Abstract
In most species, survival relies on the hypothalamic control of endocrine axes that regulate critical functions such as reproduction, growth, and metabolism. For decades, the complexity and inaccessibility of the hypothalamic–pituitary axis has prevented researchers from elucidating the relationship between the activity of endocrine hypothalamic neurons and pituitary hormone secretion. Indeed, the study of central control of endocrine function has been largely dominated by ‘traditional’ techniques that consist of studying in vitro or ex vivo isolated cell types without taking into account the complexity of regulatory mechanisms at the level of the brain, pituitary and periphery. Nowadays, by exploiting modern neuronal transfection and imaging techniques, it is possible to study hypothalamic neuron activity in situ, in real time, and in conscious animals. Deep-brain imaging of calcium activity can be performed through gradient-index lenses that are chronically implanted and offer a ‘window into the brain’ to image multiple neurons at single-cell resolution. With this review, we aim to highlight deep-brain imaging techniques that enable the study of neuroendocrine neurons in awake animals whilst maintaining the integrity of regulatory loops between the brain, pituitary and peripheral glands. Furthermore, to assist researchers in setting up these techniques, we discuss the equipment required and include a practical step-by-step guide to performing these deep-brain imaging studies.
Keywords: neuroendocrinology, whole animal physiology, deep-brain imaging, neuronal activity, conscious animals
Introduction
For almost a century, neuroendocrinologists have worked towards characterising the central regulation of endocrine axes that ensure critical functions such as reproduction, growth, and metabolism. While research questions have for many years focused firmly on the neural control of the pituitary gland, the neuroendocrinology field has grown wider and now includes studying the effect of centrally produced hormones on various brain areas, as well as the role of several peripherally born peptides that affect neuroendocrine systems controlling metabolism. An unavoidable feature of all neuroendocrine systems is that they generate rhythms and rely on these rhythms to function optimally. These hormone oscillations dynamically regulate gene transcription and synaptic transmission, and changes in these oscillations are observed in a variety of (patho)physiological states (Le Tissier et al. 2017).
Although it has long been postulated that neuroendocrine neurons have the ability to generate hormone rhythms via the patterning of their electrophysiological activity, the regulatory mechanisms underlying this rhythmic mode of hormone secretion have remained hazy. One reason for this is that research efforts in this area have simply been hindered by the complexity and inaccessibility of the hypothalamic–pituitary system. Another reason is that the study of neuroendocrine function has been largely dominated by techniques that consist of studying isolated cell types in in vitro or ex vivo preparations. This insular approach has led to an inevitable bottleneck where data on cellular and biochemical processes within specific cell types or nuclei have multiplied without a clear/tangible link to physiological function. Similarly, recent efforts in single-cell transcriptomics of the hypothalamus (Romanov et al. 2017, Wang & Ma 2019) have documented a highly complex heterogenous hypothalamus, but the implication of neuropeptide expression for physiological function remains difficult to interpret without appropriate tools. Nevertheless, technological developments in genetics and systems neuroscience have enabled specific neurons to be manipulated in vivo. Chemogenetics and optogenetics (Fenno et al. 2011) tools have made it possible to study the hypothalamus in ways that were previously unimaginable, shedding light on gonadotropin pulse generation (Campos & Herbison 2014, Han et al. 2015, Voliotis et al. 2019) and brain control of appetite for example (Atasoy et al. 2012, Betley et al. 2015). Likewise, genetically encoded calcium indicators (GECIs) (Pérez Koldenkova & Nagai 2013) are now frequently used to monitor neuronal activity in living animals. Their characteristics making them an excellent proxy for electrical activity and a versatile tool that facilitates diverse imaging techniques. For example, fibre photometry experiments that consist of monitoring the average calcium activity of a neuronal population in vivo have shown that arcuate kisspeptin neurons are responsible for the generation of pulses of LH (Clarkson et al. 2017).
Despite the valuable insights these techniques have provided, none of them have allowed researchers to study neuronal activity across a population at the single-cell level – characterising for example cell-to-cell heterogeneity or synchronicity – and in turn relate this activity to specific functions. Fortunately, deep-brain single-cell imaging can now be achieved using gradient-index (GRIN) lenses that are chronically implanted and permit imaging of multiple (10–100s) neurons within the population (Barretto et al. 2009). Depending on the research question and image quality required, visualisation of neuronal activity can be carried out in head-fixed configuration using a bench-top microscope (Kim et al. 2008, Bocarsly et al. 2015) or in freely moving configuration using a miniature head-mounted microscope (Ghosh et al. 2011). Importantly, using these techniques, it becomes possible to correlate the impact of neuronal activity within a network on other functions. This methodology has been successfully used to study arcuate nucleus and amygdala control of feeding behaviour in freely moving mice (Betley et al. 2015, Jennings et al. 2015). Similarly, head-fixed microscopy through GRIN lenses has made it possible to image and manipulate pituitary cells over a period of days to weeks in awake mice (Hoa et al. 2019) and while it is yet to be published, one can easily imagine combining this technique with serial blood sampling to understand the link between the activity of specific hypothalamic neurons and the resulting peripheral hormonal release (Fig. 1). In the near future, in vivo deep-brain imaging could become critical when combined with other emerging strategies. For example, researchers now have the ability to visualise in vivo the effect on neuronal activity of individual genomic variants identified from human genomes, thus filling a gap in our general view of neuroendocrine systems (Fig. 1).
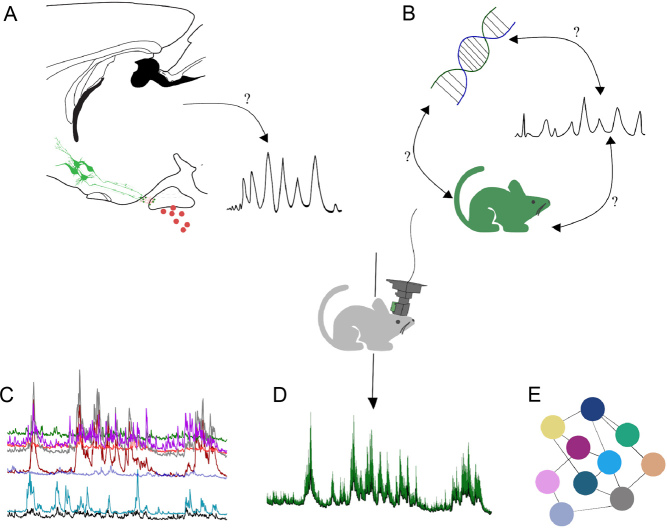
Figure 1.
Deep-brain imaging as a powerful tool to understand neuroendocrine functioning. Unknowns such as the relationship between the activity of specific neurons and the dynamics of peripheral hormonal secretion (A) or the way newly discovered genetic mutations result in phenotypic changes (B) can be elucidated using in vivo deep brain imaging. Researchers will greatly benefit from real-time visualisation of single neuron calcium activity (C), population calcium activity (D), and will gain insight into the network activity of genetically defined neurons (E).
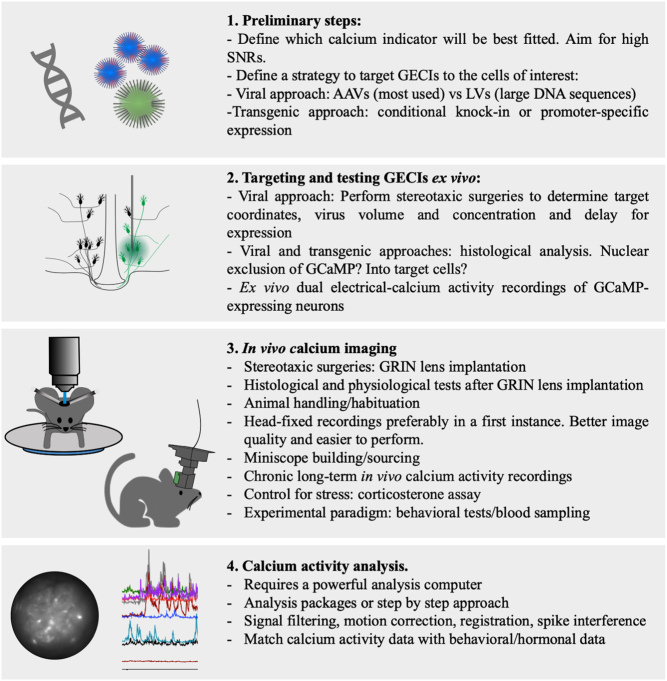
In this review, we will discuss deep-brain imaging techniques that enable the study of endogenous brain activity from neuroendocrine populations in awake animals whilst maintaining the integrity of regulatory loops between the brain, pituitary and peripheral glands. Rather than cataloguing all available methodologies, we will focus on the most widely used and accessible systems. We will present a step by step approach toward in vivo deep-brain imaging (Fig. 2) and provide advice on neuronal activity indicators, neuronal targeting, microscopy techniques, analysis, animal handling and physiological/behavioural assessments so that researchers will be able to set up these techniques to address their own research questions.
Figure 2.
Typical step-by-step approach toward in vivo deep-brain calcium imaging.
Monitoring neuronal activity with genetically encoded indicators
In order to understand brain processing, scientists have tried to decrypt the action potential (AP) firing of groups of neurons and relate this to specific physiological functions. Traditional methods such as intracellular patch-clamp limit the number of neurons that can be studied at the same time, are invasive and difficult to perform in vivo. Extracellular recordings are easier to make, but do not give precise information about the electrical activity of individual neurons and do not enable the study of specific neuronal firing within a population. Looking to overcome these issues, Francis Crick proposed in 1999 that ‘One way-out suggestion is to engineer these neurons so that when one of them fires it would emit a flash of light of a particular wave length’ (Crick 1999); and indeed, the development of genetically encoded optical indicators of neuronal activity has enabled progress towards this goal to an extent that was unimaginable two decades ago. Nowadays, optical imaging enables thousands of neurons to be simultaneously observed in vitro or in vivo, whilst voltage and calcium indicators reveal spatiotemporal activity patterns within neurons such as dendritic integration, voltage propagation, or dendritic spiking (Lin & Schnitzer 2016). Although microscopy requires optical access to the tissue of interest, many techniques allow minimally invasive optical access or placement of optical probes up to millimetres away from the imaged cells, thus allowing the immediate vicinity of cells under study to be left unperturbed (Ghosh et al. 2011, Betley et al. 2015).
Calcium indicators
Calcium is a universal second messenger, playing an essential role in excitable cells and signal transduction. In neurons, APs trigger large and rapid calcium (Ca2+) influx through voltage-gated channels. AP firing can therefore be assessed by measuring changes in concentrations of intracellular Ca2+ (Yasuda et al. 2004). Similarly, activation of neurotransmitter receptors during synaptic transmission causes Ca2+ transients in dendritic spines, and with appropriate indicators, imaging intracellular Ca2+ dynamics can be used to measure neuronal spiking and synaptic activity across populations of neurons in vitro and in vivo. GECIs and small-molecule calcium-sensitive dyes (Cobbold & Rink 1987) such as fura-2 (Grynkiewicz et al. 1985) are both used to report changes in intracellular Ca2+ concentration; but GECIs have the advantage that they enable long-term, repeated non-invasive imaging of specific cells and compartments (Mao et al. 2008). State-of-the-art GECIs include Förster Resonance Energy Transfer (FRET)-based sensors (Fiala et al. 2002, Palmer & Tsien 2006, Mank et al. 2008), but for in vivo experiments particularly, the single-wavelength sensor GCaMP family has become the default tool (Tian et al. 2009). GCaMPs are based on circularly permuted green fluorescent protein (cpGFP), calmodulin (CaM), and the Ca2+/CaM-binding ‘M13’ peptide. Ca2+ binding to the calmodulin domain results in a structural shift that enhances the fluorescence by deprotonation of the fluorophore. Since the original GCaMP sensor was published (Nakai et al. 2001), many variants have been developed with the purpose of performing calcium imaging in vivo (Ohkura et al. 2005, Tallini et al. 2006, Tian et al. 2009). The experimenter will be burdened with picking the appropriate GECI from a broad assortment, but generally speaking, imaging from large populations of neurons in densely labelled samples is helped by lower baseline fluorescence indicators, which reduces the background signal from neuropil and inactive neurons (Dana et al. 2019). Moreover, low baseline fluorescence becomes especially important when working in vivo where large tissue volumes contribute to the background signal. For the reasons mentioned above, we and others have worked with GCaMP6s,m,f (Chen et al. 2013) and are now experimenting with JGCaMP7f (Dana et al. 2019); all offer high signal-to-noise ratio (SNR) and GCaMP6m and JGCaMP7 show improved detection of individual spikes (Chen et al. 2013, Dana et al. 2019). It is also worth noting the development of red-shifted GECIs (e.g. jRCaMP1a, jRCaMP1b, jRGECO1a, jRGECO1b) (Akerboom et al. 2013, Dana et al. 2016, Montagni et al. 2019); these allow the simultaneous use of optogenetic activation of channel rhodopsins using blue light while recording calcium activity (Akerboom et al. 2013).
Voltage indicators
Voltage imaging can directly display membrane potential dynamics and has been used to measure neural dynamics for decades (Bando et al. 2019). Genetically encoded voltage indicators (GEVIs) can be categorised into three classes based on their molecular structure and voltage-sensing mechanism: voltage-sensitive domain-based sensors, rhodopsin-based sensors, and rhodopsin-FRET sensors (see Bando et al. (2019) for a comparative review on GEVIs). While imaging voltage using fluorescent-based sensors appears to be an ideal technique to probe neural circuits with high spatiotemporal resolution, due to insufficient SNR, imaging membrane potential is still challenging, and in order to achieve single action potential resolution in vivo, brighter voltage indicators will need to be developed (Aharoni & Hoogland 2019).
Directing GECIs to neurons of interest
Viral vectors
Viruses can be used to target Ca2+ indicators to specific cells in vivo. Adeno-associated virus (AAV) and lentivirus (LV) are the most common viral vectors used today for neuroscience applications and result in robust expression of the desired gene throughout the cell. Due to their low immunogenicity, AAVs and LVs are well tolerated and highly expressed over long periods of time without any reported adverse effects (Kaplitt et al. 1994, Sakuma et al. 2012, Samulski & Muzyczka 2014) in various species (Vasileva & Jessberger 2005). AAVs are the most widely used by the scientific community; their efficiency has been evaluated for many brain regions and cell types, and most of them are commercially available from facilities such as Addgene, Penn Vector Core, or ETH Zurich. It is very common to deliver a Cre-dependent viral vector in a Cre-line mutant mouse. With this approach, Ca2+ indicator cell-type specificity is typically determined by the genetically controlled presence of a recombinase (Cre) in the specific cells of interest. The viral approach has several advantages, including topographic specificity and the ability to control the amount of protein transduced (adjustment of volume and concentration of viral vectors). However, viral neuronal transfection relies on stereotaxic injection and therefore every animal is unique. Viral vectors also have to be carefully selected and require rigorous post hoc histological validation.
AAVs
AAVs are small particles (20 nm) allowing them to spread very efficiently within tissues and are particularly well-adapted to brain cell transfection. The choice of the appropriate AAV needs be based on multiple factors, from the physical location of the viral injection in the brain to the serotype/cell-type affinity of the chosen viral vector, as well as the molecular and genetic machinery driving its replication/integration. AAVs present as different serotypes meaning that their capsid proteins differ as well as DNA sequences (inverted terminal repeats, ITRs) that result in variations in speed of viral gene expression onset and target cell specificity. Overall, AAV1, 2, 5, 6, 8, and 9 have different affinities for different cells but should all be able to transfect neurons (Aschauer et al. 2013, Hammond et al. 2017). When in doubt, AAV-DJ (Grimm et al. 2008) or AAV DJ8 (Hashimoto et al. 2017) may be good options to test out transfection of neurons that have not been studied before due to their alleged increased tissue tropism. Several ubiquitous promoters are available to drive transgene expressions with AAVs; although not exhaustive, Table 1 summarizes serotypes and promoters used to successfully transfect various hypothalamic neuronal populations.
Table 1.
Virus database for the transfection of hypothalamic neurons.
| Serotype | Promoter | Transgene | Host Specie | Brain area | Neuronal Identity | References |
|---|---|---|---|---|---|---|
| 9 | EF1a | ChR2 | mouse, rat | MS, rPOA, AHA | GnRH | (Campos & Herbison 2014) and unpublished data |
| 9, 5, 2 | EF1a, CAG | ChR2, GCaMP6s | mouse | ARN | GHRH | Unpublished data |
| 9, 5, 2 | EF1a, CAG | ChR2, GCaMP6s | mouse, rat | PVN, LH | CRH | (Pomrenze et al. 2015, Füzesi et al. 2016, Romanov et al. 2017) |
| 9, 8, 5, 2 | EF1a, CAG, Syn | ChR2, GCaMP6s GCaMP6m, hM3Dq |
mouse | PVN, | TRH | (Krashes et al. 2014) and unpublished data |
| 9 | EF1a | ChR2, GCaMP6s, ArchR, HaloR hM3Dq, hM4Di |
mouse | RP3V, ARN | Kisspeptin | (Han et al. 2015, Clarkson et al. 2017) |
| 5 | CAG | hM4Di | mouse | ARN | POMC | (Atasoy et al. 2012) |
| 1, 8 | Syn | ChR2, ArchR, GCamp6s, hM4Di | mouse | ARN | AgRP | (Atasoy et al. 2012, Krashes et al. 2014, Betley et al. 2015) |
| 1, DJ,9, 5 | Syn, Ef1a, | GCamp6s, ChETA, hM3Dq, eYFP | mouse | LH, ARN | GABAergic | (Jennings et al. 2015, Resendez et al. 2016, Silva et al. 2019) |
| 1 | Syn | GCamp6s | mouse | VMH | Galanergic | (Viskaitis et al. 2017) |
| 1 | Syn | GCamp6s | mouse | VMH | Estrogen receptor 1 | (Remedios et al. 2017) |
Brain area abbreviations: AHA, anterior hypothalamus; ARN, arcuate nucleus; LH, lateral hypothalamus; MS, medial septum; PVN, paraventricular nucleus; RP3V, rostral periventricular region of the third ventricle; rPOA, rostral preoptic area; VMH, ventro-medial hypothalamus.
Neuronal identity abbreviations: AgRP, Agouti-related peptide; CRH, corticotropin-releasing hormone; GABA: gamma-aminobutyric acid; GHRH, growth hormone-releasing hormone; GnRH, gonadotropin-releasing hormone; POMC, pro-opiomelanocortin; TRH, thyrotropin-releasing hormone.
Lentiviruses
LVs are big particles (80–120 nm) and can carry inserts as large as 10 kb (as opposed to the limited 4.5 kb capacity of AAVs), which is particularly pertinent when working with a promoter-specific virus. Although they are less commonly used than AAVs, LVs are more efficient at transfecting cells in vitro, have similar capabilities in vivo (but do not spread as widely as AAVs) and do not require making a choice of serotype (Cannon et al. 2011).
Transgenics
In order to work with animals with similar levels of transgene expression within the targeted neuronal phenotype, transgenic lines have been generated. Essentially, two approaches exist.
In the first type of transgenic line, GCaMP is expressed under the control of specific promoters. For example, the Thy1-GCaMP3 (Chen et al. 2012), 6s and 6f (Dana et al. 2014) transgenic mouse lines express GCaMP in Thy1-expressing neurons across the entire brain and have been used for in vitro and in vivo Ca2+ imaging. Similarly GCaMP2 expression under the Kv3.1 potassium-channel promoter has been used to perform Ca2+ imaging of the glomerular layer of the mouse olfactory bulb (Fletcher et al. 2009). Finally, transgenic rat lines have been developed and Thy1-GCaMP6f rats have been used to record cortical activity with a head-mounted microscope (Scott et al. 2018).
The second and more common approach is to generate transgenic animals bearing GCaMP expression in specific cell types, by crossing a Cre-driver with a floxed-GCaMP mouse (Hsiang et al. 2017, Zimmerman et al. 2019). Several floxed-GCaMP mice lines are now commercially available from Jackson Laboratories and have been characterised by users. Nevertheless, potential concerns have to be kept in mind if working with transgenics and attention will need to be given to potential ectopic expression, drift and loss of specificity of the transgene, or even disrupted Ca2+ dynamics within GCaMP-expressing neurons (Steinmetz et al. 2017). After years of working with GECIs or genetically-addressable opsins, many labs including our own have favoured the use of viruses. Overall, viral vectors lead to a greater level of expression of the desired transgene, which is essential in vivo, and generating transgenic lines has been an expensive, time-consuming process that has not always resulted in success.
Histological validation of GCaMP expression
Regardless of the technique used to target GCaMP to specific cells, it is essential to confirm the extent of transgene expression, spread, and location over the experimental timeline using histology methodologies. Typically, animals will be terminally anesthetized and transcardially perfused with a 4% paraformaldehyde solution, and brains will be removed, sectioned and processed for histological analysis. Native GCaMP expression should be bright enough to visualize in fixed sections with epifluorescence or confocal microscopy, with fluorescence signal limited to the cell membrane. Filled nuclei and/or misshapen neurons can be a sign of overexpression, which can be overcome by reducing viral vector concentration.
In order to identify transfected neurons with immunohistochemistry techniques, GCaMP labelling can be achieved using a GFP antibody; this is especially useful in situations where GCaMP cannot be clearly detected without enhancement. Alternatively, it is possible to perfuse the animal with calcium-containing DPBS (Dulbecco’s phosphate buffer saline). Since DPBS contains a saturating concentration of Ca2+ (0.9 mM), GCaMP brightness will be maximal, and the experimenter may therefore be able to visualize GCaMP fluorescence without antibodies (Dana et al. 2014).
Imaging deep brain areas
While cortical activity can be recorded through cranial windows, hypothalamic neuroendocrine neurons are located deep in the brain (from half a centimetre deep for mice to up to a centimetre deep for rats) and it is therefore necessary to find tools that allow the experimenter to see ‘deep inside’ the brain. Fortunately, in parallel to the development of Ca2+ indicators, instrumentation has been optimized to render Ca2+ imaging possible in subcortical brain areas.
GRIN lenses
GRIN lenses resemble simple glass needles, but they are in fact complex lenses with a radially varying index of refraction; this causes an optical ray to follow a sinusoidal propagation path through the lens. GRIN lenses combine refraction at the end surfaces along with continuous refraction within the lens. Their characteristics allow them to relay optical images from one end to the other, with very low aberrations for objects near the optical axis. For these reasons, GRIN lenses are the perfect tool to perform in vivo imaging in deep-brain regions, relaying signals to a point above the skull surface where image acquisition can take place (Barretto et al. 2009). GRIN lenses exist in different lengths and diameters. For brain regions such as the dorsal striatum (Zhang et al. 2019), prelimbic cortex (Pinto & Dan 2015) or hippocampus (Ziv et al. 2013), a 1 mm diameter GRIN lens can be used to increase the field of view. For deep-brain areas such as the amygdala (Li et al. 2017) or hypothalamus (Jennings et al. 2015), a lens of approximatively 0.5 mm diameter can be used in order to minimize tissue damage during lens implantation. For neuroendocrine hypothalamic areas (e.g. arcuate nucleus, paraventricular nucleus, rostral preoptic area), we have successfully used 0.6 mm diameter 7.4 mm long lenses for mice, and 0.8 mm diameter 10.8 mm long lenses for rats.
Prism probes
Side-view GRIN lenses or ‘prism-probes’ exist. These are composed of a prism fixed to the bottom of a cylindrical GRIN relay lens. The slanted surface of the prism is coated with metal (aluminium or silver for high reflectivity) to reflect by 90° the light from the microscope to excite GCaMP cells located along the imaging face of the prism probe. The emitted light from the cells also reflects off the hypotenuse of the prism and can be collected through the objective of the microscope. These probes have been used for multi-layer cortical imaging (Gulati et al. 2017) and could be suitable for populations of hypothalamic neurons that are spatially distributed along the z-axis.
GRIN lens implantation
Unravelling the intricacies of neuroendocrine function with in vivo imaging studies lasting days to weeks requires optical access to the structure of interest while maintaining both its integrity and that of the surrounding tissue. First of all, before performing GRIN lens implantation in animals, it is sensible to use a ‘phantom brain’ preparation where the GRIN lens is implanted into an agar/fluorescent microbead preparation (Kim et al. 2017). This preparation can be looked at under any epifluorescence microscope in order to verify the working distance of the GRIN lens. GRIN lenses have to be placed above the targeted structure and the space needed between the lens and the neurons is determined by the working distance (50–350 μm) of the imaging end of the GRIN.
In order to gain optical access to the hypothalamus, a GRIN lens has to be guided through the cortex towards the ventral side of the brain. To overcome the challenge of crossing the meninges and going through the brain tissue without increasing pressure and causing excessive damage, the GRIN lens can be inserted into the lumen of a needle, which can then be retracted once the GRIN lens is located correctly. Practically, the GRIN lens is placed inside a small gauge needle, with movement along the needle restricted by placing a metal rod above it. The needle is then lowered to a point just above the target, and then removed with the metal rod kept in place, ensuring the GRIN lens stays in place. Finally, the metal rod is removed and the GRIN lens fixed in position with dental cement (Hoa et al. 2019). An alternative is to first create a path to the target structure with a needle or a dissection knife (Gulati et al. 2017), and to then lower the naked GRIN lens along this path; in this method, the lens can be carefully held using a bulldog clamp or a pipette tip trimmed to match the diameter of the lens. Another interesting method consists of placing the top part of the GRIN lens inside a glass tube and fixing it using Super Glue. The glass tube can then be held with an electrode holder on the stereotaxic arm. Once the GRIN lens has been positioned in the brain and cemented in place, acetone can be carefully introduced into the glass tube in order to dissolve the Super Glue, thus freeing the GRIN lens (Zhang et al. 2019).
GRIN lens implantations are invasive procedures that cause inflammatory responses in the brain, during which the neurons do not behave normally. It is recommended to wait at least 3 weeks after implantation before starting imaging sessions; overall, we have had the most success imaging Ca2+ activity from 5 weeks to several months after GRIN lens implantation. It is also necessary to ensure that GRIN lens implantations do not result in long-term inflammatory responses that could alter physiological function. Attention therefore needs to be given to possible behavioural/hormonal changes, and post hoc histological validation is necessary.
Sourcing GRIN lens
Currently, to our knowledge, there are two manufacturers of GRIN lenses suited to brain imaging applications. The first is GRINTECH, who hold a strategic partnership with the neurotechnology company Inscopix. All GRINTECH lenses used in the field of non-confocal, single photon epi-fluorescence imaging for non-human neuroscience application must be purchased through Inscopix. The second manufacturer is GoFoton, who are the largest manufacturer of GRIN lenses in the world. Although their GRIN lens production has been focused on telecommunication applications and fibre coupling, they have been working over the past year at developing GRIN lenses specifically for brain imaging applications. Hopefully, GoFoton will be able to provide high-quality GRIN lenses for microscopic applications in the near future.
Imaging in conscious animals
Deep-brain imaging of Ca2+ activity can be performed through GRIN lenses that are chronically implanted; these relay fluorescence captured inside the brain to the surface of the skull, which can be observed with conventional microscopy techniques. Depending on the research question and the image quality needed, deep-brain imaging can be performed with a bench top microscope on head-restrained animals or with the help of a head-mounted miniature microscope for freely-moving animals.
Head-fixed configuration
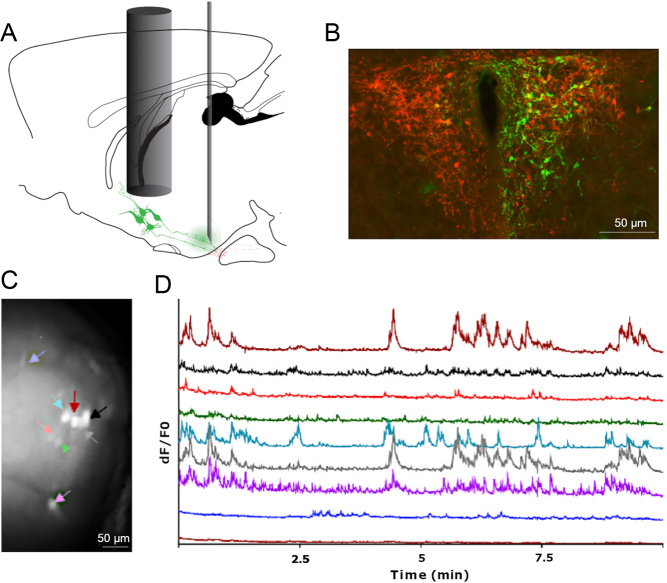
In order to perform deep-brain imaging in conscious animals, rodents can be temporarily restrained under a microscope with the help of a metal piece chronically fixed to their skull. Animals retain the ability to move on a wheel or a cylinder in order to give an impression of movement and reduce stress during the experiments. This approach has been used to perform calcium imaging in deep-brain areas (Bocarsly et al. 2015), and even in two areas at the same time (Lecoq et al. 2014). While most of these studies have been performed using two-photon microscopes, we have successfully performed 1-photon calcium imaging in the hypothalamus of head-fixed mice with a stereomicroscope fitted with a 20x objective (for detailed methodology see Hoa et al. (2019)). Using this set-up, we investigated the endogenous activity of hypophysiotropic TRH neurons across several days. To do so, we worked with T rh-IRES-Cre mice (Krashes et al. 2014) (kindly gifted by Prof. B Lowell, Beth Israel Deaconess Medical Center & Harvard Medical School) that had been injected with a Cre-dependent AAV driving GCaMP6s expression. We recorded the activity of several TRH neurons at single-cell resolution level in head-fixed mice (Fig. 3). This experimental design enabled us to study how hypophysiotropic TRH neurons interact with each other within local networks and modulate their activity throughout the day (P Campos and P Mollard, unpublished observations). A great benefit of deep-brain imaging in head-fixed configuration is that bench top microscopes offer excellent imaging quality, and it is therefore possible to record the activity of sub-cellular structures (Meng et al. 2019). Indeed, we have successfully imaged the calcium activity of assemblies of TRH neuron terminals in the median eminence, although 1-photon microscopy could not spatially resolve individual boutons (P Campos and P Mollard, unpublished observations).
Figure 3.
In vivo calcium imaging of TRH neurons in conscious mice. (A) Trh-IRES-Cre mice were injected in the median eminence with a viral vector (AAV5.CAG.Flex.GCaMP6s.WPRE.SV40) and TRH neurons were visualised through a GRIN lens placed above the PVN. (B) Immunofluorescence image showing GCaMP6s expression (green) in TRH neurons (red) located in the PVN. (C) GRIN lens view of a field of the TRH neurons expressing GCAMP6m. Regions of interest corresponding to individual neurons are indicated by coloured arrows. (D) Time-lapse recordings of calcium (GCAMP6s) activity of eight hypophysiotropic TRH neurons in an adult Trh-IRES-Cre male mouse. Scale bars 50 μm.
Miniscopes
In 2011, Mark Schnitzer’s group first reported the miniature microscope (miniscope) with epifluorescent light source (Ghosh et al. 2011), which was small, light, and easy to mount on the head of a mouse for studying complex behaviours in freely moving animals. Combined with a GRIN lens, the miniscope imaging system enables recording of calcium activity from hundreds of neurons in deep-brain regions for months, with imaging quality comparable to benchtop microscopes. Over the last 5–10 years, several miniscope designs have emerged, but currently, two miniscopes in particular appear to be widely used: the Inscopix nVista miniscope and the UCLA miniscope. These two miniature microscopes share the same core features. The optical design includes an achromatic lens which focuses near-collimated light from an objective GRIN lens onto a CMOS sensor and a set of optical filters. The plastic housing can be machined or 3D printed, and custom electronics are used to control and read-out the excitation LED light source and CMOS imaging sensor. Miniscopes are connected to a data acquisition (DAQ) box that relays data to a computer. A base plate is attached to the skull of the animal with dental cement and allows magnetic latching of the miniscope to the baseplate.
nVista Inscopix miniscope
The Inscopix company was founded in 2011 by Kunal Ghosh, Mark Schnitzer and Abbas El Gamal in order to commercialise their miniature microscope. Some years later, their technology has evolved and the Inscopix miniature microscope, called nVista, is now widely used. The characteristics of the current version of the nVista microscope are presented in Table 2. It possesses a software-controlled electronic focusing mechanism (300 μm) that does not require any manual intervention. The miniscope is connected to a DAQ box which has the capacity to store data on an SSD and/or an SD card. The DAQ box is also equipped with ethernet and WIFI connectivity, which allows remote control of the miniscope and data streaming through Web-based acquisition software.
Table 2.
Comparison of the characteristics of the Inscopix nVista miniature microscope and UCLA miniscope.
| System | UCLA miniscope-3rd generation | nVista 4th generation |
|---|---|---|
| Weight (g) | 3 | 1.8 |
| Size L × W × H (mm) | 16.3 × 13 × 22.5 | 8.8 × 15 × 22 |
| Footprint on scull (Baseplate) (mm2) | 75 | 57.44 |
| Resolution (pixels) | 752 × 480 | 1280 × 800 |
| Field of view (µm) | 700 × 450a | 650 × 900 |
| Sample-rate (Hz) | 60 | 50 |
| Focusing mechanism | Manual (300 μm) - Slider | Electronic (300 μm) - Liquid lens |
aThe field of view given for the 3rd generation UCLA miniscope is based on imaging superficial brain structures. The addition of an objective GRIN lens that is required to image deep-brain structures reduces this field of view. To our knowledge, precise field of view measurements for deep brain imaging using this miniscope are not known.
On purchasing the nVista system, researchers will receive a full support package, which makes it probably the easiest way to start working with deep-brain imaging with a miniscope. Researchers will benefit from field scientific consultants that will, through site visits and online communication, help them set up the technology and troubleshoot experimental and conceptual challenges (from Ca2+ indicator selection, to data analysis) in order to ensure the success of the research project. It is worth highlighting that the nVista miniscope comes with a 1-year manufacturer’s warranty that can be prolonged to 5 years. For damaged scopes sent back to the company, turn-around time is typically around 1 week. In our own experience, this warranty has been valuable; we have had to return damaged nVista miniscopes to Inscopix 3–4 times per year for repair. This support package comes at a price of course; currently, the nVista system (consisting of one nVista 3.0 scope + accessories + 1-year warranty + help + software) costs in the region of 70–80,000 USD.
UCLA miniscope
The UCLA miniscope is also based on the design pioneered by Mark Schnitzer’s Lab, but this miniscope is open-source. The characteristics of the UCLA miniscope version 3 (V3) are presented in Table 2. It also uses a single, flexible coaxial cable to carry power, communication, and data transmission to a custom open source DAQ box and software. The default version of the UCLA miniscope only allows cortical imaging. For imaging deeper brain areas, an objective GRIN lens can be mounted inside the miniscope and positioned above a GRIN relay lens chronically implanted above the target cells. Focusing adjustment (300 μm) occurs through a linear slider that can be manually adjusted in height; although it preserves the orientation of neurons between imaging sessions, it can be tricky to use, especially when the miniscope is head mounted.
The UCLA miniscope has had a significant impact on the neuroscience community, with at least 400 labs around the world building and using these imaging devices for their research studies over the past 3 years. This success is likely due to a broad dissemination policy. The ‘miniscope project’ began as a collaboration between the Golshani Lab, Silva Lab, and Khakh Lab at the University of California, Los Angeles, and provides documentation, tutorials on part procurement, assembly and experimental application guides, both online and through workshops. The miniscope website (http://miniscope.org) provides a centralized hub for users to share design files, source code, and other relevant information related to this imaging technique.
Building a UCLA miniscope is cost-effective (DAQ + 1 scope < $1000 USD). It is not complicated but does require soldering and hands-on assembly. A main advantage is that it can be tuned to fit the research project requirements, and additionally by building it, researchers understand how to fix it quickly. For those that do not feel confident building the miniscope, LabMaker (www.labmaker.org), based in Berlin, Germany, sell the UCLA miniscope as a kit that does not require any soldering for approximately $2000 USD (includes DAQ + 1 scope).
Improvements of the UCLA miniscope
It is worth noting that this is a rapidly evolving field, with technological improvements in miniature microscopes emerging all the time. For example, at the time of writing, a new version (V4) of the UCLA miniscope has just been released (http://miniscope.org). Compared to the UCLA V3 miniscope, the V4 miniscope is lighter (2.6 g), utilises a smaller baseplate (25 μm footprint), has a field of view that can be set based on the combination of lenses used when assembling the miniscope (1000 μm-diameter or more), has an improved CMOS that requires just a fifth of the excitation power of previous systems, and is expected to have improved resolution (0.5 MP) and faster imaging speed (120FPS) (Daniel Aharoni, 2019 Neuro Open-source Workshop, January 2019). In addition, the manual focusing mechanism of V3 has been replaced by an electronic system. Indeed, similar to the nVista system, the V4 miniscope benefits from liquid lenses that consist of an interface of two immiscible liquids which, under voltage, deform and act as a lens allowing for electronic focusing without the need for manual adjustment. This should make focusing much easier, and because liquid lenses can be adjusted rapidly, it may become possible to perform interleaved recording from different focal planes (Aharoni & Hoogland 2019). Finally, in order to allow for simultaneous two-colour fluorescence imaging, the objective GRIN lenses present in the previous version of the UCLA miniscopes that created chromatic aberrations, have been replaced in V4 miniscopes by achromatic lenses (Aharoni & Hoogland 2019). However, since deep-brain imaging necessitates the implantation of GRIN lenses in the brain, this new feature does not yet represent an advantage for the study of deeply located neuroendocrine cells. It is important to note that although the UCLA V4 miniscope possesses promising improvements and can be fitted with relay GRIN lenses for deep brain imaging, to our knowledge, they have not yet been tested for structures deeper than the cortex. The V4 is expected to be released as a kit in 2020.
Alternatives and next-generation miniscopes
Wire-free scopes
Whilst tethered systems are currently the most effective method for in vivo recording/control of freely moving animals, wireless (Liberti et al. 2017) and wire-free (Shuman et al. 2020) open-source miniscopes have been developed with the latest being used to record CA1 place cells during maze navigation in epileptic mice. These recent studies have demonstrated the feasibility of using a lightweight wireless miniature microscope system to transmit Ca2+ imaging data to a receiver (Liberti et al. 2017) or save data directly to a microSD card positioned on the miniscope (Shuman et al. 2020). Both designs are powered by onboard lithium polymer batteries that can be attached to the microscope body or mounted on the back of the animal, consequently adding weight to the microscope design (~2 g for a 50 mAh battery). Alternatively, wireless powering of a miniaturized design with low power consumption is thought to be feasible with near-field wireless power transfer similar to those that have already been implemented for wireless optogenetic stimulation (Aharoni & Hoogland 2019). While power consumption (which scales with data bandwidth) is one of the limiting factors in designing a miniaturized wireless microscope due to battery size and weight requirements, power-efficient CMOS imaging sensors can be used along with pixel binning or pixel subsampling to minimize power consumption while maintaining a comparable field of view, albeit at a lower resolution than is possible using tethered systems (Aharoni & Hoogland 2019). The open-source nature of ongoing miniscope projects allows for the rapid sharing of designs, modifications and ideas across laboratories, and it is expected that wireless systems will rapidly improve and that their use will soon escalate within the neuroscience community. Of particular interest here is Open-ePhys, a non-profit initiative encompassing a large team of scientists and developers, which has released a kit to convert the wired UCLA miniscope (version 3) into a wire-free system.
Two-photon imaging
Two-photon (2P) excitation improves optical sectioning of samples and thereby ensures that the light collected is only from the cellular and subcellular structures that lie along the focal plane of 2P excitation. While 2P brain imaging in animal models usually requires that the animal is head fixed, there have been several successful attempts at building a 2P miniscope (see Helmchen et al. 2013 for review) with some designs used to image in freely moving rodents (Zong et al. 2017, Ozbay et al. 2018). A major disadvantage to this technique is that it requires expensive table-top lasers coupled to optical fibres to achieve 2P excitation (Aharoni & Hoogland 2019). Furthermore, a recent study compared data acquired with a 2P benchtop microscope with one-photon images acquired with a miniscope and found that orientation tuning of the same identified neurons was comparable irrespective of the type of fluorescence excitation used (Glas et al. 2019).
Animal handling
Regardless of the imaging technique, it is important to take the time to handle animals to ensure they become familiar with the experimenter and being moved in and out of their cage. It is possible to quickly and briefly anaesthetise the animal when attaching the miniscope or placing the animal in the head-attached set-up, but it is not recommended. Indeed, as discussed later in this review, anaesthetics, even when used for a very short period of time, may affect neuronal dynamics, lead to changes in hormone secretion, or alter animal behaviour.
Head-fixed habituation
For head-fixed experiments, it is useful to place the animal under the microscope for a few minutes every day leading up to the experiment. Attention should be paid to details such as lighting, electrical equipment, and ventilation in order for the animal to acclimatise to the exact conditions that will be used for the experiment. It is also important to protect the animal’s eyes from the microscope’s excitation light by covering the front part of the head with suitable opaque material. We have found that animals never get completely used to being head restrained for long periods of time, but studies have reported self-initiated head-fixation system for functional imaging in mice. For example, mice have been shown to self-initiate head-fixation in order to get water, enabling imaging to be performed through cranial windows (Murphy et al. 2016, Aoki et al. 2017). Although it has never been used for deep-brain imaging, this habituation technique seems promising and shows that with patience and good experimental design, it is indeed feasible to perform imaging in ‘low stress’ head-fixed animals.
Miniscope habituation
For miniscope experiments, the acclimatisation process is more straightforward. Animals should first habituate to their imaging arena for a few minutes each day leading up to the experiment. Then, it is important for the animals to get used to carrying the miniscope, which can weigh about 10% of the weight of an adult mouse. We use ‘dummy’ miniscopes to train our animals; they are of the exact same weight and size as the real miniscope and are connected using the same type of cable. We have found that animals carrying dummies for a few days soon behave in a completely normal way. It is also useful to use a dummy miniscope so the animals get used to the microscope cable. Indeed, mice will try to chew on the cable, and we have found that coating the dummy cable with bitter nail polish usually prevents mice from chewing the real cable during experiments. We have yet to try this methodology with rats.
Evaluating stress
Together with handling and habituation, it is recommended to evaluate the level of stress of the animals throughout the experiments by measuring corticosterone concentration. Commercially-available ELISA kits are widely used to quantify corticosterone levels for the assessment of stress in laboratory animals. Some of them, such as the AssayMax corticosterone ELISA kit (AssayPro), only require small volumes of whole blood (3–6 μL per sample) that can be collected using a minimally invasive technique such as tail-tip blood sampling (Guillou et al. 2015). Although the precision in determining true values of corticosterone is low for commercial ELISA kits, they have proven to be useful to determine relative differences within studies (e.g. head-fixed animals vs freely moving animals vs sham animals).
Analysis
In vivo Ca2+ imaging using GRIN lenses and 1-photon-based microscopes enables the study of neural activities in behaving animals which would be impossible using other techniques. However, the high and fluctuating background fluorescence, inevitable movement and distortion of the imaging field, and extensive spatial overlaps of fluorescent signals emitted from imaged neurons, present major challenges for extracting neuronal signals from the raw imaging data (Lu et al. 2018). Overall, the experimenter will need to identify the spatial outline of each neuron in the field of view, untwine spatially-overlapping neurons, and finally deconvolve the spiking activity of each neuron from the dynamics of the Ca2+ indicator.
Enhancement and motion correction
Overall, several steps are necessary to obtain valuable signals. First, the data needs to be ‘enhanced’ by applying a spatial filter that enhances the signal intensity and removes as much background noise as possible. It is then necessary to perform movement correction in order to align (or register) images. This step not only helps correct for movement that can occur when animals are grooming, chewing etc., but also helps with brain tissue movement in areas close to ventricles. Indeed, we have observed that the brain shows movement even when animals are static, which we hypothesise is due, at least in part, to cerebral spinal fluid flowing through the ventricles. Non-rigid motion-correction algorithms that run on Fiji (such as Moco (Dubbs et al. 2016)) or Matlab (such as NoRMCorre (Pnevmatikakis & Giovannucci 2017)) have proven useful in solving large-scale image registration problems.
Extraction of calcium signals in regions of interest
When images have been treated and motion corrected, they are ready to be analysed. Fluorescence in regions of interest (ROI) can be measured over time. The selection of ROI can easily be done by hand (ROI Manager in Fiji) in very scattered populations, but for large data sets that contain a dense populations of neurons, automated cell-sorting algorithms may be better suited to extract spatial and temporal properties of individual cellular Ca2+ signals (Mukamel et al. 2009, Pnevmatikakis et al. 2016). In brief, most cell identification algorithms run principal component analysis (PCA) to remove signals that mostly encode noise. Dimension reduction is then followed by independent component analysis (ICA) to identify the spatial and temporal properties of visualized cells by segmenting the data into statistically independent spatial and temporal signals. PCA/ICA relies on statistical independence of signals and fluorescent signals will be identified as cells only if their activities are not synchronised and if they do not have the same pixel coordinates. The extracted signals can then be manually sorted to confirm that they represent cellular Ca2+ transients from spatially defined GCaMP-expressing cells. This technique is particularly helpful for the analysis of dense populations and allows the rapid identification of thousands of neurons (Ziv et al. 2013, Jennings et al. 2015), but has also been used to successfully extract individual cellular signals from relatively sparse neuronal populations such as AGRP neurons located within the arcuate nucleus (Betley et al. 2015). Alternatively, constrained nonnegative matrix factorization (CNMF) approaches have been developed for in vivo Ca2+ recording analysis (Zhou et al. 2018) that have proven to be more accurate than PCA/ICA methods. The main advantage is that CNMF methods have the capability of demixing spatially overlapping neurons. The CNMF-E (Constrained Nonnegative Matrix Factorization for microEndoscopic data) variant has an added model to estimate and account for time-varying background fluctuations in fluorescence (Zhou et al. 2018, Aharoni & Hoogland 2019). It is however important to note that all computational methods also have limitations that can reduce the accuracy of data interpretation (Resendez et al. 2016), and it is currently unclear which method is best suited for Ca2+ indicator data from epifluorescence deep-brain imaging. For a complete review of Ca2+ imaging data analysis and alternative techniques see (Pnevmatikakis 2019).
Spike detection
Spike inference methods aim to estimate the spike times of a neuron given its isolated fluorescence trace. After ROIs of each active neuron have been identified, δF/F traces can be extracted then deconvolved to approximate spiking activity of each segment. This process can be challenging due to the unknown and often non-linear nature of the indicator dynamics, the presence of measurement noise, and the relatively low imaging rate of typical recordings (Pnevmatikakis 2019). While CNMF methods have been successfully used to infer spikes, it should be emphasized that the signal reported by a fluorescent indicator is a convolution of the Ca2+ transients and the indicator response. Although a good temporal relationship between neuronal firing and GCaMP signals has been reported for several neuronal cell types (Chen et al. 2013, Dana et al. 2019), this relationship cannot be taken for granted and Ca2+ transients should not be seen as electrical recordings. In order to help with the interpretation of in vivo GCaMP fluorescence dynamics in specific neuronal populations, it can be informative to undertake dual electrical-Ca2+ imaging experiments in targeted neurons in vitro before performing in vivo experiments.
Analysis packages
To facilitate the management of big data sets as well as data analysis by non-experts, open access analysis packages have become available and are constantly improving. The following three packages have been used successfully by the miniscope community: (1) CaImAn, a computational toolbox for large scale calcium imaging analysis, which includes movie handling, motion correction, source extraction, spike deconvolution and result visualisation (Giovannucci et al. 2019), which is the most up-to-date package; (2) MiniscoPy, a python-based package adapted from CaImAn specifically to analyse miniscope recordings (Zhou et al. 2018); and (3) MiniscopeAnalysis, an updated version of the initial miniscope analysis package developed for the miniscope project. As an alternative to these packages, Lu and colleagues (Lu et al. 2018) have developed a fully automated algorithm called the miniscope 1-photon imaging pipeline (MIN1PIPE), which contains several stand-alone modules and can handle a wide range of imaging conditions and qualities with minimal parameter tuning, and automatically and accurately isolate spatially localized neural signals. We have worked with MIN1PIPE and found it efficient and easy to use and adapt to our needs without having to be a specialist in data analysis.
Inscopix clients will benefit from the user-friendly ‘Inscopix Data Processing’ software that performs all steps of the analysis (filter, motion correction, cell registration for longitudinal studies, extraction of the signal) with very limited parameter tuning. We have found it very easy to use and convenient for recordings with good SNR and limited motion artefacts.
Computer/hard disk specifications
The high-definition data acquired during Ca2+ imaging sessions require a significant amount of hard disk space during recording (acquiring 30 min of data at the frame rate of 20 Hz with the nVista miniscope requires approximately 110 GB of hard disk space) as well as a substantial amount of space for data storage. This problem should be thought through before starting any recordings. Moreover, in order to perform longitudinal studies, movies from different recording sessions will need to be concatenated into a single large file, and powerful computers with numerous cores will be required for analysis.
Readout and physiological consequence of the signals recorded
Once the recordings have been analysed and spatial and temporal data have been extracted, it can still be a challenge to understand the signals. Indeed, within a defined neuronal population, subgroups or individual neurons may be active at different time points, so it is critical to find ways to distinguish the activity we are interested in from the rest.
Behavioural/environmental changes
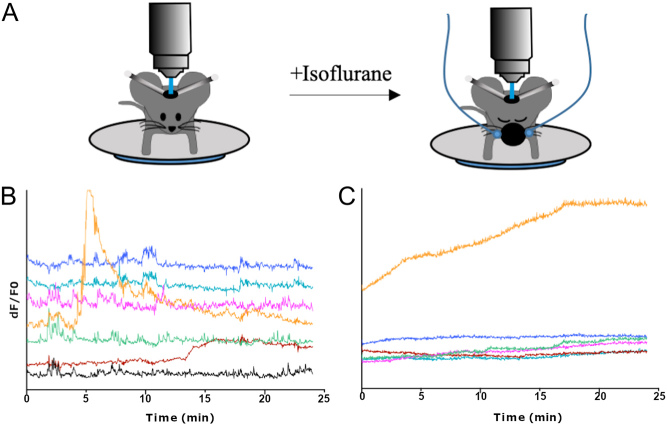
One of the most notable benefits provided by in vivo imaging of neuronal activity in conscious animals is that it is possible to correlate the signal with contextual changes in environment (e.g. time of the day, temperature, light intensity), behaviour (e.g. environmental exploration, time-locked stimulus presentations), or pharmacological induced alterations of network activity. Indeed, the ability to image cell type-specific activity during naturalistic animal behaviour allows this imaging protocol to be applied to a wide array of experimental manipulations, not only to answer questions that could not be tackled using previous methods for monitoring of neural activity, but to provide clues about the significance of the recorded calcium signals. With the ability to record activity across several days/weeks, each animal can become its own control. But what is perhaps more valuable is that because individual neurons can be registered and therefore studied over several recording sessions, each neuron can become its own control. We have used this experimental design to study the effect of isoflurane on hypothalamic neuronal activity. General anaesthetics, especially isoflurane, are routinely used to induce a sleep-like state, allowing otherwise painful or stressful procedures to be performed, but have been long known to alter hormonal secretion. For example, in rats fed adlibitum, isoflurane increases glycemia, glucagon and GH levels but causes decreases in insulin, ACTH and corticosterone levels (Saha et al. 2005). Similarly, pulsatile LH secretion and LH concentrations are diminished in anaesthetised mouse models (Campos & Herbison 2014). How exactly isoflurane works in the brain is not fully understood (Campagna et al. 2003), and although its effect on neurons has not been studied in real-time in vivo, studies have reported disrupted calcium activity in astrocytes (Thrane et al. 2012) and altered blood flow in the brain (Rungta et al. 2017) following isoflurane anaesthesia. Armed with the ability to image hypothalamic neurons in situ, we have been able to characterise the effect of isoflurane on the Ca2+ dynamics of arcuate nucleus neurons. We performed Ca2+ imaging in head-restrained mice first in awake conditions, and then for a 25-min period following anaesthesia induction. We monitored the same neurons in both conditions and found that isoflurane greatly altered the Ca2+ activity of the recorded neurons (Fig. 4) (P Campos and P Mollard, unpublished observations).
Figure 4.
Isoflurane changes the calcium activity of arcuate nucleus neurons (A) 25-min-long in vivo recordings of arcuate nucleus neurons were performed in head-fixed mice, mice were then anaesthetised with isoflurane and the same neurons were recorded for another 25 min. (B) Time-lapse recordings of calcium (GCaMP6s) activity of 7 arcuate nucleus neurons in an awake adult C57Bl6 male. (C) Time-lapse recordings of calcium activity of the same 7 arcuate nucleus neurons in the same anaesthetised adult male.
Blood sampling
An important advantage of working with neuroendocrine circuits is that the final readout of the network and its physiological relevance can in many cases be readily assessed through changes in circulating hormone concentrations. The recent advance in ultra-sensitive hormone assays for pituitary hormones (Steyn et al. 2011, 2013, Guillou et al. 2015) have allowed researchers to increase the sampling frequency while decreasing blood volumes needed to get a high-resolution reading of hormonal dynamics. Two main techniques can be effectively combined with in vivo imaging of hypothalamic neurons.
Tail-tip method
The tail-tip technique involves cutting the last 1 mm of tail tip and then collecting small 3–5 μL volumes of blood that can be analysed for hormone concentration using ultra-sensitive assays. The blood flow is encouraged by gently massaging the tail and stops spontaneously when stroking is stopped. Because of the size of the samples this technique results in minimal blood loss and has been shown to be very efficient in characterising LH and GH profiles in mice (Steyn et al. 2011, 2013). To succeed in using this blood sampling method thorough training is necessary, particularly as animals will be either tethered or head-restrained. Animals need to get used to staying in the hand of the experimenter, being restrained by holding the base of the tail, and having their tail massaged. It is important to note that rats are more sensitive than mice with their tail, and we have failed to measure LH surges in female rats after taking frequent blood samples using this technique.
Automated blood sampling
To collect a series of blood samples at high frequency, animals can be implanted with vascular cannula(s) and connected to an automated blood sampling (ABS) system. These systems withdraw small blood samples via the vascular cannula, which are sent to a fraction collector where they are deposited into collection tubes. The advantage of ABS systems is that the blood sampling procedure can be carried out with minimal disturbance to the animal, and the sampling frequency can be specified according to the goal of the study. These experiments are considered stress-free for the animals and have been used extensively to study the regulation of the hypothalamic-pituitary-adrenal axis (Windle et al. 1998, Walker et al. 2012). There are, however, some disadvantages to this technique. First, the animals need to be implanted with a vascular cannula placed in the jugular vein. Because of the difficulty of this surgery, these experiments, although possible in mice (Adams et al. 2015) are better-suited to larger rodents such as rats. Second, the sample volumes are typically greater than those collected using the tail-tip technique. Third, this technique is particularly suited for the measurement of stable hormones such as corticosterone (Walker et al. 2012). Finally, as animals need to be tethered to perform ABS studies, attention is required to ensure the cable from the miniscope does not become tangled.
Conclusions
Deep-brain imaging techniques have the potential to produce data that were long thought impossible to obtain because of the inaccessibility of the hypothalamic-pituitary system. Overall, being able to study the activity of specific hypothalamic neurons in awake animals and in real time offers an incredible and invaluable opportunity to broaden our understanding of how the brain controls endocrine function. It also gives us the opportunity to study why endocrine functions become disrupted in pathophysiological conditions, and how these changes may lead to the development or even protection from pathological consequences. Furthermore, in vivo brain imaging in conscious animals permits longitudinal studies, thus offering the capability to use animals (and even individual neurons) as internal controls prior to a challenge (e.g. stress, pregnancy, circadian perturbation), which is in-line with 3Rs guidelines and compares favourably with approaches involving numerous animals to provide adequate n numbers in test and control groups. While these techniques were long thought to be available only to laboratories specialised in optics and engineering, or to laboratories with sufficient funding to purchase ready-made equipment, they are now assessible to the wider endocrine field with the help of open source resources. It is expected that high-quality research and high-impact publications emerging from these methodologies will benefit the scientific and medical communities and open up new research opportunities. While this review has focused on imaging Ca2+ dynamics within neurons, many other indicators exist. Of particular interest are Flamindo (Odaka et al. 2014) and R-FlincA (Ohta et al. 2018), which are single-wavelength indicators that permit imaging of 3′5′-cyclic adenosine monophosphate (cAMP) dynamics in living organisms. Likewise iATPSnFRS allows imaging of extracellular and cytosolic ATP (ATP) in astrocytes and neurons (Lobas et al. 2019). Intensive efforts have also resulted in the development of modified G-protein-coupled receptors in which a permuted GFP has been inserted into the third loop in order to monitor various signalling molecules. While this technology currently only works for the detection of small neurotransmitters such as acetylcholine (Jing et al. 2018) and dopamine (Patriarchi et al. 2018), one can hope that these tools will soon be suitable for in vivo detection of peptides and neuropeptides.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
P C and J J W acknowledge financial support from the Medical Research Council (fellowship MR/N008936/1 to J J W) and the Engineering and Physical Sciences Research Council (grant EP/N014391/1 to J J W). P C and P M acknowledge financial support from the Agence Nationale de la Recherche (ANR-15-CE14–0012-01, ANR-18-CE14–0017-01), France-Bioimaging (INBS10-GaL/AR-11/12), Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Université de Montpellier, and Fondation pour la Recherche Médicale (DEQ20150331732).
Acknowledgments
The authors acknowledge Prof. Bradley Lowell for kindly providing Trh-IRES-Cre mice.
References
- Adams JM, Otero-Corchon V, Hammond GL, Veldhuis JD, Qi N, Low MJ. 2015. Somatostatin is essential for the sexual dimorphism of GH secretion, corticosteroid-binding globulin production, and corticosterone levels in mice. Endocrinology 156 1052–1065. ( 10.1210/en.2014-1429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni D, Hoogland TM. 2019. Circuit investigations with open-source miniaturized microscopes: past, present and future. Frontiers in Cellular Neuroscience 13 141 ( 10.3389/fncel.2019.00141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Carreras Calderón N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger MB, Severi KE, Macklin JJ, et al 2013. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Frontiers in Molecular Neuroscience 6 2 ( 10.3389/fnmol.2013.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki R, Tsubota T, Goya Y, Benucci A. 2017. An automated platform for high-throughput mouse behavior and physiology with voluntary head-fixation. Nature Communications 8 1196 ( 10.1038/s41467-017-01371-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S. 2013. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS ONE 8 e76310 ( 10.1371/journal.pone.0076310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. 2012. Deconstruction of a neural circuit for hunger. Nature 488 172–177. ( 10.1038/nature11270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando Y, Sakamoto M, Kim S, Ayzenshtat I, Yuste R. 2019. Comparative evaluation of genetically encoded voltage indicators. Cell Reports 26 802.e4–813.e4. ( 10.1016/j.celrep.2018.12.088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto RPJ, Messerschmidt B, Schnitzer MJ. 2009. In vivo fluorescence imaging with high-resolution microlenses. Nature Methods 6 511–512. ( 10.1038/nmeth.1339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. 2015. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521 180–185. ( 10.1038/nature14416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocarsly ME, Jiang WC, Wang C, Dudman JT, Ji N, Aponte Y. 2015. Minimally invasive microendoscopy system for in vivo functional imaging of deep nuclei in the mouse brain. Biomedical Optics Express 6 4546–4556. ( 10.1364/BOE.6.004546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, Forman SA. 2003. Mechanisms of actions of inhaled anesthetics. New England Journal of Medicine 348 2110–2124. ( 10.1056/NEJMra021261) [DOI] [PubMed] [Google Scholar]
- Campos P, Herbison AE. 2014. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. PNAS 111 18387–18392. ( 10.1073/pnas.1415226112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Sew T, Montero L, Burton EA, Greenamyre JT. 2011. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Experimental Neurology 228 41–52. ( 10.1016/j.expneurol.2010.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Cichon J, Wang W, Qiu L, Lee SJ, Campbell NR, Destefino N, Goard MJ, Fu Z, Yasuda R, et al 2012. Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron 76 297–308. ( 10.1016/j.neuron.2012.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499 295–300. ( 10.1038/nature12354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, et al 2017. Definition of the hypothalamic GnRH pulse generator in mice. PNAS 114 E10216–E10223. ( 10.1073/pnas.1713897114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold PH, Rink TJ. 1987. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochemical Journal 248 313–328. ( 10.1042/bj2480313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. 1999. The impact of molecular biology on neuroscience. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences 354 2021–2025. ( 10.1098/rstb.1999.0541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Chen TW, Hu A, Shields BC, Guo C, Looger LL, Kim DS, Svoboda K. 2014. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS ONE 9 e108697 ( 10.1371/journal.pone.0108697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, et al 2016. Sensitive red protein calcium indicators for imaging neural activity. eLife 5 e12727 ( 10.7554/eLife.12727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, et al 2019. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nature Methods 16 649–657. ( 10.1038/s41592-019-0435-6) [DOI] [PubMed] [Google Scholar]
- Dubbs A, Guevara J, Yuste R. 2016. Moco: fast motion correction for calcium imaging. Frontiers in Neuroinformatics 10 6 ( 10.3389/fninf.2016.00006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. 2011. The development and application of optogenetics. Annual Review of Neuroscience 34 389–412. ( 10.1146/annurev-neuro-061010-113817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala A, Spall T, Diegelmann S, Eisermann B, Sachse S, Devaud JM, Buchner E, Galizia CG. 2002. Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons. Current Biology 12 1877–1884. ( 10.1016/s0960-9822(02)01239-3) [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Masurkar AV, Xing J, Imamura F, Xiong W, Nagayama S, Mutoh H, Greer CA, Knöpfel T, Chen WR. 2009. Optical imaging of postsynaptic odor representation in the glomerular layer of the mouse olfactory bulb. Journal of Neurophysiology 102 817–830. ( 10.1152/jn.00020.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. 2016. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nature Communications 7 11937 ( 10.1038/ncomms11937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. 2011. Miniaturized integration of a fluorescence microscope. Nature Methods 8 871–878. ( 10.1038/nmeth.1694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci A, Friedrich J, Gunn P, Kalfon J, Brown BL, Koay SA, Taxidis J, Najafi F, Gauthier JL, Zhou P, et al 2019. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8 e38173 ( 10.7554/eLife.38173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas A, Hübener M, Bonhoeffer T, Goltstein PM. 2019. Benchmarking miniaturized microscopy against two-photon calcium imaging using single-cell orientation tuning in mouse visual cortex. PLoS ONE 14 e0214954 ( 10.1371/journal.pone.0214954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. 2008. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. Journal of Virology 82 5887–5911. ( 10.1128/JVI.00254-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry 260 3440–3450. [PubMed] [Google Scholar]
- Guillou A, Romanò N, Steyn F, Abitbol K, Le Tissier P, Bonnefont X, Chen C, Mollard P, Martin AO. 2015. Assessment of lactotroph axis functionality in mice: longitudinal monitoring of PRL secretion by ultrasensitive-ELISA. Endocrinology 156 1924–1930. ( 10.1210/en.2014-1571) [DOI] [PubMed] [Google Scholar]
- Gulati S, Cao VY, Otte S. 2017. Multi-layer cortical Ca2+ imaging in freely moving mice with prism probes and miniaturized fluorescence microscopy. Journal of Visualized Experiments 124 e55579 ( 10.3791/55579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SL, Leek AN, Richman EH, Tjalkens RB. 2017. Cellular selectivity of AAV serotypes for gene delivery in neurons and astrocytes by neonatal intracerebroventricular injection. PLoS ONE 12 e0188830 ( 10.1371/journal.pone.0188830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SY, McLennan T, Czieselsky K, Herbison AE. 2015. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. PNAS 112 13109–13114. ( 10.1073/pnas.1512243112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Mizushima T, Chijiwa T, Nakamura M, Suemizu H. 2017. Efficient production of recombinant adeno-associated viral vector, serotype DJ/8, carrying the GFP gene. Virus Research 238 63–68. ( 10.1016/j.virusres.2017.05.017) [DOI] [PubMed] [Google Scholar]
- Helmchen F, Denk W, Kerr JND. 2013. Miniaturization of two-photon microscopy for imaging in freely moving animals. Cold Spring Harbor Protocols 2013 904–913. ( 10.1101/pdb.top078147) [DOI] [PubMed] [Google Scholar]
- Hoa O, Lafont C, Fontanaud P, Guillou A, Kemkem Y, Kineman RD, Luque RM, Fiordelisio Coll T, Le Tissier P, Mollard P. 2019. Imaging and manipulating pituitary function in the awake mouse. Endocrinology 160 2271–2281. ( 10.1210/en.2019-00297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang JC, Johnson KP, Madisen L, Zeng H, Kerschensteiner D. 2017. Local processing in neurites of VGluT3-expressing amacrine cells differentially organizes visual information. eLife 6 e31307 ( 10.7554/eLife.31307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, et al 2015. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160 516–527. ( 10.1016/j.cell.2014.12.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M, Zhang P, Wang G, Feng J, Mesik L, Zeng J, Jiang H, Wang S, Looby JC, Guagliardo NA, et al 2018. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nature Biotechnology 36 726–737. ( 10.1038/nbt.4184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, During MJ. 1994. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nature Genetics 8 148–154. ( 10.1038/ng1094-148) [DOI] [PubMed] [Google Scholar]
- Kim P, Puoris’haag M, Côté D, Lin CP, Yun SH. 2008. In vivo confocal and multiphoton microendoscopy. Journal of Biomedical Optics 13 010501 ( 10.1117/1.2839043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Nagarajan N, Pastuzyn E, Jenks K, Capecchi M, Shepherd J, Menon R. 2017. Deep-brain imaging via epi-fluorescence computational cannula microscopy. Scientific Reports 7 44791 ( 10.1038/srep44791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al 2014. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507 238–242. ( 10.1038/nature12956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tissier P, Campos P, Lafont C, Romanò N, Hodson DJ, Mollard P. 2017. An updated view of hypothalamic-vascular-pituitary unit function and plasticity. Nature Reviews: Endocrinology 13 257–267. ( 10.1038/nrendo.2016.193) [DOI] [PubMed] [Google Scholar]
- Lecoq J, Savall J, Vučinić D, Grewe BF, Kim H, Li JZ, Kitch LJ, Schnitzer MJ. 2014. Visualizing mammalian brain area interactions by dual-axis two-photon calcium imaging. Nature Neuroscience 17 1825–1829. ( 10.1038/nn.3867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, Murthy VN, Dulac C. 2017. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171 1176.e17–1190.e17. ( 10.1016/j.cell.2017.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti WA, Perkins LN, Leman DP, Gardner TJ. 2017. An open source, wireless capable miniature microscope system. Journal of Neural Engineering 14 045001 ( 10.1088/1741-2552/aa6806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MZ, Schnitzer MJ. 2016. Genetically encoded indicators of neuronal activity. Nature Neuroscience 19 1142–1153. ( 10.1038/nn.4359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobas MA, Tao R, Nagai J, Kronschläger MT, Borden PM, Marvin JS, Looger LL, Khakh BS. 2019. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nature Communications 10 711 ( 10.1038/s41467-019-08441-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Li C, Singh-Alvarado J, Zhou ZC, Fröhlich F, Mooney R, Wang F. 2018. MIN1PIPE: A miniscope 1-photon-based calcium imaging signal extraction pipeline. Cell Reports 23 3673–3684. ( 10.1016/j.celrep.2018.05.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank M, Santos AF, Direnberger S, Mrsic-Flogel TD, Hofer SB, Stein V, Hendel T, Reiff DF, Levelt C, Borst A, et al 2008. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nature Methods 5 805–811. ( 10.1038/nmeth.1243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, O’Connor DH, Scheuss V, Nakai J, Svoboda K. 2008. Characterization and subcellular targeting of GCaMP-type genetically-encoded calcium indicators. PLoS ONE 3 e1796 ( 10.1371/journal.pone.0001796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Liang Y, Sarsfield S, Jiang WC, Lu R, Dudman JT, Aponte Y, Ji N. 2019. High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo. eLife 8 e40805 ( 10.7554/eLife.40805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagni E, Resta F, Conti E, Scaglione A, Pasquini M, Micera S, Mascaro ALA, Pavone FS. 2019. Wide-field imaging of cortical neuronal activity with red-shifted functional indicators during motor task execution. Journal of Physics D 52 074001 ( 10.1088/1361-6463/aaf26c) [DOI] [Google Scholar]
- Mukamel EA, Nimmerjahn A, Schnitzer MJ. 2009. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 63 747–760. ( 10.1016/j.neuron.2009.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Boyd JD, Bolaños F, Vanni MP, Silasi G, Haupt D, LeDue JM. 2016. High-throughput automated home-cage mesoscopic functional imaging of mouse cortex. Nature Communications 7 11611 ( 10.1038/ncomms11611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. 2001. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature Biotechnology 19 137–141. ( 10.1038/84397) [DOI] [PubMed] [Google Scholar]
- Odaka H, Arai S, Inoue T, Kitaguchi T. 2014. Genetically-encoded yellow fluorescent cAMP indicator with an expanded dynamic range for dual-color imaging. PLoS ONE 9 e100252 ( 10.1371/journal.pone.0100252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura M, Matsuzaki M, Kasai H, Imoto K, Nakai J. 2005. Genetically encoded bright Ca2+ probe applicable for dynamic Ca2+ imaging of dendritic spines. Analytical Chemistry 77 5861–5869. ( 10.1021/ac0506837) [DOI] [PubMed] [Google Scholar]
- Ohta Y, Furuta T, Nagai T, Horikawa K. 2018. Red fluorescent cAMP indicator with increased affinity and expanded dynamic range. Scientific Reports 8 1866 ( 10.1038/s41598-018-20251-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbay BN, Futia GL, Ma M, Bright VM, Gopinath JT, Hughes EG, Restrepo D, Gibson EA. 2018. Three dimensional two-photon brain imaging in freely moving mice using a miniature fiber coupled microscope with active axial-scanning. Scientific Reports 8 8108 ( 10.1038/s41598-018-26326-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Tsien RY. 2006. Measuring calcium signaling using genetically targetable fluorescent indicators. Nature Protocols 1 1057–1065. ( 10.1038/nprot.2006.172) [DOI] [PubMed] [Google Scholar]
- Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, Folk RW, Broussard GJ, Liang R, Jang MJ, et al 2018. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360 eaat4422 ( 10.1126/science.aat4422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Koldenkova V, Nagai T. 2013. Genetically encoded Ca(2+) indicators: properties and evaluation. Biochimica et Biophysica Acta 1833 1787–1797. ( 10.1016/j.bbamcr.2013.01.011) [DOI] [PubMed] [Google Scholar]
- Pinto L, Dan Y. 2015. Cell-type-specific activity in prefrontal cortex during goal-directed behavior. Neuron 87 437–450. ( 10.1016/j.neuron.2015.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnevmatikakis EA. 2019. Analysis pipelines for calcium imaging data. Current Opinion in Neurobiology 55 15–21. ( 10.1016/j.conb.2018.11.004) [DOI] [PubMed] [Google Scholar]
- Pnevmatikakis EA, Giovannucci A. 2017. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. Journal of Neuroscience Methods 291 83–94. ( 10.1016/j.jneumeth.2017.07.031) [DOI] [PubMed] [Google Scholar]
- Pnevmatikakis EA, Soudry D, Gao Y, Machado TA, Merel J, Pfau D, Reardon T, Mu Y, Lacefield C, Yang W, et al 2016. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron 89 285–299. ( 10.1016/j.neuron.2015.11.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De Guglielmo G, Crawford E, et al 2015. A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Frontiers in Neuroscience 9 487 ( 10.3389/fnins.2015.00487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedios R, Kennedy A, Zelikowsky M, Grewe BF, Schnitzer MJ, Anderson DJ. 2017. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550 388–392. ( 10.1038/nature23885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Jennings JH, Ung RL, Namboodiri VMK, Zhou ZC, Otis JM, Nomura H, McHenry JA, Kosyk O, Stuber GD. 2016. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nature Protocols 11 566–597. ( 10.1038/nprot.2016.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpár A, Mulder J, Clotman F, Keimpema E, et al 2017. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nature Neuroscience 20 176–188. ( 10.1038/nn.4462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungta RL, Osmanski BF, Boido D, Tanter M, Charpak S. 2017. Light controls cerebral blood flow in naive animals. Nature Communications 8 14191 ( 10.1038/ncomms14191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. 2005. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Experimental Biology and Medicine 230 777–784. ( 10.1177/153537020523001012) [DOI] [PubMed] [Google Scholar]
- Sakuma T, Barry MA, Ikeda Y. 2012. Lentiviral vectors: basic to translational. Biochemical Journal 443 603–618. ( 10.1042/BJ20120146) [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Muzyczka N. 2014. AAV-mediated gene therapy for research and therapeutic purposes. Annual Review of Virology 1 427–451. ( 10.1146/annurev-virology-031413-085355) [DOI] [PubMed] [Google Scholar]
- Scott BB, Thiberge SY, Guo C, Tervo DGR, Brody CD, Karpova AY, Tank DW. 2018. Imaging cortical dynamics in GCaMP transgenic rats with a head-mounted widefield macroscope. Neuron 100 1045.e5–1058.e5. ( 10.1016/j.neuron.2018.09.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman T, Aharoni D, Cai DJ, Lee CR, Chavlis S, Page-Harley L, Vetere LM, Feng Y, Yang CY, Mollinedo-Gajate I, et al 2020. Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nature Neuroscience 23 229–238. ( 10.1038/s41593-019-0559-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Desroziers E, Hessler S, Prescott M, Coyle C, Herbison AE, Campbell RE. 2019. Activation of arcuate nucleus GABA neurons promotes luteinizing hormone secretion and reproductive dysfunction: Implications for polycystic ovary syndrome. EBioMedicine 44 582–596. ( 10.1016/j.ebiom.2019.05.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Buetfering C, Lecoq J, Lee CR, Peters AJ, Jacobs EAK, Coen P, Ollerenshaw DR, Valley MT, de Vries SEJ, et al 2017. Aberrant cortical activity in multiple GCaMP6-expressing transgenic mouse lines. eNeuro 4 e0207-17. ( 10.1523/ENEURO.0207-17.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. 2011. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology 152 3165–3171. ( 10.1210/en.2011-0253) [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. 2013. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 154 4939–4945. ( 10.1210/en.2013-1502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, et al 2006. Imaging cellular signals in the heart in vivo: cardiac expression of the high-signal Ca2+ indicator GCaMP2. PNAS 103 4753–4758. ( 10.1073/pnas.0509378103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M. 2012. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. PNAS 109 18974–18979. ( 10.1073/pnas.1209448109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian LS, Hires A, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. 2009. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods 6 875–881. ( 10.1038/nmeth.1398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva A, Jessberger R. 2005. Precise hit: adeno-associated virus in gene targeting. Nature Reviews: Microbiology 3 837–847. ( 10.1038/nrmicro1266) [DOI] [PubMed] [Google Scholar]
- Viskaitis P, Irvine EE, Smith MA, Choudhury AI, Alvarez-Curto E, Glegola JA, Hardy DG, Pedroni SMA, Paiva Pessoa MR, Fernando ABP, et al 2017. Modulation of SF1 neuron activity coordinately regulates both feeding behavior and associated emotional states. Cell Reports 21 3559–3572. ( 10.1016/j.celrep.2017.11.089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voliotis M, Li XF, De Burgh R, Lass G, Lightman SL, O’Byrne KT, Tsaneva-Atanasova K. 2019. The origin of GnRH pulse generation: an integrative mathematical-experimental approach. Journal of Neuroscience 39 9738–9747. ( 10.1523/JNEUROSCI.0828-19.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JJ, Spiga F, Waite E, Zhao Z, Kershaw Y, Terry JR, Lightman SL. 2012. The origin of glucocorticoid hormone oscillations. PLoS Biology 10 e1001341 ( 10.1371/journal.pbio.1001341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ma W. 2019. Hypothalamic and pituitary transcriptome profiling using RNA-sequencing in high-yielding and low-yielding laying hens. Scientific Reports 9 10285 ( 10.1038/s41598-019-46807-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Wood SA, Lightman SL, Ingram CD. 1998. The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology 139 4044–4052. ( 10.1210/endo.139.10.6238) [DOI] [PubMed] [Google Scholar]
- Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, Svoboda K. 2004. Imaging calcium concentration dynamics in small neuronal compartments. Science’s STKE 2004 pl5 ( 10.1126/stke.2192004pl5) [DOI] [PubMed] [Google Scholar]
- Zhang L, Liang B, Barbera G, Hawes S, Zhang Y, Stump K, Baum I, Yang Y, Li Y, Lin DT. 2019. Miniscope GRIN lens system for calcium imaging of neuronal activity from deep brain structures in behaving animals. Current Protocols in Neuroscience 86 e56 ( 10.1002/cpns.56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Resendez SL, Rodriguez-Romaguera J, Jimenez JC, Neufeld SQ, Giovannucci A, Friedrich J, Pnevmatikakis EA, Stuber GD, Hen R, et al 2018. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 7 e28728 ( 10.7554/eLife.28728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Huey EL, Ahn JS, Beutler LR, Tan CL, Kosar S, Bai L, Chen Y, Corpuz TV, Madisen L, et al 2019. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 568 98–102. ( 10.1038/s41586-019-1066-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. 2013. Long-term dynamics of CA1 hippocampal place codes. Nature Neuroscience 16 264–266. ( 10.1038/nn.3329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W, Wu R, Li M, Hu Y, Li Y, Li J, Rong H, Wu H, Xu Y, Lu Y, et al 2017. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nature Methods 14 713–719. ( 10.1038/nmeth.4305) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a