Abstract
Early after pediatric cardiac arrest, families and care providers struggle with the uncertainty of long-term neurologic prognosis. Cardiac arrest characteristics such as location, intra-arrest factors and post-arrest events have been associated with outcome. We paid particular attention to post-arrest modalities that have been shown to predict neurologic outcome. These modalities include neurologic examination, somatosensory evoked potentials (SEPs), electroencephalogram (EEG), and neuroimaging. There is no one modality that accurately predicts neurologic prognosis. Thus, a multi-modal approach should be undertaken by both neurologists and intensivists to present a clear and consistent message to families. Methods used for the prediction of long-term neurologic prognosis need to be specific to prevent false positives in identifying poor outcome. Evidence is discussed that evaluated children with coma, each with various etiologies of cardiac arrest, outcome measures, and timing of follow-up.
Keywords: Pediatric cardiac arrest, prognostication, SEP, EEG, MRI
Introduction:
Pediatric cardiac arrest survivors are a heterogeneous population with substantial variation in outcomes. Neurologic injury is a leading cause of morbidity and mortality in this patient population. Determining long-term neurologic prognosis early after cardiac arrest is a challenge frequently encountered by clinicians. Multiple modalities have been reported to assist clinicians in determining prognosis. However, studies evaluating these modalities have utilized various pediatric populations with neurologic injury and have heterogeneous outcome measures and time points. As our ability to predict these longer-term outcomes is limited, information care providers relay to family members is cautious. Short-term outcomes and tools that are commonly employed to prognosticate neurologic outcome are discussed in detail below. Modalities described include neurologic examination, somatosensory evoked potentials (SEPs), electroencephalogram (EEG), head computed tomography (HCT), and magnetic resonance imaging (MRI).
Neurologic outcome measures
The definition of a meaningful recovery or quality of life after cardiac arrest will vary across individual families. Having an outcome measure that includes level of recovery and mortality is paramount when reviewing literature on prediction of prognosis. In addition, incorporating a broad definition of poor outcome is important in capturing different meaningful recoveries across cultures and individuals. Outcomes presented in the pediatric cardiac arrest literature range from survival to discharge, specific scales at discharge to more functional outcomes (Table). One of the most common scales to assess level of consciousness is the Glasgow Coma Scale (GCS). This 15-point practical scale has been widely used since Teasdale and Jennett created it, as it has an ease of use across specialties and disciplines. 1 Similarly, the Glasgow Outcome Scale (GOS) is a simple to use measure that includes good recovery (1), moderate disability (2), severe disability (3), vegetative state (4) and death (5). This outcome scale encompasses many features of the scales to follow, in that it rates level of consciousness and independence in activities of daily living. 2 However, this scale does not account for developmental age variability nor neurobehavioral outcomes.
Table :
Pediatric post-arrest modalities with evidence to predict poor outcome
| Modality | Reference | Predictors of Poor Outcomes |
Study Type, N | Poor Outcome Measure |
Timeframe of Outcome |
Predictive Value or Association with Outcome |
|---|---|---|---|---|---|---|
| Exam | Bratton et al 1994 26 | No purposeful movements at 24 hours | Retrospective, 44 children | Severe disability or death | 6 months | PPV 100% |
| Mandel et al 2002 27 | Absence of spontaneous respirations at 24 hours, GCS <5 at 24 hours, absence of pupil reactivity at 24 hours | Prospective, 42 children | PCPC 4-6 | 3 years | PPV 100%, 100%, 100% respectively | |
| Carter et al 2005 28 | Absence of pupil reactivity at 2 days | Prospective, 36 children | GOS 1-3 | 5 years | PPV 50% | |
| Abend et al 2012 3 | Absence of pupil reactivity at 24 hours | Prospective, 35 children | PCPC 4-6 | Hospital discharge | PPV 100% | |
| SEP | Zandbergen et al 1998 41 | No early cortical SEP within 1 week of ROSC | Systematic review | PVS or death | N/A | +LR 12 |
| Carter et al 1999 40 | Bilateral absent N20 | Prospective, 105 children | GOS 1-3 | 5 years | PPV 91% | |
| Carter et al 2005 28 | Bilateral absent N20 | Prospective, 36 children | GOS 1-3 | 5 years | Specificity 100% | |
| EEG | Nishisaki et al 2007 47 | Discontinuous/isoelectric | Retrospective, 33 children | Change in PCPC >1 or death | Hospital discharge | PPV 90% |
| Kessler et al 2011 49 | Unreactive and continuous or discontinuous/lack of cerebral activity | Prospective, 35 children | PCPC 4-6 | PICU discharge | PPV 91% | |
| Topjian et al 2013 45 | Status epilepticus | Prospective, 200 children | Worsened PCPC score from baseline or death | PICU discharge | OR 17.3 | |
| Ostendorf et al 2016 44 | Early seizures, myoclonic status epilepticus, burst-suppression or suppression | Retrospective, 73 children | PCPC 4-6 or change of PCPC by > 1 from baseline | Hospital discharge | P=0.05, P=0.17, PPV 100% respectively | |
| Topjian et al 2016 48 | Discontinuous/burst-suppression, voltage attenuation | Retrospective, 128 children | PCPC 3-6 or change of PCPC by > 1 from baseline | Hospital discharge | PPV 82% | |
| Fung et al 2019 46 | Model of EEG background, stage 2 sleep, variability, reactivity | Prospective, 89 children | PCPC 4-6 | PICU discharge | PPV 86% | |
| Imaging | Dubowitz et al 1998 59 | MRI T2: focal or generalized hyperintensity , abnormal basal ganglia, abnormal cortex | Retrospective, 22 children | CPC 3-5 | Undefined | PPV 100%, 100%, 100% respectively |
| Christophe et al 2002 60 | Abnormal MRI | Retrospective, 40 children | Developmental outcomes | 1 month | PPV 82% | |
| Rafaat et al 2008 57 | Abnormal repeat HCT | Retrospective, 101 children | GOS 1-2 | 6 months | 96% (23/24 patients) | |
| Oualha et al 2013 61 | Abnormal MRI DWI/ADC, cortical edema, basal ganglia edema, cerebellar edema | Retrospective, 20 children | PCPC 4-6 | Undefined | PPV 53%, P=0.02, P=0.0005, P=0.05 respectively | |
| Fink et al 2013 62 | MRI T2: basal ganglia injury | Retrospective, 28 children | GOS 1-3 | Hospital discharge | PPV 90% | |
| Starling et al 2015 58 | HCT less than 24 hours with diffuse loss of GWD, basilar cistern effacement, sulcal effacement | Retrospective, 78 children | PCPC 4-6 or change from baseline ≥1 | Hospital discharge | PPV 91%, 93%, 100% respectively | |
| Manchester et al 2016 63 | Global decrease MRI ADC | Retrospective, 14 children | PCPC 4-6 | Hospital discharge | P=0.02 | |
| Yacoub et al 2019 64 | Global MRI ADC threshold | Retrospective, 26 children | PCPC 3-6 or worsening from baseline | 6 months | PPV 100% |
Legend: GWD=grey white differentiation; HCT=head computed tomography; MRI=magnetic resonance imaging; DWI=diffusion-weighted imaging; ADC= apparent diffusion coefficient; EEG= electroencephalogram; PPV=positive predictive value; +LR=positive likelihood ratio; GCS=Glasgow Coma Scale; SEP=somatosensory evoked potentials; PVS=persistent vegetative state; ROSC=return of spontaneous circulation; PICU=pediatric intensive care unit; N/A= not applicable.
Outcome scales are as follows: Pediatric Cerebral Performance Category (PCPC) 1=normal, 2=mild disability, 3=moderate disability, 4=severe disability, 5=coma or vegetative state, 6=brain death 4 ; Glasgow Outcome Scale (GOS) 1=dead, 2= vegetative state, 3=severe disability, 4=moderate disability, 5=good recovery 2 ; Cerebral Performance Category (CPC) 1=good with mild deficit, 2=moderate disability, 3=severe disability, 4=coma or vegetative state, 5=brain death. 2
Outcome measures are likely enhanced when unfavorable outcome is more precisely defined rather than just survival. 3 Unfavorable outcomes in the literature are most commonly defined as Pediatric Cerebral Performance Category (PCPC) 3-6 or moderate disability, severe disability, coma/vegetative state or brain death. 4 In Slomine et al, they incorporated two definitions of favorable outcome with the first being PCPC 1 (normal) or 2 (mild disability) and the second being PCPC 1-3 and compared each to Vineland Adaptive Behavior Scales, Second Edition (VABS-II) twelve-month outcomes. The accuracy of both classification groups were similar. 5 Whether to include moderate disability after pediatric cardiac arrest in a dichotomized favorable outcome category is debatable. A PCPC of 3 translates to a child attending a special education classroom with or without a learning deficit but able to perform independent activities of daily living. 4 It is important to note that families’ perceptions of good neurologic outcomes may not be aligned with clinical prediction models utilizing PCPC 1-3 for favorable outcomes. Thus, when communicating neurologic findings and prognosis to family members at the bedside, clinicians should be clear about the details of a poor neurologic outcome.
PCPC and Pediatric Overall Performance Category (POPC) both have very good interrater reliabilities (r=0.88-0.96) and have been validated for short-term outcomes when prospectively studied in all children admitted to a pediatric intensive care unit (PICU) in a 1 year period. 4 Both the PCPC and POPC were created to identify short-term functional morbidity and the POPC was designed to capture overall health status in addition to neurologic outcome. Both PCPC and POPC corresponded to other reliable measures of short-term morbidity: length of PICU stay, total hospital costs, discharge care needs, and Pediatric Risk of Mortality Score (PRISM). In comparison to the similar Cerebral Performance Category (CPC), PCPC has an additional category to capture mild disability. 2,4,6 Intelligent, adaptive, and developmental quotients all decreased with increasing PCPC scores, which provides further evidence that both PCPC and POPC are validated assessments when more detailed ones are not feasible. 6 The ease of obtaining PCPC and POPC is a tradeoff for the limitation of using these measures across younger age groups as it limits detailed behavioral outcomes. Even though originally developed and validated in 1,469 patients with median age of 3.1 years, scoring children less than schoolage on the PCPC requires some amount of estimation, especially when discriminating between severe and moderate disability. 4 This task becomes even more challenging when deciphering through charts in a retrospective observational study. Lack of precision at the higher severity of disability in the PCPC has been shown in comparison to the Functional Status Scale (FSS) and VABS-II. 6,7 Despite its validation with functional short-term outcomes in children, PCPC and POPC remain rudimentary measures, and have not been age-validated.
A more robust assessment of functional status is VABS-II, which is an age-corrected score of 4 functional domains (mean 100, SD 15). 8 Weaknesses of VABS-II include that it is a caregiver report and it may be less sensitive in assessing younger aged patients. 6 Slomine et al showed that 3-month and 12-month VABS-II assessments after pediatric cardiac arrest were strongly correlated, although this correlation was weaker in the less than 1 year of age cohort (r=0.88 in out-of-hospital cardiac arrest and r=0.66 in in-hospital cardiac arrest). 5
Specific to cardiac arrest, Pediatric Resuscitation after Cardiac Arrest (PRCA) was developed. 9 PRCA was adapted from the Pediatric Stroke Outcome Measure (PSOM), while incorporating a broader range of functional areas including focal exam features, language, behavior and cognition. Scores range from 0-21 with 21 representing maximal deficit. VABS-II and cognitive scores were strongly correlated with PRCA category (r=−0.88, P<0.0001 and r=−0.72, P<0.0001, respectively). 9 Strengths of the PRCA scale include having a global score with the ability to localize deficits as well as two different versions based on age group. Weaknesses of the PRCA scale include requiring a neurologist to perform a detailed examination and the subjective nature of grading the severity of function.
Functional Status Scale (FSS) is an outcome measure designed for hospitalized pediatric patients that assesses six functional domains: mental status, sensory functioning, communication, motor functioning, feeding and respiratory status. Each domain is scored from a 1 to 5-point scale. FSS was validated against the Adaptive Behavior Assessment System II in 836 children across 7 institutions. 10 Strengths of the FSS include incorporating activities of daily living as well as motor function and mental status. As compared to other measures, FSS is more objective.
In summary, there are a wide variety of outcome measures that are used to study neuroprognostication tools after pediatric cardiac arrest. Guidelines for uniform reporting of pediatric advance life support recommend reporting outcome measures that are “ageappropriate” and “validated in children.” 11 Furthermore, a discussion of any observational cardiac arrest study will carry with it the limitation of self-fulfilling prophecy, where the clinical team has used the information being studied in the research setting to withdraw technological support prematurely, thus leading to a bias of unfavorable neurologic outcomes. One of the leading causes of death for this patient population is withdrawal of life-sustaining therapies (WLST) for poor neurologic prognosis. This has been demonstrated in the Therapeutic Hypothermia after Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) trial with 42% of deaths attributed to WLST. 12 This is vitally important to bear in mind when applying this data to individual patients at the bedside. 13,14
Epidemiology of pediatric cardiac arrest
Pediatric cardiac arrest has an incidence of 0.7-2% of all hospital admissions and 1-5.5% of all PICU admissions. 15,16,17,18 Out-of-hospital pediatric cardiac arrest (OHCA) has an incidence of 8.04 per 100,000 child person-years. 19 Neurologic outcomes after pediatric cardiac arrest can depend on location with in-hospital cardiac arrest (IHCA) favorable outcomes, defined as PCPC 1-2 at hospital discharge or no change from baseline, ranging from 47-69% and OHCA 24-35%. 9,20 In a cohort of pediatric cardiac arrest patients with GCS motor scores less than 5, a multivariate model of outcome prediction evaluating 12-month outcomes with VABS-II demonstrated cardiac arrest characteristics alone were weak predictors of long-term outcome (R2=0.21). In comparison, a model that also incorporated post-cardiac arrest factors such as seizures, length of hospital stay and neurologic disability at hospital discharge was superior in predicting 12 month outcomes (R2=0.63). 9
Survival after pediatric cardiac arrest has increased over time. Girotra et al examined trends of survival to discharge after return of spontaneous circulation (ROSC) from IHCA in 1031 children across 12 hospitals over a 9-year period. This population included patients with arrests that occurred in the neonatal intensive care unit and excluded patients with arrests that occurred in the operating room or the emergency department (ED). Over this time period, risk-adjusted survival rates to hospital discharge increased from 14% to 43.4% (P=0.02). A similar increase in survival was seen across all age groups, sex, and initial cardiac arrest rhythm. This overall increase in survival was attributed to improved survival during the acute resuscitation period, defined as ROSC for at least 20 minutes, as this increased from 42.9% to 81.2% (P=0.006). There was no significant increase in survivors with neurologic disability (PCPC 4-6) over this time period. 21 Thus, an increase in cardiac arrest survivors secondary to improved resuscitative measures without increasing the number of patients with severe neurologic disability is encouraging to continue research and quality improvement measures in both intra-arrest and post-arrest factors to improve survival rates and decrease neurologic disability.
In addition to location, etiology of arrest has correlated with outcome. Respiratory etiology of arrest (as compared to cardiac etiology) and longer duration of chest compressions were associated with poor one year outcomes in a prospective cohort of 179 pediatric IHCA and OHCA survivors. 9 Furthermore, in 329 children enrolled in the prospective Therapeutic Hypothermia after Pediatric Cardiac Arrest In-Hospital (THAPCA-IH) trial, many cardiac arrest factors were shown to be associated with survival and neuropsychological outcomes (VABS-II) after 12 months. Etiology of cardiac arrest did not correlate with outcomes in this specific cohort. Asystole as an initial rhythm (OR 0.09, 95% CI 0.02,0.43, P=0.002), higher post-arrest blood lactate concentration (OR 0.94, 95% CI 0.89,0.98, P=0.008), and more than four doses of epinephrine (OR 0.52, 95% CI 0.30,0.92, P=0.026) were independently associated with lower 12-month survival in a logistic regression model. Requiring extracorporal membrane oxygenation (ECMO) (OR 0.52, 95% CI 0.29,0.94, P=0.030) and higher post-arrest blood lactate concentration (OR 0.92, 95% CI 0.88,0.97, P=0.003) were independently associated with a 15-point decrease in VABS-II from pre-arrest functioning. 22 Performing open chest compressions, typically in patients already with sternotomy, was independently associated with a greater 12-month survival (OR 2.21, 95% CI 1.12,4.34, P=0.022). 22
In a single institution retrospective study of 101 children after OHCA, cardiac arrest factors during the acute resuscitative period either before arrival or in the ED were demonstrated to be useful in predicting outcomes. In a multiple logistic regression model, patients who had basic or no cardiopulmonary resuscitation (CPR) pre-hospital (OR 0.032; 95% CI 0.004,0.25; P<0.001) and a shorter duration of resuscitation (OR 0.058; 95% CI 0.010,0.33; P=0.001) were independently associated with survival to hospital discharge. In this cohort, requiring pre-hospital advanced CPR is associated with a longer interval between arrest and arriving at the hospital, accounting for the predictive value of basic or no CPR during pre-hospital care. 23
Age may be a further independent factor associated with outcome, as suggested by Meaney et al who evaluated 464 children after IHCA in four age groups: less than 1 month (newborn), 1 month to 1 year (infants), 1 year to 8 years, 8 years to 21 years. When adjusting for arrest confounding factors, newborns and infants have almost a fivefold and threefold odds of surviving to discharge (OR 4.9, 3.1, respectively) compared with the older aged groups. There was no significant difference in favorable outcome (PCPC 1-3) of survivors between age groups. 24 In summary, cardiac arrest characteristics that have independent merit in predicting poor outcomes after pediatric cardiac arrest include: OHCA, initial rhythm of asystole, higher post-arrest blood lactate, more than four doses of epinephrine, and requiring ECMO.
Modalities of neuroprognostication (Table)
Neurologic examination
A comatose state is one in which a person cannot be woken with adequate stimulation. 25 Factors such as medications and nonconvulsive status epilepticus can cloud an examination and contribute to this state. As such, it is necessary to consider confounders when examining a comatose child after cardiac arrest. Frequent neurologic examinations are essential to determine level of arousal, localization and extent of injury.
A retrospective study of serial neurologic examinations evaluating 44 children with abnormal mental status after a near-drowning event described that examinations 24 hours after injury correlated with outcome assessments at a minimum of 6-month follow-up. Specifically, a good outcome (mild or no deficits) was associated with purposeful movements (sensitivity 100%, specificity 100%, positive predictive value 100%, and negative predictive value 100%). All children without purposeful movements had a poor outcome (death or severe deficits). 12% had WLST for poor neurologic prognosis on day 4 or 5 after injury. Limited univariate analysis and no multivariate analysis were reported. 26 In a prospective cohort, 42 comatose children after a nontraumatic cardiac arrest were serially evaluated for outcome predictors, with outcomes assessed at three years after injury. GCS, pupillary responses, and spontaneous respirations at admission were not predictive. Absence of spontaneous respirations at 24 hours and GCS less than 5 at 24 hours after admission were associated with poor outcome of PCPC 4-6 (positive predictive value 100%, 100%, sensitivity 27%, 54%, specificity 100%, 100% respectively). Furthermore, absence of pupillary responses at 24 hours had positive predictive value (PPV) 100%, sensitivity 35% and specificity 100% towards prediction of poor outcome. 27 Carter et al serially evaluated 36 non-traumatic comatose children to determine the prediction power of motor and pupillary responses towards outcome at 5 years post-injury using GOS. Etiologies of coma for this patient population were not explicitly stated. Bilateral absence of motor responses after 2 days post-injury had specificity of 25% for unfavorable outcomes (severely disabled, vegetative state, or death) with sensitivity 33%, PPV 50% and negative predictive value (NPV) 14%. Comparatively, bilateral absence of pupillary responses after 2 days post-injury had specificity for poor outcomes of 82% (sensitivity 25%, PPV 50% and NPV 61%). 28 Based on the evidence provided above, relying on admission GCS, motor responses, pupillary responses or spontaneous respirations for prognosis is not advised.
Using simple exam maneuvers of testing for pupillary light responses and stimulation testing motor movements, Abend et al in 2012 prospectively showed in pediatric survivors after cardiac arrest treated with therapeutic hypothermia, once patients were normothermic for 24 hours, absent motor and pupillary responses were predictive of unfavorable outcomes (PCPC 4-6) with PPV 100%. 3 Excluding children who received paralytics improved the PPV of absent motor and pupillary responses for unfavorable outcomes at time points closer to ROSC and during or shortly after therapeutic hypothermia. 3
A study from 2014 addressed accuracy of physicians in predicting neurologic outcome after pediatric cardiac arrest at different time points in the clinical course. There was no difference in accuracy between pediatric neurologists or intensivists. Furthermore, predictive accuracy improved over time. The most influential factor when physicians scored outcomes was the exam at day 5-7. Other important factors included brain MRI and EEG for neurologists and cardiac arrest factors and brain MRI for intensivists. 29
Specifically in the adult literature, absent pupillary responses, absent corneal reflexes and no motor responses to pain or motor extension 3 days after cardiac arrest have been predictive of poor neurologic outcomes. 30,31 Greer et al prospectively studied 200 comatose cardiac arrest adult patients with outcomes assessed at 6 months by Modified Ranken Scale with poor outcome scored as moderate, severe disability or death. At 6 months, absence of pupillary and corneal reflexes on Day 3 proved reliable for predicting poor outcomes (sensitivity 24%, 49%, specificity 100%,100% respectively). For patients with extensor or no motor responses, specificity for poor outcome was 70% with sensitivity 81%. 32 For comatose adults, American Heart Association (AHA) 2015 guidelines recommend that the earliest time to prognosticate via examination is 72 hours after ROSC, in patients not treated with targeted temperature management (TTM). In those treated with TTM, timing of prognostication examination may be 72 hours after normothermia is achieved. 33
In summary, although the examination can be confounded, if performed later in a child’s hospital course it can be helpful with prognostication of poor outcome, especially when assessing simple exam maneuvers such as pupil reactivity, respiratory drive, and motor responses. However, the weaknesses of the studies above are apparent in that they are either retrospective or small prospective cohorts with a fair proportion of patients with poor outcome having WLST and with rare multivariate analyses, thus not accounting for sedation effects. The AHA 2019 Scientific Statement on pediatric cardiac arrest recommends that early examination after ROSC be interpreted for prognostication with caution and that the predictive ability improves with serial examinations and time. 34
Somatosensory evoked potentials
There is a large body of literature in both pediatric and adults evaluating SEPs and outcome prediction after severe brain injury. SEPs can be used to test the response in the somatosensory cortex to peripheral sensory stimulation, thus assessing integrity of the afferent pathway to the cortex, expressed via the N20 peak. In contrast with examination and EEG, certain heavy sedation and muscle relaxants such as phenobarbital, midazolam, pentobarbital, thiopental and fentanyl have been shown to not confound results in a clinically significant manner. 35-39 Similar to the physical examination, SEPs are noninvasive and able to be done at the bedside.
In a prospective outcome study of 105 comatose children after severe traumatic and non-traumatic brain injury, outcomes at five years were assessed using GOS with favorable outcomes defined as normal, mild or moderate disability. Children with bilateral absent SEPs had poor outcomes with sensitivity 61%, specificity 95%, PPV 91% and NPV 74%. This demonstrates a fairly good predictive ability of bilateral absent SEPs, although in a cohort with a wide array of coma etiologies. 40 In a systematic review of patients older than 10 years of age (including adult patients) fourteen variables of either examination or electrophysiological studies were evaluated for predictors of poor neurologic outcome (death or vegetative state) in patients with hypoxic-ischemic coma, excluding traumatic causes. Three prognostic indices had a specificity of 100%: absence of pupillary responses on day 3, absent motor responses to pain on day 3 and bilateral absence of early cortical SEPs within the first week after injury. SEPs had a pooled positive likelihood ratio for poor outcome of 12 and had the lowest 95% CI of pooled false-positive test rates: 0-2%. Although including adults, this study compares SEPs with other reliable predictors, which demonstrates at least comparable predictive value with motor and pupillary responses. 41 In the prospective Carter et al study described above, serial evaluations were undertaken during the first 9 days after injury. Bilateral absent SEPs were greater than or equal to the predictive power of the last motor examination for unfavorable outcome at 5 years using GOS (sensitivity 90%, 93%, specificity 100%, 50% respectively). This study’s strengths included utilizing measured outcomes 5 years after brain injury and demonstrating at least comparable predictive abilities to motor responses. 28
The predictive power of SEPs in comatose adult patients after cardiac arrest show similar results. In 66 comatose adult patients after cardiac arrest, the predictive power of evoked potentials was determined based on outcomes at hospital discharge with favorable outcome measured using CPC for good or moderate disability. None of the 22 patients with loss of cortical N20 survived with specificity 100%, sensitivity 45%, PPV 100% and NPV 39% for poor outcomes. However, the presence of a N20 peak did not correlate with favorable outcome in this cohort. Sedatives (benzodiazepines and opioids) were commented on in this study but not factored into assessing SEPs predictive value. 42 In a prospective study of 162 comatose patients, none of the 46 patients without bilateral N20 peaks survived with sensitivity 37%, specificity 100%, PPV 100%, NPV 31% for poor outcome (severe disability, vegetative state or death). 43 Furthermore, the American Academy of Neurology Practice Parameter in 2006 and the 2015 AHA guideline update support accurate prediction of poor outcome in adult survivors after cardiac arrest with bilateral absence of cortical N20 peak when performed at least 24 hours after ROSC. 30,33
In conclusion, the studies presented above demonstrate that SEPs can be helpful in predicting poor outcome in comatose children after cardiac arrest. However, the lack of a homogeneous pediatric cardiac population evaluated in the studies presented is a concerning weakness. Similar to the neurologic examination, utilizing SEPs as a single modality for neurological prognostication after pediatric cardiac arrest is not recommended. Thus, the AHA 2019 Scientific Statement on pediatric cardiac arrest recommends the routine use of SEPs for neurological prognostication in children after cardiac arrest be done with extreme caution. 34
Electroencephalography
EEG is a non-invasive bedside test that requires skilled interpretation and knowledge of clinical factors for accurate interpretation and clinical correlation. Electrographic seizures, status epilepticus, and abnormal background on EEG after cardiac arrest have all been associated with poor neurologic outcomes. 44-50 Ostendorf et al retrospectively studied 73 children after cardiac arrest and found all 9 patients with seizures in the first hour of recording (P=0.05) and all 6 patients with myoclonic status epilepticus had poor outcomes (P=0.17), defined by PCPC 4-6 or increase in PCPC by more than 1 from admission to hospital discharge. 44 In a prospective study of 200 children who underwent EEG monitoring for acute encephalopathy, of which 50 had hypoxic ischemic encephalopathy (HIE), 21.5% (43/200) had status epilepticus. Status epilepticus was associated with an increased risk of death (OR 5.1) and unfavorable neurologic outcome (OR 17.3). Of this cohort, 20.5% had electrographic seizures and this was not associated with worsened outcomes. The broad inclusion of etiologies of HIE exclude this study from direct application to post-arrest patients, but was helpful in demonstrating that status epilepticus and isolated seizures may have different influences on outcomes after acute brain injuries. 45 The utility of many EEG features including seizures and status epilepticus for prognostication were measured by Fung et al in a prospective observational single center study of 89 children admitted to an ICU post-cardiac arrest. Unfavorable outcomes were defined as PCPC scores 4-6 at discharge from the PICU. In this cohort, neither seizures nor status epilepticus were associated with poor neurologic outcomes (P = 0.44). 46 Thus, the use of seizures and/or status epilepticus to predict outcome after pediatric cardiac arrest remains uncertain based on current literature.
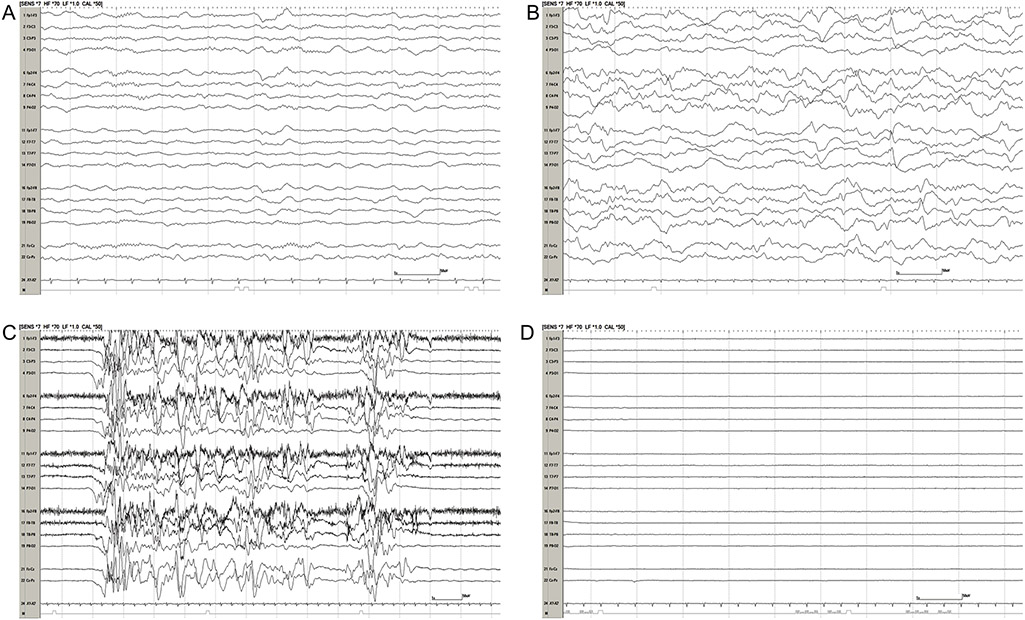
Besides these electrographic events, “malignant” background patterns (discontinuous or burst-suppression, or attenuated-featureless) have been shown to be associated with poor neurological outcomes after pediatric cardiac arrest. 44,46-49 In a retrospective cohort of 33 children with EEG monitoring performed within 7 days after IHCA (median 1 day, IQR 0-2 days), discontinuous or isoelectric EEG backgrounds had sensitivity 53%, specificity 94%, PPV 90% and NPV 65% for poor neurologic outcomes. Poor neurologic outcome was determined at hospital discharge and defined by an increase in PCPC of more than 1 from admission or death.47 This EEG background terminology has fairly high inter-rater agreement. In 74 EEG tracings of 37 children survivors after cardiac arrest, 30 minutes of hypothermia and 30 minutes of normothermia epochs were evaluated. High inter-rater agreements between neurophysiologists were found with patterns of burst suppression (kappa 0.73), continuity (kappa 0.69), sleep architecture (kappa 0.8) and overall impression score (kappa 0.65). Seizure occurrence, epileptiform discharges and beta activity had less agreement (kappa 0.46, 0.4, 0.38, respectively). 51 In the Ostendorf et al study described above, all 25 children with EEG backgrounds of burst-suppression or voltage suppression had poor outcomes with sensitivity 50%, specificity 100%, PPV 100%, and NPV 48%. When evaluated in a multivariate model, normal or slow EEG background within one hour of recording had OR of good outcome of 4.1 (95% CI 1.1,15.9, P=0.04). 44 In a retrospective cohort of 128 children with EEG monitoring after ROSC, outcomes were defined at hospital discharge as poor if PCPC 3-6 or more than 1 increase in PCPC from baseline. 82% of children with EEG backgrounds of discontinuous-burst suppression or voltage attenuation had unfavorable neurologic outcomes with sensitivity 74%, specificity 78%, PPV 82%, and NPV 69%. In a multivariate model with each worsening of EEG background category, the odds of death were 3.1 (95% CI 2.18,6.0, P<0.001) and odds of poor outcome were 4.4 (95% CI 2.51,7.17, P=0.001). In this cohort, 26% of patients died from WLST.48 In a prospective cohort of 35 children who underwent therapeutic hypothermia after cardiac arrest with EEG monitoring post-arrest, outcomes were assessed upon discharge from PICU with poor outcome defined as PCPC 4-6. During the normothermic period, EEG backgrounds of unreactive but continuous, or discontinuous/lack of cerebral activity were associated with poor outcomes (P=0.006, OR 27 or P=0.02, OR 18 respectively). EEG backgrounds that were continuous but unreactive, or discontinuous, burst suppressed or voltage attenuated during normothermia had sensitivity 83%, specificity 82%, PPV 91%, NPV 69% for poor neurologic outcomes. 49 Figure 1 displays examples of these background EEG categories.
Figure 1: Post-arrest EEG Monitoring.
(Two-column image): Four EEGs all within 1 day after cardiac arrest displayed in a longitudinal bipolar montage. All EEGs are displayed with the same sensitivity at 7 uV/mm. (A) Continuous background with symmetric sleep spindles; (B) slow-disorganized background; (C) discontinuous/burst-suppression background; (D) severe voltage attenuation. EEG = electroencephalogram.
In the Fung et al study described above, they also evaluated numerous EEG features in their cohort to find the combination that most accurately predicts outcome. EEG features analyzed included: background category, seizures, symmetry, frequency, continuity, voltage, stage 2 sleep transients, reactivity, and variability. The optimal model for determining poor neurologic outcome included: EEG background category, stage 2 sleep transients and variability and/or reactivity with AUC 0.78 (95% CI 0.68,0.87), specificity 95%, sensitivity 26%, PPV 86%, and NPV 55%. 46
In comparison to standard visual interpretation of EEG tracings, quantitative EEG (QEEG) technology allows for a computed measure from clinically obtained EEGs which may provide a more standardized, objective approach. Lee et al prospectively evaluated ability of QEEG features to predict outcomes in 69 children after cardiac arrest using a machine learning algorithm. They found the best model of 8 QEEG features predicted clinical outcomes with sensitivity 84%, specificity 75%, PPV 79% and NPV 80%. Unfavorable outcomes were defined as PCPC 4-6 at discharge from PICU. 52 While a promising tool, QEEG remains very much in the research stages.
Furthermore, the presence of sleep spindles has been suggested to correlate with favorable neurologic outcomes after pediatric cardiac arrest in a retrospective cohort study of 34 children. Ten children had spindles present in the first 24 hours of EEG monitoring with the first spindle observed at a median time of 12.2 hours. Of those 10, 8 had a favorable outcome (PCPC 1, 2) or no change from baseline 6 months post arrest (P=0.001). Presence of normal or abnormal spindles had sensitivity 80%, specificity 92%, PPV 80%, NPV 92% for a favorable outcome. Of 24 children with poor outcomes, 10 had mild or moderately abnormal EEG backgrounds with 8/10 having absent spindles. All 14 patients with poor outcome and severely abnormal EEG background had absent spindles. 50 Thus, considering spindles and whether they are present, absent or normal may be an underappreciated part of interpreting EEG tracings in assessing for outcome in cardiac arrest survivors.
In a recent prospective, multi-institutional study of 346 comatose adult patients after cardiac arrest, multiple modalities, including EEG, were evaluated within 24 hours for predictive power of poor neurological outcome. EEG background patterns were evaluated for continuity, epileptiform discharges, low-voltage, burst-suppression, suppression, and isoelectric. Malignant patterns were defined as isoelectric or burst-suppression. Outcomes were assessed at hospital discharge and at six months using CPC scale of vegetative state or brain death for poor outcomes. Malignant EEG patterns had sensitivity 25%, specificity 100%, PPV 100%, and NPV 46% for poor outcome at 6 months. This study has several strengths including a lack of WLST at study sites, early EEGs within 24 hours after ROSC, and functional outcomes measured at 6 months. 53 The 2015 AHA adult post-cardiac arrest guidelines cautiously supports using persistent absence of EEG reactivity at 72 hours after ROSC or re-warming, persistent status epilepticus with absence of reactivity, and/or burst-suppression at 72 hours after ROSC, along with other predictors to evaluate likelihood of poor outcome. 33
In conclusion, the evidence above supports using EEG as a post-arrest modality predictor of both favorable and unfavorable outcomes at PICU or hospital discharge. The majority of quality evidence supports using background EEG features for these outcome measures. The current pediatric literature is limited by study size and retrospective study design. In 2015, the American Clinical Neurophysiology Society published a consensus statement on indications for continuous EEG in critically ill children. In this statement, continuous EEG for 24 hours is recommended to identify nonconvulsive seizures or status epilepticus in critically ill children with altered mental status after acute brain injury post-cardiac arrest. In addition, evidence is provided to recommend assessing for seizures if a critically ill child is being treated with neuromuscular blockade and at risk for seizures. This statement also mentions that EEG can aid in prognosis in children with hypoxic-ischemic injury after cardiac arrest. 54 The AHA Pediatric Advanced Life Support 2015 guidelines state that EEG recordings done within 7 days after ROSC can be helpful in prognostication at time of hospital discharge but should not be the only modality used. 55
Imaging
Patterns of injury on neuroimaging performed after cardiac arrest have been shown to have predictive value. 56-64 Rafaat et al retrospectively described 101 comatose and non-comatose children after a near-drowning cardiac arrest with HCT imaging. Outcomes were measured by GOS at a minimum of 6 months after the events. All 28 patients with abnormal initial HCT did not survive. Twenty-four out of seventy-three children with normal initial CT had an abnormal repeat image and outcome was poor (vegetative state or death) in 96% (23/24) of these children. The majority of patients with poor outcome and abnormal HCT had injury patterns of diffuse loss of gray-white matter differentiation and hypodensities in the basal ganglia. The homogeneous population of pediatric cardiac arrest and 6 month outcomes are strengths of this study. 57 In a retrospective cohort study of 78 children after OHCA who had HCT performed within 24 hours after ROSC, children who died were significantly more likely to have loss of gray-white matter differentiation (PPV 91%), basilar cistern (PPV 93%) or sulcal effacement (PPV 100%). 58 Thus, early HCT findings of basal ganglia injury and edema resulting in loss of gray-white matter differentiation, sulcal and basilar cistern effacement have been associated with poor neurologic outcome after pediatric cardiac arrest (Figure 2).
Figure 2: Post-arrest Imaging.
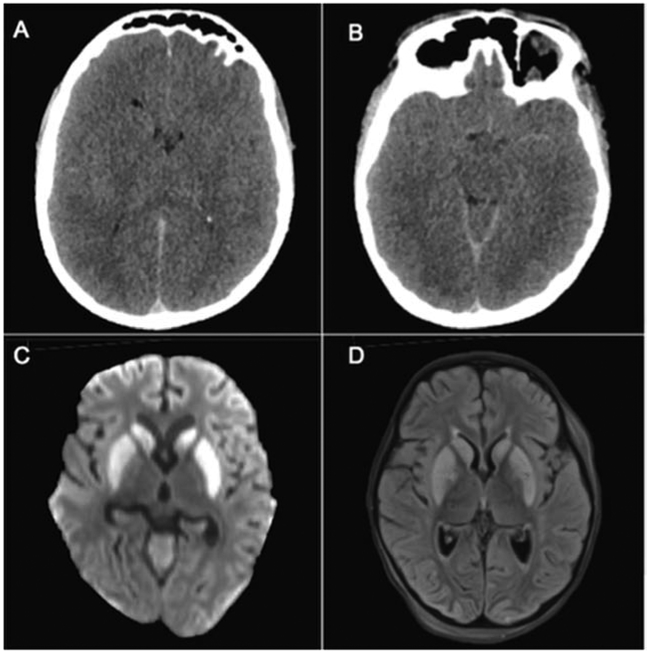
(Two-column image): (A,B) 16-year-old boy with HCT performed within 24 hours after cardiac arrest from respiratory arrest. HCT displays effacement of the sulci, occipital horns of the ventricles, and basilar cisterns, indicating diffuse cytotoxic edema secondary to hypoxic ischemic injury. (C,D) 3-year-old boy with MRI performed 2 days after cardiac arrest from respiratory arrest. DWI (C) and FLAIR (D) demonstrate diffusion abnormalities and hyperintensities in bilateral caudate and putamen consistent with hypoxic ischemic injury; HCT = head computed tomography; MRI = magnetic resonance imaging; DWI = diffusion weighted imaging; FLAIR = fluid-attenuated inversion recovery.
MRI and MR spectroscopy (MRS) findings across multiple time points after ROSC were described in a retrospective cohort study of 22 comatose children after a near-drowning cardiac arrest. In this study, imaging patterns that correlated with poor outcome (vegetative state or death) included focal or generalized T2 hyperintensity (sensitivity 94%, specificity 100%, PPV 100%, NPV 86%), abnormal T2 signal in the basal ganglia (sensitivity 100%, specificity 100%, PPV 100%, NPV 100%) and abnormal T2 signal in the cortex (sensitivity 69%, specificity 100%, PPV 100%, NPV 55%). In all 16 patients with poor outcomes MRS were abnormal. MRI and MRS after 3 days had the best correlation with outcome. 59 Christophe et al described an MRI scoring system in 40 comatose children after hypoxic injury and compared this to neurodevelopmental outcomes at a minimum of 1 month after discharge. Their non-validated neurodevelopmental outcome system assessed for motor and cognitive function, as well as presence of seizures. Abnormal MRI was established by assessing ischemic injury in T1 and T2 sequences in watershed and basal ganglia areas. Using these metrics, they found that having an abnormal MRI predicted poor outcome with sensitivity 96%, specificity 50%, PPV 82% and NPV 86%. 60
While hypoxic-ischemic injury patterns on MRI evolve with time, diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) sequences have a higher sensitivity for acute ischemic injury. 65,66 A retrospective study of 20 children described DWI/ADC MRI results within the first week after a cardiac arrest. Poor outcomes were delineated by PCPC 4-6 at an undefined follow-up timeframe. Fifteen children had abnormal MRIs and all 8 patients with poor outcome had abnormal DWI (sensitivity 100%, specificity 42%, PPV 53%, NPV 100%). Specific regions of abnormality associated with poor outcome included basal ganglia, cerebral cortex, and cerebellum (P=0.005, 0.02, 0.05 respectively). Notably, two children with favorable neurologic outcomes and normal DWI had EEG backgrounds of burst-suppression or attenuation or loss of reactivity and two children with poor neurologic outcomes and abnormal DWI had EEG with normal background patterns. This continues to support a multi-modal approach to neuroprognostication. 61 Similarly, Fink et al retrospectively looked at 28 children after cardiac arrest who underwent a MRI within 2 weeks after ROSC. Outcome was determined by GOS at hospital discharge with favorable outcome defined as good recovery or moderate disability. Basal ganglia lesions on T2 sequences predicted poor outcomes with sensitivity 64%, specificity 93%, PPV 90%, and NPV 72%. There was an overall association between lesions in multiple brain lobes and worse outcomes (P<0.01). Radiographic abnormalities on DWI sequences were significantly associated with poor outcomes, most frequently in the occipital, parietal and frontal lobes (P=0.02). 62
Manchester et al prospectively studied 14 children after cardiac arrest with MRIs performed within 2 weeks of injury to quantify regional cerebral blood flow and diffusion changes on MRI. Greater ADC values were found in the frontal lobes and the lowest (more diffusion restriction) were in the occipitoparietal cortex, thalamus, and putamen. Outcomes were determined via PCPC at hospital discharge with PCPC 4-6 classified as poor outcome. Children with poor outcomes significantly correlated with globally decreased ADC (P=0.02). Also, the following regions with decreased ADC correlated with increased cerebral blood flow, more frequently in children with poor outcomes: genu, caudate, putamen, occipital gray matter, substantia nigra, and middle cerebellar peduncles (r>0.6). 63 Recently, Yacoub et al retrospectively evaluated whole brain ADC values in 26 children after cardiac arrest. Primary outcome measured was PCPC 6 months after ROSC with poor outcome defined as PCPC 3-6 or worsening from baseline. MRIs were performed within 2 weeks after ROSC. They concluded that there are two global ADC thresholds both with specificity 100%, sensitivity 80%, PPV 100%, NPV 89% for poor outcome. It should be noted that there was a high rate of patients with WLST (60%) in this study. 64
In the adult cardiac arrest study by Scarpino et al described above, HCTs within 24 hours after ROSC were also obtained. Gray matter to white matter ratio threshold was established to represent severity of edema and had specificity of 100%, sensitivity 48%, and PPV 100% for poor outcome. 53 In a meta-analysis, 44 studies were systematically reviewed for predictive power of neuroimaging in adult cardiac arrest survivors. Reduction in gray-white ratio (GWR) on HCT had specificity 97%, sensitivity 44%, and positive likelihood ratio [LR+] 13.8 for predicting poor outcome. DWI sequence on MRI was also useful in predicting poor outcome with specificity 92%, sensitivity 77%, LR+ 9.2. Limitations of the studies included in the metaanalysis were inconsistent timing in obtaining images and variable outcome assessments with the most common being the CPC scale. 67 In the 2015 AHA guidelines for prediction of prognostication after adult cardiac arrest, both HCT and MRI are listed in their recommendations. Specifically, for HCT completed within 2 hours after ROSC in a patient without TTM, clinicians can use reduction in GWR to predict poor outcome. In addition, “extensive” diffusion restriction on MRI when done 2-6 days after ROSC can be used in combination with other modalities to predict unfavorable outcome. 33
In summary, MRI and HCT can be useful in prognostication after pediatric cardiac arrest. The current literature is limited by study size, variable timing of imaging, modalities of imaging, and inconsistency of the findings evaluated in each study. Further work should focus on comparison of regional injury patterns with whole brain analysis for prognostication. Trends in the literature above suggest that regional injury in the basal ganglia, deep gray and occipital lobes are associated with poor outcomes (Figure 2). The AHA 2019 Scientific Statement on pediatric cardiac arrest recommends HCT as a useful tool to identify treatable intracranial injury but evidence is insufficient to support use for prognostication, whereas brain MRI with DWI sequences done in the first 3-7 days after ROSC may be useful to supplement other prognostication criterion. 34
Conclusion:
Optimal prediction of neurological outcomes after pediatric cardiac arrest requires a multi-modal approach incorporating the available data including cardiac arrest characteristics, neurological examination at least 24 hours from ROSC or normothermia, EEG background features, SEP responses and neuroimaging. Advanced techniques including quantitative measures on EEG and MRI may provide new objective data from modalities that have been measured qualitatively previously. This review also highlights the lack of standardization in outcome measures and timing of assessments which will be essential for moving the field of pediatric cardiac arrest forward.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [R01 NS097721] (SHF).
Footnotes
Declaration of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Teasdale G, Jennett B. ASSESSMENT OF COMA AND IMPAIRED CONSCIOUSNESS: A Practical Scale. The Lancet. 1974;304(7872):81–84. doi: 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 2.Jennett B, Bond M. ASSESSMENT OF OUTCOME AFTER SEVERE BRAIN DAMAGE: A Practical Scale. The Lancet. 1975;305(7905):480–484. doi: 10.1016/S0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 3.Abend NS, Topjian AA, Kessler SK, et al. Outcome prediction by motor and pupillary responses in children treated with therapeutic hypothermia after cardiac arrest*: Pediatr Crit Care Med. 2012;13(1):32–38. doi: 10.1097/PCC.0b013e3182196a7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. doi: 10.1016/S0022-3476(05)82544-2 [DOI] [PubMed] [Google Scholar]
- 5.Slomine BS, Silverstein FS, Page K, et al. Relationships between three and twelve month outcomes in children enrolled in the therapeutic hypothermia after pediatric cardiac arrest trials. Resuscitation. March 2019. doi: 10.1016/j.resuscitation.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 6.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of Pediatric Overall Performance Category and Pediatric Cerebral Performance Category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments: Crit Care Med. 2000;28(7):2616–2620. doi: 10.1097/00003246-200007000-00072 [DOI] [PubMed] [Google Scholar]
- 7.Pollack MM, Holubkov R, Funai T, et al. Relationship Between the Functional Status Scale and the Pediatric Overall Performance Category and Pediatric Cerebral Performance Category Scales. JAMA Pediatr. 2014;168(7):671. doi: 10.1001/jamapediatrics.2013.5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparrow S, Balla D. Vineland Adaptive Behavior Scales: Survey Forms Manual. 2nd Ed. 2005. [Google Scholar]
- 9.Ichord R, Silverstein FS, Slomine BS, et al. Neurologic outcomes in pediatric cardiac arrest survivors enrolled in the THAPCA trials. Neurology. 2018;91(2):e123–e131. doi: 10.1212/WNL.0000000000005773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: New Pediatric Outcome Measure. PEDIATRICS. 2009;124(1):e18–e28. doi: 10.1542/peds.2008-1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: The Pediatric Utstein Style: A statement for Healthcare Professionals from a Task Force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Resuscitation. 1995;30(2):95–115. doi: 10.1016/0300-9572(95)00884-V [DOI] [PubMed] [Google Scholar]
- 12.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic Hypothermia after Out-of-Hospital Cardiac Arrest in Children. N Engl J Med. 2015;372(20):1898–1908. doi: 10.1056/NEJMoa1411480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wartenberg KE, Hwang DY, Haeusler KG, et al. Gap Analysis Regarding Prognostication in Neurocritical Care: A Joint Statement from the German Neurocritical Care Society and the Neurocritical Care Society. Neurocrit Care. 2019;31(2):231–244. doi: 10.1007/s12028-019-00769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou SE, Maciel CB, Ormseth CH, Beekman R, Gilmore EJ, Greer DM. Distinct predictive values of current neuroprognostic guidelines in post-cardiac arrest patients. Resuscitation. 2019;139:343–350. doi : 10.1016/j.resuscitation.2019.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suominen P, Olkkola KT, Voipio V, Korpela R, Palo R, Räsänen J. Utstein style reporting of in-hospital paediatric cardiopulmonary resuscitation. Resuscitation. 2000;45(1):17–25. doi: 10.1016/S0300-9572(00)00167-2 [DOI] [PubMed] [Google Scholar]
- 16.Reis AG, Nadkarni V, Perondi MB, Grisi S, Berg RA. A Prospective Investigation Into the Epidemiology of In-Hospital Pediatric Cardiopulmonary Resuscitation Using the International Utstein Reporting Style. PEDIATRICS. 2002;109(2):200–209. doi: 10.1542/peds.109.2.200 [DOI] [PubMed] [Google Scholar]
- 17.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and Outcomes of Cardiopulmonary Resuscitation in Pediatric Intensive Care Units. Crit Care Med. 2016;44(4):798–808. doi: 10.1097/CCM.0000000000001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slonim AD, Patel KM, Ruttimann UE, Pollack MM. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med. 1997;25(12):1951–1955. [DOI] [PubMed] [Google Scholar]
- 19.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and Outcomes From Out-of-Hospital Cardiac Arrest in Children: The Resuscitation Outcomes Consortium Epistry–Cardiac Arrest. Circulation. 2009;119(11):1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: A multicenter cohort study*: Crit Care Med. 2009;37(7):2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS. Survival Trends in Pediatric In Hospital Cardiac Arrests: An Analysis from Get With The Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6(1):42–49. doi: 10.1161/CIRCOUTCOMES.112.967968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meert K, Telford R, Holubkov R, et al. Paediatric in-hospital cardiac arrest: Factors associated with survival and neurobehavioural outcome one year later. Resuscitation. 2018;124:96–105. doi: 10.1016/j.resuscitation.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindler MB, Bohn D, Cox PN, et al. Outcome of Out-of-Hospital Cardiac or Respiratory Arrest in Children. N Engl J Med. 1996;335(20):1473–1479. doi: 10.1056/NEJM199611143352001 [DOI] [PubMed] [Google Scholar]
- 24.Meaney PA, Nadkarni VM, Cook EF, et al. Higher Survival Rates Among Younger Patients After Pediatric Intensive Care Unit Cardiac Arrests. Pediatrics. 2006;118(6):2424–2433. doi: 10.1542/peds.2006-1724 [DOI] [PubMed] [Google Scholar]
- 25.Posner JB, Saper CB, Schiff ND, Plum F. Plum and Posner’s Diagnosis of Stupor and Coma. 4th ed. New York: Oxford University Press; 2007. [Google Scholar]
- 26.Bratton SL. Serial Neurologic Examinations After Near Drowning and Outcome. Arch Pediatr Adolesc Med. 1994;148(2):167. doi: 10.1001/archpedi.1994.02170020053008 [DOI] [PubMed] [Google Scholar]
- 27.Mandel R, Martinot A, Delepoulle F, et al. Prediction of outcome after hypoxic-ischemic encephalopathy: A prospective clinical and electrophysiologic study. J Pediatr. 2002;141(1):45–50. doi: 10.1067/mpd.2002.125005 [DOI] [PubMed] [Google Scholar]
- 28.Carter BG, Butt W. A prospective study of outcome predictors after severe brain injury in children. Intensive Care Med. 2005;31(6):840–845. doi: 10.1007/s00134-005-2634-0 [DOI] [PubMed] [Google Scholar]
- 29.Kirschen MP, Topjian AA, Hammond R, Illes J, Abend NS. Neuroprognostication After Pediatric Cardiac Arrest. Pediatr Neurol. 2014;51(5):663–668.e2. doi: 10.1016/j.pediatrneurol.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice Parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–210. doi: 10.1212/01.wnl.0000227183.21314.cd [DOI] [PubMed] [Google Scholar]
- 31.Booth CM, Boone RH, Tomlinson G, Detsky AS. Is This Patient Dead, Vegetative, or Severely Neurologically Impaired?: Assessing Outcome for Comatose Survivors of Cardiac Arrest. JAMA. 2004;291(7):870–879. doi: 10.1001/jama.291.7.870 [DOI] [PubMed] [Google Scholar]
- 32.Greer DM, Yang J, Scripko PD, et al. Clinical examination for prognostication in comatose cardiac arrest patients. Resuscitation. 2013;84(11):1546–1551. doi: 10.1016/j.resuscitation.2013.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post–Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 suppl 2):S465–S482. doi: 10.1161/CIR.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topjian Alexis A., de Caen Allan, Wainwright Mark S., et al. Pediatric Post–Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019;140(6):e194–e233. doi: 10.1161/CIR.0000000000000697 [DOI] [PubMed] [Google Scholar]
- 35.Hume AL, Cant BR. Central somatosensory conduction after head injury. Ann Neurol. 1981;10(5):411–419. doi: 10.1002/ana.410100503 [DOI] [PubMed] [Google Scholar]
- 36.Sutton LN, Frewen T, Marsh R, Jaggi J, Bruce DA. The effects of deep barbiturate coma on multimodality evoked potentials. J Neurosurg. 1982;57(2):178–185. doi: 10.3171/jns.1982.57.2.0178 [DOI] [PubMed] [Google Scholar]
- 37.Newlon PG, Greenberg RP, Enas GG, Becker DP. Effects of Therapeutic Pentobarbital Coma on Multimodality Evoked Potentials Recorded from Severely Head-injured Patients. Neurosurgery. 1983;12(6):613–619. doi: 10.1227/00006123-198306000-00003 [DOI] [PubMed] [Google Scholar]
- 38.McPherson RW, Sell B, Traystman RJ. Effects of Thiopental, Fentanyl, and Etomidate on Upper Extremity Somatosensory Evoked Potentials in Humans. Anesthesiol J Am Soc Anesthesiol. 1986;65(6):584–589. [DOI] [PubMed] [Google Scholar]
- 39.Sloan TB, Fugina ML, Toleikis JR. EFFECTS OF MIDAZOLAM ON MEDIAN NERVE SOMATOSENSORY EVOKED POTENTIALS †. Br J Anaesth. 1990;64(5):590–593. doi: 10.1093/bja/64.5.590 [DOI] [PubMed] [Google Scholar]
- 40.Carter BG, Taylor A, Butt W. Severe brain injury in children: long-term outcome and its prediction using somatosensory evoked potentials (SEPs). Intensive Care Med. 1999;25(7):722–728. doi: 10.1007/s001340050936 [DOI] [PubMed] [Google Scholar]
- 41.Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A. Systematic review of early prediction of poor outcome in anoxicischaemic coma. The Lancet. 1998;352(9143):1808–1812. doi: 10.1016/S0140-6736(98)04076-8 [DOI] [PubMed] [Google Scholar]
- 42.Madl C, Grimm G, Kramer L, et al. Early prediction of individual outcome after cardiopulmonary resuscitation. The Lancet. 1993;341(8849):855–858. doi: 10.1016/0140-6736(93)93061-5 [DOI] [PubMed] [Google Scholar]
- 43.Madl C, Kramer L, Domanovits H, et al. Improved outcome prediction in unconscious cardiac arrest survivors with sensory evoked potentials compared with clinical assessment: Crit Care Med. 2000;28(3):721–726. doi: 10.1097/00003246-200003000-00020 [DOI] [PubMed] [Google Scholar]
- 44.Ostendorf AP, Hartman ME, Friess SH. Early Electroencephalographic Findings Correlate With Neurologic Outcome in Children Following Cardiac Arrest: Pediatr Crit Care Med. 2016;17(7):667–676. doi: 10.1097/PCC.0000000000000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus Is Associated With Mortality and Worse Short-Term Outcome in Critically Ill Children*: Crit Care Med. 2013;41(1):215–223. doi: 10.1097/CCM.0b013e3182668035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fung FW, Topjian AA, Xiao R, Abend NS. Early EEG Features for Outcome Prediction After Cardiac Arrest in Children: J Clin Neurophysiol. 2019;36(5):349–357. doi: 10.1097/WNP.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishisaki A, Sullivan J, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years*: Pediatr Crit Care Med. 2007;8(1):10–17. doi: 10.1097/01.pcc.0000256621.63135.4b [DOI] [PubMed] [Google Scholar]
- 48.Topjian AA, Sánchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early Electroencephalographic Background Features Predict Outcomes in Children Resuscitated From Cardiac Arrest*: Pediatr Crit Care Med. 2016;17(6):547–557. doi: 10.1097/PCC.0000000000000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessler SK, Topjian AA, Gutierrez-Colina AM, et al. Short-Term Outcome Prediction by Electroencephalographic Features in Children Treated with Therapeutic Hypothermia After Cardiac Arrest. Neurocrit Care. 2011;14(1):37–43. doi: 10.1007/s12028-010-9450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ducharme-Crevier L, Press CA, Kurz JE, Mills MG, Goldstein JL, Wainwright MS. Early Presence of Sleep Spindles on Electroencephalography Is Associated With Good Outcome After Pediatric Cardiac Arrest: Pediatr Crit Care Med. 2017;18(5):452–460. doi: 10.1097/PCC.0000000000001137 [DOI] [PubMed] [Google Scholar]
- 51.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver Reproducibility of Electroencephalogram Interpretation in Critically Ill Children: J Clin Neurophysiol. 2011;28(1): 15–19. doi: 10.1097/WNP.0b013e3182051123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S, Zhao X, Davis KA, Topjian AA, Litt B, Abend NS. Quantitative EEG predicts outcomes in children after cardiac arrest. Neurology. 2019;92(20):e2329–e2338. doi: 10.1212/WNL.0000000000007504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarpino M, Lolli F, Lanzo G, et al. Neurophysiology and neuroimaging accurately predict poor neurological outcome within 24 hours after cardiac arrest: The ProNeCA prospective multicentre prognostication study. Resuscitation. 2019;143:115–123. doi: 10.1016/j.resuscitation.2019.07.032 [DOI] [PubMed] [Google Scholar]
- 54.Herman ST, Abend NS, Bleck TP, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part I: Indications. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2015;32(2):87–95. doi: 10.1097/WNP.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015; 132(18 suppl 2):S526–S542. doi: 10.1161/CIR.0000000000000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor SB, Quencer RM, Holzman BH, Naidich TP. Central nervous system anoxic-ischemic insult in children due to near-drowning. Radiology. 1985;156(3):641–646. doi: 10.1148/radiology.156.3.4023222 [DOI] [PubMed] [Google Scholar]
- 57.Rafaat KT, Spear RM, Kuelbs C, Parsapour K, Peterson B. Cranial computed tomographic findings in a large group of children with drowning: Diagnostic, prognostic, and forensic implications*: Pediatr Crit Care Med. 2008;9(6):567–572. doi: 10.1097/PCC.0b013e31818c8955 [DOI] [PubMed] [Google Scholar]
- 58.Starling RM, Shekdar K, Licht D, Nadkarni VM, Berg RA, Topjian AA. Early Head CT Findings Are Associated With Outcomes After Pediatric Out-of-Hospital Cardiac Arrest*: Pediatr Crit Care Med. 2015;16(6):542–548. doi: 10.1097/PCC.0000000000000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubowitz DJ, Bluml S, Arcinue E, Dietrich RB. MR of Hypoxic Encephalopathy in Children after Near Drowning: Correlation with Quantitative Proton MR Spectroscopy and Clinical Outcome. Am J Neuroradiol. 1998;19(9):1617–1627. [PMC free article] [PubMed] [Google Scholar]
- 60.Christophe C, Fonteyne C, Ziereisen F, et al. Value of MR Imaging of the Brain in Children with Hypoxic Coma. AJNR Am J Neuroradiol. 2002;23(4):716–723. [PMC free article] [PubMed] [Google Scholar]
- 61.Oualha M, Gatterre P, Boddaert N, et al. Early diffusion-weighted magnetic resonance imaging in children after cardiac arrest may provide valuable prognostic information on clinical outcome. Intensive Care Med. 2013;39(7):1306–1312. doi: 10.1007/s00134-013-2930-z [DOI] [PubMed] [Google Scholar]
- 62.Fink EL, Panigrahy A, Clark RSB, et al. Regional Brain Injury on Conventional and Diffusion Weighted MRI is Associated with Outcome After Pediatric Cardiac Arrest. Neurocrit Care. 2013; 19(1):31–40. doi: 10.1007/s12028-012-9706-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manchester LC, Lee V, Schmithorst V, Kochanek PM, Panigrahy A, Fink EL. Global and Regional Derangements of Cerebral Blood Flow and Diffusion Magnetic Resonance Imaging after Pediatric Cardiac Arrest. J Pediatr. 2016;169:28–35.e1. doi: 10.1016/j.jpeds.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yacoub M, Birchansky B, Mlynash M, et al. The prognostic value of quantitative diffusion-weighted MRI after pediatric cardiopulmonary arrest. Resuscitation. 2019;135:103–109. doi : 10.1016/j.resuscitation.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 65.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11(3):423–429. [PMC free article] [PubMed] [Google Scholar]
- 66.Hajnal JV, Bryant DJ, Kasuboski L, et al. Use of fluid attenuated inversion recovery (FLAIR) pulse sequences in MRI of the brain. J Comput Assist Tomogr. 1992;16(6):841–844. doi: 10.1097/00004728-199211000-00001 [DOI] [PubMed] [Google Scholar]
- 67.Lopez Soto C, Dragoi L, Heyn CC, et al. Imaging for Neuroprognostication After Cardiac Arrest: Systematic Review and Meta-analysis. Neurocrit Care. September 2019. doi: 10.1007/s12028-019-00842-0 [DOI] [PubMed] [Google Scholar]