Abstract
Objective
First-line chemotherapy in metastatic neuroendocrine carcinomas (NECs) is based on etoposide and platinum. However, there is no standard concerning second-line treatment. The objective of this study was to evaluate efficacy and tolerance of dacarbazine or temozolomide in metastatic digestive NEC as post first-line treatment.
Material and methods
This study included patients with a metastatic NEC of digestive or unknown primary site. All patients received platinum-etoposide as first-line chemotherapy. Primary endpoint was progression-free survival (PFS). Secondary endpoints were clinical/morphological responses, toxicity, and overall survival (OS).
Results
Twenty-seven patients were included: 17 received dacarbazine and 10 temozolomide as post-first line treatments. Median PFS was 3.0 (95%CI (2.2;3.7)) months. There was no significant difference between dacarbazine and temozolomide on PFS. Clinical and morphological responses were found in 12 and 9 patients, respectively. Median OS was 7.2 (95%CI (2.2;12.2)) months. The toxicity profile was that expected with such treatments.
Conclusion
LV5FU2-dacarbazine or temozolomide-capecitabine chemotherapies allow a temporary clinical response for almost half of patients and/or a morphological response for a third of patients.
Keywords: dacarbazine, temozolomide, alkylating agents, neuroendocrine carcinoma, neuroendocrine tumours.
Introduction
Poorly differentiated neuroendocrine carcinomas (NEC) are a group of neuroendocrine neoplasms with an incidence of 0.18–0.36 per 100,000 inhabitants (1). These tumours have a poor prognosis, and the median overall survival (OS) for metastatic NEC without chemotherapy is 5–7 months (2, 3, 4). First-line chemotherapy is well-defined and based on platinum and etoposide (5). This treatment extends OS up to 11–19 months, with a median duration of response of 8–9 months (2, 3, 4, 6). Then, three regimens for second-line chemotherapy are usually prescribed: FOLFIRI (3, 7), FOLFOX (3, 8), or temozolomide-based (6, 9, 10) regimens. However, survival remains poor under these treatments with a median progression-free survival (PFS) lower than 3–4 months (3, 6, 7, 8, 9, 10). 5-Fluorouracil (5FU) with dacarbazine is an alternative i.v. regimen to oral temozolomide-capecitabine (TEMCAP) regimen for well-differentiated neuroendocrine tumours (NET) (11). This i.v. regimen may be an alternative treatment for NEC, but its efficacy and tolerance have never been studied specifically in NEC. Therefore, we decided to evaluate efficacy and tolerance of dacarbazine- or temozolomide-based chemotherapy, after failure of platinum-etoposide regimen in metastatic NEC of digestive or unknown primary sites.
Patients and methods
Patients
Patients were retrospectively included from five centres of the French Group of Endocrine Tumours (GTE). We included all consecutive patients with a digestive or unknown primary metastatic NEC, treated from 2006 to 2019, who underwent a dacarbazine- or temozolomide-based chemotherapy as second- or further-line treatment. Non-digestive NEC, grade 3 but well-differentiated neuroendocrine tumours (NET), and mixed neuroendocrine-non neuroendocrine neoplasms (MiNEN) were not included. All patients also had to have received platinum-etoposide as first-line chemotherapy, and all were metastatic (synchronous or metachronous) when alkylating agents were initiated.
Patient records were reviewed to collect tumour characteristics, prior treatments, and information on the dacarbazine/temozolomide treatment line (dose, toxicity, and efficacy). The final pathology reports were reviewed to collect tumour characteristics such as location, size, Ki67 proliferation index, grade, and NEN World Health Organization (WHO) classification (12). When the pathologists were not able to distinguish small-cell from large-cell morphology, these were categorised as ‘undefined NEC’. All samples were analysed as part of the routine workflow of the pathology departments, in the EURACAN or ENETS Centers of Excellence for the 19 and 3 patients from Lyon and Gustave-Roussy Institute, respectively. The same technique was used in all centres. Samples were fixed for 24 h in pH-adjusted formalin (pH = 7) followed by paraffin embedment. Ki67 immunohistochemistry (clone MIB1 DAKO, ref M7240, 1/100 dilution) was performed on Ventana benchmark GX automates (Roche). Ki67 index were evaluated according to the ENETS guidelines by manual counting in hot spot areas (percentage of 2000 cells in areas of highest nuclear labeling) (13).
Endpoints
The primary endpoint was PFS. Secondary endpoints were clinical response, biological response, morphological response, toxicity profile, and OS. Clinical response was defined as resolution of tumour-related symptoms (paraneoplastic fever, occlusive syndrome, and pain) or as an improvement of the performance status (PS) by a decrease of at least 1 point. Biological response was reported for both neuron-specific enolase (NSE) and alkaline phosphatase (ALP), in patients with high baseline levels of NSE/ALP, as a >30% decrease in level compared to baseline. Morphological response was based on the RECIST criteria v1.1 (14). Recorded adverse events were defined using the Common Terminology Criteria for Adverse Event version 4.03 (CTCAE v4.03).
Statistical analysis
Categorical variables are expressed as number (percentage). Continuous variables are presented as median (range). PFS and OS were estimated using the Kaplan–Meier method; PFS was calculated from the date of temozolomide- or dacarbazine-based regimen initiation to the date of first progression according to RECIST criteria v1.1, or the beginning of a new anti-tumour treatment, or disease-related death for PFS; and OS to the date of death or last follow-up for OS. We explored the baseline factors potentially influencing PFS by univariate analyses using the Log-rank test for each variable of interest. For continuous parameters, the threshold was defined as the median value of the population. Multivariate analyses using a Cox proportional hazards regression model were performed to identify factors independently associated with prognosis. All significant factors from the univariate analysis (Log-rank P-value <0.10) were included in the multivariate analyses; a P-value of <0.05 was considered statistically significant. The results from the survival analyses are presented with the effect estimates, hazard ratios (HR), and 95% CI. All statistical analyses were performed using IBM-SPSS version 21 (IBM Corporation).
Results
Patients
A total of 27 patients with poorly differentiated NEC were included: 18 males, median age of 64 (range 20–82) years, with a PS1 in 12/27 patients. Among all patients studied, 4/27 had a paraneoplastic syndrome: Cushing syndrome (n = 2), polyneuropathy (n = 1), and paraneoplastic hypercalcemia (n = 1). Primary sites were pancreatic (n = 10), colorectal (n = 7), biliary (n = 5), gastric (n = 3), or unknown (n = 2); the median Ki67 was 80% (range 50–100). All patients were re-evaluated by CT-scan, and 14/27 patients underwent an 18-FDG PET-scan. All patients had metastatic disease, with a median of two sites, mainly in the liver (20/27), distant lymph nodes (15/27), and the lung (5/27) (Table 1).
Table 1.
Patient and tumour baseline characteristics.
| Dacarbazine based (n = 17) | Temozolomide based (n = 10) | P-value | Total (n = 27) | |
|---|---|---|---|---|
| Female, n | 4 | 5 | 0.16 | 9 |
| Median age, years (range) | 59 (20–74) | 68 (44–82) | 0.23 | 64 (20–82) |
| Performance status (PS), n/N | 0.80 | |||
| PS1 | 6/11 | 6/10 | 12/21 | |
| PS2 | 3/11 | 2/10 | 5/21 | |
| PS3 | 2/11 | 2/10 | 4/21 | |
| Paraneoplastic syndromea, n | 4 | 0 | 0.10 | 4 |
| Primary site, n | 0.18 | |||
| Stomach | 3 | 0 | 3 | |
| Colorectal | 4 | 3 | 7 | |
| Pancreatic | 6 | 4 | 10 | |
| Biliary tract | 2 | 3 | 5 | |
| Unknown | 2 | 0 | 2 | |
| Stage at diagnosis, n | 0.19 | |||
| II | 0 | 1 | 1 | |
| III | 1 | 3 | 4 | |
| IV | 16 | 6 | 22 | |
| Synchronous metastases, n | 16 | 6 | 0.03 | 22 |
| Median Ki67, % (range) | 80 (50–100) | 68 (50–90) | 0.92 | 80 (50–100) |
| WHO classification, n | 0.33 | |||
| Large cells NEC | 4 | 4 | 8 | |
| Small cells NEC | 7 | 5 | 12 | |
| Undefined cell size NEC | 6 | 1 | 7 | |
| Median number of metastatic sites (range) | 1 (1–3) | 2 (1–3) | 0.35 | 2 (1–3) |
| Metastatic sites, n | ||||
| Liver | 11 | 9 | 0.15 | 20 |
| Distant lymph nodes | 11 | 4 | 0.21 | 15 |
| Lung | 2 | 3 | 0.24 | 5 |
| Peritoneum | 3 | 1 | 0.53 | 4 |
| Bone | 2 | 0 | 0.26 | 2 |
| Brain | 0 | 1 | 0.18 | 1 |
| Other | 1 | 2 | 0.26 | 3 |
| 18-FDG PET-scan positive, n/N | 9/9 | 5/5 | 14/14 | |
| Median NSE in x ULN (range)b | 3.4 (2.3–90.3) | 3.3 (1.0–59.2) | 0.76 | 3.4 (1.0–90.3) |
| Median ALP in x ULN (range)b | 2.1 (1.0–5.1) | 1.0 (1.0–3.3) | 0.23 | 1.3 (1.0–5.1) |
aCushing syndrome (n = 2), polyneuropathy (n = 1), and hyperparathyroidism (PTHrp, n = 1). bBaseline NSE and ALP were available in 14 and 21 patients, respectively.
ALP, alkaline phosphatase; N, number of patients with available data for this variable; NEC, neuroendocrine carcinoma; NSE, neuron specific enolase; ULN, upper limit of normal; WHO, World Health Organization.
All patients received first-line platin-etoposide chemotherapy, for a median of 6 (range 1–13) cycles; objective response was obtained in 16/27 patients, and the median PFS was 6.2 (95%CI (3.9;8.5)) months. Prior to dacarbazine- or temozolomide-based chemotherapy, 13/27 patients received FOLFIRI as second-line chemotherapy for a median of 5 (range 2–29) cycles; objective response was obtained in 4/13 patients, and the median PFS was 4.7 (95%CI (1.0;8.5)) months (Table 2).
Table 2.
Prior treatment characteristics.
| Dacarbazine based (n = 17) | Temozolomide based (n = 10) | P-value | Total (n = 27) | |
|---|---|---|---|---|
| Primary tumour resection, n | 4 | 6 | 0.06 | 10 |
| First-line chemotherapy | ||||
| Platin-etoposide, n | 17 | 10 | 1 | 27 |
| Median number of cycles (range) | 6 (1–12) | 6 (3–13) | 0.33 | 6 (1–13) |
| Objective response, n | 9 | 7 | 0.38 | 16 |
| Median PFS, months (95%CI) | 4.1 (0.6; 7.6) | 5.7 (2.8; 8.6) | 0.52 | 5.7 (4.2; 7.2) |
| Second-line chemotherapy, n | ||||
| Folfiri, n | 8 | 5 | 0.88 | 13 |
| Median number of cycles (range) | 6 (2–15) | 5 (3–29) | 0.17 | 5 (2–29) |
| Objective response, n/N | 2/8 | 2/5 | 0.57 | 4/13 |
| Median PFS, months (95%CI) | 3.7 (0.0; 7.7) | 6.3 (0.0; 15.6) | 0.30 | 4.7 (1.0; 8.5) |
| Number of prior systemic treatments, n | 0.51 | |||
| 1 | 9 | 5 | 14 | |
| 2 | 7 | 4 | 11 | |
| ≥3 | 1 | 1 | 2 |
N, number of patients with available data for this variable.
OS, overall survival; PFS, progression-free survival.
Chemotherapy regimens
Among the 27 patients included, 17 received a dacarbazine-based regimen and 10 a temozolomide-based regimen. In the dacarbazine group, 12/17 patients received the LV5FU2-dacarbazine regimen (11) and 5/17 patients received the FED regimen (15) (5FU, epirubicine, and dacarbazine). In the temozolomide group, 7/10 patients received the TEMCAP regimen (11), 2/10 patients received temozolomide alone, and 1/10 patients received an association of temozolomide, capecitabine, and bevacizumab. The median number of cycles was 4 for both dacarbazine- and temozolomide-based regimens. Nine out of twenty-seven patients received granulocyte-colony stimulating factor (G-CSF, Table 3). There were no significant differences in characteristics between the two groups, except that the dacarbazine population presented with significantly more synchronous metastasis (Tables 1, 2 and 3).
Table 3.
Treatment characteristics and results of dacarbazine-based or temozolomide-based chemotherapy.
| Dacarbazine based (n = 17) | Temozolomide based (n = 10) | P-value | Total (n = 27) | |
|---|---|---|---|---|
| Median time from diagnosis to first cycle, months (range) | 9.8 (0.2–25.0) | 9.5 (0.6–37.2) | 0.80 | 9.8 (0.2–37.2) |
| Median duration of treatment, months (range) | 2.9 (0.1–13.1) | 4.2 (0.1–8.0) | 0.63 | 3.0 (0.1–13.1) |
| Median number of cycles of tem- or dacarbazine (range) | 4 (1–6) | 4 (1–8) | 0.22 | 4 (1–8) |
| Median number of cycles of 5FU (range) | 4 (1–6) | 3 (0–6) | 0.15 | 3 (0–6) |
| Patient receiving G-CSF, n | 7 | 2 | 0.48 | 9 |
| Clinical response, n/N | 9/16 | 3/10 | 0.19 | 12/26 |
| Biological response, n/N | ||||
| NSE | 2/4 | 1/3 | 0.08 | 3/7 |
| ALP | 4/12 | 1/8 | 0.44 | 5/20 |
| Objective response, n | 7 | 2 | 0.26 | 9 |
| Median progression-free survival, months (95%CI) | 2.9 (2.0;3.8) | 3.4 (1.2;5.6) | 0.42 | 3.0 (2.2;3.7) |
| Median overall survival, months (95%CI) | 5.0 (1.6;8.4) | 8.6 (0.5;16.7) | 0.85 | 7.2 (2.2;12.2) |
N, number of patients with available data for this variable.
5FU, 5-fluorouracil; ALP, alkaline phosphatase; G-CSF, granulocyte-colony stimulating factor; NSE, neuron specific enolase; Tem, temozolomide.
Progression-free survival
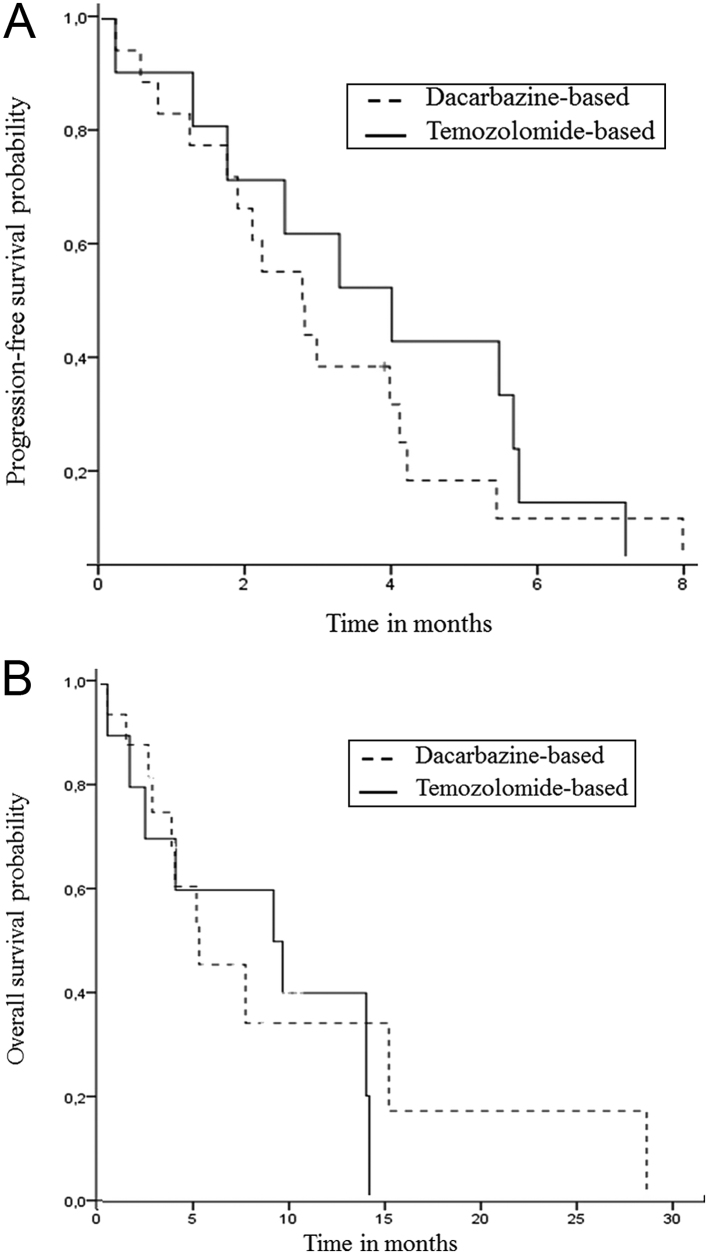
The median PFS was 3.0 (95%CI (2.2;3.7)) months. There was no significant difference (HR 1.40; 95%CI (0.61;3.21); P = 0.42) between the dacarbazine and temozolomide groups (Fig. 1A and Tables 3, 4). Male gender, Ki67 below 80%, absence of bone metastasis, and only one prior systemic therapy were associated (P < 0.1) with better PFS and were included in the multivariate analysis; then, only the absence of bone metastasis remained associated with an increased PFS (HR 0.05; 95%CI (0.005–0.43); P = 0.007; Table 4). For patients with an objective response (n = 9), PFS was significantly longer (5.5 months; 95%CI (3.6;7.3)) than for non-responders (2.1 months; 95%CI (1.4;2.8); P = 0.003); this was not included in the multivariate analysis because it was not available at chemotherapy initiation.
Figure 1.
Progression-free survival (A) and overall survival (B) from the beginning of dacarbazine- or temozolomide-based chemotherapy.
Table 4.
Factors associated with progression-free survival in univariate and multivariate analyses.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | (95%CI) | P-value | Hazard ratio | (95%CI) | P-value | |
| Age, <60 vs ≥60 years | 1.80 | (0.76;4.27) | 0.18 | |||
| Gender, female vs male | 2.79 | (1.08;7.19) | 0.03 | 2.99 | (0.93;9.56) | 0.065 |
| Performance Status, 0–1 vs ≥2 | 0.61 | (0.24;1.53) | 0.29 | |||
| Primary site, other vs pancreas | 0.49 | (0.21;1.16) | 0.11 | |||
| Ki67, <80% vs ≥80% | 0.47 | (0.19;1.13) | 0.09 | 0.58 | (0.22;1.54) | 0.271 |
| Number of metastatic sites, 1 vs >1 | 1.63 | (0.68;3.95) | 0.27 | |||
| Liver Metastasis, no vs yes | 0.95 | (0.39;2.30) | 0.91 | |||
| Lymph nodes metastasis, no vs yes | 1.24 | (0.55;2.79) | 0.60 | |||
| Lung Metastasis, no vs yes | 0.94 | (0.35;2.52) | 0.90 | |||
| Peritoneum metastasis, no vs yes | 0.61 | (0.21;1.82) | 0.38 | |||
| Bone metastasis, no vs yes | 0.07 | (0.01;0.51) | 0.01 | 0.05 | (0.005;0.43) | 0.007 |
| Prior systemic therapy, 1 vs ≥2 | 0.45 | (0.19;1.04) | 0.06 | 0.64 | (0.24;1.69) | 0.37 |
| Dacarbazine- vs temozolomide-based chemotherapy | 1.40 | (0.61;3.21) | 0.42 | |||
Secondary endpoints
A clinical response was obtained in 12/26 patients: ten on PS and two on tumour-related symptoms (resolution of an occlusive syndrome and paraneoplastic fever). A biological response on ALP was obtained in 5/20 patients. Objective response (OR) was obtained in 9/27 patients: 7/17 in the dacarbazine group and 2/10 in the temozolomide group. The median OS was 7.2 (95%CI (2.2–12.2)) months, without significant difference between both regimens (Fig. 1B and Table 3). For patients with an objective response (n = 9), OS was significantly longer (13.2 months; 95%CI (2.0;24.3)) than for non-responders (4.8 months; 95%CI (1.2;8.5); P = 0.032).
There was no treatment-related death. Eight out of twenty-seven patients experienced adverse events (AE) of grade ≥3 based on CTCAE v4.03. The most frequent AE were fatigue for 21/24 patients, anaemia for 18/23 patients, and thrombopenia for 12/23 patients. The most frequently G3-4 AE were neutropenia (2/23), thrombopenia (2/23), and fatigue (2/24). No patients experienced febrile neutropenia. Patients treated by temozolomide-based chemotherapy experienced significantly more neutropenia and thrombocytopenia than those treated by dacarbazine-based chemotherapy (Table 5).
Table 5.
Treatment-related adverse eventsa.
| Dacarbazine-based (n = 17) | Temozolomide-based (n = 10) | P-value | Total (n = 27) | |||||
|---|---|---|---|---|---|---|---|---|
| All grades, n/N | Grades 3–4, n/N | All grades, n/N | Grades 3–4, n/N | All grades | Grades 3–4 | All grades, n/N | Grades 3–4, n/N | |
| Fatigue | 12/14 | 1/14 | 9/10 | 1/10 | 0.36 | 0.83 | 21/24 | 2/24 |
| Nausea/vomiting | 7/13 | 0/13 | 2/9 | 1/9 | 0.15 | 0.05 | 9/22 | 1/22 |
| Diarrhoea | 7/15 | 1/15 | 2/9 | 0/9 | 0.58 | 0.57 | 9/24 | 1/24 |
| Mucositis | 1/13 | 1/13 | 2/9 | 0/9 | 0.16 | 0.08 | 3/22 | 1/22 |
| Palmar-plantar erythrodysesthesia syndrome | 1/13 | 0/13 | 4/9 | 1/9 | 0.12 | 0.58 | 5/22 | 1/22 |
| Anaemia | 10/14 | 0/14 | 8/9 | 1/9 | 0.49 | 0.25 | 18/23 | 1/23 |
| Neutropenia | 1/14 | 1/14 | 5/9 | 1/9 | 0.04 | 0.12 | 6/23 | 2/23 |
| Febrile neutropenia | 0/14 | 0/14 | 0/9 | 0/9 | - | - | 0/23 | 0/23 |
| Thrombocytopenia | 4/14 | 1/14 | 8/9 | 1/9 | 0.03 | 0.58 | 12/23 | 2/23 |
| Otherb | 4/15 | 0/15 | 2/9 | 0/9 | 0.73 | - | 6/24 | 0/24 |
N, number of patients with available data for this variable.
aAdverse events were graded according to the Common Terminology Criteria for Adverse Event version 4.03 (CTCAE v4.03). bOther: constipation (n = 3), abdominal pain (n = 1), acute renal failure (n = 1), and dysesthesia (n = 1).
Among the 17 patients treated by dacarbazine-based chemotherapy, 5 of them received the FED regimen and 12 the LV5FU2-dacarbazine. For the FED regimen, OR was obtained for 3/5 patients, median PFS was 5.5 (95%CI (0.7;10.3)) months and OS 14.3 (95%CI (0.0;32.4)) months; while in the LV5FU2-dacarbazine group, OR was significantly less observed (4/12; P = 0.03), median PFS was lower (2.1 months; 95%CI (0.4;3.7); P = 0.04), and OS was 4.8 (95%CI (3.2;6.4); P = 0.30) months. There was no significant difference in toxicity profiles between both regimens, except for mucositis with 1/5 patient who had a grade 3 event.
Discussion
NECs represent a rare type of tumour with poor prognosis. This rarity explains the few clinical trials investigating these tumours and the low level of proof from recommendations which are mainly based on small retrospective studies. This study confirms the poor prognosis of metastatic NEC during post first-line treatment, with a PFS of 3.0 months and an OS of 7.2 months under dacarbazine- or temozolomide-based regimens. However, these regimens seem to offer some benefit for patients as shown by the temporary clinical response found in half of patients and a morphological response in a third of them.
In the present study, PFS was relatively low, although in line with that reported under other regimens used as post first-line treatments: OR was 24–31% and median PFS 2.9–4.0 months for FOLFIRI (3, 7); OR was 16–29% and PFS 2.3–4.3 months for FOLFOX (3, 8). The Nordic group was the first to report their experience in post first-line chemotherapy using temozolomide-based regimens: OR and median PFS were 0–33% and 2.4–6.0 months (6, 9, 10). Therefore, based on these results and the ones presented herein, dacarbazine- or temozolomide-based chemotherapy seems to be a possible alternative to FOLFOX or FOLFIRI regimens for metastatic NEC after first-line platinum-etoposide chemotherapy. There is currently no argument to choose one of these four regimens over the others, but a study comparing FOLFIRI and TEMCAP regimens as post-first line treatment of metastatic NEC is ongoing (NCT03387592). These poor results, however, highlight the need to find more effective new treatments against NEC. Some studies are ongoing, testing targeted therapies such as bevacizumab in association with FOLFIRI (17) or everolimus (NCT02113800), or immunotherapy (NCT03591731 and NCT03866382).
The comparisons between regimens herein have to be interpreted with caution because of the retrospective nature of the study, the possible selection bias, and the small sample size. No significant differences were found between dacarabazine-based and temozolomide-based chemotherapies in terms of efficacy and toxicity, except that patients under temozolomide-based regimen experienced more neutropenia and thrombocytopenia. A higher rate of grade 3/4 events was found in the present study when compared to the study by De Mestier et al. which compared the same regimens in a well-differentiated NET population (11). This difference could be explained by the study population, since all patients herein had a poorly differentiated NEC, which are known to present with more symptoms and a much worse prognosis (2, 3, 4). Of note, as reported by De Mestier et al., who found 8.5% and 24.7% grade 3/4 events for LV5FU2-dacarbazine and TEMCAP, respectively, we also show a favourable toxicity profile for LV5FU2-dacarbazine. In addition, five patients herein received a FED regimen, all treated before 2010. This regimen has been described for NET (15), with an acceptable toxicity profile: all grade 3/4 events were haematologic; febrile neutropenia occurred in 8% of patients. This regimen is not recommended in European guidelines (5) and its use in NEC has never been reported. This association in the present study seems to underline a positive outcome, with a longer PFS compared to LV5FU2-dacarbazine regimen and a comparable toxicity except for the higher frequency of mucositis. However, these results should be taken carefully, due to the very small sample size and a possible selection bias. This should be confirmed in further studies.
Factors associated with better outcome under dacarbazine- and temozolomide-based chemotherapy were also explored in the present study. Patients who achieved OR had a longer PFS, but this finding should be considered more an early marker of disease control, available 2 months after the beginning of chemotherapy, than a predictive marker of response. The only marker available at baseline significantly associated with shorter PFS in multivariate analysis was the presence of bone metastasis. However, since only two patients had bone metastasis and the diagnosis was not standardised (half of the patients underwent a 18FDG-PET, whereas the others only underwent CT-scan), this result must be analysed carefully. Therefore, the present population with bone metastasis may be underestimated, leading to a measurement bias in symptomatic patients. Of note, the present study did not include a control group without treatment, preventing us to distinguish prognostic vs predictive factors. Bone metastasis is certainly more a prognostic factor as already reported (16), rather than a predictive factor for response to a specific treatment. Currently, the most advanced predictive factor for response to alkylating agents remains the O6-methylguanine-DNA methyltransferase (MGMT) status, but this was not explored herein. MGMT promoter methylation or loss of MGMT protein expression seems to be associated with increased OR and survival under alkylating agents in NET (3, 18, 19), but this has not yet been demonstrated in a prospective study, which is the aim of the ongoing MGMT-NET study (19). Moreover, this biomarker has been poorly explored in NEC patients: in the study reported by Welin et al., only 1 out of 20 patients had MGMT methylation and a negative MGMT expression upon IHC; this patient had an OR for 15 months and an OS of 22 months, which is much longer than that of the whole population (9). If confirmed, dacarbazine- or temozolomide-based chemotherapy could become the standard second-line chemotherapy in patients presenting a NEC with MGMT methylation.
In conclusion, this study confirms a poor prognosis of metastatic NEC during post first-line treatment and underlines the urgent need for new therapies. LV5FU2-dacarbazine or temozolomide-capecitabine seem to be an alternative chemotherapy for second-line treatment of metastatic digestive NEC, as it provides a temporary clinical response in half of patients and/or a morphological response in a third of them.
Declaration of interest
T W received financial support from IPSEN, Novartis, Keocyt, and Roche to conduct clinical trials and/or ancillary studies. C L B received financial support from IPSEN, Novartis, Keocyt, and AAA. The other authors have nothing to disclose.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Statement of ethics
This retrospective study was conducted according to the World Medical Association Declaration of Helsinki. The database was approved by the national data protection commission (Commission nationale de l'informatique et des libertés, CNIL) on 6 November 2015 (n°15-111). A consent has been obtained from each alive patient after full explanation of the purpose and nature of all procedures used.
Author contribution statement
T C and T W contributed to the study design. T C, P G, C F, J H, T L, K V, E B, C L-B, and T W collected the data and followed the patients. T W made the statistical analyses. All authors have read the manuscript and have agreed with its submission.
Acknowledgements
The authors thank Véréna Landel, from DRCI (Hospices Civils de Lyon), for help in editing and manuscript preparation.
References
- 1.Korse CM, Taal BG, van Velthuysen M-LF, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. European Journal of Cancer 2013. 49 1975–1983. ( 10.1016/j.ejca.2012.12.022) [DOI] [PubMed] [Google Scholar]
- 2.Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D, Walter T, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocrine-Related Cancer 2015. 22 657–664. ( 10.1530/ERC-15-0119) [DOI] [PubMed] [Google Scholar]
- 3.Walter T, Tougeron D, Baudin E, Le Malicot K, Lecomte T, Malka D, Hentic O, Manfredi S, Bonnet I, Guimbaud R, et al. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: are they really heterogeneous? Insights from the FFCD-GTE national cohort. European Journal of Cancer 2017. 79 158–165. ( 10.1016/j.ejca.2017.04.009) [DOI] [PubMed] [Google Scholar]
- 4.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014. 120 2814–2823. ( 10.1002/cncr.28721) [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, et al. Enets consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016. 103 186–194. ( 10.1159/000443172) [DOI] [PubMed] [Google Scholar]
- 6.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Annals of Oncology 2013. 24 152–160. ( 10.1093/annonc/mds276) [DOI] [PubMed] [Google Scholar]
- 7.Hentic O, Hammel P, Couvelard A, Rebours V, Zappa M, Palazzo M, Maire F, Goujon G, Gillet A, Levy P, et al. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide–platinum combination in patients with neuroendocrine carcinomas grade 3. Endocrine-Related Cancer 2012. 19 751–757. ( 10.1530/ERC-12-0002) [DOI] [PubMed] [Google Scholar]
- 8.Hadoux J, Malka D, Planchard D, Scoazec JY, Caramella C, Guigay J, Boige V, Leboulleux S, Burtin P, Berdelou A, et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocrine-Related Cancer 2015. 22 289–298. ( 10.1530/ERC-15-0075) [DOI] [PubMed] [Google Scholar]
- 9.Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Öberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011. 117 4617–4622. ( 10.1002/cncr.26124) [DOI] [PubMed] [Google Scholar]
- 10.Olsen IH, Sørensen JB, Federspiel B, Kjaer A, Hansen CP, Knigge U, Langer SW. Temozolomide as second or third line treatment of patients with neuroendocrine carcinomas. Scientific World Journal 2012. 2012 170496 ( 10.1100/2012/170496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Mestier L, Walter T, Brixi H, Evrard C, Legoux JL, de Boissieu P, Hentic O, Cros J, Hammel P, Tougeron D, et al. Comparison of temozolomide-capecitabine to 5-fluorouracile-dacarbazine in 247 patients with advanced digestive neuroendocrine tumors using propensity score analyses. Neuroendocrinology 2019. 108 343–353. ( 10.1159/000498887) [DOI] [PubMed] [Google Scholar]
- 12.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Modern Pathology 2018. 31 1770–1786. ( 10.1038/s41379-018-0110-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense RT, et al. Enets consensus guidelines for the standards of care in neuroendocrine tumors: pathology – diagnosis and prognostic stratification. Neuroendocrinology 2017. 105 196–200. ( 10.1159/000457956) [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009. 45 228–247. ( 10.1016/j.ejca.2008.10.026) [DOI] [PubMed] [Google Scholar]
- 15.Walter T, Bruneton D, Cassier PA, Hervieu V, Pilleul F, Scoazec JY, Chayvialle JA, Lombard-Bohas C. Evaluation of the combination 5-fluorouracil, dacarbazine, and epirubicin in patients with advanced well-differentiated neuroendocrine tumors. Clinical Colorectal Cancer 2010. 9 248–254. ( 10.3816/CCC.2010.n.037) [DOI] [PubMed] [Google Scholar]
- 16.Trikalinos NA, Tan BR, Amin M, Liu J, Govindan R, Morgensztern D. Effect of metastatic site on survival in patients with neuroendocrine neoplasms (NENs). An analysis of SEER data from 2010 to 2014. BMC Endocrine Disorders 2020. 20 44 ( 10.1186/s12902-020-0525-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter T, Malka D, Hentic O, Lombard-Bohas C, Le Malicot K, Smith D, Ferru A, Assenat E, Cadiot G, Lievre A, et al. Evaluating bevacizumab in combination with FOLFIRI after the failure of platinum-etoposide regimen in patients with advanced poorly differentiated neuroendocrine carcinoma: the PRODIGE 41–BEVANEC randomized phase II study. Digestive and Liver Disease 2018. 50 195–198. ( 10.1016/j.dld.2017.11.020) [DOI] [PubMed] [Google Scholar]
- 18.Campana D, Walter T, Pusceddu S, Gelsomino F, Graillot E, Prinzi N, Spallanzani A, Fiorentino M, Barritault M, Dall'Olio F, et al. Correlation between MGMT promoter methylation and response to temozolomide-based therapy in neuroendocrine neoplasms: an observational retrospective multicenter study. Endocrine 2018. 60 490–498. ( 10.1007/s12020-017-1474-3) [DOI] [PubMed] [Google Scholar]
- 19.Lemelin A, Barritault M, Hervieu V, Payen L, Péron J, Couvelard A, Cros J, Scoazec JY, Blin S, Villeneuve L, et al. O6-methylguanine-DNA methyltransferase (MGMT) status in neuroendocrine tumors: a randomized phase II study (MGMT-NET). Digestive and Liver Disease 2019. 51 595–599. ( 10.1016/j.dld.2019.02.001) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a