space, and mask-constrained volumes were extracted.
4.4. Tissue Culture
Fibroblasts were cultured as described previously [29]. In brief, all 10 control and all 10 sAD lines were cultured in EMEM-based media (Corning) incubated at 37 °C in a 5% carbon dioxide atmosphere. Sodium pyruvate (1%) (Sigma Aldrich), non-essential amino acids (1%) (Lonza), penicillin and streptomycin (1%) (Sigma Aldrich), multi-vitamins (1%) (Lonza), Fetal Bovine Serum (10%) (Biosera) and 50µg/mL uridine (Sigma Aldrich) were added to the media. All experiments described were performed on all 20 cell lines. Fibroblasts were plated at a density of 5000 cells per well in a white 96 well plate for ATP assays. For MMP assays fibroblasts were plated at a density of 2500 cells per well in a black 96 well plate. Each assay was performed on three separate passages of fibroblasts; cells between passages 5–10 were used. All experiments were performed on passage-matched cells.
4.5. Intracellular ATP levels
Cellular adenosine triphosphate (ATP) levels were measured using the ATPlite kit (Perkin Elmer) as described previously [65]. ATP levels were corrected for cell number by using CyQuant (ThermoFisher) measurements, as previously described [29]. These values were then normalised to control levels.
4.6. Mitochondrial Membrane Potential
MMP was measured using tetramethlyrhodamine (TMRM) staining of live fibroblasts, as previously described [29]. Cells were plated in a black 96 well plate, incubated at 37 °C for 48 h. TMRM dye was added one hour prior to imaging on an InCell Analyzer 2000 high-content imager (GE Healthcare).
4.7. Extracellular Lactate Measurement
Extracellular lactate was measured from confluent flasks of fibroblasts using an L-Lactate assay kit (Abcam ab65331). The assay was performed according to the manufacturer’s instructions.
4.8. Metabolic Flux Assay
4.8.1. Mito Stress Test
Oxygen consumption rates (OCR) were measured using a 24-well Agilent Seahorse XF analyzer as described previously [29]. In brief, cells were plated at a density of 65,000 cells per well 48 h prior to measurement. Three measurements were taken at the basal point: after the addition of oligomycin (0.5 µM), after the addition of carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) (0.5 µM) and after the addition of rotenone (1 µM). OCR measurements were normalised to cell count as described in [29]. Measurements of basal mitochondrial respiration, maximal mitochondrial respiration and MSRC were also calculated.
4.8.2. Glycolysis Stress Test
Cells were plated in a Seahorse XF24 Cell Culture Microplate (Agilent) at a density of 65,000 cells per well 48 h prior to measurement. The glycolysis stress test standard protocol was used [66]. This is a completely separate assay to the mitochondrial stress test, and allows for the thorough interrogation of glycolysis via the addition of supplements and inhibitors of the glycolytic pathway. Three measurements were taken at the basal point: after the addition of glucose (10 µM), after the addition of oligomycin (1.0 µM) and after the addition of 2-deoxyglucose (50 µM). The glycolysis stress test can measure several aspects of cellular glycolysis. These include the basal glycolytic rate of the cell, the maximum level of glycolysis the cell can achieve and the glycolytic reserve which refers to the difference between maximum level of glycolysis and the basal level of glycolysis. The different aspects of glycolysis are calculated by measuring the extracellular acidification rate (ECAR). ECAR rates were normalised to cell count as described above.
4.9. Statistical Analysis
For comparing each neuropsychological, neuroimaging and metabolic functional markers, a Student’s t-test was used, comparing the means of the control group for each parameter to the disease group. Statistics were calculated using GraphPad Prism Software (V7.02). Analyses of covariance were also used for group comparisons including confounding variables. For covariate analysis when comparing control and disease group grey matter volumes, or when correlating neuropsychological and neuroimaging sAD markers with fibroblast functional markers, the IBM SPSS Statistics suite (Version 26; https://www.ibm.com/uk-en/products/spss-statistics) was used as this function was not available in the GraphPad Prism Software. Significance levels were adjusted to account for multiple comparisons.
5. Conclusions
These data highlight how in-depth analysis of mitochondrial and glycolytic function in sAD fibroblasts identifies metabolic abnormalities that parallel changes seen in neuropsychological features distinctive of the early stages of sporadic Alzheimer’s disease. This model system could be used to develop biomarkers useful in early detection as well as in the development of novel therapeutic approaches for sAD.
Acknowledgments
We would like to thank all research participants involved in this study. This research was supported by the Wellcome 4ward North Academy and ARUK Yorkshire Network Centre Small Grant Scheme (to SMB), Parkinson’s UK (to HM, F1301) and the NIHR Sheffield Biomedical Research Centre. This is a summary of independent research carried out at the NIHR Sheffield Biomedical Research Centre (Translational Neuroscience). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care (DHSC). Support of the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 601055, VPH-DARE@IT to AV is acknowledged. PJS is supported by an NIHR Senior Investigator award. The support of the NIHR Clinical Research Facility—Sheffield Teaching Hospital is also acknowledged.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4426/10/2/32/s1, Figure S1: Quantification of hippocampal volumes from T1-weighted MRI scans.
Author Contributions
Conceptualization, S.M.B., D.J.B., H.M., L.F., A.V., M.D.M. and P.J.S.; Methodology, S.M.B., M.D.M., K.B., H.M. and A.V.; Software, S.M.B., and M.D.M.; Validation, S.M.B., D.J.B., K.B., H.M., L.F., A.V., M.D.M. and P.J.S.; Formal Analysis, S.M.B., D.J.B., K.B., H.M., L.F., A.V., M.D.M. and P.J.S.; Investigation, S.M.B., M.D.M., and K.B.; Resources, S.M.B., D.J.B., K.B., H.M., L.F., A.V., M.D.M. and P.J.S.; Data Curation, S.M.B., D.J.B., K.B., H.M., L.F., A.V., M.D.M. and P.J.S.; Writing—Original Draft Preparation, S.M.B.; Writing—Review and Editing, S.M.B., D.J.B., K.B., H.M., L.F., A.V., M.D.M. and P.J.S.; Visualization, S.M.B., D.J.B., K.B., H.M., L.F., A.V., M.D.M. and P.J.S.; Supervision, H.M., P.J.S., A.V., L.F. and D.J.B.; Project Administration, S.M.B.; Funding Acquisition, S.M.B., D.J.B., K.B., H.M., L.F., A.V. and P.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Wellcome 4ward north (216340/Z/19/Z), ARUK Yorkshire Network Centre Small Grant Scheme (Ref: ARUK-PCRF2016A-1), Parkinson’s UK (F1301), European Union Seventh Framework Programme (FP7/2007 – 2013) under grant agreement no. 601055, NIHR Sheffield Biomedical Research Centre award.
Conflicts of Interest
The authors have no financial or personal relationships with other people/organizations that could inappropriately influence (bias) their work.
References
- 1.Wimo A., Guerchet M., Ali G.-C., Wu Y.-T., Prina A.M., Winblad B., Jönsson L., Liu Z., Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R., Corrada M.M., Curriero F.C., Kawas C. Survival following a diagnosis of Alzheimer disease. Arch. Neurol. 2002;59:1764–1767. doi: 10.1001/archneur.59.11.1764. [DOI] [PubMed] [Google Scholar]
- 4.Perl D.P. Neuropathology of Alzheimer’s Disease. Mt. Sinai J. Med. 2010;77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy J., Selkoe D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science. 2002;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Honig L.S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M., Hager K., Andreasen N., Scarpini E., Liu-Seifert H., et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018;378:321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 7.Cummings J.L., Morstorf T., Zhong K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ALZFORUM Topline Result for First DIAN-TU Clinical Trial: Negative on Primary. [(accessed on 29 March 2020)]; Available online: https://www.alzforum.org/news/research-news/topline-result-first-dian-tu-clinical-trial-negative-primary.
- 9.Savva G.M., Wharton S.B., Ince P.G., Forster G., Matthews F.E., Brayne C. Age, neuropathology, and dementia. N. Engl. J. Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 10.Le Couteur D.G., Hunter S., Brayne C. Solanezumab and the amyloid hypothesis for Alzheimer’s disease. BMJ. 2016;355:i6771. doi: 10.1136/bmj.i6771. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 13.Haroutunian V., Schnaider-Beeri M., Schmeidler J., Wysocki M., Purohit D.P., Perl D.P., Libow L.S., Lesser G.T., Maroukian M., Grossman H.T. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch. Neurol. 2008;65:1211–1217. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintraub S., Wicklund A.H., Salmon D.P. The neuropsychological profile of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magistretti P.J., Allaman I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Ewers M., Brendel M., Rizk-Jackson A., Rominger A., Bartenstein P., Schuff N., Weiner M.W. Alzheimer’s Disease Neuroimaging I Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. NeuroImage. Clin. 2013;4:45–52. doi: 10.1016/j.nicl.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalpouzos G., Eustache F., de la Sayette V., Viader F., Chételat G., Desgranges B. Working memory and FDG–PET dissociate early and late onset Alzheimer disease patients. J. Neurol. 2005;252:548–558. doi: 10.1007/s00415-005-0685-3. [DOI] [PubMed] [Google Scholar]
- 18.Ishii K., Sasaki M., Yamaji S., Sakamoto S., Kitagaki H., Mori E. Demonstration of decreased posterior cingulate perfusion in mild Alzheimer’s disease by means of H215O positron emission tomography. Eur. J. Nucl. Med. 1997;24:670–673. doi: 10.1007/BF00841407. [DOI] [PubMed] [Google Scholar]
- 19.Morgen K., Frolich L. The metabolism hypothesis of Alzheimer’s disease: From the concept of central insulin resistance and associated consequences to insulin therapy. J. Neural Transm. 2015;122:499–504. doi: 10.1007/s00702-015-1377-5. [DOI] [PubMed] [Google Scholar]
- 20.Yao J., Irwin R.W., Zhao L., Nilsen J., Hamilton R.T., Brinton R.D. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2009;106:14670. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blass J.P., Sheu R.K., Gibson G.E. Inherent abnormalities in energy metabolism in Alzheimer disease. Interaction with cerebrovascular compromise. Ann. N. Y. Acad. Sci. 2000;903:204–221. doi: 10.1111/j.1749-6632.2000.tb06370.x. [DOI] [PubMed] [Google Scholar]
- 22.Baik S.H., Kang S., Lee W., Choi H., Chung S., Kim J.I., Mook-Jung I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019;30:493–507.e496. doi: 10.1016/j.cmet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Swerdlow R.H., Khan S.M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med. Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Hartl D., Schuldt V., Forler S., Zabel C., Klose J., Rohe M. Presymptomatic Alterations in Energy Metabolism and Oxidative Stress in the APP23 Mouse Model of Alzheimer Disease. J. Proteome Res. 2012;11:3295–3304. doi: 10.1021/pr300021e. [DOI] [PubMed] [Google Scholar]
- 25.Gan X.Q., Huang S.B., Wu L., Wang Y.F., Hu G., Li G.Y., Zhang H.J., Yu H.Y., Swerdlow R.H., Chen J.X., et al. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochim. Biophys. Acta Mol. Basis Dis. 2014;1842:220–231. doi: 10.1016/j.bbadis.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosconi L., Tsui W.H., De Santi S., Li J., Rusinek H., Convit A., Li Y., Boppana M., de Leon M.J. Reduced hippocampal metabolism in MCI and AD: Automated FDG-PET image analysis. Neurology. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 27.Vlassenko A.G., Vaishnavi S.N., Couture L., Sacco D., Shannon B.J., Mach R.H., Morris J.C., Raichle M.E., Mintun M.A. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc. Natl. Acad. Sci. USA. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlassenko A.G., Gordon B.A., Goyal M.S., Su Y., Blazey T.M., Durbin T.J., Couture L.E., Christensen J.J., Jafri H., Morris J.C., et al. Aerobic glycolysis and tau deposition in preclinical Alzheimer’s disease. Neurobiol. Aging. 2018;67:95–98. doi: 10.1016/j.neurobiolaging.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell S.M., Barnes K., Clemmens H., Al-Rafiah A.R., Al-Ofi E.A., Leech V., Bandmann O., Shaw P.J., Blackburn D.J., Ferraiuolo L., et al. Ursodeoxycholic Acid Improves Mitochondrial Function and Redistributes Drp1 in Fibroblasts from Patients with Either Sporadic or Familial Alzheimer’s Disease. J. Mol. Biol. 2018;430:3942–3953. doi: 10.1016/j.jmb.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickart L., Vasquez-Soltero J.M., Margolina A. The Effect of the Human Peptide GHK on Gene Expression Relevant to Nervous System Function and Cognitive Decline. Brain Sci. 2017;7:20. doi: 10.3390/brainsci7020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desler C., Hansen T.L., Frederiksen J.B., Marcker M.L., Singh K.K., Juel Rasmussen L. Is There a Link between Mitochondrial Reserve Respiratory Capacity and Aging? J. Aging Res. 2012;2012:192503. doi: 10.1155/2012/192503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martino Adami P.V., Quijano C., Magnani N., Galeano P., Evelson P., Cassina A., Do Carmo S., Leal M.C., Castaño E.M., Cuello A.C., et al. Synaptosomal bioenergetic defects are associated with cognitive impairment in a transgenic rat model of early Alzheimer’s disease. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017;37:69–84. doi: 10.1177/0271678X15615132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capitani E., Rosci C., Saetti M.C., Laiacona M. Mirror asymmetry of Category and Letter fluency in traumatic brain injury and Alzheimer’s patients. Neuropsychologia. 2009;47:423–429. doi: 10.1016/j.neuropsychologia.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Doody R.S., Massman P., Dunn J.K. A method for estimating progression rates in Alzheimer disease. Arch. Neurol. 2001;58:449–454. doi: 10.1001/archneur.58.3.449. [DOI] [PubMed] [Google Scholar]
- 36.Scahill R.I., Schott J.M., Stevens J.M., Rossor M.N., Fox N.C. Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proc. Natl. Acad. Sci. USA. 2002;99:4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boller B., Mellah S., Ducharme-Laliberte G., Belleville S. Relationships between years of education, regional grey matter volumes, and working memory-related brain activity in healthy older adults. Brain Imaging Behav. 2017;11:304–317. doi: 10.1007/s11682-016-9621-7. [DOI] [PubMed] [Google Scholar]
- 38.Rzezak P., Squarzoni P., Duran F.L., de Toledo Ferraz Alves T., Tamashiro-Duran J., Bottino C.M., Ribeiz S., Lotufo P.A., Menezes P.R., Scazufca M., et al. Relationship between Brain Age-Related Reduction in Gray Matter and Educational Attainment. PLoS ONE. 2015;10:e0140945. doi: 10.1371/journal.pone.0140945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada C.N., Natelson Love M.C., Triebel K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern Y. Cognitive reserve: Implications for assessment and intervention. Folia Phoniatr. Logop. Off. Organ. Int. Assoc. Logop. Phoniatr. IALP. 2013;65:49–54. doi: 10.1159/000353443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schniertshauer D., Gebhard D., Bergemann J. Age-Dependent Loss of Mitochondrial Function in Epithelial Tissue Can Be Reversed by Coenzyme Q10. J. Aging Res. 2018;2018:6354680. doi: 10.1155/2018/6354680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sriskanthadevan S., Jeyaraju D.V., Chung T.E., Prabha S., Xu W., Skrtic M., Jhas B., Hurren R., Gronda M., Wang X., et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood. 2015;125:2120–2130. doi: 10.1182/blood-2014-08-594408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadava N., Nicholls D.G. Spare Respiratory Capacity Rather Than Oxidative Stress Regulates Glutamate Excitotoxicity after Partial Respiratory Inhibition of Mitochondrial Complex I with Rotenone. J. Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortiboys H., Johansen K.K., Aasly J.O., Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- 45.Tan W., Pasinelli P., Trotti D. Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2014;1842:1295–1301. doi: 10.1016/j.bbadis.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carling P.J., Mortiboys H., Green C., Mihaylov S., Sandor C., Schwartzentruber A., Taylor R., Wei W., Hastings C., Wong S., et al. Deep phenotyping of peripheral tissue facilitates mechanistic disease stratification in sporadic Parkinson’s disease. Prog. Neurobiol. 2020;187:101772. doi: 10.1016/j.pneurobio.2020.101772. [DOI] [PubMed] [Google Scholar]
- 47.Milanese C., Payán-Gómez C., Galvani M., Molano González N., Tresini M., Nait Abdellah S., van Roon-Mom W.M., Figini S., Marinus J., van Hilten J.J., et al. Peripheral mitochondrial function correlates with clinical severity in idiopathic Parkinson’s disease. Mov. Disord. 2019;34:1192–1202. doi: 10.1002/mds.27723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gottlieb E., Armour S.M., Harris M.H., Thompson C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–717. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 49.Grogan A., Green D.W., Ali N., Crinion J.T., Price C.J. Structural correlates of semantic and phonemic fluency ability in first and second languages. Cereb. Cortex. 2009;19:2690–2698. doi: 10.1093/cercor/bhp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venneri A., Jahn-Carta C., De Marco M., Quaranta D., Marra C. Diagnostic and prognostic role of semantic processing in preclinical Alzheimer’s disease. Biomark. Med. 2018;12:637–651. doi: 10.2217/bmm-2017-0324. [DOI] [PubMed] [Google Scholar]
- 51.Baldo J.V., Schwartz S., Wilkins D., Dronkers N.F. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J. Int. Neuropsychol. Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- 52.Liang W.S., Reiman E.M., Valla J., Dunckley T., Beach T.G., Grover A., Niedzielko T.L., Schneider L.E., Mastroeni D., Caselli R., et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl. Acad. Sci. USA. 2008;105:4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 54.Ramanoël S., Hoyau E., Kauffmann L., Renard F., Pichat C., Boudiaf N., Krainik A., Jaillard A., Baciu M. Gray Matter Volume and Cognitive Performance During Normal Aging. A Voxel-Based Morphometry Study. Front. Aging Neurosci. 2018;10:235. doi: 10.3389/fnagi.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maynard S., Hejl A.M., Dinh T.S.T., Keijzers G., Hansen Å.M., Desler C., Moreno-Villanueva M., Bürkle A., Rasmussen L.J., Waldemar G., et al. Defective mitochondrial respiration, altered dNTP pools and reduced AP endonuclease 1 activity in peripheral blood mononuclear cells of Alzheimer’s disease patients. Aging. 2015;7:793. doi: 10.18632/aging.100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisar Z., Hroudová J., Hansíková H., Lelková P., Wenchich L., Jirák R., Zeman J., Martásek P., Raboch J. Mitochondrial Respiration in the Platelets of Patients with Alzheimer’s Disease. Curr. Alzheimer Res. 2016;13:930–941. doi: 10.2174/1567205013666160314150856. [DOI] [PubMed] [Google Scholar]
- 57.Martin-Maestro P., Gargini R., Garcia E., Perry G., Avila J. Slower Dynamics and Aged Mitochondria in Sporadic Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2017;2017:9302761. doi: 10.1155/2017/9302761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonntag K.C., Ryu W.I., Amirault K.M., Healy R.A., Siegel A.J., McPhie D.L., Forester B., Cohen B.M. Late-onset Alzheimer’s disease is associated with inherent changes in bioenergetics profiles. Sci. Rep. 2017;7:14038. doi: 10.1038/s41598-017-14420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Mertens J., Paquola A.C., Ku M., Hatch E., Böhnke L., Ladjevardi S., McGrath S., Campbell B., Lee H., Herdy J.R., et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015;17:705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wakefield S.J., McGeown W.J., Shanks M.F., Venneri A. Differentiating normal from pathological brain ageing using standard neuropsychological tests. Curr. Alzheimer Res. 2014;11:765–772. doi: 10.2174/156720501108140910121631. [DOI] [PubMed] [Google Scholar]
- 62.De Marco M., Duzzi D., Meneghello F., Venneri A. Cognitive Efficiency in Alzheimer’s Disease is Associated with Increased Occipital Connectivity. J. Alzheimers Dis. 2017;57:541–556. doi: 10.3233/JAD-161164. [DOI] [PubMed] [Google Scholar]
- 63.Cardoso M.J., Leung K., Modat M., Keihaninejad S., Cash D., Barnes J., Fox N.C., Ourselin S. STEPS: Similarity and Truth Estimation for Propagated Segmentations and its application to hippocampal segmentation and brain parcelation. Med. Image Anal. 2013;17:671–684. doi: 10.1016/j.media.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Vijayakumar A., Vijayakumar A. Comparison of hippocampal volume in dementia subtypes. ISRN Radiol. 2013;2013:174524. doi: 10.5402/2013/174524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mortiboys H., Thomas K.J., Koopman W.J., Klaffke S., Abou-Sleiman P., Olpin S., Wood N.W., Willems P.H., Smeitink J.A., Cookson M.R. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann. Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agilent Technologies, Agilent Seahorse XF Glycolysis Stress Test Kit. [(accessed on 29 March 2020)]; Available online: https://www.agilent.com/cs/library/usermanuals/public/XF_Glycolysis_Stress_Test_Kit_User_Guide.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.