Abstract
Background:
Controlled drug challenge studies provide valuable information about the acute behavioral effects of drugs, including individual differences that may affect risk for abuse. One question that arises in such studies is whether a single administration of a drug (and placebo) provides an accurate measure of response to the drug.
Methods:
Here, we examined data from two studies, one with alcohol and one with amphetamine, in which participants received two administrations of the drug and placebo. In this analysis we assess the stability of acute subjective and cardiovascular responses to the drugs across the two administrations. We examine i) systematic increases or decreases to the drugs from the first to the second administration, ii) test-retest reliability within individuals and iii) the accuracy of the acute drug responses to predict drug choice in a later session.
Results:
Responses were largely stable across sessions, although on the second session amphetamine “liking” was higher, and subjective responses to placebo including “liking” and “want more” decreased in both studies. Test-retest reliability within individuals was high. Responses during the first drug administration were as accurate in predicting drug choice as responses during both administrations combined.
Conclusions:
Our findings indicate that a single administration of drug (and placebo) provides a good index of an individual’s responses to alcohol or amphetamine, when participants are tested under controlled experimental conditions.
Keywords: amphetamine, alcohol, healthy volunteer, subjective, drug, choice
1. Introduction
Clinical pharmacology and substance abuse researchers often assess acute drug effects in human volunteers under controlled laboratory conditions. These studies characterize the subjective, behavioral and physiological effects of drugs, often to identify individual differences that may predict risk for excessive use or abuse of the drugs. To maximize experimental control, researchers administer the drugs at fixed doses compared to placebo, under double blind conditions, with minimal information about the identity of the drugs to reduce expectancies. Often, the drug and placebo are administered on two or more occasions to minimize the influence of day-to-day variations in mood and to obtain a more accurate profile of an individual’s response to a drug. However, the extent of this variability, or the test-retest reliability of the drug administrations, is not fully known. There is some evidence that subjects’ responses to drugs are stable across weeks (Lutz and Childs, 2017; Mundt et al., 1997) and even years (King et al., 2016; Schuckit and Smith, 1996). Yet, responses may also vary across drug administrations due to pharmacological factors such as tolerance and sensitization (Childs and De Wit, 2013), or mood states before the drug is administered (Mitchell et al., 1996). From a practical point of view for future research, it is valuable to know whether a single administration of a drug is as informative as two administrations to investigate individual differences in drug responses.
Individual differences in drug responses may contribute to substance abuse. A behavioral drug preference procedure (Chutuape and De Wit, 1994; de Wit et al., 1987; H. de Wit et al., 1986; Holdstock and Wit, 2001) assesses individual differences in the reinforcing effects of drugs and isolates factors that are related to drug preference. In this procedure, subjects are first exposed to drug and placebo during sampling sessions and then, in one or more choice sessions, they choose either the drug or placebo to indicate their preference. Some individuals consistently choose drug, while others prefer placebo (Crane et al., 2018; H de Wit et al., 1986; Uhlenhuth et al., 1981; Weafer et al., 2019). Those who subsequently choose amphetamine, for example, report that the drug produces greater vigor, elation, arousal, and decreased fatigue and sedation than those who choose placebo (H de Wit et al., 1986; Gabbay, 2003; Johanson et al., 1983). However, most of these studies include two or more sampling sessions, and it is not known whether responses from a single experience with a drug (and placebo) predict drug preference as well as more than one administration. Multiple administrations are often included to reduce day-to-day variability.
In the analyses presented here, we re-examined data from two studies (Crane et al., 2018; Weafer et al., 2019), one with alcohol and one with amphetamine, to determine the stability of subjects’ responses to the drugs across two administrations. The dependent measures were subjective ratings of mood and drug effects, as well as physiological indices of the drugs’ effects. In each study, subjects received drug and placebo in alternating order, under double blind conditions, on two occasions (i.e., four sessions total). The subjects then also participated in a choice session in which they had the opportunity to choose which substance (drug or placebo) they preferred. It was expected that subjective responses to the drugs during the acute administration(s) (e.g., drug liking ratings) would predict drug choice. Three primary analyses were conducted: first we examined whether the subjective responses to the drugs changed systematically across the two sampling administrations. Second, we examined the degree to which subjects’ responses on the first administration of drug were correlated with their responses on the second administration. Third, we examined the predictive value of either the first drug administration or both drug administrations, to predict choice during the choice session
2. Methods
2.1. Design
Healthy young adults participated in two five-session drug choice studies, one with alcohol (n = 95; Weafer et al., 2019) and one with amphetamine (n = 102; Crane et al., 2018). In both studies, participants first sampled drug and placebo under double blind conditions, and drug effects were measured at regular intervals over several hours. Then on a choice session, subjects chose which of the two substances (i.e., drug or placebo) they preferred. In this analysis we addressed two questions: i) what is the stability of subjective and cardiovascular drug effects across repeated drug administrations, and ii) in relating subjective drug responses to drug choice, does including a second drug administration add significantly more information than a single drug administration.
2.2. Alcohol Study
2.2.1. Participants
Healthy men (n = 51) and women (n = 44) aged 21–29 years were recruited from the university and surrounding community. Participants were moderate drinkers (7–30 drinks per week) who reported at least one binge drinking episode (5 or more drinks on a single occasion for men; 4 for women) in the last month. Exclusion criteria included any serious medical problems, flushing reaction to alcohol, serious psychiatric disorders including psychotic symptoms, PTSD or severe substance use disorder (APA, 2013), pregnancy or lactation, less than high school education, lack of fluency in English and BMI outside the range of 18–26. For blinding purposes, participants were told that the beverages used in the study might contain a stimulant, sedative/tranquilizer, alcohol, or placebo. The study was approved by the Institutional Review Board of the University of Chicago, and was carried out in accordance with the Declaration of Helsinki.
2.2.2. Procedure
Participants attended five experimental sessions separated by 2–7 days. For 24 hrs before the sessions, they were instructed to abstain from alcohol and drugs, other than their usual amounts of caffeine and nicotine. They were tested for drug use at each session. Sessions took place from 3:00 p.m. to 8:00 p.m. On sampling sessions, after compliance tests and baseline measures, participants consumed color-coded beverages containing placebo or alcohol (0.8 g/kg for men and 0.68 g/kg for women). The doses were selected to achieve a peak breath alcohol content (BrAC) of 80mg/100ml (Fillmore, 2001; Mulvihill et al., 1997). The beverages were consumed in four equal serving cups over 15 min. Alcohol or placebo was provided in a consistent cup color, in alternating order (sessions 1 and 3 or 2 and 4). Thus, they received alcohol and placebo twice, first on sessions 1 and 2 (“Pair 1”) and second on sessions 3 and 4 (“Pair 2”). Participants were informed that the same drug was associated with the same color and asked to attend to the drugs’ effects to inform their choice. On the fifth session, participants chose to ingest the beverage they preferred, by color, and the number of cups (from 1–4) they wished to ingest. Subjective and cardiovascular measures and breath alcohol levels were obtained at half-hour intervals during each session.
2.2.3. Drug
0.8 g/kg oral alcohol (190-proof, 95% ethanol; Everclear, Luxco, Inc., St. Louis, MO) was divided into 4 servings of 0.2 g/kg each. The 0.8 g/kg dose is equivalent to 4 standard drinks, where a standard drink is defined as one 12 oz beer, one 5 oz glass of wine, or one 1.5 oz shot of 80 proof alcohol. Women received a reduced dose (0.68 g/kg) to account for sex differences in total body water (Frezza et al., 1990; Sutker et al., 1983). Beverages were served in a 10% solution by volume with the subjects’ preferred fruit juice. The placebo beverage consisted of fruit juice plus 3 ml ethanol added as a taste mask. All beverages were sprayed with an alcoholic mist to provide a strong alcoholic scent.
2.2.4. Measures
Subjective measures:
Several self-report measures were obtained, but we focus here on the Drug Effects Questionnaire (DEQ). The DEQ consists of four questions on a 100 mm visual analog scale (VAS): “Do you feel any drug effect?” (“Not at all” to “A lot”); “Do you like the effects that you are feeling now?” (“Not at all” to “A lot”); “Do you dislike the effects that you are feeling now?” (“Not at all” to “A lot”); and “Would you like more of what you consumed, right now?” (“Not at all” to “Very much”).
Physiological measures:
Heart rate (HR) and blood pressure (BP) were obtained by blood pressure monitor (Omron Healthcare, Inc., Lake Forest, IL). BrAC was measured via breathalyzer (Intoximeters Inc., St. Louis, MO).
Drug choice:
On the fifth session, participants were given a choice of which color beverage they wished to ingest (i.e., alcohol or placebo), and the amount of the beverage from 1 to 4 cups. For this analysis, we separated subjects only on whether they chose alcohol or placebo (not preferred dose).
2.2.5. Statistical Analyses
Similarity of responses to alcohol across sessions:
The first aim of the study was to determine whether subjects’ responses to alcohol differed between Pair 1 and Pair 2 (i.e., sessions 1 and 2 compared to sessions 3 and 4). Test-retest reliability was estimated by intraclass correlation coefficients (ICC). ICC estimates and their 95% confidence intervals were calculated using SPSS statistical package version 25 (SPSS Inc, Chicago, IL) based on an absolute-agreement, 2-way mixed-effects model. In addition, we conducted linear mixed effects models for repeated measures (Hedeker and Gibbons, 2006) in SPSS 25 to test whether responses to alcohol varied systematically (i.e., increased or decreased) across the two administrations. The degree to which visit pair interacted with drug (alcohol vs placebo) and time (30-minute intervals across a single session) were assessed. Pair × drug interactions predicted measures of subjective and cardiovascular responses – specifically ratings of DEQ “Feel Dug” “Like Drug” “Dislike Drug” and “Want More” as well as heart rate and blood pressure. The models included random intercept, drug, and time effects to allow for individual differences in drug response and time trends, and to account for the correlation between repeated measurements. Drug order (drug or placebo administered first), BMI, age, and sex were included as covariates. The primary effects of interest were the three-way interactions among visit pair, drug, and time (linear and quadratic trends).
Test-retest reliability within individuals:
We calculated test-retest Pearson correlations across subjects’ responses to alcohol on the first Pair and the second Pair. We calculated the peak change from pre-beverage within sessions, and subtracted placebo session scores from alcohol scores for each subject to obtain a single measure of response to alcohol, and then examined the correlation between Pair 1 and Pair 2. Peak change scores provide a good summary measure of the magnitude of drug effects, taking into account minor variations in pre-drug values and in timing of the drug effects (Crane et al., 2018; Weafer et al., 2019). Peak change scores are highly correlated with area under the curve summary measures in secondary analyses of both data sets.
Choice prediction:
The second aim of the study was to examine the relationship between subjective responses to the drug and drug choice, to determine whether responses during Pair 1 were equally predictive of choice as both Pairs combined (all 4 sessions). Subjects were divided into two groups, choosers (n = 55) and non-choosers (n = 40), based on their choice of alcohol or placebo on session 5. We then examined the relationship between drug (vs placebo) choice and responses during Pair 1 and both Pairs combined. We examined acute subjective responses to alcohol (peak change scores of alcohol-minus-placebo) in choosers and non-choosers using t-tests, using either Pair 1 data or Pairs 1 and 2 combined. As an extension to this analysis, in Supplementary materials, we also compared responses during Pair 1 and Pair 2 separately, as predictors of drug choice, using both alcohol-minus-placebo scores and alcohol scores alone.2
2.3. Amphetamine Study
2.3.1. Participants
Healthy men (n = 60) and women (n = 52) aged 21–29 years were recruited in a similar manner to the alcohol study. The same inclusion criteria as the alcohol study applied, except that there were no minimum drinking criteria, and alcohol flushing response was not an exclusion criterion. Participants were told that the capsules used in the study might contain a stimulant, sedative/tranquilizer, or placebo.
2.3.2. Procedure
Participants attended five experimental sessions according to the same procedure as the alcohol study above (e.g., separated by 2–7 days), except that sessions took place from 9:00 a.m. to 1:00 p.m. and participants ingested colored capsules rather than beverages. The color-coded capsules contained either d-amphetamine (5 mg each; total of 20 mg, Barr Pharmaceuticals, Pomona NY) or placebo and were administered under double blind conditions in alternating order over the four sampling sessions. On the fifth session, participants chose to ingest the colored capsules (drug or placebo), and number of capsules from 1 to 4. We focus here only on whether they chose drug or placebo.
2.3.3. Drug
20 mg oral d-amphetamine (Barr Pharmaceuticals, Pomona NY; 5 mg tablets) was placed in opaque size 00 capsules with dextrose filler. Placebo capsules contained only dextrose. This dose was chosen because it produces reliable rewarding effects, and it has been safely administered to similar samples of healthy young adults in previous studies (Kirkpatrick et al., 2013; White et al., 2006).
2.3.4. Measures
Participants completed several measures, but here we focus on the DEQ and cardiovascular measures of drug effects. Participants completed the DEQ at 30 min intervals, and HR and BP were obtained as described above. On the fifth session, subjects chose which color capsule they wished to ingest (i.e., amphetamine or placebo).
2.3.5. Statistical Analyses
The statistical analyses for this study parallel the alcohol study above. Briefly, ICC estimates and their 95% confidence intervals estimated test-retest reliability based on an absolute-agreement, 2-way mixed-effects model. Linear mixed effects models for repeated measures were used to test the degree to which subjects’ responses to the drug vs placebo varied systematically across the two Pairs of sessions, and correlations were conducted to examine the concordance in individual subjects’ responses. We also examined the extent to which subjective responses during the Pair 1 predicted choice of amphetamine on Session 5 as well as Pairs 1 and 2 combined. We compared the ratings of subjective drug effects of choosers (n = 81) compared to non-choosers (n = 31) using t-tests, using either Pair 1 data or Pairs 1 and 2 combined. Additionally, direct comparisons of Pair 1 and Pair 2 are included in the supplementary material.3
3. Results
3.1. Alcohol Study
3.1.1. Group responses to alcohol across session pairs.
Our first aim was to determine whether subjects’ responses differed across the two pairs of alcohol and placebo sessions (Pair 1 compared to Pair 2). The test-retest reliability between each pair of sessions was estimated by intraclass correlation coefficients (ICC), which are presented in Table 1. ICC compares variability within a group to variability across the group. Test-retest reliability is generally considered excellent when ICC is between 0.75 and 1, good when between 0.6 to 0.74, fair when between 0.4 to 0.59 and poor when < 0.4 (Cicchetti, 1994). Excellent reliability occurred between the first and second alcohol session for all subjective measures, i.e., “Feel Drug,” “Dislike Drug,” and “Want More,” aside from “Like Drug,” which showed good reliability. Between placebo sessions, only “Feel Drug” and “Dislike Drug” showed good test-retest reliability, with fair reliability for “More Drug” and poor reliability for “Like Drug”. Cardiovascular measures demonstrated fair to poor reliability across alcohol session or placebo session days.
Table 1.
ICC test-retest reliability. Intraclass correlation coefficient (ICC) estimates and their 95% confidence intervals are shown. Values are derived from an absolute-agreement, 2-way mixed-effects model.
| Alcohol Study | |||||||
|---|---|---|---|---|---|---|---|
| Alcohol | Placebo | ||||||
| 95% Confidence Interval | 95% Confidence Interval | ||||||
| ICC | Lower Bound | Upper Bound | ICC | Lower Bound | Upper Bound | ||
| Feel | 0.812 | 0.716 | 0.875 | Feel | 0.667 | 0.493 | 0.780 |
| Like | 0.707 | 0.559 | 0.805 | Like | 0.394 | 0.093 | 0.596 |
| Dislike | 0.745 | 0.616 | 0.830 | Dislike | 0.626 | 0.433 | 0.752 |
| More | 0.811 | 0.715 | 0.874 | More | 0.459 | 0.169 | 0.646 |
| HR | 0.220 | −0.178 | 0.483 | HR | 0.366 | 0.043 | 0.580 |
| SYS | 0.434 | 0.157 | 0.621 | SYS | 0.075 | −0.388 | 0.384 |
| DIA | 0.358 | 0.030 | 0.574 | DIA | −0.013 | −0.521 | 0.326 |
| Amphetamine Study | |||||||
| Amphetamine | Placebo | ||||||
| 95% Confidence Interval | 95% Confidence Interval | ||||||
| ICC | Lower Bound | Upper Bound | ICC | Lower Bound | Upper Bound | ||
| Feel | 0.825 | 0.744 | 0.880 | Feel | 0.765 | 0.659 | 0.838 |
| Like | 0.816 | 0.731 | 0.874 | Like | 0.717 | 0.571 | 0.811 |
| Dislike | 0.708 | 0.576 | 0.800 | Dislike | 0.681 | 0.537 | 0.781 |
| More | 0.832 | 0.755 | 0.884 | More | 0.717 | 0.581 | 0.808 |
| HR | 0.355 | 0.061 | 0.557 | HR | 0.451 | 0.202 | 0.623 |
| SYS | 0.445 | 0.192 | 0.619 | SYS | 0.322 | 0.018 | 0.532 |
| DIA | 0.418 | 0.156 | 0.598 | DIA | 0.497 | 0.267 | 0.654 |
Supplementary Table 1 presents results from the linear mixed effects models that tested the degree to which session pairs interacted with drug and time to predict measures of subjective and cardiovascular responses. There were no drug × time × session interactions. Figure 1 shows that alcohol increased subjective ratings of DEQ “Feel Drug,” “Like Drug,” “Dislike Drug,” and “Want More” across both Pairs of sessions. Covariates included in the analysis (drug order, sex, age, and BMI) did not significantly affect any of the subjective ratings. However, cardiovascular responses were affected by sex and BMI. In general, the responses to alcohol were stable across the two sessions although responses to placebo declined during the second placebo session. Post hoc tests of drug × session interactions indicated that ratings on all four DEQ scales were lower during the second placebo administration compared to the first (paired t-tests of peak change from baseline: “Feel Drug” 18.96, 13.04, p=0.004; “Like Drug” 34.29, 18.47, p<0.0005; “Dislike Drug” 26.97, 18.23, p=0.005; “Want More” 35.71, 19.34, p<0.0005; n=95). Responses to alcohol remained stable across the two alcohol sessions. Alcohol increased heart rate and systolic blood pressure (Fig 2; drug × time), and this remained stable across the two pairs of sessions.
Figure 1.
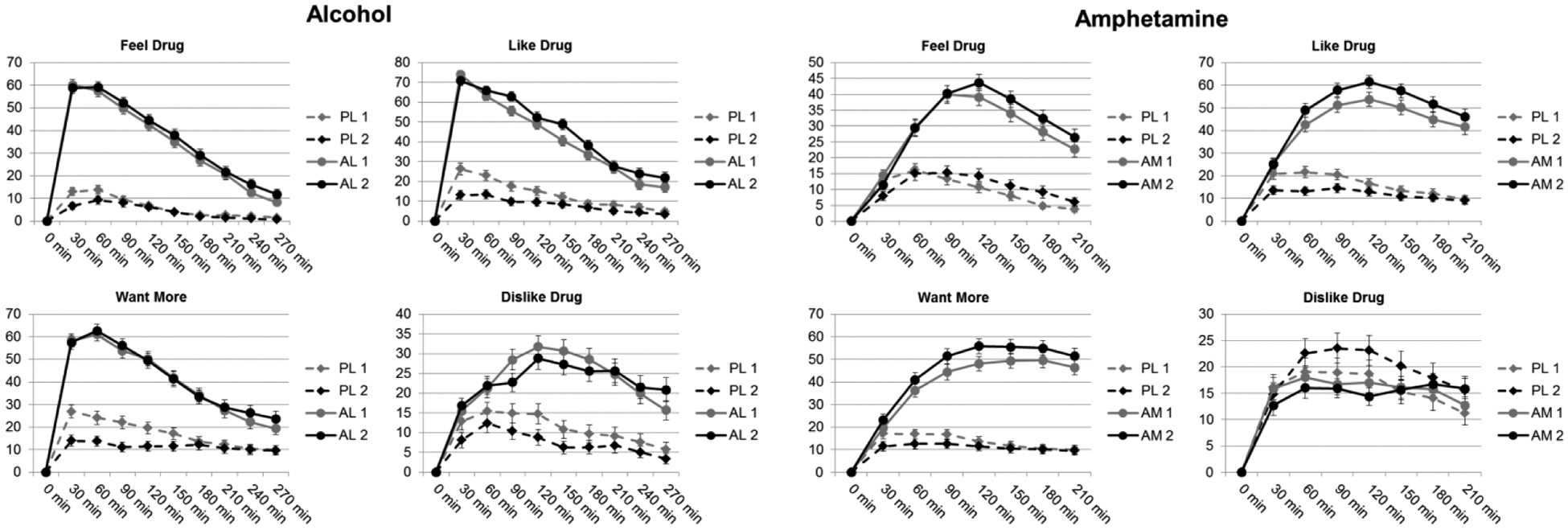
Mean ± SEM scores on scales of the drug effects questionnaire (DEQ) after alcohol vs placebo (left) and amphetamine versus placebo (right) during the two successive administrations of the drugs. Time 0 refers to the time at which the drug was administered. The DEQ was not administered before drug administration, and was completed at half-hour intervals. Responses are shown for “Feel Drug” “Like Drug” “Want More” and “Dislike Drug” across first and second placebo (PL 1, PL 2), alcohol (AL 1, AL 2) or amphetamine (AM 1, AM 2) sessions.
Figure 2.
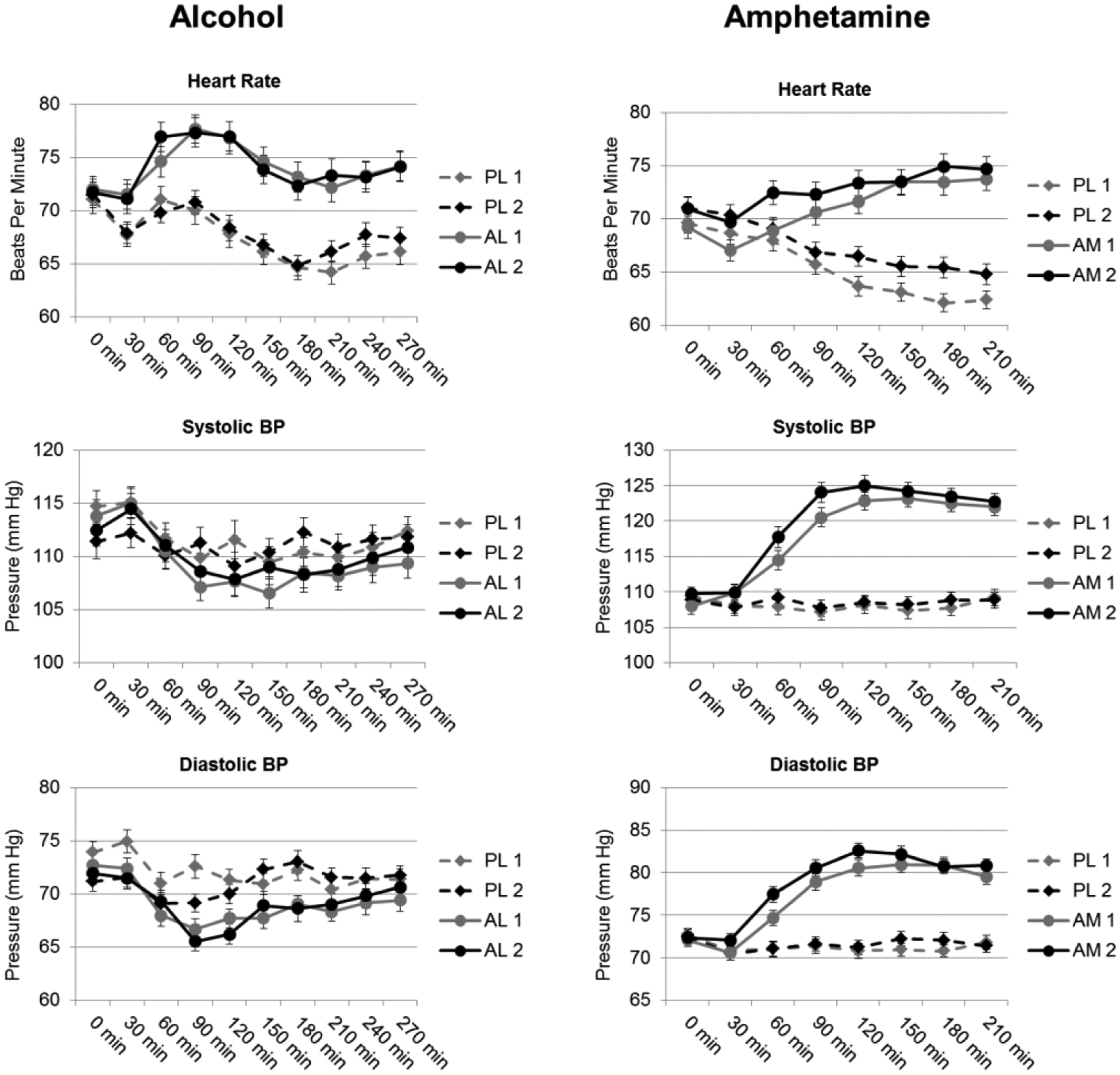
Mean ± SEM scores on scales of blood pressure (BP) and heart rate after alcohol vs placebo (left) and amphetamine versus placebo (right) across the two administrations of the drugs. Cardiovascular measures were attained at half-hour intervals alongside subjective measures. PL 1, PL 2 = first and second placebo sessions; AL 1, AL 2 = first and second alcohol sessions; AM 1, AM 2 = first and second amphetamine sessions.
3.1.2. Individual responses to alcohol across session pairs.
We next assessed the reliability of individual differences in responses to alcohol across the two Pairs of sessions. Peak change scores on placebo sessions were subtracted from alcohol sessions for each subject, separately for Pair 1 and Pair 2, and correlations were calculated. Subjects’ ratings on all scales of the DEQ (n=95) were significantly correlated during Pair 1 and Pair 2 (“Feel Drug” r=0.442, p<0.0005; “Like Drug” r=0.235, p=0.022; “Dislike Drug” r=0.5. p<0.0005; “Want More” r=0.333, p=0.001). However, cardiovascular responses were not significantly correlated across the two Pairs.
3.1.3. Acute responses to alcohol in relation to alcohol choice: Pair 1 vs Pairs 1 and 2 combined.
The second aim of our study was to determine whether responses to alcohol and placebo during Pair 1 predicted alcohol choice as effectively as responses from Pairs 1 and 2 combined, as studies often combine administrations to minimize variation. Acute responses to alcohol (Pair 1 or Pairs 1 and 2 averaged) were compared in choosers (n = 55) vs non-choosers (n = 40) using T-tests (Figures 3, 4). The demographic characteristics of the choosers and non-choosers were similar on sex, age, BMI, education, current and lifetime drug use, and personality (Table 2). When comparing acute responses (alcohol-minus-placebo) between the groups to predict alcohol choice, we found that non-choosers rated “Dislike Drug” higher than choosers. This effect was apparent in both the Pair 1 analysis and Pairs 1 and 2 combined analysis (effect sizes of d=0.612 and d=0.764). Choosers and non-choosers did not differ on ratings of “Feel Drug” or “Like Drug” on either Pair 1 or both Pairs combined. The only measure on which the combination of Pairs 1 and 2 differed was on “Want More”: the alcohol choosers wanted more alcohol than the non-choosers only when both Pairs were combined. Similarly, the direct comparison of Pair 1 to Pair 2 also resulted in non-choosers rating “Dislike Drug” higher than choosers, without differences in ratings on “Feel Drug,” and with Pair 1 differing from Pair 2 on higher “Want More” ratings in choosers. In this case, Pair 1 also differed on “Like Drug” with higher ratings by choosers in Pair 2 (Supp. Fig 1). However, comparing Pairs 1 and 2 using scores from the alcohol condition alone (without subtracting placebo responses) resulted in matched predictions of choice across each subjective response. Specifically, Pairs 1 and 2 each showed that non-choosers rated “Dislike Drug” higher than choosers, while choosers rated “Like Drug” and “More Drug” higher than non-choosers, with no difference in ratings of “Feel Drug” between choosers and non-choosers (Supp. Fig 2). Together, these data are consistent with response stability in the prediction of alcohol choice.
Figure 3.
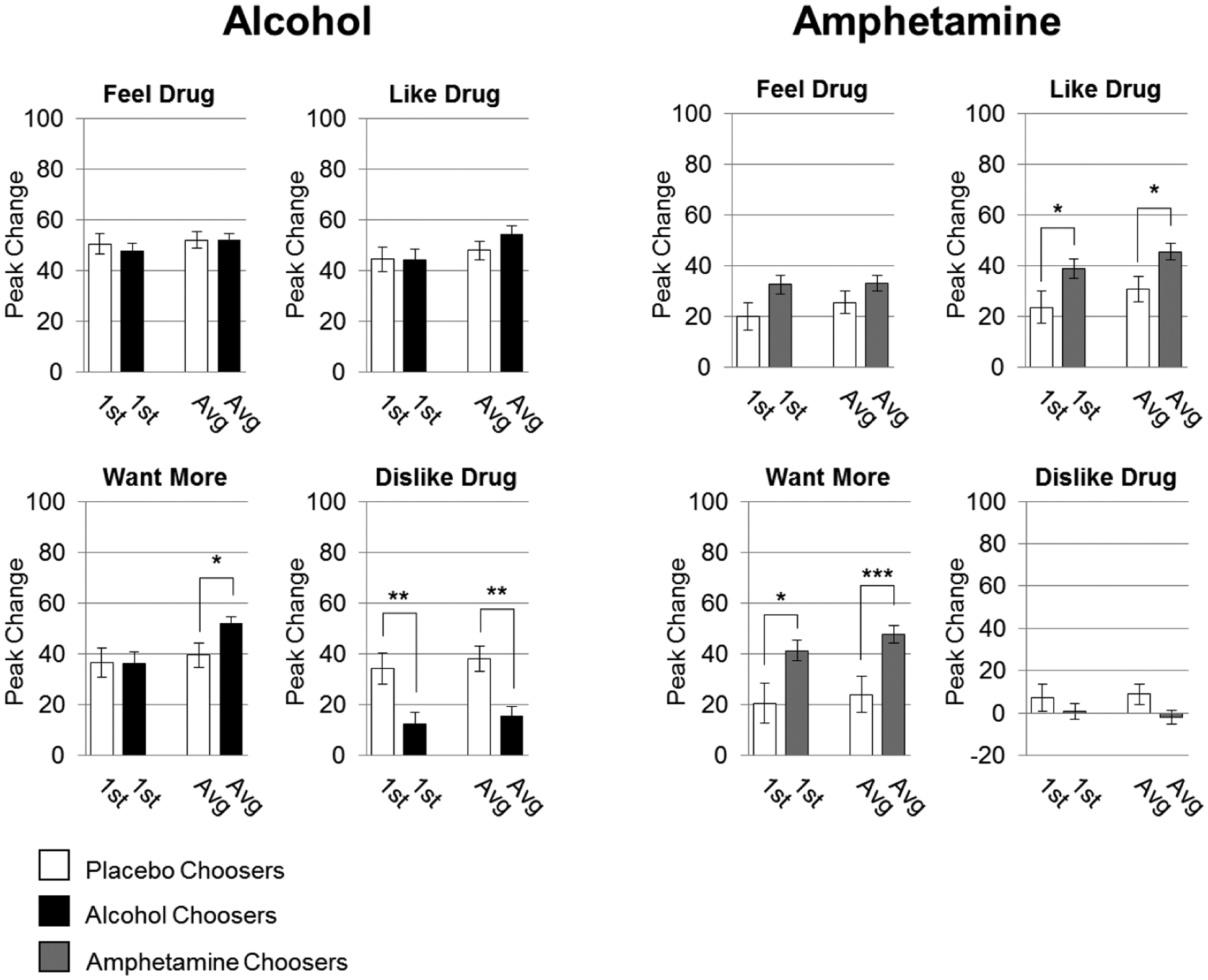
DEQ responses in relation to choice between Pair 1 and Pairs 1 and 2 combined. Data are expressed as mean (drug-minus-placebo peak change from baseline) ± SEM (n=55,40 alcohol and placebo choosers respectively, n=81,31 amphetamine and placebo choosers respectively; unpaired t-tests; *p<0.05; **p<0.01; ***p<0.001).
Figure 4.
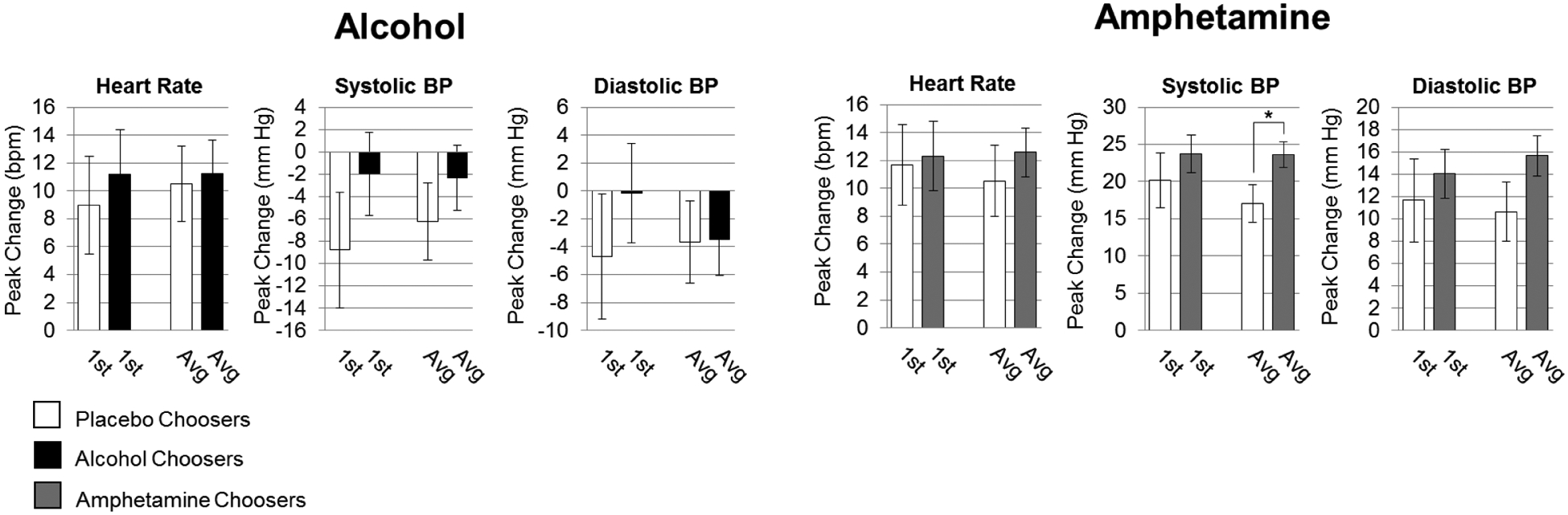
Cardiovascular responses in relation to choice between Pair 1 and Pairs 1 and 2 combined. Data are expressed as mean (drug-minus-placebo peak change from baseline) ± SEM (n=55,40 alcohol and placebo choosers respectively, n=81,31 amphetamine and placebo choosers respectively; unpaired t-tests; *p<0.05).
Table 2.
Demographic information for participants who chose alcohol or amphetamine (Choosers) and participants who chose placebo (Non-choosers) during the choice session of the alcohol and amphetamine studies. “Binges” correspond to 5 or more drinks on a single occasion for men, 4 for women.
| Alcohol | Amphetamine | |||
|---|---|---|---|---|
| Choosers | Non-choosers | Choosers | Non-choosers | |
| Sex (M,F) | (31, 24) | (19, 21) | (47, 34) | (13, 18) |
| Age | 24.27 (2.6) | 24.03 (2.8) | 24.92 (2.9) | 25.29 (3.0) |
| BMI | 23.30 (2.3) | 23.33 (2.3) | 23.12(1.9) | 22.22(1.9) |
| Education years | 15.42(1.6) | 15.55(1.4) | 15.23(1.5) | 15.75(1.4) |
| Current Drug Use | ||||
| Alcohol (days last month) | 16.13(5.1) | 14.50 (5.4) | 10.15(6.6) | 8.81 (5.8) |
| Alcohol (binges last month) | 4.18(3.2) | 4.95 (2.7) | 1.85 (2.8) | 1.65 (2.6) |
| Alcohol (drinks per occasion) | 3.54(1.6) | 3.68(1.6) | 2.95(1.87) | 2.48(1.0) |
| Alcohol (total drinks last 28 days) | 51.51 (19.3) | 49.78 (20.3) | 27.93 (24.2) | 24.77 (22.3) |
| Tobacco (% used in last month) | 27% | 30% | 36% | 29% |
| Cannabis (% used in last month) | 69% | 63% | 46% | 39% |
| Lifetime Drug Use | ||||
| Opiate (% reported use) | 16% | 20% | 31% | 26% |
| Stimulant(% reported use) | 44% | 55% | 20% | 16% |
| Personality (MPQ) | ||||
| Positive Emotionality | 52.61 (9.8) | 53.79 (8.6) | 55.28 (9.5) | 55.26 (9.2) |
| Negative Emotionality | 45.47 (9.0) | 43.38 (8.8) | 42.59 (9.4) | 44.06 (9.3) |
| Constraint | 41.51 (8.7) | 39.69 (7.1) | 41.37 (8.2) | 42.71 (8.0) |
3.2. Amphetamine Study
3.2.1. Group responses to amphetamine across session pairs.
The test-retest reliability between each pair of sessions was estimated by ICC, presented in Table 1. Excellent reliability occurred across all subjective measures between amphetamine sessions aside from “Dislike Drug,” which showed good reliability. Between placebo sessions, excellent reliability occurred for “Feel Drug,” with good reliability on all other subjective measures. Cardiovascular measures demonstrated fair to poor reliability between amphetamine and placebo session days.
Supplementary Table 14 presents linear mixed effects model results testing the degree to which amphetamine responses change across the two administrations of the drug. Covariates included in the analysis (drug order, sex, age, and BMI) did not affect any subjective rating except for “Want More” due to BMI (estimate=1.64, p=0.04), indicating that higher BMI increased responding to “Want More” in the absence of all other variables including drug. In addition, cardiovascular responses were influenced by both sex and BMI. Figure 1 shows that amphetamine increased subjective ratings of DEQ “Feel Drug,” “Like Drug,” and “Want More,” during both Pairs of sessions (drug × time interactions). Although there were no drug × time × session interactions, responses to placebo declined during the second pair: Ratings of “Like Drug” and “Want More” were lower during the second placebo administration compared to the first (Drug × session interaction; paired t-tests of peak change from baseline: “Like Drug” 30.52, 22.01, p<0.0005; “Want More” 24.59, 17.46, p=0.044; n=112). In addition, ratings of “Like Drug” were higher during the second amphetamine session compared to the first (paired t-test: “Like Drug” 65.63, 70.17, p=0.002; n=112). Post hoc testing revealed no statistically significant differences in cardiovascular responses between the first and second sessions (paired t-test: systolic blood pressure, p=0.16; n=112). Figure 2 shows that amphetamine increased heart rate, systolic, and diastolic blood pressure for both session Pairs (drug × time), but these effects were not different across the two pairs.
3.2.2. Individual responses to amphetamine across session pairs.
Individuals participants’ responses to amphetamine were highly correlated across the two pairs for all DEQ scales (n=112; “Feel Drug” r=0.58, p<0.0005; “Like Drug” r=0.51, p<0.0005; “Dislike Drug” r=0.27, p=0.003; “Want More” r=0.56, p<0.0005) and diastolic blood pressure (r=.27, p=0.004).
3.2.3. Acute responses to amphetamine in relation to amphetamine choice: Pair 1 vs Pairs 1 and 2 combined.
DEQ responses on “Want Drug” and “Like Drug” scales were significantly related to choice using data from only Pair 1 or data from Pairs 1 and 2 combined. Choosers (n = 81) and non-choosers (n = 31) were similar on most demographic characteristics, except that choosers included more men and reported higher current and lifetime drug use (Table 2). Choosers reported higher ratings of amphetamine liking and wanting more than non-choosers, and this was evident using data from either Pair 1 or both Pairs combined (Figure 3), as well as data from Pair 2 (Supplementary Figures 1, 2).5 The chooser/non-chooser groups did not differ on “Feel Drug” or “Dislike Drug.” Subjects who chose amphetamine exhibited a greater increase in systolic blood pressure than non-choosers only when all four sessions were averaged (Figure 4).
4. Discussion
This investigation assessed the value of including more than a single drug administration to determine acute responses in drug challenge procedures. Using data from two studies, one with alcohol and one with amphetamine, we examined whether responses to the drugs changed systematically across two administrations (i.e., either increased or decreased), whether participants’ responses were stable across two administrations, and whether a second administration improved the ability to predict drug choice, compared to a single administration. Subjects’ responses were stable across the two sessions for both drugs, and highly correlated within subjects. Further, one administration of the drug was as good as two administrations in predicting drug choice. These findings provide valuable guidance for future drug challenge studies.
In general, subjects’ responses to both drugs were stable across the two administrations, with excellent test-retest reliability. The finding that responses to alcohol are stable is consistent with previous studies (Lutz and Childs, 2017; Mundt et al., 1997) showing that responses to alcohol neither increase nor decrease during the second administration. With regard to amphetamine, responses were mainly stable across the administrations, with one exception: subjects reported greater drug liking during the second administration, compared to the first. Interestingly, we previously reported (Childs and De Wit, 2013) that positive responses to amphetamine increased during the second administration. However, in the Childs et al. study, this increase in response may have been affected by Pavlovian conditioning: In that study amphetamine and placebo were administered in different and distinctive environments, creating the optimal conditions for Pavlovian conditioning. Pavlovian conditioning is considered integral to the process of sensitization (Boileau et al., 2006; Robinson and Berridge, 1993; Stewart and Vezina, 1991). The present findings indicate that there may be some increase in positive responses to amphetamine even without creating distinctive conditioning environments. Another minor difference in subjects’ responses across the two administrations was related to responses to placebo. That is, subjects’ responses during the placebo sessions declined during the second pair of sessions in both the alcohol and amphetamine studies. In the alcohol study, ratings on all DEQ scales were lower during the second placebo session compared to the first. In the amphetamine study as well, there was a decline on ratings of “Like Drug” and “Want More” between the first and second placebo session. These declines in placebo responses are likely a result of the participants’ increasing experience with active vs inactive substances in the studies. Notably, decreases in placebo response have the potential of inflating the apparent drug response when drug-minus-placebo values are used. On the other hand, it could also be argued that the decrease in placebo response gives a more accurate estimate of the true pharmacological effect of the drug. Future studies in which it is important to provide an accurate assessment of a drug effect might benefit from prior placebo treatment to reduce unwanted variability.
We also examined the extent to which individual subjects’ responses were consistent across the two drug administrations. In the alcohol study, we found that subjective ratings on all scales of the DEQ, but not cardiovascular responses, were significantly correlated between session pairs. This is consistent with previous reports showing individual stability in subjective responses to alcohol after an average of 21 days (Mundt et al., 1997) or 5 years (King et al., 2016). Additionally, others have shown that behavioral scores, but not heart rate or diastolic blood pressure, remained stable in individuals over a one-month interval between alcohol sessions (Wilson and Nagoshi, 1987). In the amphetamine study, we also found significant correlations between session pairs on all scales of the DEQ as well as diastolic blood pressure. This is consistent with stable subjective responses to amphetamine over one or two-week intervals (Kegeles et al., 1999; Narendran et al., 2013). Together, we conclude that individual differences in response to alcohol and amphetamine are reliably reproduced between first to second administrations.
Finally, we examined subjective responses to the drugs during the sampling sessions as predictors of preference on the choice session. As described previously (H de Wit et al., 1986; Gabbay, 2003; Johanson et al., 1983), subjective responses to drugs during the sampling sessions are predictive of drug choice on the choice session. In the present study, participants with higher ratings of “Want More” after alcohol were more likely to choose alcohol, and in the amphetamine study, those who reported higher ratings of “Like Drug” and “Want More” were more likely to choose amphetamine. The question we examined in this analysis was whether drug choice could be predicted equally well from a single drug administration, or whether a second administration substantially improved the ability to predict choice. We determined that for both alcohol and amphetamine, a single administration was as effective as two administrations. That is, subjects’ ratings of “Like Drug” or “Want More” on the first administration predicted choice about as well as their ratings on the two administrations combined. This finding is useful for future studies, justifying the use of a single determination to characterize individual differences in responses to drugs.
5. Conclusions
Together, our findings indicate that responses to single doses of these two drugs, amphetamine and alcohol, are stable across two administrations, when healthy young adults are tested under highly controlled conditions. That is, normal day-to-day fluctuations in mood or physiological states do not appreciably alter the subjective or cardiovascular effects of these drugs. It is likely that responses would be less stable in non-laboratory settings, as the contexts of drug use outside the laboratory vary widely due to numerous factors such as social conditions, sensory stimulation, emotional states and expectations. Moreover, the volunteers in the present studies were homogeneous with regard to age, weight, education and absence of psychiatric symptomatology. Nevertheless, these findings are relevant to laboratory-based studies examining individual differences in the direct pharmacological effects, showing that the responses are stable and can be well characterized in a single administration.
Supplementary Material
Highlights.
A single drug administration provides a good estimate of responses to drugs
Retested responses are consistent at the group level and within individuals
Responses to placebo decline with repeated experience
Subjective responses to a single administration predict drug preference
Acknowledgements
This research was supported by the National Institutes of Health [DA02812]. JW was supported by the National Institutes of Health [K01AA024519]
Role of funding sources
Nothing declared.
Footnotes
Declarations of interest
None.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi…
References
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C, 2006. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch. Gen. Psychiatry 63, 1386–95. 10.1001/archpsyc.63.12.1386 [DOI] [PubMed] [Google Scholar]
- Childs E, De Wit H, 2013. Contextual conditioning enhances the psychostimulant and incentive properties of d-amphetamine in humans. Addict. Biol 18, 985–992. 10.1111/j.1369-1600.2011.00416.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MAD, De Wit H, 1994. Relationship between subjective effects and drug preferences: ethanol and diazepam. Drug Alcohol Depend. 34, 243–251. 10.1016/0376-8716(94)90163-5 [DOI] [PubMed] [Google Scholar]
- Cicchetti DV, 1994. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess 6, 284–290. 10.1037/1040-3590.6.4.284 [DOI] [Google Scholar]
- Crane NA, Gorka SM, Weafer J, Langenecker SA, De Wit H, Phan KL, 2018. Neural activation to monetary reward is associated with amphetamine reward sensitivity. Neuropsychopharmacology 43, 1738–1744. 10.1038/s41386-018-0042-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Hedeker D, McCracken SG, Johanson CE, 1986. Lack of preference for diazepam in anxious volunteers. Arch. Gen. Psychiatry 43, 533–41. 10.1001/archpsyc.1986.01800060023004 [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE, 1986. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend 16, 341–360. 10.1016/0376-8716(86)90068-2 [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE, 1987. Individual Differences in Behavioral and Subjective Responses to Alcohol. Alcohol. Clin. Exp. Res 11, 52–59. 10.1111/j.1530-0277.1987.tb01263.x [DOI] [PubMed] [Google Scholar]
- Fillmore MT, 2001. Cognitive preoccupation with alcohol and binge drinking in college students: alcohol-induced priming of the motivation to drink. Psychol. Addict. Behav 15, 325–32. [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS, 1990. High Blood Alcohol Levels in Women: The Role of Decreased Gastric Alcohol Dehydrogenase Activity and First-Pass Metabolism. N. Engl. J. Med 322, 95–99. 10.1056/NEJM199001113220205 [DOI] [PubMed] [Google Scholar]
- Gabbay FH, 2003. Variations in affect following amphetamine and placebo: markers of stimulant drug preference. Exp. Clin. Psychopharmacol 11, 91–101. 10.1037//1064-1297.11.1.91 [DOI] [PubMed] [Google Scholar]
- Hedeker DR, Gibbons RD, 2006. Longitudinal data analysis. Wiley-Interscience. [Google Scholar]
- Holdstock L, Wit H, 2001. Individual Differences in Responses to Ethanol and d-Amphetamine: A Within-Subject Study. Alcohol. Clin. Exp. Res 25, 540–548. 10.1111/j.1530-0277.2001.tb02248.x [DOI] [PubMed] [Google Scholar]
- Johanson CE, Kilgore K, Uhlenhuth EH, 1983. Assessment of dependence potential of drugs in humans using multiple indices. Psychopharmacology (Berl). 81, 144–149. 10.1007/BF00429009 [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Zea-Ponce Y, Abi-Dargham A, Rodenhiser J, Wang T, Weiss R, Van Heertum RL, Mann JJ, Laurelle M, 1999. Stability of [123I]IBZM SPECT measurement of amphetamine-induced striatal dopamine release in humans. Synapse 31, 302–308. [DOI] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D, 2016. A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol. Psychiatry 79, 489–498. 10.1016/j.biopsych.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Johanson CE, De Wit H, 2013. Personality and the acute subjective effects of d-amphetamine in humans. J. Psychopharmacol 27, 256–264. 10.1177/0269881112472564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz JA, Childs E, 2017. Test–retest reliability of the underlying latent factor structure of alcohol subjective response. Psychopharmacology (Berl). 234, 1209–1216. 10.1007/s00213-017-4535-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, Laurent CL, De Wit H, 1996. Interaction of expectancy and the pharmacological effects of d-amphetamine: Subjective effects and self-administration. Psychopharmacology (Berl). 125, 371–378. 10.1007/BF02246020 [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M, 1997. Alcohol and the ability to inhibit behavior in men and women. J. Stud. Alcohol 58, 600–605. 10.15288/jsa.1997.58.600 [DOI] [PubMed] [Google Scholar]
- Mundt JC, Perrine MW, Searles JS, 1997. Individual differences in alcohol responsivity: Physiological, psychomotor and subjective response domains. J. Stud. Alcohol 58, 130–140. 10.15288/jsa.1997.58.130 [DOI] [PubMed] [Google Scholar]
- Narendran R, Himes M, Mason NS, 2013. Reproducibility of Post-Amphetamine [11C]FLB 457 Binding to Cortical D2/3 Receptors. PLoS One 8, 1–6. 10.1371/journal.pone.0076905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 1993. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Schuckit M, Smith T, 1996. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry 53, 202–210. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P, 1991. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav. Pharmacol 2, 65–71. [PubMed] [Google Scholar]
- Sutker PB, Tabakoff B, Goist KC, Randall CL, 1983. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol. Biochem. Behav 18, 349–354. 10.1016/0091-3057(83)90198-3 [DOI] [PubMed] [Google Scholar]
- Uhlenhuth EH, Johanson CE, Kilgore K, Kobasa SC, 1981. Drug preference and mood in humans: Preference for d-amphetamine and subject characteristics. Psychopharmacology (Berl). 74, 191–194. 10.1007/BF00432692 [DOI] [PubMed] [Google Scholar]
- Weafer J, Phan KL, de Wit H, 2019. Poor inhibitory control is associated with greater stimulation and less sedation following alcohol. Psychopharmacology (Berl). 10.1007/s00213-019-05420-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Lott DC, De Wit H, 2006. Personality and the subjective effects of acute amphetamine in healthy volunteers. Neuropsychopharmacology 31, 1064–1074. 10.1038/sj.npp.1300939 [DOI] [PubMed] [Google Scholar]
- Wilson JR, Nagoshi GT, 1987. One-month repeatability of alcohol metabolism, sensitivity and acute tolerance. J. Stud. Alcohol 48, 437–442. 10.15288/jsa.1987.48.437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


