Abstract
Introduction
Inactivating mutations in CYP24A1, encoding vitamin D-24-hydroxylase, can lead to an accumulation of active vitamin D metabolites and consequent hypercalcaemia. Patient (infantile and adult) presentation is varied and includes mild-severe hypercalcaemia, hypercalciuria, nephrocalcinosis and nephrolithiasis. This study aimed to characterize the clinical and biochemical phenotypes of a family with two CYP24A1 missense variants.
Methods
The proband and seven family members underwent detailed clinical and biochemical evaluation. Laboratory measurements included serum calcium, intact parathyroid hormone (iPTH), vitamin D metabolites and urine calcium and creatinine.
Results
The proband presented during the second trimester of a planned pregnancy with flu-like symptoms. Laboratory tests showed elevated adjusted calcium of 3.27 (upper reference limit (URL: 2.30) mmol/L), suppressed iPTH (<6 ng/L), elevated 25(OH)D (264 (URL: 55) nmol/L) and elevated 1,25(OH)D (293 (URL: <280) pmol/L). Ionized calcium was 1.55 (URL: 1.28) mmol/L. Sanger sequencing revealed two heterozygous missense variants in the CYP24A1: p.(Arg439Cys), R439C and p.(Trp275Arg), W275R. The proband’s brother and sister had the same genotype. The brother had intermittent hypercalcaemia and hypervitaminosis D. Only the sister had a history of nephrolithiasis. The proband’s daughter and two nephews were heterozygous for the R439C variant. The proband and her brother frequently had elevated 25(OH)D:24,25(OH)2D ratios (>50) during follow-up.
Conclusions
W275R is a new pathogenic CYP24A1 mutation in compound heterozygotic form with R439C in this family.
Keywords: vitamin D, CYP24A1 mutation, hypercalcaemia, hypervitaminosis D, genetic mutation, phenotype
Introduction
Vitamin D is a fat soluble vitamin (1) essential for calcium and phosphate homeostasis, bone metabolism (2) and possibly the prevention of chronic diseases such as cardiovascular disease and cancer (3). Vitamin D is obtained when 7-dehydrocholesterol in the skin is exposed to UV B (UVB) radiation (vitamin D3) and from diet and/or dietary supplements (primarily vitamin D2 but also vitamin D3) (4). Vitamin D3, by itself, is not an active substance and must first undergo a series of reactions in the liver and kidneys to generate the active form (5). In the liver, vitamin D3 is hydroxylated to form the prehormone 25-hydroxycholecalciferol D (25(OH)D3) catalysed primarily by CYP2R1 with CYP27A1 possibly contributing (6). 25(OH)D3 is then transported to the proximal tubules of the kidney bound to vitamin D binding protein where it is hydroxylated to form the active 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), catalysed by CYP27B1. Finally, 25(OH)D3 and 1,25(OH)2D3 are catabolised by CYP24A1, a key physiological regulator, to form 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) and 1,24,25-trihydroxyvitamin D3 (1,24,25(OH)3D3) (Supplementary Fig. 1, see section on supplementary materials given at the end of this article), the first step toward the final inactive end product, calcitroic acid, which is excreted (7). Vitamin D2 undergoes similar metabolism and catabolism to vitamin D3 through similar pathways (6).
Loss-of-function mutations in CYP24A1 inhibit the breakdown of 25(OH)D3, 25(OH)D2, 1,25(OH)2D3 and 1,25(OH)2D2 leading to an accumulation of active vitamin D metabolites and consequent hypercalcaemia (8), nephrocalcinosis and nephrolithiasis (9). Therefore, mutations in CYP24A1 should be considered as a potential cause of vitamin D-mediated parathyroid hormone (PTH)-independent hypercalcaemia. To date, cases of both adults and children with CYP24A1 mutations and hence increased sensitivity to vitamin D loading have been reported. Classically, infants tend to present with hypercalcaemia (10), whereas older children and adults usually present with nephrolithiasis, hypercalciuria and nephrocalcinosis (11) with hypercalcaemia and suppressed PTH. We recently identified a 32-year-old pregnant female (G1P0) who presented with symptomatic hypercalcaemia, hypercalciuria, hypervitaminosis D and suppressed intact PTH (iPTH). Symptom onset occurred at 17 weeks of gestation coinciding with trimester 2 of pregnancy. DNA sequencing identified two CYP24A1 variants.
This study aimed to characterize the clinical and biochemical phenotypes of a family with heterozygous and compound heterozygous variants in CYP24A1 following identification of two variants in the proband and to describe the proband’s unique presentation.
Methodology
Ethical approval for this study was granted by the Research Ethics Committee, Galway University Hospitals (Reference No – C.A. 1927; approval date 13-02-2018). Following informed written consent, DNA samples from the proband and the proband’s family were examined using targeted exon PCR of all coding exons of CYP24A1 followed by Sanger sequencing. Clinical and biochemical phenotypes were correlated with CYP24A1 sequence information in the proband, her parents, siblings (n = 2), her daughter and two nephews.
The proband (II.2) presented during the second trimester of a planned pregnancy with flu-like symptoms and was discovered following identification of asymptomatic hypercalcaemia on routine blood work. The proband (II.2), her father (I.1), mother (I.2), brother (II.1), sister (II.3), daughter (III.3) and two nephews (III.1, III.2) were recruited to this study. Detailed medical histories were recorded, and clinical examinations were performed. Family members had DNA samples tested for CYP24A1 mutations by direct sequencing at the Northern Molecular Genetics Service, Newcastle upon Tyne, UK. Participants also had routine clinical biochemistry measured including calcium (Ca2+) (mmol/L), albumin (g/L), phosphate (mmol/L), vitamin D metabolites (25(OH)D, 1,25(OH)2D, 24,25(OH)2D), iPTH, fibroblast growth factor 23 (FGF-23), creatinine (estimated glomerular filtration rate (eGFR) using the CKD-Epidemiology Collaboration (CKD-EPI) formula (12)) and alkaline phosphatase (ALP) (U/L). Adult participants had urinary Ca2+ measured (spot and/or 24-hour urine collections). II.3 was unable to attend in person and only had DNA analysis for the CYP24A1 variants. II.1 and II.2 had serial measurements of Ca2+, 25(OH)D and iPTH.
Measurement of vitamin D metabolites
Serum 25(OH)D concentrations were measured using liquid chromatography tandem mass spectrometry (LC-MS/MS) on the Agilent HPLC 1290; Agilent 6460 Triple quadrupole MS/MS in the Clinical Biochemistry Laboratory at Galway University Hospitals (ISO 15189:2012 standards) (13, 14). Vitamin D was reported as total serum 25(OH)D concentration (the sum of 25(OH)D2 and 25(OH)D3 concentrations). The limits of quantification for 25(OH)D2 and 25(OH)D3 were 5 nmol/L and 8 nmol/L, respectively. Assay precision was ≤8.0% and bias was ≤5.0% at 25(OH)D2 levels of 42.2 nmol/L and 94.4 nmol/L and 25(OH)D3 levels of 39.9 nmol/L and 94.4 nmol/L. The Clinical Biochemistry Laboratory meets the performance targets set by the DEQAS (Vitamin D External Quality Assessment Scheme) advisory panel. Serum 24,25(OH)2D (total 24,25(OH)2D2 and 24,25(OH)2D3) and serum 1,25(OH)2D (total 1,25(OH)2D2 and 1,25(OH)2D3) were measured using LC-MS/MS on the Micromass Quattro Ultima Pt electrospray ionisation (ESI) tandem mass spectrometer (Waters Corp., Milford, MA, USA) at the Norfolk and Norwich University Hospital NHS Foundation Trust, Colney Lane, Norwich, UK (15). For serum 24,25(OH)2D2 and 24,25(OH)2D3, the limits of quantification were 0.1 and 0.8 nmol/L, respectively. The inter-assay coefficients of variability (CVs) for both serum 24,25(OH)2D2 and 24,25(OH)2D3 were between 7.9 and 11% (15). For 1,25(OH)2D, the limit of quantification was 10 pmol/L with CVs of 8.0–13.0%.
Results
Clinical history: II.2 and III.3
The proband (II.2) presented during a planned pregnancy and was followed for 4 years. Of white European ethnicity, the proband was healthy, active and took over the counter (OTC) vitamin D supplements (1000 units per day; Protego Witamina D 100 food supplement and/or Mama DHA food supplement) for 2 months prior to conception. Past medical history included mitral valve prolapse (21 years previous), surgery for deviated nasal septum, migraine and abnormal liver function tests secondary to isotretinoin use for acne. She was an ex-smoker for 5 years (5 pack per year history). There was no alcohol consumption up to or during pregnancy. Previous laboratory tests were within normal range (adjusted (adj.) Ca2+ (reference interval (RI): 2.15–2.55 mmol/L). Serum 25(OH)D concentrations or other vitamin D metabolites were not previously measured.
At 17 weeks of gestation, the proband attended her first antenatal visit. Clinical examination was unremarkable (blood pressure (BP) 122/78 mmHg). Electrocardiograph (ECG) was normal. Routine blood tests revealed elevated adj. Ca2+ (3.27 mmol/L (trimester-specific upper reference limit (URL): <2.30 mmol/L) with an ionized Ca2+ of 1.44 mmol/L (trimester-specific URL: 1.25 mmol/L), suppressed iPTH <6 ng/L (RI: 18–25 ng/L), elevated cholecalciferol (25(OH)D) 264 nmol/L (URL: 55 nmol/L) and elevated calcitriol (1,25(OH)2D) 293 pmol/L (URL: <280 pmol/L) with evidence of hypercalciuria (Table 1). While undergoing an extensive workup for hypercalcaemia of pregnancy, the proband was treated aggressively with i.v. fluids with a resultant fall in Ca2+. Relevant negative laboratory indices included: parathyroid hormone-related protein (PTHrP) <1.0 pmol/L (RI: 0–1.8 pmol/L), lactate dehydrogenase (LDH) 100 U/L (RI: 135–214 U/L), urine protein: creatinine ratio (PCR), serum protein electrophoresis (SPEP), serum free light chains (SFLC), urine protein electrophoresis (UPEP), vitamin A 1.54 µmol/L (RI: 0.0–3.84 µmol/L) and calcitonin 1.0 ng/L (RI: 0.0–10.0 ng/L). Spot urine Ca2+: creatinine molar ratio and 24-hour urine Ca2+ were elevated at 0.82 mmol/mmol (RI: 0.25–0.75 mmol/mmol) and 9.85 mmol/24 h (RI: 0.30–6.9 mmol/24 h), respectively. Chest radiography, pelvis and thyroid echocardiography were unremarkable. Of note, abdominal ultrasound showed no evidence of urinary stone disease or nephrocalcinosis. The OTC vitamin D supplements which II.2 was taking prior to admission were examined and quantitated at the Public Analyst Laboratory Cork, Ireland (accredited service: ISO 17025). HPLC was the methodology used to determine the vitamin D content – Protego Witamina D 100 food supplement, vitamin D3, label 25 µg/capsule, result of analysis 27.5 µg/capsule; Mama DHA food supplement vitamin D3 label 25 µg/2 capsule, result of analysis 16.8 µg/2 capsule.
Table 1.
Demographics, clinical, biochemical and genetic analyses on first contact.
| Parameter | II.2a | RIb | II.2c | I.1 | I.2 | II.1 | II.3 | RId | III.1e | III.2e | III.3e |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 32 | - | 33 | 58 | 58 | 36 | 32 | - | 2 | 7 | 1 |
| Gender (F/M) | F | - | F | M | F | M | F | - | M | M | F |
| Mutation present A±B | A/B | - | A/B | A/A | B | A/B | A/B | - | A | A | A |
| Total Ca2+ (mmol/L) | 3.15 | 2.05–2.25 | 2.45 | 2.47 | 2.41 | 2.51 | n/a | 2.15–2.55 | 2.68 | 2.44 | 2.45 |
| Adj. Ca2+ (mmol/L) | 3.27 | 2.10–2.30 | 2.41 | 2.42 | 2.37 | 2.46 | n/a | 2.18–2.55 | n/a | n/a | n/a |
| Albumin (g/L) | 36 | 26–45 | 47 | 48 | 47 | 48 | n/a | 39–51 | 46 | 50 | 42 |
| Phosphate (mmol/L) | 1.04 | 0.87–1.48 | 0.93 | 1.37 | 1.2 | 1.1 | n/a | 0.87–1.45 | 1.61 | 1.46 | 1.81 |
| iPTH (ng/L) | <6 | 18–25 | 15.5 | 54.9 | 37.6 | 18 | n/a | 15–65 | 23.5 | 21.8 | 46.3 |
| 25(OH)D (nmol/L) | 264 | 25–55 | 105 | 57 | 35 | 148 | n/a | 75–125 | 141 | 81 | 93 |
| 1,25(OH)2D (pmol/L) | 293 | 72–280 | 83 | 125 | 94 | 143 | n/a | 55–139 | n/a | n/a | n/a |
| 24,25(OH)2D (nmol/L) | n/a | - | 3.3 | n/a | n/a | 2.1 | n/a | 1.3–13.5 (13) | n/a | n/a | n/a |
| 25(OH)D:24,25(OH)2D ratio | n/a | - | 32 | n/a | n/a | 70 | n/a | 7–23 (13) | n/a | n/a | n/a |
| 1,25(OH)2D:24,25(OH)2D ratio | n/a | - | 25 | n/a | n/a | 68 | n/a | 11–62 | n/a | n/a | n/a |
| FGF-23 (RU/mL) | n/a | - | 299 | 45 | 129 | 155 | n/a | <100 | n/a | n/a | n/a |
| Creatinine (µmol/L) | 90 | 35–71 | 94 | 111 | 82 | 102 | n/a | F: 49–90 M: 60–110 |
28 | 42 | 24 |
| eGFR (mL/min/1.73 m2) | 63 | 60–120 | 59 | 63 | 68 | 72 | n/a | 60–120 | n/a | n/a | n/a |
| ALP (U/L) | 51 | 25–126 | 35 | 72 | 55 | 68 | n/a | 35–104 | 367 | 235 | 263 |
| fUr. Ca2+:creatinine molar ratio | 0.82 | 0.25–0.75 | n/a | 0.21 | 0.21 | n/a | n/a | 0.25–0.75 | n/a | n/a | n/a |
| Ur. Ca2+ (mmol/24h) | 9.85 | 0.3–6.9 | n/a | n/a | n/a | 17.4 | n/a | 2.5–7.5 | n/a | n/a | n/a |
A or B, heterozygous; A, p.(Arg439Cys); A/A, homozygous; A/B, compound heterozygous, a17/40 weeks of gestation; B, p.(Trp275Arg); bspecific for trimester 2 of pregnancy; cindicates results from sample collected 3 months postpartum; dnon-pregnant adult; eall values for P6, P7 and P8 are within age-defined RIs; F, female; fspot/random urine sample; I.1, father; I.2, mother; II.1, brother; II.2, proband; II.3, sister; III.1, nephew; III.2, nephew; III.3, proband’s daughter; M, male; n/a, not available; RI, reference interval.
During pregnancy, the proband was treated with a combination of i.v. fluids, a diet low in calcium and vitamin D and oral corticosteroids. II.2 required numerous prolonged hospital admissions to maintain Ca2+ levels within normal limits. The baby (III.3) had regular fetal scans with occipitofrontal diameter, head circumference, abdominal circumference and fetal weight within normal limits. At 36+3 weeks of gestation, II.2 developed a headache with a BP of 177/80 mmHg and commenced on labetalol 200 mg bd. At 36+4 weeks of gestation, BP increased to 190/100 mmHg with clonus on clinical examination. Pre-eclampsia was diagnosed. She was given betamethasone and BP was managed with the addition of hydralazine peripartum.
The baby (III.3) was delivered at 36+4 weeks of gestation by emergency C-section. III.3’s ionized Ca2+ was just above the upper limit of normal (1.35 mmol/L (RI: 1.15–1.33 mmol/L)). The baby was alert with normal vitals (afebrile, heart rate 150 (100–159) b.p.m, respiratory rate 50 (31–60) breaths per minute, oxygen 98 (>95)%. Placental histology, on microscopic examination of villi, pointed to segmental areas of impaired maternal blood flow within the placenta. However, this did not appear to have compromised the overall placental function in relation to fetal growth and oxygenation. Post-delivery, there was a spike in II.2’s adj. Ca2+ (3.29 (RI: 2.17–2.51) mmol/L) and ionized Ca2+ (1.35 (RI arterial: 1.15–1.28)mmol/L) (Fig. 1A). This was aggressively treated with i.v. fluids and i.v. hydrocortisone followed by an oral prednisolone taper with a resultant normalisation of adj. Ca2+. Post-partum, BP remained difficult to control and required an increase in labetalol and the addition of nifedipine and methyldopa with a resultant normalisation in BP. Antihypertensives were tapered over time and eventually stopped – BP remained normal. II.2 developed an acute kidney injury post-delivery (eGFR decreased from a baseline 63 mL/min/1.73 m2 to 44 mL/min/1.73 m2). Kidney function returned to baseline following aggressive hydration. Work-up for secondary hypertension revealed normal aldosterone:renin ratio and plasma metanephrines. Computerized tomography (CT) of thorax, abdomen and pelvis did not reveal any potential source of hypercalcaemia. At 3 months post-partum, the proband’s 24,25(OH)2D and 25(OH)D measured 3.3 nmol/L (RI: 1.1–13.5 (15)) and 105 nmol/L, respectively, providing for an elevated 25(OH)D:24,25(OH)2D ratio of 32 (RI: 7–23 (15)). While acknowledging that the magnitude of the 25(OH)D:24,25(OH)2D ratio was below currently published cut-offs (>50 and mostly >80), indicative of CYP24A1 mutations (16), the dual measurements (absolute 25(OH)D and 24,25(OH)2D concentrations) together with the abnormal 25(OH)D:24,25(OH)2D ratio warranted further investigation. As substrate depletion, bone disorders or pathological conditions associated with an increased concentration of FGF23 were not clinically implicated, follow-up examinations focused on 24-hydroxylase under activity and possible genetic abnormalities of CYP24A1. Sequencing of the proband’s DNA was carried out which revealed two CYP24A1 genetic variants: R439C and W275R. The baby (III.3) was heterozygous for the known pathogenic mutation R439C.
Figure 1.
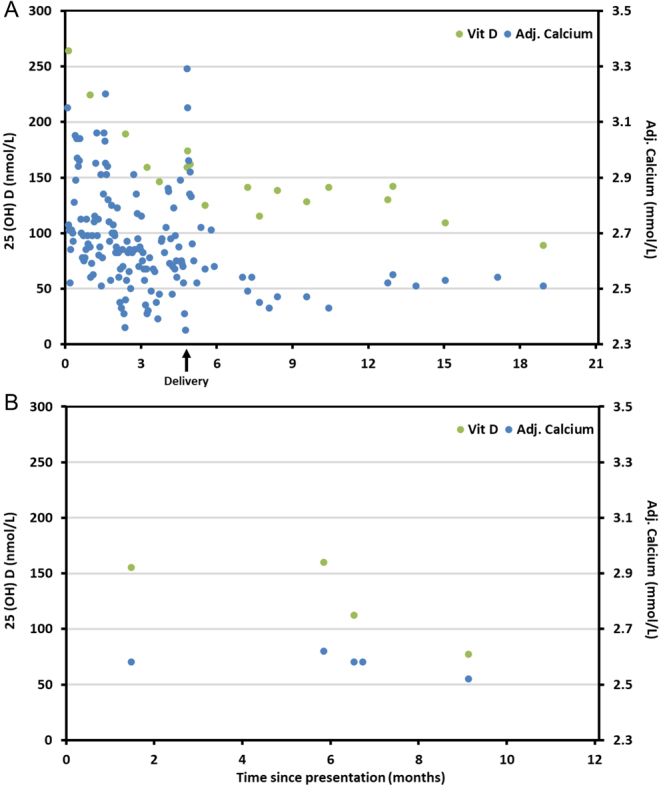
25(OH)D and adj. Ca2+ trends post presentation in the (A) proband (II.2) and (B) brother (II.1).
II.2 is being followed closely in our outpatient department. II.2 has been advised to apply sunscreen daily, be cautious regarding sun exposure and keep hydrated. A dietician has prescribed a low calcium/vitamin D diet. The trends in II.2’s 25(OH)D and adj. Ca2+ are presented in Fig. 1A.
Clinical history: II.1, III.1 and III.2
The proband’s brother (II.1) presented as part of our familial screening program and was compound heterozygous for CYP24A1 variants: W275R and R439C. At his initial visit, II.1 had normal adj. Ca2+ (2.46 mmol/L), 24,25(OH)2D (2.1 nmol/L) and iPTH (18 ng/L) and elevated 25(OH)D (148 nmol/L), 25(OH)D:24,25(OH)2D ratio of 70 (RI: 7–23 (15)), 1,25(OH)2D (143 pmol/L) and 1,25:24,25(OH)2D ratio of 68 (RI:11–62) (Table 2). The trends in II.1s 25(OH)D and adj. Ca2+ over the following 12 months are presented in Fig. 1B. II.1 had intermittent hypercalcaemia with adj. Ca2+ between 2.51 and 2.65 mmol/L. Like II.2, he was advised to apply sunscreen daily, be cautious regarding sun exposure, keep hydrated and have a low calcium/vitamin D diet. His children III.1 and III.2 are heterozygous for the R439C mutation.
Table 2.
Biochemical analyses and vitamin D metabolite profiling during follow-up: proband (II.2) and brother (II.1).
| Parameter | Proband (aII.2) | aII.1 | Reference interval | |||||
|---|---|---|---|---|---|---|---|---|
| F | M | |||||||
| Month/year | b05/16 | 04/17 | 06/17 | 11/18 | 05/19 | 05/16 | 07/17 | - |
| Total Ca2+ (mmol/L) | 2.45 | 2.50 | 2.53 | 2.59 | 2.54 | 2.51 | 2.65 | 2.15–2.55 |
| Adj. Ca2+ (mmol/L) | 2.41 | 2.51 | 2.49 | 2.55 | 2.52 | 2.46 | 2.60 | 2.17–2.51 |
| Albumin (g/L) | 47 | 44 | 47 | 47 | 46 | 48 | 48 | 39–51 |
| Phosphate (mmol/L) | 0.93 | 1.10 | 1.19 | 1.47 | 1.21 | 1.10 | 1.16 | 0.87–1.45 |
| iPTH (ng/L) | 15.5 | <6.0 | 12.3 | 10.2 | 9.6 | 18.0 | 14.6 | 15–65 |
| 25(OH)D (nmol/L) | 105 | 94 | 104 | 106 | 89 | 148 | 136 | 75–125 |
| 1,25(OH)2D (pmol/L) | 83 | 124 | 135 | 101 | 124 | 143 | 192 | 55–139 |
| 24,25(OH)2D (nmol/L) | 3.3 | 1.3 | 1.7 | 2.5 | 1.3 | 2.1 | 2.6 | 1.3–13.5 |
| 25(OH)D:24,25(OH)2D ratio | 32 | 72 | 61 | 42 | 68 | 70 | 52 | 7–23 |
| 1,25(OH)2D:24,25(OH)2D ratio | 25 | 95 | 79 | 40 | 95 | 68 | 74 | 11–62 |
| FGF-23 (RU/mL) | 299 | n/a | 216 | n/a | n/a | 155 | 240 | <100 |
| Creatinine (µmol/L) | 90 | 75 | 76 | 72 | 87 | 102 | 108 | F: 49–90M: 60–110 |
| eGFR (mL/min/1.73 m2) | 63 | >90 | 88 | >90 | 74 | 72 | 75 | 60–120 |
| ALP (U/L) | 51 | 46 | 48 | 37 | 43 | 68 | 72 | 35–104 |
| cUr. Ca2+:creatinine molar ratio | 0.82 | n/a | 1.32 | n/a | n/a | n/a | n/a | 0.25–0.75 |
| Ur. Ca2+ (mmol/24h) | 9.85 | n/a | n/a | n/a | n/a | 17.4 | n/a | 2.5–7.5 |
acompound heterozygous (A/B): A, p.(Arg439Cys); B, p.(Trp275Arg); eGFR was calculated using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) formula; F, female; M, male; b3 months post-partum (not breastfeeding); n/a, not available; cspot/random urine sample.
Clinical history: I.1, I.2 and II.3
Following enrolment in our study, the proband’s sister (II.3) was identified as being compound heterozygous with a history of nephrolithiasis during childhood for which no cause was identified. Following identification of her mutations, she presented with hypercalcaemia and nephrolithiasis during pregnancy to a hospital in a different country. The recent identification of her genotype facilitated rapid management. II.2’s mother (I.2) was heterozygous for the W275R variant and her father (I.1) was homozygous for the R439C mutation. Baseline laboratory indices were not indicative of hypercalcaemia or hypervitaminosis D (Table 1).
Genetic testing
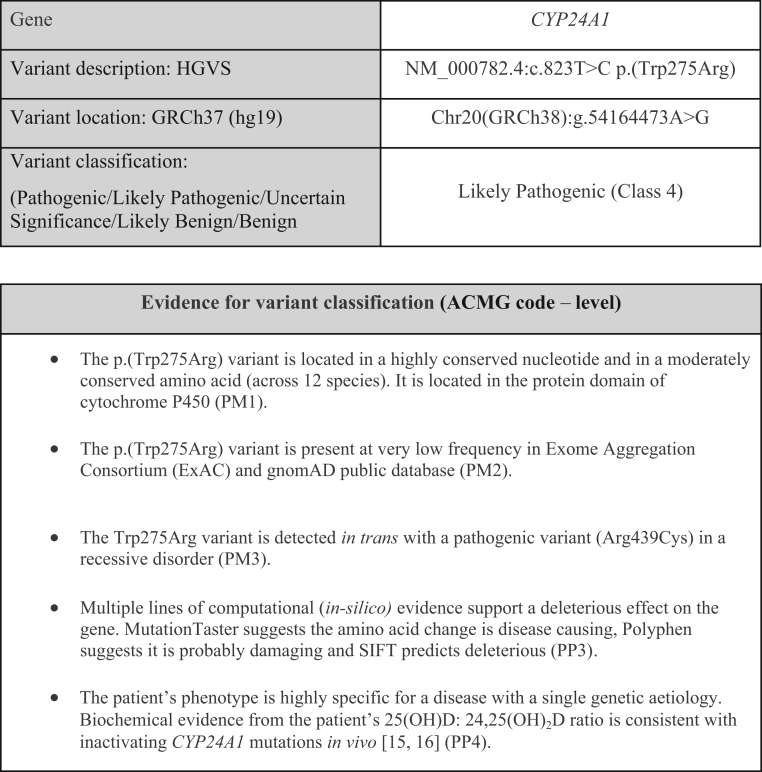
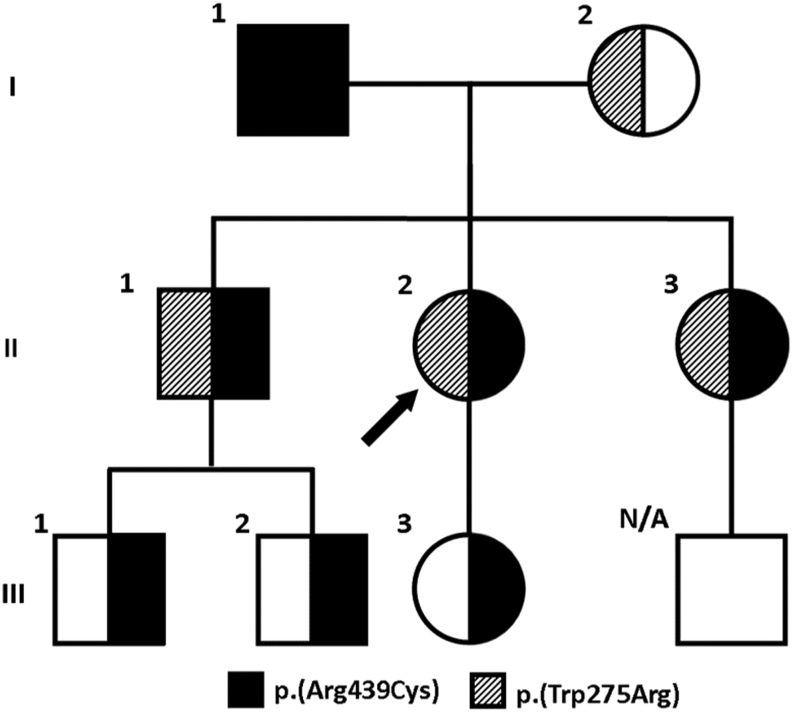
Two heterozygous CYP24A1 missense variants were identified in the proband (II.2). R439C is a known pathogenic mutation resulting in a C to T transition in exon 10, c.1315C>T; p.(Arg439Cys) and was previously shown to be a loss of function mutation by site directed mutagenesis (16). W275R is a novel missense variant resulting in a T to C transition in exon 6, c.823T>C; p.(Trp275Arg) first identified by our group (15). The W275R variant is not previously reported in ClinVar (NIH Clinical Genomic Resource) or LOVD (Leiden Open Variation database) and is present at very low frequency in gnomAD and Exome Aggregation consortium (ExAC) public databases. Investigations into the pathogenicity of W275R show that the substitution occurs in a highly conserved amino acid within the protein domain of Cytochrome P450. An online genetic variant tool predictor (17) was used to grade the evidence accumulated for W275R. Variant classification was based on the American College of Medical Genetics (ACMG) Guidelines (18) and the Association for Clinical Genomic Science (ACGS) Best Practice Guidelines for Variant Classification (19). Predicted pathogenicity using in silico evidence (MutationTaster, Polyphen and SIFT) support a deleterious effect on the CYP24A1 protein (Fig. 2). Based on current evidence, the W275R variant is classified as likely pathogenic (class 4). Stronger evidence from functional studies or new cases will be required to upgrade its pathogenicity. Sanger sequencing of parental bloods confirmed the inheritance of both mutations in trans in the proband. The probands father (I.1) is homozygous for R439C and her mother (I.2) is heterozygous for the W275R mutation. Segregation analysis in other family members was performed. The family pedigree is presented in Fig. 3. Participants demographics, clinical and biochemical analyses on first contact and genotype are presented in Table 1. Of note, only the probands sister had a history of nephrolithiasis (II.3).
Figure 2.
Evidence for CYP24A1 c.823T>C; p.(Trp275Arg) variant classification. The ACMG utilises a series of evidence criteria in support of a pathogenic (P) or benign (B) classification. The different types of evidence (functional, genetic, population, in-silico etc) are stratified according to the level of evidence (supporting, moderate, strong or very strong) and a pathogenicity classification (pathogenic, likely pathogenic, variant of unknown significance (VUS), likely benign or benign) assigned according to a set of ‘combined criteria’ provided in the guidelines (19). PM, moderate evidence of pathogenicity; PP, supporting evidence of pathogenicity. The level of evidence is then categorised numerically: PM1, located in functional domain; PM2, absent from controls; PM3, recessive disorder and present in trans with a pathogenic mutation; PP4, functional biochemical evidence; PP3, in silico evidence supporting pathogenicity.
Figure 3.
Family pedigree showing inheritance of CYP24A1 mutations. Arrow indicates the position of the proband in the family. I.1, father; I.2, mother; II.1, brother; II.2, proband; II.3, sister; III.1, nephew; III.2, nephew; III.3, proband’s daughter.
Follow-up 25(OH)D:24,25(OH)2D ratio determinations in the proband (II.2) and brother (II.1)
A total of five separate 25(OH)D:24,25(OH)2D ratio determinations were performed on serum samples collected from the proband. The first was carried out at 3 months post-partum and the remaining four over the following 3 years (Table 2). Of note, ratios in two of these five analyses were 32 and 42, respectively, and inconsistent with current criteria for biallelic CYP24A1 mutations (16). However, the remaining three 25(OH)D:24,25(OH)2D ratio results varied between 61 and 72 (Table 2) and are in keeping with published decision thresholds for biallelic CYP24A1 mutations (16). Furthermore, the proband’s brother (II.1), who harbours the same CYP24A1 genotype, had 25(OH)D:24,25(OH)2D ratios measured on two occasions and both results were consistent with published cut-offs indicative of biallelic CYP24A1 mutations (16). Biochemical results, together with the population data and in silico assessment (see Fig. 2), support the contention that this novel W275R variant is likely a mutation and not a polymorphism.
Discussion
This study reports two CYP24A1 mutations R439C and W275R identified in a pregnant female during the second trimester. The proband’s father (I.1) was homozygous for the R439C pathogenic mutation but did not have hypercalcaemia, which may be due to the variable penetrance of this mutation together with external factors that mitigate the development of hypercalcemia, such as dietary calcium intake, vitamin D levels, volume status and so on. Family members who were compound heterozygous for R439C and W275R mutations had hypercalcaemia and elevated concentrations of vitamin D metabolites. Only one family member had nephrolithiasis. The proband first presented during pregnancy, suggesting that patients with CYP24A1 mutations can maintain normal Ca2+ levels during steady state but may develop hypercalcaemia with the challenge of pregnancy when 1,25(OH)2D levels are physiologically elevated. Variable presentations and severity of CYP24A1 mutations have been reported with only eight cases reported during the perinatal period (Table 3) (11, 16, 20, 21, 22, 23, 24, 25).
Table 3.
Clinical, biochemical and genetic findings in eight reported cases of maternal hypercalcaemia due to CYP24A1 pathogenic variants.
| Case Report | Molin 2015 (16) | Dinour 2015 (20) | Shah 2015 (11) | Woods 2016 (23) | Hedberg 2019 (24) | McBride 2019 (21, 22) | MacDonald 2019 (25) | |
|---|---|---|---|---|---|---|---|---|
| Genotype | Compound heterozygous | Homozygous | Compound heterozygous | Homozygous | Compound heterozygous | Homozygous | Compound heterozygous | Homozygous |
| Mutation | p.E322A/p.L409S | p.E143del | p.E143del/p.R396W | p.S334Vf*9 | p.R396W/p.L148P | p.R396W | p.E143del/p.K351Nfs*21 | p.E143del |
| Age/years | NS | 35 | 28 | 20 | 23 | 21 | 27 | Mid 20s |
| Gestation week/PP | 32 | 32 | 14 | 1–5 days PP | 38 | 15 | 5–7 days PP | 13 |
| Ethnicity | NS | Moroccan | NS | Mexican | Swedish | Caucasian | NS | |
| PMHx | NS | Renal stones | Renal stones | Hypertension | Renal stones | Renal stones | Renal calculus | |
| Medullary nephrocalcinosis | NS | Yes | Yes | NS | Yes | Yes | Yes | NS |
| Symptoms | NS | Weakness and confusion |
Fatigue and constipation |
Altered mental status and acute pancreatitis | Epigastric pain and HTN |
PP Headache Nausea and HTN |
Symptomatic hypercalcaemia | Symptomatic hypercalcaemia |
| Calcium (mmol/L) | 3.3 | 3.00 to 3.68 | 2.38 to 3.05 | 3.50 | 4.29 | 2.87 | 3.63 | 2.90 |
| PTH (ng/L) | Suppressed | <3 | <3 | <6 | 5 | <10 | 5.6 | 6.6 |
| 25(OH)D (nmol/L) | NS | 70 | 159–185a | 113–133 | 98 | 267 | 77 | 116 |
| 1,25(OH)2D (pmol/L) | 441 | 214 | 422–509 | 396–468 | 187 | 386 | 250 | 380 |
| 24,25(OH)2D (nmol/L) | NS | NS | 1.85a | Not detected | NS | NS | NS | 0.1 |
| 25(OH)D: 24,25(OH)2D ratio | NS | NS | 86a | NS | NS | NS | NS | 594 |
| Ur calcium:creatinine ratio | NS | NS | NS | NS | NS | NS | 0.22 | 2.09 |
| 24-h Ur calcium (mmol/day) | NS | 10.53 | 9.43 | 6.91 | 15.5 | 5.7 | NS | NS |
| Vitamin D supplements | NS | 750 IU/day | NS | Yes | NS | NS | 250 IU/day | 50,000 IU/month |
| Medication/over the counter | NS | Calcium bicarbonate | Prenatal vitamin | Amlodipine | Citalopram | NS | NS | NS |
aValues used to derive 25(OH)D to 24,25(OH)2D ratio.
HTN: hypertension; NS: not stated; PP: post-partum.
Vitamin D is essential for calcium homeostasis and bone metabolism (2). Calcium homeostasis is altered in normal pregnancy (24). In healthy women, total 1,25-(OH)2D levels were more than double early in pregnancy due to increased production of renal and placental 1-α hydroxylases (26) and remained elevated until the end of pregnancy. In addition, the absorption of calcium from the intestine more than doubles and bone resorption occurs to provide calcium for breast milk (27). Hypercalcaemia is exacerbated in pregnant women with inactivating CYP24A1 mutations and is normally associated with 1,25(OH)2D levels in the upper normal range or slightly raised and concentrations of 25(OH)D that can be low, normal or mildly elevated (28). Vitamin D supplements recommended during pregnancy have the potential to aggravate hypercalcaemia in women with loss-of-function CYP24A1 mutations. The vitamin D supplements taken by the proband during pregnancy likely explain the markedly elevated 25-hydroxyvitamin D concentration observed at presentation. Castanet et al. reported that a child who received higher dose vitamin D supplements (1900 U/day) as a baby developed hypercalcaemia, hypercalciuria and resultant nephrocalcinosis compared to his brother who only received 400 U/day – both had a homozygous CYP24A1 mutation (29) – suggesting that vitamin D dose may have an effect. Careful consideration should be given to the dosage of vitamin D supplementation and to monitoring adj. Ca2+ and vitamin D levels in pregnant females.
As highlighted by our case (II.2), investigation of non-PTH mediated hypercalcaemia remains challenging, especially in pregnancy. The commonest causes (90%) of hypercalcaemia are hyperparathyroidism and malignancy (30). The other 10% of cases result from hypercalcaemia related to vitamin D (granulomatous disease and vitamin D intoxication), endocrine disorders such as thyrotoxicosis, medications (thiazide diuretic, milk-alkali syndrome and vitamin A), renal (acute renal failure, chronic renal failure or renal transplant) or other rarer causes such as CYP24A1 mutations. The choice of imaging modality is limited in pregnancy as ionizing radiation poses risks to both mother and fetus (31). Therefore, initial investigations are typically biochemical and haematological. PTH related peptide (PTHrP) is raised in humoral hypercalcaemia of malignancy (32). In the context of low PTHrP, measurement of 1,25(OH)2D can be used to screen for vitamin D-mediated hypercalcaemia which can be seen with lymphoma. If both PTHrP and 1,25(OH)2D levels are low, hypercalcaemia secondary to osteolytic metastases should be considered (32). There is often a considerable delay in obtaining PTHrP or 1,25(OH)2D results as they are usually only performed in specialised centres. When there is clinical suspicion of malignancy, imaging (typically CT) or invasive procedures, such as bone marrow biopsy, can be required to identify the source. Delaying such investigations (a risk benefit analysis is required in pregnancy) contributes to anxiety and stress in the pregnant female.
The utility of the ratio of serum 25(OH)D to 24,25(OH)2D concentration has been promulgated as a useful biochemical screening tool to assess vitamin D catabolic status. Persistent hypercalcaemia together with a suppressed PTH, elevated 1,25(OH)2D level and a raised 25(OH)D:24,25(OH)2D ratio are consistent with a CYP24A1 loss-of-function mutation (33). Molin et al. suggest that a 25(OH)D:24,25(OH)2D ratio of >50 and usually >80 is indicative of inactivating CYP24A1 mutations in vivo (16). Previous work reveals that patients with biallelic CYP24A1 pathogenic variants have a significantly elevated ratio, whether they exhibit hypercalcemia or not (16). In the current study, the proband had 25(OH)D:24,25(OH)2D ratios measured on five occasions and in two, the ratio while above the reference interval of 23, were well below published cut-offs indicative of biallelic CYP24A1 mutations. We can only surmise that the observed fluctuations in the ratio occurred in tandem with the proband adhering to medical advice: minimizing unprotected/direct exposure to sunlight and avoiding diets rich in vitamin D or vitamin D supplements. Identifying CYP24A1 variants, as in II.3 pre-pregnancy, facilitates a more nuanced approach to the management of hypercalcaemia.
Consensus regarding the management of hypercalcaemia secondary to CYP24A1 pathogenic variants during pregnancy (or indeed hypercalcaemia during pregnancy due to other causes) do not currently exist. Based on our experience and reports in other case studies, for patients with known CYP24A1 pathogenic variants considering pregnancy, we advocate avoiding supplements containing vitamin D or calcium, avoiding calcium bicarbonate, remaining well hydrated, generous daily application of sunscreen and reduced exposure to sunshine. Referral to a dietician for advice on a low calcium and vitamin D diet is recommended. Close monitoring of adj. Ca2+, 25(OH)D and iPTH before, during and after pregnancy is essential. If Ca2+ remains elevated, aggressive hydration with i.v. fluids (saline) is recommended. There are several reports on the use of oral corticosteroids in managing hypercalcaemia due to CYP24A1 pathogenic variants during pregnancy (22); they may be of benefit as they inhibit 1-α-hydroxylase conversion of 25(OH)D to 1,25(OH)2D thereby lessening intestinal calcium absorption (34). Calcitonin has been used cyclically (22) or as a single dose (23); calcitonin decreases hypercalcaemia by inhibiting osteoclast activity, enhances renal excretion of calcium (35) and does not cross the placenta (27). There are limited reports on the use of cinacalcet (36, 37) and bisphosphonates (38, 39, 40, 41, 42) during pregnancy or for treatment of hypercalcaemia of pregnancy due to other causes. Regarding bisphosphonates, Losada et al. reported that 20% of children of women treated with bisphosphonates close to or during pregnancy had congenital malformations (42); therefore, their use in pregnancy cannot be recommended.
Rifampicin (43), ketoconazole (44, 45, 46) and fluconazole (47) have been reported as successful treatments for hypercalcaemia due to CYP24A1 mutations in the non-pregnant state. Rifampicin has been used to treat tuberculosis during pregnancy (48); however, there are no reports on its effectiveness or safety in treating hypercalcaemia during pregnancy. Depending on the dose and duration of treatment, ketoconazole and fluconazole are potentially teratogenic and should only be used if the benefit to the fetus clearly outweighs the risk (49). Regarding any pharmacotherapy, a risk benefit analysis must be undertaken on a patient-by-patient basis.
Most laboratory systems do not have the digital functionality to deal with appropriate RIs in pregnancy. At our institution, the RI for total Ca2+ is 2.18 to 2.55 mmol/L in males and non-pregnant females. In pregnancy, this changes to 2.10–2.30 mmol/L during the second trimester and 2.05–2.43 mmol/L during the third trimester. In non-pregnant females, RIs are typically compiled from the local population at each institution. This is not the case for RIs in pregnancy. Robust trimester specific RIs for PTH and vitamin D metabolites are not available. In the absence of appropriate RIs associated with laboratory results for each trimester, the physician could potentially miss mild elevations in adj. Ca2+ and, therefore, miss the opportunity to instigate appropriate management strategies at an early stage.
Conclusions
In this family, only patients with the compound heterozygous mutations (R439C/W275R) had elevated Ca2+ and vitamin D metabolites, while only the proband’s sister had nephrolithiasis. The proband became symptomatic during pregnancy, suggesting that these mutations may be associated with a variable phenotype or incomplete penetrance, supporting the view that pregnancy can unmask an otherwise ‘silent genetic disorder’. Hypercalcaemia due to CYP24A1 mutations in pregnancy may be associated with late onset hypertension/pre-eclampsia and warrants further study to determine causation. Accurate variant classification is critical as interpretation impacts directly on both patient and family care/management. Hypercalcaemia due to CYP24A1 mutations in pregnancy has also been associated with adverse maternal and baby outcomes in many of the reported cases and necessitates aggressive management. Our case highlights the difficulties associated with the investigation and management of hypercalcaemia in pregnancy and the importance of vitamin D profiling and genetic testing to investigate kindred of patients with known CYP24A1 mutations. Of importance, for positive mother and baby outcomes, all laboratory systems should as a rule and not as an exception have the appropriate digital functionality to deal with RIs in pregnancy. Identifying even a mild elevation in Ca2+ early in the gestation period can prevent or lessen the complications associated with hypercalcaemia for mother and baby.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
T P G is supported by a Hardiman Scholarship from the College of Medicine, Nursing and Health Science, National University of Ireland, Galway and a bursary from the Irish Endocrine Society/Royal College of Physicians of Ireland. J A S is supported by Kidney Research UK and the Northern Counties Kidney Research Fund.
Ethical approval
Ethical approval was granted by the Clinical Research Ethics Committee, Galway University Hospitals (Reference No – C.A. 1927; approval date 13-02-2018).
Guarantor
P M O S.
Author contribution statement
T P G, J A S, M B and P M O S performed study design. T P G, C J, J A S, M B and P M O S conducted the study. T P G, C J, S A, L M B, D T O K, D B, M N I, M C D, J E G, J J M, T O B, J A S, M B and P M O S performed data collection. T P G, C J and P M O S performed data analysis. T P G, C J, S A, L M B, D T O K, D B, M N I, M C D, J E G, J J M, T O B, J A S, M B and P M O S performed data interpretation. T P G, C J and P M O S drafted the manuscript. D T O K, D B, M N I and J A S revised the manuscript content. T P G, C J, S A, L M B, D T O K, D B, M N I, M C D, J E G, J J M, T O B, J A S, M B and P M O S approved the final version of the manuscript. P M O S takes responsibility for the integrity of the data analysis.
Acknowledgements
The authors wish to express their gratitude to the family who made this study possible. The authors wish to extend a special thanks to the scientific, nursing and medical staff at the Centre for Endocrinology, Diabetes and Metabolism, the Department of Clinical Biochemistry, Saolta University Health Care Group (SUHCG), Galway University Hospitals & Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom. The authors would like to acknowledge Jonathan C.Y. Tang & Professor William D. Fraser (Bioanalytical Facility (BAF), Norwich Medical School, University of East Anglia) for providing the Vitamin D metabolite profiling results.
References
- 1.Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence-based review. Journal of the American Board of Family Medicine 2009. 22 698–706. ( 10.3122/jabfm.2009.06.090037) [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF, Schnoes HK. Metabolism and mechanism of action of vitamin D. Annual Review of Biochemistry 1976. 45 631–666. ( 10.1146/annurev.bi.45.070176.003215) [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D: a d-lightful solution for health. Journal of Investigative Medicine 2011. 59 872–880. ( 10.2310/JIM.0b013e318214ea2d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilz S, Marz W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, Grant WB, Pludowski P, Hiligsmann M, Trummer C, et al. Rationale and plan for vitamin D food fortification: a review and guidance paper. Frontiers in Endocrinology 2018. 9 373 ( 10.3389/fendo.2018.00373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyton AC, Hall JE. Textbook of Medical Physiology, 10th ed. Philadelphia, PA, USA: Saunders Elsevier. [Google Scholar]
- 6.Tuckey RC, Cheng CYS, Slominski AT. The serum vitamin D metabolome: what we know and what is still to discover. Journal of Steroid Biochemistry and Molecular Biology 2019. 186 4–21. ( 10.1016/j.jsbmb.2018.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle D. Vitamin D: production, metabolism, and mechanisms of action. In Endotext. Eds Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, et al. South Dartmouth, MA, USA: MDText.com Inc., 2000. (available at: https://www.ncbi.nlm.nih.gov/books/NBK278935/) [PubMed] [Google Scholar]
- 8.Sayers J, Hynes AM, Srivastava S, Dowen F, Quinton R, Datta HK, Sayer JA. Successful treatment of hypercalcaemia associated with a CYP24A1 mutation with fluconazole. Clinical Kidney Journal 2015. 8 453–455. ( 10.1093/ckj/sfv028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colussi G, Ganon L, Penco S, De Ferrari ME, Ravera F, Querques M, Primignani P, Holtzman EJ, Dinour D. Chronic hypercalcaemia from inactivating mutations of vitamin D 24-hydroxylase (CYP24A1): implications for mineral metabolism changes in chronic renal failure. Nephrology, Dialysis, Transplantation 2014. 29 636–643. ( 10.1093/ndt/gft460) [DOI] [PubMed] [Google Scholar]
- 10.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Broking E, Fehrenbach H, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. New England Journal of Medicine 2011. 365 410–421. ( 10.1056/NEJMoa1103864) [DOI] [PubMed] [Google Scholar]
- 11.Shah AD, Hsiao EC, O'Donnell B, Salmeen K, Nussbaum R, Krebs M, Baumgartner-Parzer S, Kaufmann M, Jones G, Bikle DD, et al. Maternal hypercalcemia due to failure of 1,25-dihydroxyvitamin-D3 catabolism in a patient With CYP24A1 mutations. Journal of Clinical Endocrinology and Metabolism 2015. 100 2832–2836. ( 10.1210/jc.2015-1973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine 2009. 150 604–612. ( 10.7326/0003-4819-150-9-200905050-00006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin TP, Wall D, Blake L, Griffin DG, Robinson S, Bell M, Mulkerrin EC, O'Shea PM. Higher risk of vitamin D insufficiency/deficiency for rural than urban dwellers. Journal of Steroid Biochemistry and Molecular Biology 2020. 197 105547 ( 10.1016/j.jsbmb.2019.105547) [DOI] [PubMed] [Google Scholar]
- 14.Griffin TP, Wall D, Blake L, Griffin DG, Robinson SM, Bell M, Mulkerrin EC, O'Shea PM. Vitamin D status of adults in the community, in outpatient clinics, in hospital and in nursing homes in the West of Ireland. Journals of Gerontology: Series A, Biological Sciences and Medical Sciences 2020. [epub]. ( 10.1093/gerona/glaa010) [DOI] [PubMed] [Google Scholar]
- 15.Tang JCY, Nicholls H, Piec I, Washbourne CJ, Dutton JJ, Jackson S, Greeves J, Fraser WD. Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method. Journal of Nutritional Biochemistry 2017. 46 21–29. ( 10.1016/j.jnutbio.2017.04.005) [DOI] [PubMed] [Google Scholar]
- 16.Molin A, Baudoin R, Kaufmann M, Souberbielle JC, Ryckewaert A, Vantyghem MC, Eckart P, Bacchetta J, Deschenes G, Kesler-Roussey G, et al. CYP24A1 mutations in a cohort of hypercalcemic patients: evidence for a recessive trait. Journal of Clinical Endocrinology and Metabolism 2015. 100 E1343–E1352. ( 10.1210/jc.2014-4387) [DOI] [PubMed] [Google Scholar]
- 17.Kleinberger J, Maloney KA, Pollin TI, Jeng LJ. An openly available online tool for implementing the ACMG/AMP standards and guidelines for the interpretation of sequence variants. Genetics in Medicine 2016. 18 1165 ( 10.1038/gim.2016.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine 2015. 17 405–424. ( 10.1038/gim.2015.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellard S, Baple E, Callaway A, Berry I, Forrester N, Turnbull C, Owens M, Eccles D, Abbs S, Scott R, et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2020, Vol 2020. London, UK: Association for Clinical Genomic Science, 2020. (available at: https://www.acgs.uk.com/quality/best-practice-guidelines/#VariantGuidelines) [Google Scholar]
- 20.Dinour D, Davidovits M, Aviner S, Ganon L, Michael L, Modan-Moses D, Vered I, Bibi H, Frishberg Y, Holtzman EJ. Maternal and infantile hypercalcemia caused by vitamin-D-hydroxylase mutations and vitamin D intake. Pediatric Nephrology 2015. 30 145–152. ( 10.1007/s00467-014-2889-1) [DOI] [PubMed] [Google Scholar]
- 21.McBride L, Crosthwaite A, Houlihan C, Stark Z, Rodda C. Rare cause of maternal and neonatal hypercalcaemia. Journal of Paediatrics and Child Health 2019. 55 232–235. ( 10.1111/jpc.14219) [DOI] [PubMed] [Google Scholar]
- 22.McBride L, Houlihan C, Quinlan C, Messazos B, Stark Z, Crosthwaite A. Outcomes following treatment of maternal hypercalcemia due to CYP24A1 pathogenic variants. Kidney International Reports 2019. 4 888–892. ( 10.1016/j.ekir.2019.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods GN, Saitman A, Gao H, Clarke NJ, Fitzgerald RL, Chi NW. A young woman with recurrent gestational hypercalcemia and acute pancreatitis caused by CYP24A1 deficiency. Journal of Bone and Mineral Research 2016. 31 1841–1844. ( 10.1002/jbmr.2859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedberg F, Pilo C, Wikner J, Torring O, Calissendorff J. Three sisters with heterozygous gene variants of CYP24A1: maternal hypercalcemia, new-onset hypertension, and neonatal hypoglycemia. Journal of the Endocrine Society 2019. 3 387–396. ( 10.1210/js.2018-00337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macdonald C, Upton T, Hunt P, Phillips I, Kaufmann M, Florkowski C, Soule S, Jones G. Vitamin D supplementation in pregnancy: a word of caution. Familial hypercalcaemia due to disordered vitamin D metabolism. Annals of Clinical Biochemistry 2020. 57 186–191. ( 10.1177/0004563219897691) [DOI] [PubMed] [Google Scholar]
- 26.Gertner JM, Coustan DR, Kliger AS, Mallette LE, Ravin N, Broadus AE. Pregnancy as state of physiologic absorptive hypercalciuria. American Journal of Medicine 1986. 81 451–456. ( 10.1016/0002-9343(86)90298-6) [DOI] [PubMed] [Google Scholar]
- 27.Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinology and Metabolism Clinics of North America 2011. 40 795–826. ( 10.1016/j.ecl.2011.08.002) [DOI] [PubMed] [Google Scholar]
- 28.Cappellani D, Brancatella A, Kaufmann M, Minucci A, Vignali E, Canale D, De Paolis E, Capoluongo E, Cetani F, Jones G, et al. Hereditary hypercalcemia caused by a homozygous pathogenic variant in the CYP24A1 gene: a case report and review of the literature. Case Reports in Endocrinology 2019. 2019 4982621 ( 10.1155/2019/4982621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castanet M, Mallet E, Kottler ML. Lightwood syndrome revisited with a novel mutation in CYP24 and vitamin D supplement recommendations. Journal of Pediatrics 2013. 163 1208–1210. ( 10.1016/j.jpeds.2013.04.056) [DOI] [PubMed] [Google Scholar]
- 30.Minisola S, Pepe J, Piemonte S, Cipriani C. The diagnosis and management of hypercalcaemia. BMJ 2015. 350 h2723 ( 10.1136/bmj.h2723) [DOI] [PubMed] [Google Scholar]
- 31.Sadro CT, Dubinsky TJ. CT in pregnancy: risks and benefits. Applied Radiology 2013. 42 6–16. [Google Scholar]
- 32.Mirrakhimov AE. Hypercalcemia of malignancy: an update on pathogenesis and management. North American Journal of Medical Sciences 2015. 7 483–493. ( 10.4103/1947-2714.170600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin TP, Murray H, Blake L, Griffin DG, Bell M, Mulkerrin E, O'Shea PM. Normocalcemia in the face of marked hypervitaminosis D: the utility of vitamin D metabolite profiling. Journal of Applied Laboratory Medicine 2019. 4 264–269. ( 10.1373/jalm.2018.026849) [DOI] [PubMed] [Google Scholar]
- 34.Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Therapeutics and Clinical Risk Management 2015. 11 1779–1788. ( 10.2147/TCRM.S83681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rey E, Jacob CE, Koolian M, Morin F. Hypercalcemia in pregnancy – a multifaceted challenge: case reports and literature review. Clinical Case Reports 2016. 4 1001–1008. ( 10.1002/ccr3.646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horjus C, Groot I, Telting D, van Setten P, van Sorge A, Kovacs CS, Hermus A, de Boer H. Cinacalcet for hyperparathyroidism in pregnancy and puerperium. Journal of Pediatric Endocrinology and Metabolism 2009. 22 741–749. ( 10.1515/jpem.2009.22.8.741) [DOI] [PubMed] [Google Scholar]
- 37.Nadarasa K, Bailey M, Chahal H, Raja O, Bhat R, Gayle C, Grossman AB, Druce MR. The use of Cinacalcet in pregnancy to treat a complex case of parathyroid carcinoma. Endocrinology, Diabetes and Metabolism Case Reports 2014. 2014 140056 ( 10.1530/EDM-14-0056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stathopoulos IP, Liakou CG, Katsalira A, Trovas G, Lyritis GG, Papaioannou NA, Tournis S. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones 2011. 10 280–291. ( 10.14310/horm.2002.1319) [DOI] [PubMed] [Google Scholar]
- 39.Djokanovic N, Klieger-Grossmann C, Koren G. Does treatment with bisphosphonates endanger the human pregnancy? JOGC 2008. 30 1146–1148. ( 10.1016/S1701-2163(16)34026-9) [DOI] [PubMed] [Google Scholar]
- 40.Levy S, Fayez I, Taguchi N, Han JY, Aiello J, Matsui D, Moretti M, Koren G, Ito S. Pregnancy outcome following in utero exposure to bisphosphonates. Bone 2009. 44 428–430. ( 10.1016/j.bone.2008.11.001) [DOI] [PubMed] [Google Scholar]
- 41.Ornoy A, Wajnberg R, Diav-Citrin O. The outcome of pregnancy following pre-pregnancy or early pregnancy alendronate treatment. Reproductive Toxicology 2006. 22 578–579. ( 10.1016/j.reprotox.2006.05.009) [DOI] [PubMed] [Google Scholar]
- 42.Losada I, Sartori L, Di Gianantonio E, Zen M, Clementi M, Doria A. Bisphosphonates in patients with autoimmune rheumatic diseases: can they be used in women of childbearing age? Autoimmunity Reviews 2010. 9 547–552. ( 10.1016/j.autrev.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 43.Hawkes CP, Li D, Hakonarson H, Meyers KE, Thummel KE, Levine MA. CYP3A4 induction by rifampin: an alternative pathway for vitamin D inactivation in patients With CYP24A1 mutations. Journal of Clinical Endocrinology and Metabolism 2017. 102 1440–1446. ( 10.1210/jc.2016-4048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. Journal of Clinical Endocrinology and Metabolism 1992. 75 1446–1452. ( 10.1210/jcem.75.6.1464646) [DOI] [PubMed] [Google Scholar]
- 45.Nguyen M, Boutignon H, Mallet E, Linglart A, Guillozo H, Jehan F, Garabedian M. Infantile hypercalcemia and hypercalciuria: new insights into a vitamin D-dependent mechanism and response to ketoconazole treatment. Journal of Pediatrics 2010. 157 296–302. ( 10.1016/j.jpeds.2010.02.025) [DOI] [PubMed] [Google Scholar]
- 46.Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y, Foreman JW, Chelminski PR, Kumar R. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. Journal of Clinical Endocrinology and Metabolism 2012. 97 E423–E427. ( 10.1210/jc.2011-1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayers J, Hynes AM, Srivastava S, Dowen F, Quinton R, Datta HK, Sayer JA. Successful treatment of hypercalcaemia associated with a CYP24A1 mutation with fluconazole. Clinical Kidney Journal 2015. 8 453–455. ( 10.1093/ckj/sfv028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bothamley G. Drug treatment for tuberculosis during pregnancy: safety considerations. Drug Safety 2001. 24 553–565. ( 10.2165/00002018-200124070-00006) [DOI] [PubMed] [Google Scholar]
- 49.Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O, Charlier C. Antifungal drugs during pregnancy: an updated review. Journal of Antimicrobial Chemotherapy 2015. 70 14–22. ( 10.1093/jac/dku355) [DOI] [PubMed] [Google Scholar]
- 50.Griffin TP, Bell M, Robinson S, Mulkerrin E, O’Shea PM. Vitamin D and vitamin D deficiency in Ireland – a call to action. Update Endocrinology and Diabetology 2017. 37–40. (available at: https://www.medicalindependent.ie/update-endocrinology-diabetology-2017/) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a